Abstract
Time-resolved characterization of T7 RNA polymerase pausing and terminating at a class II termination site has been carried out using site-specifically tethered chemical nucleases. The data indicate that T7RNAP normally moves uniformly down the template as a rigid body. However, at the class II site this movement is interrupted, and the leading edge of the polymerase moves further along the DNA than the trailing edge. This discontinuous movement may persist until it can no longer be accommodated by conformational changes in the elongation complex, at which point the polymerase can either pause or terminate. Termination, but not pausing, is abrogated by introduction of a disulfide bond between the polymerase fingers and thumb subdomains. The introduced cysteines disrupt a thumb–fingers salt-bridge and, under reducing conditions, this mutant enzyme shows reduced processivity coincident with extension of the RNA to 5 nt. These observations suggest that termination requires that the thumb and fingers subdomains move apart, in a reversal of a conformational change important for initially forming a stable transcription complex.
Keywords: RNA polymerase/T7 RNA polymerase/transcription initiation/transcription termination
Introduction
Transcription is controlled not only at the point of initiation, but also during elongation, when signals encoded in the DNA direct the polymerase to pause or terminate. For T7 RNA polymerase—the most well-studied member of the single-subunit class of RNAPs—two distinct pause or termination signals have been characterized. One of these, the class I signal, is comprised of a U-rich element immediately downstream of a sequence which can form a stable G–C-rich hairpin when transcribed into RNA (Macdonald et al., 1994; Hartvig and Christiansen, 1996). It is similar to the intrinsic (rho-independent) terminators of Escherichia coli RNA polymerase. In fact, T7RNAP will terminate at intrinsic E.coli RNAP terminators (Jeng et al., 1990, 1992) and termination mechanisms at such terminators may be similar for both single- and multi-subunit RNAPs. T7RNAP termination at class I sites appears to depend on formation of RNA secondary structure, inasmuch as termination at these sites occurs even if the non-template DNA strand is removed, but is eliminated by incorporation of base-pair destabilizing inosine monophosphate (IMP) into the RNA (Hartvig and Christiansen, 1996).
The other T7RNAP terminator has been dubbed the class II site (Macdonald et al., 1994). It is comprised of a conserved 8 bp sequence (PyATCTGTT in the non-template strand; He et al., 1998; Lyakhov et al., 1998). Pausing and termination usually occur 7–8 bp downstream of this element. A class II site is found at the junction of the DNA concatemers produced during T7 genome replication, and it has been suggested that pausing or termination of T7RNAP at this site is important for processing and maturation of the phage DNA (Lyakhov et al., 1997; Zhang and Studier, 1997). The class II element does not display any obvious RNA secondary structure and incorporation of IMP into the RNA does not affect termination at this element (Hartvig and Christiansen, 1996), although removal of upstream non-template strand DNA reduces termination (He et al., 1998). For this reason, it has been proposed that pausing and termination at the class II site are DNA mediated (Hartvig and Christiansen, 1996), perhaps involving specific interactions between the RNAP and the conserved DNA sequence element (He et al., 1998). Studies have also revealed that the size of the transcription bubble contracts at the class II site (Song and Kang, 2001), and it has been suggested that termination at this site might involve a reversal of the conformational changes undergone during transcription initiation (Lyakhov et al., 1998; Song and Kang, 2001). However, no direct evidence for a conformational change in the polymerase during termination has been obtained, and the mechanism of pausing and termination at the class II site remains obscure.
To better understand this process we introduced cysteines at different positions into a polymerase from which most of the endogenous cysteines had been eliminated (Mukherjee et al., 2002). A chemical nuclease [iron (S)-1-(p-bromoacetamidobenzyl)EDTA or Fe-BABE; Greiner et al., 1997] was conjugated to the introduced cysteines and elongation complexes halted by NTP limitation, or paused at a class II terminator, were formed with the conjugated enzymes. Addition of peroxide to these complexes led to hydroxyl radical formation and nucleic acid cleavage in the vicinity of the conjugate, allowing the disposition of different parts of the polymerase relative to the DNA to be assessed. The cleavage data indicate that the elongation complex normally moves uniformly as a rigid body along the DNA, but at the class II site this uniform movement is disrupted and the leading (downstream) elements of the polymerase move further downstream than the trailing (upstream) elements. Introduction of a disulfide crosslink between two mobile domains of the polymerase abrogates termination, but not pausing, at the class II site. Together with other data these observations suggest a termination mechanism in which an interaction between the class II site and upstream elements of the RNAP limits movement of the polymerase even as transcript extension persists, resulting in DNA scrunching or conformational changes in the polymerase to accommodate the growing RNA. When this process reaches its steric limits, the polymerase pauses, and any further transcript extension drives a conformational change in the enzyme which results in termination.
Results
Suitability of Fe-BABE for probing a transient complex
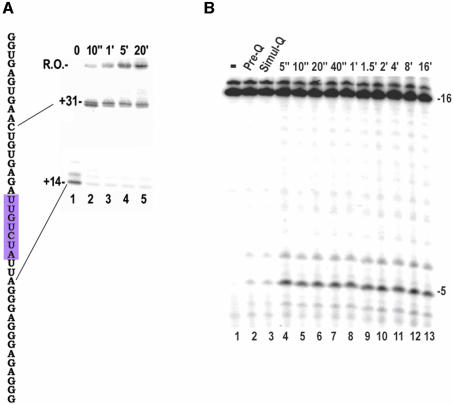
The paused/terminating elongation complex (EC) that forms at the class II site has a limited lifetime, so it was first necessary to determine if cleavage by the hydroxyl radicals generated at the iron center in the Fe-BABE conjugate would be fast enough to detect this complex. Figure 1A shows the sequence of the transcript made on the template used in these studies and a kinetic analysis of termination and pausing on this template. The conserved element of the class II site extends from +16 to +23. Transcription on this template can be halted at +14 by omission of UTP and CTP (Figure 1A, gel lane 1). Upon addition of UTP and CTP the transcript is rapidly extended to +30 where most of the complexes pause (lane 2). A minute after initiation of the chase most of the +30 complex has either been extended to +31 or escaped to form runoff transcript (lane 3). Most of the complexes that halt at +31 terminate there, as evidenced by the fact that these transcripts are not extended over the next 20 min (lanes 4 and 5) and are released into solution as assessed by ultrafiltration (not shown). The paused/terminating complexes at +30/+31 therefore appear to have lifetimes in the order of half a minute. To assess whether Fe-BABE could be used to probe these complexes we evaluated the amount of transcript cleavage in reactions quenched before (Figure 1B, lane 2), simultaneous with (Figure 1B, lane 3), or varying amounts of time after (lanes 4–13), addition of H2O2 to halted ECs formed with a polymerase containing Fe-BABE conjugated to residue 388. A conjugate at this position cleaves the transcript 5 nt upstream of the RNA 3′-end (Figure 1B; Mukherjee et al., 2002). The quenching agent is seen to reduce cleavage by 90% if added before or simultaneous with the H2O2. The amount of cleavage obtained in reactions quenched 5 s after initiation of cleavage is identical to that seen if the reaction is allowed to proceed for 16 min. Thus, Fe-BABE cleavage appears to have the time resolution required to characterize transient complexes that have half-lives of ≥5 s.
Fig. 1. (A) Left: transcript sequence of the 66 bp template used in these studies (the sequence from –1 to –17 is that of a consensus T7 promoter). The invariant class II element is highlighted in magenta. Right: gel analysis of transcription on this template. Lane 1: GTP, ATP and [α-32P]GTP only added; transcription halts at +14, misincorporation allows some transcription to +15. Lanes 2–5: UTP+CTP chase of reaction from lane 1 for 10 s to 20 min, as indicated. R.O.: runoff. (B) Kinetics of Fe-BABE transcript cleavage. Halted ECs containing a 16 nt transcript labeled with [α-32P]CMP at its 3′-end were formed with T7RNAP conjugated with Fe-BABE at residue 388 (lane 1). Transcript cleavage was induced by addition of H2O2 and ascorbate to generate hydroxyl radicals either after (lane 2), simultaneous with (lane 3), or varying times before (5 s to 16 min as indicated), addition of quenching buffer.
Cleavage patterns in paused/terminating complexes
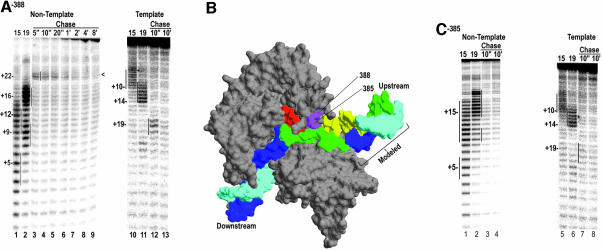
Figure 2A shows the cleavage patterns obtained with an EC halted at either +15 (lane 1) or +19 (lane 2), and bearing Fe-BABE conjugated to residue 388. In the +15 complex, cleavage extends from +3 to +10 on the template strand (lane 10), and on the non-template strand two centers of cleavage are seen centered on +12 and +5 (lane 1). Cleavage in the +19 complex is similar, except that cleavages are shifted downstream by 4 nt (lanes 2 and 11). These cleavage positions are consistent with the crystal structures of a T7RNAP EC (Figure 2B; Tahirov et al., 2002; Yin and Steitz, 2002). Lanes 3–9 show the time course of cleavage of the non-template strand following chasing of complexes halted at +14. A single weak site of cleavage around +22 is apparent 5 s after initiation of the chase (lane 3), and this decays over time until, by 8 min (lane 9), no cleavage can be detected. On the template strand, cleavage around +19 is detected 10 s after initiation of the chase (lane 12), and is almost undetectable 10 min later (lane 13). We attribute the +22 and +19 centered cleavages in the 10 s chase reaction to paused/terminating complexes because their appearance and decay track the appearance and disappearance or release of the 30/31 nt transcripts in assays like those shown in Figure 1A. In addition we also examined cleavage by a conjugate to residue 385 (Figure 2C). This residue is directly adjacent to 388 on the same side of helix N of the thumb subdomain, and conjugates at 388 and 385 exhibit similar cleavage patterns in the +15 and +19 complexes (Figure 2C, lanes 1, 2, 5 and 6). However, we previously showed that mutation of aa 385 reduces pausing and termination at a class II site to ∼10% of wild-type levels (Brieba et al., 2001), so we expect to see little or no cleavage corresponding to formation of a paused or terminating complex with this conjugate. The undetectable (lanes 3 and 4), or very weak (lanes 7 and 8), cleavage in the chase reactions with the 385 conjugate is consistent with this expectation, and reinforces the conclusion that the cleavage detected with the 388 conjugate is due to paused or terminating complexes. It may be noted that, even with the 388 conjugate, the cleavage seen in the chased complexes is weaker than in the complexes halted at +15 or +19. At least two factors may account for this. First, the 388 mutation has a partial termination defect: termination at the class II site for a D388A mutant is ∼40% of the wild-type level (Brieba et al., 2001). Second, even for the wild-type enzyme, the pause/termination site is expected to be only partially occupied by a polymerase since, at any given time after the chase, a fraction of the complexes will have already moved past this site or will have terminated and released the transcript (Figure 1A).
Fig. 2. (A) Non-template (lanes 1–9) or template (lanes 10–13) strand cleavage by Fe-BABE conjugated to aa 388 in ECs halted at +15 (lanes 1 and 10), or +19 (lanes 2 and 11), or chased from +14 with UTP+CTP for the indicated times. Cleavage sites were mapped by reference to G+A ladders prepared with the identical DNAs and are highlighted by vertical lines. (B) Structure of the T7RNAP EC (Yin and Steitz, 2002; pdb:1msw) with the template strand in cyan, the non-template strand in blue, and the RNA in red. Residues 388 and 385 are in magenta and regions of the template and non-template strands cut by the 388 conjugate (in the +15 and +19 halted complexes) are colored yellow and green, respectively. Note that the DNA labeled as ‘Modeled’ was added onto the crystal structure by extending the upstream DNA. (C) As in (A), but for complexes with the conjugate at residue 385.
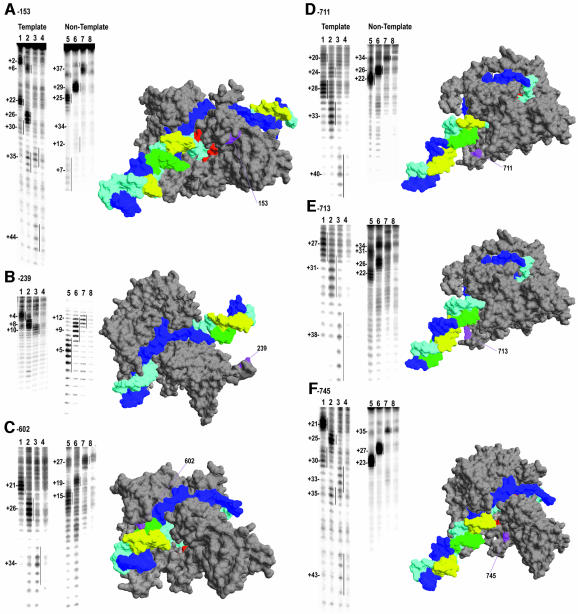
For the latter reason, only conjugates which gave strong cleavage in a halted EC also gave a reliable signal in the pausing/termination reaction. Of 11 single-site Cys substitutions constructed previously (Mukherjee et al., 2002), only four (at residues 153, 239, 388 and 745) gave reliable cleavage signals in the paused/terminating complexes when conjugated with Fe-BABE. Another three Cys substitutions (at residues 602, 711 and 713) were therefore constructed. Figure 3 presents representative cleavage data for all of these conjugates on both the template (lanes 1–4) and non-template (lanes 5–8) strands, for complexes halted at +15 (lanes 1 and 5) or +19 (lanes 2 and 6) and at 10 s (lanes 3 and 7) or 10 min (lanes 4 and 8) after being chased from +14. The cleavage patterns in the +15 complex are all consistent with the T7RNAP EC crystal structure and with the determinants of Fe-BABE cleavage observed previously, which indicated that accessibility to the hydroxyl radical source determines whether cleavage is observed, while proximity determines the intensity of cleavage (Mukherjee et al., 2002; Mukherjee and Sousa, 2003). For example, in the +15 complex, the 153 conjugate shows strong cleavage around +22 and +25 on the template and non-template strands, respectively (Figure 3A, lanes 1 and 5), corresponding to the DNA regions which are both accessible and nearest to hydroxyl radicals generated at 153 (see RNAP structure in Figure 3A). However, this strong cleavage is bracketed by two weaker sites centered at +30/+2 and +34/+6 on the template and non-template strands, respectively, corresponding to the downstream and upstream DNA elements which are accessible to, but more distant from, diffusible hydroxyl radicals generated at 153 (Figure 3A). The cleavage patterns of the +19 complex are very similar to those of the +15 complex, except that the former are all shifted downstream by 4 nt relative to the latter, as was also seen for the 385 and 388 conjugates (Figure 2). However, cleavage patterns in the 10 s chase complexes (lanes 3) do not all shift in a concerted manner. Cleavage positions of conjugates (153, 602, 711, 713, 745) which cleave downstream of the RNA 3′-end are seen to shift by 7–9 nt relative to cleavage in the +19 complex, while upstream cleavage by 239 (Figure 3B) shifts by only 2–3 nt. Upstream cleavage by 388/385 was seen to shift by an intermediate amount—5–6 nt—between the +19 and chased complexes (Figure 2). Thus, the shifts in cleavage position between +15 and +19 indicate that the RNAP translocates as a rigid body between these sites, with upstream and downstream regions of the polymerase moving in concert along the DNA by an amount equal to the length of the RNA extension. However, upon encounter of the class II site, this concerted movement is disrupted and the downstream regions of the polymerase appear to move further along the DNA than the upstream regions, and all of the cleavage positions shift by less than the 11 nt expected for extension of the RNA from +19 to the predominant pause site at +30.
Fig. 3. As in Figure 2A, but for conjugates at residues 153 (A), 239 (B), 602 (C), 711 (D), 713 (E) and 745 (F). Conjugate positions are labeled and highlighted in magenta on the polymerase structures, and cleavage positions (for the +15 and +19 complexes) are in yellow and green for the template and non-template strand, respectively. Note that, with the exception of the structure in (C), DNA extensions have been modeled onto the EC structure to allow display of cleavage positions lying up- or downstream of the visible DNA in the EC crystal structure.
An extended, back-tracked DNase I footprint in the paused complex
The Fe-BABE cleavage data suggest that the EC paused at the class II site should cover a larger area of DNA than a halted complex, as well as being back-tracked by ∼3 nt. To evaluate this we used DNase I footprinting. Since the pause site is only transiently occupied, we first optimized the duration of the +30 pause by varying chase time and [CTP]. From this analysis we estimated that if complexes halted at +14 were chased for 10 s at 10 µM CTP, followed by a 10 s DNase I digestion, then ∼60% of the DNAs would exhibit a complex paused at +30 for the duration of the digest. Complexes halted at +15 and +19 were similarly treated with a 10 s DNase I digest. Complexes halted at +15 exhibited strong template strand protection from ∼–2 to +18, and enhanced cleavage at –3/–4 (Figure 4B and A, lane 3), in agreement with previous DNase I footprinting of halted ECs (Shi et al., 1988). An additional 10–15 downstream bases are partially protected from DNase I, consistent with the EC crystal structure which reveal that DNA well downstream of the RNA 3′-end interacts with, but is exposed on, the surface of the RNAP (Tahirov et al., 2002; Yin and Steitz, 2002). Complexes halted at +19 exhibited a similar footprint that was shifted downstream by 4–5 nt (Figure 4B and A, lane 2). In the paused complex, a region of partial (>50%) protection is observed, extending from ∼+3 to +31, with enhanced cleavage detected around +1 (Figure 4D and A, lane 4).
Fig. 4. (A) Denaturing PAGE of the 5′-end labeled template used in these studies. Lane 1: DNase I-treated DNA alone. Lane 2: DNase I-treated DNA with T7RNAP EC halted at +19. Lane 3: DNase I-treated DNA with T7RNAP halted at +15. Lane 4: DNase I-treated DNA with T7RNAP paused at the class II site. Lane 5: DNA alone (no DNase I). (B) Superimposed scans of lanes 1 (black) and 3 (red) from (A). Horizontal line indicates a region of >80% protection. (C) Superimposed scans of lanes 1 (black) and 2 (green) from (A). Horizontal line indicates a region of >80% protection. (D) Superimposed scans of lanes 1 (black) and 4 (blue) from (A). Horizontal line indicates region of >50% protection. Arrows in B–D indicate positions of enhanced DNase I sensitivity.
Fe-BABE cleavage of RNA in the paused complex
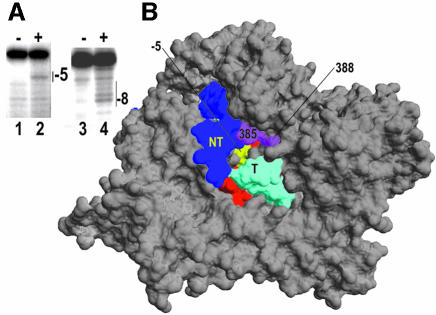
If the paused complex is back-tracked we also expect to see changes in the position of transcript cleavage. Of the conjugates used in this study only those at 388 and 385 give sufficiently strong transcript cleavage in an EC, and both of these cleave the RNA five bases away from its 3′-end (Figure 1B; Mukherjee et al., 2002). This strong and sharply delimited cleavage (which is distinct from the more typical, broader Gaussian patterns) is consistent with EC crystal structures (Tahirov et al., 2002; Yin and Steitz, 2002) which reveal that hydroxyl radicals generated at 385/388 would only be accessible to the nearby –5 base of the transcript through a pore between the thumb and N-terminal domains (Figure 5B). To compare transcript cleavage in paused versus non-paused ECs in a similar context we used a template like that in Figure 1A, but containing a U21G change which eliminates pausing and termination. Complexes halted at +31 and containing transcripts internally labeled at +19 were formed on this template with 388 conjugated RNAP by using GTP, ATP and UTP at 0.5 mM, and [α-32P]CTP and 3′-dCTP at a 1:2 ratio; while complexes paused predominately at +30 (again containing transcripts labeled at +19) were formed on the class II template with GTP, ATP and UTP at 0.5 mM, and [α-32P]CTP. Addition of peroxide and ascorbate to the halted complexes resulted in a single predominant cleavage 5 nt away from the transcript 3′-end (Figure 5A, lanes 1 and 2), while cleavage in the paused complex extended from 5 to 10 nt away from the transcript 3′-end, with the strongest cleavage site 8 nt away (Figure 5A, lanes 3 and 4). The differences between the cleavage patterns of the 388 conjugate in a paused versus halted EC are therefore consistent with back-tracking of ∼3 nt in the paused complex.
Fig. 5. (A) Halted (lanes 1 and 2) or paused (lanes 3 and 4) complexes containing transcripts internally labeled at +19 were formed on a template containing a non-functional class II element (lanes 1 and 2) or a functional class II template (lanes 3 and 4). Halting on the non-functional class II template was effected by incorporation of 3′-dCMP at +31. Peroxide and ascorbate were added to induce cleavage by the conjugate at residue 388 (lanes 2 and 4, labeled ‘+’). The transcript in the halted complex is cleaved 5 nt away from the 3′-end (lane 2), while in the paused complex it is cleaved 8 nt away. (B) Crystal structure of the T7RNAP EC with NT (non-template) strand, T (template) strand and residues 385, 388 colored as in Figure 2B. The –5 nt of the transcript is highlighted in yellow.
A disulfide bond between the thumb and fingers subdomains blocks termination
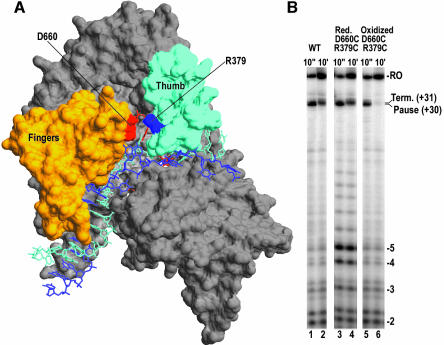
The Fe-BABE cleavage data suggested that during pausing or termination polymerase elements defining the upstream boundaries of the EC (the thumb and N-terminal domain) do not move along the DNA in concert with elements defining the more downstream boundaries (fingers), either because the DNA scrunches and/or because the polymerase changes conformation. To evaluate the importance of discontinuous movements of upstream and downstream polymerase domains, we took advantage of the existence of a salt-bridge in the EC between D660 of the fingers subdomain and R379 of the thumb subdomain (Figure 6B). Both residues were mutated to cysteines. SDS–PAGE under reducing and non-reducing conditions (not shown) revealed that dialysis of this mutant protein into buffer lacking reducing agents and containing 2 mM oxidized glutathione resulted in formation of a disulfide bond between the two introduced cysteines. Single-round transcription reactions carried out with reduced wild-type protein shows low amounts of abortive transcripts and complexes paused at +30 following a 10 s chase of complexes halted at +14 (Figure 6B, lane 1). Runoff transcripts and transcripts terminated at +31 are seen after 10 min (lane 2). Reactions carried out with reduced D660C/R379C enzyme exhibit a wild-type pattern of pausing and termination (lanes 3 and 4), but show a large increase in abortive transcription, especially of 5 nt and longer transcripts (increases in 2–4 nt abortive transcripts are due to more rounds of abortive cycling due to the increased tendency to abort RNAs ≥5 nt in length). Reactions carried out with oxidized mutant enzyme containing a disulfide bond between cysteines at 660 and 379 (lanes 5 and 6) show a normal pattern of abortive transcription, indicating that formation of the disulfide bond corrects the increased abortive transcription due to loss of the salt-bridge between the fingers and thumb domains. The intramolecularly disulfide-linked enzyme also pauses normally at +30 (lane 5), indicating that the disulfide bond does not block recognition of the class II site. However, termination at +31 is almost completely eliminated by the disulfide bond (lane 6), suggesting that termination requires that the thumb move away from the fingers. We also examined termination at a class I (hairpin) terminator (not shown), and found that both the reduced and disulfide-linked mutant enzyme showed normal termination, indicating that termination at class I terminators works through a conformationally distinct mechanism. Storage of either the mutant or wild-type proteins in non-reducing buffers for a week or more led to slow inactivation, possibly due to formation of intermolecular disulfide crosslinks. The presence of oxidized glutathione concentrations of 8 mM or more in transcription reactions with the wild-type enzyme was also inhibitory. However, the conditions used to form and assay the D660C/R379C disulfide-linked enzyme (overnight dialysis into 0 mM DTT + 2 mM oxidized glutathione) had no effect on transcription by the wild-type enzyme, indicating that the observed effects were not due to differences in assay conditions. (Supplementary data are available at The EMBO Journal Online).
Fig. 6. (A) Structure of the T7RNAP EC (pdb:1msw) with the thumb and fingers subdomains in cyan and orange, respectively. Residues D660 and R379, which form a salt-bridge between the fingers and thumb, are labeled and highlighted in red and blue respectively. (B) Denaturing PAGE of transcription reactions run by forming complexes halted at +14 and then chased with CTP and UTP for 10 s (odd numbered lanes) or 10 min (even numbered lanes) with either wild-type protein (lanes 1 and 2), D660C/R379C double mutant under reducing conditions where the 379–660 disulfide bond is absent (lanes 3 and 4), or the double mutant under conditions which induce formation of the disulfide bond (lanes 5 and 6).
Requirement for high [CTP] to drive termination
It has been previously reported that termination at class II sites is more efficient at high NTP concentrations (Song and Kang, 2001). This observation is contrary to behavior with other terminators or other RNAPs, where low NTP levels tend to increase termination, presumably because they slow elongation and thereby extend the time window during which the polymerase may terminate (Reynolds et al., 1992; McDowell et al., 1994). To investigate these [NTP] effects in greater detail we examined the consequences of varying the concentrations of individual NTPs. Lanes 1–6 of Figure 7 reveal the kinetics of pausing and termination for complexes chased from +14 with 1 mM CTP and 0.5 mM UTP. At 10 s most of the complexes have been chased to +31, though a fraction is paused at +30 (lane 1). Most of the latter have disappeared by 60 s (lane 4), but ∼50% of the 31 nt transcripts are terminated as they remained unextended even after 5 min (lane 6), and are released from the template as assessed by ultrafiltration (not shown). If the +14 halted complexes are chased with a low concentration of the NTP required for extension from +30→+31 (10 µM CTP), then most of the complexes pause at +30 (lane 7) and are slowly extended to form runoff transcript, so that only ∼15% of the 30 nt transcripts remain unextended by 5 min (lane 12; the +30 pause under these conditions follows single-exponential decay kinetics with a half-life of ∼35 s). Though the observation that low CTP levels prolong the +30 pause is expected, what is surprising is that low CTP levels eliminate pausing/termination at +31. As a consequence, runoff transcripts actually accumulate more rapidly at low [CTP] (lanes 7–12) than at high [CTP] (lanes 1–6), even though low [CTP] increases pausing at +30. It was previously suggested that such effects are due to a requirement for high NTPs levels to maintain a termination competent conformation of the RNAP which forms ∼4 bp before the polymerase actually reaches the termination site (Song and Kang, 2001). To assess this, we chased +14 halted complexes with low [UTP] and low [CTP] (lanes 13–18), and with high [CTP] and low [UTP] (lanes 19–24). If high NTP levels are required to maintain the pause/termination competent conformation then we might expect that low UTP (which is used during extension from +29→+30) would reduce pausing/termination at +30, just as low CTP reduces pausing/termination at +31. At 10 µM [UTP] we can observe, as expected, some complexes paused at +29 over the 10–40 s time window (lanes 13–15 and 18–21). However, in the reaction with both low CTP and UTP (lanes 13–18), pausing at +30 is similar to that seen in the reaction with low CTP only (lanes 7–12), although the accumulation of the 30 nt transcript at low UTP is slowed due to pausing at +29. Similarly, in the reaction with low UTP and high CTP (lanes 19–24), pausing at +30 and pausing/termination at +31 occur in a manner similar to that seen when both UTP and CTP concentrations are high (lanes 1–6), the major difference being a slower accumulation of the +31 transcript due to pausing at +29. Furthermore, the reduction in [UTP] reduces, as we would expect, the rate of accumulation of runoff transcript (compare lanes 19–24 to 1–6, or lanes 13–18 to 7–12), while reduced [CTP] increases the rate of accumulation of runoff transcript (compare lanes 13–18 with lanes 19–24). Thus the effects of low [CTP] are specific and cannot be duplicated by reducing the [NTP] used in the +29→+30 extension step.
Fig. 7. Complexes halted at +14 were chased for the indicated times either with 1 mM CTP and 0.5 mM UTP (lanes 1–6),10 µM CTP and 0.5 mM UTP (lanes 7–12), 10 µM CTP and UTP (lanes 13–18) or 1 mM CTP and 10 µM UTP (lanes 19–24). R.O., runoff.
Discussion
The paused and terminating complexes which form at the class II site are, necessarily, transient species. There is a paucity of methods for time-resolved structural characterization of such short-lived states, whether they occur during transcription or other macromolecular reactions. We previously used tethered chemical nucleases to characterize structural transitions during transcription initiation by T7RNAP (Mukherjee et al., 2002; Mukherjee and Sousa, 2003). This approach revealed movements of nucleic acids and protein conformational changes during initiation, and predicted a large scale reorganization of the transcription complex upon transition to elongation, which was subsequently confirmed and greatly amplified by crystallographic studies (Tahirov et al., 2002; Yin and Steitz, 2002). However, this previous study characterized either stable ECs or cycling (abortive initiation) reactions in which a specific complex dominated the steady-state population distribution. We have now applied this approach in a time-resolved fashion. In manual mixing experiments the method was shown to have a time resolution of ≤5 s (Figure 1B), sufficient for characterization of the paused/terminating complex which has a half-life of a few tens of s (Figure 1A). Quench-flow approaches could allow for even better time resolution.
The chemical nuclease cleavage patterns for two ECs halted at either +15 or +19 are consistent with recently described EC crystal structures (Tahirov et al., 2002; Yin and Steitz, 2002), and the difference between the cleavage positions in the two complexes (4–5 nt) equals the difference in transcript length (Figures 2 and 3). Similarly placed cleavage patterns (relative to the position of the RNA 3′-end) are also seen for an EC halted at +14 on a different template (Mukherjee et al., 2002, and unpublished observations). These observations imply that the EC normally moves uniformly down the template as a rigid body, with DNA and RNA bound in the complex as revealed in the EC crystal structures. However, at the class II site this uniform movement is disrupted. The cleavage data reveal that the leading edge of the polymerase (as defined by conjugates which cleave downstream of the RNA 3′-end) backtracks by 2–3 nt (Figure 3), while the trailing edge (defined by conjugates cleaving upstream of the RNA 3′-end) shifts upstream by 5–6 (conjugates 385 and 388; Figure 2) or 8–9 (conjugate 239; Figure 3) nt, relative to the RNA 3′-end, and as compared with ECs halted at +15 or +19. Though the transient nature of the paused/terminating complex allows for only partial DNase I protection, the extended DNase I footprint of the paused/terminating complex is in agreement with the Fe-BABE cleavage data (Figure 4). In particular, DNase I digestion provides a positive signal in the form of a DNase I hypersensitive site previously shown to occur ∼18 nt upstream of the RNA 3′-end in a halted T7RNAP EC (Shi et al., 1988). In the complex paused at +30 this hypersensitive site occurs around +1, well upstream of where it would be expected to occur if the paused complex had normal EC structure.
Significantly, conjugates at residues 385 and 388 cleave between +18 and +22 in the paused EC (Figure 2), within the invariant class II element which extends from +16 to +23. Mutation of either of these residues, especially 385, reduces recognition of the class II element (Brieba et al., 2001). While deletion/insertion mutations between aa 163–174 have also been shown to reduce class II termination, such mutations also disrupt proper RNA displacement and transcription bubble resolution in the EC (Lyakhov et al., 1997). Since proper RNA displacement is important for recognition of the class II element (He et al., 1998), these mutations may affect class II termination indirectly, by disrupting RNA displacement or proper resolution of the transcription bubble. Mutation of residues 385 or 388 does not disrupt RNA displacement (Brieba et al., 2001), and the location of these residues close to the upstream DNA in the EC (Figure 2), makes them likely candidates for amino acids directly involved in interaction with the class II element. However, if the EC paused at +30 had a normal structure, residues 385 and 388 would be positioned close to nt +24/+25 (Figure 2), too far away to contact the class II element, which extends from +23 to +16. The apparent 5–6 nt upstream shift in the position of these residues in the paused complex would place them close to nt +19/20, in the center of the class II element. This suggests a mechanism in which the residues involved in interaction with the class II element first establish an interaction with this sequence when the EC reaches ∼+25, corresponding to the point at which residues 385/388 would reach +19/20 in an EC of normal structure (Figure 8.1, +25 EC). Once this interaction is established we suggest that it persists, even as transcript extension continues, with the additional intervening DNA between the moving active site and the static upstream interaction accommodated by a combination of DNA compaction (‘scrunching’; Cheetham and Steitz, 1999) and/or conformational changes in the polymerase (Figure 8). That structural changes in the EC begin before the polymerase actually reaches the bases at which it pauses or terminates is supported by the observation that collapse of the trailing edge of the transcription bubble begins after the polymerase has passed the classed II element, but while it is still 4–7 nt upstream of the termination point (Song and Kang, 2001). An analogy may be made to T7RNAP transcription initiation, when upstream promoter interactions are maintained even as the RNA is extended from +1 to +8 (Cheetham and Steitz, 1999; Temiakov et al., 2000; Brieba and Sousa, 2001; Mukherjee et al., 2002).
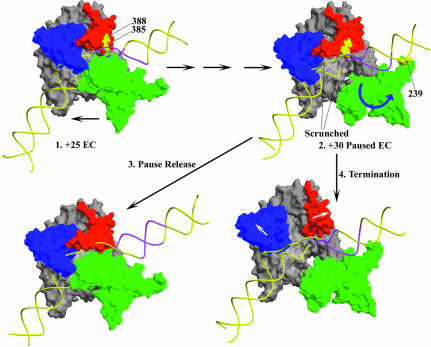
Fig. 8. A model for class II termination. 1. EC crystal structure (pdb: 1HW38; duplex DNA extensions are modeled onto the DNA from the crystal structure and the RNA is omitted for clarity). Polymerase movement is in the direction of the lower arrow. The thumb (aa 330–410, red), fingers (aa 560–680, blue) and N-terminal (aa 60–255, green) domains form a box around the DNA which, barring a conformational change, effectively precludes template release. If the EC is at +25 on the template used in these studies, then the region highlighted in magenta corresponds to the conserved class II element at +16 to +23. 2. Hypothetical +30 Paused EC structure. Interactions between 385/388 and the class II element, first established in the +25 EC, may persist as the RNA is extended to +30. The additional DNA is accommodated in the EC by a combination of ‘scrunching’ and/or conformational changes in the polymerase, and, after reaching +30, the polymerase backtracks ∼3 nt to form the stably paused complex. The N-terminal domain may shift and rotate as suggested by the blue arrow, so that an Fe-BABE conjugate at 239 would cleave upstream of the class II element. This movement of the N-terminal domain could also create room to allow the trailing edge of the transcription bubble to become duplex, as has been observed during pausing at the class II site (Song and Kang, 2001). 3. Spontaneous release of the class II element leads to escape from the pause and recovery of the normal EC structure (now shown at +31). 4. Strain in the paused complex may cause the thumb and fingers to move apart as suggested by the white arrows, so the RNAP can release DNA and terminate transcription.
And just as during initiation, there is likely to be a limit to how far the RNA can be extended before the upstream interaction must be released. On the template used here this appears to occur when the RNA has been extended to +30. At this point the polymerase pauses and appears to backtrack ∼3 nt (Figure 8.2, +30 Paused EC). If the NTP required for extension to +31 (CTP) is present at low concentrations, this pause is prolonged, but all of the polymerases which eventually escape the +30 pause form runoff transcript (Figures 7 and 8.3, Pause release). However, at high [CTP] the polymerase is more rapidly driven to extend the transcript to +31, where a large fraction of the polymerases terminate, in a step that appears to require that the thumb and fingers subdomains move apart (Figures 6, 7 and 8.4, Termination). It has previously been observed that class II termination is more efficient at high NTP concentrations, and it was suggested that this was due to a requirement for high NTP concentrations to maintain a termination-prone conformation of the enzyme (Song and Kang, 2001). While this cannot be ruled out, it appears less likely in light of the observation that reduced termination specifically requires low [CTP], and is not seen when low [UTP] (required for extension from +29 to +30) is used. We suggest that low [CTP] reduces termination because it prolongs the +30 pause, thus extending the time during which the polymerase may release the upstream interaction, while high [CTP] drives the polymerase rapidly to +31 while it is still engaged with the class II element, and where increased strain can then drive a conformational change that leads to termination.
It has been proposed that class II termination may, in some ways, be considered a ‘reversal’ of the transcription initiation process (Lyakhov et al., 1998; Song and Kang, 2001). The effect of the thumb–fingers crosslink on termination could be interpreted in this light. It should be emphasized that the two residues targeted for mutagenesis to form this crosslink (D660, R379) are at the very tips of the fingers and thumb subdomains, ∼9 Å away from the nearest DNA or RNA, so the effects of the D660C/R379C mutations are unlikely to be due to direct loss of interaction with nucleic acid. In the apoenzyme, residues 345–383 are disordered (Jeruzalmi and Steitz, 1998), so the salt-bridge between D660 and R379 is unlikely to be present, at least not stably so, until the polymerase binds DNA. Our observations reveal that loss of this potential salt-bridge leads to increased abortive transcription and reduced processivity coincident with extension of the RNA to 5 nt (Figure 6B). This observation is provocative, since steric arguments (Cheetham and Steitz, 1999; Tahirov et al., 2002; Yin and Steitz, 2002), analysis of mutant T7RNAPs (He et al., 1997; Brieba et al., 2001), and chemical nuclease cleavage (Mukherjee et al., 2002) have suggested that a conformational change in the thumb occurs coincident with extension of the RNA to ∼5 nt. It is tempting to identify establishment of the D660–R379 interaction with this transition, but this would be counter to the observation that the D660–R379 salt-bridge is seen in the crystal structure of an initiation complex with a 3 nt RNA (Cheetham and Steitz, 1999). It is possible that this salt-bridge forms early, but only becomes important for complex stability when the RNA reaches 5 nt in length. Alternatively, the presence of the salt-bridge in the 3mer initiation complex crystal structure may be an artifact, since it has been shown that the thumb’s conformation is sensitive to crystal packing contacts (Sousa et al., 1993, 1994). In any case, formation of the D660C–R379C crosslink corrects the complex instability conferred by the mutations in their oxidized forms, as well as abrogating termination at the class II site (Figure 6B). This reinforces the conclusion that the effect of these mutations reflects either loss or gain of interaction between thumb and fingers, rather than loss of direct interactions with DNA or RNA. To the extent that the establishment of the D660–R379 interaction appears to be important for stabilizing transcription complexes with ≥5 nt RNAs, while disruption of the 660–379 contact appears to be important for class II termination, one may describe termination as a reversal of one aspect of the initiation process. However, this description needs to be limited to class II sites, since the D660C/R379C mutations, in either oxidized or crosslinked forms, do not affect class I termination.
The working model of class II pausing and termination presented here (Figure 8) is still incomplete. Tyrosine 385 is the most likely candidate for a residue involved in class II element recognition, but other side-chains are almost certainly involved. Recognition of the class II element may involve the template DNA strand (He et al., 1998), but no specific side-chain:base contacts have been defined and it is unclear if this DNA element is recognized as a canonical B-form duplex, or whether some sequence-dependent altered DNA conformation also plays a role. And while the DNA sequence downstream of the class II element can be varied, termination is more efficient if this sequence is T-rich (Lyakhov et al., 1998). It therefore appears that while the conserved element is absolutely required to cause pausing, termination is enhanced if the RNA:DNA hybrid immediately downstream of this element is unstable. Finally, it was recently shown that class II termination is affected by replacing the catalytic Mg2+ with Mn2+ (Boudvillain et al., 2002). Because the latter co-factor is less stringent in its requirements for alignment of reactive groups during catalysis, it was suggested that this meant that pausing and termination involve changes in active site geometry that misalign the reactive groups. Consistent with this, we have found that an active site mutation (H784A) that enhances the extension of RNA:DNA mispairs (Huang et al., 2000), also reduces pausing and termination at the class II site (unpublished observations). The involvement of ‘active site events’ involving misalignment of reactive groups during pausing or termination is not at odds with a mechanism that proposes an primary role for more upstream interactions with the class II element. The latter may communicate an allosteric signal which results in active site reorganization. Alternatively, the misalignment of reactive groups (i.e. primarily the 3′-OH of the RNA) may be a direct consequence of DNA ‘scrunching’ or the accumulation of strain in the EC, and may be further exacerbated by A–U richness in the RNA:DNA hybrid.
Our results show that tethered chemical nucleases can provide time-resolved structural information on complexes that form transiently during a transcription reaction. They suggest that pausing and termination at the class II element begins with establishment of a sequence-specific interaction between RNAP and DNA which keeps the polymerase from moving down the template, even as transcript extension persists. This interaction is analogous to, but distinct from, the promoter interaction since it involves a different DNA sequence and a different recognition surface of the RNAP. The pausing and termination reaction then ends when the polymerase releases the class II element to form runoff transcript (analogous to promoter release) or when the complex terminates by releasing the transcript (analogous to release of the transcript during an abortive initiation event). The degree to which conformational changes during pausing and termination either recapitulate or reverse some of the conformational changes undergone during promoter-directed initiation has yet to be fully illuminated.
Materials and methods
Preparation of mutant polymerases, Fe-BABE conjugation and disulfide bond formation
Single cysteine substitutions were engineered into a T7RNAP in which seven of the 12 endogenous cysteines had been mutated to serines using PCR-mediated mutagenesis as described previously (Mukherjee et al., 2002; Mukherjee and Sousa, 2003). The D660C/R379C double mutant was prepared similarly, by introduction of one cysteine at a time into a wild-type T7RNAP background. Polymerase expression, purification and conjugation with Fe-BABE (Dojindo Laboratories) were carried out as described previously (Mukherjee et al., 2002; Mukherjee and Sousa, 2003). To form the disulfide bond in the D660C/R379 double mutant, wild-type or D660C/R379C polymerase at 5 mg/ml in storage buffer (50 mM Tris pH 8.0, 1 mM EDTA, 5 mM DTT, 0.5 M NaCl, 50% glycerol) was dialyzed at 4°C overnight into storage buffer lacking DTT and with 2 mM oxidized glutathione added. Formation of the disulfide bond was monitored by SDS–PAGE under reducing or non-reducing conditions (the enzyme with the disulfide bond ran slightly slower than the reduced enzyme). After dialysis, RNAPs were assayed immediately in standard transcription reactions except that DTT was omitted from the reaction buffer. Reactions with enzymes that had not been dialyzed into oxidizing buffer were carried out in parallel.
DNA cleavage with conjugated RNAPs
All reactions were carried out with a synthetic duplex 66 bp template whose sequence from –17 to –1 corresponds to the T7 promoter consensus, and whose +1 to +40 sequence is as given in Figure 1A. From +1 to +14 the transcript from this template incorporates only G and A, allowing halted ECs to be formed at +14 by addition of only GTP and ATP to the transcription reaction, or terminated complexes to be formed at +15 or +19 by addition of either GTP, ATP and 3′-dUTP or GTP, ATP, UTP and 3′-dCTP, respectively. ECs were formed at room temperature (r.t.) in transcription buffer (Mukherjee et al., 2002; Mukherjee and Sousa, 2003) with 3 × 10–8 M template labeled with 32P at the 5′-end of either the template or non-template strand, and with 10–7 M Fe-BABE conjugated T7RNAP and NTPs at 0.5 mM. After a 10 min incubation, heparin was added to 0.2 mg/ml, and cleavage was carried out by addition of Na ascorbate and H2O2 as described previously (Mukherjee et al., 2002; Mukherjee and Sousa, 2003) except that cleavage reactions were quenched after 5 s by addition of one reaction volume of 95% formamide, 50 mM EDTA, 0.01% xylene cyanol (stop buffer). To map cleavage by paused/terminating RNAPs, complexes halted at +14 were first formed by incubating reactions containing GTP and ATP for 5 min at r.t., followed by addition of heparin, CTP and UTP. Peroxide and ascorbate were then added 10 s to 10 min after addition of CTP and UTP, as specified in different figures. All cleavage reactions were quenched 5 s after ascorbate/peroxide addition. DNAs were resolved by electrophoresis on 15% acrylamide, 0.8% bis-acrylamide, 8 M urea, 1× Tris–Borate–EDTA and visualized on a Molecular Dynamics phosphorimager. All cleavage experiments were repeated at least three times to ensure reproducibility. Cleavage positions were mapped by reference to Maxam–Gilbert G+A ladders prepared as described (Mukherjee et al., 2002; Mukherjee and Sousa, 2003).
DNase I footprinting of halted and paused Ecs
Halted complexes were formed using DNA labeled at the 5′-end of the template strand using GTP, ATP and 3′-dUTP (+15 complex) or GTP, ATP, UTP and 3′-dCTP as described above, but with wild-type, unconjugated RNAP. After addition of heparin to 0.2 mg/ml, these complexes were treated with 0.04 u/µl DNase I for 10 s at r.t. and DNase I digestion was stopped by addition of one reaction volume of stop buffer. DNAs without added RNAP, but containing all other reaction components, were treated similarly. Paused complexes were footprinted by chasing complexes halted at +14 (GTP+ATP only) with 0.2 mg/ml heparin, 0.5 mM UTP and 10 µM CTP for 10 s, followed by DNase I treatment for 10 s. DNAs were resolved, visualized and cleavage sites mapped as described above for reactions with conjugated polymerases.
RNA cleavage with RNAP conjugated with Fe-BABE at residue 388
ECs were formed on either unlabeled 66 bp synthetic template or BglII linearized pPK5. The latter template contains a single consensus T7 promoter and generates a transcript containing only G and A from +1 to +14 and a C and U at +15 and +16, respectively. Transcription reactions were carried out for 10 min at r.t. with this template (at 5 × 10–8 M) and Fe-BABE conjugated RNAP (10–7 M) with 0.5 mM GTP, ATP, 3′-dUTP and 1% v/v 10 mCi/ml, 800 mCi/mM [α-32P]CTP to allow formation of a stably terminated EC containing a 16 nt transcript labeled to high specific activity at its 3′-end. Cleavage was initiated by addition of peroxide/ascorbate and quenched by addition of stop buffer 5 s to 16 min later, as specified in Figure 1B. Transcript cleavage reactions on the synthetic template were carried out by forming complexes halted at +14 which were then chased by addition of heparin, 0.5 mM UTP and 1% v/v 10 mCi/ml, 800 mCi/mM [α-32P]CTP. This allows the pause (30 nt) transcript, which incorporates as single C at +19 before the major pause at +30, to be labeled to very high specific activity at a single internal position at +19. Twenty s after initiation of the chase, cleavage was induced and quenched after 5 s. Cleaved transcripts were resolved and visualized as described above.
Kinetics of pausing and termination
ECs halted at +14 on the 66 bp synthetic template (at 10–7 M) were formed by incubating transcription reactions (w/RNAP at 3 × 10–7 M) for 5 min at r.t. with 0.5 mM ATP, 0.1 mM GTP and 1% v/v 10 mCi/ml, 800 mCi/mM [α-32P]GTP. Reactions were then chased by addition of heparin to 0.2 mg/ml and UTP and CTP at concentrations as indicated in individual figures. Reactions were stopped by addition of one reaction volume of stop buffer and resolved and visualized as described above.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgement
This work was supported by NIH Grant GM 52522 and Welch Grant AQ1486 (to R.S.).
References
- Boudvillain M., Schwartz,A. and Rahmouni,A.R. (2002) Limited topological alteration of the T7 RNA polymerase active center at intrinsic termination site. Biochemistry, 4, 3137–3146. [DOI] [PubMed] [Google Scholar]
- Brieba L.G. and Sousa,R. (2001) T7 promoter release mediated by DNA scrunching. EMBO J., 20, 6826–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieba L.G., Gopal,V. and Sousa,R. (2001) Scanning mutagenesis reveals roles for helix n of the bacteriophage T7 RNA polymerase thumb subdomain in transcription complex stability, pausing, and termination. J. Biol. Chem., 276, 10306–10313. [DOI] [PubMed] [Google Scholar]
- Cheetham G. and Steitz,T.A. (1999) Structure of a transcribing T7 RNA polymerase initiation complex. Science, 286, 2305–2309. [DOI] [PubMed] [Google Scholar]
- Greiner D.P., Miyake,R., Moran,J.K., Jones,A.D., Negishi,T., Ishihama,A. and Meares,C.F. (1997) Synthesis of the protein cutting reagent iron (S)-1-(p-bromoacetamidobenzyl)ethylenediaminetetra acetate and conjugation to cysteine side chains. Bioconjug. Chem., 8, 44–48. [DOI] [PubMed] [Google Scholar]
- Hartvig L. and Christiansen,J. (1996) Intrinsic termination of T7 RNA polymerase mediated by either RNA or DNA. EMBO J., 15, 4767–4774. [PMC free article] [PubMed] [Google Scholar]
- He B., Rong,M., Durbin,R.K. and McAllister,W.T. (1997) A mutant T7 RNA polymerase that is defective in RNA binding and blocked in the early stages of transcription. J. Mol. Biol., 265, 275–288. [DOI] [PubMed] [Google Scholar]
- He B., Kukarin,A., Temiakov,D., Chin-Bow,S.T., Lyakhov,D.L., Rong,M., Durbin,R.K. and McAllister,W.T. (1998) Characterization of an unusual, sequence-specific termination signal for T7 RNA polymerase. J. Biol. Chem., 273, 18802–18811. [DOI] [PubMed] [Google Scholar]
- Huang J., Brieba,L.G. and Sousa,R. (2000) Misincorporation by wild-type and mutant T7 RNA polymerases: identification of interactions that reduce misincorporation rates by stabilizing the catalytically incompetent open conformation. Biochemistry, 39, 11571–11580. [DOI] [PubMed] [Google Scholar]
- Jeng S.T., Gardner,J.F. and Gumport,R.I. (1990) Transcription termination by bacteriophage T7 RNA polymerase at rho-independent terminators. J. Biol. Chem., 265, 3823–3830. [PubMed] [Google Scholar]
- Jeng S.T., Gardner,J.F. and Gumport,R.I. (1992) Transcription termination in vitro by bacteriophage T7 RNA polymerase. The role of sequence elements within and surrounding a rho-independent transcription terminator. J. Biol. Chem., 267, 19306–19312. [PubMed] [Google Scholar]
- Jeruzalmi D. and Steitz,T.A. (1998) Structure of T7 RNA polymerase complexed to the transcriptional inhibitor T7 lysozyme. EMBO J., 17, 4101–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyakhov D.L., He,B., Zhang,X., Studier,F.W., Dunn,J.J. and McAllister,W.T. (1997) Mutant bacteriophage T7 RNA polymerases with altered termination properties. J. Mol. Biol., 269, 28–40. [DOI] [PubMed] [Google Scholar]
- Lyakhov D.L., He,B., Zhang,X., Studier,F.W., Dunn,J.J. and McAllister,W.T. (1998) Pausing and termination by bacteriophage T7 RNA polymerase. J. Mol. Biol., 280, 201–213. [DOI] [PubMed] [Google Scholar]
- Macdonald L.E., Durbin,R.K., Dunn,J.J. and McAllister,W.T. (1994) Characterization of two types of termination signal for bacteriophage T7 RNA polymerase. J. Mol. Biol., 238, 145–158. [DOI] [PubMed] [Google Scholar]
- McDowell J.C., Robert,J.W., Jin,D.J. and Gross,C. (1994) Determination of intrinsic transcription termination efficiency by RNA polymerase elongation rate. Science, 266, 822–825. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. and Sousa,R. (2003) Structural transitions mediating transcription initiation by T7 RNA polymerase. Biol. Procedures Online, 5, 78–89. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Brieba,L.G. and Sousa,R. (2002) Use of site specifically tethered chemical nucleases to study macromolecular reaction. Cell, 110, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R., Bermudez-Cruz,R.M. and Chamberlin,M.J. (1992) Parameters affecting transcription termination by Escherichia coli RNA polymerase. I. Analysis of 13 rho-independent terminators. J. Mol. Biol., 224, 31–51. [DOI] [PubMed] [Google Scholar]
- Shi Y., Gamper,H. and Hearst,J.E. (1988) Interaction of T7 RNA polymerase with DNA in an elongation complex arrested at a specific psoralen adduct site. J. Biol. Chem., 263, 527–534. [PubMed] [Google Scholar]
- Song H. and Kang,C. (2001) Sequence-specific termination by T7 RNA polymerase requires formation of paused conformation prior to the point of RNA release. Genes Cells, 6, 291–301. [DOI] [PubMed] [Google Scholar]
- Sousa R., Chung,Y.J., Rose,J. and Wang,B.C. (1993) Crystal structure of bacteriophage T7 RNA polymerase at 3.3 Å resolution. Nature, 364, 593–599. [DOI] [PubMed] [Google Scholar]
- Sousa R., Rose,J. and Wang,B.C. (1994) The thumb’s knuckle. Flexibility in the thumb subdomain of T7 RNA polymerase is revealed by the structure of a chimeric T7/T3 RNA polymerase. J. Mol. Biol., 244, 6–12. [DOI] [PubMed] [Google Scholar]
- Tahirov T.H., Temiakov,D., Anikin,M., Patlan,V., McAllister,W.T., Vassylyev,D.G. and Yokoyama,S. (2002) Structure of a T7 RNA polymerase elongation complex at 2.9 Å resolution. Nature, 420, 43–50. [DOI] [PubMed] [Google Scholar]
- Temiakov D., Mentesanas,P.E., Ma,K., Mustaev,A., Borukhov,S. and McAllister,W.T. (2000) The specificity loop of T7 RNA polymerase interacts first with the promoter and then with the elongating transcript, suggesting a mechanism for promoter clearance. Proc. Natl Acad. Sci. USA, 97, 14109–14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y.W. and Steitz,T.A. (2002) Structural basis for the transition from initiation to elongation transcription in T7 RNA polymerase. Science, 298, 1387–1395. [DOI] [PubMed] [Google Scholar]
- Zhang X. and Studier,F.W. (1997) Mechanism of inhibition of bacteriophage T7 RNA polymerase by T7 lysozyme. Sequence-specific termination by T7 RNA polymerase requires formation of paused conformation prior to the point of RNA release. J. Mol. Biol., 269, 10–27. [DOI] [PubMed] [Google Scholar]