Abstract
Background
Racial/ethnic differences in the pediatric population in insulin dynamics have been documented. Additionally, girls tend to be more insulin resistant than boys. Although the mechanism driving these differences is unclear, diet may be a contributor.
Objective(s)
The objective of this study was to evaluate the contribution of reported macronutrient intake on insulin dynamics and determine if diet composition may account for racial/ethnic and sex differences in insulin response/action.
Methods
Participants were 250 African- (n = 84), European- (n = 105), or Hispanic-American (n = 61) children 7–12 years, pubertal stage ≤3. An intravenous glucose tolerance test was used to derive the insulin sensitivity index and acute insulin response to glucose (AIRg) diet by two 24 h recalls, and body composition by dual-energy x-ray absorptiometry (DXA).
Results
Reported energy intake from fat was positively related to fasting insulin (P < 0.05) and AIRg (P = 0.05). Reported energy from carbohydrate was inversely associated with fasting insulin (P < 0.05), and reported energy from protein was inversely associated with AIRg (P < 0.05). The interaction terms between ethnicity and diet, and sex and diet were not significant for any outcome variables.
Conclusion
Dietary intake influences insulin dynamics; however, the racial/ethnic and sex differences in insulin dynamics in this population are not accounted for by macronutrient intake. Pubertal status is likely to play a role in the interaction between diet, race/ethnicity, sex and insulin dynamics. Longitudinal studies are needed to determine if the contribution of diet to insulin dynamics strengthens with reproductive maturation.
Keywords: diet, insulin sensitivity, pediatric obesity, race/ethnicity
Introduction
The dramatic rise in the prevalence of obesity among the pediatric population over the past two decades has closely paralleled the increased incidence of type 2 diabetes (T2D) (1–4). Furthermore, racial/ethnic differences exist such that inherent differences in the physiology of insulin response and action in combination with an obesogenic environment may put African-American (AA) and Hispanic-American (HA) children at greater risk for developing T2D compared with their European-American (EA) counterparts. Sex differences have also been noted with female children exhibiting more insulin resistance than male children (5). Evidence from longitudinal studies has demonstrated the pathogenic importance of low insulin sensitivity and high insulin secretion in the development of T2D (6,7). Although racial/ethnic differences in insulin dynamics suggestive of increased risk for T2D have been clearly demonstrated, the mechanisms that manifest these differences remain unclear. One of the possible factors that are likely to influence these mechanisms is diet quality. It is plausible that dietary composition adds to or exacerbates genetic or physiological differences between racial/ethnic and sex groups in regards to insulin dynamics.
Beginning with the work of Ancel Keys in the 1960s, many studies implicated dietary fat as the “villain” in health outcomes. More recent studies in adults suggest that excess carbohydrates (CHO) may intensify the insulin response and lead to more adverse metabolic outcomes than the ingestion of a high fat diet (8–11). Nevertheless, results of studies investigating the influence of dietary composition on insulin dynamics have not clearly indicated that manipulation of dietary components results in improvements in glucose metabolism (12–15). Thus, although there is theoretical support, significant evidence implicating a specific macronutrient has not been established.
In children, fewer studies have been conducted, with varied results. For example, some researchers have shown a positive relationship between dietary intake of CHO and fat in AA (16–18), but not European children (19). Further, a relationship between fat intake (but not CHO intake) and the insulin sensitivity index (SI) in EA and HA adolescents has been observed (20), but neither dietary fat nor CHO was associated with SI in a prepubertal subset of their cohort (21), implying the possibility of an effect of maturation status. More recently, Davis et al. (22) showed that increased total sugar intake was associated with lower SI among HA adolescents. Taken together, it remains unclear whether diet composition influences insulin dynamics in early pubertal children and if diet may account for differences in insulin response and action.
We have previously observed racial/ethnic as well as sex differences in insulin dynamics in this population (23). The objectives of this study were to evaluate whether dietary intake is associated with insulin dynamics across racial/ethnic and sex groups, and to determine if racial/ethnic and sex differences in insulin dynamics in this cohort could be accounted for, at least in part, by dietary intake.
Materials and methods
Subjects
A total of 250 children self-identified as AA (n = 84), EA (n = 105), or HA (n = 61) and aged 7–12 years were recruited from the Birmingham, Alabama area for an on-going study investigating racial/ethnic differences in metabolic outcomes. The children were pubertal stage ≤3, as assessed by a pediatrician according to the criteria of Marshall and Tanner (24). Children were excluded if they had prior major illness, including type 1 or type 2 diabetes, or took medication or had a condition known to influence body composition, insulin sensitivity or insulin secretion (e.g., glucocorticoid or thyroid therapy). Before participating in the study, the nature, purpose, and possible risks of the study were carefully explained to the parents and children. The children and parents provided informed assent and consent, respectively, to the protocol, which was approved by the Institutional Review Board for human subjects at the University of Alabama at Birmingham (UAB). All measurements were performed at the General Clinical Research Center (GCRC) and the Department of Nutrition Sciences at UAB between 2005 and 2008.
Protocol
Subjects completed two testing sessions. In the first session, pubertal status, body mass index (BMI), and body composition were assessed and a dietary recall was obtained. In the second session, a second dietary recall was obtained. Subjects were admitted to the GCRC in the late afternoon for an overnight visit. All participants were offered the same meal and snack foods. After 20.00 h, only water and/or non-caloric decaffeinated beverages were permitted until after the morning testing. Upon completing the overnight fast, an insulin-modified, frequently sampled intravenous glucose tolerance test (IVGTT) was performed.
Dietary intake
Dietary composition was determined by two 24 hour dietary recalls using the multiple pass method with cup and bowl sizes provided to help gauge portion sizes. Recalls were always performed in person in the presence of at least one parent, which has been validated in this population (25,26). One recall was performed at each visit (approximately 1 week apart). Prior to testing, parents were asked about the current health status of the child and to report aberrations from typical dietary intake. All recalls used in this analysis were, according to parental report, taken in the healthy state and reflected typical intake. A trained dietitian coded and entered data into the computerized Nutrition Data System for Research (NDSR), a dietary analysis program designed for the collection and analyses of 24 h dietary recalls. The mean of the individual daily intakes for each macronutrient was used in subsequent analyses.
Assessment of body composition
Children arrived at the department of Nutrition Sciences for the first testing session. Body composition (total body fat mass and non-bone lean tissue mass) was measured by dual-energy x-ray absorptiometry (DXA) using a GE Lunar Prodigy densitometer (GE LUNAR Radiation Corp., Madison, WI), as described elsewhere (23).
Intravenous glucose tolerance test (IVGTT)
Following the overnight fast, a topical anesthetic was applied to the antecubital space of both arms, and flexible intravenous catheters were placed in both arms for an IVGTT, as described elsewhere (27). Acute insulin response to glucose (AIRg), an approximation of first phase insulin secretion, was calculated as the incremental area under the curve for insulin during the first 10 minutes after glucose injection using trapezoidal methodology (28). Glucose and insulin values were entered into the MINMOD Millennium version computer program for determination of the derived insulin sensitivity index (SI).
Assay of glucose and insulin
Glucose and insulin were measured in 10 µl sera using an Ektachem DT System (Johnson and Johnson Clinical Diagnostics), as previously described (29).
Socioeconomic status (SES)
Socioeconomic status (SES) was measured with the Hollingshead 4-factor index of social class (30), which combines the educational attainment and occupational prestige for the number of working parents in the child’s family. Scores on the questionnaire range from 8 to 66, with higher scores indicating higher theoretical social status.
Statistical analyses
Racial/ethnic and sex differences in descriptive statistics were examined using ANOVA with Duncan’s post-hoc analysis. Additional analyses were performed on the total sample using AN-COVA, adjusting for relevant covariates (race/ethnicity, sex, total fat mass, total lean mass, SES, Tanner stage) to prevent potential confounding. Relevant covariates were determined by exploratory stepwise regression with P-value for inclusion into the model set at P < 0.10. Analyses evaluating racial/ethnic differences included sex as a covariate, whereas analyses evaluating sex differences included race/ethnicity as a covariate.
Multiple linear regression models (n = 250) were tested for contributions of the independent variables (total energy, energy from macronutrients) to the dependent variables (fasting insulin, SI and AIRg). All models were adjusted for pubertal stage, sex, SES, total fat and lean mass and race/ethnicity. Because SI is a significant determinant of AIRg, all analyses with AIRg as the dependent variable included SI as a covariate. The first models evaluated the effect of diet on insulin dynamic outcomes using the entire sample. In models analyzing the total sample in which ethnicity and/or sex were significant covariates, additional models, testing the effect of the interaction of ethnicity and sex (independently) with dietary variables were evaluated. Statistical significance was set at P < 0.05. Total minutes engaged in daily physical activity did not differ between boys and girls or racial/ethnic group nor was it a contributor to any dependent variable. As such, it was not included in final analyses.
To conform to the assumptions of linear regression, all statistical models were evaluated for residual normality and logarithmic transformations were performed when appropriate. All data were analyzed using SAS 9.1 software.
Results
Descriptive statistics (Table I)
Table I.
characteristics for the total sample and according to ethnicity and sex.
| Total sample | Ethnicity | Sex | ||||
|---|---|---|---|---|---|---|
| n = 250 | EA (n = 105) |
AA (n = 84) |
HA (n = 61) |
Males (n = 134) |
Females (n = 116) |
|
| Age | 9.6±0.1 | 9.7±0.1 | 9.7±0.2 | 9.3±0.2 | 9.8±0.11 | 9.4±0.12 |
| Females (%) | 53.6% | 52.5 | 55.8 | 52.6 | ||
| SES | 39.6±0.8 | 49.4±1.0a | 38.4±1.1b | 25.6±1.2c | 39.5±1.2 | 39.7±1.2 |
| Pubertal stage | 1 | 1a | 2b | 1a | 1 | 2 |
| BMI (kg/m2) | 18.5±0.2 | 17.9±0.3a | 18.4±0.3a | 19.4±0.3b | 18.5±0.2 | 18.4±0.3 |
| BMI percentile | 65.8±1.5 | 60.1±2.3a | 63.0±2.5a | 78.1±2.9b | 65.7±2.1 | 65.7±2.2 |
| Weight (kg) | 36.5±0.6 | 35.6±0.9 | 37.5±1.0 | 36.9±1.1 | 37.0±0.7 | 36.1±0.8 |
| Height (cm) | 139.8±0.6 | 140.3±1.0ab | 141.6±1.1a | 137.0±1.2b | 140.3±0.8 | 139.3±0.9 |
| Total fat mass (kg) | 8.8±0.3 | 8.2±0.5a | 8.2±0.7a | 10.6±0.7b | 8.1±0.51 | 9.5±0.52 |
| Percent fat (%) | 23.1±0.6 | 22.4±0.8a | 20.5±1.0a | 28.2±1.1b | 20.7±0.81 | 25.8±0.82 |
| % >85th percentile BMI | 27.8 | 19.8a | 28.8ab | 36b | 25.9 | 19.9 |
| Total lean mass (kg) | 25.7±0.3 | 25.4±0.5a | 27.3±0.5b | 24.3±0.7a | 26.7±0.41 | 24.7±0.52 |
| Fasting insulin (µIU/ml) | 12.4±0.4 | 10.1±0.4a | 13.1±0.6b | 13.9±1.0b | 11.5±0.51 | 13.3±0.52 |
| SI (× 10−4 min−1/(µIU/ ml) | 5.8±0.2 | 7.0±0.3a | 4.6±0.4b | 5.5±0.4b | 6.4±0.31 | 5.3±0.32 |
| AIRg (µlU/ml × 10 min) | 889.1±44.2 | 619.7±63.1a | 1 222.3±70.2b | 878.9±82.8c | 857.2±60.8 | 919.6±3.5 |
| Total energy (kcal/d) | 1 884.4±27.3 | 1 870.1±42.5 | 1 891.7±47.7 | 1 890.2±53.4 | 1 959.5±37.01 | 1 797.0±39.02 |
Superscripts indicate significant differences between racial/ethnic groups (p < 0.05).
Superscripts indicate significant differences by sex (p < 0.05).
Note: Significant differences between groups did not change when adjusted for covariates (race/ethnicity, sex, body composition, SES, and Tanner stage; AIRg model also included SI as a covariate).
EA = European American; AA = African American; HA = Hispanic American; SES = socioeconomic status; BMI = body mass index; SI = insulin sensitivity index; AIRg = acute insulin response to glucose.
There were no differences in age or weight between the racial/ethnic groups. EA had higher SI (PAA < 0.01; PHA < 0.05), lower fasting insulin (P < 0.05, AA and HA) and AIRg (PAA < 0.001; PHA < 0.01), and a higher SES compared with AA and HA (P < 0.001, AA and HA). AA were leaner (PEA < 0.05; PHA < 0.01), assessed at a higher Tanner stage (P < 0.01, EA and HA), and had a higher AIRg (PEA < 0.001; PHA < 0.01) than EA and HA. AA were taller (P < 0.05) and reported a higher SES (P < 0.001) than HA. HA had a greater adiposity than both EA and AA (P < 0.05, both). HA were more likely than EA (P < 0.05) to exceed the 85th percentile for BMI.
There were no sex differences in terms of SES, weight, height, or AIRg. However, girls were younger (P < 0.05), but assessed at a higher Tanner stage (P < 0.05). In addition, females had greater adiposity (P < 0.05), higher fasting insulin (P < 0.05), less lean mass (P < 0.01) and lower SI (P < 0.05) than boys. There was no difference in the percentage of boys and girls exceeding the 85th percentile for BMI.
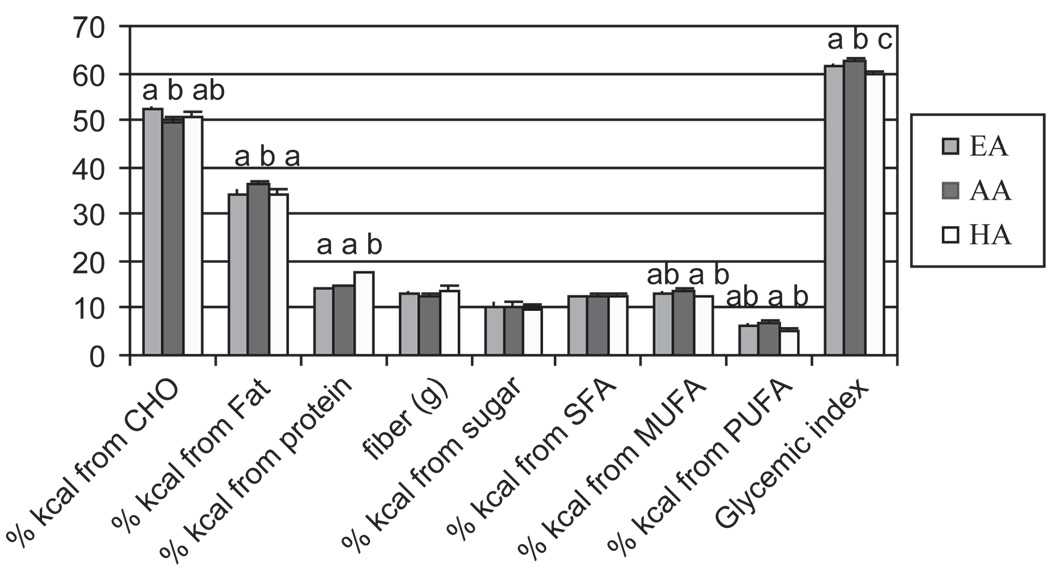
There were no differences between racial/ethnic groups in total daily energy intake (Table I). When macronutrients were compared according to percentage of total intake and composition (Figure 1a), EA reported a greater intake of energy from carbohydrate than AA (P < 0.05), whereas AA reported a greater intake of energy from dietary fat than EA and HA (P < 0.01, 0.05, respectively), and HA reported a greater intake of energy from protein than EA and AA (p < 0.001, for both). AA consumed more calories from monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) than HA and consumed foods, with a greater glycemic index than EA (P < 0.05) and HA (P < 0.01). The glycemic index of reported foods from EA was greater than that of HA (P < 0.05).
Figure 1.
Figure 1a. Dietary intake as reported by two 24 h recalls. EA = European Americans (light gray); AA = African Americans (dark gray); HA = Hispanic Americans (white). a–cSuperscripts indicate significant differences in reported intake between racial/ethnic groups (P < 0.05).
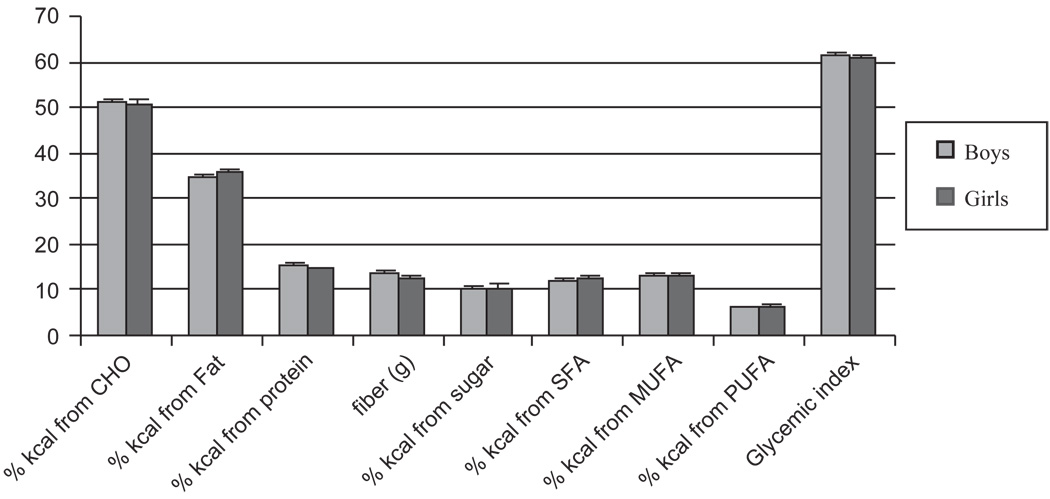
Figure 1b. Dietary intake as reported by two 24 h recalls. Boys (light gray); girls (dark gray).
Boys consumed more total energy per day than girls (p < 0.01), but there were no differences in the intake of energy from any macronutrient. There were also no differences in CHO or fat quality or in glycemic index between boys and girls (Figure 1b).
Using multiple linear regression analyses, several models were tested to determine the independent contributions of reported total energy intake, energy from macronutrients and diet quality to the dependent variables fasting insulin, SI and AIRg (Table II). Total energy intake did not contribute to insulin response or action. There was an association between intake of energy from fat and AIRg, such that a greater intake of energy from fat was related to greater AIRg (P = 0.05). A positive relationship between energy from dietary fat and fasting insulin also approached significance (P = 0.06), suggesting that a greater intake of energy from fat may lead to hyperinsulinemia. Intake of energy from protein was inversely associated with AIRg, such that a greater protein intake was related to a lower post challenge insulin response (P = 0.02). Energy from CHO was inversely related to fasting insulin (P = 0.04), such that a greater intake of energy from CHO was associated with lower fasting insulin.
Table II.
Relationship between reported dietary intake and insulin dynamics.
| Fasting Insulin | SI | AIRg | ||||
|---|---|---|---|---|---|---|
| Standardized parameter estimate |
p-value | Standardized parameter estimate |
p-value | Standardized parameter estimate |
p-value | |
| Total daily energy (kcal) | 0.02 | 0.66 | 0.02 | 0.65 | 0.04 | 0.44 |
| % Energy from fat | 0.10 | 0.06 | −0.06 | 0.21 | 0.10 | 0.05 |
| % energy from CHO | −0.10 | 0.04 | 0.04 | 0.41 | 0.30 | 0.60 |
| % energy from protein | 0.03 | 0.61 | 0.01 | 0.83 | −0.13 | 0.02 |
| Fiber intake (g) | −0.01 | 0.86 | −0.02 | 0.68 | −0.00 | 0.96 |
| % Energy from Sugar | 0.09 | 0.10 | −0.07 | 0.21 | 0.06 | 0.28 |
| % Energy from SFA | 0.10 | 0.06 | −0.02 | 0.60 | 0.06 | 0.26 |
| % Energy from MUFA | 0.07 | 0.18 | −0.09 | 0.13 | −0.03 | 0.51 |
| % Energy from PUFA | 0.04 | 0.51 | −0.03 | 0.65 | 0.13 | 0.02 |
| GI (glucose) | −0.05 | 0.36 | −0.09 | 0.13 | −0.03 | 0.60 |
Bolded P-values represent significant associations. Values in italics represent trends toward significance 0.05 <P ≤0.10.
All models included total fat mass, total lean mass, SES, sex, race/ethnicity, and Tanner stage as covariates. Models evaluating the independent variable AIRg also included SI as a covariate.
In terms of fat and CHO quality, the relationships between both energy from total sugar, and energy from saturated fatty acids and fasting insulin approached significance (P = 0.10, P = 0.06, respectively), suggesting that a high intake of sugar and saturated fat may contribute to hyperinsulinemia. There was also a positive relationship between intake of energy from PUFA and AIRg (P = 0.02). Reported dietary fiber intake was relatively low and did not contribute to insulin dynamics.
Neither the interaction terms between racial/ethnic group and dietary variables nor the interaction terms between sex and dietary variables were significant for any of the dependent variables, suggesting that the contribution of diet to racial/ethnic and sex differences in insulin dynamics was minimal in this sample.
Discussion
Evidence implicating a specific component of the diet that is most related to increased risk of T2D and obesity remains controversial. Racial/ethnic and sex differences in insulin dynamics exist (29,31,32). In this study, there was a relationship between diet and insulin-related outcomes; however, reported macronutrient intake or quality did not account for racial/ethnic or sex differences in insulin dynamics.
Dietary intake of the children in our sample reflected national trends (33), exceeding recommendations for simple sugars and saturated fat. In our sample, intake of simple sugar and saturated fat tended to be associated with fasting insulin, as has been reported (33). In contrast, in our sample, an inverse relationship between CHO and fasting insulin was observed, unlike that which has been noted in samples of older children (34). Between-study differences in dietary CHO quality, subject maturation status, or the ethnic composition of the subjects may explain different results among studies. In this study, a greater intake of energy from PUFA was associated with greater AIRg. Although PUFA intake is often associated with beneficial health effects, an imbalance in the n-6:n-3 has been associated with hyperinsulinemia and increased insulin secretion (35). A greater intake of energy from PUFA was associated with greater AIRg in this sample. Our subjects consumed on average an n-6:n-3 of 12:1 (far exceeding the ‘optimal’ 2:1 or 4:1 recommendation), perhaps explaining this observed relationship (data not shown) (35). Although regional and ethnic variations in the types of foods consumed are apparent in our sample as well as national samples, the macronutrient composition of the diet remains very similar (33). As such, consumption of “Westernized” diets by most children in the United States may explain why there were no interactions in the dietary and insulin dynamics models (33).
The association between diet composition and insulin outcomes may become apparent with age or maturation. Dietary fat (20) was associated with SI in adolescents, but not younger children. Similar to our results, Lindquist (17) found no association with any macronutrient and composition and SI in young children. Dietary sugar (22) was associated with SI among overweight subjects who were on average Tanner stage 2. Puberty is a critical period characterized by insulin resistance and significant changes in body composition and metabolism (29). Pubertal status could play a role in the interaction between diet and insulin dynamics. To minimize the influence of puberty on insulin-related outcomes, recruitment for our study was limited to individuals between ages 7–12 and ≤pubertal stage 3, which is the time at which the hypothalamic-pituitarygonadal axis matures (36).
Puberty is also a sensitive period for fat accumulation and fat accumulation may be influenced by both diet and insulin dynamics. HA in our sample had the greatest adiposity. The contribution of diet to insulin response and action has been observed in older obese HA adolescents (22) and in AA adolescents, but not EA adolescents (17,20,21). We have previously demonstrated that AA girls (who had greater AIRg), gained more weight over the pubertal transition than their EA counterparts (37). It has been hypothesized that over time greater insulin secretion may promote greater fat accumulation, which leads to the development of an even greater degree of insulin resistance (38). We propose that the unique metabolic characteristics that may lead to the pathogenesis of type 2 diabetes are likely to be established during the pubertal transition. Although racial/ethnic differences in insulin dynamics are inherent and are influenced at least in part by a genetic component, it is possible that the obesogenic and insulinogenic diet consumed by the pediatric population adds to this pathogenesis (39). Although the mechanism by which puberty initiates unique metabolic activity has yet to be elucidated, unhealthy dietary habits, especially during this critical period may have an additive effect over time. Moreover, the additive effect may exhibit a differential impact according to race/ethnicity and sex, and an inter-individual threshold that may differ between racial/ethnic groups. For example, just as other contributors (e.g., intra-abdominal adipose tissue) to adverse metabolic outcomes (e.g., insulin sensitivity) that track over time (40–42) may have differential impact among individuals of various racial/ethnic groups, so too may dietary intake. Thus, the multi-factorial perfect storm that occurs over the pubertal transition exacerbates the inherent racial/ethnic differences in insulin dynamics. Although there were few racial/ethnic differences in reported dietary intake, the contribution of diet may have a differential impact on individuals of various racial/ethnic backgrounds with maturation.
In this study, protein intake was associated with lower acute insulin response to glucose. This observation may appear counterintuitive in that protein is a potent insulin secretogogue on an acute basis (43). However, it is possible that relatively higher chronic protein intake may attenuate the insulin response to glucose. Further, subjects who ate relatively more protein presumable ate relatively less fat and carbohydrate, which may have resulted in an attenuated insulin response to glucose.
Strengths of this study included robust measures of insulin dynamics and body composition. A limitation was its cross-sectional nature preventing the establishment of a cause and effect relationship; longitudinal data will be required to determine if diet influences insulin dynamics over time. In addition, the sample was relatively small and included only healthy, primarily normal-weight (<85th percentile) subjects limiting the generalizablity of the findings. Further, though the sample size was sufficient to detect relationships using the entire sample, when stratified by ethnicity the sample size was limited, thereby possibly restricting the ability to detect ethnic-specific relationships.
In summary, diet likely plays a role in insulin dynamics, though the effect may be additive or interactive with obesity, maturity, and ethnicity. Although diet did not account for racial/ethnic and sex differences in insulin dynamics in our early pubertal children, it is possible that, with maturation and fat deposition, the contribution of diet to insulin dynamics may change in an ethnic-specific manner. These changes may play a role in the increased burden of type 2 diabetes noted among AA and HA relative to EA. Longitudinal studies are needed to determine if diet, via insulin sensitivity or secretion, contributes to disparities in T2D and if the contribution of diet to insulin dynamics strengthens with reproductive maturity.
Acknowledgements
Funded by: R01 DK067426-01, M01 RR00032.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Flegal KM, Tabak CJ, Ogden CL. Overweight in children: definitions and interpretation. Health Educ Res. 2006;21:755–760. doi: 10.1093/her/cyl128. [DOI] [PubMed] [Google Scholar]
- 2.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 4.Pinhas-Hamiel O, Dolan LM, Daniels SR, et al. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128:608–615. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- 5.Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44:3–15. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- 6.Osei K, Rhinesmith S, Gaillard T, et al. Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre-diabetic African Americans: implications for primary diabetes prevention. Diabetes Care. 2004;27:1439–1446. doi: 10.2337/diacare.27.6.1439. [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Hanson RL, Tataranni PA, et al. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49:2094–2101. doi: 10.2337/diabetes.49.12.2094. [DOI] [PubMed] [Google Scholar]
- 8.Katcher HI, Legro RS, Kunselman AR, et al. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr. 2008;87:79–90. doi: 10.1093/ajcn/87.1.79. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig DS. Dietary glycemic index and the regulation of body weight. Lipids. 2003;38:117–121. doi: 10.1007/s11745-003-1040-x. [DOI] [PubMed] [Google Scholar]
- 10.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23:447–456. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Rivellese AA, Auletta P, Marotta G, et al. Long term metabolic effects of two dietary methods of treating hyperlipidaemia. BMJ. 1994;308:227–231. doi: 10.1136/bmj.308.6923.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebbeling CB, Leidig MM, Feldman HA, et al. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 13.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 14.Lau C, Faerch K, Glumer C, et al. Dietary glycemic index, glycemic load, fiber, simple sugars, and insulin resistance: the Inter99 study. Diabetes Care. 2005;28:1397–1403. doi: 10.2337/diacare.28.6.1397. [DOI] [PubMed] [Google Scholar]
- 15.Nordmann AJ, Nordmann A, Briel M, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–293. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 16.Arslanian SA. Metabolic differences between Caucasian and African-American children and the relationship to type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2002;15 Suppl 1:509–517. [PubMed] [Google Scholar]
- 17.Lindquist CH, Gower BA, Goran MI. Role of dietary factors in ethnic differences in early risk of cardiovascular disease and type 2 diabetes. Am J Clin Nutr. 2000;71:725–732. doi: 10.1093/ajcn/71.3.725. [DOI] [PubMed] [Google Scholar]
- 18.Weigensberg MJ, Ball GD, Shaibi GQ, et al. Dietary fat intake and insulin resistance in black and white children. Obes Res. 2005;13:1630–1637. doi: 10.1038/oby.2005.200. [DOI] [PubMed] [Google Scholar]
- 19.Scaglioni S, Sala M, Stival G, et al. Dietary glycemic load and macronutrient intake in healthy Italian children. Asia Pac J Public Health. 2005;17:88–92. doi: 10.1177/101053950501700205. [DOI] [PubMed] [Google Scholar]
- 20.Sunehag AL, Toffolo G, Treuth MS, et al. Effects of dietary macronutrient content on glucose metabolism in children. J Clin Endocrinol Metab. 2002;87:5168–5178. doi: 10.1210/jc.2002-020674. [DOI] [PubMed] [Google Scholar]
- 21.Sunehag AL, Toffolo G, Campioni M, et al. Effects of dietary macronutrient intake on insulin sensitivity and secretion and glucose and lipid metabolism in healthy, obese adolescents. J Clin Endocrinol Metab. 2005;90:4496–4502. doi: 10.1210/jc.2005-0626. [DOI] [PubMed] [Google Scholar]
- 22.Davis JN, Alexander KE, Ventura EE, et al. Associations of dietary sugar and glycemic index with adiposity and insulin dynamics in overweight Latino youth. Am J Clin Nutr. 2007;86:1331–1338. doi: 10.1093/ajcn/86.5.1331. [DOI] [PubMed] [Google Scholar]
- 23.Casazza K, Gower BA, Willig AL, et al. Physical Fitness, Activity, and Insulin Dynamics in Early Pubertal Children. 2008 doi: 10.1123/pes.21.1.63. In press Edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 25.Biro G, Hulshof KF, Ovesen L, et al. Selection of methodology to assess food intake. Eur J Clin Nutr. 2002;56 Suppl 2:S25–S32. doi: 10.1038/sj.ejcn.1601426. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery C, Reilly JJ, Jackson DM, et al. Validation of energy intake by 24-hour multiple pass recall: comparison with total energy expenditure in children aged 5–7 years. Br J Nutr. 2005;93:671–676. doi: 10.1079/bjn20051405. [DOI] [PubMed] [Google Scholar]
- 27.Goran MI, Shaibi GQ, Weigensberg MJ, et al. Deterioration of insulin sensitivity and beta-cell function in overweight Hispanic children during pubertal transition: a longitudinal assessment. Int J Pediatr Obes. 2006;1:139–145. doi: 10.1080/17477160600780423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe RM, Steil GM, Bergman RN. Critical evaluation of the combined model approach for estimation of prehepatic insulin secretion. Am J Physiol. 1998;274:E172–E183. doi: 10.1152/ajpendo.1998.274.1.E172. [DOI] [PubMed] [Google Scholar]
- 29.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50:2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 30.Cirino PT, Chin CE, Sevcik RA, et al. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment. 2002;9:145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- 31.Arslanian S. Insulin secretion and sensitivity in healthy African-American vs American white children. Clin Pediatr (Phila) 1998;37:81–88. doi: 10.1177/000992289803700204. [DOI] [PubMed] [Google Scholar]
- 32.Gower BA, Fernandez JR, Beasley TM, et al. Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes. 2003;52:1047–1051. doi: 10.2337/diabetes.52.4.1047. [DOI] [PubMed] [Google Scholar]
- 33.Nicklas TA, Hayes D. Position of the American Dietetic Association: nutrition guidance for healthy children ages 2 to 11 years. J Am Diet Assoc. 2008;108 doi: 10.1016/j.jada.2008.04.005. 1038-7. [DOI] [PubMed] [Google Scholar]
- 34.Davis JN, Ventura EE, Weigensberg MJ, et al. The relation of sugar intake to beta cell function in overweight Latino children. Am J Clin Nutr. 2005;82:1004–1010. doi: 10.1093/ajcn/82.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry EM. Are diets high in omega-6 polyunsaturated fatty acids unhealthy? 2001 [Google Scholar]
- 36.Phillip M, Lazar L. Precocious puberty: growth and genetics. Horm Res. 2005;64 Suppl 2:56–61. doi: 10.1159/000087760. [DOI] [PubMed] [Google Scholar]
- 37.Casazza K, Goran MI, Gower BA. Associations among Insulin, Estrogen, and Fat Mass Gain over the Pubertal Transition in African-American and European-American Girls. J Clin Endocrinol Metab. 2008;93:2610–2615. doi: 10.1210/jc.2007-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han JC, Rutledge MS, Kozlosky M, et al. Insulin resistance, hyperinsulinemia, and energy intake in overweight children. J Pediatr. 2008;152:612–617. doi: 10.1016/j.jpeds.2007.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lustig RH. The‘skinny’ on childhood obesity: how our western environment starves kids’ brains. Pediatr Ann. 2006;35 doi: 10.3928/0090-4481-20061201-08. 898-7. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi T, Boyko EJ, McNeely MJ, et al. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57:1269–1275. doi: 10.2337/db07-1378. [DOI] [PubMed] [Google Scholar]
- 41.Pascot A, Despres JP, Lemieux I, et al. Contribution of visceral obesity to the deterioration of the metabolic risk profile in men with impaired glucose tolerance. Diabetologia. 2000;43:1126–1135. doi: 10.1007/s001250051503. [DOI] [PubMed] [Google Scholar]
- 42.St-Pierre J, Lemieux I, Vohl MC, et al. Contribution of abdominal obesity and hypertriglyceridemia to impaired fasting glucose and coronary artery disease. Am J Cardiol. 2002;90:15–18. doi: 10.1016/s0002-9149(02)02378-0. [DOI] [PubMed] [Google Scholar]
- 43.Tremblay F, Lavigne C, Jacques H, et al. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr. 2007;27:293–310. doi: 10.1146/annurev.nutr.25.050304.092545. [DOI] [PubMed] [Google Scholar]