Abstract
The role played by the β-galactoside-binding lectin galectin-3 (Gal-3) in airway remodeling, a characteristic feature of asthma that leads to airway dysfunction and poor clinical outcome in humans, was investigated in a murine model of chronic allergic airway inflammation. Wild-type (WT) and Gal-3 knock-out (KO) mice were subjected to repetitive allergen challenge with ovalbumin (OVA) up to 12 weeks and bronchoalveolar lavage fluid (BALF) and lung tissue collected after the last challenge were evaluated for cellular features associated with airway remodeling. Compared to WT mice, chronic OVA challenge in Gal-3 KO mice resulted in diminished remodeling of the airways with significantly reduced mucus secretion, sub-epithelial fibrosis, smooth muscle thickness, and peribronchial angiogenesis. The higher degree of airway remodeling in WT mice was associated with higher Gal-3 expression in the BALF as well as lung tissue. Cell counts in BALF and lung immunohistology demonstrated that eosinophil infiltration in OVA-challenged Gal-3 KO mice was significantly reduced compared to WT mice. Evaluation of cellular mediators associated with eosinophil recruitment and airway remodeling revealed that levels of eotaxin-1, IL-5, IL-13, FIZZ1 and TGF-β were substantially lower in Gal-3 KO mice. Finally, leukocytes from Gal-3 KO mice demonstrated decreased trafficking (rolling) on vascular endothelial adhesion molecules compared to WT cells. Overall, these studies demonstrate that Gal-3 is an important lectin that promotes airway remodeling via airway recruitment of inflammatory cells, specifically eosinophils, and the development of a Th2 phenotype as well as increased expression of eosinophil-specific chemokines, pro-fibrogenic and angiogenic mediators.
Introduction
Airway remodeling is a characteristic feature of chronic asthma leading to airway dysfunction and a poorer clinical outcome. Chronic allergic airway inflammation with increased number of inflammatory cells, especially eosinophils, and elevated levels of Th2 cytokines and chemokines as seen in asthma leads to structural changes in the airways. These include subepithelial fibrosis, increased smooth muscle mass, neovascularization, and epithelial alterations leading to the thickening of airway walls and consequently to mucous hypersecretion, airway edema, airway narrowing and bronchial hyperresponsiveness (1). The damaged airway epithelium and recruited inflammatory cells contribute to airway remodeling through the production of various proinflammatory cytokines, growth factors, and other mediators (2, 3). An obligatory role in airway remodeling has been demonstrated for factors that induce eosinophilic inflammation, such as IL-5, IL-13, CCR-3 and NF-κB (4).
Human eosinophils, epithelial cells, endothelial cells (EC), fibroblasts, B cells, T cells, monocyte/macrophages, dendritic cells, neutrophils and mast cells express Galectin-3 (Gal-3), a member of a family of β-galactoside-binding lectins that is known to be involved in many aspects of immune response such as cell adhesion, migration, survival and even activation (5–8). Previous studies have shown that Gal-3 plays an important role in allergic inflammation and in eosinophil recruitment in murine models of acute allergen-induced asthma and atopic dermatitis (9, 10). Studies from our laboratory demonstrated that Gal-3 functions as an adhesion molecule to support human eosinophil rolling and adhesion under conditions of flow (11). Further, the suppression of Gal-3 expression was found to attenuate infiltration of eosinophils and other inflammatory cells in a mouse model of allergic rhinitis (12). Overall, these latter findings suggest that Gal-3 can promote eosinophil recruitment and allergic inflammatory responses.
Eosinophil-mediated damage to the respiratory system is a major mechanism underlying the pathogenesis of chronic asthma. Eosinophil-derived growth factors and cytokines are considered to be a major contributor to the development of remodeled lungs in chronic asthma (13–15) and eosinophil-deficient mice are protected from pulmonary mucus accumulation, peribronchial collagen deposition and increases in airway smooth muscle (16, 17). Since Gal-3 promotes eosinophil trafficking (11) and their recruitment to the airways during acute allergic inflammation (9), the role played by this lectin in mediating airway remodeling during episodes of chronic allergic inflammation was investigated in the present study using WT and Gal-3 deficient mice.
Materials and Methods
Murine model of chronic allergic airway inflammation
Gal-3 knock-out (KO) mice were generated as described (18). These mice were backcrossed to C57BL/6 mice for nine generations and interbreeding of gal3+/− F9 resulted in KO mice in the C57BL/6 background, which were used in this study. Gal-3 KO and wild-type (WT) mice (8–12 weeks old) were sensitized with 50 µg ovalbumin (OVA) (Grade V, Sigma Chemical Co., St Louis, MO) in 0.5 mg aluminum hydroxide by subcutaneous injections on days 0, 7, 14 and 21 and then challenged with OVA (20 µg/mouse) intranasally (i.n.) on days 23, 25, 28 as described previously(19). This was followed by additional i.n. challenges with OVA twice a week for 8 weeks. Control mice were administered PBS instead of OVA for sensitization and challenges. All studies involving mice were performed following standards and procedures approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Bronchoalveolar lavage fluid (BALF) and lung tissue collection
Mice were sacrificed 24 hours after the last allergen challenge and BALF (1.1 ± 0.2 ml) was pooled after three washes with saline (0.5 ml each). Total and differential cell counts were determined and BALF supernatants were stored at −70°C for further evaluation. Right lungs were snap-frozen and left lungs were perfused with 4% paraformaldehyde to preserve pulmonary structure, fixed in 4% paraformadehyde and paraffin-embedded.
Measurement of lung eoatxin-1 by quantitative real-time PCR (qPCR) and ELISA
Total RNA from lung tissue of control and OVA-exposed WT and Gal-3 KO mice was extracted using TRIzol® Reagent (Invitrogen) according to the manufacturer’s recommendations. Equal amounts of the isolated RNA (1 µg) from each sample was used for reverse transcription, which was carried out using random primers and SuperScript® III Reverse Transcriptase (Invitrogen) as recommended by the manufacturer. Quantitative PCR was performed using the PerfeCTa™ SYBR® Green FastMix™ for iQ™ (Quanta Biosceinces, Gaithersburg, MD). cDNA obtained by reverse transcription of total RNA from each sample was amplified using SYBR® Green PCR Master Mix (Quanta Biosciences) and forward and reverse primers for Eotaxin-1, MCP-1, or β–actin (Integrated DNA Technologies, Coralville, IA). The primer sequences for eotaxin-1 and MCP-1 were designed using NCBI primer blast program, while the primer sequence for β–actin was derived from a previous study (20). The reaction was carried out in the iQ™5 multicolor real-time PCR detection System (BioRad, Hercules, CA) under the following conditions: initial denaturation at 95°C for 10 min followed by 45 cycles of 95°C for 10 sec and 60°C for 1 min. After PCR amplification, a melting curve was generated for every PCR product to check the specificity of the PCR reaction. All samples were run in duplicate. The relative amount of mRNA for each sample was calculated based on its threshold cycle, Ct, suggested by the software (IQ™5 Optical System software) in comparison to the Ct of the housekeeping gene β-actin. The results were expressed as fold change (2−ΔΔCT) in expression after subtraction of internal β-actin control. BALF eotaxin-1 levels were measured using an ELISA kit (R & D Systems, Minneapolis, MN) according to the manufacturer’s recommendations..
Immunohistology
Paraffin-embedded tissue sections (4 µm thick) were stained with Harris Modified Hematoxylin and Shandon Instant Eosin (Thermo Fisher Scientific Co., Pittsburgh, PA) to determine cellular infiltration. For all immunohistological analysis, tissue sections were subjected to antigen retrieval followed by quenching of endogenous peroxidase activity prior to staining with specific antibodies and sections were briefly counterstained (5 sec) with hematoxylin at the end of the procedure. VECTASTAIN ABC kits containing biotinylated secondary antibodies (anti-goat, anti-rat, anti-rabbit or anti-mouse) and avidin-biotin horseradish peroxidase complex (Vector Laboratories, Burlingame, CA), and the Peroxidase AEC (3-amino-9-ethylcarbazole) substrate kit (Vector Laboratories) were used for detection. Stained slides were analyzed using either a Leica DME light microscope or a Nikon Microphot EPI-FL microscope (magnification of ×100 or ×400) and images were captured with an Olympus DP71 camera. Histological analysis for infiltrated eosinophils was performed by immunohistochemical staining for eosinophil-specific major basic protein (MBP) with rat mAb against murine MBP as described earlier (21). MBP-positive cells in five randomly chosen non-overlapping microscopic fields were counted and expressed as the average number of cells/field. Gal-3 expression in lung tissue was detected with rabbit anti-human Gal-3 antibody (1:1000)(22). Found in inflammatory zone 1 (FIZZ1) protein expression was detected using goat polyclonal anti mouse FIZZ1 antibody (resistin-like α [E19], 2 µg/ml, Santa Cruz Biotechnology) and quantitated using ImageJ image analysis system (23) as described earlier (21). Results were expressed as FIZZ1 positive area (µm2) per 100 µm basement membrane length. Both large and small airways were evaluated. TGF-β1 expression was detected with polyclonal antibodies against TGF-β1 (4 µg/ml, Santa Cruz Biotechnology, Santa Cruz, CA). Expression of α-smooth muscle actin (SMA) was evaluated using mAb against murine SMA (4 µg/ml, Sigma Chemical Co.) and CD31 expression was detected with rabbit polyclonal antibodies against murine CD31 (2 µg/ml, Abcam, Cambridge, MA). Expression of eotaxin-1 was detected using rat mAb against murine eotaxin-1 (20 µg/ml, R & D Systems). Rat IgG (for MBP and eotaxin-1), goat IgG (for FIZZ1), rabbit IgG (for Gal-3, TGFβ-1 and CD31) or mouse IgG (for SMA) were used as control antibodies.
Measurement of Gal-3 and TGF-β1 by Western blotting
Lung tissue from control and OVA-exposed Gal-3 KO and WT mice was homogenized in RIPA buffer (Santa Cruz Biotechnology) and estimated for total protein (BCA Protein Assay Kit, Pierce Biotechnology, Rockford, IL). BALF (20 µl/lane/sample) or lung lysates (25 µg [for Gal-3] or 50 µg [for TGF-β1] protein/lane/sample) were electrophoresed on 10–20% Tricine gels (Invitrogen, Carlsbad, CA) under reduced conditions, transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA), blocked (5% nonfat milk in PBS with 0.1% Tween 20) and exposed to polyclonal antibodies against human Gal-3 (1:1000)(22) or TGF-β1 (1:200, Santa Cruz Biotechnology) followed by HRP-conjugated goat anti-rabbit IgG (1:8000, Cell Signaling Technology, Danvers, MA). Bound antibodies were detected using ECL Western Blotting Substrate (Pierce Biotechnology). HRP-conjugated anti-mouse β-actin (1:5000, Santa Cruz Biotechnology) was used to monitor levels of β-actin expression in lung tissues as an internal control. Bands were visualized on X-ray films, which were scanned and images were subsequently analyzed using ImageJ to quantitate the integrated density (pixels) of the bands. Density of Gal-3 and TGF-β1 bands in lung tissue lysates were normalized against β-actin.
Airway remodeling analysis
Airway mucus production, peribronchial fibrosis, peribronchial smooth muscle layer area and peribronchial angiogenesis were analyzed in both large and small airways. To detect airway mucus production, lung sections were stained with periodic acid-Schiff’s (PAS) reagent (Sigma Chemical Co.) as described previously (24). PAS-stained goblet cells were enumerated by light microscopy (magnification of ×400). For each lung section, the number of PAS-positive cells in horizontally sectioned airways was counted and expressed as a percentage of the total number of epithelial cells in that airway. Masson’s trichrome staining (Sigma Chemical Co.) was used for assessment of subepithelial fibrosis (24). The trichrome stained fibrotic area was outlined and quantitated using ImageJ and expressed as area of fibrosis (µm2) per µm basement membrane length. SMA immunostaining (described above) was used to evaluate airway smooth muscle hypertrophy/hyperplasia. Area of the peribronchial smooth muscle layer was quantitated as described in the case of trichrome staining. Results were expressed as SMA-positive area (µm2) per mm basement membrane length. Vascular density around the airways was assessed by immunostaining with rabbit polyclonal antibody against CD31 (described above). The number of CD31-positive blood vessels with a diameter less than 15 µm (small blood vessels) present within the 150 µm area surrounding the epithelial basement membrane of each airway were counted and expressed as the number of small blood vessels per airway.
Measurement of lung cytokines by cytometric bead array (CBA)
Lung tissue of control and OVA-exposed WT and Gal-3 KO mice was homogenized in lysis buffer (PBS containing 1% Triton X-100, 1 mM PMSF and protease inhibitor cocktail). After measuring protein concentration (BCA Protein Assay Kit) of the lysates, Th1 (IL-2 and IFN-γ) and Th2 (IL-4, IL-5) cytokine levels as well as that of IL-13 in the lung lysate supernatants was analyzed using mouse Th1/Th2 cytokine and IL-13 CBA kits (Cat. No. 551287 and 558349, BD PharMingen). Level of each cytokine was expressed as pg cytokine/mg protein.
Expression and purification of recombinant mouse (rm) VCAM-1 and Gal-3
Mouse Gal-3 and VCAM-1 cDNA clones were obtained from Open Biosystems (Huntsville, AL). Full length Gal-3 and VCAM-1 were generated by PCR amplification (DNA Engine Thermal Cycler®, BioRad) using the following primers: AAATGGATCCATGGCAGACAGCTTTTCGCT (upstream) and AAATGAATTCTTAGATCATGGCGTGGTTAG (downstream) for Gal-3, and AAGCATTGAATTCAATGCCTGTGAAGATGGTCGCGG (upstream) and AAAGCATTCTCGAGTGGATTTCTGTGCCTCCACCAG (downstream) for VCAM-1. Gal-3 and VCAM-1 clones were digested with BamH1/EcoRI and EcoR1/Xho1 (New England BioLabs, Ipswich, MA), respectively, subcloned into pGEX6p2 vector systems (GE Healthcare Life Sciences, Piscataway, NJ), and expressed in BL21(DE3)pLysS E.Coli (Clontech Laboratories, Mountainview, CA). GST fusion proteins were purified as recommended by the manufacturer. The GST recombinant proteins were dialyzed against PBS and stored at −80°C. The purity of the recombinant proteins was assessed by Western blot analysis prior to use.
Confocal microscopy
Cells recovered from BALF of allergen-challenged WT mice by cytocentrifugation were fixed for 20 min at room temperature using 4% paraformaldehyde, washed with PBS and then permeabilized with 0.2% Triton X-100 in PBS for 10 min at RT. To prevent nonspecific binding, cells were first treated with TruStain fcX™ Fc blocking antibody (anti-mouse CD16/32 at 5 µg/ml, BioLegend, San Diego, CA) for 30 minutes at 4°C and then incubated overnight with rat mAb against murine MBP (2.5 µg/ml) and rabbit anti-human Gal-3 antibodies (10 µg/ml) (22) at 4°C. Bound antibodies were detected using FITC-conjugated goat anti-rat IgG (6 µg/ml, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and Rhodamine Red-X-conjugated goat anti-rabbit IgG (3 µg/ml, Jackson ImmunoResearch Laboratories, Inc.), respectively, for 1 h at RT. Mounting medium with DAPI (Vector Laboratories) was used to visualize the cells. Slides were observed at ambient temperature using a FLUOVIEW FV1000/BX61 - Confocal Laser Scanning Biological Microscope equipped with an UPlanSApo lens (20×/0.85 [oil]) and a PlanApo N lens (60 ×/1.42 [oil]). FV10-ASW 2.0 software was used for image acquisition (Olympus, Melville, NY).
Flow cytometry
Cell surface expression of Gal-3 was assessed using rabbit anti-human Gal-3 antibodies (22). Non-permeabilized total bone marrow leukocytes or BALF cells (1 × 106) reconstituted in 1% BSA in PBS were first incubated with 1 µg of TruStain fcX™ Fc blocking antibody (BioLegend) for 20 min prior to treatment with total IgG purified from rabbit anti-Gal-3 serum at 10 µg/ml. Rabbit IgG (Calbiochem, Gibbstown, NJ) was used as a control. After washing, cells were incubated with PE-conjugated goat anti-rabbit IgG (10 µg/ml, Jackson ImmunoResearch Laboratories, Inc.), washed and immediately analyzed on a FACScan flow cytometer (Beckton Dickinson, San Jose, CA) equipped with FlowJo flow cytometry analysis software for data acquisition.
Flow Chamber Studies
Rolling of bone marrow cells collected from the femurs of WT and Gal-3 KO mice on endothelial adhesion molecules under conditions of flow was evaluated in an in vitro parallel plate flow chamber as described in our previous studies (11, 21). Interaction of bone marrow cells (2 × 105) with rmVCAM-1-, rmGal-3-, ICAM-1 (10 µg/ml in PBS, 200 µl/cover slip) or PBS (control)-coated glass cover slips at a flow rate of 1 ml/min (wall shear stress, 1.0–2.0 dynes/cm2) was evaluated using a Leitz Wetzlar inverted microscope and recorded to manually determine the number of interacting cells. Results were expressed as the number of rolling cells/min. In some cases, cells were pre-incubated with lactose (3 mM, Sigma Chemical Co.), an inhibitor of the biological activity of Gal-3 (25), or maltose (ICN Biochemicals, Aurora, OH) as the negative control for 30 min prior to infusion into the flow chamber.
Statistical Analysis
Each complete experiment was repeated 2–3 times. Results are expressed as the mean ± SE. Statistical significance was determined by on-tailed unpaired Student’s t-test using Microsoft Excel. A p value <0.05 was considered as significant.
Results
Chronic allergic airway inflammation is associated with increased levels of Gal-3 in the lungs
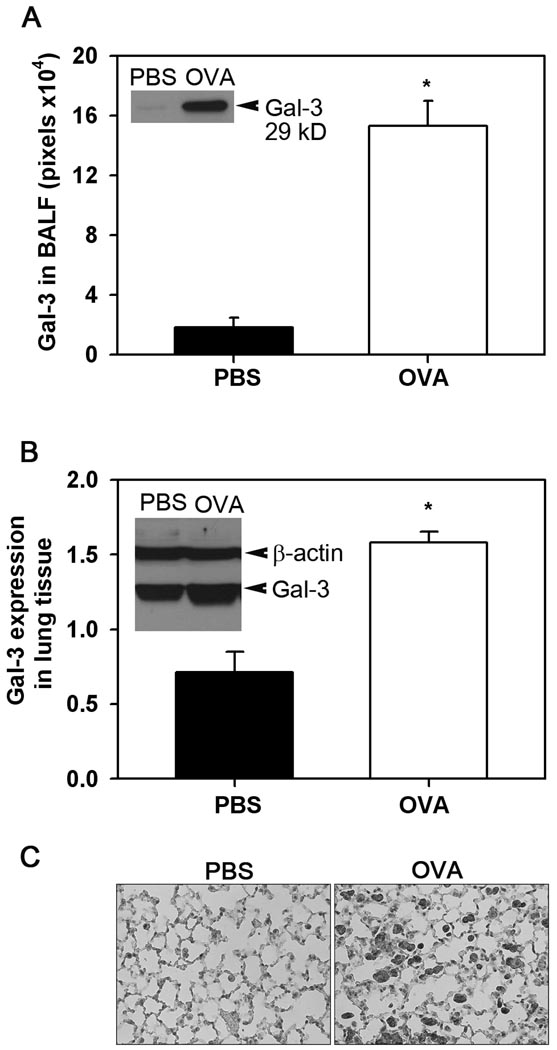
To determine whether expression of Gal-3 in the lungs is altered during allergic airway inflammation, the BALF and lung tissue of WT mice exposed to chronic allergen challenge were analyzed for Gal-3 expression. Western blot analysis demonstrated significantly elevated levels of soluble Gal-3 (p <0.05) in the BALF of OVA-challenged WT mice, while Gal-3 levels in PBS-exposed WT control mice were almost undetectable (Fig. 1, A). In addition, Gal-3 levels in lung tissue lysates of OVA-challenged WT mice were also significantly higher (p <0.05) than in lysates of control mice (Fig. 1, B). As expected, no Gal-3 was detected in the BALF and lung lysates of Gal-3 KO mice (data not shown). Further, immunohistology demonstrated that the increased Gal-3 expression observed in the lungs of OVA-challenged mice was largely associated with the alveolar macrophages (Figure 1, C, right panel).
Fig. 1. Gal-3 expression is up-regulated during chronic airway allergic inflammation.
Gal-3 levels in BALF (A) and total lung tissue lysates (B) from control (PBS-exposed) and OVA-challenged WT mice were determined by Western blot analysis using rabbit antibodies against human Gal-3. Blots with lung tissue lysates were probed with HRP-conjugated anti-mouse β-actin to monitor levels of β-actin expression as an internal control. Bands were visualized on X-ray films, scanned and analyzed using ImageJ to quantitate the density (pixels) of the bands. Results (mean ± SE) were normalized for β-actin expression in the case of lung lysates (n = 4 mice/group). Inset in (A) and (B) show Gal-3 expression in BALF and lung lysate from representative control and OVA-challenged mice, respectively. *p < 0.05 compared with WT control mice. Immunohistochemical staining was performed on lung sections from control and OVA-challenged mice using rabbit antibodies against human Gal-3 as described in Materials and Methods. Representative images from each group at a magnification of ×200 are shown (C).
Gal-3 deficient mice exhibit reduced cellular infiltration of the airways in response to chronic allergen-challenge
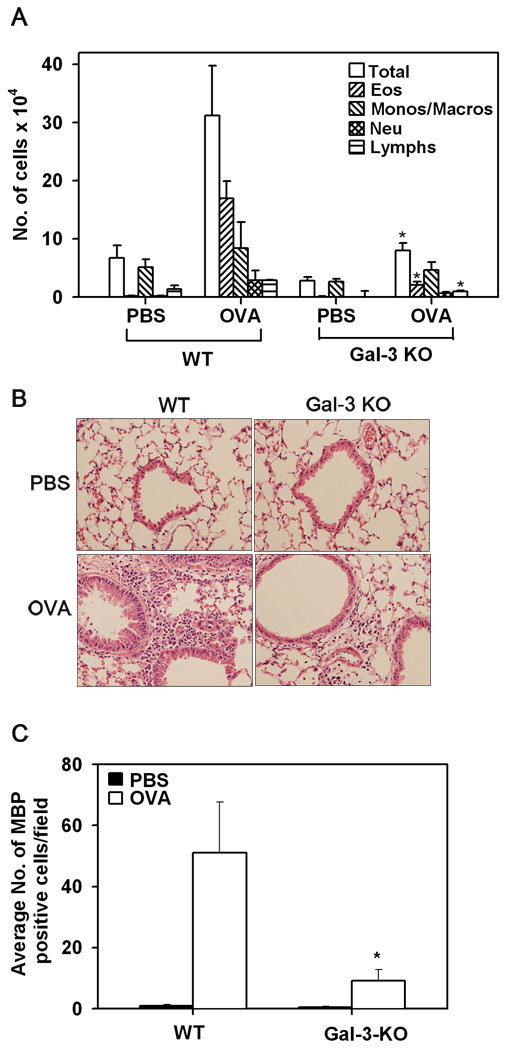
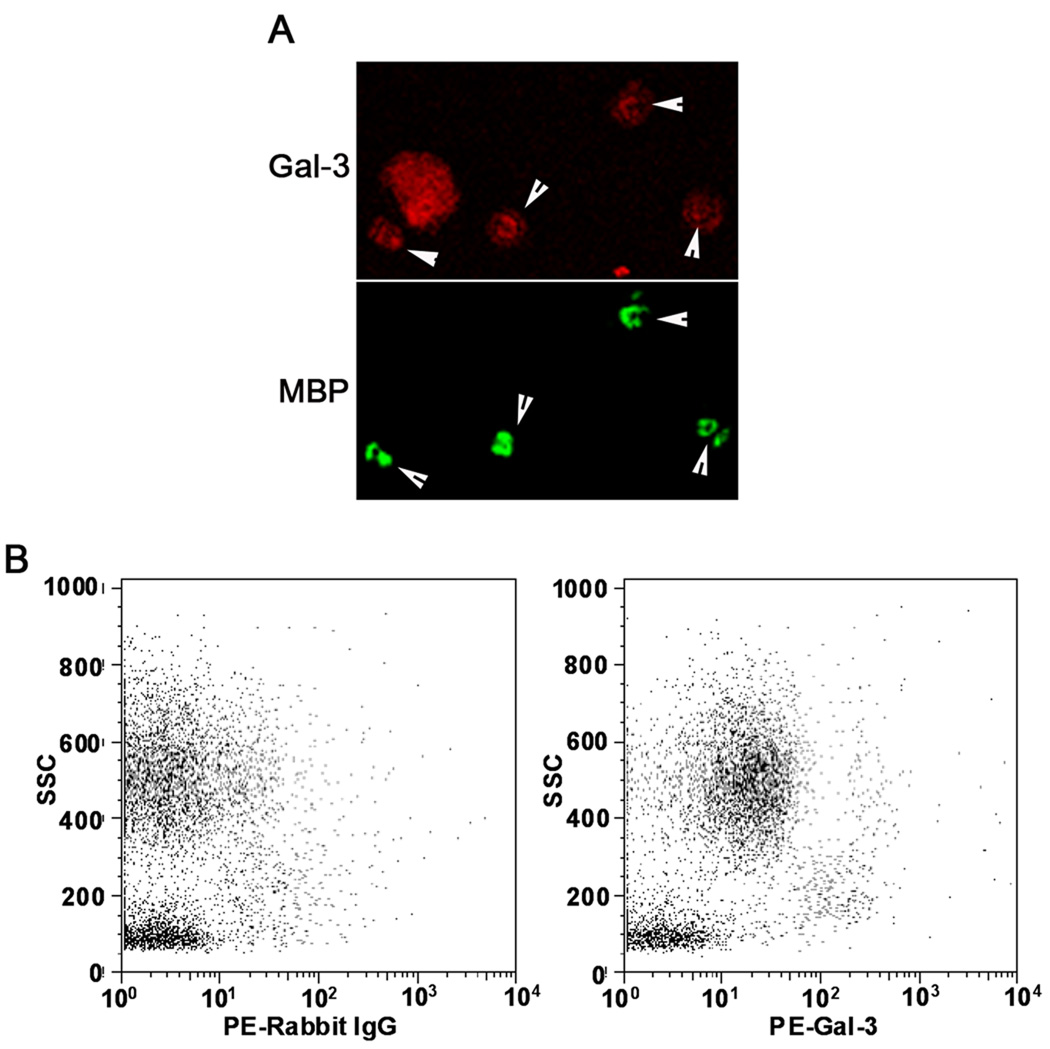
Infiltration by inflammatory cells, especially eosinophils, is a characteristic feature of allergic airway inflammation. Accumulation of inflammatory cells in the BALF as well as lung tissue of chronic allergen-challenged WT and Gal-3 KO mice was evaluated. As expected, OVA-challenged WT mice demonstrated a significant increase (p <0.05) in the recruitment of inflammatory cells to the airways compared to control WT mice, as observed by the total number of cells recovered from the BALF (Fig. 2, A) and the cellular infiltration of lung tissue visualized by H&E staining (Fig. 2, B). While OVA-challenged Gal-3 KO mice demonstrated an increase in the number of inflammatory cells recovered from the BALF compared to control Gal-3 KO mice, it was significantly lower (p <0.05) than that observed in allergen-challenged WT mice. Further, analysis of differential cells counts demonstrated a marked reduction (p < 0.05) in eosinophil and lymphocyte recruitment, while a moderate, but statistically insignificant, reduction was observed in monocyte/macrophage and neutrophil infiltration of the airways in allergen-challenged Gal-3 KO mice compared to the WT counterparts (Fig. 2, A). Lung tissue eosinophils were quantitated by immunohistology using mAb specific for murine MBP. While only a negligible number of MBP-positive eosinophils were present in PBS-exposed lung sections, an increased number of MBP-positive cells were present in lung sections from OVA-exposed WT mice as expected (Fig. 2, C). In contrast, the number of MBP-positive eosinophils in the lungs of allergen-challenged Gal-3 KO mice was markedly reduced (p <0.05) compared to that of OVA-challenged WT mice (Fig. 2, C). These findings suggest that Gal-3 plays an important role in the recruitment of eosinophils to the airways during chronic allergic airway inflammation. To confirm that eosinophils in the airways of OVA-challenged mice express Gal-3, confocal microscopy studies were performed with BALF cells that were collected from WT OVA-challenged mice and dual stained with rat mAb specific for murine MBP and rabbit anti-Gal-3 (Fig. 3, A). These studies demonstrated that several of the Gal-3 positive cells in the BALF were MBP-positive confirming that eosinophils in the airways of WT OVA-challenged mice express Gal-3. The Gal-3-positive MBP-negative cells are most likely macrophages and neutrophils that are also known to express Gal-3 (26–28). Additionally, flow cytometry studies demonstrated that the granulocyte population in the BALF of WT OVA-challenged mice, which consists largely of eosinophils (>90% based on differential cell counts), express Gal-3 on the cell surface (Fig. 3, B).
Fig. 2. Cellular infiltration of the airways in response to chronic allergen challenge is inhibited in Gal-3 KO mice.
BALF collected from control and OVA-challenged Gal-3 KO and WT mice 24 hours after the last challenge was evaluated for total as well as differential cell counts by microscopic evaluation of cytocentrifuged slides (n = 8 for control groups and 9 for OVA-challenged groups) and expressed as mean ± SE of the number of cells × 104 (A). Cellular infiltration of lung tissue was evaluated by H&E staining of paraformaldehyde-fixed lung tissue sections from control and allergen-challenged mice. Representative images from each group at a magnification of ×200 are shown (B). Lung tissue eosinophils were evaluated by immunohistochemical staining of lung sections with eosinophil-specific MBP using rat mAb against murine MBP. MBP-positive cells in five randomly selected non-overlapping microscopic fields were counted (magnification of ×400) and results were expressed as the average number (mean ± SE) of cells/field (n = 9 mice/group) (C). *p <0.05 when compared with WT OVA mice.
Fig. 3. Eosinophils recruited to the airways in response to allergen challenge express Gal-3.
BALF cells from OVA-challenged WT mice were dual stained with rat mAb against murine MBP and rabbit anti-human Gal-3 antibodies followed by FITC-conjugated goat anti-rat IgG and Rhodamine Red-X-conjugated goat anti-rabbit IgG to detect the bound primary antibodies. Cells were evaluated by confocal microscopy (magnification of ×600). Arrow heads indicate cells in a representative field that were positive for MBP as well as Gal-3 (A). BALF cells (non-permeabilized) from WT OVA-challenged mice were analyzed for surface Gal-3 expression by flow cytometry after treatment with Fc blocking antibody using total IgG purified from rabbit anti-Gal-3 serum as the primary antibody. Rabbit IgG was used as a control. PE-conjugated goat anti-rabbit IgG was used as the secondary antibody. Side-scatter profiles for control IgG (left) and anti-Gal-3 antibody (right) are shown (B).
OVA-challenged Gal-3 deficient mice display lower IL-5 and IL-13 levels in the lungs
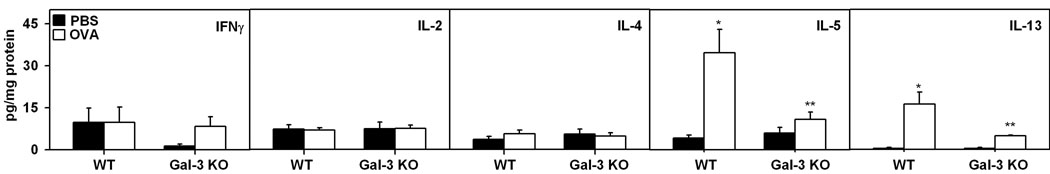
Since allergic airway inflammation is largely a Th2-driven phenomenon, and cytokines such as IL-5 and IL-13 are known to play a role in airway remodeling (4), Th2 (IL-4, IL-5 and IL-13) as well as Th1 (IL-2 and IFN-γ) cytokine levels in the lung tissue of control and allergen-challenged WT and Gal-3 KO mice were determined by CBA (Fig. 4). There was a significant increase (p <0.05) in IL-5 and IL-13 levels in the lung tissue of OVA-challenged WT mice compared to PBS-exposed controls. Importantly, expression of these two cytokines in allergen-challenged Gal-3 KO mice was substantially lower than in allergen-challenged WT mice and comparable to the levels observed in PBS-exposed Gal-3 KO mice in the case of IL-5. No significant differences were detected in IFNγ, IL-2 and IL-4 levels between control and OVA-challenged WT or Gal-3 KO mice.
Fig. 4. Th2 cytokine levels are down-regulated in the lungs of Gal-3 KO mice in response to chronic allergen challenge.
Key Th1 (IL-2 and IFN-γ) and Th2 (IL-4, IL-5 and IL-13) cytokine levels in lung lysates of control and chronic allergen-challenged WT and Gal-3 KO mice (n = 5–8/group) was determined by CBA. Results were expressed as mean ± SE of pg/mg protein for each cytokine. *p < 0.05 compared with WT PBS mice. **p <0.05 compared to WT OVA.
Allergen-challenged Gal-3 deficient mice exhibit decreased eotaxin-1 levels in the lungs
Eotaxin-1 plays a fundamental role in the development of allergic responses by regulating the recruitment and activation of eosinophils. To understand the basis for the reduced eosinophil infiltration in OVA-challenged Gal-3 KO mice, eotaxin-1 levels in the BALF and lung tissue of control and allergen-challenged WT and Gal-3 KO mice was determined. Chronic allergen exposure resulted in an increase in eotaxin-1 levels in the BALF of WT mice, which was significantly impaired in Gal-3 KO mice (Fig. 5, A). In addition, immunostaining of lung sections demonstrated that while eotaxin-1 expression in control PBS-exposed WT and Gal-3 KO mice is largely observed in the airway epithelium and type 2 alveolar epithelial cells, expression of this chemokine in the lungs of OVA-challenged WT mice is seen in alveolar macrophages and other infiltrating cells in addition to the airway epithelium and type 2 cells. More importantly, expression of eotaxin-1 by infiltrating cells (but not airway epithelial or type 2 cells) in lung sections of OVA-challenged Gal-3 KO mice was markedly lower (Fig. 5, B). Further, eotaxin-1 mRNA levels in lung tissue of OVA-challenged Gal-3 KO mice determined by qPCR was significantly lower than in allergen-challenged WT mice and similar to that observed in control mice (Fig. 5, C).
Fig. 5. Allergen-challenged Gal-3 KO mice exhibit decreased eotaxin-1 levels.
Eotaxin-1 levels in the BALF of control and OVA-challenged WT and Gal-3 KO mice (n= 7–9/group) were measured by ELISA. Concentration of eotaxin-1 in the BALF was determined against a generated standard curve (range 7.8 to 250 pg/ml) and expressed as pg/ml of BALF (mean ± SE) (A). Eotaxin-1 expression in lung tissue of control and chronic OVA-challenged WT and Gal-3 KO mice was evaluated by immunostaining using rat mAb against murine eotaxin-1. Representative images from each group at a magnification of ×100 are shown (B). Expression of eotaxin-1 mRNA level in lung tissue of control and OVA-challenged WT and Gal-3 KO mice (n = 7/group) was detected by qPCR. Results were expressed as mean ± SE of fold change (2−ΔΔCT) in eotaxin-1 expression after subtraction of internal β-actin control (C). *p < 0.05 compared with WT OVA mice.
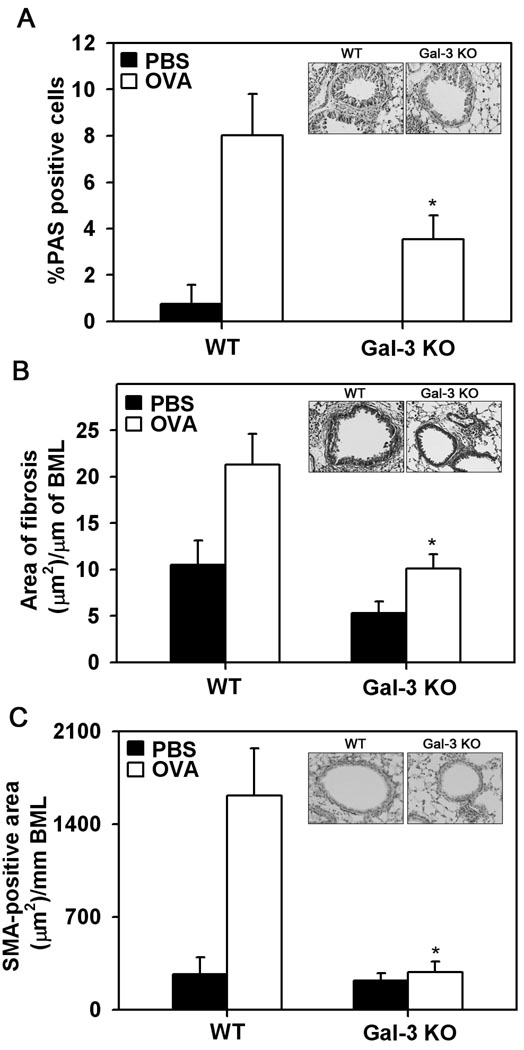
Chronic allergen-challenged Gal-3 deficient mice exhibit reduced airway remodeling
Mucus hypersecretion, subepithelial fibrosis and increased smooth muscle mass around airways are characteristic features of asthmatic airways. Prolonged allergen exposure associated structural changes in the lung were evaluated in OVA-challenged WT and Gal-3 KO mice. WT mice exhibited an increase in the number of PAS-positive mucus-producing cells in the bronchial epithelium compared to PBS-exposed WT mice (Fig. 6, A). In contrast, allergen-challenged Gal-3 KO mice demonstrated only a small number of PAS-positive cells which was significantly lower (p <0.05) than that observed in allergen-challenged WT mice (Fig. 6, A and inset). Peribronchial fibrosis was determined by trichrome staining. Both groups of OVA-challenged mice demonstrated increased peribronchial trichrome staining compared to their respective PBS-challenged controls. However, the area of peribronchial trichrome staining in allergen-challenged Gal-3 KO mice was significantly lower (p <0.05) compared to that of allergen-challenged WT mice (Fig. 6, B and inset). Likewise, while chronic allergen challenge caused a significant increase in the thickness of the peribronchial smooth muscle layer in the lungs of WT mice, the smooth muscle layer in lungs of Gal-3 KO mice was significantly thinner (p <0.05) and similar to that observed in PBS-challenged mice (Fig. 6, C and inset). Overall, these studies demonstrate that Gal-3 deficiency results in decreased remodeling in chronic allergen-exposed lungs.
Fig. 6. Allergen-induced airway remodeling is attenuated in Gal-3 KO mice.
Mucus secretion in the airways of control and chronic allergen-challenged WT and Gal-3 KO mice (n=6 mice/group) was quantitated by PAS staining of lung sections. The number of PAS-positive goblet cells was expressed as a percentage of the total number of epithelial cells in each airway (mean ± SE) (A). Peribroncial fibrosis was evaluated by staining lung sections with Masson’s trichrome stain, quantitated by image analysis using Image J and expressed as area of fibrosis (µm2)/µm basement membrane length (BML) (B). Thickness of the smooth muscle layer in lung sections was quantitated by immunohistochemical staining for SMA and expressed as SMA-positive area (µm2)/mm BML (C). The inset in each case shows representative images from OVA-challenged WT (left) and Gal-KO (right) mice at a magnification of ×200. *p < 0.05 when compared with WT OVA mice.
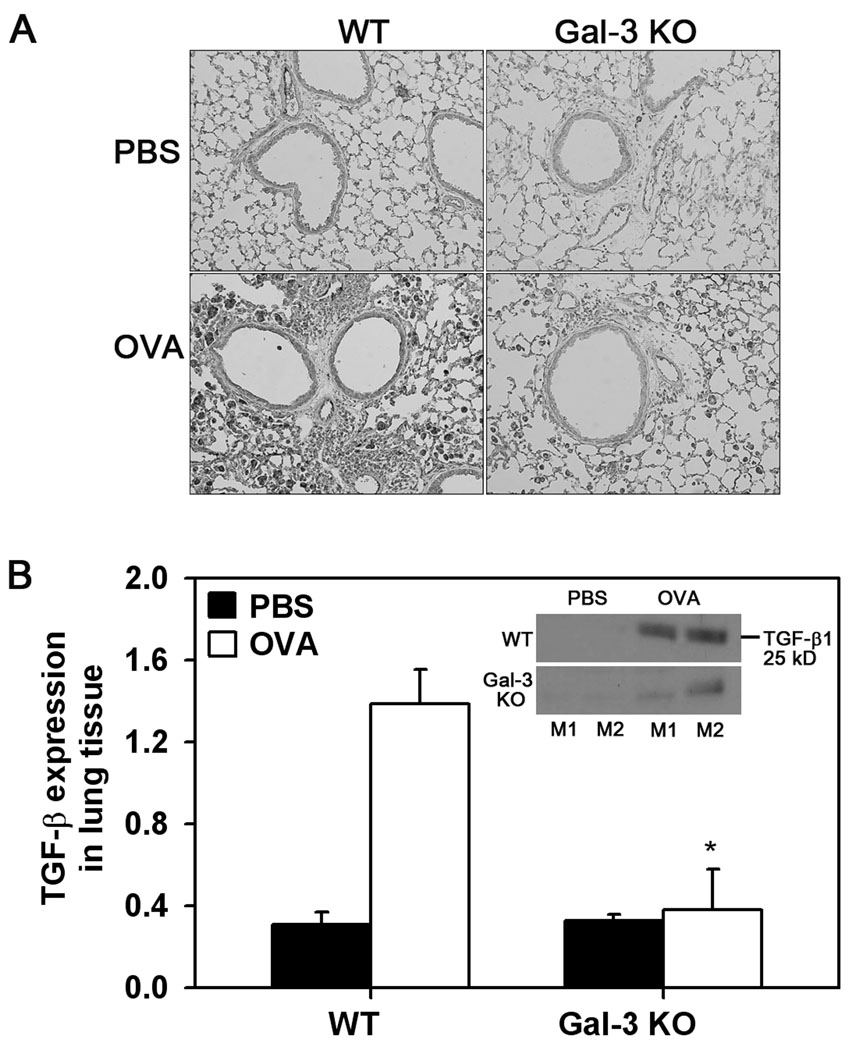
Lung TGF-β levels are decreased in allergen-challenged Gal-3 deficient mice
Since TGF-β1 is an important mediator of peribronchial fibrosis and airway smooth muscle proliferation (4, 29), the expression of TGF-β1 in the lungs of WT and Gal-3 KO mice was investigated by immunostaining. WT control mice demonstrated minimal TGF-β1 expression largely confined to EC and airway epithelial cells. Exposure to OVA challenge resulted in an apparent increase in TGF-β1 expression by alveolar macrophages and other inflammatory cells around the blood vessels and airways in WT mice. Lung sections of allergen-challenged Gal-3 KO mice, on the other hand, demonstrated lower TGF-β1 expression (Fig. 7, A). This observation was further confirmed by Western blot analysis of total lung lysates with rabbit antibodies against TGF-β1 followed by densitometry of the 25 kD band which corresponds to the mature biologically active homodimeric form of TGF-β1. As expected, expression of mature TGF-β1 was up-regulated in WT OVA-challenged mice compared to WT control mice. Importantly, OVA-challenged Gal-3 KO mice displayed significantly lower (p <0.05) mature TGF- β1 expression compared to WT OVA-challenged mice (Fig. 7, B).
Fig. 7. TGF-β1 expression is decreased in allergen-challenged Gal-3 KO mice.
TGF-β1 expression in lung tissue of control and OVA-challenged WT mice and Gal-3 KO mice (n = 6/group) was detected by immunohistochemistry with polyclonal antibodies against TGF-β1. Representative images from each group at a magnification of ×100 are shown (A). Level of expression of mature TGF-β1 (25 kD) was quantitated from densitometric analysis of Western blots of lung tissue lysates from control and OVA-challenged WT and Gal-3 KO mice (n = 4/group) after normalizing for β-actin expression and expressed as mean ± SE (B). *p < 0.05 when compared with WT OVA mice.
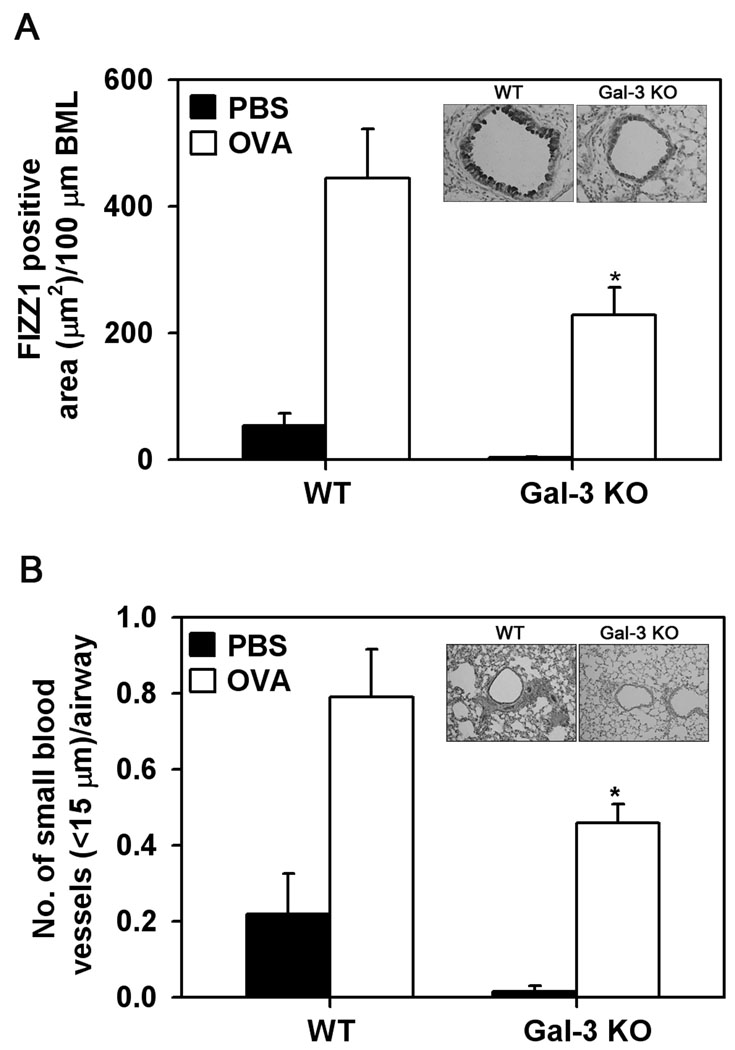
Allergen-challenged Gal-3 deficient mice exhibit decreased FIZZ1 expression
FIZZ1 is a protein known to be expressed in the airways under allergic conditions and have potent inflammatory and remodeling activity including pulmonary fibrosis (30, 31) as well as angiogenesis (32). Expression of FIZZ1 was evaluated in chronic allergen-exposed Gal-3 KO and WT mice (Fig. 8, A). PBS-exposed WT and Gal-3 KO mice displayed low levels of FIZZ1 expression in the airway epithelium. WT mice repetitively challenged with OVA demonstrated a significant increase in FIZZ1 expression compared to control mice (p<0.05). While OVA-challenged Gal-3 KO mice also demonstrated an increase in FIZZ1 expression compared to the respective control group, it was significantly lower (p <0.05) compared to FIZZ1 expression observed in OVA-challenged WT mice (Fig. 8, A and inset). In addition, immunohistology studies with CD31 specific antibodies demonstrated that WT mice exposed to chronic allergen challenge exhibited increased peribronchial angiogenesis with a significant increase in the number of CD31-positive small blood vessels (<15 µm) compared to PBS-challenged control mice (p <0.05). In contrast, allergen-challenged Gal-3 deficient mice exhibited significantly fewer (p <0.05) CD31-positive small blood vessels in response to allergen challenge (Fig. 8, B and inset). Overall, the level of FIZZ1 expression in allergen-challenged WT and Gal-3 KO mice correlated with the airway remodeling and angiogenesis observed in these mice.
Fig. 8. Allergen-induced angiogenesis is inhibited in Gal-3 KO mice.
FIZZ1 expression in lung tissue of control and OVA-challenged WT and Gal-3 KO mice (n = 6/group) was evaluated by immunohistochemistry using goat polyclonal antibodies against murine FIZZ1. Airway FIZZ1 expression was quantitated by image analysis using Image J and expressed as FIZZ1-positive area (µm2)/100 µm BML (A) Peribronchial angiogenesis was evaluated by immunohistochemical staining of lung sections with antibodies against CD31. The number of blood vessels with a diameter less than 15 µm in the area surrounding the airways (150 µm) was quantitated and expressed as the number of blood vessel per airway (B). Data represent mean ± SE. The inset shows representative images from OVA-challenged WT (left) and Gal-KO (right) mice at a magnification of ×200. *p < 0.05 when compared with WT OVA mice.
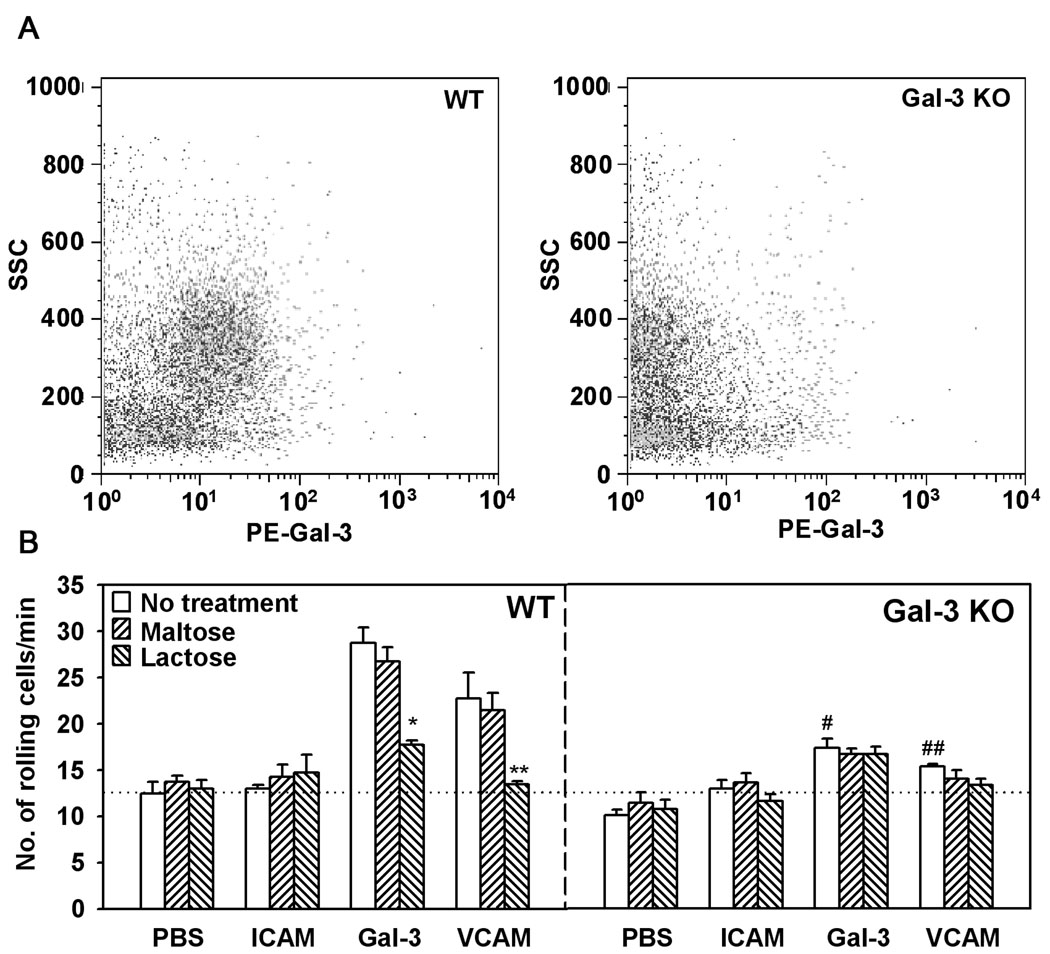
Bone marrow-derived leukocytes from Gal-3 KO mice exhibit decreased rolling on vascular adhesion molecules VCAM-1 and Gal-3
Our previous studies have demonstrated that both human eosinophil- and endothelial-expressed Gal-3 support eosinophil rolling under conditions of flow (11). Leukocyte-endothelial interactions (rolling and adhesion) constitute an important step in the cascade of events leading to the eventual recruitment of inflammatory cells to sites of inflammation. To evaluate the involvement of murine leukocyte-expressed Gal-3 in the first and rate limiting step of this cascade, i.e. ability to roll on the endothelium, trafficking of bone marrow leukocytes from WT and Gal-3 KO mice on endothelial adhesion molecules such as VCAM-1 and Gal-3 was investigated under conditions of flow. First, bone marrow leukocytes from WT and Gal-3 KO mice were evaluated for the expression of Gal-3 by flow cytometry. Based on side scatter analysis, the granulocyte population in the bone marrow of WT, but not Gal-3 KO mice, was found to express Gal-3 on the cell surface (Fig. 9, A). Next, flow chamber studies with bone marrow leukocytes demonstrated that cells from WT mice exhibited substantial rolling on cover-slips coated with rmGal-3 and rmVACM-1, but not ICAM-1 or PBS alone. In contrast, rolling of bone marrow leukocytes from Gal-3 KO mice on these endothelial adhesion molecules was significantly lower (p <0.05) (Fig. 9, B). Further, pretreatment of bone marrow leukocytes from WT mice with lactose prior to infusion into the flow chamber inhibited rolling of these cells on both Gal-3 and VCAM-1 to levels similar to that observed with cells from Gal-3 KO mice. Maltose, which was used as a negative control, had no effect. Treatment of bone marrow leukocytes from Gal-3 KO mice with lactose had no further effect on the already reduced rolling of these cells on Gal-3 and VCAM-1. These data suggest that Gal-3 is required for murine leukocyte rolling under conditions of flow and may facilitate their trafficking to sites of inflammation, such as the airways, during chronic allergen exposure.
Fig. 9. Bone marrow leukocytes from Gal-3 KO mice exhibit decreased rolling on endothelial adhesion molecules.
Bone marrow leukocytes (non-permeabilized) from WT and Gal-3 KO mice were analyzed for surface Gal-3 expression by flow cytometry using total IgG purified from rabbit anti-Gal-3 serum (10 µg/ml) as the primary antibody after treatment with Fc blocking antibody. PE-conjugated goat anti-rabbit IgG (10 µg/ml) was used as the secondary antibody. Side-scatter profiles for bone marrow leukocytes from WT (left) and Gal-3 KO (right) mice are shown (A). Single cell suspensions of bone marrow leukocytes from WT and Gal-3 KO mice were infused into a flow chamber containing cover-slips coated with rmGal-3 or rmVCAM-1 at a flow rate of 1 ml/minute for 5 minutes. ICAM-1 was used as a negative control. In some experiments, bone marrow leukocytes were pre-incubated with lactose or maltose (as a control) at a concentration of 3 mM before infusion. Results were expressed as the number of rolling cells per minute (mean ± SE) (B). *p <0.01 versus rolling of untreated WT BM cells on rmGal-3; **p <0.01 versus rolling of untreated WT BM cells on rmVCAM-1; #p <0.01 versus rolling of untreated WT BM cells on rmGal-3; ## p <0.05 versus rolling of untreated WT BM cells on rmVCAM-1.
Discussion
A role for Gal-3 in the pathogenesis of acute allergic airway inflammation has been demonstrated previously (9). In these studies, acute allergen-challenged Gal-3 deficient mice developed less airway hyperresponsiveness and significantly fewer eosinophils as well as decreased Th2 cytokines in the airways compared to WT mice. These studies, together with our previous studies demonstrating a role for eosinophil- and endothelial-expressed Gal-3 in eosinophil trafficking under conditions of flow(11), show that Gal-3 plays a role in eosinophil recruitment and in the development of acute allergic airway inflammation. Herein, we have extended these studies by evaluating the involvement of Gal-3 in airway remodeling associated with chronic and repetitive allergen exposure using a murine model of OVA-induced allergic airway inflammation with mice deficient in Gal-3. Chronic exposure of WT mice to OVA for 12 weeks resulted in sustained expression of elevated levels of Gal-3 in the BALF and lung tissue associated with a classical asthmatic response characterized by eosinophil infiltration into the airways, elevated levels of Th2 cytokines (IL-5 and IL-13), eotaxin-1 and other inflammatory mediators (TGF-β1 and FIZZ1) as well as structural changes such as peribronchial fibrosis, increased smooth muscle mass, peribronchial angiogenesis and increased mucus secretion in the airways. All of these features were attenuated in allergen-exposed Gal-3 KO mice with an overall suppression of the asthma phenotype indicating an important role for Gal-3 in the chronic phase of asthma.
In addition to the elevated levels of soluble Gal-3 in the BALF of chronic OVA-challenged WT mice (Fig. 1, A), a marked increase in Gal-3 expression was observed in lung tissue largely by alveolar macrophages (Fig. 1, B and C). Gal-3 is known to play a significant role in monocyte-macrophage differentiation and also promote chemotaxis of monocyte/macrophages (25, 33). While there was no significant increase in the number of macrophages recovered from the BALF after chronic allergen exposure compared to control mice in our study (Fig. 2, A), it is likely that increased Gal-3 expression in the lung tissue and BALF may be a consequence of macrophage activation. Recent studies report that Gal-3 potentiates alternative macrophage activation which is associated with increased Gal-3 expression and secretion and that disruption of the Gal-3 gene affects this pathway (34). The alternative pathway of macrophage activation is stimulated by Th2 cytokines, such as IL-4 or IL-13 (35). IL-13 levels are elevated in WT mice in response to chronic allergen challenge (Fig. 4) and may stimulate macrophage activation and release of Gal-3.
The reduction in total cellular recruitment observed in the airways (BALF) of chronic allergen-challenged Gal-3 KO mice was largely attributable to the significant decrease in eosinophil and lymphocyte recruitment (Fig. 2, A), although a moderate, but statistically insignificant, reduction in monocyte/macrophages and neutrophils was also observed. Further, there was a drastic reduction in lung tissue eosinophils (Fig. 2, C). Reduced infiltration of airways by eosinophils was associated with a reduction in IL-5 and IL-13 levels in lung lysates of chronic allergen-challenged Gal-3 KO mice (Fig. 4). IL-5, a cytokine released predominantly by Th2 cells, is known to support trafficking of eosinophils from the bone marrow to the lung by regulating eosinophil proliferation, differentiation and release from the bone marrow (36). Expression of IL-13, another Th2 cell-derived cytokine, is also linked to an eosinophilic inflammatory response (37). Reduced IL-5 and IL-13 levels in allergen-challenged Gal-3 KO mice may contribute to the reduced eosinophilia observed in these mice. In addition, IL-13 can direct leukocyte trafficking by regulating the expression of VCAM-1 on EC (38). Our previous studies have demonstrated that Gal-3, by binding to α4β1 on the cell surface of human eosinophils, can function as an adhesion molecule to support rolling and adhesion on vascular EC by interacting with VCAM-1 and homotypically with Gal-3 (11). In the present study, eosinophils in the BALF of WT OVA-challenged mice were found to express Gal-3 (Fig. 3, A and B) which may play a role in mediating recruitment of these cells to the airways. This is further substantiated by the observation that bone marrow leukocytes from Gal-3 KO mice demonstrated decreased rolling on endothelial adhesion molecules such as VCAM-1 and Gal-3 compared to WT leukocytes that expressed Gal-3, indicating the importance of cell surface-expressed Gal-3 in mediating leukocyte trafficking (Fig. 9), and accounting for decreased recruitment of inflammatory leukocytes, especially eosinophils, to the airway (Fig. 2). However, in addition to this important role of Gal-3 in mediating eosinophil trafficking, α4-VCAM-1 and β2-ICAM-1 interactions as well as other adhesion molecules such as P-selectin (39) may independently contribute to eosinophil recruitment accounting for the residual recruitment of these cells to the airways of mice deficient in Gal-3..
The involvement of Gal-3 in the development of chronic allergen-induced Th2 inflammatory responses was investigated. Recent studies in an atopic dermatitis model suggest that Gal-3 exerts a regulatory effect on Th development at the dendritic cell and T cell levels causing the development of a Th1 polarized response in its absence (10). In addition, previous studies in an acute model of allergic airway inflammation with Gal-3 KO mice demonstrated a lower Th2 (IL-4), but a higher Th1 (IFN-γ) response. However, in the present study, while Th2 (IL-5 and IL-13) levels in allergen-challenged Gal-3 KO mice were substantially reduced compared to WT mice, Th1 levels remained the same in the two groups (Fig. 4). Gal-3 may promote the development of a Th2 phenotype also through its ability to mediate eosinophil trafficking and thus, their recruitment to sites of inflammation. Both murine and human eosinophils are constitutively able to express IL-13, which is rapidly released in response to inflammatory stimuli (40, 41). Apart from mediating leukocyte trafficking, a role for Gal-3 in promoting migration has been demonstrated in a study using the mouse air pouch model, where injection of Gal-3 into air pouches was found to induce eosinophil recruitment, probably indirectly by inducing eosinophil chemoattractants (25). In addition, in the present study, there was a significant reduction in lymphocyte recruitment to the airways after OVA challenge in Gal-3 KO mice (Fig. 2, A) suggesting that Gal-3 promotes recruitment of these cells to the airways which in turn may contribute to elevated IL-5 and IL-13 levels. Gal-3 is thought to regulate T cell functions by inhibiting apoptosis, promoting cell growth and regulating TCR signal transduction(42). Several of these functions may also be affected in Gal-3 deficient mice accounting for the decreased responses after OVA challenge in these mice. In addition, Gal-3 deficient mice may develop a tolerogenic T cell response after chronic allergen exposure. While this needs further investigation, studies have shown that regulatory T cells are elevated in Gal-3 deficient mice in experimental autoimmune encephalomyelitis(43) which is largely a Th1-driven disease. Contrary to our findings, gene therapy studies with Gal-3 have demonstrated inhibition of bronchial obstruction and inflammation in antigen-challenged rats through IL-5 gene downregulation (44) as well as attenuation of chronic airway inflammation and remodeling (45). This divergence may reflect differences in the effects of endogenous versus exogenously delivered Gal-3. The activity of recombinant Gal-3 artificially delivered into the lungs using plasmids may lead to functional effects that are quite distinct from those observed with endogenous lung expression of Gal-3.
Since eosinophil recruitment to the airways of chronic allergen-challenged Gal-3 KO mice was significantly attenuated, the expression of eotaxin-1, the major eosinophil chemoattractant, in the setting of chronic allergic inflammation was analyzed. In WT mice, chronic allergen-induced eotaxin-1 expression was largely observed in alveolar macrophages as well as alveolar and airway epithelial cells (Fig. 5, B, lower left panel). In allergen-challenged Gal-3 KO mice, eotaxin-1 levels were significantly lower in the lung tissue (Fig. 5, B, lower right panel and C) and BALF (Fig. 5, A). Eotaxin can be produced by EC, smooth muscle cells, fibroblasts, epithelial cells, alveolar macrophages and eosinophils themselves (46) and IL-13 has been shown to regulate the production of eotaxin-1 by lung fibroblasts and airway epithelial cells (37, 47, 48). Reduced eotaxin-1 levels in the lungs of allergen-challenged Gal-3 KO mice may be due to reduced infiltration of the airways by inflammatory cells that release eotaxin-1 such as Th2 cells, alveolar macrophages and/or eosinophils themselves, or due to decreased IL-13-induced production of this chemokine by lung cells. Chemokines synthesized by Th2 cells and other cell types are involved in the development of eosinophilic inflammation in bronchial asthma and studies using a Th2-cell-dependent murine model of asthma have shown that infiltration of eosinophils and antigen-specific Th2 cells is associated with eotaxin, MCP-3, RANTES and MCP-1 in the lungs of Th2-cell-transferred mice after antigen provocation (49). In the present study, in addition to eotaxin-1, expression of MCP-1 in the lung tissue of chronic allergen-challenged Gal-3 KO mice was also inhibited (data not shown).
Once recruited, eosinophils can cause damage to the airway mucosa during chronic allergic airway inflammation and several studies have demonstrated an important role for eosinophils in airway remodeling. Mice deficient in IL-5 exhibit significantly less peribronchial fibrosis as well as reduced smooth muscle compared to WT mice in response to OVA challenge (24). Mice with a total ablation of the eosinophil lineage were protected from peribronchiolar collagen deposition, airway epithelial mucus production and increases in airway smooth muscle mass in response to allergen challenge (16, 17). In the present study, allergen-challenged Gal-3 KO mice with diminished eosinophil recruitment also demonstrated a significant reduction in peribronchial fibrosis, smooth muscle mass, mucus secretion and peribronchial angiogenesis compared to WT mice (Fig. 6). Eosinophils are an important source of the profibrotic cytokine IL-13 as well as modulators of remodeling such as TGF-β1, vascular endothelial growth factor, fibroblast growth factor, epidermal growth factor and nerve growth factor(13, 50). IL-13 has been shown to induce subepithelial fibrosis as well as goblet cell hyperplasia and mucus secretion (37). In addition, IL-13 can induce the production of TGF-β1 and also activate stored latent TGF-β1(51) which is widely implicated in the development of allergic airway inflammation and airway remodeling in mice as well as humans (4, 52). Indeed Gal-3 KO mice with decreased eosinophil infiltration also demonstrate significantly decreased IL-13 and TGF-β1 expression compared to WT mice after allergen exposure (Figs. 4 and 7). Further, previous studies have demonstrated a role for Gal-3 in TGF-β-mediated myofibroblast activation as well as matrix production in the liver. Disruption of the Gal-3 gene blocked myofibroblast activation and procollagen (I) expression (53). Thus, it is possible that the disruption of the Gal-3 gene inhibits the profibrogenic effect of TGF-β. Recent studies suggest that TGF-β promotes development of Th17 cells (54). Th17-derived cytokines such as IL-17F have a pro-inflammatory role in asthma (55). Decreased expression of TGFβ-1 in the lungs of OVA-challenged Gal-3 KO mice may influence Th17 development and Th17-mediated effects including eosinophilic airway inflammation. Finally, enzymes such as eosinophil peroxidase and myeloperoxidase released extracellularly react with proteoglycans resulting in the localized release of hypobromus acid which in turn causes degradation of the ECM contributing to tissue damage associated with airway inflammatory diseases such as asthma (56). Overall, a reduction in eosinophil recruitment in allergen-challenged Gal-3 deficient mice was clearly associated with significant attenuation of airway remodeling.
Another molecule that is expressed by bronchial epithelial and alveolar type II cells during allergic inflammation is FIZZ1 (31, 57, 58). This molecule has been shown to induce VEGF production by murine epithelial cells (59) as well as proliferation of EC, correlating with angiogenesis in a murine model of asthma (32). Further, FIZZ1 has been shown to stimulate myofibroblast differentiation (30) and enhance production of collagen type I and SMA by lung fibroblast cell lines (31). In the current study, FIZZ1 expression dramatically increased in the lungs of chronic allergen-challenged WT mice, with airway epithelial cells staining positive for this protein, while Gal-3 deficiency resulted in a significant reduction in FIZZ1 expression (Fig. 8, A and inset). Reduction of FIZZ1 expression may contribute to the decreased fibrosis and angiogenesis observed in allergen-challenged Gal-3 KO mice. FIZZ1 gene expression is known to be regulated by Th2 cytokines such as IL-4 and IL-13 (60) and reduced IL-13 levels in allergen-challenged Gal-3 KO mice may in part be responsible for the decreased FIZZ1 expression in these mice. Interestingly, FIZZ1 has also been shown to induce expression of VCAM-1 by EC (61) which supports eosinophil trafficking by interacting with α4β1 as well as Gal-3 on the cell surface of human eosinophils (11). Finally, Gal-3 itself may contribute directly to airway remodeling. In hepatic stellate cells, expression of Gal-3 is upregulated during transdifferentiation into myofibroblasts as well as during fibrosis in the liver and can also induce proliferation of these cells (62). Further evidence for a direct role for Gal-3 in remodeling is presented in recent studies demonstrating that Gal-3 mediates post-ischemic tissue remodeling by stimulating the proliferation of EC and neural progenitor cells in the ischemic brain (63).
Collectively, our studies demonstrate an important role for Gal-3 in chronic allergen-induced airway inflammation and airway remodeling. Gal-3 deficiency resulted in inhibition of airway inflammation with significantly decreased eosinophil recruitment to the airways and the development of a Th2 phenotype (inhibition of IL-5 and IL-13 expression) associated with decreased expression of eosinophil-specific chemokines (eotaxin) as well as pro-fibrogenic (TGF-β1) and antigenic (FIZZ1) mediators leading to significant inhibition of airway remodeling.
Acknowledgements
The authors wish to thank Dr. Riaz I. Zuberi, Torrey Pines Institute for Molecular Studies, San Diego, CA, for helpful discussions regarding this study. The authors also wish to thank Cari M. Calhoun and Yana G. Greenberg for excellent technical assistance.
Footnotes
This work was supported by National Institutes of Health grants AI35796, U19-AI70535 and HL0793041 to P. Sriramarao.
References
- 1.Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc. 2009;6:301–305. doi: 10.1513/pats.200808-089RM. [DOI] [PubMed] [Google Scholar]
- 2.Tagaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int. 2007;56:331–340. doi: 10.2332/allergolint.R-07-152. [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra AK, Henderson WR., Jr The role of leukotrienes in airway remodeling. Curr Mol Med. 2009;9:383–391. doi: 10.2174/156652409787847209. [DOI] [PubMed] [Google Scholar]
- 4.Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Current Opinion in Immunology. 2007;19:676–680. doi: 10.1016/j.coi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994;269:4424–4430. [PubMed] [Google Scholar]
- 6.Sano H, Hsu DK, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu FT. Critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003;112:389–397. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 Activates the NADPH-Oxidase in Exudated but not Peripheral Blood Neutrophils. Blood. 1998;91:3430–3438. [PubMed] [Google Scholar]
- 9.Zuberi RI, Hsu DK, Kalayci O, Chen HY, Sheldon HK, Yu L, Apgar JR, Kawakami T, Lilly CM, Liu FT. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am J Pathol. 2004;165:2045–2053. doi: 10.1016/S0002-9440(10)63255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saegusa J, Hsu DK, Chen H-Y, Yu L, Fermin A, Fung MA, Liu F-T. Galectin-3 Is Critical for the Development of the Allergic Inflammatory Response in a Mouse Model of Atopic Dermatitis. Am J Pathol. 2009;174:922–931. doi: 10.2353/ajpath.2009.080500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao SP, Wang Z, Zuberi RI, Sikora L, Bahaie NS, Zuraw BL, Liu F-T, Sriramarao P. Galectin-3 Functions as an Adhesion Molecule to Support Eosinophil Rolling and Adhesion under Conditions of Flow. J Immunol. 2007;179:7800–7807. doi: 10.4049/jimmunol.179.11.7800. [DOI] [PubMed] [Google Scholar]
- 12.Han JL, Ding RY, Zhao L, Ren Z, Jiang XJ. Rosiglitazone attenuates allergic inflammation and inhibits expression of galectin-3 in a mouse model of allergic rhinitis. J Int Med Res. 2008;36:830–836. doi: 10.1177/147323000803600426. [DOI] [PubMed] [Google Scholar]
- 13.Kariyawasam HH, Robinson DS. The role of eosinophils in airway tissue remodelling in asthma. Curr Opin Immunol. 2007;19:681–686. doi: 10.1016/j.coi.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Fattouh R, Jordana M. TGF-beta, eosinophils and IL-13 in allergic airway remodeling: a critical appraisal with therapeutic considerations. Inflamm Allergy Drug Targets. 2008;7:224–236. doi: 10.2174/187152808786848388. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, Takatsu K, Delespesse G, Nagai H. Role of Interleukin-5 and Eosinophils in Allergen-Induced Airway Remodeling in Mice. Am. J. Respir. Cell Mol. Biol. 2004;31:62–68. doi: 10.1165/rcmb.2003-0305OC. [DOI] [PubMed] [Google Scholar]
- 16.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 17.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 18.Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–1083. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, Croft M, Zhang M, Varki A, Broide DH. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, Aronica M, Li X. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol. 2009;182:1631–1640. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuberi RI, Ge X, Jiang S, Bahaie NS, Kang BN, Hosseinkhani RM, Frenzel EM, Fuster MM, Esko JD, Rao SP, Sriramarao P. Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation. J. Immunol. 2009;183:3971–3979. doi: 10.4049/jimmunol.0901604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- 23.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 24.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113:551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, Hirashima M, Liu FT. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000;165:2156–2164. doi: 10.4049/jimmunol.165.4.2156. [DOI] [PubMed] [Google Scholar]
- 26.Alves CMOS, Silva DAO, Azzolini AECS, Marzocchi-Machado CM, Carvalho JV, Pajuaba ACAM, Lucisano-Valim YM, Chammas R, Liu F-T, Roque-Barreira MC, Mineo JR. Galectin-3 plays a modulatory role in the life span and activation of murine neutrophils during early Toxoplasma gondii infection. Immunobiology. 2010 doi: 10.1016/j.imbio.2009.08.001. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Komai-Koma M, Gilchrist DS, Hsu DK, Liu F-T, Springall T, Xu D. Galectin-3 Is a Negative Regulator of Lipopolysaccharide-Mediated Inflammation. J Immunol. 2008;181:2781–2789. doi: 10.4049/jimmunol.181.4.2781. [DOI] [PubMed] [Google Scholar]
- 28.Frigeri LG, Liu FT. Surface expression of functional IgE binding protein, an endogenous lectin, on mast cells and macrophages. J Immunol. 1992;148:861–867. [PubMed] [Google Scholar]
- 29.Xie S, Sukkar MB, Issa R, Khorasani NM, Chung KF. Mechanisms of induction of airway smooth muscle hyperplasia by transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293:L245–L253. doi: 10.1152/ajplung.00068.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung MJ, Liu T, Ullenbruch M, Phan SH. Antiapoptotic effect of found in inflammatory zone (FIZZ)1 on mouse lung fibroblasts. The Journal of Pathology. 2007;212:180–187. doi: 10.1002/path.2161. [DOI] [PubMed] [Google Scholar]
- 31.Dong L, Wang SJ, Camoretti-Mercado B, Li HJ, Chen M, Bi WX. FIZZ1 plays a crucial role in early stage airway remodeling of OVA-induced asthma. J Asthma. 2008;45:648–653. doi: 10.1080/02770900802126941. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Wang J, Li H, Han X. Found in Inflammatory Zone 1 Induces Angiogenesis in Murine Models of Asthma. Lung. 2008;186:375–380. doi: 10.1007/s00408-008-9099-1. [DOI] [PubMed] [Google Scholar]
- 33.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- 34.MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of Alternative Macrophage Activation by Galectin-3. J Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 35.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 36.Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jahnsen FL, Brandtzaeg P, Haye R, Haraldsen G. Expression of functional VCAM-1 by cultured nasal polyp-derived microvascular endothelium. Am J Pathol. 1997;150:2113–2123. [PMC free article] [PubMed] [Google Scholar]
- 39.Broide DH, Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunol. Reviews. 2001;179:163–172. doi: 10.1034/j.1600-065x.2001.790116.x. [DOI] [PubMed] [Google Scholar]
- 40.Gessner A, Mohrs K, Mohrs M. Mast Cells, Basophils, and Eosinophils Acquire Constitutive IL-4 and IL-13 Transcripts during Lineage Differentiation That Are Sufficient for Rapid Cytokine Production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 41.Spencer LA, Szela CT, Perez SAC, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu DK, Chen HY, Liu FT. Galectin-3 regulates T-cell functions. Immunol Rev. 2009;230:114–127. doi: 10.1111/j.1600-065X.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H-R, Al Rasebi Z, Mensah-Brown E, Shahin A, Xu D, Goodyear CS, Fukada SY, Liu F-T, Liew FY, Lukic ML. Galectin-3 Deficiency Reduces the Severity of Experimental Autoimmune Encephalomyelitis. J Immunol. 2009;182:1167–1173. doi: 10.4049/jimmunol.182.2.1167. [DOI] [PubMed] [Google Scholar]
- 44.del Pozo V, Rojo M, Rubio ML, Cortegano I, Cardaba B, Gallardo S, Ortega M, Civantos E, Lopez E, Martin-Mosquero C, Peces-Barba G, Palomino P, Gonzalez-Mangado N, Lahoz C. Gene Therapy with Galectin-3 Inhibits Bronchial Obstruction and Inflammation in Antigen-challenged Rats through Interleukin-5 Gene Downregulation. Am. J. Respir. Crit. Care Med. 2002;166:732–737. doi: 10.1164/rccm.2111031. [DOI] [PubMed] [Google Scholar]
- 45.Lopez E, del Pozo V, Miguel T, Sastre B, Seoane C, Civantos E, Llanes E, Baeza ML, Palomino P, Cardaba B, Gallardo S, Manzarbeitia F, Zubeldia JM, Lahoz C. Inhibition of Chronic Airway Inflammation and Remodeling by Galectin-3 Gene Therapy in a Murine Model. J Immunol. 2006;176:1943–1950. doi: 10.4049/jimmunol.176.3.1943. [DOI] [PubMed] [Google Scholar]
- 46.Hemelaers L, Louis R. Eotaxin: an important chemokine in asthma. Rev Med Liege. 2006;61:223–226. [PubMed] [Google Scholar]
- 47.Fritz DK, Kerr C, Botelho F, Stampfli M, Richards CD. Oncostatin M (OSM) primes IL-13- and IL-4-induced eotaxin responses in fibroblasts: Regulation of the type-II IL-4 receptor chains IL-4R[alpha] and IL-13R[alpha]1. Experimental Cell Research. 2009;315:3486–3499. doi: 10.1016/j.yexcr.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Xia Y, Nguyen A, Lai YH, Feng L, Mosmann TR, Lo D. Effects of Th2 Cytokines on Chemokine Expression in the Lung: IL-13 Potently Induces Eotaxin Expression by Airway Epithelial Cells. J Immunol. 1999;162:2477–2487. [PubMed] [Google Scholar]
- 49.Mori A, Ogawa K, Kajiyama Y, Suko M, Kaminuma O. Th2-cell-mediated chemokine synthesis is involved in allergic airway inflammation in mice. Int Arch Allergy Immunol. 2006;140:55–58. doi: 10.1159/000092712. [DOI] [PubMed] [Google Scholar]
- 50.Bloemen K, Verstraelen S, Van Den Heuvel R, Witters H, Nelissen I, Schoeters G. The allergic cascade: Review of the most important molecules in the asthmatic lung. Immunology Letters. 2007;113:6–18. doi: 10.1016/j.imlet.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 51.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohn L, Elias JA, Chupp GL. Asthma: Mechanisms of Disease Persistence and Progression. Annual Review of Immunology. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 53.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin H, Wang L, Feng T, Elson CO, Niyongere SA, Lee SJ, Reynolds SL, Weaver CT, Roarty K, Serra R, Benveniste EN, Cong Y. TGF-{beta} Promotes Th17 Cell Development through Inhibition of SOCS3. J Immunol. 2009;183:97–105. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawaguchi M, Kokubu F, Fujita J, Huang SK, Hizawa N. Role of interleukin-17F in asthma. Inflamm Allergy Drug Targets. 2009;8:383–389. doi: 10.2174/1871528110908050383. [DOI] [PubMed] [Google Scholar]
- 56.Rees MD, McNiven TN, Davies MJ. Degradation of extracellular matrix and its components by hypobromous acid. Biochem J. 2007;401:587–596. doi: 10.1042/BJ20061236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV, Jr, Shelton DL, Hébert CC. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELM{alpha}, a Novel Hypoxia-Induced Mitogenic Factor in Lung With Vasoconstrictive and Angiogenic Properties. Circ Res. 2003;92:1065–1067. doi: 10.1161/01.RES.0000073999.07698.33. [DOI] [PubMed] [Google Scholar]
- 59.Tong Q, Zheng L, Lin L, Li B, Wang D, Huang C, Li D. VEGF is upregulated by hypoxia-induced mitogenic factor via the PI-3K/Akt-NF-kappaB signaling pathway. Respir Res. 2006;7:37. doi: 10.1186/1465-9921-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stutz AM, Pickart LA, Trifilieff A, Baumruker T, Prieschl-Strassmayr E, Woisetschlager M. The Th2 Cell Cytokines IL-4 and IL-13 Regulate Found in Inflammatory Zone 1/Resistin-Like Molecule {alpha} Gene Expression by a STAT6 and CCAAT/Enhancer-Binding Protein-Dependent Mechanism. J Immunol. 2003;170:1789–1796. doi: 10.4049/jimmunol.170.4.1789. [DOI] [PubMed] [Google Scholar]
- 61.Tong Q, Zheng L, Lin L, Li B, Wang D, Li D. Hypoxia-Induced Mitogenic Factor Promotes Vascular Adhesion Molecule-1 Expression via the PI-3K/Akt-NF-{kappa}B Signaling Pathway. Am. J. Respir. Cell Mol. Biol. 2006;35:444–456. doi: 10.1165/rcmb.2005-0424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maeda N, Kawada N, Seki S, Arakawa T, Ikeda K, Iwao H, Okuyama H, Hirabayashi J, Kasai K-i, Yoshizato K. Stimulation of Proliferation of Rat Hepatic Stellate Cells by Galectin-1 and Galectin-3 through Different Intracellular Signaling Pathways. Journal of Biological Chemistry. 2003;278:18938–18944. doi: 10.1074/jbc.M209673200. [DOI] [PubMed] [Google Scholar]
- 63.Yan YP, Lang BT, Vemuganti R, Dempsey RJ. Galectin-3 mediates post-ischemic tissue remodeling. Brain Res. 2009;1288:116–124. doi: 10.1016/j.brainres.2009.06.073. [DOI] [PubMed] [Google Scholar]