Abstract
Mice lacking the immunoregulatory cytokine, IL-10, develop necrotizing hepatitis following infection with Trichinella spiralis, and inflammation is dependent on migration of intestinally activated CD4+ T cells into the liver. Hepatic production of IL-4 is elevated in these mice, and we hypothesized that it plays a role in the development of hepatic pathology. Wildtype (WT), IL-10 knockout (KO), IL-4 KO, and IL-10/IL-4 KO mice were orally infected, and disease progression was followed by histology, alanine aminotransferase (ALT) assays, and flow cytometric analysis of hepatocellular content. Both IL-10 KO and IL-10/IL-4 KO mice experienced hepatocellular injury, but only IL-10 KO mice advanced to a necrotic phase. Hepatic CD4+ T cells were the major source of IL-4, and IL-10 regulated the number of intestinally-derived CD4+IL-4+ cells. Sequestration of activated neutrophils in the liver required IL-4, and neutrophil depletion prevented progression to overt necrosis. Adoptive transfer of intestinal WT CD4+ T cells inhibited neutrophil accumulation and inflammation, but their regulatory effects did not require IL-10 signaling.
Conclusion
The absence of IL-10 led to hepatocyte injury during infection, but IL-4 was necessary for the development of neutrophil-dependent necrosis. These studies provide new insight into the combinatorial role of these cytokines and their targets in the generation and progression of hepatic inflammation.
Keywords: Trichinella spiralis, CD4+ T cell, neutrophil, hepatitis, cytokine
The liver performs many crucial metabolic activities, but it also functions in immune defense (1). Its constant exposure to harmless and potentially harmful antigens requires effective discrimination and the generation of appropriate responses. Failure can lead to inadequate immunity and tissue injury. Inflammatory hepatic diseases, which can be distinguished by heterogeneous etiologies, often share common mechanisms of tissue injury (2, 3). For example, T cell-mediated hepatopathies include viral hepatitis and autoimmune hepatitis, diseases which are instigated by different antigens (4, 5). Moreover, immune-mediated damage can be facilitated by cells such as granulocytes and macrophages that become activated and migrate into the liver in an antigen non-specific manner (6, 7). The cytokine environment shapes their activities, and a better appreciation of the interactions between cytokine producing cells and their targets will shed light on key aspects of the pathogenesis of inflammatory diseases of the liver (2).
Previously, we established an infection model to investigate the role of IL-10 in the liver (8, 9). In contrast to WT animals, we found that IL-10 KO mice developed severe multifocal hepatic inflammation and necrosis after oral infection with the parasitic nematode, Trichinella spiralis. This pathogen matures and reproduces in the intestine. The juvenile form, or newborn larva, enters the mesenteric vasculature and is carried to the liver by the portal vein. Beyond the liver, newborn larvae (NBL) circulate systemically and may penetrate skeletal muscle cells where they grow and await ingestion of the host to renew the life cycle. In our current studies, we have begun to investigate the mechanisms underlying hepatocyte injury and death. Our results demonstrated that progression of initial hepatocyte damage into organized regions of necrosis was controlled by the prevailing cytokine environment. While the absence of IL-10 led to cellular injury during infection, IL-4 was required for evolution to necrotizing hepatitis. These results support a critical role for IL-4 in controlling the progression of hepatic inflammation following enteric parasitic infection and illustrate the importance of the enterohepatic cytokine balance for appropriate hepatic immune function.
Experimental Procedures
Mice
C57BL/6 and IL-10 KO (on a C57BL/6 background) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). IL-4 KO and IL-10/IL-4 KO mice were a generous gift from Dr. Tom Wynn at the National Institutes of Health. PHIL mice were provided by Dr. Jamie Lee at the Mayo Clinic. These mice were bred onto an IL-10 KO background, and transgenic mice were identified as described (10). Disruption of the IL-10 locus was confirmed by PCR, using primer sequences previously listed (8). Animals were bred and housed at Cornell University, a facility accredited by the American Association for Accreditation of Laboratory Animal Care. Studies were approved by the Institutional Animal Care and Use Committee.
Parasites and infections
T. spiralis first-stage larvae (L1) were recovered from the muscles of irradiated Albino Oxford (AO) rats by digestion with 1% pepsin in acidified water as described previously (11). Experimental mice were administered 600 L1 by gavage.
Neutrophil depletion
In some experiments, mice were given a control rat IgG or were rendered neutropenic by injecting an α-Gr-1 antibody (clone RB6-8C5) as described previously (12) or clone NIMP-R14, a kind gift from Dr. Fabienne Tacchini-Cottier (13). RB6.8C5 recognizes Gr-1 which is expressed by other cell types in addition to neutrophils, albeit at lower levels (14). NIMP-R14 recognizes a 25–30 kDa protein present on the neutrophil surface and is reported to be specific (13). To confirm neutropenia, differential cell counts were performed on blood smears obtained from individual mice at the time of euthanasia.
Isolation of hepatic leukocytes
Hepatic leukocytes were recovered as described previously (9). Cells were analyzed by flow cytometry, cultured for cytokine determination, or were centrifuged onto glass slides using a Shandon Cytospin 2 (Thermo Fisher Scientific, Waltham, MA) for differential cell counting (300 cells per sample counted).
Flow cytometry
Cells were restimulated ex vivo, stained, and analyzed as described (9, 12). Antibodies employed were specific for: CD4 (clone RM4-5), CD62L (clone MEL-14), CD11b (clone M1/70), CCR9 (clone CD-1.2) (eBioscience, San Diego, CA), α4β7 (clone DATK32), Ly6-G (clone 1A8), IL-4 (clone 11B11) (BD Pharmingen, San Jose, CA), and F4/80 (clone BM8) (Caltag, Carlsbad, CA). Appropriate isotype matched clones served as controls and were used to set analysis gates.
Cytokine ELISA
Hepatic leukocytes were restimulated in vitro with medium or somatic larval antigens at 10μg/well. After 3 days, supernatants were collected, and IL-4 levels were determined by ELISA as described (9).
Measurement of ALT activity
ALT activity was measured in individual serum samples, using a commercially available kit from Pointe Scientific (Canton, MI).
Histology
Liver tissue was fixed in 10% neutral-buffered formalin and embedded in paraffin. 6 μm sections were stained with hematoxylin and eosin (H & E) for microscopic examination. Photomicrographs were created using an Olympus BX51 microscope and Olympus DP12 digital camera system (Center Valley, PA).
Adoptive transfer of CD4+ T cells
Mesenteric lymph nodes from WT mice were obtained 5 days following oral infection. CD4+ T cells were purified by negative selection using the CD4+ T cell isolation kit from Miltenyi Biotec (Auburn, CA). The percentage of CD4+ cells was determined to be ≥ 95% by flow cytometry. 2 × 106 cells in 0.5 ml of PBS or PBS alone were injected i.p. into IL-10 KO recipients one day prior to their infection. In some groups of recipients, a control IgG or α-IL-10R (clone 1B1.3a; 300 μg i.p. every other day, beginning one day before infection) antibody was administered. Other groups included mice that were given cells and PBS or PBS only. To aid in interpretation of the effects of α-IL-10R treatment, we also included a group of WT recipients that were given the same dose and regimen as IL-10 KO mice. Twelve days later, mice were evaluated for ALT activity, liver histology, hepatic leukocyte content (total, CD4+α4β7+ cells, and Ly6-G+F4/80−cells), and cytokine production.
Statistical analysis
Each experiment was performed 3–5 times, and each group contained 3–5 mice. Means and SD's were calculated from values obtained from individual mice in a treatment group. Means were compared by the Student's t test or ANOVA followed by an appropriate posttest using GraphPad Prism software (San Diego, CA). Significance was assessed at p < 0.05.
Results
The capacity to produce IL-4 correlates with disease severity
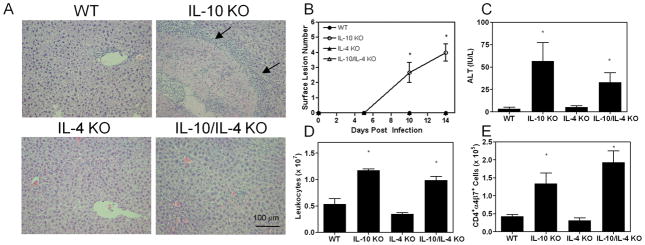
In previous studies, we found that oral infection resulted in hepatic inflammation and necrosis in IL-10 KO mice (8). TUNEL staining and histopathological examination of liver tissue indicated that cell death was necrotic and not apoptotic in nature. Hepatic injury was mediated by intestinally-derived CD4+ T cells during infection and could be mitigated by blocking their entry into the liver (9). Moreover, transfer of these cells from IL-10 KO mice to RAG2 KO animals reproduced the disease, suggesting that this lymphocyte subset alone was sufficient for inciting injury. Type 2 cytokine production, particularly IL-4 synthesis, was prominent in the infected IL-10 KO liver (9). Therefore, we hypothesized that IL-4 promoted inflammation and necrosis during infection in IL-10 KO mice. Infection of singly and doubly deficient animals revealed that lesion development was dependent on IL-4 in IL-10 KO mice (Figure 1A). While multifocal lesions were grossly and histologically visible in IL-10 KO animals, they were completely absent in IL-10/IL-4 KO mice. Lesions were characterized by central necrosis that was surrounded by mononuclear and polymorphonuclear cells. While liver tissue from infected IL-10/IL-4 KO mice appeared to contain more leukocytes than that from WT animals, areas of hepatocellular necrosis were not detected. Neither WT nor IL-4 KO mice acquired hepatic lesions (Figure 1B). As we reported previously, serum ALT activity at 12 days post infection was significantly greater in infected IL-10 KO mice compared to WT mice (Figure 1C) (9). Infection did not lead to an increase in ALT values in IL-4 KO mice, indicating a lack of hepatocyte damage. In contrast, ALT levels in infected IL-10/IL-4 KO mice rose significantly above WT levels but were not different from IL-10 KO mice. When considered with the histological evidence, the results suggested that initial hepatocyte injury occurred in the absence of IL-10, but the evolution of organized necrotic lesions required IL-4. We considered, however, that differences in parasite burden between IL-10 KO and IL-10/IL-4 KO mice might affect lesion development. Accordingly, we counted intestinal worm numbers as an indication of the load the liver received during the acute phase of infection and found no differences, suggesting that the disparity in hepatic response was not due to parasite burden but rather reflected differences in immunity (data not shown). Immune-mediated hepatic injury is the result of effector leukocyte recruitment and activity, and we find enumeration of hepatic leukocytes to be a sensitive indicator of inflammation. Both infected IL-10 KO and IL-10/IL-4 KO mice had elevated numbers of hepatic leukocytes compared to WT mice, implying that IL-10 regulated the total leukocyte content within in the liver independently of IL-4 (Figure 1D). Additionally, the number of CD4+α4β7+ cells in the liver was increased in both IL-10 KO and IL-10/IL-4 KO mice, suggesting that IL-10 also controlled intestinally-derived CD4+ cell infiltration of the liver in a manner unaffected by IL-4 (Figure 1E). Comparison of other lymphocyte subsets between IL-10 KO and IL-10/IL-4 KO mice revealed only a slight and variable decrease in CD8+ T cell numbers in IL-10 KO animals (data not shown). Overall, the data supported the contention that IL-10 prevented hepatocyte injury and accumulation of intestinally-derived CD4+ cells while IL-4 was required for the development of hepatic necrosis.
Figure 1.
IL-4 is required for the development of hepatic necrosis in infected IL-10 KO mice. (A) Representative liver sections obtained 12 days following oral infection. Arrows distinguish the margins of a lesion in an IL-10 KO mouse. (B) Number of surface lesions. (C) ALT activity. (D) Number of intrahepatic leukocytes. (E) Number of CD4+α4β7+ cells. Asterisks indicate a statistically significant difference relative to WT values.
CD4+ T cells are the major source of IL-4 in the liver during infection
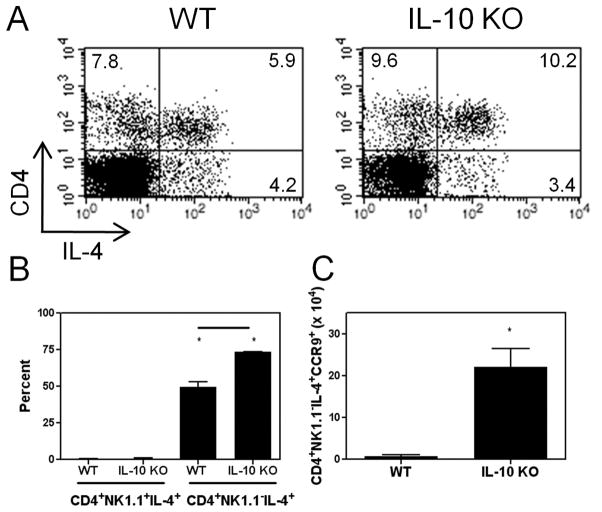
To investigate further the role of IL-4 in the liver during infection, we sought to determine which cell type(s) produced it. The majority of IL-4+ cells were CD4+; however, the percentage of CD4+IL-4+ cells in IL-10 KO mice was approximately twice that in WT mice (Figure 2A). Most CD4+ cells in the liver are conventional CD4+ T cells, but some classical NK T cells also express CD4. To distinguish between contributions from these two cell types, we stained cells for CD4, IL-4, and NK1.1. IL-4+ cells were gated, and the percentages of IL-4 expressing conventional CD4+ T cells versus NK T cells are shown in Figure 2B. Almost all of the IL-4+ cells colocalized with the CD4+NK1.1− population. Thus, CD4+ T cells were the major source of IL-4. Additionally, this population was expanded in IL-10 KO animals compared with WT mice. Since we previously discovered that an intestinal immune response was a prerequisite for hepatic inflammation, we asked if any CD4+NK1.1−IL-4+ cells were gut-derived. CCR9, like α4β7, is upregulated on lymphocytes after activation within gut-associated lymphoid tissue (GALT) and is used as a marker of intestinal origin (15). Infected IL-10 KO animals had significantly more IL-4+, intestinally-derived CD4+ T cells compared to WT mice (Figure 2C).
Figure 2.
IL-10 suppresses the accumulation of CD4+IL-4+ T cells in the liver. Twelve days post infection livers were obtained for flow cytometric analysis. (A) Hepatic CD4 and IL-4 expression are shown for representative animals. (B) IL-4+ cells were gated, and the percentages of CD4+NK1.1+IL-4+ and CD4+NK1.1−IL-4+ cells per strain are shown. Asterisks denote significant differences between cell types per strain, and the line indicates a difference between WT and IL-10 KO values. (C) Numbers of hepatic CD4+NK1.1−IL-4+CCR9+ cells. Asterisk designates a significant difference between WT and IL-10 KO values.
Infected eosinophil deficient IL-10 KO mice develop hepatic inflammation and necrosis
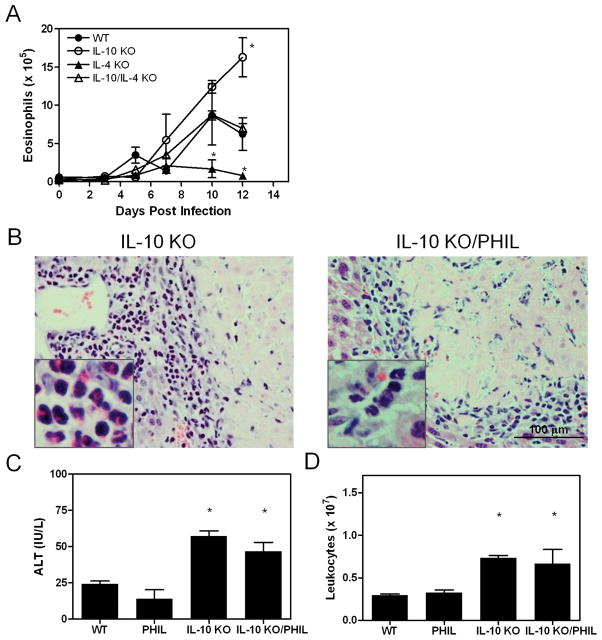
Previously, we noted that lesions in infected IL-10 KO mice contained an abundance of granulocytes, including neutrophils and eosinophils. IL-4 promotes eosinophil proliferation, recruitment, and effector functions, and its expression is elevated by T. spiralis infection (16). This led us to ask if eosinophils were involved in the development of hepatic necrosis. We compared eosinophil infiltration in singly and doubly deficient mice following infection (Figure 3A). As expected, IL-4 KO mice displayed reduced eosinophilia compared to WT animals. In contrast, eosinophil numbers were higher in infected IL-10 KO mice compared to WT animals. Hepatic eosinophil content in IL-10/IL-4 KO mice was similar to that in WT mice. Hence, eosinophil accumulation in the liver was inhibited by IL-10 and promoted by IL-4. We tested whether eosinophils were essential in the development of hepatic necrosis by mating IL-10 KO animals to eosinophil deficient (PHIL) mice to generate mice lacking both IL-10 and eosinophils. Subsequent infection revealed that both IL-10 KO and IL-10 KO/PHIL mice formed hepatic lesions (Figure 3B). Eosinophils were abundant in areas juxtaposed to lesions in IL-10 KO animals; however, they were absent in IL-10 KO/PHIL mice, demonstrating that eosinophils were not critical in the development of hepatic necrosis. Results of ALT activity assays and hepatic leukocyte counts corroborated this interpretation (Figure 3C and D).
Figure 3.
Eosinophils do not mediate hepatic necrosis. Leukocytes were isolated at the indicated times by density gradient centrifugation. Total cells were enumerated, and an aliquot from each mouse was spun onto a glass slide and stained for differential cell counting (A). The average number of eosinophils ± SD/group/time point is shown. Asterisks indicate a significant difference relative to WT values at the same time point. (B) Representative liver sections from day 12 infected IL-10 KO and IL-10 KO/PHIL mice. The inset in the left panel is a higher magnification of an area around the lesion showing eosinophils. A similar inset is shown for a IL-10 KO/PHIL mouse to demonstrate the absence of eosinophils. (C) ALT activity. (D) Hepatic leukocytes. Asterisks indicate significant differences from WT values.
IL-10 suppresses hepatic infiltration of neutrophils
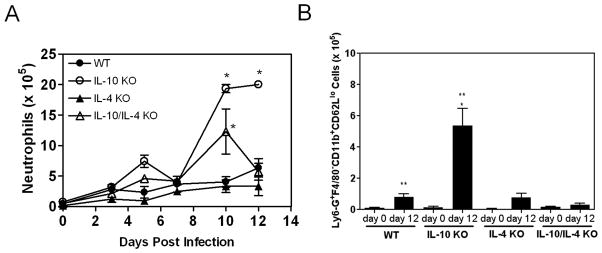
During infection, neutrophils were significantly increased in the livers of IL-10 KO animals compared to WT mice with peak numbers (day 10) occurring just prior to the time of maximal lesion size (days 12–14) (Figure 4A). Numbers remained low in both WT and IL-4 KO mice while those in IL-10/IL-4 KO animals initially rose but then fell, never achieving values observed in IL-10 KO mice. This was confirmed by flow cytometric analysis of hepatic leukocytes (data not shown). Additionally, the prevalence of neutrophils in the liver was suppressed by IL-10. For example, neutrophils represented 14 ± 1.7% of total leukocytes in IL-10 KO livers on day 12 compared to 9 ± 1% in WT animals (p< 0.05). Loss of endogenous IL-4 decreased the prevalence to 2.7 ± 1.5% in IL-10/IL-4 KO mice. Activated neutrophils can release cytotoxic mediators, suggesting their potential participation in lesion development. We used expression of CD11b and CD62L to determine if IL-10 and IL-4 influenced neutrophil activation state in the liver. Infection resulted in a significant increase in the number of Ly6-G+F4/80− cells (markers of neutrophils) with an activated CD11b+CD62Llo phenotype in WT and IL-10 KO mice compared to naive animals (Figure 4B). Upon infection, only the number of activated neutrophils in IL-10 KO mice differed significantly from WT animals. Taken together, the data suggested that IL-10 and IL-4 may have differential effects on neutrophil trafficking and activation state.
Figure 4.
The capacity to produce IL-10 is associated with reduced infiltration of activated neutrophils. (A) At indicated times, hepatic leukocytes were recovered, and the number of neutrophils was determined. Asterisks indicate significant differences relative to WT mice. (B) Hepatic leukocytes obtained 12 days post infection were stained for expression of Ly6-G, F4/80, CD62L, and CD11b. The average number of Ly6-G+F4/80−CD11b+CD62Llo cells ± SD/liver is displayed. The single asterisk denotes a significant difference between day 12 WT and IL-10 KO values while the double asterisks indicate significant differences between naïve and infected mice.
Neutrophils mediate hepatic necrosis but are not involved in initiating hepatic injury
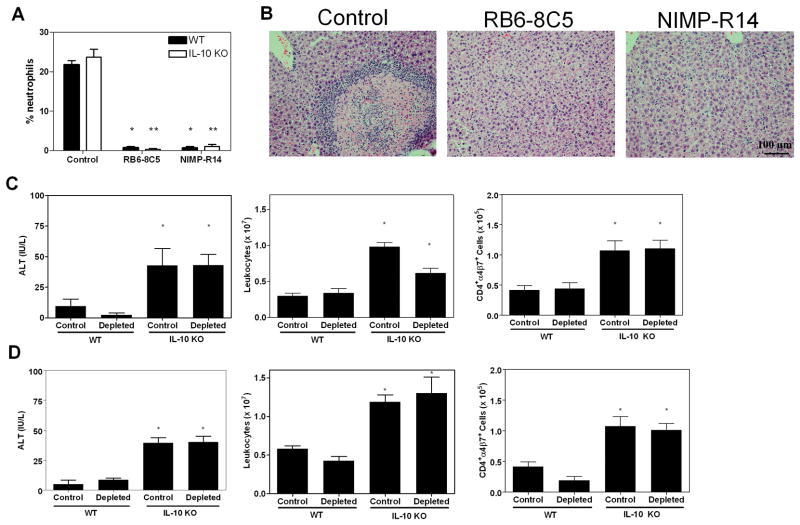
To determine whether neutrophils played a role in initial hepatocyte injury and/or subsequent development of hepatic necrosis, we depleted mice of neutrophils with either of two monoclonal antibodies. Figure 5A shows the effect of antibody administration on peripheral neutrophils. Both depleting antibodies reduced the prevalence of neutrophils to below 2%. Control antibody-treated and neutropenic IL-10 KO mice had greater ALT values and hepatic leukocyte numbers compared to WT mice after infection (Figure 5C–D). However, only control antibody-treated IL-10 KO mice developed hepatic necrosis (Figure 5B). The number of CD4+α4β7+ cells was significantly increased in both control and depleted IL-10 KO mice as compared with WT mice, corresponding to the elevated serum ALT activity in these animals (Figure 5C–D). Thus, in the absence of IL-10 and presence of IL-4, neutrophils were necessary for the development of hepatic necrosis but were not required for the initiation of hepatocyte injury.
Figure 5.
Neutrophil depleted mice are protected from hepatic necrosis. Mice (5 mice/group) were given either a control IgG antibody or were depleted of neutrophils with clone RB6.8C5 (C) or clone NIMP-R14 (D). (A) Percentages of peripheral blood neutrophils. Single asterisks designate significant differences between control and depleted WT values, and double asterisks denote differences between control and depleted IL-10 KO numbers. (B) Representative photomicrographs of control and depleted IL-10 KO liver tissue (day 12). (C and D) ALT activity and CD4+α4β7+ cell numbers. Asterisks indicate significant differences relative to WT values.
CD4+ T cells generated in an IL-10 sufficient environment do not require IL-10 to protect mice
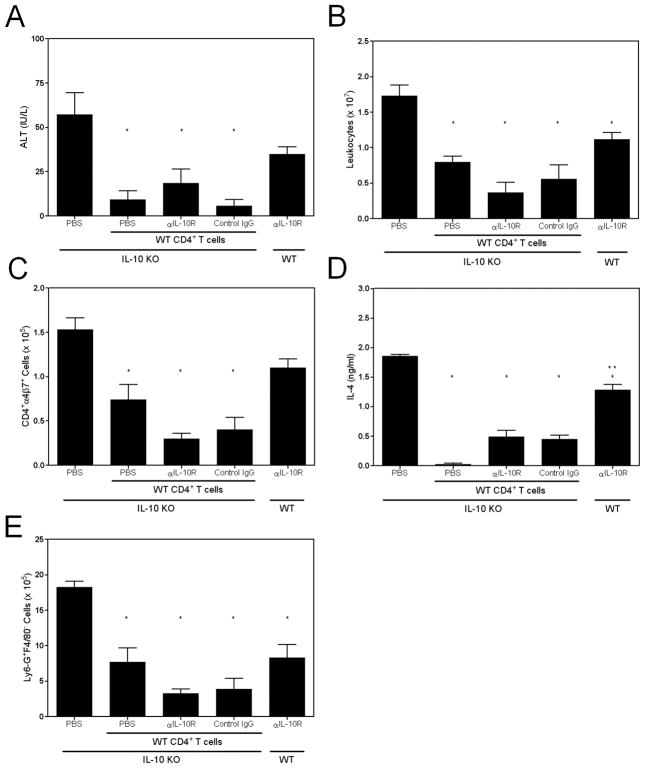
Our prior studies showed that infection of RAG2 KO mice, that are deficient in lymphocytes but possess neutrophils, did not lead to hepatocyte injury or necrosis, but transfer of intestinal CD4+ T cells from infected IL-10 KO animals induced necrotizing hepatitis upon infection (9). Moreover, neutrophil or CD4+ cell depletion prevented necrosis in infected IL-10 KO mice (9) (Figure 5). Thus, our data support a model in which, in the absence of IL-10, CD4+ T cells activated within GALT migrate to the liver and elaborate cytokines that regulate both neutrophil accumulation and state of activation. In support of this, we reported that adoptive transfer of intestinal CD4+ T cells from infected IL-10 KO mice to WT mice led to a mild hepatitis upon infection, while transfer of WT cells to IL-10 KO recipients was protective (9). To determine whether IL-10 was required for protective activity, we transferred WT CD4+ T cells into IL-10 KO mice that had received PBS, an irrelevant antibody, orα-IL-10R antibody. Animals that received WT CD4+ T cells had decreased ALT activity and hepatic leukocyte content (total and intestinally-derived CD4+ cells) compared to IL-10 KO mice which did not receive cells (Figure 6). Additionally, the development of necrotic lesions was suppressed in IL-10 KO recipients that received cells as compared to those given PBS (data not shown). Interestingly, cultured hepatic leukocytes from adoptively transferred mice released less IL-4, suggesting that the transferred WT CD4+ T cells controlled IL-4 production (Figure 6D). In vivo blockade of the IL-10R did not compromise protection, suggesting that IL-10 was important during T cell activation in GALT rather than for T cell function in the liver. Since neutrophil depletion blocked the development of hepatic necrosis, we hypothesized that transfer of intestinal CD4+ T cells from WT mice would reduce neutrophil numbers and decrease hepatic necrosis. Indeed, IL-10 KO recipients accumulated significantly fewer Ly6-G+F4/80− cells in the liver (Figure 6E). Furthermore, blockade of IL-10 signaling did not reverse this effect. To aid in interpretation of these results, we included a group of WT recipients which were administered α-IL-10R antibodies. These animals experienced hepatocellular damage and an influx of CD4+α4β7+ cells similar to IL-10 KO mice. Hepatic IL-4 levels were greater in WT mice compared to IL-10 KO mice that received cells but less than in PBS-injected IL-10 KO animals. Additionally, two-thirds of WT mice developed small necrotic foci (data not shown). Thus, the α-IL-10R antibody preparation antagonized the effects of IL-10.Overall, our data indicated that intestinally-derived CD4+ T cells, activated in an IL-10 sufficient environment, can protect the liver against hepatic injury and necrosis by regulating effector cell trafficking and function.
Figure 6.
CD4+ T cells generated in an IL-10 sufficient environment do not require IL-10 to protect mice. CD4+ T cells from infected WT mice were transferred to IL-10 KO recipients. The recipients received WT cells plus PBS, a control rat IgG, or an antibody that blocks IL-10 from binding to its receptor. Additionally, one group received only PBS as a positive control. Another group consisted of WT mice given α-IL-10R antibodies. All mice were infected with 600 L1 and were evaluated on day 12. (A) ALT activity. (B) Hepatic leukocytes. (C) CD4+α4β7+ cell numbers. (D) IL-4 was measured in supernatants from cultured hepatic leukocytes which were restimulated with parasite antigens. No IL-4 was detected from cells cultured only in medium. (E) Number of Ly6-G+F4/80− cells. Single asterisks indicate significant differences relative to the positive control group, and the double asterisk signifies a difference between the indicated group and all others that received donor cells.
Discussion
Clinically significant liver disease may result from a multitude of insults, including infection, alcohol, drugs, and ischemia/reperfusion. Inflammation is a common feature of most forms of liver disease and is a normal process that may rid the host of an insulting agent and often is a necessary precursor to regenerative tissue repair. However, if the inflammatory response is severe and prolonged, hepatic necrosis may eventually lead to extensive loss of parenchyma and irreversible tissue fibrosis.
Neutrophils are capable of migrating rapidly to foci of infection or inflammation. Infiltration and contact with inflammatory mediators can reprogram cells to alter effector responses. Chakravarti et al. recently described a subset of human blood neutrophils that became long-lived, expressed HLA-DR, CD80, and CD49d de novo, and alternatively produced leukotrienes, superoxide anions, and cytokines upon exposure to GM-CSF, TNF-α, and IL-4 (17). Thus, the microenvironment can reprogram cells that traditionally have been thought of as terminally differentiated, and this can impact disease progression. Here, we show that IL-4 was necessary for the full development of hepatic necrosis in infected IL-10 KO mice, and CD4+ T cells constituted a major source of IL-4 in the liver, a proportion of which were activated within GALT. Further, our data indicated that neutrophils played a critical role in the progression from hepatocellular injury to necrosis. Accumulation of neutrophils was inhibited in the absence of IL-4 concomitant with altered expression of key activation molecules, highlighting a role for this cytokine in the management of neutrophil function. These data define a critical balance between IL-10 and IL-4 in the hepatic response to enteric infection and suggest a role for CD4+ T cells and IL-4 in regulation of neutrophil activity during hepatic injury. Our results also demonstrated the utility of this in vivo system for investigation not only of the specific roles of IL-10 and IL-4 in the hepatic response to infection with this parasite but also for broader inquiry into the coordination of enteric and hepatic immune mechanisms.
In several experimental models of liver injury, IL-4 has been shown alternatively to be protective or deleterious. For example, IL-4 protects mice from damage induced by ischemia/reperfusion, but it promotes hepatitis following concanavalin-A injection (18, 19). While IL-4 and neutrophils are known to participate in the pathogenesis of certain liver diseases, very little is established about how IL-4 directly or indirectly influences neutrophil activity. Interestingly, Huang et al. recently reported that IL-4 stimulated the expression of CXCL-8, CD62E, VEGF, and iNOS by equine pulmonary artery endothelial cells resulting in neutrophil migration in vitro (20). In our studies, the capacity to produce IL-4 influenced expression of neutrophil adhesion molecules and sequestration in the liver. Activated neutrophils may release proteases and oxidants that further damage hepatic cells, leading to necrosis. Elucidation of the mechanisms by which this cytokine modulates neutrophil function in the liver as well as means of communication between T cells and neutrophils currently are centers of effort in our laboratory.
IL-10 is hepatoprotective in conditions such as alcoholic hepatitis and fatty liver disease (21, 22). Moreover, the importance of IL-10 in protecting against pathogen-induced liver damage has been demonstrated in several models (23, 24). Our prior work with T. spiralis infected mice revealed that IL-10 abrogated liver injury, and experiments showed that it was necessary during T cell activation in GALT to prevent the development of a subset of CD4+ T cells that migrated to the liver to induce damage (9). Here, we provided evidence that IL-10 was not required for control of hepatic inflammation when WT CD4+ T cells were transferred to IL-10 KO recipients. Taken together, these results strongly implicate intestinal CD4+ T cells in the initial hepatocellular injury that occurs following infection in the context of IL-10 deficiency. Whether this early hepatic damage is caused directly by these cells, or whether CD4+ T cell dependent injury is mediated indirectly through a secondary non-lymphocyte effector cell remains unclear. Interestingly, Alford et al. reported that cross-linking of CD46 on the surface of CD4+ T cells resulted in their ability to mediate cytotoxicity through perforin and granzyme B (25). Following initial injury, the manner by which T cells regulate neutrophil activity in our system has not been discovered. We have considered the potential effects of IL-10 and IL-4 on IL-17 production. Although we did not find a significant difference in IL-17A production by CD4+ T cells between mouse strains in preliminary studies, the possible influence of this cytokine requires further testing. Clarification of the functional differences between CD4+ T cells primed in the intestine in the presence and absence of IL-10 and their effects on the liver would constitute a significant advance in our understanding of enterohepatic immune regulation. Furthermore, given the dominance of neutrophils in many inflammatory liver diseases, a greater understanding of how the microenvironment alters neutrophil phenotype and function would likely advance development of targeted disease interventions.
Acknowledgments
The authors would like to thank Drs. B. Tennant and S. Bliss for critical review of the manuscript.
Financial Support was provided by National Institutes of Health (AI14490 to J.A.A. and DK67290 to S.K.B.)
Abbreviations
- WT
wildtype
- KO
knockout
- NBL
newborn larvae
- MAdCAM1
mucosal vascular addressin cell adhesion molecule 1
- L1
first-stage larvae
- AO
Albino Oxford
- ALT
alanine aminotransferase
- VEGF
vascular endothelial growth factor
- iNOS
inducible nitric oxide synthase
Contributor Information
Diana B. Douglas, Email: dlb338@cornell.edu.
Daniel P. Beiting, Email: beiting@sas.upenn.edu.
John P. Loftus, Email: jpl249@cornell.edu.
Judith A. Appleton, Email: jaa2@cornell.edu.
Susan K. Bliss, Email: spk2@cornell.edu.
References
- 1.Bowen DG, McCaughan GW, Bertolino P. Intrahepatic immunity: a tale of two sites? Trends in Immunology. 2005;26:512. doi: 10.1016/j.it.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Eksteen B, Afford S, Wigmore SJ, Holt AP, Adams DH. Immune-mediated liver injury. Semin Liver Dis. 2007:351. doi: 10.1055/s-2007-991512. [DOI] [PubMed] [Google Scholar]
- 3.Ramaiah SK, Jaeschke H. Role of Neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007;35:757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- 4.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annual Review of Pathology: Mechanisms of Disease. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 5.Tiegs G. Cellular and cytokine-mediated mechanisms of inflammation and its modulation in immune-mediated liver injury. Z Gastroenterol. 2007;45:63–70. doi: 10.1055/s-2006-927397. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2005;289:G969–976. doi: 10.1152/ajpgi.00223.2005. [DOI] [PubMed] [Google Scholar]
- 7.Zhai Y, Shen X-d, Hancock WW, Gao F, Qiao B, Lassman C, Belperio JA, et al. CXCR3+CD4+ T cells mediate innate immune function in the pathophysiology of liver ischemia/reperfusion injury. J Immunol. 2006;176:6313–6322. doi: 10.4049/jimmunol.176.10.6313. [DOI] [PubMed] [Google Scholar]
- 8.Bliss SK, Alcaraz A, Appleton JA. IL-10 prevents liver necrosis during murine infection with Trichinella spiralis. J Immunol. 2003;171:3142–3147. doi: 10.4049/jimmunol.171.6.3142. [DOI] [PubMed] [Google Scholar]
- 9.Bliss SK, Bliss SP, Beiting DP, Alcaraz A, Appleton JA. IL-10 regulates movement of intestinally-derived CD4+ T cells to the liver. J Immunol. 2007;178:7974–7983. doi: 10.4049/jimmunol.178.12.7974. [DOI] [PubMed] [Google Scholar]
- 10.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 11.Gagliardo LF, McVay CS, Appleton JA. Molting, ecdysis, and reproduction of Trichinella spiralis are supported in vitro by intestinal epithelial cells. Infect Immun. 2002;70:1853–1859. doi: 10.1128/IAI.70.4.1853-1859.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect Immun. 2001;69:4898–4905. doi: 10.1128/IAI.69.8.4898-4905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McFarlane E, Perez C, Charmoy M, Allenbach C, Carter KC, Alexander J, Tacchini-Cottier F. Neutrophils contribute to development of a protective immune response during onset of infection with Leishmania donovani. Infect Immun. 2008;76:532–541. doi: 10.1128/IAI.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunay IR, DaMatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1+ inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson-Lindbom B, Svensson M, Wurbel M-A, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothenberg ME, Hogan SP. The eosinophil. Annual Review of Immunology. 2006:24. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarti A, Rusu D, Flamand N, Borgeat P, Poubelle PE. Reprogramming of a subpopulation of human blood neutrophils by prolonged exposure to cytokines. Lab Invest. 2009 doi: 10.1038/labinvest.2009.74. [DOI] [PubMed] [Google Scholar]
- 18.Jaruga B, Hong F, Sun R, Radaeva S, Gao B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- 19.Kato A, Yoshidome H, Edwards MJ, Lentsch AB. Reduced hepatic ischemia/reperfusion injury by IL-4: potential anti-inflammatory role of STAT6. Inflamm Res. 2000;49:275–279. doi: 10.1007/PL00000207. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Lavoie-Lamoureux A, Moran K, Lavoie J-P. IL-4 stimulates the expression of CXCL-8, E-selectin, VEGF, and inducible nitric oxide synthase mRNA by equine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1147–1154. doi: 10.1152/ajplung.00294.2006. [DOI] [PubMed] [Google Scholar]
- 21.Kim H-J, Higashimori T, Park S-Y, Choi H, Dong J, Kim Y-J, Noh H-L, et al. Differential effects of interleukin-6 and -10 on Skeletal Muscle and Liver Insulin Action In Vivo. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 22.LeMoine O, Marchant A, DeGroote D, Azar C, Golman M, Deviere J. Role of defective monocyte interleukin-10 release in tumor necrosis factor-alpha overproduction in alcoholics cirrhosis. Hepatology. 1995;22:1436–1439. [PubMed] [Google Scholar]
- 23.Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes KA, Andrew EM, Newton DJ, Tramonti D, Carding SR. A subset of IL-10-producing γδ T cells protect the liver from Listeria-elicited, CD8+ T cell-mediated injury. European Journal of Immunology. 2008;38:2274–2283. doi: 10.1002/eji.200838354. [DOI] [PubMed] [Google Scholar]
- 25.Alford SK, Longmore GD, Stenson WF, Kemper C. CD46-induced immunomodulatory CD4+ T cells express the adhesion molecule and chemokine receptor pattern of intestinal T cells. J Immunol. 2008;181:2544–2555. doi: 10.4049/jimmunol.181.4.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]