Abstract
Objective/Hypothesis
The minimum airflow necessary to initiate stable vocal fold vibration, phonation threshold flow (PTF), may increase as exposure to dry air increases. A critical period of dehydration after which phonation can no longer be initiated may exist.
Method
PTF data were collected for eleven excised canine larynges mounted on a bench apparatus. Trials consisted of cycles of ten seconds of phonation followed by three seconds of rest. During the experimental trials, subglottal flow of comparatively dry air was increased until phonation was initiated, and phonation was sustained for the remainder of the ten second period. The subglottal flow was then decreased until phonation ceased. No saline was applied during the dehydration trials. During the control trials, subglottal airflow was humidified and saline was applied frequently to the vocal folds.
Results
PTF increased as exposure to dry air increased during the experimental trials (p = 0.010); this relationship was not statistically significant in control trials. A point existed after which phonation could not be initiated.
Conclusions
Knowledge of the effect of exposure to dry air on PTF could be useful in the clinical assessment and prevention of dehydration. Further exploration of this relationship in vivo could evaluate the effectiveness of current hydration therapies and provide theoretical support for the development of new ones.
Keywords: phonation threshold flow, excised larynx, dehydration, phonation
INTRODUCTION
The relationship between dehydration and injury to the laryngeal tissue has been studied; deviations from the normal viscosity level of the laryngeal mucosa may predispose laryngeal tissue to injury during phonation by decreasing the resistive ability of the mucosa against intervocal fold impact stress (1). Hydration level is inversely related to PTP as a physiological measure of phonatory effort (2,3). Verdolini suggested that these effects may be the result of a reduction in the vocal fold tissue viscosity or an increase in fluid extravasation from lesioned cells (4). In addition, hydration therapies have been correlated to improvements in both voice and laryngeal appearance (4). Previous studies of ex vivo larynges by Jiang et al. have found that dehydration of the vocal fold surface increases phonation threshold pressure (PTP) and mean flow rate (5). This suggests that dehydration may be correlated with an increased difficulty in phonation. In another study by Finkelhor et al., it was found that with decreased hydration, the range of vocal fold oscillation decreased (6).
While the aforementioned studies suggest that vocal fold dehydration impedes phonation, none have thoroughly examined the effects of vocal fold surface dehydration on phonation threshold flow (PTF). This study experimentally investigated the relationship between the degree of surface dehydration and PTF in an ex vivo canine larynx model, using the bench apparatus described by Jiang and Titze (7). By inducing phonation in excised canine larynges using airflow at a low humidity level, the in vivo dehydrating effect of prolonged air passage over the vocal folds during phonation was simulated. Although the viscosity of the laryngeal mucosa was not measured directly, the flow of comparatively dry air over the tissue was presumed to induce water loss from the tissue surface. We hypothesized that PTF would increase as exposure to comparatively dry airflow increased.
Phonation threshold flow (PTF), defined as the minimum airflow necessary to initiate stable vocal fold vibration, is an aerodynamic parameter of voice production that has been shown to be sensitive to variations in the biomechanical properties of the larynx (8). Jiang and Tao have described the effects of changes in these tissue properties on PTF as
where L is the vocal fold length, B is the tissue viscosity, c is the mucosal wave velocity, xo is the prephonatory glottal half-width, T is the vocal fold thickness, and ρ is the air density. This equation predicts that PTF is increased by increasing the value of tissue viscosity (8).
MATERIALS AND METHODS
Larynges
Eleven larynges were harvested postmortem from canines sacrificed for other studies. The larynges were excised according to the procedure described by Jiang and Titze (7). After excision, the larynges were inspected for pathologies or trauma, placed in a 0.9% saline solution, and frozen. Eight larynges were used in exclusively dehydration trials while two larynges were used exclusively as controls. One larynx underwent a control trial followed by a dehydration trial.
Apparatus
Immediately before experimental use, the epiglottis, corniculate cartilages, cuneiform cartilages, and ventricular folds of each larynx were dissected away to expose the vocal folds. The superior cornua and posterosuperior parts of the thyroid cartilage were dissected away to facilitate insertion of lateral micrometers into the arytenoids. The larynx was then mounted as specified by Jiang and Titze (7). The trachea was fastened by a metal pull clamp to a pipe which was connected to the pseudolung of the apparatus. Through the insertion of two 3-pronged micrometer devices, the arytenoid cartilages were stabilized bilaterally, allowing for precise control of vocal fold adduction and abduction (Figure 1). The laryngeal prominence was sutured to a third micrometer, allowing for precise control of vocal fold elongation. The vocal fold elongation and adduction remained constant throughout all trials. The apparatus used to initiate phonation in these trials was designed to simulate the human respiratory system. In the control trials, pressurized airflow was passed through two Concha Therm III humidifiers (Fisher & Paykel Healthcare Inc., Laguna Hills, CA) in series to humidify and warm the air. The vocal folds were kept hydrated by frequent application of 0.9% saline solution between trials. During the dehydration trials, the humidifiers were removed from the flow circuit. No saline solution was applied to the vocal folds during these trials. The airflow was measured using an Omega airflow meter (model FMA-1601A; Omega Engineering Inc., Stamford, CT) and flow was manually controlled throughout the experiment with a valve. Pressure measurements were taken directly below the larynx using a Heise digital pressure meter (901 series; Ashcroft Inc., Stratford, CT). Acoustic data were collected by a Sony microphone (model ECM-88; Sony Electronics Inc., New York, NY); to reduce acoustic noise produced by turbulent airflow, the microphone was positioned at a 45° angle to the vertical axis of the vocal tract and approximately 10.0 cm from the glottis. The acoustic signal was amplified using a Symetrix pre-amplifier (model 302; Symetrix Inc., Mountlake Terrace, WA). Using a National Instruments data acquisition board (model AT-MIO-16; National Instruments Corp., Austin, TX) and customized LabVIEW 8.2.1 software (National Instruments Corp., Austin, TX), airflow, pressure, and acoustic data were recorded simultaneously on a personal computer at a sampling rate of 1000 Hz. All trials were conducted in a triple-walled, sound-proof room to reduce background noise and stabilize humidity levels and temperatures. This experimental setup is depicted in Figure 2.
Figure 1.
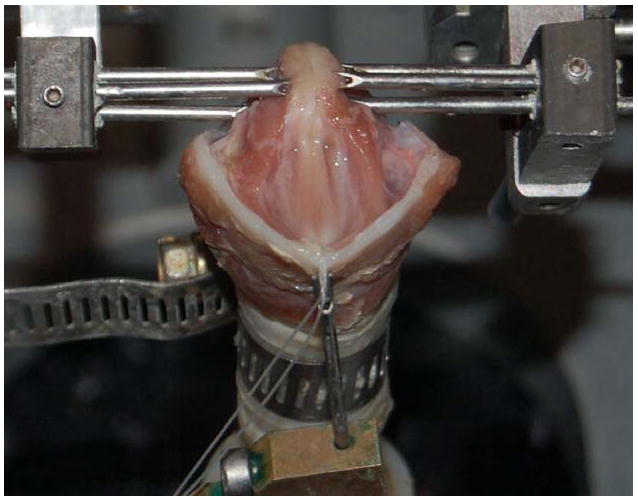
Digital photograph of an excised canine larynx mounted on the experimental bench apparatus.
Figure 2.
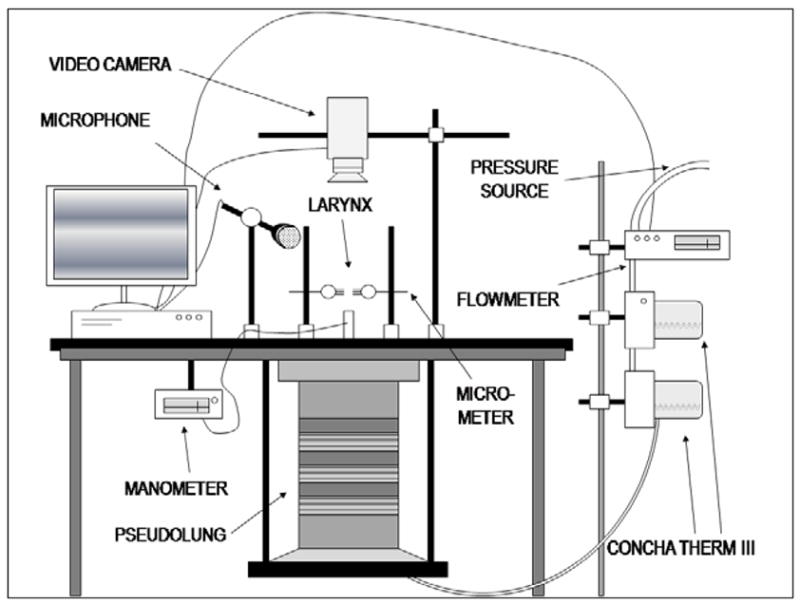
The experimental setup used to initiate and sustain phonation under dehydrating and control treatments.
Experimental Procedure
Dehydration trials were conducted as a sequence of ten-second periods of dry airflow through the vocal folds, followed by three-second periods of rest. During each ten-second drying period, comparatively dry (non-humidified: 25% ± 3% relative humidity) airflow through the larynx was manually increased slowly and consistently until phonation ensued. The measured values of airflow and air pressure at the time of phonation onset were recorded as the onset PTF and onset PTP, respectively. The sequence of drying and resting periods was repeated on the same larynx in succession for a total of twenty-three cycles or approximately five minutes (Figure 3). No saline solution was applied to the vocal folds during this time. Two of these larynges were exposed to dry air until phonation was no longer possible to determine if a critical point existed at which dehydration no longer increased PTF but prevented phonation.
Figure 3.
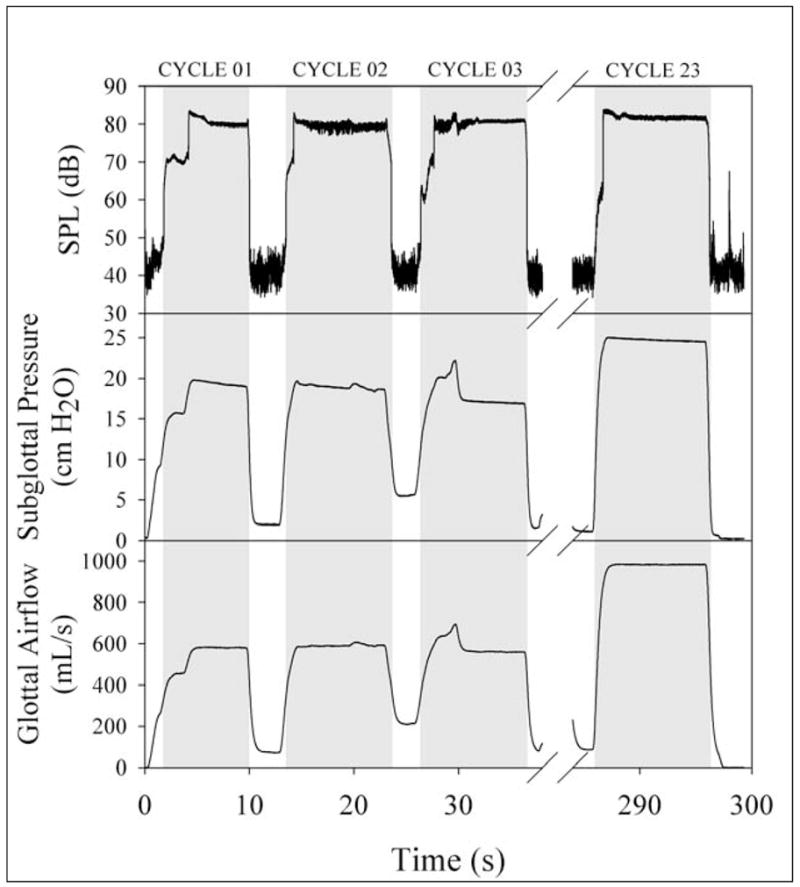
Depictions of typical acoustic, pressure, and airflow signals. The shaded area marks phonation and the left edge marks the corresponding onset phonation threshold flow (PTF). Larynges underwent dehydration trials which were comprised of twenty-three cycles of a ten-second phonation period followed by a three-second rest period.
Control Larynges
The hydration trials for the two control larynges consisted of the same sequence of ten-second periods of phonation using humidified air (100% relative humidity), followed by three seconds of rest. During each resting period, 0.9% saline solution was applied to the vocal folds to maintain hydration. As in the dehydration trials, the sequence of phonation and resting periods was repeated for a total of twenty-three cycles. An additional larynx underwent a control trial followed by a dehydration trial to ensure that confounding variables among different larynges were not affecting the results.
Data Analysis
After the control and experimental trials were completed, PTF and PTP were determined manually using a custom LabVIEW 8.0 program. This software graphically displayed the acoustic, airflow, and pressure signals as a function of time for each trial; the corresponding airflow and pressure values at the time phonation onset occurred were recorded as the PTF and PTP, respectively. A Student’s t-test was used to determine if a statistically significant difference existed between the mean initial and mean final PTF in the dehydration and control trials. A one-way ANOVA on ranks was applied to the aggregate PTF values in the dehydration trials to determine if the PTF of cycles prior to the final cycle were also affected by dehydration. Using data from the larynx that underwent both experimental and control treatments, dehydration and normal PTF values were compared pair-wise using a Wilcoxon signed-rank test.
RESULTS
The mean initial and mean final PTF values of the dehydration and control trials are provided in Table 1. Typical PTF data for a single larynx over twenty-three dehydrating phonation cycles are shown in Figure 4. The mean PTF values for all cycles during dehydration are shown in Figure 5. A Student’s t-test suggested that there was a statistically significant difference between the initial and final PTF values across all dehydrated larynges (p=0.010). One-way ANOVA on ranks suggested that the PTF values of all dehydration trials also exhibited statistically significant differences between each cycle (p=0.032), indicating that the PTF of each cycle was dependent to some degree on dehydration. The initial PTF values for the dehydrated larynges ranged from 133.9 to 661.8 mL/s, and the final PTF values ranged from 196.5 to 1219.2 mL/s. The increase in PTF across all larynges ranged from a minimum of 62.65 mL/s to a maximum of 735.61 mL/s.
Table 1.
PHONATION THRESHOLD FLOW DURING PHONATION OF LARYNGES
| Dehydration Trials PTF (mL/s) | Control Trials PTF (mL/s) | |
|---|---|---|
| Initial | 406.71 (±166.21) | 147.00 (±20.85) |
| Final | 780.11 (±316.49) | 143.75 (±34.49) |
| p-value | 0.010 | 0.920 |
Figure 4.
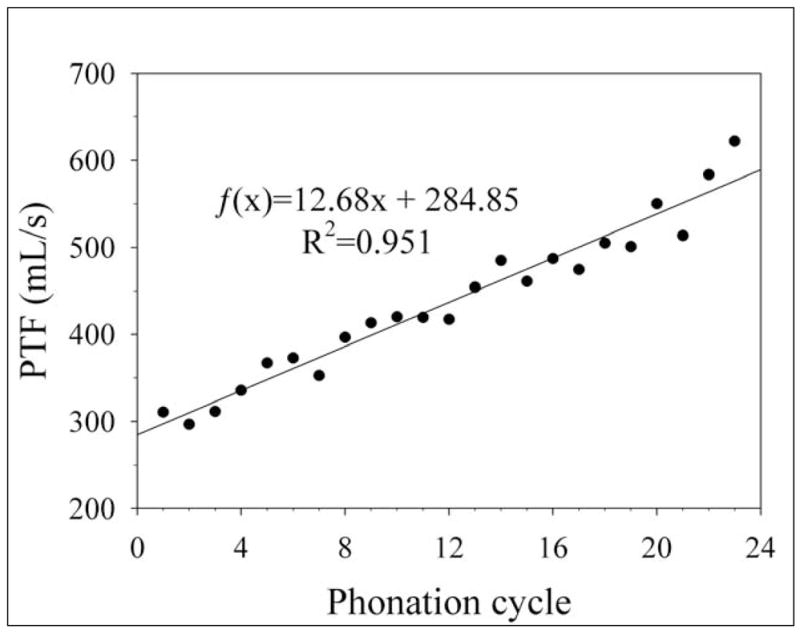
Typical phonation threshold flow (PTF) data from a single excised canine larynx during dehydration over twenty-three phonation cycles. PTF increased in subsequent phonation cycles.
Figure 5.
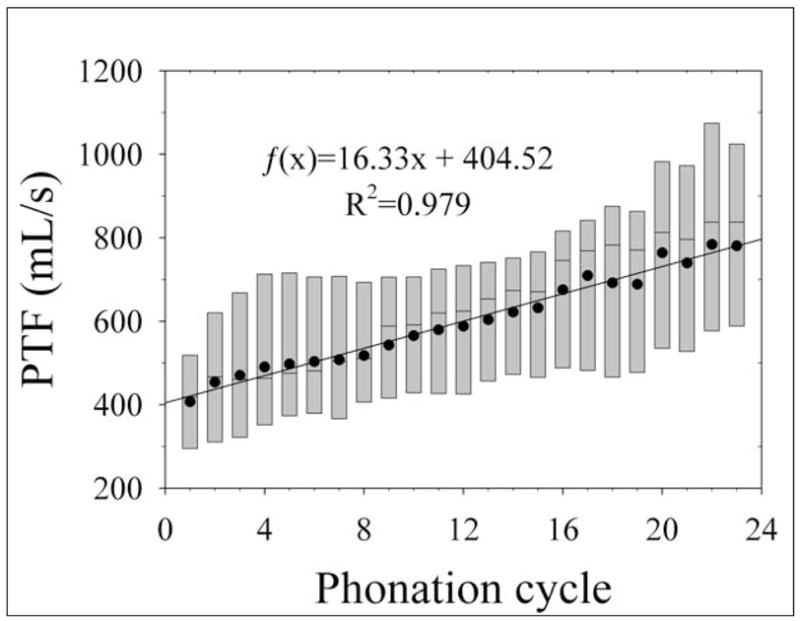
Aggregate phonation threshold flow (PTF) data in excised canine larynges during dehydration. The lower and upper edges of the box represent the 25th and 75th percentiles, respectively, and the line dividing the box represents the median. The points overlaying the boxes represent the mean PTF values for each cycle. Subsequent cycles had increasingly greater PTF. A linear regression is shown on the graph.
According to the results of the two larynges that underwent dehydration for longer than twenty-three cycles, there appears to be a critical length of time of exposure to dry air after which the vocal folds are no longer able to initiate or sustain phonation. The mean length of time after which the vocal folds could no longer phonate was 543 seconds. Figure 6 shows the increases in PTF for these larynges.
Figure 6.
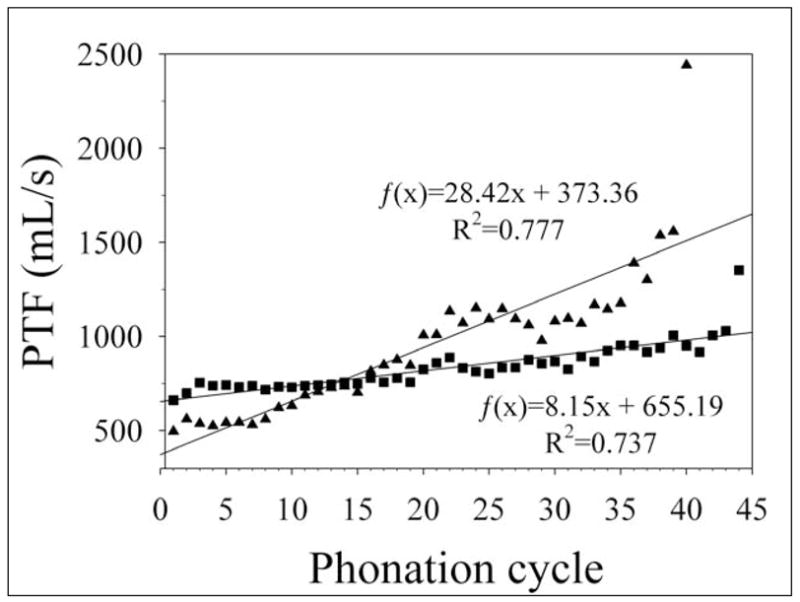
Phonation threshold flow (PTF) data in excised canine larynges for extended dehydration trials. The final point in both data sets is the last phonation cycle in which phonation could be initiated and sustained. Linear regressions are shown on the graph.
In the hydrated trials, the difference between the initial and final PTF of the control larynges was not statistically significant according to a Student’s t-test (p=0.920). The mean PTF values for all cycles during the control trials are shown in Figure 7. A single larynx underwent a control trial followed by a dehydration trial to directly compare the changes in PTF between hydration and dehydration treatments in the same larynx. In the control trial the difference between the initial and final PTF was 7.69 mL/s. The difference between the initial and final PTF in the dehydration trial was 201.86 mL/s. A Wilcoxon signed-rank test for paired groups indicated that the difference between the control and dehydration trials was significant (p=0.032), indicating that the changes in PTF during continuous exposure to dry air were not due to variables introduced by conducting trials on different larynges.
Figure 7.
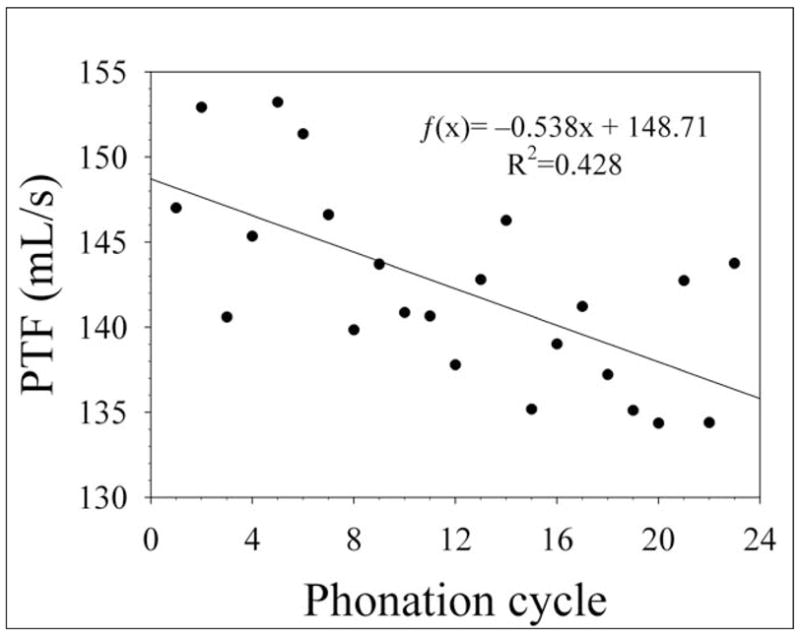
Aggregate phonation threshold flow (PTF) data in excised canine larynges during hydrated control trials. The points represent the mean PTF for each cycle. A linear regression is shown on the graph
DISCUSSION
In a recent study by Hottinger et al., the PTF and PTP were measured in variably abducted excised canine larynges. The results suggested PTF was more sensitive than PTP to changes in posterior glottal width (9). The greater sensitivity of PTF to changes in the geometry of the laryngeal system was explained by a modified one-mass model by Tao and Jiang (10). Their results showed that, in contrast to PTP, PTF always significantly increased as prephonatory glottal width increased, even when the glottal gap is large (10). In a study by Regner et al., the offset PTF was found to be dependent on the onset PTF (11). The dependence of PTF on biomechanical changes in the larynx, along with the ease of measurement of PTF have demonstrated its potential clinical application as an indicator of possible laryngeal dysfunction. Before PTF can be routinely used in the assessment of laryngeal health, more research on the relationship between it and laryngeal function is needed.
After inducing phonation by passing dry air over the vocal folds for approximately five minutes, the PTF values increased significantly by an average value of 373.4 mL/s. A positive, proportional relationship was observed between the degree of dehydration and PTF. Our findings suggest that the increases between the initial and subsequent cycles in the experimental group were proportional to the time of exposure to dry air. Because dehydration affects PTF, observing abnormally high PTF may indicate that laryngeal function is likely abnormal, and possibly dehydrated. These results support the findings by Jiang et al. (5), in which airflow over the vocal folds appeared to desiccate the surface epithelium, and supplement the findings of Verdolini et al. (2) in which systemic dehydration in humans caused similar effects. The change in PTF for the control larynges was not statistically significant, indicating that the changes observed in the experimental trials are unlikely to have been caused by variables other than the dehydration process. We hypothesized that the observed increase in PTF in the dehydration trials was most likely due to the dessication of the lamina propria of the vocal folds.
Although the results of this study indicate that one method of vocal fold dehydration is superficial, the experimentally employed drying effects ex vivo are not directly comparable to those observed in living organisms. In vivo laryngeal systems utilize several biological hydration mechanisms that are absent in the experimental specimen. In the experimental trials, the lubricating layer of mucous, which appears to be critical for normal phonation, was not replenished during the phonation period. The vibratory undulation of mucous on the surface of the vocal fold is observed in clinically healthy larynges (12). When this mucosal wave was impeded, as in dehydration, phonation required increased pulmonary exertion; a higher PTF was required. The initiation of phonation may be affected by the viscosity of vocal fold mucosa, which is increased by hydration (5). The absence of mucous production may have exacerbated the effect of dehydration on PTF. In living animals, airflow is produced by the lungs where it is saturated with moisture before passing through the vocal folds. The humidification of air reduces the osmotic gradient between the air and the vocal fold surface layer, but some exosmosis still occurs. The movement of water from epithelial cells into the interstitial spaces increases ion concentration in the cells and creates a negative osmotic gradient between those cells and the underlying tissue, and blood circulation adjacent to the basement membrane restores fluid to those cells (5). Although an increase in PTF may be caused in vivo by the passage of comparatively dry airflow through the laryngeal system, the simulated effect in the excised larynx model was probably exaggerated because the systemic hydration mechanisms were not functional in the ex vivo larynges.
Despite the limitations of the ex vivo laryngeal model, similar effects have been observed in humans exhibiting systemic dehydration. Previous studies on humans demonstrated that diuretic-induced dehydration may increase the perceived difficulty of phonation measured physiologically by the aerodynamic parameter of PTP (3,4). This study showed a similar decrease in the ease of phonation of excised larynges due to dehydration when using PTF as a physiological measure. Dehydration, either functional or organic, can cause vocal pathologies such as laryngitis sicca, in which a deficiency in lubrication of the lamina propria causes irritation, coughing, and mild inflammation (13). Physicians routinely recommend that voice patients suffering from laryngeal dysfunction avoid dehydrating situations such as dry climates, inhalation of first- and second-hand smoke, intake of caffeine or alcohol, and use of diuretic prescription drugs (1,12,13). They also typically advise against use of nasal sprays, decongestants, and breathing through the mouth (1,14,15). Numerous hydration therapies have been designed to assist in the prevention and remediation of dehydration, including ambient humidification, direct steam inhalations, mucolytic drug administration, and nasal breathing (1,13,14,16). The two experimental trials in which the larynx was dehydrated until phonation could not be initiated indicated that extensive dehydration may cause dysphonia; hydration treatments may prevent degradation of voice production by counteracting dehydration. The results of this study suggest that hydration treatments may increase the ease of phonation.
The relationship between PTF and hydration has potential clinical application, as PTF may then be an indicator of possible laryngeal dysfunction. It has been theorized that PTF is sensitive to changes in tissue properties and configuration of the larynx (8,9,10). Both theory and ex vivo modeling indicate that PTF is adversely affected by dehydration. Techniques used to measure PTF are less invasive than those that measure many other aerodynamic parameters such as PTP, making its use as an assessment parameter more clinically feasible. Whereas direct PTP measurements require a transducer placed subglottally, PTF can be measured directly and noninvasively by an extraoral flow meter. More research is needed before clinical use of PTF is possible, but because of its dependence on the biomechanical properties of the vocal folds PTF may be used to indicate that laryngeal function is likely abnormal.
Although the results of this study suggest that the dehydration of epithelial cells in ex vivo larynges causes an increase in PTF proportional to the extent of dehydration, further studies are necessary to determine if this relationship is observed in in vivo laryngeal systems. By determining the relationship between dehydration and PTF in living humans, it may also be possible to compare the difference between the effects of surface and systemic dehydration on PTF. Future studies could also be conducted to determine the effect of rehydration of vocal folds on PTF.
CONCLUSION
The data from these experiments suggest that surface dehydration of the vocal folds caused a statistically significant increase (p=0.010) in phonation threshold flow, a parameter dependant upon the biomechanical properties of the larynx. Laryngeal pathologies sometimes are manifested in the dehydration of the superficial layer of the vocal folds; PTF may have clinical applications as an indicator of laryngeal function. Further studies should be conducted to determine the possible differences between the effects of surface and systemic dehydration on PTF and the effect of rehydration of vocal folds on PTF.
Acknowledgments
This research was supported by NIH Grant No. R01 DC008153 and R01 DC05522 from the National Institute of Deafness and other Communication Disorders.
References
- 1.Verdolini K. Practice good vocal health and prevent those voice disorders. Choristers Guild Lett. 1988;2:40–44. [Google Scholar]
- 2.Verdolini K, Titze IR, Druker DG. Changes in phonation threshold pressure with induced conditions of hydration. J Voice. 1990;4:142–151. [Google Scholar]
- 3.Verdolini K, Titze IR. Dependence of phonatory effort on hydration level. J Speech and Hearing Research. 1994;37(5):1001–1007. doi: 10.1044/jshr.3705.1001. [DOI] [PubMed] [Google Scholar]
- 4.Verdolini K, Sandage M, Titze IR. Effect of hydration treatments on laryngeal nodules and polyps and related voice measures. J Voice. 1994;8(1):30–47. doi: 10.1016/s0892-1997(05)80317-0. [DOI] [PubMed] [Google Scholar]
- 5.Jiang JJ, Verdolini K, Ng J, Aquino B, Hanson D. Effects of dehydration on phonation in excised canine larynges. Ann Otol Rhinol Laryngol. 2000;109:568–575. doi: 10.1177/000348940010900607. [DOI] [PubMed] [Google Scholar]
- 6.Finkelhor BK, Titze IR, Durham PL. The effect of viscosity changes in the vocal folds on the range of oscillation. J Voice. 1988;1(4):320–325. [Google Scholar]
- 7.Jiang JJ, Titze IR. A methodological study of hemilaryngeal phonation. Laryngoscope. 1993;103:872–882. doi: 10.1288/00005537-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Jiang JJ, Tao C. The minimum glottal airflow to initiate vocal fold oscillation. J Acoust Soc Am. 2007;121(5):2873–2881. doi: 10.1121/1.2710961. [DOI] [PubMed] [Google Scholar]
- 9.Hottinger DG, Tao C, Jiang JJ. Comparing phonation threshold flow and pressure by abducting excised larynges. Laryngoscope. 2007;117:1695–1699. doi: 10.1097/MLG.0b013e3180959e38. [DOI] [PubMed] [Google Scholar]
- 10.Tao C, Jiang JJ. The phonation critical condition in rectangular glottis with wide prephonatory gaps. J Acoust Soc Am. 2008;123(3):1–5. doi: 10.1121/1.2832328. [DOI] [PubMed] [Google Scholar]
- 11.Regner MF, Tao C, Zhuang P, Jiang JJ. Onset and offset phonation threshold flow in excised canine larynges. Laryngoscope. 2008 doi: 10.1097/MLG.0b013e31816e2ec7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastian RW, Lawrence VL. Hoarseness in singers. NATS Bull. 1984;40:26–27. [Google Scholar]
- 13.Sataloff RT. The professional voice: part III. Common diagnoses and treatments. J Voice. 1987;1:283–292. [Google Scholar]
- 14.Bradley M. Prevention and correction of vocal disorders in singers. NATS Bull. 1980;36:38–41. [Google Scholar]
- 15.Sataloff RT, Baron BC, Brodnitz FS, Lawrence VL, Rubin W, Spiegel J, Woodson G. Discussion: Acute medical problems of the voice. J Voice. 1988;2(4):345–353. [Google Scholar]
- 16.Lawrence VL. Handy household hints: to sing or not to sing. NATS Bull. 1981;37:23–25. [Google Scholar]


