Abstract
Most soluble lysosomal proteins bind the mannose 6-phosphate receptor (M6P-R) to be sorted to the lysosomes. However, the lysosomes of I-cell disease (ICD) patients, a condition resulting from a mutation in the phosphotransferase that adds mannose 6-phosphate to hydrolases, have near normal levels of several lysosomal proteins, including the sphingolipid activator proteins (SAPs), GM2AP and prosaposin. We tested the hypothesis that SAPs are targeted to the lysosomal compartment via the sortilin receptor. To test this hypothesis, a dominant-negative construct of sortilin and a sortilin small interfering RNA (siRNA) were introduced into COS-7 cells. Our results showed that both the truncated sortilin and the sortilin siRNA block the traffic of GM2AP and prosaposin to the lysosomal compartment. This observation was confirmed by a co-immunoprecipitation, which demonstrated that GM2AP and prosaposin are interactive partners of sortilin. Furthermore, a dominant-negative mutant GGA prevented the trafficking of prosaposin and GM2AP to lysosomes. In conclusion, our results show that the trafficking of SAPs is dependent on sortilin, demonstrating a novel lysosomal trafficking.
Keywords: lysosomes/prosaposin/sortilin/sorting/traffic
Introduction
To exit from the Golgi apparatus, soluble lysosomal proteins must bind to a trans-membrane sorting receptor containing a cytoplasmic tail that interacts with adaptor proteins (Lobel et al., 1989). This interaction results in the recruitment of clathrin and ARF, and the formation of cargo vesicles (Lobel et al., 1989). Integral lysosomal membrane proteins do not require sorting receptors since their cytoplasmic tails interact directly with multimeric adaptor proteins (Peden et al., 2002). Multimeric adaptor proteins are involved in several trafficking processes such as endocytosis, basolateral targeting and lysosomal targeting (Robinson and Bonifacino, 2001). Multimeric adaptors (AP-1, AP-2, AP-3 and AP-4) are composed of four polypeptide chains that form a functional complex (Robinson and Bonifacino, 2001; Peden et al., 2002). AP-3 has been shown to traffic integral lysosomal membrane proteins, such as LAMPs, to the lysosome (Robinson and Bonifacino, 2001).
The mannose 6-phosphate receptor (M6P-R) is a well-characterized trans-membrane protein receptor that binds and routes to the lysosomal compartment most soluble hydrolases including several cathepsins (Lobel et al., 1989; Nielsen et al., 2001). A novel class of adaptor proteins, the monomeric GGAs (Golgi-localizing, γ-adaptin ear homology domain, ARF-binding proteins), have been shown to bind the cytosolic tail of the M6P-R (Puertollano et al., 2001a; Zhu et al., 2001) and to recruit the components needed for the formation of clathrin-coated vesicles. The GGAs are monomeric adaptor proteins composed of four domains: a VHS domain that binds to the tail of the sorting receptor via a specific protein motif, a GAT domain which interacts with ARF (Puertollano et al., 2001b; Zhu et al., 2001), and the hinge and ear domains that bind clathrin and accessory proteins to form coated vesicles (Hirst et al., 2000). The use of dominant-negative GGA constructs lacking the hinge and ear domains abolished the recruitment of clathrin and caused the retention of M6P-R in the Golgi, preventing proper intracellular sorting of several lysosomal proteins (Puertollano et al., 2001a).
Analyses of human fibroblasts from patients with I-cell disease (ICD) showed that sphingolipid activator proteins (SAPs) use an alternative targeting mechanism to reach the lysosomes (Rijnboutt et al., 1991). ICD results from a mutation in the phosphotransferase that adds mannose 6-phosphate to hydrolases that must be delivered to the lysosomes via the M6P-R (Reitman et al., 1981). SAPs are five non-enzymatic cofactors required for the lysosomal degradation of glycosphingolipids with short oligosaccharide chains (Bierfreund et al., 2000). Four of them (saposins A, B, C and D) are small homologous glycoproteins derived from a common precursor protein (prosaposin) encoded by a single gene (Hiraiwa et al., 1997). Saposins increase the catalytic rate of lysosomal hydrolases by forming water-soluble complexes with specific sphingolipids, hence lysosomal hydrolases can cleave sugar residues to yield ceramide, which is deacylated to sphingosine. The fifth activator, the GM2 activator protein (GM2AP), is the product of a separate gene and is an essential cofactor of β-hexosaminidase A in the degradation of GM2 to GM3 ganglioside (Yamanaka et al., 1994). The lysosomal trafficking of prosaposin is dependent on the D domain along with its highly conserved C-terminal region (Zhao and Morales, 2000). Deletion of either of these regions causes the misrouting of prosaposin to the secretory pathway (Zhao and Morales, 2000). Sphingomyelin, present in the inner leaflet of the Golgi membrane, is also required to target prosaposin to the lysosomes (Lefrancois et al., 2002). Depletion of this lipid with specific inhibitors causes the misrouting of prosaposin to the secretory pathway (Lefrancois et al., 1999). Based on these results, we have hypothesized that the D domain of prosaposin interacts with sphingomyelin to bring this protein into close contact with the Golgi membrane and to facilitate the binding of its C-terminal region to an unknown sorting receptor (Lefrancois et al., 2002). Little is known about the trafficking mechanism of GM2AP other than that it is M6P-R independent (Glombitza et al., 1997).
Recent evidence suggests that the trans-membrane Golgi protein, sortilin, may be implicated in the transport of proteins to the lysosome. Chimeric constructs containing the luminal domain of the M6P-R linked to the trans-membrane and cytosolic portion of sortilin restored the lysosomal trafficking of soluble hydrolases in M6P-R-deficient cells (Nielsen et al., 2001). While the M6P-R binds the majority of soluble hydrolases, no lysosomal proteins are known to interact with sortilin (Nielsen et al., 2001). Interestingly, the cytosolic portion of sortilin and M6P-R contain an ‘acidic cluster–dileucine motif’ that interacts with the monomeric adaptor proteins (Nielsen et al., 2001). In this report, we are testing the hypothesis that sortilin is the trafficking receptor of prosaposin and GM2AP. Although it has been suggested that prosaposin is targeted to the lysosomal compartment via secretion and endocytosis through the lipoprotein receptor-related protein (LRP) receptor (Hiesberger et al., 1998), we present evidence for a direct trafficking of both prosaposin and GM2AP from the trans-Golgi network (TGN) to the lysosomal compartment via a sortilin-mediated mechanism.
Results
Expression and localization of truncated sortilin
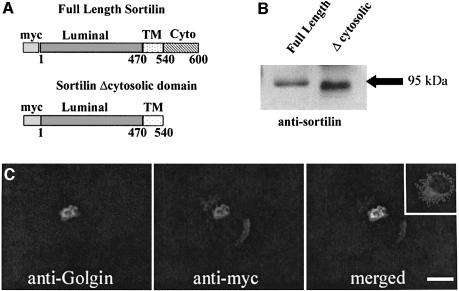
Deletion of the acidic cluster–dileucine signal from the cytoplasmic tail of the M6P-R abolished its interaction with the VHS domain of GGA proteins and its subsequent sorting to the lysosomes. Thus, we used a similar approach to investigate the role of sortilin in the transport of prosaposin and GM2AP. To this effect, a full-length (wild-type) and a truncated sortilin lacking the acidic cluster–dileucine signal (Δcytosolic) (Figure 1A) were in vitro translated, run on a 12.5% acrylamide gel and transferred to nitrocellulose paper. The membrane was blotted and reacted with an anti-sortilin antibody. The full-length sortilin produced a band at ∼95 kDa while the truncated sortilin was slightly lower (Figure 1B). COS-7 cells were then transiently transfected with the truncated construct to examine its intracellular localization. The cells were stained for the construct with an anti-myc antibody and for a Golgi marker, anti-Golgin. The construct was retained in the Golgi apparatus and localized to the same compartment as the Golgi marker (Figure 1C, merged). Non-transfected COS-7 cells were stained for full-length sortilin (Figure 1C, inset). The staining pattern for full-length sortilin was not restricted to the perinuclear region, but extended into punctate structures.
Fig. 1. Expression and localization of truncated sortilin. Myc-tagged full-length and a truncated sortilin construct that lacked its cytosolic domain (A) (residues 540–600) were in vitro translated and run on a 12.5% SDS–polyacrylamide gel. The full-length construct ran to the standard 95 kDa (B, lane 1) while the truncated construct was slightly smaller (B, lane 2). The truncated construct was transfected into COS-7 cells and localized in the perinuclear region (C, anti-myc). This staining pattern was merged to the Golgi staining with anti-Golgin, indicating that the truncated sortilin was being retained in the Golgi apparatus (merged). The inset shows the immunofluorescent staining of full-length sortilin. Scale bar = 10 µm.
Truncated sortilin abolished the transport of prosaposin and GM2AP
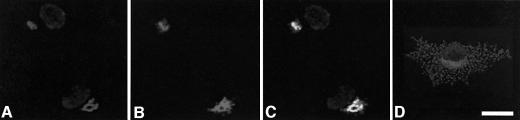
In this experiment, COS-7 cells were transfected with a dominant-negative sortilin construct lacking the cytoplasmic region implicated in the binding of GGAs (Nielsen et al., 2001). The construct was linked to a myc tag to discriminate between transfected and non-transfected cells and to distinguish truncated from endogenous sortilin. The cells were immunostained with an anti-prosaposin (Figure 2A, C and D), anti-GM2AP (Figure 3A, C and D) or anti-cathepsin B (Figure 4A and C) antibody, followed by incubation with a secondary antibody conjugated to Alexa 568. To detect the construct, the same culture cells were immunostained with an anti-myc antibody followed by a secondary antibody conjugated to Alexa 488. Non-transfected cells (Figure 2D and 3D) showed the characteristic anti-prosaposin and anti-GM2AP immunostaining in the perinuclear region as well as in cytoplasmic punctate structures. The transfected cells (Figures 2 and 3) displayed a perinuclear immunostaining with the anti-prosaposin, anti-GM2AP and the anti-myc antibodies. However, no punctate structures were seen in transfected cells, suggesting that overexpression of truncated sortilin competed for the binding of prosaposin and abolished its transport to the lysosomal compartment. The immuno staining pattern of the anti-cathepsin B antibody was not different in the non-transfected (not shown) and transfected cells (Figure 4).
Fig. 2. Effect of dominant-negative sortilin lacking its cytoplasmic domain in COS-7 cells immunostained with anti-prosaposin antibody. COS-7 cells were immunostained with anti-prosaposin antibody following transfection with truncated sortilin (A). COS-7 cells were also stained for the truncated sortilin construct (B). The merged image shows prosaposin in a perinuclear staining pattern in the same compartment as the truncated sortilin construct (C). Immunostaining for endogenous prosaposin demonstrates its normal distribution in non-transfected COS-7 cells (D). Scale bar = 20 µm.
Fig. 3. Effect of dominant-negative sortilin lacking its cytoplasmic domain in COS-7 cells immunostained with anti-GM2AP antibody. COS-7 cells were immunostained with anti-GM2AP antibody following a transfection with truncated sortilin (A). COS-7 cells were also stained for the truncated sortilin construct (B). The merged image shows GM2AP in a perinuclear staining pattern in the same compartment as the truncated sortilin construct (C). Immunostaining for endogenous GM2AP demonstrates its normal distribution in non-transfected COS-7 cells (D). Scale bar = 20 µm.
Fig. 4. Effect of dominant-negative sortilin lacking its cytoplasmic domain in COS-7 cells immunostained with anti-cathepsin B antibody. While anti-cathepsin B antibody produced a punctate staining in the cytoplasm of non-transfected cells (not shown), the truncated construct (B and C) had no effects on the staining pattern of anti-cathepsin B antibody (A and C). Scale bar = 20 µm.
Sortilin inhibition prevents the normal sorting of prosaposin
To confirm the involvement of sortilin in the sorting of prosaposin, sortilin mRNA was targeted with six small interfering RNA (siRNA) constructs. Two siRNAs were able to reduce the amount of sortilin mRNA isolated from COS-7 cells (Figure 5A). The level of β-actin mRNA was similar in all samples (Figure 5B). Based on this result, we transfected siRNA 1 into COS-7 cells to examine its effect on the expression of the protein. Western blot analysis revealed that siRNA 1 reduced the amount of sortilin (Figure 5C). We also used siRNA 1, in conjunction with immunofluorescence, to examine its effect on the expression of sortilin and the sorting of prosaposin and GM2AP to lysosomes. Compared with control cells (Figure 5D and E insets), the prosaposin and GM2AP staining was greatly reduced and showed no punctate staining. The Golgi apparatus was stained with anti-Golgin antibody. To determine the fate of activator proteins after sortilin inhibition, COS-7 were targeted with the siRNA 1 construct, pulsed with [35S]methionine/cysteine and chased with Dulbecco’s modified Eagle’s medium (DMEM) containing 5 mM cold methionine/cysteine. Cell lysates (not shown) and media were immunoprecipitated 30, 60 and 120 min after the initial labeling with anti-prosaposin antibody, resolved by SDS–PAGE and visualized by autoradiography. The results showed that ablation of sortilin increases the secretion of prosaposin into the culture media (Figure 5G).
Fig. 5. Use of siRNAs to knock-down sortilin. (A) Six siRNAs were tested for their ability to knock-down sortilin mRNA in COS-7 cells. siRNA 1 and 3 (lanes 1 and 3) were able to reduce sortilin mRNA, while the others could not (lanes 2, 4, 5 and 6). (B) β-Actin is present in all samples, showing that the drop in sortilin mRNA is specific and not dependent on a total decrease in mRNA. (C) Western blot of cell lysate from COS-7 cells transfected or not with siRNA 1. (D) COS-7 cells transfected with sortilin siRNA and stained for anti-Golgin and anti-prosaposin. An untransfected cell is shown in the inset. (E) COS-7 cells transfected with sortilin siRNA and stained for anti-Golgin and anti-GM2AP. The inset shows a non-transfected cell. (F) A COS-7 cell transfected with sortilin siRNA and stained for anti-Golgin and anti-cathepsin B. Scale bar =10 µm. (G) A representative pulse–chase analysis of COS-7 cells transfected with the sortilin siRNA 1 (bottom) shows that inactivation of sortilin mRNA increases the secretion of prosaposin into the culture medium. The top panel shows basal secretion of prosaposin in control cells. The quantitative values in optical density support this observation. Scale bar = 10 µm.
Co-immunoprecipitation shows association of sortilin and SAPs
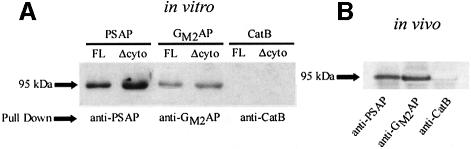
Full-length sortilin, truncated sortilin, prosaposin and GM2AP were in vitro translated and used in a co-immunoprecipitation (Co-IP) assay. The binding of sortilin and truncated sortilin to prosaposin or GM2AP was tested using an anti-prosaposin or anti-GM2AP antibody and protein G-conjugated Sepharose beads. Prosaposin was able to pull-down full-length (Figure 6A, lane 1) and truncated sortilin (95 kDa band) (Figure 6A, lane 2). This demonstrated that the luminal domain of sortilin binds prosaposin, and hence substantiated the functional studies in COS-7 cells transfected with the dominant-negative form of sortilin. Similarly, GM2AP was able to pull-down full-length (Figure 6A, lane 3) and truncated sortilin (Figure 6A, lane 4), demonstrating that sortilin interacts with both SAPs. On the other hand, cathepsin B did not pull-down either truncated or full-length sortilin (Figure 6A, lanes 5 and 6). In vivo Co-IP confirmed the in vitro data as prosaposin and GM2AP were able to pull-down sortilin from COS-7 cell lysates (Figure 6B, lanes 1 and 2). On the other hand, cathepsin B could not pull-down sortilin (Figure 6B, lane 3).
Fig. 6. Co-immunoprecipitation showing the association of sortilin with SAPs. (A) In vitro translated full-length and truncated sortilin were incubated with SAPs or cathepsin B. Lanes 1 and 2 demonstrate that prosaposin pulls-down both full-length and truncated sortilin. Lanes 3 and 4 show that GM2AP pulls-down full-length and truncated sortilin, while lanes 5 and 6 demonstrate that cathepsin B is unable to bind sortilin. (B) In vivo co-immunoprecipitation using COS-7 cell lysates. Lanes 1 and 2 demonstrate the ability of prosaposin and GM2AP to pull-down sortilin, while cathepsin B was not able to pull-down sortilin (lane 3).
Dominant-negative GGA abolishes the transport of SAPs
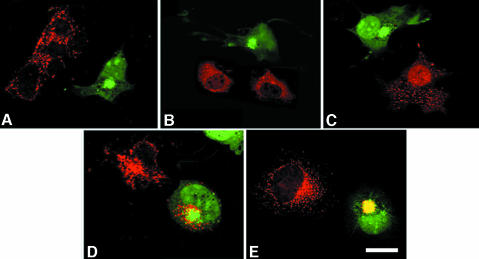
To substantiate our experimental evidence showing that prosaposin and GM2AP use sortilin to traffic to the lysosomes, COS-7 cells transfected with the dominant-negative GGA3 construct were immunostained with anti-prosaposin (Figure 7A), anti-GM2AP (Figure 7B), anti-cathepsin B (Figure 7C), anti-LAMP-2 antibodies (Figure 7D) or anti-myc (sortilin) and stained with a secondary antibody conjugated to Alexa 568. The GGA3 construct lacking the ‘hinge and ear’ domains was linked to the green fluorescent protein (GFP). Thus, transfected cells were recognized from non-transfected cells by their green fluorescence. While transfected cells did not exhibit immunostaining for anti-cathepsin B, anti-prosaposin or anti-GM2AP antibodies, the anti-LAMP-2 antibody produced a strong granular and perinuclear immunostaining (Figure 7A–D). Non-transfected COS-7 cells were immunostained by the four antibodies and showed punctate lysosomal staining. Sortilin staining was also abolished from punctate structures and remained in the perinuclear region of cells transfected with GFP–GGA3 (Figure 7E).
Fig. 7. Effect of a dominant-negative GGA3–GFP construct in COS-7 cells immunostained with anti-prosaposin (A), anti-GM2AP (B), anti-cathepsin B (C), anti-LAMP-2 antibody (D) or anti-sortilin (E). GGA3–GFP-transfected cells are recognized by their green fluorescence. While anti-prosaposin, anti-GM2AP and anti-cathepsin B antibodies yielded an intense punctate staining, transfected cells (green fluorescence) are not stained. LAMP-2 staining is not affected, while that of sortilin is reduced to the perinuclear area. Scale bar = 15 µm.
Discussion
Sortilin belongs to a growing family of multiligand type-1 receptors with homology to the yeast receptor Vps10p (Nielsen et al., 2001). Based on structural features and experimental evidence, sortilin was proposed to traffic lysosomal proteins from the TGN to the lysosomes (Nielsen et al., 2001). Chimeric sortilin containing the dileucine motifs linked to the luminal domain of the M6P-R restored lysosomal trafficking of soluble hydrolases in M6P-R-deficient cells (Nielsen et al., 2001). However, no lysosomal proteins are known to interact with sortilin. In this investigation, we tested the hypothesis that sortilin is involved in the sorting of SAPs (prosaposin and GM2AP) and that the lysosomal trafficking of these proteins requires monomeric adaptor protein GGA.
The lysosomal targeting mechanism for sphingolipid activator proteins (prosaposin and GM2AP) has not been identified conclusively. Earlier reports from several laboratories have demonstrated that both SAPs can reach the lysosomes in an M6P-R-independent manner (Rijnboutt et al., 1991; Rigat et al., 1997). Immunocytochemical analysis of tunicamycin-treated cells demonstrated that non-glycosylated prosaposin can be targeted more efficiently to lysosomes, and biochemical analysis revealed that prosaposin is associated with Golgi membrane fractions and that this association is not disrupted by free mannose 6-phosphate (Igdoura et al., 1996). A carbohydrate-independent pathway of native GM2AP has been characterized recently (Rigat et al., 1997). Taken together, all these reports indicate the existence of an alternative mechanism of sorting and transport of lysosomal proteins. Although it has been suggested that the alternative mechanism of transport of these lysosomal proteins is that of secretion and internalization by other receptors (Hiesberger et al., 1998), our data do not support this model as a general mechanism. First, we only find the 65 kDa form of prosaposin in the lysosomal compartment as opposed to the 70 kDa form. If the mechanism of transport to the lysosome consisted primarily of the secreted form, the 70 kDa form should be more abundant, not the 65 kDa form. Also, unpublished data from our laboratory of cells that had a non-functional LRP receptor showed that they contained prosaposin in their lysosomes. These results indicate the existence of a novel intracellular route.
Three approaches were used to test our first hypothesis (i.e. that sortilin is involved in the sorting of SAPs): a dominant-negative competition experiment; siRNA; and Co-IP assays. The dominant-negative competition experiment was performed using a truncated sortilin overexpressed in COS-7 cells. The truncated sortilin lacked the cytoplasmic domain necessary to bind GGAs and exit from the Golgi apparatus (Nielsen et al., 2001). This protein could, however, compete and bind its specific ligands. Overexpression of truncated sortilin abolished the punctate cytoplasmic immunostaining of anti-prosaposin and anti-GM2AP antibody but not the Golgi-like perinuclear staining. This result indicates that truncated sortilin competed for and probably retained a fraction of prosaposin and GM2AP in the Golgi apparatus. However, pulse–chase analysis demonstrated that the bulk of prosaposin is secreted to the culture medium (data not shown). This result suggests that after saturation of sortilin receptors, prosaposin enters a default secretory pathway. As expected, overexpression of truncated sortilin had no effect on cathepsin B immunostaining since this hydrolase is trafficked to the lysosomal compartment via the M6P-R (Mort and Buttle, 1997). To confirm further the requirement for sortilin in the sorting of prosaposin, RNAi technology was used to knock-down sortilin mRNA in COS-7 cells. Sortilin-deficient cells were not able to route prosaposin to the lysosomal compartment. Interestingly, pulse–chase analysis showed that ablation of sortilin increased the secretion of the mature form of prosaposin into the culture medium, demonstrating the requirement for sortilin in the trafficking of prosaposin to the lysosome.
To confirm whether prosaposin or GM2AP were interactive partners of sortilin, an in vitro Co-IP assay was conducted. Truncated and wild type-sortilin as well as prosaposin, GM2AP or cathepsin B were translated and labeled in a reticulocyte lysate system. Each lysosomal protein was incubated with full-length or truncated sortilin, and immunoprecipitated with anti-prosaposin, anti-GM2AP or anti-cathepsin B antibody. The immunoprecipitated complex was pulled-down with protein G-coated Sepharose beads, electrophoresed, transferred to nitrocellulose paper and visualized with a non-radioactive detection assay. The results demonstrated that both full-length and truncated sortilin co-immunoprecipitated with both prosaposin and GM2AP but not with cathepsin B. Finally, in vivo Co-IP demonstrated that unlike cathepsin B, prosaposin and GM2AP were able to pull-down sortilin.
To examine our second hypothesis (i.e. that the lysosomal trafficking of SAPs requires monomeric adaptor protein GGA), we tested the effect of mutant adaptor proteins on the transport of prosaposin and GM2AP. Two classes of adaptor proteins have been implicated in the transport of lysosomal proteins from the Golgi apparatus to the lysosomes, namely the GGAs and AP-3. GGAs have been shown to bind sortilin (Nielsen et al., 2001). To investigate the involvement of GGAs in the transport of prosaposin from the Golgi to the lysosomes, a truncated form of GGA lacking the hinge and ear domains was transfected and overexpressed in COS-7 cells. Overexpression of this mutant created a dominant-negative competition with the endogenous GGA. Since the transfected construct lacked the clathrin-binding subunits, all lysosomal proteins whose transport is dependent on sorting receptors that interact with GGAs were misrouted away from lysosomes. Our results demonstrated that prosaposin and GM2AP immunostaining were absent in the lysosomes of transfected cells, indicating that their transport is dependent on the presence of GGAs. The absence of immunostaining in the lysosomes with anti-cathepsin B antibody was expected because GGAs are required to traffic M6P-R-bound proteins including cathepsin B (positive control).
In a recent study, the selective deletion of prosaposin functional domains demonstrated that the C-terminus was required for its targeting to lysosomes. When the D domain and the C-terminus of prosaposin were added to albumin, this chimeric construct was rerouted to lysosomes (Zhao and Morales, 2000). Based on these results, it was hypothesized that prosaposin interacts with membrane lipids via its D domain and with a sorting receptor, other than the M6P-R, via its C-terminus (Lefrancois et al., 2002).
The C-terminus of prosaposin contains a saposin-like motif that forms a short α-helix stabilized by two conserved disulfide bonds (Zhao et al., 1998). This region is significantly similar (66%) to the N-terminus of surfactant-B protein (SP-B) (Zaltash and Johansson, 1998). SP-B requires the presence of the N-terminal region for its transient routing to multivesicular and lamellar bodies in type II neumocytes (Lin et al., 1996; Stahlman et al., 2000).
Members of the superfamily of aspartic proteinases also contain a common saposin-like domain (Kervinen et al., 1999). Other proteins containing saposin-like domains are acyloxyacyl hydrolase and acid sphingomyelinase (Muniz and Riezman, 2000). Evidence from several laboratories suggests that saposin-like motifs in aspartic proteinases promote membrane–lipid binding and vacuolar targeting (Ponting and Russell, 1995).
To our surprise, the overexpression of truncated sortilin abolished the transport of the GM2AP to the lysosomes. Although the GM2AP does not contain a saposin-like motif, a striking feature of its structure is the presence of a short α-helix stabilized by a disulfide bridge between C112 and C138. Patients with a C138 substitution are associated with the AB variant form of GM2 gangliosidosis characterized by the absence of GM2AP in lysosomes. Taken together, all these results indicate the existence of an alternative mechanism of sorting and transport of lysosomal proteins in addition to the M6P-R pathway (Lefrancois et al., 2002).
In conclusion, our data disclosed a novel mechanism of lysosomal sorting and trafficking mediated by sortilin. Although sortilin is involved in the sorting and trafficking of both prosaposin and GM2AP, this receptor may be responsible for the targeting of other soluble lysosomal proteins.
Materials and methods
Cell culture and transfection
COS-7 cells were cultured in DMEM (Sigma, St Louis, MO) supplemented with 10% fetal bovine serum (FBS) and antibiotics. The COS-7 cells were harvested then plated on coverslips (5 × 105/10 ml) in NuSerum supplemented medium overnight in preparation for a transient transfection. The cells were transfected using a DEAE–dextran/chloroquine phosphate protocol (Zhao and Morales, 2000) and then fed with DMEM supplemented with 10% FBS and antibiotics overnight.
Immunofluorescent staining
The cells were washed three times in phosphate-buffered saline (PBS) and, in the case of some cells, also incubated in 60 nM LysoTracker (Molecular Probes Inc., Eugene, OR) for 30 min then fixed with 3.8% paraformaldehyde (Sigma) for 30 min at room temperature followed by two washes in PBS. The cells were treated with 0.5% Triton X-100 (Boehringer Mannheim, Indianapolis, IN) for 30 min at room temperature and then blocked with 100 µl of 10% goat serum for 1 h, followed by 100 µl of primary antibody diluted 1:200 in PBS overnight at 4°C. The cells were then washed in 0.05% Tween-20 (Sigma) three times for 5 min each. The appropriate Alexa-conjugated secondary antibody (Molecular Probes) was diluted to 1:200 and 100 µl of the antibody solution was placed on each coverslip for 1 h at room temperature. The cells were then washed with 0.05% Tween-20 three times for 5 min each, followed by a rinse with distilled water. The coverslips were mounted face down on microscope slides with VectaShield (Vector Labs, Burlingame, CA) and sealed with nail polish to be viewed on a Zeiss 410 confocal microscope (Carl Zeiss, Germany). The slides were stored in a light-proof black box.
Plasmids and antibodies
The mutant GGA3–GFP construct is from Dr Juan Bonifacino’s lab (CBMB, NIH, Bethesda, MD). The full-length sortilin construct was from Dr Claus Petersen (University of Aarhus, Denmark). The truncated form of sortilin was generated in our lab. It included the luminal and trans-membrane regions without the cytosolic portion subcloned into the pcDNA3.1B expression vector. The anti-cathepsin B antibody was a generous gift from Dr John S.Mort (Shriner’s Hospital, Montreal, Quebec). The anti-GM2AP antibody and plasmid were a generous gift from Dr Don Mahuran (Hospital for Sick Children, Toronto, Ontario). The anti-prosaposin antibody was generated in our laboratory against the N-terminus and the functional domains A–B of prosaposin. The characterization and specificity of the prosaposin antibody were discussed in a previous report (Zhao and Morales, 2000). The anti-myc antibody was purchased from Invitrogen (Mississauga, Ontario). The anti-sortilin antibody was purchased from BD Biosciences (Toronto, Ontario).
RNAi
A silencer siRNA construction kit was purchased from Ambion (Austin, TX) and used to produce six siRNAs to human sortilin with 3′-overhanging uridine dimers. The siRNAs were chosen to correspond to sequences located in the 5′, 3′ or medial regions of sortilin mRNA. The siRNAs were transfected into COS-7 cells using the DEAE–dextran/chloroquine phosphate protocol. Total RNA isolation and analysis was performed using Qiagen’s Rneasy kit (Mississauga, Ontario) after a 72 h incubation with the siRNA. The mRNA levels were quantified by spectrophotometry. The expression level of sortilin RNA in both target and control was determined by RT–PCR using primers designed in our lab. First-strand cDNA was synthesized from total RNA with the Omniscript Reverse Transcriptase Kit (Qiagen) using oligo(dT) primer. Then, 2 µg of total RNA sample was added in a 20 µl reaction. For PCR, 2 µl of cDNA was used as a template in a volume of 25 µl of reaction mixture. The PCR mixture contained cDNA, 2.5 µl of 10× PCR buffer (20 mM MgCl2), 1 µl of a dNTP mixture, 0.5 U of Taq (Qiagen) and 1 µl of each primer. Amplification was performed under the following conditions: 30 cycles at 94°C during 1 min to denature, 55°C during 1 min to anneal, 72°C for 2 min for extension, and a final extension for another 10 min at 72°C. The PCR products were separated by electrophoresis in a 1% (w/v) agarose gel, and 0.4% ethidium bromide was added during the gel preparation.
Pulse–chase labeling
COS-7 cells were plated to 50% confluency in 100 mm dishes 1 day before transfection and maintained in DMEM containing 10% FBS. After 24 h transfection with a sortilin siRNA, the cells were washed with PBS twice and incubated in methionine/cysteine-free DMEM containing 5% dialyzed FBS for 40 min at 37°C. Then, the cells were pulse-labeled with 0.8 mCi of [35S]methionine/cysteine (Promix AGQ0080, Amersham, Boston, MA) in methionine/cysteine-free DMEM for 15 min at 37°C. Subsequently, the cells were washed with warm PBS twice and chased in 3 ml of chase medium (DMEM containing 5 mM cold methionine/cysteine) for 0, 30, 60 and 120 min. Untransfected COS-7 cells were used as control. Finally, the cells were harvested, lysed and spun down to remove cellular debris. The supernatant was collected and pre-cleared with 50 µl of protein A–beads during 1 h (50% slurry). Then the supernatant was incubated with anti-prosaposin antibody at 4°C overnight followed by a 1 h incubation in 50 µl of a 50% slurry of protein A–beads at 4°C. After spinning down at 10 000 r.p.m. for 1 min, the supernatant was discarded and the pellet washed with IP buffer (150 mM NaCl, 1.0% NP-40, 0.5% DOC, 0.1% SDS, 50 mM Tris pH 8.0) three times, and then with 50 mM Tris pH 8.0. The immunoprecipitates were resolved by SDS–PAGE and visualized by autoradiography.
Co-immunoprecipitation assays
Proteins were in vitro translated using Promega’s TnT Coupled Reticulocyte Lysate System (Promega, Madison, WI). Briefly, prosaposin, GM2AP, sortilin and truncated sortilin plasmids were incubated with the rabbit reticulocyte lysate from the kit. Proteins were labeled using the Transcend Non-Radioactive Translation Detection System (Promega) for visualization. The proteins were then incubated together with the appropriate antibody. The complex was pulled-down using protein A-coated Sepharose beads and washed using MatchMaker Co-IP Kit solutions (Clontech, Palo Alto, CA). The proteins were then suspended in reducing sample buffer and boiled at 100°C for 5 min. The samples were subsequently run on a 12.5% acrylamide gel, transferred to nitrocellulose paper, and visualized with the Transcend Non-Radioactive Translation Detection System (Promega). Conversely, cell lysates were prepared from COS-7 cells using TNE (50 mM Tris, 2 mM EDTA, 150m M NaCl, 1% Triton X-100). After incubating the cells for 10 min in the above buffer on ice, the cell debris was pelleted at 14 000 r.p.m. for 30 min. The cell lysates were immunoprecipitated with prosaposin, GM2AP or cathepsin B antibody for 2 h. A 50 µl aliquot of protein G-coated Sepharose beads was added and incubated for 2 h, and spun down at 8000 r.p.m. for 5 min. The immunoprecipitated fractions were run on a 12% polyacrylamide gel and transferred to nitrocellulose paper. The blot was then probed for sortilin using an anti-sortilin antibody.
Acknowledgments
Acknowledgements
We thank Rosa Puertollano, John Mort and Claus Petersen for reagents. This investigation was supported by an Operating grant from the Canadian Institutes of Health Research. S.L. was supported by a studentship from CIHR.
References
- Bierfreund U., Kolter,T. and Sandhoff,K. (2000) Sphingolipid hydrolases and activator proteins. Methods Enzymol., 311, 255–276. [DOI] [PubMed] [Google Scholar]
- Glombitza G.J., Becker,E., Kaiser,H.W. and Sandhoff,K. (1997) Biosynthesis, processing and intracellular transport of GM2 activator protein in human epidermal keratinocytes. The lysosomal targeting of the GM2 activator is independent of a mannose-6-phosphate signal. J. Biol. Chem., 272, 5199–5207. [DOI] [PubMed] [Google Scholar]
- Hiesberger T., Huttler,S., Rohlmann,A., Schneider,W., Sandhoff,K. and Herz,J. (1998) Cellular uptake of saposin (SAP) precursor and lysosomal delivery by the low density lipoprotein receptor-related protein (LRP). EMBO J., 17, 4617–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa M., Martin,B.M., Kishimoto,Y., Conner,G.E., Tsuji,S. and O’Brien,J.S. (1997) Lysosomal proteolysis of prosaposin, the precursor of saposins (sphingolipid activator proteins): its mechanism and inhibition by ganglioside. Arch. Biochem. Biophys., 341, 17–24. [DOI] [PubMed] [Google Scholar]
- Hirst J., Lui,W.W., Bright,N.A., Totty,N., Seaman,M.N. and Robinson,M.S. (2000) A family of proteins with γ-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol., 149, 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igdoura S.A., Rasky,A. and Morales,C.R. (1996) Trafficking of sulfated glycoprotein-1 (prosaposin) to lysosomes or to the extracellular space in rat Sertoli cells. Cell Tissue Res., 283, 385–394. [DOI] [PubMed] [Google Scholar]
- Kervinen J., Tobin,G.J., Costa,J., Waugh,D.S., Wlodawer,A. and Zdanov,A. (1999) Crystal structure of plant aspartic proteinase prophytepsin: inactivation and vacuolar targeting. EMBO J., 18, 3947–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois S., Michaud,L., Potier,M., Igdoura,S. and Morales,C.R. (1999) Role of sphingolipids in the transport of prosaposin to the lysosomes. J. Lipid Res., 40, 1593–1603. [PubMed] [Google Scholar]
- Lefrancois S., May,T., Knight,C., Bourbeau,D. and Morales,C.R. (2002) The lysosomal transport of prosaposin requires the conditional interaction of its highly conserved d domain with sphingomyelin. J. Biol. Chem., 277, 17188–17199. [DOI] [PubMed] [Google Scholar]
- Lin S., Phillips,K.S., Wilder,M.R. and Weaver,T.E. (1996) Structural requirements for intracellular transport of pulmonary surfactant protein B (SP-B). Biochim. Biophys. Acta, 1312, 177–185. [DOI] [PubMed] [Google Scholar]
- Lobel P., Fujimoto,K., Ye,R.D., Griffiths,G. and Kornfeld,S. (1989) Mutations in the cytoplasmic domain of the 275 kd mannose 6-phosphate receptor differentially alter lysosomal enzyme sorting and endocytosis. Cell, 57, 787–796. [DOI] [PubMed] [Google Scholar]
- Mort J.S. and Buttle,D.J. (1997) Cathepsin B. Int. J. Biochem. Cell Biol., 29, 715–720. [DOI] [PubMed] [Google Scholar]
- Muniz M. and Riezman,H. (2000) Intracellular transport of GPI-anchored proteins. EMBO J., 19, 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.S., Madsen,P., Christensen,E.I., Nykjaer,A., Gliemann,J., Kasper,D., Pohlmann,R. and Petersen,C.M. (2001) The sortilin cytoplasmic tail conveys Golgi–endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J., 20, 2180–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden A.A., Rudge,R.E., Lui,W.W. and Robinson,M.S. (2002) Assembly and function of AP-3 complexes in cells expressing mutant subunits. J. Cell Biol., 156, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting C.P. and Russell,R.B. (1995) Swaposins: circular permutations within genes encoding saposin homologues. Trends Biochem. Sci., 20, 179–180. [DOI] [PubMed] [Google Scholar]
- Puertollano R., Aguilar,R.C., Gorshkova,I., Crouch,R.J. and Bonifacino,J.S. (2001a) Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science, 292, 1712–1716. [DOI] [PubMed] [Google Scholar]
- Puertollano R., Randazzo,P.A., Presley,J.F., Hartnell,L.M. and Bonifacino,J.S. (2001b) The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell, 105, 93–102. [DOI] [PubMed] [Google Scholar]
- Reitman M.L., Varki,A. and Kornfeld,S. (1981) Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5′-diphosphate-N-acetylglucosamine:glycoprotein N-acetylglucosaminylphosphotransferase activity. J. Clin. Invest., 67, 1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigat B., Wang,W., Leung,A. and Mahuran,D.J. (1997) Two mechanisms for the recapture of extracellular GM2 activator protein: evidence for a major secretory form of the protein. Biochemistry, 36, 8325–8331. [DOI] [PubMed] [Google Scholar]
- Rijnboutt S., Aerts,H.M., Geuze,H.J., Tager,J.M. and Strous,G.J. (1991) Mannose 6-phosphate-independent membrane association of cathepsin D, glucocerebrosidase and sphingolipid-activating protein in HepG2 cells. J. Biol. Chem., 266, 4862–4868. [PubMed] [Google Scholar]
- Robinson M.S. and Bonifacino,J.S. (2001) Adaptor-related proteins. Curr. Opin. Cell Biol., 13, 444–453. [DOI] [PubMed] [Google Scholar]
- Stahlman M.T., Gray,M.P., Falconieri,M.W., Whitsett,J.A. and Weaver,T.E. (2000) Lamellar body formation in normal and surfactant protein B-deficient fetal mice. Lab. Invest., 80, 395–403. [DOI] [PubMed] [Google Scholar]
- Yamanaka S., Johnson,O.N., Lyu,M.S., Kozak,C.A. and Proia,R.L. (1994) The mouse gene encoding the GM2 activator protein (Gm2a): cDNA sequence, expression and chromosome mapping. Genomics, 24, 601–604. [DOI] [PubMed] [Google Scholar]
- Zaltash S. and Johansson,J. (1998) Expression and characterization of saposin-like proteins. J. Protein Chem., 17, 523–524. [PubMed] [Google Scholar]
- Zhao Q. and Morales,C.R. (2000) Identification of a novel sequence involved in lysosomal sorting of the sphingolipid activator protein prosaposin. J. Biol. Chem., 275, 24829–24839. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Bell,A.W., El-Alfy,M. and Morales,C.R. (1998) Mouse testicular sulfated glycoprotein-1: sequence analysis of the common backbone structure of prosaposins. J. Androl., 19, 165–174. [PubMed] [Google Scholar]
- Zhu Y., Doray,B., Poussu,A., Lehto,V.P. and Kornfeld,S. (2001) Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science, 292, 1716–1718. [DOI] [PubMed] [Google Scholar]