I. INTRODUCTION
The association between children’s exposure to marital conflict and externalizing behavior has been thoroughly documented in cross-sectional and longitudinal studies with diverse methodologies (for a review, see Cummings & Davies, 2002). The current “second generation” of marital conflict research aims to investigate the mechanisms explaining the impact of marital conflict on children’s externalizing behaviors and other adjustment problems (Davies, Harold, Goeke-Morey, & Cummings, 2002). Emotional security (Davies & Cummings, 2006), social learning (Bandura, 1977), cognitive–contextual (Grych & Fincham, 1990), and specific emotions (Crockenberg & Langrock, 2001) theories have each elucidated processes through which marital conflict can promote aggressive and dysregulated behavior. However, few studies have attempted to account for individual differences in the relation between marital conflict and child adjustment, aside from investigations of age and gender as moderators, and even fewer studies have examined physiological activity as a moderator of effects in this context.
Despite the relatively robust nature of the association between marital conflict and externalizing problems, many children exposed to marital conflict do not develop externalizing problems, and even among children who do exhibit externalizing behavior in the context of marital conflict, significant variability exists (Cummings & Davies, 2002). Steinberg and Avenevoli (2000) posited that individual differences in physiological responding might modulate the type and degree of maladjustment among children exposed to environmental stress such as marital conflict. That is, certain patterns of arousal and regulation, both inherited and acquired through experience, may operate as vulnerability or protective factors in the context of marital conflict. In this monograph, we focus on the two main branches of the autonomic nervous system (ANS): the sympathetic and parasympathetic nervous systems (SNS and PNS). The SNS and PNS are key components of the human stress response system and may be individual difference variables that increase or decrease susceptibility to externalizing problems associated with marital conflict (El-Sheikh, 2005a). Indeed, although influenced by environmental stress during early childhood, recent studies indicate moderate stability in ANS responding by middle childhood, including evidence for stability of PNS activity (respiratory sinus arrhythmia [RSA]; El-Sheikh, 2005b) and SNS activity (skin conductance level [SCL]; El-Sheikh, 2007). Accordingly, ANS functioning is a potential individual difference factor at the level of physiological responding that might moderate (rather than mediate) the effect of marital conflict on child adjustment (see Calkins & Keane, 2004).
There are particular gaps and inconsistencies in the study of relations between marital conflict, ANS responding, and children’s externalizing problems. This monograph is designed to address these gaps in a series of studies, advancing explanatory models for externalizing behavior problems in middle childhood. Consistent with these aims, we examine interactions among marital conflict, SNS activity, and PNS activity in the prediction of child externalizing problems (see our conceptual model, Figure 1). Compared with the study of the activity of either system alone, we expect that simultaneously considering the activity of both ANS branches will better account for the influence of marital conflict and physiological stress responses on behavioral adjustment. Indeed, investigations of physiological systems as independent entities are inevitably limited because physiological systems do not operate in isolation from one another. Rather, multiple physiological systems are in a perpetual process of coordinated fine-tuning to meet individual and environmental needs (Bauer, Quas, & Boyce, 2002).
Figure 1.
Conceptual Model.
Coordinated action of physiological systems serves homeostatic functions under ideal circumstances. However, under conditions of intense or chronic stress in which stress response systems are excessively activated, physiological systems may become dysregulated and uncoordinated, contributing to psychiatric disorders and the behavioral precursors to such disorders, such as child externalizing problems (Bauer et al., 2002). Exposure to marital conflict activates children’s stress response systems, including sympathetic and parasympathetic branches of the ANS (El-Sheikh, 2005b; El-Sheikh, Harger, & Whitson, 2001). Moreover, the premise of this monograph is that individual differences in the joint action of these physiological systems might shape the effects of marital conflict on children. As such, the current studies advance models that have linked patterns of SNS and PNS activity with adjustment or performance across behavioral and psychological domains (Beauchaine, Gatzke-Kopp, & Mead, 2007; Berntson, Cacioppo, & Quigley, 1991; Porges, 2007). Specifically, this monograph investigates SNS × PNS interactions and examines whether the association between marital conflict and child externalizing behavior is moderated by these multisystem interactions.
SNS ACTIVITY: SCL AND REACTIVITY
The SNS is activated during times of stress, equipping the body for a fight-or-flight response by increasing heart rate and oxygen flow throughout the body (Boucsein, 1992). However, there are individual differences in the types of stressors that elicit SNS activity, as well as individual differences in intensity and duration of SNS activation (Fowles, Kochanska, & Murray, 2000). In general, SNS activation in response to stress is considered adaptive because it promotes coping in the face of threat or environmental challenge. However, chronic and prolonged SNS reactivity incurs “wear and tear” on the body’s stress response system and has been associated with multiple negative health and adjustment outcomes (McEwen, 1998; McEwen & Seeman, 1999; Porges, 1997).
Skin conductance refers to electrodermal activity caused by the activity of sweat glands. These sweat glands are innervated solely by the SNS component of the ANS. SNS activity can be assessed by observing baseline levels or changes in SCL from baseline to challenge conditions, referred to as SCL reactivity (SCL-R).
Prior research suggests the importance of studying baseline levels of SNS activity (e.g., baseline SCL) in relation to children’s externalizing symptoms. For example, children with disruptive behavior disorder have lower baseline SCL than do controls (van Goozen, Matthys, Cohen-Kettenis, Buitelaar, & van Engeland, 2000). This association persists into adolescence (van Bokhoven, Matthys, van Goozen, & Engeland, 2005). However, few studies have examined the role of baseline SCL in the context of family discord or in relation to baseline activity or reactivity of the PNS. This is an important gap in research that the present studies address.
SCL-R has been used in numerous investigations of stress reactivity among children, adolescents, and adults. For example, consistent relations have emerged linking SCL-R and internalizing symptoms among children. Greater SCL-R in response to mildly frightening stimuli is associated with self-reported symptoms of anxiety in adolescents (Weems, Zakem, Costa, Cannon, & Watts, 2005), and greater SCL-R has been linked to greater shyness and inhibition (Kagan, Reznick, & Snidman, 1987) and to internalizing symptoms in children (El-Sheikh, 2005a). The literature linking SCL-R with childhood externalizing behaviors has been less consistent (Lorber, 2004; Scarpa & Raine, 1997). Some childhood studies report that higher levels of SCL-R are associated with children’s reactive aggression (Hubbard et al., 2002) and externalizing problems (El-Sheikh, 2005a), but more studies have found that lower levels of SCL-R are associated with child externalizing problems (Fung et al., 2005; Herpertz et al., 2005; McBurnett, 1992; Snoek, van Goozen, Matthys, Buitelaar, & van Engeland, 2004; van Goozen, Matthys, Cohen-Kettenis, Gispen-de Wied, & van Engeland, 1998).
According to the results of a recent meta-analysis (Lorber, 2004), individuals with (nonaggressive) conduct problems exhibit lower resting electrodermal activity, and lower electrodermal reactivity to tasks, as compared with individuals without conduct problems. Longitudinal research is also supportive of the association between electrodermal underarousal and conduct problems. For example, adult criminals showed significantly lower electrodermal arousal during middle adolescence as compared with adults without a criminal record (Raine, Venables, & Williams, 1990). In addition, Beauchaine and colleagues have shown that preschoolers, elementary-age children, and adolescents with clinical levels of conduct problems exhibit attenuated baseline levels of SNS-linked cardiac activity (i.e., lengthened cardiac preejection periods [PEPs]) at baseline and during reward conditions (Beauchaine et al., 2007; Crowell et al., 2006).
Several theories have been put forth to explain the association between low SNS arousal and externalizing behaviors. Stimulation-seeking theory posits that low arousal is perceived as an unpleasant physiological state (Zuckerman, 1969, 1974). Individuals with abnormally low arousal levels therefore engage in risky and antisocial behaviors to increase their arousal to normal levels. Fearlessness theory, an alternative perspective, suggests that low arousal in stressful circumstances indicates low sensitivity to punishment or aversive consequences (Raine, 2002) and corresponding failure to inhibit antisocial behavior.
It is possible that different findings concerning the association between electrodermal arousal and externalizing behaviors can be reconciled by considering characteristics of the samples under investigation. Many studies finding evidence for electrodermal underarousal among children with conduct problems have been conducted with males who have diagnosable mental disorders or a history of criminal activities (Raine et al., 1990). For example, Fung et al. (2005) showed that psychopathy-prone adolescent males had lower skin conductance responding than control participants in anticipation and response to white-noise bursts. Herpertz et al. (2005) found that boys with conduct disorder and conduct disorder plus attention-deficit/hyperactivity disorder (ADHD) reported lower levels of emotional response to aversive stimuli and lower electrodermal responding than children with ADHD or no diagnosis. Studies with subclinical samples, in contrast, have more consistently shown evidence for a link between heightened SCL-R and externalizing problems, particularly aggression (El-Sheikh, 2005a; Hubbard et al., 2002).
A potential subtype of children with antisocial behavior and callous-unemotional traits (e.g., lack of guilt and empathy, constricted emotional expression) can be characterized by attenuated sympathetic arousal in response to stress. In contrast, another subtype of children who exhibit impulsive, dysregulated antisocial behavior (e.g., reactive aggression) but not callous-unemotional traits can be characterized by heightened sympathetic reactivity (Frick et al., 2003; Frick & Ellis, 1999). Indeed, Blair (1999) found that children with “emotional and behavioral difficulties” (EBD) plus psychopathic traits (i.e., callous-unemotional traits) showed SCL hyporesponsivity to distress cues, as compared with control children. In contrast, children with EBD and low psychopathic tendencies did not show SCL hyporesponsivity.
Whereas sample characteristics have been discussed as potential sources of the inconsistent findings (El-Sheikh, 2005a), it is also possible that evaluating SCL and SCL-R in the context of family stress may clarify discrepant findings. That is, as a potential marker of biological sensitivity to context (Boyce & Ellis, 2005), increased SCL and SCL-R may emerge as vulnerability factors for externalizing problems particularly in the context of high marital conflict and other family disruptions. To our knowledge, however, there are only two existing studies that have examined associations among children’s SCL-R in the context of marital conflict and their behavioral adjustment. El-Sheikh (2005a) examined SCL-R in response to an interadult argument as a mediator and a moderator of the relation between marital conflict and children’s (ages 6–12) externalizing problems. In this study, higher SCL-R served as a vulnerability factor for girls’ externalizing problems associated with marital conflict. That is, marital conflict predicted greater child adjustment problems for girls with higher levels of SCL-R.
In a longitudinal study (El-Sheikh, Keller, & Erath, 2007) with the same sample that participated in El-Sheikh (2005a), SCL-R significantly interacted with marital conflict and child gender in predicting changes in externalizing behaviors 2 years later.1 Marital conflict predicted increased externalizing problems over time for all girls (but especially those with higher SCL-R) and for boys with lower SCL-R. Boys with higher SCL-R exhibited increased externalizing behaviors over time regardless of exposure to marital conflict. Thus, for girls, higher SCL-R operated as a vulnerability-reactive factor for externalizing symptoms (Luthar, Cicchetti, & Becker, 2000), such that the disadvantages of higher SCL-R were exacerbated under conditions of higher risk (e.g., marital conflict). For boys, higher SCL-R operated as a vulnerability-stable factor for externalizing behaviors (Luthar et al., 2000), such that the disadvantages of higher SCL-R were stable across varying levels of risk (e.g., marital conflict). These findings highlight the heightened vulnerability for maladjustment over time for children and young adolescents who not only live in homes characterized by higher marital conflict but who also have particularly higher or lower levels of SNS activation in response to challenges and stressors.
Another avenue for understanding risk for externalizing problems involves considering the joint influence or interaction of sympathetic and parasympathetic activity. Indeed, both systems are activated by environmental stress. Considering only the SNS may fail to account for the full influence of the ANS and limit progress in this important area of inquiry.
PNS ACTIVITY: VAGAL TONE AND VAGAL REACTIVITY
Vagal tone (indexed by RSA) and vagal reactivity to challenge (RSA-R) are two commonly used measures of PNS functioning (Bornstein & Suess, 2000; Calkins 1997). Vagal tone refers to baseline functioning, and vagal reactivity refers to changes in RSA from baseline to challenge conditions. Vagal reactivity may be characterized as vagal withdrawal (i.e., decreased RSA) or vagal augmentation (i.e., increased RSA).
Vagal tone reflects the status of the PNS at rest and perhaps the ability to sustain attentional focus, engage in social communication, and maintain homeostasis under normal circumstances (Porges, 1991, 2007). Low vagal tone is characteristic of both externalizing and internalizing problems and thus has been viewed as a nonspecific index of emotion dysregulation in children (Beauchaine, 2001). Vagal withdrawal represents parasympathetic inhibition and reflects awareness of environmental challenge and the mobilization of physiological and attentional resources to mount an active stress response (Bornstein & Suess, 2000; Huffman et al., 1998; Porges, 1996, 2007). Vagal withdrawal accelerates heart rate and increases metabolic output, facilitating engagement or attempts to cope with environmental demands. Vagal augmentation, or parasympathetic activation, in the context of environmental stress may indicate a failure to generate physiological resources that promote engagement with environmental demands. Whereas vagal augmentation is linked with negative adjustment outcomes, vagal withdrawal in stressful circumstances may promote adaptive coping and emotion regulation and appears to be the more adaptive response to environmental challenges (Porges, 2007).
Vagal measures are influenced by various environmental experiences and can be directly associated with child outcomes. For example, lower vagal withdrawal is related to maternal negative and controlling behavior (Calkins, Smith, Gill, & Johnson, 1998), parental marital conflict (El-Sheikh et al., 2001), child internalizing and externalizing problems (Calkins & Dedmon, 2000; El-Sheikh & Whitson, 2006), and child sleep disruptions (El-Sheikh & Buckhalt, 2005). Conversely, greater vagal withdrawal to an audiotaped argument has been found to predict decreased externalizing problems concurrently among 8–11-year-olds (El-Sheikh et al., 2001) and decreased internalizing problems over a 2-year period among 6–14-year-olds (El-Sheikh & Whitson, 2006). Whereas moderate vagal withdrawal appears adaptive, extremely intensive or prolonged vagal withdrawal may be a marker of over-reactivity (Beauchaine, 2001).
In addition to its direct association with child adjustment, there is growing evidence that vagal withdrawal functions as a protective factor against, and vagal augmentation functions as a vulnerability factor for, child adjustment problems in the context of marital conflict (El-Sheikh & Whitson, 2006; El-Sheikh et al., 2001; Katz & Gottman, 1997; Whitson & El-Sheikh, 2003). For example, vagal withdrawal to a simulated argument protected elementary-age boys against externalizing and health problems associated with verbal and physical marital conflict, respectively (El-Sheikh et al., 2001), and protected elementary-age girls and boys against internalizing problems associated with psychological and physical marital conflict (Whitson & El-Sheikh, 2003). Furthermore, with the same sample of children that participated in Whitson and El-Sheikh (2003), a 2-year longitudinal follow-up indicated that vagal withdrawal to a simulated argument protected girls against internalizing problems associated with earlier exposure to marital conflict (El-Sheikh & Whitson, 2006).2 An additional longitudinal investigation showed that vagal withdrawal buffered children from the negative effects of exposure to marital conflict in physical health and academic domains (Katz & Gottman, 1997). Although moderation or interaction effects are difficult to replicate (Jaccard, Wan, & Turrisi, 1990), the aforementioned findings indicate consistency regarding the protective role of vagal withdrawal, or the vulnerability function of vagal augmentation, in relation to externalizing problems in the context of marital conflict.
Higher baseline vagal tone has also been shown to buffer the negative influence of parental problem drinking and marital conflict on children’s externalizing behaviors in several studies (Blandon & Calkins, 2007; El-Sheikh, 2005a; El-Sheikh et al., 2001; Katz & Gottman, 1995, 1997). Lower vagal tone has been found in children and adolescents with clinical levels of internalizing and externalizing problems (Beauchaine, 2001; Beauchaine et al., 2007), but direct relations between children’s vagal tone and externalizing behaviors have not emerged in several studies using community samples (Calkins, Graziano, & Keane, 2007; El-Sheikh, 2001, 2005a; El-Sheikh et al., 2001; Graziano, Keane, & Calkins, 2007; Whitson & El-Sheikh, 2003). Given these inconsistencies, an intriguing hypothesis is that considering the joint influence of vagal activity along with sympathetic activity may clarify the role of vagal tone as a predictor of externalizing behavior (Beauchaine, 2001).
INTERACTIONS AMONG PHYSIOLOGICAL SYSTEMS
Both skin conductance and vagal functioning have been useful as predictors of child adjustment and, more recently, as moderators of child maladjustment in the context of marital conflict (El-Sheikh et al., 2001, 2007). However, the specificity of hypotheses that can be drawn on the basis of this research has been limited to expecting positive or negative outcomes in association with skin conductance or vagal functioning separately (Beauchaine, 2001). Notably, the two branches of the ANS generally operate concurrently and perform opposing functions: Activation of the PNS decelerates heart rate and reduces physiological arousal, whereas activation of the SNS accelerates heart rate and increases physiological arousal. Considering both branches could allow researchers to characterize stress responses and child adjustment outcomes with greater specificity and appreciation for the sophistication of functioning. Several theories and conceptual models bearing on the coinfluence of sympathetic and parasympathetic branches of the ANS are instructive.
POLYVAGAL THEORY
The Polyvagal Theory (Porges, 1995b, 1997, 1998, 2007) describes the experience of emotion by integrating multiple physiological systems. This theory tracks the evolutionary development of various stress response systems that culminate in a three-tiered system in mammals, allowing sophisticated emotional and social response strategies. As a general principle, the theory posits that vestiges of earlier, less complex stress response systems are available in humans and are activated when more contemporary systems fail or become overwhelmed (Porges, 2007).
According to the Polyvagal Theory, the first response system to evolve was the dorsal vagal complex, or “vegetative vagus” (Porges, 1995b, 1997), which is distinguished by nonmyelinated vagal motor fibers that originate in the dorsal motor nucleus (DMNX) of the brain. It is proposed that DMNX fibers become active only when innervation from the nucleus ambiguus (NA) branch of the vagus, a more recent evolutionary adaptation (discussed below), is withdrawn. In response to threat, the vegetative vagus minimizes oxygen usage and energy demand by slowing heart rate and reallocating energy throughout the body. Thus, vegetative vagus activity results in subsequent behavioral responses characteristic of reptiles, such as freezing or feigning death in the service of avoidance.
The next evolutionary development is the SNS, which fosters mobilization. To prepare the body for action, the SNS increases cardiac output and sweat gland secretion while simultaneously inhibiting gastrointestinal tract activity (Porges, 1997). Thus, the body shifts energy from normal homeostatic functions to allow an active behavioral response. The most recent evolutionary development involves the ventral vagal complex, or “smart vagus.” This complex includes the myelinated vagus and portions of other cranial nerves originating in the NA, which project to various organs in the body (Porges, 1995a). The trigeminal and facial nerves are also commonly considered part of this complex (Porges, 1997, 1998). Collectively, this system controls facial expression, sucking, swallowing, listening, and vocalization (Kettunen, Ravaja, Naatanen, & Keltikangas-Jarvinen, 2000; Porges, 1998) and thus has been described as the social engagement system (Porges, 2007).
In addition, activity of the ventral vagal complex exerts an inhibitory influence on the heart, and its withdrawal stimulates a heart rate increase, independent of sympathetic activity. The myelination of vagal fibers originating in the NA allows for firm control and speed in responding to the environment. Thus, the “vagal brake” can be withdrawn or instated to produce rapid changes in cardiovascular output to meet environmental demands without engaging the SNS (Porges, 2007). Furthermore, the ventral vagal complex allows for a metabolically conservative response to the environment by promoting incremental changes in heart rate to support regulated emotional responses (Doussard-Roosevelt & Porges, 1999).
According to the Polyvagal Theory (Porges, 2007), when confronted with a challenge, mammals automatically respond by first orienting then disengaging the vagal brake, inhibiting parasympathetic influence. This response results in a rapid increase in heart rate that allows the individual to engage attention in the environment, gather information, and/or use appropriate social strategies (such as enlisting complex emotions) to ameliorate the threat. If the challenge diminishes, the vagal brake can quickly reengage to reduce arousal and minimize metabolic expenditure. This ability to transiently engage and disengage with the environment allows for temporary shifts in energy, such as those required for the listening and communication phases of social interaction (Porges, 1998). However, if the stressor is intense or chronic, then the SNS may be activated. This engagement allows for “fight or flight” behaviors but is consequently more metabolically demanding than the initial vagal response. Likewise, if the sympathetic response is not sufficient to meet external challenge, then the dorsal vagal complex may engage, resulting in an immobilization response such as freezing. Although this framework is helpful as a general guideline, the progression does not occur in simple discrete steps; instead, it is characterized by “transitional blends” among systems (Porges, 1998). Thus, even when the PNS is adaptively regulating arousal, one or both of the other systems may be activated. Research has firmly established that stress-induced changes in heart rate can be caused by parasympathetic withdrawal, sympathetic engagement, or a combined action of the systems (Cacioppo, Uchino, & Berntson, 1994).
This three-tiered conception of autonomic responding provided by the Polyvagal Theory leads to our assertion that individuals who are more adept at regulating arousal via the PNS may be able to produce an adaptive behavioral response (e.g., flexible, appropriately aroused, and soothable) in the context of marital conflict (a chronic stressor). In addition, these individuals avoid the metabolic expenditure and health risks associated with resorting to engagement of the SNS or the dorsal vagal complex. For example, current research suggests that during the body’s response to stress, it is excessive sympathetic reactivity that is detrimental to the organs of the body rather than the arousal stimulated by the removal of parasympathetic influence (Burns, Friedman, & Katkin, 1992; Cacioppo et al., 1995). This is asserted because the effects of stress on the heart and gastrointestinal tract are thought to be caused by the secretion of catecholamines, hormones associated with sympathetic activity (Baum, Davidson, Singer, & Street, 1987; Taggart & Carruthers, 1971; Uchino, Cacioppo, & Kiecolt-Glaser, 1996). The protective effects of vagal tone and vagal withdrawal also can be explained in part by their relation with rapid cardiovascular recovery instead of prolonged reactivity (Brosschot & Thayer, 1998). Generally, a more adaptive response to stress is described as short in latency, potent in response, and rapid in recovery that matches the demands of the environment (Brosschot & Thayer, 1998; Dienstbier, 1989; Gunnar & Donzella, 1999; Mayne, 1999). Individuals with higher vagal tone and greater vagal withdrawal are proposed to have more organized responses to stress, with shorter latency and greater magnitude of response, exhibiting a rapid transitory pattern (Porges, 1991, 1995a). In other words, the greater the physiologic variability in heart rate (a correlate of higher vagal tone), the greater the potential for the individual to react to the environment with an appropriate response and sooth aroused emotions (Porges, 1992).
AN INTEGRATED MODEL OF ANS FUNCTIONING IN PSYCHOPATHOLOGY
Despite evidence for the importance of each of the ANS subsystems, it is also clear that they do not operate alone, and each often works alongside other physiological response systems. Beauchaine’s (2001) conceptualization of interactions between sympathetic and parasympathetic systems highlights the shortcomings of using a single physiological system to predict child outcomes. As discussed by Beauchaine (2001), measures of vagal tone and vagal withdrawal have been associated with a diverse range of negative child outcomes, including both internalizing (e.g., anxiety, panic, depression) and externalizing (e.g., anger, aggression, disruptive behavior) problems. Thus, vagal functioning is best conceptualized as a general index of appropriate engagement with the environment and emotion regulation, germane to social competence and both internalizing and externalizing problems (Cole, Fox, Zahn-Waxler, Usher, & Welsh, 1996). To determine the specific behavioral form in which vagal dysregulation manifests, sympathetic response patterns must also be considered (Beauchaine, 2001).
Beauchaine posited that the behavioral activation and behavioral inhibition systems of motivation (Gray, 1987), both tightly intertwined with the SNS, interact with PNS functioning to predict child behavioral outcomes. Beauchaine reviewed empirical and theoretical support for patterns of PNS (i.e., vagal) activity in conjunction with SNS activity that are characteristic of several common psychopathologies. For example, aggression may be characterized by low SNS activity accompanied by either low vagal tone or abnormally high vagal withdrawal, which both reflect PNS inhibition.
Beauchaine and colleagues have also provided empirical evidence that children with conduct disorder plus ADHD exhibit lower electrodermal reactivity (reduced punishment sensitivity) and lower PEP (reduced reward sensitivity), both reflecting SNS inhibition, and lower vagal tone (poor emotion regulation), reflecting PNS inhibition (Beauchaine, 2001; Crowell et al., 2006). Conversely, higher electrodermal reactivity and higher vagal tone conferred partial protection from conduct problems (Shannon, Beauchaine, Brenner, Neuhaus, & Gatzke-Kopp, 2007). Beauchaine proposed that the progression of inherited impulsivity (reflected in sympathetic underarousal) to conduct problems is contingent upon family processes that affect the development of vagal regulation of emotion. High levels of conflict escalation and negative reinforcement of children’s arousal and aggression were proposed to increase risk for conduct problems via the development of poor vagal regulation of emotion (e.g., low vagal tone; Beauchaine et al., 2007).
Thus, according to this model, SNS and PNS coinhibition is the product of both inherited vulnerabilities and a high-risk familial context. The outcome in early to middle childhood is angry, impulsive, and hyperactive behaviors (i.e., symptoms of conduct disorder and ADHD) and poor vagal modulation of emotion (Beauchaine et al., 2007). By middle to late childhood, it would seem that such an autonomic response pattern, and corresponding emotion dysregulation, could become relatively stable and exacerbate the influence of marital conflict on children, as proposed in this monograph (see El-Sheikh, 2005b; El-Sheikh, 2007).
THE DOCTRINE OF AUTONOMIC SPACE
Another influential model concerning the joint action of sympathetic and parasympathetic systems was proposed by Berntson et al. (1991) and Berntson and Cacioppo (2004). These authors proposed the doctrine of autonomic space, a two-dimensional model of autonomic control, which conceptualized sympathetic and parasympathetic reactivity as flexible. That is, reactivity across the SNS and PNS can be characterized as reciprocal or nonreciprocal. Because SNS and PNS activation affect and reflect opposing levels of physiological arousal, prior conceptualizations had assumed coupled, reciprocal control, such that heightened activity in one branch was lawfully associated with decreased activity in the other branch. With regard to this assumption, Berntson et al. (1991) noted that exceptions to this pattern of autonomic control had been demonstrated, especially in response to psychological stressors experienced in daily life (Berntson & Cacioppo, 2004), warranting a more complex model of sympathetic and parasympathetic conjoint action on dually innervated organs.
Reciprocal activation refers to conditions under which both branches of the ANS promote the same directional response in a target organ or system (e.g., cardiovascular system). Reciprocal sympathetic activation involves sympathetic activation and parasympathetic inhibition, both of which upregulate physiological processes such as heart rate and cardiovascular output. By comparison, reciprocal parasympathetic activation is characterized by sympathetic inhibition and parasympathetic activation, both of which downregulate similar physiological processes, serving calming functions. Nonreciprocal activation refers to conditions under which branches of the ANS promote opposing responses in target systems. Specifically, coactivation refers to increased sympathetic and parasympathetic action, and coinhibition refers to decreased action of both branches. Because sympathetic and parasympathetic actions serve opposing physiological functions, such parallel, or nonreciprocal, activation actually produces opposing physiological outcomes.
According to Berntson et al. (1991), modes of reciprocal activation can produce strong, unidirectional changes in the system under autonomic influence. Thus, reciprocal sympathetic activation may be well suited for adjustments to challenge or stress, particularly when the necessary coping response is well defined, whereas reciprocal parasympathetic activation may be most appropriate for situations in which a calm physiological state is beneficial. Modes of nonreciprocal activation, on the other hand, yield a more ambivalent physiological response because the action of ANS branches is in opposition. Indeed, in the case of coactivation or coinhibition, it is possible that little or no change in the state of the system would occur if the relative activation of sympathetic and parasympathetic branches was equivalent. Thus, nonreciprocal modes may operate to preserve the baseline functional state of an organ or system in situations without challenge or stress. It is also possible that the nonreciprocal activation occurs when the optimal behavioral response in a novel or challenging situation is unclear to the individual (Berntson et al., 1991).
Several recent studies investigated Berntson and colleagues’ conceptualization of autonomic space. For example, Salomon, Matthews, and Allen (2000) assessed patterns of sympathetic (i.e., PEP) and parasympathetic (i.e., RSA) reactivity to several challenges, or stressors, in a sample of children and adolescents. Responses were relatively stable across tasks and the authors were able to classify participants’ responses according to Berntson and colleagues’ conceptualization. Across tasks, 75% of participants exhibited a reciprocal sympathetic response, consistent with other studies among adults showing that reciprocal sympathetic activation is a normative response to stress (e.g., Berntson et al., 1994). However, youth exhibited several different patterns of autonomic response, and these patterns were differentially associated with measures of family conflict. For example, coactivators reported lower levels of family conflict than reciprocal sympathetic responders and coinhibitors, and parents of coinhibitors reported higher levels of family conflict than parents of reciprocal sympathetic responders (Salomon et al., 2000). Alkon et al. (2003) divided 3–8-year-old children according to the same autonomic profiles in response to laboratory stressors. In this study, only a small proportion of children were characterized by a coactivation profile. Coinhibition and reciprocal parasympathetic profiles became more prevalent with age, whereas the reciprocal sympathetic profile declined with age.
Boyce and colleagues have also conducted innovative research that is informed by the Berntson et al. (1991) conceptualization. For example, Boyce et al. (2001) found that 6–7-year-old children with externalizing behavior problems exhibited coinhibition in response to laboratory challenges, consistent with findings of Beauchaine (2007). Quas, Bauer, and Boyce (2004) examined interactions between autonomic reactivity and experimentally manipulated supportiveness of an adult interviewer as predictors of children’s memory performance. In this study, an autonomic composite score was computed based on PEP and RSA scores (see Boyce et al., 2001), with higher scores indicating reciprocal sympathetic activation. Quas et al. (2004) found that higher reciprocal sympathetic activation was positively associated with correct responses in the high-support condition but negatively associated with correct responses in the low-support condition. Interestingly, these findings are consistent with the biological sensitivity to context model (Boyce & Ellis, 2005), which posits that physiologically reactive children exhibit the most adaptive outcomes in positive social contexts but the least adaptive outcomes in disadvantaged social contexts.
Collectively, these studies suggest that reciprocal sympathetic activation is the most common stress response profile and perhaps associated with the most adaptive outcomes depending on the context. In contrast, coinhibition appears less common and tends to be associated with higher levels of stress exposure and greater risk for externalizing problems. It is important to note that the autonomic space literature refers to SNS-linked cardiac reactivity (e.g., cardiac PEP), whereas we use skin conductance as the marker of SNS activity in this monograph. It is not clear that the autonomic space model can be applied to electrodermal measures such as SCL, and we later return to this issue as a direction for future research. Nevertheless, both SCL and PEP are influenced by the SNS and have been used as indices of SNS activity.
THE PRESENT STUDIES
The models reviewed above have each advanced understanding of multisystem physiological responses to stress and their links with behavioral and psychological functioning. Taken together, these perspectives have guided our views on the meaning of certain patterns of SNS and PNS activation. For example, coactivation may indicate that the parasympathetic response is insufficient for managing the stressor (as reflected in vagal augmentation rather than vagal withdrawal), prompting activation of a significant sympathetic response. Reciprocal sympathetic activation, on the other hand, may indicate an efficient parasympathetic response to stress (as reflected in vagal withdrawal) and a corresponding (moderate) sympathetic response to meet metabolic demands. Coinhibition may indicate vagal withdrawal, allowing PNS activity to meet metabolic demands, yet an insufficient sympathetic response. Reciprocal parasympathetic activation may indicate an efficient and effective calming response to mild stress by the parasympathetic system, requiring little to no sympathetic response.
It is important to note that in this monograph we examine all combinations of SCL and RSA at baseline and in response to laboratory tasks. Thus, we consider baseline and reactivity levels of ANS systems as individual characteristics that can be used collectively to describe profiles of autonomic activity and as concurrent activity patterns. The prefixes “co” and “reciprocal” are used generally to describe both cross-system profiles and simultaneous action across systems. Our inclusion of both baseline and reactivity levels of SNS and PNS activity allows a more comprehensive test of whether ANS activity patterns may moderate the association between marital conflict and child externalizing behavior. Both baseline and reactivity levels of SNS and PNS activity have been linked with various environmental stressors and child developmental outcomes. Furthermore, just as baseline levels of one branch can influence the reactivity of the same branch (law of initial values), it is likely that baseline levels of one branch may influence or interact with reactivity levels of another branch. The present studies provide an initial examination of whether it is useful to assess interactions between baseline levels of one ANS branch and reactivity levels of another ANS branch.
Prior psychophysiological research has investigated patterns of SNS and PNS activity as predictors of child behavioral adjustment, without measurement of the environmental context (for an empirical exception, see Quas et al., 2004; for a conceptual exception, see Beauchaine et al., 2007). A core principle of developmental psychopathology is that child developmental outcomes are best understood in terms of interactions among multiple individual and environmental systems (Masten, 2006). Thus, it may be more informative to examine interactions between autonomic branches as moderators of children’s exposure to environmental stress. The results of such investigations should be more directly informative in regard to autonomic patterns that increase or decrease susceptibility to behavioral maladjustment in the context of family risk. That is, the influence of environmental stress exposure may differ according to the specific pattern of autonomic activity across different autonomic systems.
One common environmental stressor in the family context that has been shown to activate children’s stress response system consistently, across several physiological domains, is exposure to marital conflict. For example, consistent with the sensitization hypothesis (Cummings, 1994), repeated exposure to family conflict is directly associated with physiological responses, including vagal reactivity (El-Sheikh et al., 2001), electrodermal reactivity (El-Sheikh, 2005a), sleep disruptions (El-Sheikh, Buckhalt, Keller, Cummings, & Acebo, 2007), and cortisol level changes (Davies, Sturge-Apple, Cicchetti, & Cummings, 2007; Pendry & Adam, 2007). In addition, as noted above, certain types of physiological responses, including vagal augmentation (El-Sheikh & Whitson, 2006; El-Sheikh et al., 2001) and SCL-R (El-Sheikh, 2007), have been associated with greater externalizing problems in the context of marital conflict. No prior research, however, has examined interactions between sympathetic and parasympathetic functioning as moderators of the relation between marital conflict and child externalizing behavior.
The studies in this monograph investigate interactions between the SNS and PNS as vulnerability and protective factors for externalizing behaviors in the context of marital conflict, representing a novel empirical test of El-Sheikh and colleagues’ developing biopsychosocial framework. Prior work guided by this framework investigated children’s physiological reactivity and regulation as important individual difference variables that moderate the link between exposure to family stress and child adjustment (e.g., El-Sheikh, 2005a; El-Sheikh & Whitson, 2006; El-Sheikh et al., 2001, 2007). This framework is advanced conceptually in the monograph by integrating multisystem psychophysiological models (Beauchaine et al., 2007; Berntson et al., 1991; Porges, 2007) with leading theories in the marital conflict literature (e.g., Emotional Security Theory), which propose that child characteristics can function as moderators of risk. Empirically, this framework is advanced by testing three-way interactions among marital conflict, parasympathetic activity, and sympathetic activity in the prediction of child externalizing problems. As such, El-Sheikh and colleagues’ biopsychosocial framework is encompassed within a broader developmental psychopathology model that conceptualizes child maladjustment as an outcome of transactions among multiple individual and environmental risk factors. Thus, collectively, we have placed this monograph in the context of important theoretical models, while at the same time advancing our own innovative biopsychosocial model conceptually and empirically.
The perspectives on autonomic activity outlined above guided our hypotheses about the patterns of autonomic activity that will operate as vulnerability or protective factors for externalizing problems in the context of marital conflict. We view interactions between SNS and PNS activity as moderately stable in middle childhood (Berntson & Cacioppo, 2004; El-Sheikh, 2005a–c; El-Sheikh, 2007) and expect that ANS interactions have implications for children’s responses to marital conflict. Maladaptive responses are expected to leave children more susceptible to externalizing problems in the context of marital conflict, through processes such as increased sensitization to conflict (Cummings & Davies, 1994) and operant reinforcement of aggression (Snyder, Schrepferman, & St. Peter, 1997; see also Beauchaine et al., 2007).
Consistent with Beauchaine’s (2001) and Beauchaine et al.’s (2007) proposition (albeit with electrodermal rather than cardiovascular measures of SNS activity), we expect that lower SCL (either baseline SCL or SCL-R) in conjunction with lower RSA (either baseline RSA or RSA withdrawal) will be associated with externalizing behaviors. We extend this perspective by considering SNS and PNS activity in the context of exposure to marital conflict and propose that coinhibition (i.e., lower SCL or SCL-R and lower RSA or RSA withdrawal) will accentuate the association between marital conflict and externalizing behaviors. Furthermore, building on Polyvagal Theory (Porges, 1997) and Berntson et al.’s (1991) framework, we propose that coactivation of the SNS and PNS, characterized here by higher SCL or SCL-R in conjunction with either higher RSA or RSA augmentation, reflects a maladaptive and ambivalent stress response and therefore will also predict externalizing behaviors. Further, this pattern of coactivation is expected to amplify the association between children’s exposure to marital conflict and their externalizing behaviors. Conversely, we anticipate that reciprocal sympathetic activation, reflected in sympathetic activation and parasympathetic inhibition (i.e., higher SCL or SCL-R with lower RSA or RSA withdrawal), and reciprocal parasympathetic activation, reflected in parasympathetic activation and sympathetic inhibition (i.e., higher RSA or RSA augmentation with lower SCL or SCL-R), will attenuate the association between marital conflict and child externalizing problems. We expect that these reciprocal patterns of autonomic activity reflect more normative and organized, directional responses to stress at the physiological level and will therefore protect against externalizing problems otherwise associated with marital conflict (see Table 1 for a summary of autonomic response profiles).
TABLE 1.
ANS Profiles
| Profile | SNS Activity | PNS Activity | Net Effect on Physiological Arousal |
|---|---|---|---|
| Reciprocal sympathetic |
Activation (high SCL or SCL-R) |
Inhibition (low RSA or RSA withdrawal) |
Increase |
| Reciprocal parasympathetic |
Inhibition (low SCL or SCL-R) |
Activation (high RSA or RSA augmentation) |
Decrease |
| Coactivation | Activation (high SCL or SCL-R) |
Activation (high RSA or RSA augmentation) |
Ambiguous |
| Coinhibition | Inhibition (low SCL or SCL-R) |
Inhibition (low RSA or RSA withdrawal) |
Ambiguous |
In three separate studies, we examine these hypotheses via three-way interactions among marital conflict, either SCL or SCL-R to lab challenges and either RSA or RSA-R to lab challenges (i.e., all combinations across the SNS and PNS were examined). Reflecting another commonality across these studies, community samples are used. Community samples foster generalization of results and also allow for study of responses covering the full range of the constructs under investigation. Multiple informants are utilized in all three studies. The samples include all socioeconomic status levels and a large representation of African Americans, providing the opportunity for examining research questions in diverse and understudied groups.
Physiologic data are drawn from laboratory stress tasks, including a simulated argument between adults and a problem-solving task. There is a recognized need in the literature to examine physiological reactivity to multiple lab challenges, and our examination of responses to a socioemotional stressor and a problem-solving stressor is responsive to this need. Facilitating comparisons across studies, we used these two identical lab challenges across the three studies. A significant question we address is whether patterns of results will replicate or otherwise be repeated across studies.
II. INTERACTIONS AMONG MARITAL CONFLICT, SYMPATHETIC, AND PARASYMPATHETIC NERVOUS SYSTEMS ACTIVITY IN THE PREDICTION OF CHILDREN’S EXTERNALIZING PROBLEMS
In this study, we examined hypotheses via three-way interactions among marital conflict, skin conductance level (SCL), or SCL reactivity (SCL-R) in conjunction with either respiratory sinus arrhythmia (RSA) or RSA reactivity (RSA-R). Participants were third-grade children and their parents, and the sample was community based, thus covering the entire range of constructs under investigation (e.g., lower and higher levels of marital conflict). Hypotheses were tested using multiple reporters, including mother, father, and child reports of marital conflict and mother, father, and teacher reports of child externalizing problems. Although sharing commonalities with other studies, facilitating study of whether findings can be repeated, Study 1 is also distinguished from other studies in the monograph by its broader coverage of specific dimensions of externalizing behaviors as dependent variables. We examined both parents’ and teachers’ reports of behavior problems in several domains, including hyperactivity/distractibility, aggression, and delinquency.
METHOD
Participants
Participants were 176 children (98 girls and 78 boys) attending the third grade at a public school in the southeastern USA and their parents. Based on information provided by schools, we contacted families. Out of those who met our inclusion criteria, 66% participated in the study, 28% refused to participate, and 6% indicated that they were too busy and asked to be called at a later date. To be included in the study, children had to live in a two-parent household. The average couple had been living together for 10 years (standard deviation [SD] = 5.47). Children’s mean age was 8.69 years (SD = 0.40). Mothers’ mean age was 34.17 years (SD = 5.70), and fathers’ mean age was 36.90 years (SD = 6.59). Families represented the complete spectrum of possible economic backgrounds (Hollingshead, 1975; M = 3.07; SD = 0.89; range: 1–5), with the median income in the US$35,000–50,000 range. European Americans comprised 69% of the sample, and the remaining 31% were African American. With respect to the socioeconomic status (SES) and ethnic composition of the sample, participants were representative of the community from which they were drawn. Families received monetary compensation for their participation.
Procedures and Measures
Families visited the laboratory located in the university campus. Parents completed consent forms while a researcher read the child an assent form. During the visit, parents completed questionnaires about themselves and their family. In addition, children participated in a psychophysiological session during which their physiological responses (i.e., RSA and SCL) were measured in the context of two stressful events: exposure to an audiotaped interadult conflict and a star-tracing task. Physiological sensors (i.e., electrodes attached to the child’s fingers, sides, and chest, and a bellows belt around the child’s chest) were placed on the child while a parent was present. The research assistant talked with both the child and parent while attaching the electrodes to help the child relax (i.e., approximately 10 min). The parent and researcher then left the room and the child was given an additional 2 min to acclimate to the laboratory setting. Following a 3-min baseline assessment, the child was presented with the two challenge conditions, each lasting 3 min, with a recovery period between conditions. The first challenge, socioemotional in nature, involved listening to an audiotaped argument through speakers, which supposedly occurred between a man and a woman next door. To increase generalizability of findings, two themes were used for the arguments: in-laws and leisure activities, and a similar number of boys and girls were exposed to each theme (RSA-R or SCL-R did not differ as a function of argument topic). The arguments were characterized by verbal expressions of anger. Similar scripts have been used in other studies and were effective in inducing RSA withdrawal (El-Sheikh et al., 2001) and SCL-R (El-Sheikh & Cummings, 1992) in children. Of note is that the arguments were used to examine children’s responses to a normative stressor. Substantial literature supports the feasibility, reliability, and validity of analogue procedures used to induce emotional and physiological arousal in children (Cummings & Davies, 2002).
A 12-min recovery period followed the argument task. Next, children completed the second challenge condition, in which the child was asked to trace a star on a piece of paper by looking at the image through a mirror (3 min; Mirror Tracer, Lafayette Instrument Company, Lafayette, Indiana, United States). A board was put across the child’s chair, and the child was given a sheet of paper with a picture of a star. The star was blocked from direct view but visible through a mirror. Children were asked to trace the star using only the mirror image as a visual guide. The examination of children’s responses to both social and nonsocial stressors can provide greater specificity regarding the role of psychophysiological responses (Chen, Matthews, Salomon, & Ewart, 2002). The star-tracing task is a well-established nonsocial laboratory challenge (Matthews, Rakaczky, Stoney, & Manuck, 1987; Matthews, Woodall, & Stoney, 1990), and prior research shows that it is related to individual differences in family risk and child functioning (El-Sheikh et al., 2007). Given our primary focus on individual differences in responding versus differential responding to the two tasks, we chose to use a fixed order of presentation of challenges in all of the monograph studies. Findings should be interpreted within this context.
RSA Data Acquisition and Reduction
Standard guidelines (Berntson et al., 1991) were followed to assess RSA. Two electrocardiography (ECG) electrodes were placed on each rib cage approximately 10–15 cm below the armpits while an additional electrode was placed in the center of the chest to ground the signal. Respiratory changes (chest expansion and compression during breathing) were assessed with a pneumatic bellows that was attached around the participant’s chest and fastened with a beaded chain. A custom bioamplifier from SA Instruments (San Diego, CA) was used during data collection, and the signal was digitized with the Snap-Master Data Acquisition System (HEM Corporation, Southfield, MI) at a sampling rate of 1,000 readings per second. To assess ECG, the bioamplifier was set for bandpass filtering with half power cutoff frequencies of 0.1 and 1,000 Hz, and the signal was amplified with a gain of 500. The Interbeat Interval (IBI) Analysis System from James Long Company (Caroga Lake, NY) was used to process the ECG signal. A pressure transducer with a bandpass of DC to 4,000 Hz was used with the bellows to ensure that no phase or time shifts were introduced in the measurement of respiration.
Identification of R-waves was provided via an automated algorithm. An interactive graphical program was used to allow manual correction of misidentified R-waves, in the rare case that this was needed. R-wave times were then converted to IBIs and resampled into equal time intervals of 125 ms. That is, the absolute times (e.g., r-waves) were determined, and the time between one r-wave to the next was computed (i.e., IBI). Considering individual variations in IBIs, data were resampled at an equal sampling interval of 125 ms. Any IBIs that span 125-ms interval are prorated. The program prorates at every eighth of a second. The prorated IBIs were stored for computation of the mean and variance of heart period as well as assessing heart period variability due to RSA. RSA during baseline and challenge conditions were computed for the entire epoch.
RSA is determined by rhythmic fluctuations in heart period that are accompanied by phases of the respiratory cycle (Grossman, Karemaker, & Wieling, 1991; Grossman & Wientjes, 1986). The peak-to-valley method was used to compute RSA, and all units were in seconds. This method is one of several acceptable methods for quantifying RSA (Berntson et al., 1997). The peak-to-valley method correlates highly with spectrally derived measures of RSA (Galles, Miller, Cohn, & Fox, 2002) and with changes in RSA as produced by pharmacological or surgical blockades, and it has the ability to assess RSA reactivity (RSA-R) during brief time periods (see Berntson et al., 1997, for further information on the advantages of the peak-to-valley method). To identify inspiration and expiration onset, a respiration signal was used. The difference in IBI readings from inspiration to expiration onset was used to compute RSA. Because baseline RSA levels could influence RSA-R (law of initial values), RSA-R was computed as a residualized change score (obtained through regressing baseline RSA on RSA during the challenge tasks). Low values for RSA-R reflect greater RSA withdrawal in response to the tasks.
SCL Data Acquisition
To assess SCL and SCL reactivity (SCL-R; changes in SCL from baseline to challenges), two Ag–AgCI skin conductance electrodes filled with BioGel (an isotonic NaCI electrolyte gel) were attached with small Velcro bands to the volar surfaces of the distal phalanges of the first and second fingers of the child’s nondominant hand (consistent with recommendations of Scerbo, Freedman, Raine, Dawson, & Venables, 1992). To control the area of gel contact, double-sided adhesive collars with a 1 cm hole in the center were used. In order to avoid biasing the electrodes, a constant sinusoidal (AC) voltage (i.e., 0.5 V rms) was used. Children’s SCL was assessed continuously throughout the session at a rate of 1,000 readings/s and was calculated using the James Long Company Software. A 16-channel A/D converter was used to digitize and amplify the signals (i.e., bio amplifier Model MME-4; James Long Company). Averages for SCL responses during the baseline, argument condition, and star-tracing task were obtained. SCL-R was computed as a residualized change score (obtained through regressing baseline SCL on SCL during the challenge tasks). All baseline SCL and SCL-R variables are expressed in microSiemens (µS).
Marital Conflict
Marital conflict was assessed using both parent and child reports. Parents reported their own and their spouses’ verbal and physical aggression in the past year on the Conflict Tactics Scale (CTS2; Straus, Hamby, Boney-McCoy, & Sugarman, 1996). Parents rated how often they used a list of 18 behaviors during conflict, as well as how often their spouse engaged in those behaviors, on a 7-point scale, ranging from 0 (never) to 6 (more than 20). The CTS has well-established reliability and validity (Straus et al., 1996). Owing to constraints placed on the study by the Internal Review Board (IRB) of the university, 10 items were deleted from the physical aggression subscale: (1) had a sprain, bruise, or small cut because of a fight; (2) used a knife or gun; (3) passed out from a hit on the head; (4) went to a doctor because of a fight; (5) choked him or her; (6) needed to see a doctor because of a fight but didn’t; (7) beat up partner; (8) had broken bone because of a fight; (9) burned or scalded partner on purpose; and (10) felt physical pain on the next day because of a fight. Items pertaining to kicking, slapping, grabbing, slamming against the wall, punching, shoving, twisting arms, and throwing objects were permitted to be included. The internal consistency for the CTS was .87 for mother reports and .86 for father reports.
Children provided reports on the Children’s Perceptions of Interparental Conflict Scale (CPIC; Grych, Seid, & Fincham, 1992). The CPIC assesses children’s perceptions and appraisals of marital conflict. The Destructive Conflict scale was used in the current study and consists of 19 items that assess children’s perceptions of the frequency, intensity, and resolution of their parents’ conflicts. Higher scores reflect higher levels of destructive interparental conflict. The CPIC has good internal consistency, test–retest reliability, and is appropriate for school-age children (Grych et al., 1992). In the present study, the internal consistency of this measure was .86.
Children’s reports on the CPIC were significantly correlated with both mothers’ and fathers’ reports of marital hostility (range: .18–.35). To reduce the number of analyses and the probability of Type 1 error, a marital conflict composite score was created by standardizing and summing parent reports on the CTS and child reports on the CPIC. Higher scores reflect higher levels of marital conflict.
Children’s Externalizing Behaviors
Mothers and fathers reported on children’s externalizing behaviors using the Personality Inventory for Children-II (PIC2; Lachar & Gruber, 2001). The PIC2 is a comprehensive revision of the original PIC (Lachar & Gruber, 1995) that is based on a body of research spanning more than 40 years and has been used in more than 4,000 studies. It provides a valuable alternative to exclusive use of the Child Behavior Checklist (CBCL; Achenbach, 1991) for the study of children’s adjustment. In particular, the PIC may be more sensitive to externalizing symptoms falling below the clinical range (El-Sheikh, 2001), making it advantageous for use with community samples. All items are rated as true or false about the child. True responses are summed and converted to T scores. The following scales were used in analyses: Delinquency and Attention-Deficit/Hyperactivity (ADH). The PIC2 Delinquency scale includes 47 items assessing antisocial behavior (e.g., stealing), dyscontrol (e.g., loses temper, becomes violent), and non-compliance (e.g., breaks rules, disobeys). The ADH scale consists of 21 items (e.g., child often forgets things, has problems waiting, jumps from one activity to another). The PIC2 has demonstrated test–retest reliability, interrater reliability, as well as discriminant and construct validity (Lachar & Gruber, 2001; Wirt, Lachar, Klinedinst, & Seat, 1990). For example, El-Sheikh (2001) found that mother-reported externalizing behavior on the PIC was correlated (r = .48, p < .001) with teacher-reported externalizing behavior on the CBCL–Teacher Report Form (Achenbach, 1991). Mother reports of delinquency on the CBCL and PIC were correlated (r = .49, p < .001), as were mother reports of ADH on the CBCL and PIC (r = .44, p < .001). In the current sample, reliability coefficients ranged from .78 to .83. The Delinquency and ADH scale can be combined to provide an overall measure of children’s externalizing symptoms. Based on this composite, 33 children in the sample were within the borderline or clinical range according to at least one parent on the PIC (i.e., T scores ≥ 60).
Teachers’ reports of externalizing behavior in the school setting were obtained through the Student Behavior Survey (SBS; Wingenfeld, Lachar, Gruber, & Kline, 1998), Child Behavior Survey (CBS; Ladd & Profilet, 1996), and the Teacher Checklist for Peer Relations (TCPR; Dodge & Coie, 1987). The SBS is the teacher report of the PIC2. Items are rated on a scale from 1 (student never displays the behavior) to 4 (student usually displays the behavior). Items are summed and converted to T scores. The SBS Conduct Problems scale includes 16 items (e.g., destroys property, starts fights). The Verbal Aggression scale includes seven items (e.g., threatens students) and the Physical Aggression scale includes five items (e.g., hits or pushes other students). Reliability coefficients ranged from .79 to .83. The additional SBS ADH scale (16 items) was also obtained. However, the reliability of this assessment was low (α =.38). Removing the following three items improved the reliability coefficient to .70: (1) daydreams or seems preoccupied; (2) misbehaves unless closely supervised; and (3) impulsive/acts without thinking. A revised score omitting these items was computed and used in analyses. Based on the SBS, seven children were within the borderline or clinical range of behavior problems (i.e., scores ≥ 60). The CBS scales assessing Aggression with Peers (7 items; α = .86) and Hyperactivity/Distractibility (4 items; α = .79) were used in analyses. In addition, teachers’ reports on the TCPR scales that assess Reactive Aggression (3 items; α = .85) and Proactive Aggression (3 items; α = .89) were used in analyses. Multiple teacher-report scales were included to assess whether findings would generalize across subtypes of aggression against peers (i.e., physical and verbal, proactive and reactive) and behavior problems in the classroom setting (oppositional conduct problems, inattentive–hyperactive behavior).
RESULTS
Three-way interaction effects among marital conflict, children’s RSA, and SCL were tested using regression analyses, according to recommendations by Aiken and West (1991). Variables were centered before creating interaction terms. In the first step of hierarchical multiple regression, child age, sex,3 ethnicity, and family SES were entered as covariates because these variables were significantly associated with at least one of the primary study variables. The ethnicity variable was coded as 0 for European American children and 1 for African American children. Because the two variables were associated in this study and others (Amano, Kanda, & Hidetoshi, 2001), analyses using baseline RSA also controlled for child body mass index (BMI). BMI was calculated using laboratory measurements of children’s height and weight (kg/m2). Main effects and all two-way interactions were included along with the three-way interaction term. Significant interactions were interpreted by plotting regression lines 1 SD above and below the mean for marital conflict and the two moderators (RSA/RSA-R, SCL/SCL-R). Outliers (± 3.29 SD) on the outcome variables were identified and deleted, according to recommendations by Tabachnick and Fidell (1996). Q–Q plots were used to examine the distribution of each outcome variable, and skewed dependent variables were log transformed. Specifically, mother and father reported Delinquency; teacher reported Conduct Problems, Verbal Aggression, and Physical Aggression on the SBS; and teacher reported Aggression with Peers and Hyperactive-Distractible on the CBS; were log transformed.
Descriptive Statistics
Means, SDs, and correlations for study variables are shown in Table 1. For clearer communication, RSA and SCL raw change scores are used for descriptive statistics and correlations in Table 2 (and all subsequent tables presenting correlations in Studies 2 and 3); however, as noted, residualized change scores are used in regression analyses. Age and BMI were not included in the correlation table due to their nonsignificant relations with most study variables. As exceptions, older age was correlated with lower marital conflict, r = − .20, p < .05, and higher BMI was correlated with lower baseline RSA, r = − .22, p < .01. As shown in Table 2, marital conflict was positively correlated with fathers’ reports of ADH, r = .23, p < .01, and Delinquency, r = .31, p < .01, on the PIC. Marital conflict was not significantly related to baseline levels of children’s RSA or SCL or with children’s RSA-R and SCL-R.
TABLE 2.
Means, Standard Deviations, and Correlations Among Variables For Study 1
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | 17. | 18. | 19. | 20. | 21. | 22. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Child sex | — | |||||||||||||||||||||
| 2. Ethnicity | −.08 | — | ||||||||||||||||||||
| 3. SES | −.06 | .26** | — | |||||||||||||||||||
| 4. Baseline RSA | −.07 | −.05 | −.03 | — | ||||||||||||||||||
| 5. Baseline SCL | .14 | −.12 | .10 | −.04 | — | |||||||||||||||||
| 6. RSA-R argument task | .10 | −.04 | −.05 | −.35** | −.10 | — | ||||||||||||||||
| 7. RSA-R star-tracing | −.04 | −.05 | .04 | −.43** | .03 | .31** | — | |||||||||||||||
| 8. SCL-R argument task | −.11 | .25** | .11 | .08 | −.02 | −.12 | .00 | — | ||||||||||||||
| 9. SCL-R star-tracing | −.16* | .47** | .15 | .10 | .01 | −.12 | −.07 | .65** | — | |||||||||||||
| 10. Marital conflict | −.08 | −.01 | .05 | .13 | −.04 | −.04 | −.09 | .14 | .09 | — | ||||||||||||
| 11. PIC Delinquencya | −.01 | −.02 | .05 | −.02 | −.14 | −.02 | .02 | .08 | −.02 | .12 | — | |||||||||||
| 12. PIC Delinquencyb | .04 | .03 | .06 | .08 | −.09 | .09 | .01 | .03 | −.08 | .31** | .65** | — | ||||||||||
| 13. PIC ADHa | −.13 | −.01 | .18* | .06 | −.12 | .03 | .03 | .07 | .02 | .17 | .71** | .51** | — | |||||||||
| 14. PIC ADHb | −.02 | .04 | .04 | .01 | −.12 | .05 | .06 | .09 | .00 | .23** | .57** | .77** | .56** | — | ||||||||
| 15. SBS Conduct Problemsc |
.14 | −.11 | −.06 | −.04 | .01 | .15 | .06 | −.04 | −.11 | .02 | .29** | .21* | .15 | .17 | — | |||||||
| 16. SBS ADHc | .18 | −.07 | .21* | .10 | .20* | .04 | −.04 | −.07 | .04 | −.12 | −.10 | −.09 | .05 | −.02 | .04 | — | ||||||
| 17. SBS Verbal Aggressionc |
.12 | −.13 | −.03 | .01 | .04 | .10 | .03 | .06 | .00 | −.07 | .24** | .12 | .05 | .29** | .56** | .09 | — | |||||
| 18. SBS Physical Aggressionc |
.38** | .00 | −.03 | −.18 | .14 | .10 | −.05 | −.11 | −.12 | −.06 | .39** | .10 | .20* | −.01 | .66** | −.01 | .18* | — | ||||
| 19. CBS Aggressive with Peersc |
−.10 | −.17 | −.01 | .00 | .05 | .12 | .09 | −.01 | −.14 | −.06 | .26* | .18 | .16 | .16 | .55** | .01 | .61** | .28** | — | |||
| 20. CBS ADHc | −.21* | −.08 | −.03 | −.02 | .04 | .00 | −.01 | .11 | .00 | .07 | .26** | .27** | .33** | .40** | .39** | −.15 | .30** | .09 | .53** | — | ||
| 21. TCPR Reactive Aggressionc |
−.09 | −.17 | −.05 | .03 | −.06 | .01 | .07 | .05 | −.11 | −.09 | .40** | .17 | .32** | .23* | .54** | −.03 | .60** | .17 | .72** | .44** | — | |
| 22. TCPR Proactive Aggressionc |
−.02 | −.11 | −.08 | .01 | −.13 | .06 | .09 | .04 | .00 | −.07 | .22* | .16 | .17 | .24* | .42** | .10 | .61** | .16 | .58** | .26** | .69** | — |
| Mean | — | — | 3.07 | .13 ms | 11.31 | −.01 ms | −.03 ms | .68 µS | 2.00 µS | .12 | 47.22 | 46.51 | 48.22 | 48.64 | 45.47 | 47.73 | 44.95 | 45.52 | 1.13 | 1.38 | 5.23 | 3.59 |
| SD | — | — | .89 | .07 ms | 3.73 µS | .03 ms | .05 ms | 1.30 µS | 2.37 µS | 6.13 | 6.12 | 6.48 | 7.06 | 8.32 | 2.71 | 3.37 | 5.17 | 2.07 | .21 | .57 | 2.78 | 1.28 |
Note. RSA-R = Respiratory Sinus Arrhythmia Reactivity; SCL-R = Skin Conductance Level Reactivity; PIC = Personality Inventory for Children; ADH = Attention-Deficit/Hyperactivity; SBS = Student Behavior Survey; CBS = Child Behavior Survey; TCPR = Teacher Checklist for Peer Relations. N = 176. ms = milliseconds; µS = microsiemens.
Mother report.
Father report.
Teacher report; sex coded as 1 = boys and 2 = girls; vagal regulation is computed as posttask–pretask levels, so that higher scores reflect augmentation.
p<.05,
p<.01.
Physiological Reactivity
Children’s RSA decreased significantly from the baseline during both the argument task, t(172) = 4.15, p < .01, and star-tracing task, t(169) = 6.56, p < .01. This indicates that, on average, both tasks elicited vagal withdrawal. Fifty-four percent and 74% of children demonstrated withdrawal in response to the argument and star-tracing tasks, respectively. Also, children’s SCL increased significantly from baseline during both the argument task, t(172) = 6.93, p < .01, and star-tracing task, t(169) = 10.97, p < .01. Seventy-five percent and 82% of children demonstrated SCL increases in response to the argument and star-tracing tasks, respectively.
Interactions Among Marital Conflict, Baseline RSA, and Either SCL or SCL-R
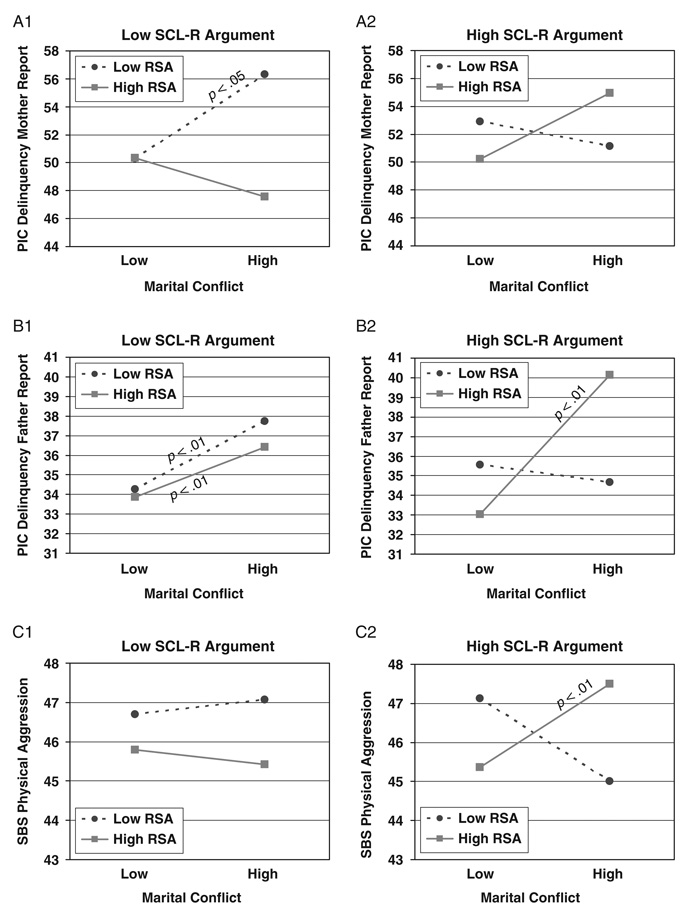
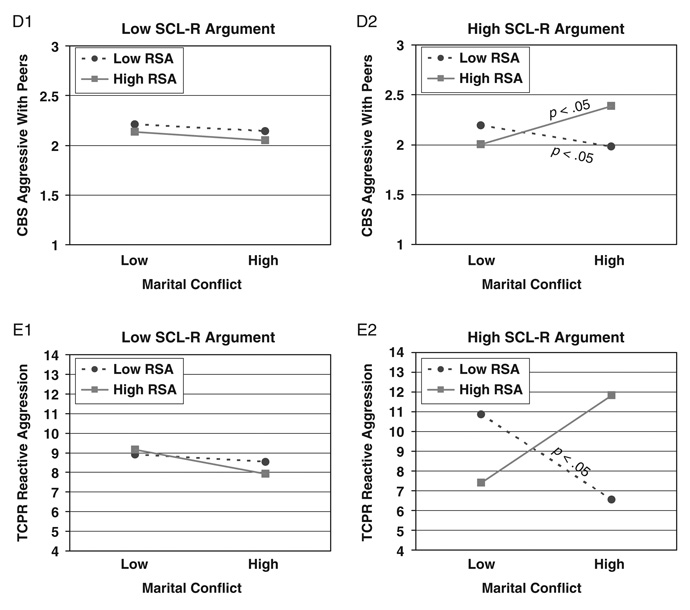
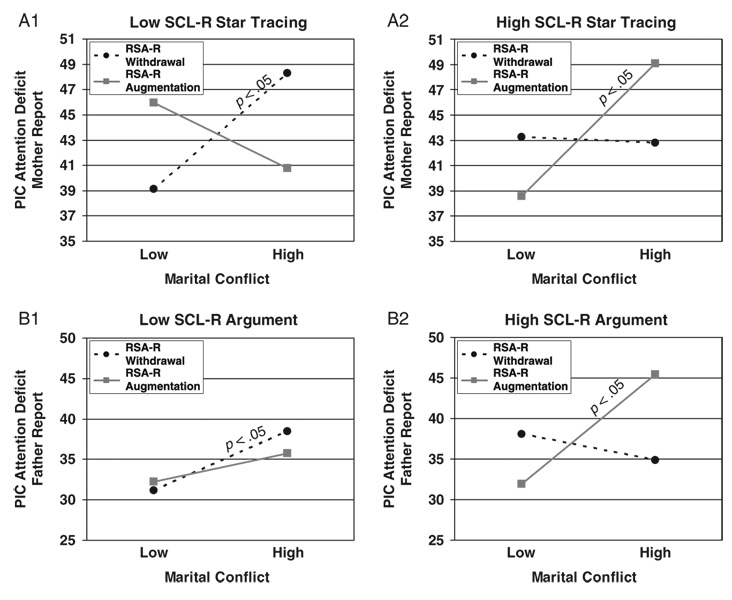
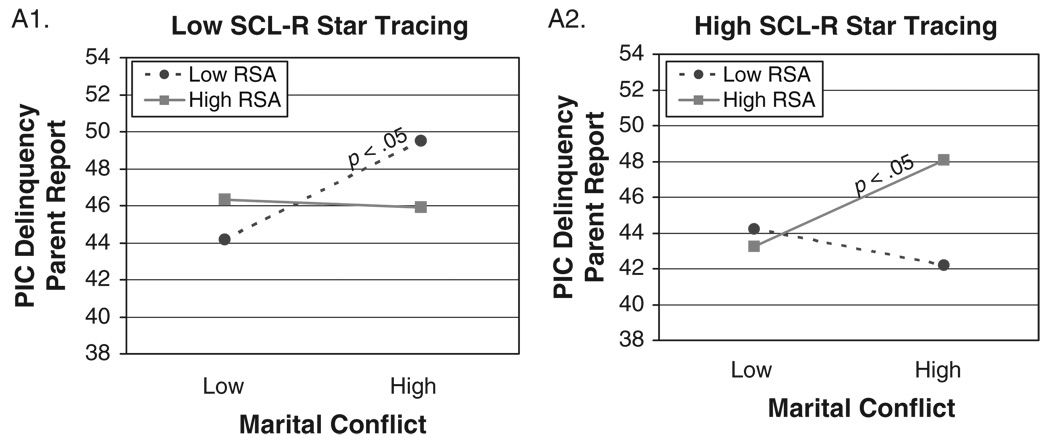
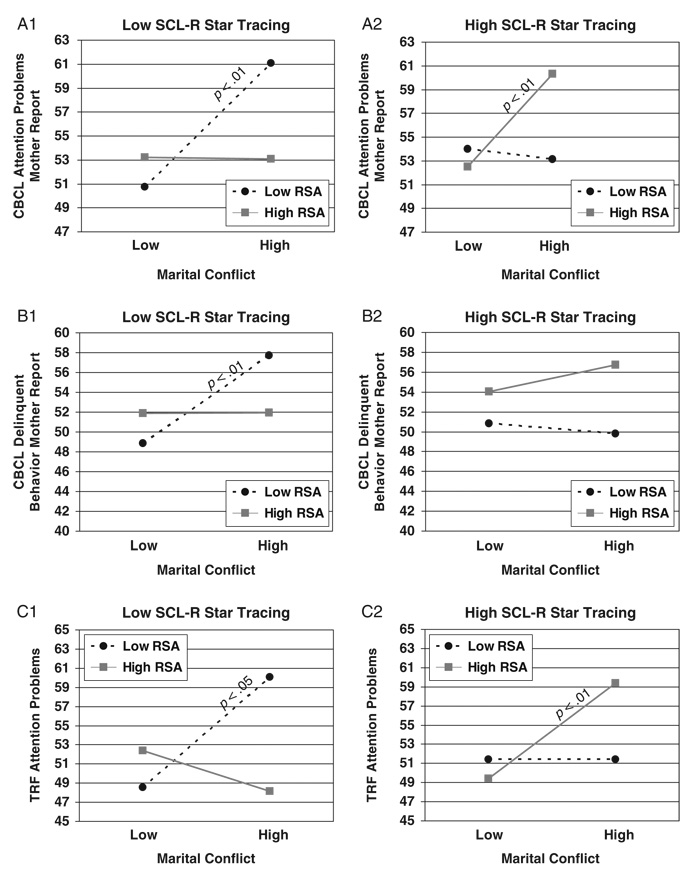
Hierarchical multiple regressions were conducted to examine baseline RSA in conjunction with either SCL or SCL-R as moderators of the association between marital conflict and child adjustment. Child age, sex, ethnicity, SES, and BMI were controlled for in the first step. Marital conflict and the main effects of the moderators were added in the second step; all two-way interactions were entered in Step 3; and the three-way interaction was included in Step 4. As shown in Table 3, 5 out of 24 possible interactions involving baseline RSA in conjunction with SCL-R were significant in predicting children’s externalizing behavior problems,4 as reported by mothers, fathers, and teachers.
TABLE 3.
Study 1: Results for Three-Way Interactions Among Marital Conflict, RSA or RSA-R, and SCL or SCL-R
| Delinquency | ADH | Aggression (TR) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRa | MRa | FRa | MR | FR | TR | Physa | Reacta | Reacta | Peers | |
| Step 1 | ||||||||||
| Age | .00 (.001) | .00 (.001) | .002 (.001) | .02 (.17) | .06 (.20) | −.05 (.08) | .00 (.00) | .00 (.01) | .00 (.004) | .001 (.001) |
| Sex | .01 (.01) | .01 (.01) | .01 (.01) | −.53 (1.48) | −.05 (1.60) | 1.82 (.66)** | .01 (.003)** | −.02 (.04) | −.02 (.04) | −.01 (.01) |
| Ethnicity | .01 (.01) | .002 (.01) | .01 (.01) | .75 (1.81) | .15 (1.81) | −1.18 (.78) | −.003 (.004) | .08 (.04)† | .08 (.04)† | .02 (.01)* |
| SES | .00 (.001) | .00 (.00) | .00 (.001) | .13 (.08)† | .03 (.08) | .06 (.03)† | .00 (.00) | .001 (.002) | .001 (.002) | .00 (.00) |
| BMI | −.002 (.001) | −.002 (.001)† | −.001 (.001) | −.13 (.14) | −.22 (.16) | −.06 (.06) | .00 (.00) | .004 (.003) | .004 (.003) | .001 (.001) |
| R2 | .03 | .03 | .03 | .04 | .02 | .14 | .22 | .05 | .05 | .07 |
| Step 2 | ||||||||||
| Marital Conflict | .001 (.001) | .001 (.001) | .003 (.001)** | .20 (.12)† | .32 (.13)* | −.08 (.06) | .00 (.00) | −.002 (.003) | −.002 (.003) | .00 (.001) |
| RSA | −.11 (.08) | .03 (.08) | −.03 (.03) | .15 (.31) | .02 (.07) | |||||
| RSA-R (AR) | 18.27 (27.02) | 1.61 (11.25) | −.05 (.60) | |||||||
| RSA-R (ST) | .02 (.10) | 8.45 (14.54) | ||||||||
| SCL | ||||||||||
| SCL-R (AR) | .01 (.004) | .001 (.004) | .43 (.56) | .34 (.23) | −.00 (.001) | −.003 (.012) | −.002 (.01) | −.001 (.003) | ||
| SCL-R (ST) | .001 (.002) | .01 (.33) | ||||||||
| R2 | .08 | .05 | .12 | .07 | .08 | .17 | .24 | .06 | .06 | .07 |
| ΔR2 | .05 | .02 | .08* | .03 | .06† | .03 | .02 | .01 | .01 | .004 |
| Step 3 | ||||||||||
| Conflict × RSA |
−.01 (.02) | .01 (.02) | .003 (.01) | .05 (.07) | .02 (.02) | |||||
| Conflict × RSA-R (AR) |
1.96 (4.15) | .83 (2.18) | .03 (.11) | |||||||
| Conflict × RSA-R (ST) |
.02 (.03) | −1.22 (4.26) | ||||||||
| Conflict × SCL | ||||||||||
| Conflict × SCL-R (AR) |
.00 (.00) | −.001 (.00) | −.12 (.07)† | .02 (.03) | .00 (.00) | .00 (.001) | −.001 (.002) | .00 (.00) | ||
| Conflict × SCL-R (ST) |
.00 (.00) | −.001 (.04) | ||||||||
| RSA × SCL | ||||||||||
| RSA × SCL-R (AR) |
.04 (.05) | .01 (.05) | .02 (.02) | −.09 (.21) | .04 (.05) | |||||
| RSA × SCL-R (ST) | ||||||||||
| RSA-R (AR) × SCL | ||||||||||
| RSA-R (ST) × SCL | ||||||||||
| RSA-R (AR) × SCL-R (AR) |
16.26 (21.71) | −3.89 (9.75) | −.59 (.51) | |||||||
| RSA-R (ST) × SCL-R (ST) |
.04 (.06) | . | −.54 (8.38) | |||||||
| R2 | .09 | .06 | .14 | .07 | .12 | .18 | .25 | .07 | .08 | .10 |
| ΔR2 | .01 | .01 | .02 | .00 | .04 | .01 | .01 | .01 | .02 | .024 |
| Step 4 | ||||||||||
| Conflict × RSA × SCL | ||||||||||
| Conflict × RSA × SCL-R (AR) |
.03 (.01)* | .03 (.01)* | .01 (.00)* | .11 (.05)* | .03 (.01)* | |||||
| Conflict × RSA × SCL-R (ST) | ||||||||||
| Conflict × RSA × SCL-R (ST) | ||||||||||
| Conflict × RSA-R (ST) × SCL | ||||||||||
| Conflict × RSA-R (AR) × SCL-R (AR) |
10.27 (4.61)* | 4.38 (2.07)* | .24 (.11)* | |||||||
| Conflict × RSA- R(ST) × SCL-R (ST) |
.03 (.02)* | 4.61 (2.18)* | ||||||||
| R2 | .13 | .10 | .17 | .10 | .16 | .22 | .31 | .12 | .13 | .17 |
| ΔR2 | .04* | .04* | .03* | .04* | .04* | .04* | .06* | .05* | .05* | .07* |
Note. ADH = Attention-Deficit/Hyperactivity; TR = teacher report; MR = mother report; FR = father report; React = reactive aggression; SCL-R = skin conductance level reactivity; RSA-R=respiratory sinus arrythmia reactivity; AR = argument task; ST = star-tracing task. Unstandardized coefficients reported (standard errors reported in parentheses).
Log transformation of dependent variable used in analyses.
p<.10,
p<.05,
p<.01.
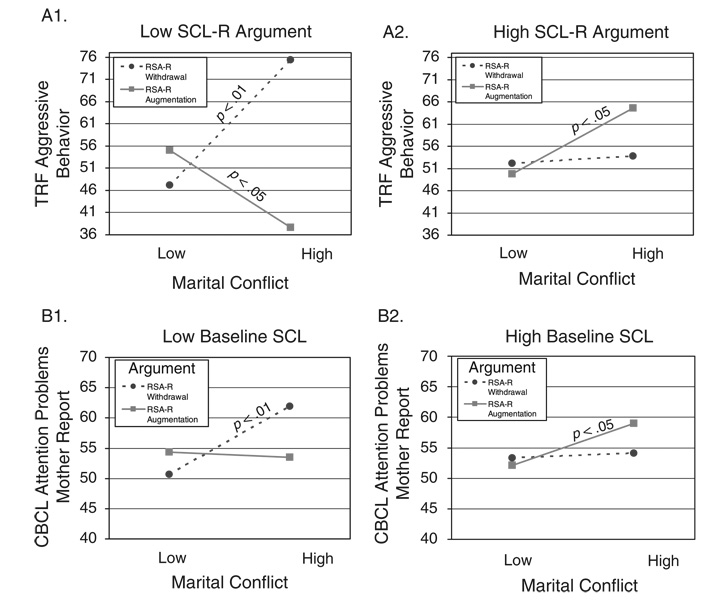
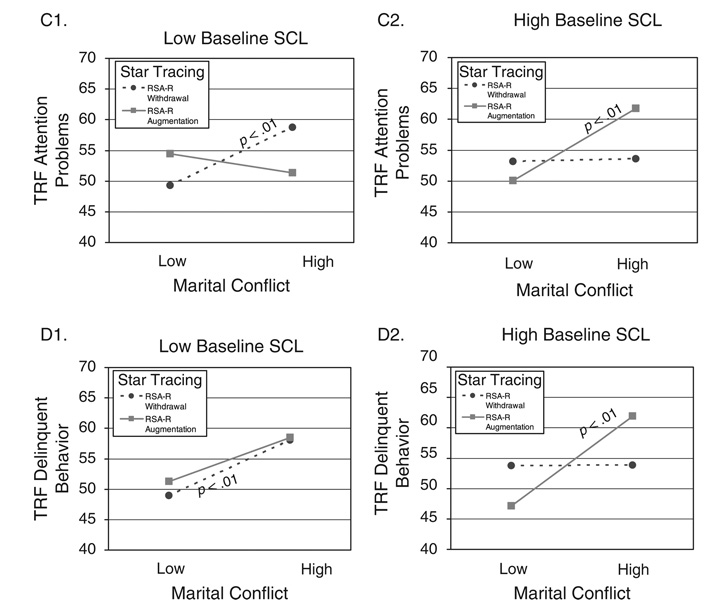
TABLE 5.
Study 2: Results for Three-Way Interactions Among Marital Conflict, RSA or RSA-R, and SCL or SCL-R as Predictors of Children’s Externalizing Problems
| ADH | Delinquency | |||
|---|---|---|---|---|
| PR | PR | PR | PR | |
| Step 1 | ||||
| Age | 0.03 (.05) | 0.02 (.04) | 0.03 (.04) | 0.02 (.04) |
| Sex | 0.72 (.94) | 0.95 (.69) | 1.12 (.69) | 0.93 (.68) |
| Ethnicity | −1.93 (1.00)† | 0.35 (.73) | 0.31 (.73) | 0.33 (.72) |
| SES | −0.05 (.05) | −0.04 (.04) | −0.04 (.04) | −0.05 (.04) |
| R2 | 0.02 | 0.02 | 0.02 | 0.02 |
| Step 2 | ||||
| Marital conflict | 0.23 (.09)* | 0.15 (.07)* | 0.17 (.07)* | 0.15 (.07)* |
| RSA | 4.39 (3.88) | |||
| RSA-R (AR) | 1.75 (7.88) | |||
| RSA-R (ST) | 11.82 (7.86) | 6.66 (5.79) | ||
| SCL | 0.17 (.08)* | |||
| SCL-R (AR) | ||||
| SCL-R (ST) | 0.16 (.11) | −0.32 (.13)* | −0.28 (.13)* | |
| R2 | 0.06 | 0.07 | 0.07 | 0.06 |
| ΔR2 | 0.04* | 0.05* | 0.04* | 0.05* |
| Step 3 | ||||
| Conflict × RSA | −0.26 (.88) | |||
| Conflict × RSA-R (AR) | −0.79 (2.02) | |||
| Conflict × RSA-R (ST) | 2.08 (2.21) | −0.58 (1.62) | ||
| Conflict × SCL | 0.03 (.03) | 0.04 (.02)† | ||
| Conflict × SCL-R (AR) | ||||
| Conflict × SCL-R (ST) | −0.02 (.03) | −0.02 (.03) | ||
| RSA × SCL | ||||
| RSA × SCL-R (AR) | ||||
| RSA × SCL-R (ST) | 2.16 (1.35) | |||
| RSA-R (AR) × SCL | −0.79 (2.66) | |||
| RSA-R (ST) × SCL | 2.07 (2.56) | |||
| RSA-R (AR) × SCL-R (AR) | ||||
| RSA-R (ST) × SCL-R (ST) | −0.56 (2.77) | |||
| R2 | 0.07 | 0.08 | 0.07 | 0.07 |
| ΔR2 | 0.01 | 0.01 | 0.003 | 0.01 |
| Step 4 | ||||
| Conflict × RSA × SCL | ||||
| Conflict × RSA × SCL-R (AR) | ||||
| Conflict × RSA × SCL-R (ST) | 1.26 (.53)* | |||
| Conflict × RSA-R (AR) × SCL | 2.29 (.81)** | |||
| Conflict × RSA-R (ST) × SCL | 1.97 (.80)* | |||
| Conflict × RSA-R (AR) × SCL-R (AR) | ||||
| Conflict × RSA-R (ST) × SCL-R (ST) | 1.81 (.83)* | |||
| R2 | 0.10 | .10 | 0.09 | 0.10 |
| ΔR2 | 0.03* | .02* | 0.02* | 0.03** |
Note. ADH = attention-deficit/hyperactivity; SCL-R = skin conductance level reactivity; AR = argument task; ST = star-tracing task; RSA-R = respiratory sinus arrythmia reactivity; PR = parent report. Unstandardized coefficients presented (standard errors presented in parentheses).
p < .10,
p < .05,
p < .01.
The pattern of results was fairly consistent. In accord with hypotheses, marital conflict predicted greater mother-reported Delinquency only for children exhibiting coinhibition (i.e., lower levels of SCL-R accompanied by lower levels of baseline RSA; see Figure 2A1 and first column of Table 3). By comparison, reciprocal parasympathetic activation (i.e., lower SCL-R accompanied by higher baseline RSA; Figure 2A1) appeared protective. Regarding father-reported Delinquency, for children with lower SCL-R, higher levels of marital conflict were predictive of higher levels of Delinquency for both children with lower and higher baseline RSA (see Figure 2B1 and third column of Table 3). However, the slope representing this association was steeper for children who exhibited coinhibition (i.e., lower baseline RSA and lower SCL-R). Conversely, when SCL-R was high, higher baseline RSA was a vulnerability factor; that is, coactivation was associated with more child behavior problems at higher levels of marital conflict according to mother and father reports (Figure 2A2 and B2). Thus, coinhibition and coactivation of sympathetic and parasympathetic nervous systems (SNS and PNS) were vulnerability factors, whereas reciprocal activation appeared protective.
Figure 2.
Study 1: Interactions among marital conflict, RSA, and SCL-R in the prediction of children’s externalizing problems.
The same pattern was found for teacher reports of children’s Physical Aggression on the SBS, Aggression with Peers scores on the CBS, and Reactive Aggression scores on the TCPR (see Figure 2, panels C–E, respectively, and columns 7, 8, and 10 of Table 3). Specifically, coactivation (i.e., higher baseline RSA and higher SCL-R) operated as a vulnerability factor, accentuating the association between marital conflict and Physical Aggression (SBS), Aggression with Peers (CBS), and Reactive Aggression (TCPR) (see Figure 2C2, D2, and E2). There were no significant interactions (0 out of 12 possible) among marital conflict, RSA, and baseline SCL predicting children’s externalizing problems.
Interactions Among Marital Conflict, RSA-R, and Either SCL or SCL-R
Hierarchical multiple regressions were conducted to examine RSA-R (i.e., RSA withdrawal or augmentation) in conjunction with either SCL or SCL-R as moderators of the association between marital conflict and child externalizing symptoms. Child age, sex, ethnicity, and SES were entered in the first step. Marital conflict and the main effects of the two moderators were included in the second step; all two-way interactions were entered in the third step; and the three-way interaction was entered in the fourth step. As shown in Table 3 (columns 2, 4, 5, 6, and 9) and Figure 3, there were significant three-way interactions among marital conflict, RSA-R, and SCL-R in predicting children’s externalizing behavior problems, as reported by mothers, fathers, and teachers; 5 out of 24 possible interactions between RSA-R and SCL-R were significant. Specifically, coinhibition (i.e., RSA withdrawal in the context of low SCL-R) served as a vulnerability factor, strengthening the association between marital conflict and ADH problems as reported by both mothers and fathers (see Figure 3A1 and B1). Likewise, coactivation (i.e., RSA augmentation in the context of high SCL-R) served as a vulnerability factor, strengthening the relation between marital conflict and ADH problems as reported by mothers and fathers (Figure 3A2 and B2). Similar patterns were found in the prediction of teacher reports of Reactive Aggression on the TCPR (Figure 3C1 and C2), ADH scores on the SBS (Figure 3D1 and D2), and mother reports of Delinquency on the PIC (Figure 3E1 and E2). There were no significant interactions (0 out of 24 possible) among marital conflict, RSA-R, and baseline SCL in predicting children’s externalizing problems.
Figure 3.
Study 1: Interactions among marital conflict, RSA, and SCL-R in the prediction of children’s externalizing problems.
Summary
Results of Study 1 support the hypothesis that either coactivation or coinhibition of the PNS and SNS poses greater vulnerability for externalizing behavior problems in the context of high marital conflict, compared with reciprocal forms of activation across the PNS and SNS. When lower SCL-R was accompanied with RSA withdrawal or lower baseline RSA (coinhibition), marital conflict was associated with greater maternal and paternal reports of Delinquency and symptoms of ADH. When higher SCL-R was accompanied with RSA augmentation or higher baseline RSA (coactivation), marital conflict was associated with greater parental reports of Delinquency and symptoms of ADH, as well as teacher reports of Physical Aggression and Aggression with Peers.
In contrast, both reciprocal parasympathetic and reciprocal sympathetic activation appeared protective in the context of high marital conflict. Specifically, under conditions of reciprocal sympathetic or parasympathetic activation, marital conflict and child externalizing behaviors were either not associated or negatively associated for all dependent variables in Study 1, with the exception of paternal reports of Delinquency under conditions of reciprocal parasympathetic activation. Thus, Study 1 provides initial support for hypotheses across multiple informants and several dimensions of externalizing behavior problems. The consistent pattern of three-way interactions observed in this study is particularly noteworthy given that statistical interactions can be difficult to find and replicate (Jaccard et al., 1990).
III. ADDITIONAL TESTING OF THREE-WAY INTERACTIONS IN AN INDEPENDENT SAMPLE
Study 1 provided support for coactivation and coinhibition of the parasympathetic and sympathetic nervous systems (PNS and SNS) as vulnerability factors for children’s externalizing problems in the context of greater marital conflict. This pattern of effects was consistent across multiple reporters of children’s maladjustment, including father, mother, and teacher reports. The results from Study 1 show clear support for the hypothesized interactions among PNS and SNS activation and marital conflict in the prediction of children’s externalizing behavior problems. However, three-way interactions among psychological variables are difficult to detect (Aguinis & Stone-Romero, 1997) and do not necessarily replicate across studies. Thus, there is some possibility that these effects were fortuitous and would not be replicated in another study. Under these circumstances, and especially given the relatively novel status of research on interactions across autonomic systems in the context of marital conflict, further study is warranted to build confidence in conclusions. Therefore, the purpose of Study 2 was to replicate the findings of Study 1 using a larger community sample that included families and children with similar demographic characteristics (e.g., child and parent age, socioeconomic status [SES], ethnic composition).
METHOD
Participants
Children (128 girls and 123 boys) and their parents were recruited from three local public schools in the southeastern USA. Children’s mean age was 8.23 years (standard deviation [SD] = 0.73). Families were eligible to participate if children were in second or third grade, two parents were present in the home, and families had been living together for at least 2 years. Exclusion criteria included physical illness, attention-deficit/hyperactivity disorder, learning disability, and mental retardation. Out of families contacted who qualified for our study, 37% participated, 18% declined participation, and 45% were interested but were not included because the desired subsample sizes had already been filled (either in relation to sex, SES, or ethnicity). We oversampled to include European and African American children across a wide SES range. All participating couples were married or had been living together for a substantial time period (M = 9.99 years, SD = 5.67), but due to misunderstandings 10 families had been living together for <2 years (M = 1.09 years, SD = .28). Mothers’ mean age was 33.35 years (SD = 5.97) and fathers’ mean age was 36.29 years (SD = 6.62) years. Most children (73%) lived with both biological parents; 24% lived with their biological mom and a step-father or mother’s live-in boyfriend, and the remaining 3% lived mostly with their biological fathers and a step-mother.
Families represented the complete spectrum of possible economic backgrounds (Hollingshead, 1975; M = 3.21; SD = 0.91; range: 1–5), with the median income in the US$35,000–50,000 range. Participants were 64% European American and 36% African American. With respect to the SES and ethnic composition of the sample, participants were representative of the community from which they were drawn. Families received monetary compensation for their participation.
Procedures and Measures
Mothers, fathers, and children visited the laboratory located on the university campus. Parents completed consent forms while a researcher read the child an assent form. Once consent and assent were obtained, the father was moved to a separate room to complete his questionnaires. Children were taken into the physiological assessment room, and their mothers were allowed to be present while an experienced researcher attached physiological sensors to the child. The researcher was instructed to explain each of the physiological tools in order to reduce any anxiety the child may have felt. Once the equipment was in place, mothers were asked to move back into an adjacent room to complete their questionnaires. Children were informed that they could stop the session at any time by raising their hands. All physiological procedures and lab challenges used in this study are identical to those used in Study 1, with minor differences noted below.
Respiratory Sinus Arrhythmia (RSA) and Skin Conductance Level (SCL) Data Acquisition and Reduction
Data acquisition and reduction were conducted exactly as described in Study 1, with one exception. The argument task was followed by a 6-min (vs. a 12-min in Study 1) recovery period before the start of the star-tracing task.
Marital Conflict
Mothers, fathers, and children reported on parental marital conflict. Similar to Study 1, mothers and fathers reported verbal and physical aggression tactics in the past year on the Conflict Tactics Scale (CTS2; Straus et al., 1996). However, because a certificate of confidentiality was obtained in this study, the complete CTS2 scale with all physical and verbal aggression items was administered; recall that severe physical aggression items were not administered in Study 1. The internal consistency of the CTS2 in this sample was high for both mothers’ (.92) and fathers’ (.96) reports.
Similar to assessments reported in Study 1, the Children’s Perceptions of Interparental Conflict Scale (CPIC; Grych et al., 1992) was completed by children via interview, and the Destructive Conflict scale was used in analyses. In the present sample, the internal consistency of the Destructive Conflict scale was .88. A marital conflict composite score, similar to that in Study 1, was created by standardizing and summing parent reports on the CTS and child reports on the CPIC. Higher scores reflect higher levels of marital conflict.
Child Externalizing Behaviors
Similar to the assessment of externalizing problems in Study 1, parents completed the Personality Inventory for Children-II (PIC2; Lachar & Gruber, 2001). The Delinquency and ADH scales, which collectively yield the Externalizing Scale, were used in analyses and had good internal consistency for mothers’ and fathers’ reports (α = .83–.84). Forty-five children were within the borderline or clinical range of externalizing problems based on at least one parent’s report on the PIC2. In this study, scores were averaged across mother and father report to reduce the number of analyses.
RESULTS
Descriptive Statistics
Means, SDs, and correlations for study variables are shown in Table 4. Age and BMI were not included in the correlation table due to their non-significant relations with most study variables. BMI was, however, correlated with higher SCL reactivity (SCL-R) to the argument task, r = .19, p < .01, and lower marital conflict, r = −.16, p < .05.
TABLE 4.
Means, Standard Deviations, and Correlations Among Variables for Study 2
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Child sex | — | |||||||||||
| 2. Ethnicity | −.04 | — | ||||||||||
| 3. SES | −.03 | .18** | — | |||||||||
| 4. Baseline RSA | −.04 | −.23** | −.06 | — | ||||||||
| 5. Baseline SCL | −.18** | −.37** | .07 | −.07 | — | |||||||
| 6. RSA-R to argument | −.03 | −.01 | −.07 | −.23** | −.00 | — | ||||||
| 7. RSA-R to star-tracing | −.06 | .15* | −.09 | −.40** | .01 | .36** | — | |||||
| 8. SCL-R to argument task | −.03 | .26** | .08 | −.03 | .35** | −.06 | −.08 | — | ||||
| 9. SCL-R to star-tracing task | −.01 | .28** | .10 | −.05 | .33** | −.01 | −.12 | .61** | — | |||
| 10. Marital conflict composite | −.02 | −.17** | −.16* | .15* | −.08 | .09 | −.10 | .03 | .02 | — | ||
| 11. PIC—Delinquencya | .07 | .03 | −.11† | .09 | .10 | .01 | .04 | −.05 | −.07 | .10 | — | |
| 12. PIC—Attention-Hyperactivea | .03 | −.11† | −.06 | .004 | .12† | .03 | .10 | −.03 | −.05 | .12† | .73** | — |
| Mean | — | — | 3.21 | .16 ms | 5.71 µS | −.01 ms | −.02 ms | 1.07 µS | 2.99 µS | −.37 | 47.14 | 49.48 |
| SD | — | — | .91 | .10 ms | 4.31 µS | .04 ms | .06 ms | 1.64 µS | 2.70 µS | 5.57 | 5.38 | 6.99 |
Note. RSA-R = respiratory sinus arrythmia activity; SCL-R = skin conductance level reactivity; PIC = Personality Inventory for Children. N = 251; ms = milliseconds; µS = microsiemens.
Parent composite report; sex coded as 0 = boys and 1 = girls; vagal regulation was computed as posttask–pretask levels, so that higher scores reflect augmentation.
p<.10,
p<.05,
p<.01.
Physiological Reactivity
Children’s RSA significantly decreased from baseline during the start-racing task, t(233) = 6.52, p < .01, but not the argument task, t(238) = 1.92, p = .06. Fifty-six percent and 74% of children demonstrated RSA withdrawal in response to the argument and star-tracing tasks, respectively. Also, children’s SCL significantly increased from baseline during both the argument task, t(234) = 10.02, p < .01, and star-tracing task, t(231) = 16.86, p < .01. Sixty-seven percent and 77% of children demonstrated SCL increases in response to the argument and star-tracing tasks, respectively.
Interactions Among Marital Conflict, Baseline RSA, and Baseline SCL or SCL-R
Similar to analyses reported for Study 1, three-way interaction effects among marital conflict, baseline RSA, and baseline SCL or SCL-R were tested using hierarchical multiple regression analyses. Child age, sex, ethnicity, and family SES were entered as covariates. In this data set, in contrast to Study 1, children’s BMI was not significantly correlated with baseline RSA and was therefore excluded from analyses (r = .12, p = .07). Child age, sex, ethnicity, and SES were entered in the first step. Marital conflict and the main effects of the moderators were added in Step 2; all two-way interactions were entered in Step 3; and the three-way interaction was included in Step 4. Significant interactions are shown in Table 5 and were interpreted by plotting regression lines 1 SD above and below the mean for marital conflict and the two moderators (e.g., baseline RSA, baseline SCL, or SCL-R).
One significant interaction out of four possible between SCL-R and baseline RSA in the prediction of child adjustment in the context of marital conflict was found (Figure 4 and column 2 of Table 5). Specifically, among children who exhibited coinhibition (low baseline RSA and low SCL-R), higher levels of marital conflict predicted higher levels of parent-reported Delinquency (Figure 4.A1), suggesting that coinhibition serves as a vulnerability factor. In contrast, no relations between marital conflict and children’s Delinquency were observed in the context of reciprocal parasympathetic activation (i.e., high baseline RSA and low SCL-R), suggesting that this pattern of activation may be protective. Among children who exhibited coactivation (high SCL-R and high baseline RSA), higher levels of child problems appeared at higher levels of marital conflict. This interaction is depicted in Figure 4.A2. The results suggest that in the context of high marital conflict, coactivation and coinhibition operate as vulnerability factors for children, accentuating the association between marital conflict and externalizing problems. No significant interactions (zero out of two possible) involving RSA in conjunction with baseline SCL were observed.
Figure 4.
Study 2: Interactions among marital conflict, baseline RSA, and SCL-R in the prediction of children’s externalizing problems.
Interactions Among Marital Conflict, RSA Reactivity (RSA-R), and Baseline SCL or SCL-R
Hierarchical multiple regressions were conducted to examine RSA-R and baseline SCL or SCL-R as moderators of the association between marital conflict and child adjustment. Child age, sex, ethnicity, and SES were entered in the first step. Marital conflict and the main effects of the two moderators were included in the second step; all two-way interactions were entered in the third step; and the three-way interaction was entered in the fourth step. As shown in column 3 of Table 5, there was one significant three-way interaction out of four possible among marital conflict, RSA-R, and SCL-R. Specifically, coinhibition (i.e., RSA withdrawal and low SCL-R) operated as a vulnerability factor, strengthening the association between marital conflict and parent-reported Delinquency problems (Figure 5.A1). Coactivation (RSA augmentation combined with high SCL-R) was also a vulnerability factor (Figure 5.A2).
Figure 5.
Study 2: Interaction among marital conflict, RSA-R, and baseline SCL or SCL-R in the prediction of children’s externalizing problems.
With regard to baseline levels of SCL, two significant three-way interactions out of four possible among marital conflict, RSA-R, and baseline SCL emerged predicting parent-reported externalizing problems (columns 1 and 4 in Table 5). Specifically, in the context of coinhibition (i.e., RSA withdrawal and low baseline SCL) and coactivation (RSA augmentation and high baseline SCL) marital conflict was related to higher levels of parent-reported Delinquency (Figure 5.B1 and 5.B2). A similar pattern was found in predicting parent-reported Attention-deficit/Hyperactivity (Figure 5.C1 and 5.C2).
Summary
Findings of Study 2 are generally consistent with the findings from Study 1 and can be interpreted to provide additional support for hypotheses. That is, consistent with hypotheses and the findings of Study 1, coinhibition or coactivation of the PNS and SNS posed greater risks for externalizing behavior problems in the context of high marital conflict, compared with reciprocal modes of activation across the PNS and SNS.
IV. ADDITIONAL CONSIDERATION OF THE ROLE OF SYMPATHETIC AND PARASYMPATHETIC NERVOUS SYSTEMS ACTIVITY IN A SAMPLE OF 6–12-YEAR-OLDS
The purpose of Study 3 is to build upon the previous two studies in replicating the significance and direction of three-way interactions among marital conflict, baseline respiratory sinus arrhythmia (RSA) or RSA reactivity (RSA-R), and baseline skin conductance level (SCL) or SCL reactivity (SCL-R). Marital conflict and physiological arousal are assessed in the same way as in the previous two studies. However, the current study differs from the previous two by (a) examining a wider age range of children, specifically 6–12-year-olds, and (b) including an alternative measure of children’s externalizing problems, namely the Child Behavior Checklist (CBCL; Achenbach, 1991) rather than the Personality Inventory for Children. Consistent with the previous two studies, it was hypothesized that marital conflict would be most strongly related to higher externalizing problems for those children demonstrating nonreciprocal activation of the sympathetic and parasympathetic nervous systems (SNS and PNS), that is, children exhibiting coactivation (high RSA or RSA augmentation in conjunction with high SCL or SCL-R) or coinhibition (low RSA or RSA withdrawal in conjunction with low SCL or SCL-R).
METHOD
Participants
Two-parent families with children in the targeted age range (6–12-year-olds) were recruited from the southeastern USA. The participants for the current study included 150 children (75 girls and 75 boys) and their parents. Children’s mean age was 9.27 years (standard deviation [SD] = 1.95). On average, parents were living together for 12.99 years (SD = 5.98). Mothers’ mean age was 37.64 (SD = 6.31), and fathers’ mean age was 39.98 (SD = 6.83) years. The racial composition of the sample was primarily European American (67%) and African American (27%) with small percentages of children from other ethnic groups. Families represented the complete spectrum of possible economic backgrounds (Hollingshead, 1975; M = 4.04; SD = 1.01; range: 1–5), with the median income in the US$35,000–50,000 range. Socioeconomic status (SES) and racial composition of the sample was similar to the area from which it was drawn. Families received monetary compensation for their participation.
Procedures and Measures
Children were accompanied by a parent (usually the mother) to the laboratory located in the university campus. Mothers were asked to complete a consent form while a researcher read the assent form to the child. Once assent and consent were obtained, children were taken into the physiological assessment room. With mothers present, researchers attached physiological sensors to child (identical to those in Studies 1 and 2). The researcher and mother left the room and the child was given 6 min to adjust to their surroundings before a baseline (3 min) measure was taken. Following the baseline assessment, children’s responses to two laboratory challenges were measured. As was the case for the previous two studies, children listened to an audiotaped mild interadult argument (3 min in length) transmitted through speakers located in the room with them. The child was led to believe that the argument was occurring outside the assessment room. Two similar argument scripts (i.e., leisure activities and in-laws issues) were used and counterbalanced by age and sex. The scripts were identical across all three studies. After the argument, there was a recovery period (5 min) and then a researcher introduced the challenge task. Similar to the previous two studies, during the second challenge task (3 min), children were asked to trace a star on a sheet of paper while looking in a mirror. Owing to ethical guidelines, at the end of the physiological session children listened to a resolution of the argument they heard previously. After all physiological equipment was removed, children were taken into an adjacent room by an experienced researcher to complete an interview regarding marital conflict and child adjustment.
Mothers completed questionnaires regarding marital conflict and child adjustment. For 10.6% of the sample, the father accompanied the child to the lab visit and completed the questionnaires instead of the mother. To simplify, the measures completed during the laboratory session are referred to as mothers’ ratings. Questionnaires regarding marital conflict were sent to fathers for completion (or mothers in 10.6% of the sample). To facilitate the return of the questionnaires sent home, we provided a self-addressed and stamped envelope.
RSA and SCL Data Acquisition and Reduction
Physiological data acquisition and reduction were performed using the same procedures and equipment as those described for Studies 1 and 2.
Marital Conflict
Mothers and fathers completed the psychological/verbal and physical aggression subscales of an earlier version of the Conflict Tactics Scale (CTS) than was used in Studies 1 and 2 (Straus & Gelles, 1990). Good internal consistency for the scales was found for mother and father reports (α = .93 and .94, respectively). Further, and similar to Studies 1 and 2, children completed the Destructive Conflict Scale of the Children’s Perceptions of Interparental Conflict Scale (CPIC; α = .90).
Children’s reports on the CPIC and parents’ reports on the CTS were significantly associated (r = .18–.80, ps < .05). Thus, a marital conflict composite score was created by standardizing and summing parent reports on the CTS and child reports on the CPIC.
Child Externalizing Behavior
Mothers reported on children’s adjustment using the CBCL (Achenbach, 1991), whereas teachers were asked to complete the Teacher Report Form (TRF; Achenbach, 1991). The Attention Problems, Delinquency, and Aggression subscales of the CBCL, which have well-established reliability and validity (Achenbach, 1991), were included in this study. Mothers were asked to indicate whether a statement was true, sometimes true, or never true about their child using a 3-point scale. The Attention Problems subscale includes items such as daydreams, stares, and cannot concentrate. Items on the Delinquency subscale include steals, has no guilt, sets fires, and truant. Items assessing Aggression included fights, attacks, argues a lot, and has a bad temper. According to mothers’ reports on the CBCL, 30 children in this sample were within the borderline or clinical range of externalizing problems (i.e., scores ≥ 60). Portions of the TRF, which is a well-established teacher report measure of child adjustment (Achenbach, 1991), were used in this study. Specifically, the Attention Problems (e.g., difficulty following directions), Delinquent Behavior (e.g., breaks school rules), and Aggressive Behavior (e.g., gets in many fights) subscales were used in analyses. Teachers were asked to rate on a 3-point scale how true a statement was about the child (not true, somewhat or sometimes true, very true or often true).
RESULTS
Descriptive Statistics
Means, SDs, and correlations are shown in Table 6. Age is not shown in the correlation table due to its nonsignificant relation with all study variables. Note that marital conflict was significantly correlated with higher levels of mother- and teacher-reported externalizing problems (r = .23–.62, ps < .05).
TABLE 6.
Means, Standard Deviations, and Correlations Among Study Variables for Study 3
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Child sex | — | |||||||||||||||
| 2. Ethnicity | −.06 | — | ||||||||||||||
| 3. SES | −.10 | .33** | — | |||||||||||||
| 4. Baseline RSA | −.19* | −.05 | .07 | — | ||||||||||||
| 5. Baseline SCL | .12 | .01 | −.07 | −.06 | — | |||||||||||
| 6. RSA-R to argument |
−.02 | .02 | −.01 | −.22* | .02 | — | ||||||||||
| 7. RSA to star- tracing |
.20* | .00 | −.03 | −.48** | .12 | .44** | — | |||||||||
| 8. SCL-R to argument |
−.21* | .05 | .03 | −.06 | .22** | −.01 | .06 | — | ||||||||
| 9. SCL-R to star- tracing |
−.12 | .06 | .16* | −.03 | .25** | .09 | −.03 | .53** | — | |||||||
| 10. Marital conflict | .10 | −.38** | −.46** | −.02 | .16 | .08 | .05 | .09 | −.08 | — | ||||||
| 11. CBCL Attention | .06 | −.07 | −.13 | .04 | .02 | .04 | .04 | −.08 | −.03 | .31*** | — | |||||
| 12. CBCL Delinquency |
.05 | −.12 | −.11 | .18* | .07 | .01 | .03 | −.07 | −.06 | .29*** | .52*** | — | ||||
| 13. CBCL Aggression |
.05 | −.01 | −.13 | −.01 | .11 | .09 | .06 | −.10 | −.04 | .23** | .69*** | .59*** | — | |||
| 14. TRF Attention | .15 | −.08 | −.42*** | .06 | .13 | .05 | .14 | −.09 | .13 | .52*** | .52*** | .43*** | .38** | — | ||
| 15. TRF Delinquency |
.15 | −.09 | −.43*** | .23 | .06 | −.08 | .10 | .00 | .12 | .62*** | .45*** | .38** | .44*** | .68*** | — | |
| 16. TRF Aggression | .02 | −.24* | −.09 | .12 | .23* | −.11 | .08 | .04 | .07 | .33** | .11 | .28* | −.02 | .25* | .22 | — |
| Mean | — | — | 4.04 | .15ms | 13.93 µS | −.01ms | −.03ms | .28 µS | 1.12 µS | .14 | 54.11 | 53.61 | 53.52 | 52.47 | 53.16 | 52.97 |
| SD | — | — | 1.01 | .07ms | 6.95 µS | .04 ms | .06ms | .61 µS | 1.66 µS | 6.61 | 6.43 | 5.09 | 5.81 | 5.39 | 5.80 | 6.32 |
Note. RSA-R = respiratory sinus arrhythmia reactivity; SCL-R = skin conductance level reactivity; CBCL = Child Behavior Checklist; attention = attention problems; TRF = Teacher Report Form. N = 150. Sex coded as 1 = boys and 2 = girls. Vagal regulation is computed as posttask–pretask levels, so that higher scores reflect augmentation.
p < .05,
p < .01,
p < .001.
Physiological Reactivity
Children’s RSA decreased significantly from baseline in response to both the argument task, t(128) = 2.83, p < .01, and star-tracing task, t(128) = 6.82, p < .01, suggesting that on average both tasks elicited RSA withdrawal. Fifty-five percent and 68% of children demonstrated RSA withdrawal in response to the argument and star-tracing tasks, respectively. Further, children’s SCL significantly increased from baseline in response to both the argument task, t(148) = 5.65, p < .01, and star-tracing task, t(148) = 8.24, p < .01. Eighty-four and 95% of the children demonstrated increased SCL in response to the argument and star-tracing tasks, respectively.
Interactions Among Marital Conflict, Baseline RSA, and Baseline SCL or SCL-R
In an identical fashion to the corresponding analyses reported for Studies 1 and 2, hierarchical multiple regressions were conducted to examine baseline RSA and either baseline SCL or SCL-R as moderators of the association between marital conflict and children’s externalizing behaviors. Child age, sex, ethnicity, and family SES were entered as covariates in the first step of each regression equation. Marital conflict and the main effects of the moderators were added in the second step; all two-way interactions were entered in Step 3; and the three-way interaction was included in Step 4. Significant interactions are shown in Table 7 and Figure 6. Six out of 12 possible interactions involving baseline RSA and SCL-R (Table 7, columns 1–3 and 7–9) were significant. Specifically, among children who exhibited coinhibition (i.e., low baseline RSA and low SCL-R), higher levels of marital conflict predicted higher levels of mother-reported Attention Problems and Delinquency (Figure 6.A1 and 6.B1), as well as teacher-reported Attention Problems and Delinquency (Figure 6.C1–6.F1), suggesting that coinhibition is a vulnerability factor. Similar to findings from the previous two studies, no relation between marital conflict and mother-reported or teacher-reported Attention Problems and Delinquency was observed in the context of reciprocal parasympathetic activation (high baseline RSA combined with low SCL-R; Figure 6.A1, 6.B1, 6.C1, and 6.D1). By comparison, and consistent with the first two studies and our hypotheses, marital conflict was associated with higher levels of mother-reported Attention Problems (Figure 6.A2), as well as teacher-reported Attention Problems (Figure 6.C2 and 6.E2) and Delinquent Behavior (Figure 6.D2 and 6.F2) for children who exhibited coactivation (high baseline RSA combined with high SCL-R).
TABLE 7.
Study 3: Results For Three-Way Interactions Among Marital Conflict, RSA or RSA-R, and SCL or SCL-R
| Delinquent Behavior | Attention Problems | Attention Problems (Cont) |
Aggressive Behavior |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR | TR | TR | TR | TR | MR | MR | TR | TR | TR | TR | TR | |
| Step 1 | ||||||||||||
| Age | 0.001 (0.02) | 0.003 (0.03) | 0.003 (0.03) | 0.03 (0.03) | 0.003 (0.03) | 0.02 (0.02) | 0.01 (0.02) | −0.03 (0.03) | −0.03 (0.03) | −0.002 (0.03) | −0.03 (0.03) | −0.03 (0.04) |
| Sex | 0.29 (0.90) | 1.28 (1.32) | 1.28 (1.32) | 0.03 (1.08) | 1.28 (1.32) | 0.30 (1.12) | 0.05 (1.10) | 1.49 (1.31) | 1.49 (1.31) | 0.53 (1.19) | 1.49 (1.31) | 0.78 (1.54) |
| Ethnicity | 0.62 (1.0) | 0.30 (1.62) | 0.30 (1.62) | −1.0 (1.32) | 0.30 (1.62) | 0.51 (1.23) | 0.10 (1.22) | −0.48 (1.61) | −0.48 (1.61) | −1.47 (1.45) | −0.48 (1.61) | 1.43 (1.88) |
| SES | −0.02 (0.03) | −0.10 (0.05)† | −0.10 (0.05)† | −0.04 (0.04) | −0.10 (0.05)† | −0.02 (0.04) | −0.02 (0.04) | −0.17 (0.05)** | −0.17 (0.05)** | −0.12 (0.05)* | −0.17 (0.05)** | −0.14 (0.06)* |
| R2 | 0.01 | 0.09 | 0.09 | 0.42 | 0.09 | 0.01 | 0.01 | 0.17 | 0.17 | 0.36 | 0.17 | 0.12 |
| Step 2 | ||||||||||||
| Marital Conflict | 0.16 (0.08)* | 0.60 (0.09)** | 0.61 (0.10)** | 0.66 (0.11)** | 0.59 (0.11)** | 0.30 (0.10)** | 0.28 (0.09)** | 0.46 (0.11)** | 0.46 (0.11)** | 0.41 (0.13)** | 0.43 (0.12)** | 0.46 (0.14)** |
| RSA | 15.91 (6.16)* | 23.58 (7.01)** | 23.51 (7.13) | 23.91 (7.11)** | 6.01 (7.61) | 11.21 (8.19) | 9.86 (8.46) | 9.60 (8.45) | ||||
| RSA-R (AR) | 3.98 (15.15) | −11.45 (17.79) | ||||||||||
| RSA-R (ST) | 3.13 (12.98) | 7.26 (14.19) | ||||||||||
| SCL | −0.02 (0.08) | −0.03 (0.09) | −0.01 (0.08) | 0.07 (0.09) | 0.06 (0.09) | |||||||
| SCL-R (AR) | −1.08 (1.09) | −2.01 (1.27) | 0.09 (1.58) | |||||||||
| SCL-R (ST) | −0.23 (0.26) | −0.17 (0.36) | −0.12 (0.32) | 0.03 (0.42) | ||||||||
| R2 | 0.11 | 0.52 | 0.52 | 0.52 | 0.42 | 0.09 | 0.09 | 0.40 | 0.37 | 0.38 | 0.36 | 0.28 |
| ΔR2 | 0.09** | 0.44** | 0.43** | 0.10* | 0.37** | 0.08* | 0.08* | 0.23** | 0.20** | 0.02 | 0.19** | 0.16* |
| Step 3 | ||||||||||||
| Conflict × RSA | −0.55 (0.73) | 3.13 (0.81)** | 2.89 (0.79)** | 2.17 (0.85)* | 0.76 (0.94) | 3.30 (1.01)** | 1.76 (1.04)† | 0.98 (1.11) | ||||
| Conflict × RSA-R (AR) | −1.41 (4.16) | −7.11 (5.32) | ||||||||||
| Conflict × RSA-R (ST) | 8.61 (2.55)** | 4.68 (2.98) | ||||||||||
| Conflict × SCL | −0.003 (0.01) | −0.01 (0.01) | −0.002 (0.01) | 0.01 (0.02) | 0.01 (0.02) | |||||||
| Conflict × SCL-R (AR) | −0.31 (0.19) | −0.66 (0.24)** | 0.31 (0.29) | |||||||||
| Conflict × SCL-R (ST) | −0.11 (0.04)** | −0.06 (0.08) | −0.07 (0.05) | −0.01 (0.10) | ||||||||
| RSA × SCL | 1.95 (1.05)† | 2.25 (1.37) | ||||||||||
| RSA × SCL-R (AR) | 15.38 (15.39) | −43.25 (19.22)* | ||||||||||
| RSA × SCL-R (ST) | 11.48 (4.52)* | 6.28 (5.41) | 8.69 (5.80) | 1.27 (7.17) | ||||||||
| RSA-R (AR) × SCL | 3.33 (2.99) | |||||||||||
| RSA-R (ST) × SCL | −0.42 (2.05) | 2.72 (2.42) | ||||||||||
| RSA-R (AR) × SCL-R (AR) | 29.53 (56.22) | |||||||||||
| RSA-R (ST) × SCL-R (ST) | ||||||||||||
| R 2 | 0.21 | 0.67 | 0.64 | 0.65 | 0.53 | 0.10 | 0.13 | 0.52 | 0.41 | 0.44 | 0.41 | 0.32 |
| ΔR2 | 0.11** | 0.15** | 0.12** | 0.12** | 0.11* | 0.01 | 0.05 | 0.13** | 0.04 | 0.06† | 0.04 | 0.05 |
| Step 4 | ||||||||||||
| Conflict × RSA × SCL | 0.46 (0.13)** | 0.80 (0.15)** | ||||||||||
| Conflict × RSA × SCLR (AR) | 8.15 (2.33)** | 9.82 (2.93)** | ||||||||||
| Conflict × RSA × SCL-R (ST) | 2.03 (0.69)** | 2.76 (0.87)** | 3.09 (0.87)** | 4.18 (1.12)** | ||||||||
| Conflict × RSA-R (AR) × SCL | 1.41 (0.52)** | |||||||||||
| Conflict × RSA-R (ST) × SCL | 0.93 (0.37)* | 1.34 (0.42)** | ||||||||||
| Conflict × RSA-R (AR) × SCL-R (AR) | 53.44 (22.38)* | |||||||||||
| Conflict × RSA-R (ST) × SCL-R (ST) | ||||||||||||
| R 2 | 0.27 | 0.73 | 0.69 | 0.72 | 0.58 | 0.15 | 0.22 | 0.61 | 0.53 | 0.64 | 0.50 | 0.39 |
| ΔR2 | 0.06** | 0.06** | 0.06** | 0.07** | 0.05* | 0.06** | 0.09** | 0.08** | 0.12** | 0.20** | 0.10** | .07* |
Note. MR = mother report; FR = father report; TR = teacher report; RSA-R = respiratory sinus arrhythmia reactivity; AR = argument task; ST = star-tracing task; SCL-R = skin conductance level reactivity. Unstandardized coefficients reported (standard errors reported in parentheses).
p < .10,
p < .05,
p < .01.
Figure 6.
Study 3: Interaction among marital conflict, RSA, and baseline SCL or SCL-R in the prediction of children’s externalizing problems.
A similar pattern was found when examining the two out of six possible significant interactions among marital conflict, baseline RSA, and baseline SCL (Table 7, columns 4 and 10). Specifically, among children who exhibited coinhibition (low baseline RSA combined with low baseline SCL) or coactivation (high baseline RSA combined with high baseline SCL), marital conflict was related to higher levels of both teacher-reported Delinquent Behavior (Figure 6.G1 and G2) and Attention Problems (Figure 6.H1 and H2). Marital conflict was also associated with greater teacher-reported externalizing symptoms in the context of reciprocal sympathetic activation (Figure 6.G2 and 6.H2); however, this association was not as strong as in the context of coactivation.
Interactions Among Marital Conflict, RSA-R, and Baseline SCL or SCL-R
Hierarchical multiple regressions were conducted to examine RSA-R (i.e., withdrawal, augmentation) and SCL or SCL-R as moderators of the association between marital conflict and child adjustment. As shown in Table 7, there was 1 out of 12 possible significant three-way interactions among marital conflict, RSA-R, and SCL-R in predicting children’s externalizing behaviors (Table 7, column 12). Specifically, coinhibition (RSA withdrawal in the context of low SCL-R) and coactivation (RSA augmentation in the context of high SCL-R) served as vulnerability factors, strengthening the association between marital conflict and Aggressive Behaviors as reported by teachers (Figure 7.A1 and 7.A2).
Figure 7.
Study 3: Interaction among marital conflict, RSA-R, and baseline SCL or SCL-R in the prediction of children’s externalizing problems.
There were also 3 significant interactions out of 12 possible among marital conflict, RSA-R, and baseline SCL in the prediction of mother- and teacher-reported externalizing problems (Table 7, columns 5, 6, and 11). Similar to previous findings, among children who exhibited coinhibition (RSA withdrawal and low baseline SCL), marital conflict was related to higher levels of mother-reported Attention Problems (Figure 7.B1) and teacher-reported Attention Problems and Delinquent Behavior (Figure 7.C1 and D1). Additionally, among children who exhibited coactivation (high RSA-R combined with high baseline SCL), marital conflict was associated with higher levels of mother- and teacher-reported Attention Problems (Figure 7.B2 and C2) and teacher-reported Delinquent Behavior (Figure 7.D2).
Summary
Results provide additional support for the role of PNS and SNS coinhibition and coactivation as vulnerability factors for children’s externalizing symptoms in the context of marital conflict. Specifically, findings demonstrate that coinhibition and coactivation are associated with children’s vulnerability using an alternative measure of externalization (CBCL) and in a sample of 6–12-year-olds. Marital conflict was associated with mother’s report and teacher’s report of greater Attention Problems, Delinquency, and (in one case) Aggressive Behavior for children exhibiting patterns of coinhibition or coactivation. Reciprocal activation of the SNS and PNS, on the other hand, appeared to function as a protective factor in almost all cases.
V. DISCUSSION
Many children are exposed to high levels of destructive marital conflict, and the damaging effects of such exposure are well documented. An important objective for investigators, therefore, is to better understand which children face heightened vulnerability for maladjustment and why their risk is elevated. The studies included in this monograph advance this objective, investigating interactions among marital conflict and the parasympathetic and sympathetic branches of the autonomic nervous system (ANS) in the prediction of child externalizing problems. The combined results across studies support a consistent picture and provide compelling evidence in support of our biopsychosocial conceptualization of child adjustment, in which interactions between physiological systems involved in stress response moderate the association between parental marital conflict and child externalizing behaviors. More specifically, opposing action of the parasympathetic and sympathetic nervous systems (PNS and SNS) (i.e., coactivation and coinhibition) operated as a vulnerability factor for externalizing behavior in the context of marital conflict, whereas reciprocal action of the PNS and SNS (i.e., reciprocal sympathetic activation and reciprocal parasympathetic activation) operated as a protective factor. This pattern of findings emerged consistently in studies with multimethod and multi-informant designs, including mother, father, and child reports of marital conflict; mother, father, and teacher reports of various child externalizing problems; and physiological data on child responses to different laboratory stress tasks. In addition, findings held across various measures of externalizing problems, including subtypes of aggressive behavior (i.e., physical, reactive) and conduct problems (i.e., delinquent, inattentive-hyperactive).
INTEGRATION OF FINDINGS WITH CURRENT THEORY
The research in this monograph was guided by contemporary theoretical models concerning the joint action of physiological systems that underlie stress responses, as well as the implications of multisystem physiological responses for child behavioral and social adjustment (Beauchaine, 2001; Beauchaine et al., 2007; Berntson et al., 1991; Porges, 2007). Recent work guided by these models has demonstrated, for example, that low levels of both sympathetic and parasympathetic activity (i.e., coinhibition) are associated with externalizing behaviors (Boyce et al., 2001) and conduct disorder (Beauchaine et al., 2007). Other studies have shown that interactions between hypothalamic–pituitary–adrenal (HPA) and SNS activity are associated with children’s internalizing and externalizing behaviors (El-Sheikh, Erath, Buckhalt, Granger, & Mize, 2008; Gordis, Granger, Susman, & Trickett, 2006).
This monograph further tests and advances these contemporary theoretical models and empirical studies. Specifically, we have advanced our developing biopsychosocial conceptual framework by integrating multisystem psychophysiological models (Beauchaine et al., 2007; Berntson et al., 1991; Porges, 2007) with leading theories in the marital conflict literature (e.g., Emotional Security Theory). We proposed that child maladjustment is better predicted by investigating interactions between environmental stressors and multiple (rather than single) physiological systems. We tested our framework empirically by investigating interactions between PNS and SNS activity as moderators of child externalizing behavior in the context of a significant environmental stressor—marital conflict. Our findings shed light on physiological profiles that incur vulnerability or offer protection against environmental risk. As such, we have situated multisystem physiological models explicitly within a developmental psychopathology framework that conceptualizes child maladjustment as an outcome of transactions among multiple individual and environmental risk factors (Cicchetti, 2006). In the following sections, we discuss the findings, first considering the general mechanism by which patterns of autonomic reactivity may operate as vulnerability factors and then discussing subtypes of externalizing behaviors as outcome measures more specifically.
The Polyvagal Theory (Porges, 1995b, 2001, 2007) posits that stress responses are first managed by the PNS. Vagal withdrawal rapidly increases heart rate and metabolic output, facilitating an efficient and active response under conditions of stress, whereas vagal augmentation promotes attentional engagement and social communication under normal circumstances or mild challenge. Vagal withdrawal does not preclude a moderate increase in sympathetic arousal to meet environmental demands, even when a stressor is managed largely by the PNS. However, when the vagal system does not sufficiently manage the stressor, a stronger SNS response is activated, producing a significant increase in heart rate and stimulating “fight or flight” behaviors. Although a strong SNS response is quite adaptive under certain circumstances, this response is more physiologically taxing, and intense or prolonged activation of the SNS is linked with numerous health and adjustment problems.
Despite the potentially predominant response by either the parasympathetic or sympathetic system, both systems generally become active in response to stress. Berntson et al. (1991) proposed that coactivation or coinhibition of the PNS and SNS reflect opposing action across the ANS branches, which may result in an ambivalent or maladaptive physiological response to stress that does not support an organized, active voluntary response. In contrast, reciprocal sympathetic activation and reciprocal parasympathetic activation produce a consistent, unidirectional physiological change that reflects coordinated functioning of the ANS branches and may be more compatible with active coping responses. Likewise, extrapolating from Polyvagal Theory (Porges, 2007), reciprocal modes of ANS responding may indicate that more evolutionarily advanced response strategies have been effective and sufficient. Conversely, coactivation and coinhibition may suggest a breakdown in regulation, in which either the parasympathetic or sympathetic system fails to perform its adaptive function in response to stress. This is consistent with our findings, which show consistent associations between marital conflict and externalizing behaviors under conditions of coactivation and coinhibition, but few significant associations under conditions of reciprocal activation.
Our findings are also consistent with Beauchaine’s (2001) and Beauchaine et al.’s (2007) research, which suggests that children with clinical levels of externalizing problems are likely characterized by coinhibition or reduced activity of both the parasympathetic and sympathetic branches. Findings of this monograph build on Beauchaine’s work and extend it, particularly by showing that certain patterns of SNS and PNS activity can operate as vulnerability or protective factors in the context of marital conflict.
Although our work diverges from the Autonomic Space model (Berntson et al., 1991) in that we examined skin conductance level (SCL) versus cardiac measures of SNS activity, our findings are consistent with this body of work in that SCL appeared to operate like preejection period (PEP) in conjunction with PNS activity to predict child behavior. Although both SCL and PEP are influenced by the SNS, it is important for future research to further support the application of electrodermal measures to the Autonomic Space model. In addition, although the Autonomic Space literature focuses on physiological reactivity, we examined all combinations of SCL and respiratory sinus arrhythmia (RSA) at baseline and in response to laboratory tasks. Our findings provide support for the importance of considering interactions involving either ANS resting or reactivity measures.
MARITAL CONFLICT, NONRECIPROCAL ANS ACTIVITY, AND EXTERNALIZING BEHAVIOR
In the present studies, we examined externalizing problems, generally, and various dimensions of externalizing behavior, including subtypes of aggression and conduct problems. There is likely some degree of convergence (and divergence) in the subtypes of externalizing problems potentiated by different ANS response patterns. Indeed, physiological activity and reactivity are intertwined with emotion regulation (Beauchaine et al., 2007; Porges, Doussard-Roosevelt, & Maita, 1994), and “emotion dysregulation is a common dimension of most categories of psychopathology and a defining feature of many” (Cole et al., 1994, p. 77). Thus, to the extent that physiological activity is affected by and affects emotion regulation, different patterns of physiological activity can place children at risk for different forms of externalizing problems. Next, we discuss the potential manifestation of different ANS response patterns as emotional and behavioral responses and as vulnerability factors for specific forms of externalizing problems.
First, coactivation may reflect physiological overarousal given the apparent sympathetic “override” of the parasympathetic response (Porges, 1995b, 2001), and thus it is possible that coactivation promotes angry, dysregulated, “fight-or-flight” responses to conflict as well as child involvement in marital conflict. Such high emotional reactivity might set the stage for coercive exchanges between parents and their children, in which children are negatively reinforced for aggressive attempts to end conflict (Patterson, 2002). High emotional reactivity to conflict might also contribute to involvement in and increased exposure to conflict and may thereby enhance sensitization to conflict (Cummings & Davies, 1994). Both coercion and sensitization processes would be expected to increase risk for aggressive behavior. Indeed, marital conflict was significantly associated with teacher-reported general aggression, physical aggression, and reactive aggression among participants who exhibited higher vagal tone in conjunction with higher SCL reactivity (SCL-R) (i.e., coactivation) during the argument task. Likewise, coactivation operated as a vulnerability factor for maternal, paternal, and teacher reports of delinquent behavior, scales that also tap disruptive and noncompliant behavior (although these behaviors are not necessarily aggressive). In addition, marital conflict was associated with maternal, paternal, and teacher reports of attention problems for children who exhibited coactivation, as discussed in further detail below.
Coinhibition appears to reflect an ambivalent physiological response in which the parasympathetic system equips the child for action by withdrawing its inhibitory influence, whereas the sympathetic system, conversely, fails to produce the metabolic output needed for an active behavioral or emotional response. Potentially, such a physiological response promotes passive vigilance, which might result in increased exposure to marital conflict and limited efforts to reduce exposure, such as by communicating upset feelings to parents. In contrast to coactivation, coinhibition was a less consistent vulnerability factor for teacher-reported aggressive behavior. However, marital conflict was associated with maternal, paternal, and teacher reports of delinquent behavior and attention problems among children who exhibited low vagal tone or vagal withdrawal concurrently with low SCL or SCL-R (i.e., coinhibition).
It is possible that coinhibition of ANS branches is more characteristic of children with underaroused antisocial behavior (Raine, 2002) or callous-unemotional traits (Frick & Ellis, 1999). For example, low vagal tone may reflect poor emotion regulation, and diminished SNS arousal may suggest fearlessness, failure of avoidance learning, or punishment insensitivity (Raine, 2002). Indeed, many antisocial children and adults exhibit comparatively little arousal when faced with cues of punishment or other aversive stimuli, which appears to be indicative of their reduced fear of punishment or aversive consequences (Fung et al., 2005; Herpertz et al., 2005; Raine, 2002). For example, Frick and colleagues have described a group of antisocial children characterized by “callous-unemotional” traits (e.g., lack of guilt and empathy, constricted emotional expression) and attenuated sympathetic arousal in response to stress (Frick & Ellis, 1999; Frick et al., 2003). Furthermore, according to the results of a recent meta-analysis (Lorber, 2004), individuals with nonaggressive conduct problems exhibit lower resting electrodermal activity and lower electrodermal activity during tasks, as compared with individuals without conduct problems. Thus, whereas children characterized by coactivation may be more likely to exhibit dysregulated and reactive forms of externalizing behavior, children characterized by coinhibition may be more likely to exhibit callous, covert forms of externalizing behavior. Of course, both patterns of ANS response may incur vulnerability for a range of externalizing problems, and the distinctions we have drawn await further research. Whether and how autonomic response profiles map onto behavioral responses in the context of stress are important questions worthy of further inquiry.
As noted, marital conflict was also associated with maternal, paternal, and teacher reports of attention problems for children who exhibited coactivation and coinhibition. These findings are consistent with previous research examining the coupling between the SNS and PNS during mental challenge, which typically find that cognitive effort is associated with SNS activation and PNS inhibition (i.e., reciprocal sympathetic activation; Wetzel, Quigley, Morell, Eves, & Backs, 2006). Studies that have examined PNS activity in isolation from SNS activity have found that children’s RSA decreases typically during challenging mental tasks (Richards & Casey, 1991; Suess, Porges, & Plude, 1994), but not for children who demonstrate difficulties with tasks requiring sustained attention, such as autistic children (Toichi & Kamio, 2003) or children exposed to opiates during fetal development (Hickey, Suess, Newlin, Spurgeon, & Porges, 1995). Studies that have examined SNS activity in isolation from PNS activity have found that SCLs normally increase when attention is focused (Tracy et al., 2000) with response being especially strong when tasks are difficult (Gronau, Sequerra, Cohen, & Ben-Shakhar, 2006). In contrast, children suffering from Asperger’s disorder (Johnson, Yechiam, Murphy, Queller, & Stout, 2006) and attention-deficit/hyperactivity (ADH) disorder (Lawrence et al., 2005) show diminished SNS response. Taken together, these studies indicate that autonomic responses to challenge tasks are associated with an individual’s ability to sustain attention. Thus, dysregulated ANS responses to marital conflict, and generalization of such responses to other stressful or threatening circumstances, may reduce children’s ability to sustain attention and inhibit impulses across contexts.
Notably, research indicates that children who have been physically abused experience trouble concentrating on tasks following exposure to interadult anger (Pollak & Tolley-Schell, 2003). It appears that these children have developed sensitivity to negative affect and focus their attention on negative interactions rather than the task at hand (Pollak, Vardi, Putzer Bechner, & Curtin, 2005). One implication is that exposure to family violence may lead to patterns of attention regulation or dysregulation (manifest as coactivation or coinhibition) that lead to the development of ADH symptoms.
Interestingly, some models accounted for large amounts of variance in externalizing symptoms in comparison with others. After controlling for child characteristics, demographics, main effects, and two-way interactions, almost all three-way interactions accounted for < 10% of unique variance in children’s externalizing symptoms, with most accounting for 5% or less. However, in the third study, interactions predicting teacher reports of children’s ADH problems accounted for 8–20% of the variance. In addition, the full models predicting teacher reports of both delinquency and ADH symptoms accounted for 53–73% of the variance, compared with 9–31% for the other full models presented in the monograph. One possible explanation is that interactions between ANS subsystems play an especially important role in the association between marital conflict and child externalization in the school setting, especially in regard to attention and impulsivity. However, considering this third study in the context of the other studies suggests that this interpretation is not warranted, highlighting the importance of replicating findings in three independent studies. Notably, interactions predicting teacher reports of functioning did not account for such large amounts of variance in Study 1, and no associations between interactions and teacher-reported functioning were observed in Study 2. Rather, it appears that the effect sizes of interactions among marital conflict, PNS, and SNS activity are generally small, as is frequently reported in the psychological literature.
DEVELOPMENT OF ANS PROFILES AND EXTERNALIZING SYMPTOMS IN THE CONTEXT OF FAMILY STRESS
Beauchaine and colleagues (e.g., Beauchaine 2001; Beauchaine et al., 2007) proposed a developmental model in which inherited impulsivity and oppositionality, marked by low sympathetic activity and reactivity, may or may not evolve into severe conduct problems depending on emotion socialization in the family. These early childhood behaviors can be transformed into poor emotion regulation, reflected by low vagal tone, and severe conduct problems in childhood via coercive family processes in which negative effect and aggressive behavior are negatively reinforced (Patterson, 2002). Alternatively, a protective family environment characterized by consistent positive reinforcement of appropriate behavior and clear, controlled consequences for aggressive behavior can foster emotion regulation abilities that buffer impulsive children form the development of angry, aggressive behavior (Beauchaine et al., 2007). According to this model, low SNS and PNS activity, described as coinhibition in the present studies, may emerge over time as a result of inherited characteristics and family circumstances.
Raine et al. (2001) have also provided evidence that autonomic responses can change over time as a result of environmental influence. Specifically, a preschool program designed to enrich social-emotional skills and cognitive development was associated with increased amplitude and speed of electrodermal responding and recovery in late childhood, as compared with a control group. Although physiological response patterns may remain somewhat malleable throughout the life course, it is likely that these responses become more stable over time. Evidence has emerged for moderate stability in baseline and reactivity levels of SNS activity and PNS activity in middle to late childhood, and reactivity may not stabilize until middle to late childhood (Bornstein & Suess, 2000; Calkins & Keane, 2004; Doussard-Roosevelt, Montgomery, & Porges, 2003; El-Sheikh 2005b, 2007). One possibility is that physiological reactivity to stressors becomes relatively stable around late childhood or early adolescence and can then be considered an individual difference variable that exacerbates or ameliorates the risk for adjustment problems in the context of family stress (El-Sheikh, 2001; El-Sheikh et al., 2007). That is, although family influences may affect children’s physiological reactivity and regulation earlier in life, these patterns of reactivity may stabilize over time. Our proposition that family factors may exert influences on children’s reactivity more strongly in infancy and early childhood and then primarily function to interact with physiological patterns in late childhood and adolescence is a hypothesis in need of further empirical investigation. This hypothesis is similar to Barlow’s (2000) conceptualization of the development of internalizing disorders in which individual vulnerabilities associated with internalizing symptoms are fostered by environmental stressors early in life (i.e., mediation model), yet go on to amplify environmental stressors later in life (i.e., moderation model).
RECIPROCAL ACTIVATION AS A PROTECTIVE FACTOR
In contrast to opposing modes of SNS and PNS action (i.e., coactivation and coinhibition), marital conflict was positively associated with externalizing behaviors in very few (i.e., two) cases under reciprocal modes of SNS and PNS action (i.e., reciprocal sympathetic activation and reciprocal parasympathetic activation). That is, in the vast majority of analyses, no association between marital conflict and externalizing behaviors was found for children exhibiting reciprocal sympathetic and parasympathetic activation. Reciprocal sympathetic activation may reflect appropriate concern or anger yet also promote active and constructive attempts to address worries with parents or other adults, or attempts to reduce exposure to conflict. On the other hand, reciprocal parasympathetic activation and sympathetic inhibition may occur when marital conflict is not interpreted as especially threatening and is managed physiologically through vagal withdrawal, without resorting to SNS activation. This type of ANS response pattern may reflect effective self-soothing in the context of marital conflict. In several cases, a negative association between marital conflict and externalizing behaviors was found for reciprocal responders. It is thus possible that adaptive physiological regulation can even allow children to gain problem-solving or emotion regulation skills through exposure to mild marital conflict, particularly when parents use constructive conflict strategies (Cummings, Goeke-Morey, & Papp, 2003, 2004). In considering these findings, it is important to keep in mind the difference between high and optimal levels of arousal and reactivity (Eisenberg, Fabes, Guthrie, & Reiser, 2000). Indeed, although a growing body of evidence suggests that vagal withdrawal in response to challenge is adaptive (El-Sheikh et al., 2001; El-Sheikh, 2005c; Katz & Gottman, 1997), and the present studies suggest that high SNS activation in conjunction with vagal withdrawal is adaptive, there is likely a point at which too much vagal withdrawal contributes to overarousal and dysregulation and impedes effective coping (Beauchaine, 2001). An important direction for future research is to specify the degrees of SNS and PNS activation and deactivation that promote effective coping and the amounts of SNS and PNS activation associated with over- or underarousal.
The present studies did not actually link physiological response patterns with measured cognitive or behavioral coping responses, and thus our speculations must be interpreted with caution. We assume that ANS activity and reactivity are linked with emotional reactivity and regulation (Izard, Youngstrom, Fine, Mostow, & Trentacosta, 2006), but this must be confirmed empirically before conclusions can be drawn. Future research that specifically links physiological activity and reactivity with behavioral coping responses, in particular, would be informative for interventions designed to protect children from exposure to environmental stressors such as marital conflict. It is important to note that prior research has examined interactions between temperamental systems at behavioral and emotional levels, complementing the study of interactions between biologically based systems in predicting child adjustment. For example, research suggests that distinct dimensions of temperament, such as reactivity (e.g., negative emotionality) and control (e.g., attentional control) systems, interact to predict adjustment outcomes (Rothbart & Bates, 1998). There is evidence that emotional reactivity is associated with externalizing systems more strongly for children who are lower in behavioral control and self-regulation (Eisenberg et al., 1996). Likewise, behavioral regulation is more strongly predictive of prosocial behavior for children high in negative emotionality (Eisenberg et al., 2000). And negative emotionality is more strongly associated with drug use in children who are low in task orientation (Bates, Pettit, & Dodge, 1995).
In addition, at least two recent studies have examined interactions between physiological systems as predictors of child adjustment. El-Sheikh et al. (2008) recently found that the interaction between baseline HPA axis and SNS activity was associated with both externalizing and internalizing problems. The highest levels of externalizing and internalizing problems were found among children with symmetrical HPA and SNS activity—particularly among children with high baseline levels in both domains. Likewise, Gordis et al. (2006) found the highest levels of parent-reported aggression among early adolescents with symmetrical HPA and SNS reactivity (i.e., low cortisol reactivity and low SNS reactivity) and the lowest levels of aggression among early adolescents with asymmetrical HPA and SNS reactivity (i.e., high cortisol activity and low SNS activity). These findings are supportive of the proposition by Bauer et al. (2002), which suggests that redundant actions of the HPA and SNS could result in hyperarousal when both systems are high in activity or hypoarousal when both systems are low in activity. The studies included in this monograph advance these prior studies by examining interactions between physiological systems in the context of marital conflict—an environmental stressor likely to provoke responses in these physiological systems. As noted, it will be important for future research to bridge the gap between the physiological responses described in this monograph and behavioral responses to environmental stress.
CLARIFICATION OF INCONSISTENCIES IN PRIOR RESEARCH
In addition to stimulating new research, findings of the present studies may help clarify inconsistencies in the existing literature. In particular, results offer a potential explanation for some inconsistencies in the literature linking electrodermal arousal with externalizing problems, in which some studies find evidence for sympathetic underarousal among children with conduct problems (for a review, see Lorber, 2004) and other studies provide evidence for sympathetic overarousal (e.g., Hubbard et al., 2002). In the present study, higher or lower SCL or SCL-R per se did not operate as a risk factor, but lower SCL or SCL-R along with reduced PNS influence (i.e., coinhibition) and higher SCL or SCL-R along with increased PNS influence (i.e., coactivation) strengthened the association between marital conflict and externalizing problems. Conversely, low SCL-R was protective in the context of increased PNS influence (i.e., reciprocal parasympathetic activation), and high SCL-R was protective in the context of reduced PNS influence (i.e., reciprocal sympathetic activation).
Likewise, higher vagal tone and vagal withdrawal in response to stress may not be universally adaptive. Although a growing body of research provides evidence for the protective role of higher vagal tone and higher vagal withdrawal in the context of family stress (El-Sheikh et al., 2001; Katz & Gottman, 1997), results of the current studies suggest that lower vagal tone may be adaptive in the context of high sympathetic activity (i.e., reciprocal sympathetic activation) and that vagal augmentation may be adaptive in the context of sympathetic inhibition (i.e., reciprocal parasympathetic activation). These findings are consistent with recent suggestions that investigations of physiological systems as independent entities are limited because physiological systems operate concurrently, whether in cooperation or opposition (Bauer et al., 2002; Beauchaine, 2001; Berntson et al., 1991). Findings also suggest that the implications of reactivity systems are dependent on regulatory abilities, which might incur further vulnerability or provide protection (Eisenberg et al., 2000; Rothbart & Bates, 1998). It would be informative for future research to consider both branches of the ANS to better understand children’s responses to environmental stress.
BASELINE FUNCTIONING VERSUS REACTIVITY
The patterns of findings involving baseline ANS functioning versus ANS reactivity warrant some discussion. In relation to SNS functioning, the majority of significant interactions involved SCL-R (19; 10 for the argument task and 9 for the star-tracing task) rather than baseline SCL (7). Thus, these findings suggest that the role of SCL-R varies depending on PNS functioning more so than does the role of baseline SCL. In other words, in comparison with baseline SCL, there is a more pronounced effect of SCL-R that depends on PNS activity to jointly influence children’s externalizing symptoms. In relation to PNS functioning, there was a relatively even split between interactions involving RSA reactivity (RSA-R) (12; 6 for the argument task and 6 for the star-tracing task) and baseline RSA (14). These findings suggest that baseline PNS activity and PNS reactivity both have important implications for the development of externalizing symptoms in the contexts of marital conflict and SNS activity. This is consistent with prior research showing both vagal tone and vagal reactivity as moderators of the association between family discord and children’s maladjustment (e.g., El-Sheikh et al., 2001) and extends that work by incorporating two physiological systems. Interestingly, the majority of interactions involving baseline RSA were with SCL-R rather than baseline SCL (12 out of 14; the reverse is also true: the majority of interactions involving SCL-R—12 out of 19—were with baseline RSA). Overall, while the interpretation of these interactions awaits further research, the findings suggest that baseline levels of one system (SNS or PNS) can interact with reactivity of another system to predict child adjustment. Thus, our results highlight the relevance of concurrent assessment of baseline and reactivity measures across various ANS systems.
LIMITATIONS
Despite the advances made by this monograph, there are several important limitations and a need for additional research. One critical methodological limitation of each of the presented studies is their cross-sectional design. As a result, it is not clear whether the observed relations reflect interactions between family stress and physiological reactivity as causal processes in the development of children’s externalizing problems or whether externalizing problems bring about particular patterns of family stress and autonomic nervous system responding. It is noteworthy that studies examining the SNS and PNS singly do offer some support for the former case. For example, vagal tone in neonates is predictive of school-age social competence (Doussard-Roosevelt et al., 2001), and higher SCL-R and vagal augmentation serve as a vulnerability factor in the longitudinal relationship between marital conflict and children’s adjustment problems (El-Sheikh & Whitson, 2006; El-Sheikh et al., 2007). Similar findings have been reported in the context of parental problem drinking (El-Sheikh, 2005c). In addition, controlling for earlier levels of problems, higher SCL-R has been shown to predict children’s increased internalizing, externalizing, and social problems in the context of paternal depressive symptoms over a 2-year period (Cummings, El-Sheikh, Kouros, & Keller, 2007). However, studies typically do not consider the alternative direction of effects, leaving open the possibility that children’s adjustment problems lead to changes in physiological reactivity. Additional research employing more sophisticated research designs, including longitudinal research, is therefore needed.
Longitudinal research is also needed to determine potential development in the observed interactions. This monograph supports the proposed model in middle-childhood and preadolescence. However, studies of physiological reactivity within a single domain (SNS or PNS) support its role in preschool (Calkins & Keane, 2004; Cole et al., 1996), adolescence (Beauchaine, Gatzke-Kopp, & Mead, 2007; Fung et al., 2005), adulthood (Goudriaan, Oosterlaan, de Beurs, & van den Brink, 2006; Zanstra, Schellekens, Schaap, & Kooistra, 2006), and old age (Denburg, Recknor, Bechara, & Tranel, 2006; Masi, Hawkley, Rickett, & Cacioppo, 2007). Interactions between the ANS and PNS have yet to be studied during these developmental periods, and it is not clear whether interactions may play a larger role for some periods in comparison with others. As noted, low to moderate stability of baseline and reactivity levels of ANS activity have been observed in middle childhood, and some evidence suggests that reactivity levels are more susceptible to environmental factors as they appear to stabilize later than baseline levels (Bornstein & Suess, 2000; Calkins & Keane, 2004; El-Sheikh, 2001, 2005a). It is therefore likely that there are important differences in trajectories of physiological activity and reactivity and differences in the mediating or moderating role of ANS activity and reactivity over the course of development.
Additional methodological limitations are worthy of note. For example, all children in all studies were first presented with the argument task, followed by the star-tracing task. Although a recovery period was used to prevent any carryover effects from the argument task, it is possible that some carryover did occur, and findings should be interpreted within this context. Future research would benefit from a counterbalanced presentation of tasks. Also, each of the studies presented in this monograph relies on questionnaire reports of marital conflict and externalizing problems. Although the multi-informant approach, including mother, father, and child reports of marital conflict and mother, father, and teacher reports of externalizing problems, is an important strength, it is necessary to replicate findings using observations and clinical interviews. Furthermore, each of the presented studies includes a community sample, rather than families characterized by severe levels of marital aggression and clinically diagnosed externalizing problems. This may be considered a strength in the sense that findings are more likely to generalize to the broader population. However, findings require replication in more specific samples. For example, studies that consider physiological reactivity solely in terms of the SNS have found that low reactivity is characteristic of conduct disorder (Frick & Ellis, 1999; Frick et al., 2003) and substance use disorder (Bobadilla & Taylor, 2007). It is therefore possible that coinhibition will emerge as a particularly maladaptive pattern of physiological reactivity, in comparison with coactivation, when clinically diagnosed children are examined. Similarly, interactions between family stress and physiological reactivity have been shown to differ based on sex (El-Sheikh, 2005a); relations between family stress and SCLs (Lieblich, Kugelmass, & Ben-Shakhar, 1973) or reactivity (Vrana & Rollock, 1998) have been shown to differ by ethnicity. As noted, sex differences were not detected in the current studies, but these null findings must be interpreted in the context of the statistical limitations (e.g., four-way interactions) of the analyses examining sex effects. Future research considering the roles of coactivation, coinhibition, and reciprocal activation for boys, girls, and children of various ethnicities would be informative.
Findings also should be extended for multiple domains of child functioning and in differing stressful contexts. This monograph has provided evidence for interactions between the SNS and PNS for various forms of externalizing problems, including ADH symptoms, delinquency, and aggression in multiple settings. Although beyond the scope of the current investigation, further research should consider the implications of the interactions for internalizing problems, cognitive functioning, and physical health. Previous studies that consider SNS and PNS reactivity separately have found evidence that physiological reactivity predicts children’s status within each of these domains (Kagan et al., 1987; Weems et al., 2005; Whitson & El-Sheikh, 2003), and future research should establish whether physiological systems also interact to predict these outcomes. Moreover, future research should seek to determine whether more optimal patterns of physiological responding may be linked with positive outcomes, such as better problem solving or optimal coping responses and skills. Furthermore, a fruitful avenue for further research is to examine the potential role of interactions between systems in the context of multiple forms of family stress. For example, SCLs and vagal regulation can interact with parental depression (Cummings et al., 2007) and parental problem drinking (El-Sheikh, 2005c) to affect child adaptation. Very few studies have examined children’s physiological reactivity to stress in the context of parent–child conflict or child maltreatment. It is critical to understand whether the interactions documented in the current studies are specific to marital conflict or generalize to other forms of family and environmental stress (Berntson & Cacioppo, 2004).
Despite these limitations, the studies included in this monograph advance our biopsychosocial framework conceptually and provide the first evidence that interactions between the two branches of the ANS moderate the association between marital conflict and child externalizing behavior problems. We hope that these studies will encourage other researchers to consider interactions among physiological systems as risk and protective factors.
ACKNOWLEDGMENTS
This research was partially supported by National Institutes of Health Grants R01-HD046795 and R29-AA10591, a National Science Foundation Grant 0339115, and an Alabama Agricultural Experiment Station/Lindsey Foundation Grant No. ALA080-001.
We would like to thank laboratory students and staff, including Bridget Wingo and Ryan Kelly, as well as children and families for participating.
Footnotes
Data from this study were also used in Study 3 of this monograph.
Data from this study were also used in Study 3 of this monograph.
Prior research has shown that the role of physiological responses to conflict may differ based on child sex. For example, the association between marital conflict and children’s internalizing and externalizing symptoms may be stronger for girls with greater SCL-R but stronger for boys with lower SCL-R (El-Sheikh, Keller, & Erath, 2007). However, testing four-way interactions (e.g., Marital Conflict × RSA × SCL × Child Sex) requires greater power than is currently available. As an alternative test, we combined data from all three studies (N = 577) presented in this monograph and fit models including the four-way interaction terms. No significant four-way interactions were observed.
The focus of this monograph is on the prediction of children’s externalizing symptoms. However, one possibility is that coactivation and coinhibition may be associated with forms of externalizing problems that are distinguished by anxiety levels. That is, coinhibition may be related to low-anxious externalization, whereas coactivation may be related to high-anxious externalization. If this is the case, one would expect interactions between marital conflict, RSA, and SCL to be predictive of children’s anxiety. Therefore, an identical series of regressions was also run predicting children’s self-reported scores on the Revised Children’s Manifest Anxiety Scale (RCMAS; Reynolds & Richmond, 1978), which were obtained in the first and second studies reported here. Across both studies, only one significant interaction was observed. Similarly, no significant correlations between RSA or SCL and RCMAS scores were observed. These findings suggest that coinhibition and coactivation are not differentially associated with children’s anxiety in the context of marital conflict.
REFERENCES
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 profile. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Aguinis H, Stone-Romero EF. Methodological artifacts in moderated multiple regression and their effects on statistical power. Journal of Applied Psychology. 1997;82(1):192–206. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Amano M, Kanda T, Hidetoshi U. Exercise training and autonomic nervous system activity in obese individuals. Medicine and Science in Sports and Exercise. 2001;33(8):1287–1291. doi: 10.1097/00005768-200108000-00007. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social learning theory. Englewood Cliffs, NJ: Prentice-Hall; 1977. [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Bates JE, Pettit GS, Dodge KA. Family and child factors in stability and change in children’s aggressiveness in elementary school. In: McCord J, editor. Coercion and punishment in long-term perspectives. New York: Cambridge University Press; 1995. pp. 124–138. [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics. 2002;23(2):102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Baum A, Davidson LM, Singer JE, Street SW. Stress as a psychophysiological process. In: Baum A, Singer JE, editors. Handbook of psychology and health: Vol. 5. Stress. Hillsdale, NJ: Erlbaum; 1987. pp. 1–24. [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s Motivational Theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74(2):174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT. Heart rate variability: Stress and psychiatric conditions. In: Malik M, Camm AJ, editors. Dynamic electrocardiography. New York: Blackwell Futura; 2004. pp. 57–64. [Google Scholar]
- Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, Fieldstone A. Autonomic cardiac control: III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockages. Psychophysiology. 1994;31:599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Responsiveness to distress cues in the child with psychopathic tendencies. Personality and Individual Differences. 1999;27(1):135–145. [Google Scholar]
- Blandon AY, Calkins SD. Marital dissatisfaction and children’s emotional regulation trajectories: The moderating effect of vagal tone. Boston: Poster presented at Society for Research on Child Development; 2007. [Google Scholar]
- Bobadilla L, Taylor J. Relation of physiological reactivity and perceived coping to substance use disorders. Addictive Behaviors. 2007;32(3):608–616. doi: 10.1016/j.addbeh.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Suess PE. Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Developmental Psychology. 2000;36:54–65. [PubMed] [Google Scholar]
- Boucsein W. Electrodermal activity. New York: Plenum Press; 1992. [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ. Autonomic reactivity and psychopathy in middle childhood. British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: A model of the link between hostility and cardiovascular disease. Annals of Behavioral Medicine. 1998;20(4):326–332. doi: 10.1007/BF02886382. [DOI] [PubMed] [Google Scholar]
- Burns JW, Friedman R, Katkin ES. Anger expression, hostility, anxiety, and patterns of cardiac reactivity to stress. Behavioral Medicine. 1992;18(2):71–78. doi: 10.1080/08964289.1992.9935174. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Malarkey WB, Kiecolt-Glaser JK, Uchino BN, Sgoutas-Emch SA, Sheridan JF, et al. Heterogeneity in neuro-endocrine and immune responses to brief psychological stressors as a function of autonomic cardiac activation. Psychosomatic Medicine. 1995;57:154–164. doi: 10.1097/00006842-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31(4):412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology. 2000;28(2):103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74(2):144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45(3):101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Smith CL, Gill KL, Johnson MC. Maternal interactive style across contexts: Relations to emotional, behavioral and physiological regulation during toddlerhood. Social Development. 1998;7:350–369. [Google Scholar]
- Chen E, Matthews KA, Salomon K, Ewart CK. Cardiovascular reactivity during social and nonsocial stressors: Do children’s personal goals and expressive skills matter? Health Psychology. 2002;21:16–24. [PubMed] [Google Scholar]
- Cicchetti D. Development and psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Vol 1. Theory and method. 2nd ed. Hoboken, NJ: Wiley; 2006. pp. 1–23. [Google Scholar]
- Cole P, Fox N, Zahn-Waxler C, Usher BA, Welsh JD. Individual differences in emotion regulation and behavior problems in preschool children. Journal of Abnormal Psychology. 1996;105(4):518–529. [PubMed] [Google Scholar]
- Crockenberg S, Langrock A. ‘The role of specific emotions in children’s responses to interparental conflict: A test of the model. Journal of Family Psychology. 2001;15:163–182. doi: 10.1037//0893-3200.15.2.163. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Getzke-Kopp L, Sylvers P, Mead H, Chipman-Chacon J. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of Abnormal Psychology. 2006;115:174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- Cummings EM. Marital conflict and children’s functioning. Social Development. 1994;3:16–36. [Google Scholar]
- Cummings EM, Davies PT. Maternal depression and child development. Journal of Child Psychology and Psychiatry. 1994;35(1):73–122. doi: 10.1111/j.1469-7610.1994.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Davies PT. Effects of marital conflict on children: Recent advances and emerging themes in process-oriented research. Journal of Child Psychology and Psychiatry. 2002;43(1):31–63. doi: 10.1111/1469-7610.00003. [DOI] [PubMed] [Google Scholar]
- Cummings EM, El-Sheikh M, Kouros CD, Keller PS. Children’s skin conductance reactivity as a mechanism of risk in the context of parental depressive symptoms. Journal of Child Psychology and Psychiatry. 2007;48(5):436–445. doi: 10.1111/j.1469-7610.2006.01713.x. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Goeke-Morey M, Papp L. Everyday marital conflict and child aggression. Journal of Abnormal Child Psychology. 2004;32(2):191–202. doi: 10.1023/b:jacp.0000019770.13216.be. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Goeke-Morey MC, Papp LM. Children’s responses to everyday marital conflict tactics in the home. Child Development. 2003;74(6):1918–1929. doi: 10.1046/j.1467-8624.2003.00646.x. [DOI] [PubMed] [Google Scholar]
- Davies PT, Cummings EM. Interparental discord, family process, and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Vol. 3: Risk, disorder, and adaptation. 2nd ed. New York: Wiley; 2006. pp. 86–128. [Google Scholar]
- Davies PT, Harold GT, Goeke-Morey MC, Cummings EM. Child emotional security and interparental conflict. Monographs of the Society for Research in Child Development. 2002;67 (3, Serial No. 270) [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. The role of child adrenocortical functioning in pathways between interparental conflict and child maladjustment. Developmental Psychology. 2007;43:918–930. doi: 10.1037/0012-1649.43.4.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Recknor EC, Bechara A, Tranel D. Psychophysiological anticipation of positive outcomes promotes advantageous decision-making in normal older persons. International Journal of Psychophysiology. 2006;61(1):19–25. doi: 10.1016/j.ijpsycho.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Dienstbier RA. Arousal and physiological toughness: Implications for mental and physical health. Psychological Review. 1989;96:84–100. doi: 10.1037/0033-295x.96.1.84. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Coie JD. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. Journal of Personality and Social Psychology. 1987;56:1146–1158. doi: 10.1037//0022-3514.53.6.1146. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, McClenny BD, Porges SW. Neonatal cardiac vagal tone and school-age developmental outcome in very low birth weight infants. Developmental Psychobiology. 2001;38(1):56–66. [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Porges SW. The role of neurobehavioral organization in stress responses: A polyvagal model. In: Lewis M, Ramsey D, editors. Soothing and stress. Mahwah, NJ: Erlbaum; 1999. pp. 57–76. [Google Scholar]
- Eisenberg N, Fabes R, Guthrie I, Murphy B, Maszk P, Holmgren R, et al. The relations of regulation and emotionality to problem behavior in elementary school. Development and Psychopathology. 1996;8:141–162. [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Reiser M. Dispositional emotionality and regulation: Their role in predicting quality of social functioning. Journal of Personality and Social Psychology. 2000;78(1):136–157. doi: 10.1037//0022-3514.78.1.136. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Parental drinking problems and children’s adjustments: Vagal regulation and emotional reactivity as pathways and moderators of risk. Journal of Abnormal Psychology. 2001;110:499–515. doi: 10.1037//0021-843x.110.4.499. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. The role of emotional responses and physiological reactivity in the marital conflict–child functioning link. Journal of Child Psychology and Psychiatry. 2005a;46:1191–1199. doi: 10.1111/j.1469-7610.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental and Psychopathology. 2005b;46:66–74. doi: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Does poor vagal regulation exacerbate child maladjustment in the context of parental problem drinking? A longitudinal examination. Journal of Abnormal Psychology. 2005c;114:735–741. doi: 10.1037/0021-843X.114.4.735. [DOI] [PubMed] [Google Scholar]
- El Sheikh M. Children’s skin conductance level and reactivity: Are these measures stable over time and across tasks? Developmental Psychobiology. 2007;49:180–186. doi: 10.1002/dev.20171. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt J. Vagal regulation and emotional intensity predict children’s sleep problems. Developmental Psychobiology. 2005;46(4):307–317. doi: 10.1002/dev.20066. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt J, Keller PS, Cummings EM, Acebo C. Child emotional insecurity and academic achievement: The role of sleep disruptions. Journal of Family Psychology. 2007;21:29–38. doi: 10.1037/0893-3200.21.1.29. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Cummings EM. Availability of control and preschoolers’ responses to interadult anger. International Journal of Behavioral Development. 1992;15:207–226. [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology. 2008;36:601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson S. Exposure to parental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Keller PS, Erath SA. Marital conflict and risk for child maladjustment over time: Skin conductance level reactivity as a vulnerability factor. Journal of Abnormal Child Psychology. 2007;35:715–727. doi: 10.1007/s10802-007-9127-2. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: Vagal regulaion as a protective factor. Journal of Family Psychology. 2006;20:30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Fowles DC, Kochanska G, Murray K. Electrodermal activity and temperament in preschool children. Psychophysiology. 2000;37(6):777–787. [PubMed] [Google Scholar]
- Frick PJ, Cornell AH, Bodin SD, Dane HE, Barry CT, Loney BR. Callous-unemotional traits and developmental pathways to severe conduct problems. Developmental Psychology. 2003;39(2):246–260. doi: 10.1037//0012-1649.39.2.246. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ellis ML. Callous-unemotional traits and subtypes of conduct disorder. Clinical Child and Family Psychology Review. 1999;2:149–168. doi: 10.1023/a:1021803005547. [DOI] [PubMed] [Google Scholar]
- Fung MT, Raine A, Loeber R, Lynam DR, Steinhauer SR, Venables PH, et al. Reduced electrodermal activity in psychopathy-prone adolescents. Journal of Abnormal Psychology. 2005;114(2):187–196. doi: 10.1037/0021-843X.114.2.187. [DOI] [PubMed] [Google Scholar]
- Galles SJ, Miller A, Cohn JF, Fox NA. Estimating parasympathetic control of heart rate variability: Two approaches to quantifying vagal tone. Psychophysiology. 2002;39 Suppl. 1:S37. [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and [alpha]-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31(8):976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Neurocognitive functions in pathological gambling: A comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction. 2006;101(4):534–547. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- Gray JA. The psychology of fear and stress. Cambridge, MA: University Press; 1987. [Google Scholar]
- Graziano PA, Keane SP, Calkins SD. Cardiac vagal regulation and early peer status. Child Development. 2007;78(1):264–278. doi: 10.1111/j.1467-8624.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- Gronau N, Sequerra E, Cohen A, Ben-Shakhar G. The effect of novel distractors on performance in focused attention tasks: A cognitive-psychophysiological approach. Psychonomic Bulletin and Review. 2006;13(4):570–575. doi: 10.3758/bf03193964. [DOI] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic sarasympathetic cardiac control using respiratory sinus arrhythmia: The need for respiratory control. Psychophysiology. 1991;28(2):201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Wientjes K. Respiratory sinus arrhythmia and parasympathetic cardiac control: Some basic issues concerning quantification, application, and implication. In: Grosman P, Janssen K, Vaitl D, editors. Cardiorespiratory and cardiosomatic psychophysiology. New York: Plenum Press; 1986. pp. 117–138. [Google Scholar]
- Grych JH, Fincham FD. Marital conflict and children’s adjustment: A cognitive–contextual framework. Psychological Bulletin. 1990;108:267–290. doi: 10.1037/0033-2909.108.2.267. [DOI] [PubMed] [Google Scholar]
- Grych JH, Seid M, Fincham FD. Assessing marital conflict from the child’s perspective: The children’s perceptions of interparental conflict scale. Child Development. 1992;63:558–572. doi: 10.1111/j.1467-8624.1992.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Looking for the Rosetta Stone: An essay on crying, soothing, and stress. In: Ramsay MLD, editor. Soothing and stress. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. pp. 39–56. [Google Scholar]
- Herpertz SC, Mueller B, Qunaibi M, Lichterfeld C, Konrad K, Herpertz-Dahlman B. Response to emotional stimuli in boys with conduct disorder. American Journal of Psychiatry. 2005;162:1100–1107. doi: 10.1176/appi.ajp.162.6.1100. [DOI] [PubMed] [Google Scholar]
- Hickey JE, Suess PE, Newlin DB, Spurgeon L, Porges SW. Vagal tone regulation during sustained attention to boys exposed to opiates in utero. Addictive Behavior. 1995;20(1):43–59. doi: 10.1016/0306-4603(94)00044-y. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. 1975 Unpublished manuscript. [Google Scholar]
- Hubbard JA, Smithmyer CM, Ramsden SR, Parker EH, Flanagan KD, Dearing KF, et al. Observational, physiological, and self-report measures of children’s anger: Relations to reactive versus proactive aggression. Child Development. 2002;73:1101–1118. doi: 10.1111/1467-8624.00460. [DOI] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pederson FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Development. 1998;69(3):624–635. [PubMed] [Google Scholar]
- Izard CE, Youngstrom EA, Fine SE, Mostow AJ, Trentacosta CJ. Emotions and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol. 1: Theory and method. 2nd ed. Hoboken, NJ: John Wiley & Sons Inc.; 2006. pp. 244–292. [Google Scholar]
- Jaccard J, Wan CK, Turrisi R. The detection and interpretation of interaction effects between continuous variables in multiple regression. Multivariate Behavioral Research. 1990;25(4):467–478. doi: 10.1207/s15327906mbr2504_4. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Yechiam E, Murphy RR, Queller S, Stout JC. Motivational processes and autonomic responsitivity in Asperger’s disorder: Evidence from the Iowa Gambling Task. Journal of the International Neuropsychological Society. 2006;12:668–676. doi: 10.1017/S1355617706060802. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman NC. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–92. [Google Scholar]
- Katz LF, Gottman JM. Buffering children from marital conflict and dissolution. Journal of Clinical Child Psychology. 1997;26:157–171. doi: 10.1207/s15374424jccp2602_4. [DOI] [PubMed] [Google Scholar]
- Kettunen J, Ravaja N, Naatanen P, Keltikangas-Jarvinen L. The relationship of respiratory sinus arrhythmia to the co-activation of autonomic and facial responses during the Rorschach test. Psychophysiology. 2000;37:242–250. [PubMed] [Google Scholar]
- Lachar D, Gruber CP. Personality Inventory for Youth (PIY) Los Angeles: Western Psychological Services; 1995. [Google Scholar]
- Lachar D, Gruber CP. Personality Inventory for Children. Second edition (PIC-2) Los Angeles: Western Psychological Services; 2001. [Google Scholar]
- Ladd GW, Profilet SM. The Child Behavior Scale: A teacher-report measure of young children’s aggressive, withdrawn, and prosocial behaviors. Developmental Psychology. 1996;32(6):1008–1024. [Google Scholar]
- Lawrence C, Barry R, Clarke A, Johnstone S, McCarthy R, Selikowitz M, et al. Methylphenidate effects in attention deficit/hyperactivity disorder: Electrodermal and ERP measures during a continuous performance task. Psychopharmacology. 2005;183(1):81–91. doi: 10.1007/s00213-005-0144-y. [DOI] [PubMed] [Google Scholar]
- Lieblich I, Kugelmass S, Ben-Shakhar G. Psychophysiological baselines as a function of race and ethnic origin. Psychophysiology. 1973;10(4):426–430. doi: 10.1111/j.1469-8986.1973.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130(4):531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D, Becker B. The construct of resilience: A critical evaluation and guidelines for future work. Child Development. 2000;71:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh P, Beauchaine TP, Williams B. Dissociation of sad facial expressions and autonomic nervous system responding in boys with disruptive behavior disorders. Psychophysiology. 2008;45:100–110. doi: 10.1111/j.1469-8986.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi CM, Hawkley LC, Rickett EM, Cacioppo JT. Respiratory sinus arrhythmia and diseases of aging: Obesity, diabetes mellitus, and hypertension. Biological Psychology. 2007;74(2):212–223. doi: 10.1016/j.biopsycho.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS. Developmental psychopathology: Pathways to the future. International Journal of Behavioral Development. 2006;30:47–54. [Google Scholar]
- Matthews KA, Raktaczky CJ, Stoney CM, Manvck SB. Are cardiovascular responses to behavioral stressors a stable individual difference variable in childhood? Psychophysiology. 1987;24:464–473. doi: 10.1111/j.1469-8986.1987.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Woodall KL, Stoney CM. Changes in and stability of cardiovascular responses to behavioral stress: Results from a four-year longitudinal study of children. Child Development. 1990;61:1134–1144. [PubMed] [Google Scholar]
- Mayne TJ. Negative affect and health: The importance of being earnest. Cognition and Emotion. 1999;13:601–635. [Google Scholar]
- McBurnett K. Psychobiological approaches to personality and their applications to child psychopathology. Advances in Clinical Child Psychology. 1992;14:107–164. [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. The New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Patterson GR. The early development of coercive family processes. In: Reid JB, Patterson GR, Snyder J, editors. Antisocial behavior in children and adolescents: A developmental analysis and model for intervention. Washington, DC: American Psychological Association; 2002. pp. 25–44. [Google Scholar]
- Pendry P, Adam EK. Associations between parents’ marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. International Journal of Behavioral Development. 2007;31:218–231. [Google Scholar]
- Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology. 2003;112:323–338. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Vardi S, Putzer Bechner AM, Curtin JJ. Physically abused children’s regulation of attention in response to hostility. Child Development. 2005;76:968–977. doi: 10.1111/j.1467-8624.2005.00890.x. [DOI] [PubMed] [Google Scholar]
- Porges SW, editor. Vagal tone: An autonomic mediator of affect. New York: Cambridge University Press; 1991. [Google Scholar]
- Porges SW. Vagal tone: A physiologic marker of stress vulnerability. Pediatrics. 1992;90(3):498–504. [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: A physiological index of stress. Neuroscience and Biobehavioral Reviews. 1995a;19(2):225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995b;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Physiological regulation in high-risk infants: A model for assessment and potential intervention. Development and Psychopathology. 1996;8:43–58. [Google Scholar]
- Porges SW. Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. Annals of the New York Academy of Sciences. 1997;807(1):62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Love: An emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology. 1998;23(8):837–861. doi: 10.1016/s0306-4530(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42(2):123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt J, Maita AK. Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development. 1994;59(2–3) Serial No. 240. [PubMed] [Google Scholar]
- Quas JA, Baver A, Boyce WT. Physiological reactivity, social support, and memory in early childhood. Child Development. 2004;75:797–814. doi: 10.1111/j.1467-8624.2004.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. The psychopathology of crime: Criminal behavior as a clinical disorder. San Diego, CA: Academic Press; 1993. [Google Scholar]
- Raine A. Annotation: The role of prefrontal deficits, low autonomic arousal, and early health factors in the development of antisocial and aggressive behavior in children. Journal of Child Psychology and Psychiatry. 2002;43(4):417–434. doi: 10.1111/1469-7610.00034. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Dalais C, Mellingen K, Reynolds C, Mednick SA. Early educational and health enrichment at age 3–5 years is associated with increased autonomic and central nervous system arousal and orienting at age 11 years: Evidence form the Mauritius Child Health Project. Psychophysiology. 2001;38:254–266. [PubMed] [Google Scholar]
- Raine A, Venables PH, Williams M. Relationships between central and autonomic measures of arousal at age 15 years and criminality at age 24 years. Arch Gen Psychiatry. 1990;47(11):1003–1007. doi: 10.1001/archpsyc.1990.01810230019003. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. What I think and feel: A revised measure of children’s manifest anxiety. Journal of Abnormal Child Psychology. 1978;6:271–280. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- Richards JE, Casey BJ. Heart rate variability during attention phases in young Heart rate variability during attention phases in young infants. Psychophysiology. 1991;28:43–53. doi: 10.1111/j.1469-8986.1991.tb03385.x. [DOI] [PubMed] [Google Scholar]
- Rothbart M, Bates J. Temperament. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Social, emotional, and personality development. Vol. 3. New York: Wiley; 1998. pp. 105–176. [Google Scholar]
- Salomon K, Matthews KA, Allen MT. Patterns of sympathetic reactivity in a samples of children and adolescents. Psychophysiology. 2000;37:842–849. [PubMed] [Google Scholar]
- Scarpa A, Raine A. Psychophysiology of anger and violent behavior. Psychiatric Clinics of North America. 1997;20:375–394. doi: 10.1016/s0193-953x(05)70318-x. [DOI] [PubMed] [Google Scholar]
- Scerbo AS, Freedman LW, Raine A, Dawson ME, Venables PH. A major effect of recording site on measurement of electrodermal activity. Psychophysiology. 1992;29:241–246. doi: 10.1111/j.1469-8986.1992.tb01693.x. [DOI] [PubMed] [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek H, van Goozen SHM, Matthys W, Buitelaar JK, van Engeland H. Stress responsivity in children with externalizing behavior disorders. Development and Psychopathology. 2004;16:389–406. doi: 10.1017/s0954579404044578. [DOI] [PubMed] [Google Scholar]
- Snyder J, Schrepferman L, St. Peter C. Origins of antisocial behavior: Negative reinforcement and affect dysregulation of behavior as socialization mechanisms in family interaction. Behavior Modification. 1997;21:187–215. doi: 10.1177/01454455970212004. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Avenevoli S. The role of context in the development of psychopathology: A conceptual framework and some speculative propositions. Child Development. 2000;71(1):66–74. doi: 10.1111/1467-8624.00119. [DOI] [PubMed] [Google Scholar]
- Straus MA, Gelles RJ. How violent are American families? Estimates from the National Family Violence Resurvey and other studies. In: Straus MA, Gelles RJ, editors. Physical violence in American families. New Brunswick, NJ: Transaction Publishers; 1990. pp. 95–108. [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The revised Conflict Tactics Scales (CTS-2): Development and preliminary psychometric data. Journal of Family Issues. 1996;17(3):283–316. [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. New York: Harper Collins; 1996. [Google Scholar]
- Taggart P, Carruthers M. Endogenous hyperlipidaemia induced by emotional stress of racing driving. Lancet. 1971;1:363–366. doi: 10.1016/s0140-6736(71)92207-0. [DOI] [PubMed] [Google Scholar]
- Toichi M, Kamio Y. Long-term memory in high-functioning autism: Controversy on episodic memory in autism reconsidered. Journal of Autism and Developmental Disorders. 2003;33(2):151–161. doi: 10.1023/a:1022935325843. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Mohamed F, Tiver R, Pinus A, Bloomer C, Pyrros A, et al. The effect of autonomic arousal on attentional focus. Neuroreport. 2000;11(18):4037–4042. doi: 10.1097/00001756-200012180-00027. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- van Bokhoven I, Matthys W, van Goozen SHM, Engeland H. Prediction of adolescent outcome in children with disruptive behavior disorders: A study of neurobiologial, psychological and family factors. European Child and Adolescent Psychiatry. 2005;14:153–163. doi: 10.1007/s00787-005-0455-x. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Matthys W, Cohen-Kettenis PT, Buitelaar JK, van Engeland H. Hypothalamic–pituitary–adrenal axis and autonomic nervous system activity in disruptive children and matched controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1438–1445. doi: 10.1097/00004583-200011000-00019. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, van Engeland H. Salivary cortisol and cardiovascular activity during stess in oppositional-defiant disorder boys and normal controls. Biological Psychiatry. 1998;43(7):531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Rollock D. Physiological response to a minimal social encounter: Effects of gender, ethnicity, and social context. Psychophysiology. 1998;35:462–469. [PubMed] [Google Scholar]
- Weems CF, Zakem AH, Costa NM, Cannon MF, Watts SE. Physiological response and childhood anxiety: Association with symptoms of anxiety disorders and cognitive bias. Journal of Clinical Child and Adolescent Psychology. 2005;34(4):712–723. doi: 10.1207/s15374424jccp3404_13. [DOI] [PubMed] [Google Scholar]
- Wetzel JM, Quigley KS, Morell J, Eves E, Backs RW. Cardiovascular measures of attention to illusory and nonillusory visual stimuli. Journal of Psychophysiology. 2006;20(4):276–285. [Google Scholar]
- Whitson S, El-Sheikh M. Moderators of family conflict and children’s adjustment and health. Journal of Emotional Abuse: Interventions, Research and Theories of Psychological Maltreatment, Trauma and Nonphysical Aggression. 2003;3:47–73. [Google Scholar]
- Wingenfeld SA, Lachar D, Gruber CP, Kline RB. Development of the teacher-informant student behavior survey. Journal of Psychoeducational Assessment. 1998;16:226–249. [Google Scholar]
- Wirt RD, Lachar D, Klinedinst JK, Seat PD. Multidimensional description of child personality: A manual for the Personality Inventory for Children (1990 ed.) Los Angeles: Western Psychological Services; 1990. [Google Scholar]
- Zanstra YJ, Schellekens JMH, Schaap C, Kooistra L. Vagal and sympathetic activity in burnouts during a mentally demanding workday. Psychosom Med. 2006;68(4):583–590. doi: 10.1097/01.psy.0000228012.38884.49. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Theoretical formulations: I. In: Zubek JP, editor. Sensory deprivation: Fifteen years of research. New York: Appleton-Century-Crofts; 1969. pp. 407–432. [Google Scholar]
- Zuckerman M. The sensation seeking motive. In: Maher BA, editor. Progress in experimental personality research. Vol. 7. New York: Academic Press; 1974. pp. 79–148. [PubMed] [Google Scholar]