Abstract
Objective
Early-onset cannabis use has been associated with later (ab)use, mental health problems (psychosis, depression) and abnormal development of cognition and brain function. During adolescence ongoing neurodevelopmental maturation and experience shape the neural circuitry underlying complex cognitive functions such as memory and executive control. Prefrontal and temporal regions are critically involved in these functions. Maturational processes leave these brain areas prone to the potentially harmful effects of cannabis use.
Method
We performed a two-site (US and NL; pooled data) functional MRI study with a cross-sectional design, investigating the effects of adolescent cannabis use on working memory (WM) and associative memory (AM) brain function in twenty-one abstinent but frequent cannabis using boys (age 13 – 19) and compared them with twenty-four non-using peers. Brain activity during WM was assessed before and following rule-based learning (automatization). AM was assessed using a pictorial hippocampal-dependent memory task.
Results
Cannabis users performed normally on both memory tasks. During WM assessment cannabis users showed excessive activity in prefrontal regions when a task was novel, whereas automatization of the task reduced activity to the same level in users and controls. No effect of cannabis use on AM-related brain function was found.
Conclusions
In adolescent cannabis users the WM system was overactive during a novel task, suggesting functional compensation. Inefficient WM recruitment was not related to a failure in automatization, but became evident when processing continuously changing information. The results seem to confirm the vulnerability of still developing frontal lobe functioning for early-onset cannabis use.
Keywords: cannabis, adolescence, early-onset, fMRI, memory
Introduction
Early initiation of cannabis use increases the risk of later (ab)use of other drugs and drug dependence, and is associated with mental health problems, such as psychosis and depression. The strength of this association appears to be dependent on the age when cannabis use begins. 1 A major concern only recently gaining attention is the effect of early-onset cannabis use on adolescent brain function and neurodevelopment.
The still developing adolescent brain differs anatomically and neurochemically from that of adults2,3 and is likely more susceptible to drug induced adaptive neuronal plasticity. Animal studies on the neural consequences of chronic cannabis exposure during the peri-adolescent period report changes in brain structure (predominantly limbic brain regions) and altered emotional and cognitive performance in later life.4 However, these effects were mostly observed at relatively high doses of synthetic cannabinoids (Win 55,212-2; CP 55,940) and, therefore, may not be comparable to the human situation.
Studies in human cannabis using adolescents are still limited, but a number of functional Magnetic Resonance Imaging (fMRI) studies have linked adolescent cannabis use to increased parietal activation along with diminished prefrontal activation during spatial working memory, and to increased parietal and prefrontal activation during inhibition, indicating reorganization of neural networks and recruitment of additional neural resources5,6 (for review see 7–9). Throughout early and late adolescence maturational processes occur at different rates in various brain regions. Maturation is slightly delayed in the temporal and prefrontal cortex, regions critically involved in memory and learning and cognitive control.2,10 The aim of the present study was to investigate the effects of adolescent regular cannabis use on memory-related brain function, with a focus on temporal and prefrontal regions. As there is evidence for gender differences in the rate and timing of neurodevelopment11 and neurodevelopmental responses to cannabinoid exposure12, only boys were included in this study. We performed BOLD fMRI using two tasks that reliably engage prefrontal and/or temporal brain regions, i.e. a verbal working memory (WM) task and a pictorial associative memory (AM) task, and compared regular cannabis using boys with age-matched non-using peers. Previous fMRI studies from our lab in adult cannabis users and non-using controls applied the same task paradigms13,14. These studies yielded no effects on task performance. However, with regard to brain activity, adult cannabis users displayed subtle alterations in superior parietal brain activity patterns during WM13 and overall hypo-activity in a network of prefrontal and parahippocampal areas during AM.14 In the present study, therefore, we would not expect a performance deficit, as the adults (having used for several years, i.e. longer than the current adolescent users) did not exhibit a deficit. For brain activity, we anticipated two possible outcomes. For one, effects of cannabis on brain activity could mimic those in the adults, i.e. altered parietal brain activity during WM and reduced activity during AM, suggesting age-independent effects of cannabis use. Alternatively, brain activity findings could differ from those found in adult users. In this case, we hypothesized hyper-activation to compensate for cannabis-induced dysfunction, most prominently in parahippocampal and prefrontal brain areas.
Method
This study was a joint venture of the University Medical Center Utrecht (The Netherlands) and the University of Iowa (US). Data were pooled to increase statistical power.
Participants
In total, 47 boys, aged 13 – 19 years, were included in the study; 23 regular cannabis users (11 Dutch, 12 US ) with at least 200 lifetime episodes of cannabis use, the others non-using controls (12 Dutch, 12 US ). Eligibility was ascertained through a screening procedure involving questionnaires on drug use, medical history and mental health. Table 1 reports inclusion and exclusion criteria. Written consent was obtained from adolescents and their parent/legal guardian in accordance with the local IRB and conformed to the Helsinki Declaration of 2004. Adolescents were informed in advance that a parent/legal guardian had to sign informed consent and would be informed by the researchers about the group the adolescent was in (e.g. user/non-user group), without revealing any details on history and/or pattern of cannabis use or other substances. Hence, parents/guardians knew in which group their son was, but we did not share information with them on the details of cannabis use, to promote the integrity of the drug use information we obtained from the youngsters. Subjects were reimbursed for participation in gift certificates.
Table 1.
In- and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Male | Medical or neurological problems |
| Age between 12 – 19 years | Regular use of illegal drugs other than cannabis (> 10 episodes lifetime), except for alcohol or nicotine |
| Right handedness | Axis I psychiatric diagnosis, except for conduct disorder which is a common diagnosis in cannabis using boys |
| IQ-scores below 80 | |
| Use of psychotropic medication | |
| Contra-indications for MRI (claustrophobia, metal objects, full dental braces) |
MRI = Magnetic resonance imaging
Procedure
Subjects participated in two separate sessions separated by one week: The first involved screening for inclusion and exclusion criteria using self-report questionnaires on drug use history and a semi-structured psychiatric interview (NIMH Diagnostic Interview Schedule for Children; C-DISC-version IV). To estimate cannabis use parameters, a semi-structured self-report questionnaire was used, containing questions on the number of times subjects used cannabis during the last three months, the last year, and (if relevant) previous years, as well as the number of joints/blunts smoked per occasion. Lifetime number of cannabis use episodes was estimated through extrapolation. In addition, we estimated the number of joints smoked last year (number of episodes * average number of joints used per occasion) and number of joints lifetime, where we took into account periods of reported more or less frequent use.
To estimate IQ, subjects performed four subtests (similarities, block design, vocabulary and matrix reasoning) of the Wechsler Intelligence Scale for Children – 4th edition (WISC-IV). The second session, one week after the first, included neuropsychological testing (results will be reported elsewhere) and fMRI scanning. Participants abstained from cannabis, alcohol and other substances for at least 24 hours prior to the first session and remained abstinent until the second session was finished. Smoking was allowed until two hours before the scanning session (to avoid nicotine withdrawal). On both sessions, urine samples were collected for drug screening (enzyme-multiplied immunoassay for cannabis, alcohol, amphetamines, ecstasy, opiates, cocaine and benzodiazepines). Exclusion followed on positive testing on any psychoactive substance on the second test day, except for cannabis that can linger in the body for several weeks due to its lipophilic properties, and thus, can induce a positive test result even after one week of abstinence.15 Instead, cannabis-using subjects were excluded only when their urine toxicology test failed to show a decrease in quantified levels (µg/l) of cannabinoid metabolites (THCCOOH) between the first and second urine sample taken. Based on the lab results, two subjects had to be excluded from analyses. Two other subjects had a positive urine test on cannabinoids during the second session, but THCOOH-levels were decreased compared to the first measurement one week earlier, which was consistent with self-reported abstinence. All other subjects had negative urine tests on the day of testing.
Assessment of WM and AM
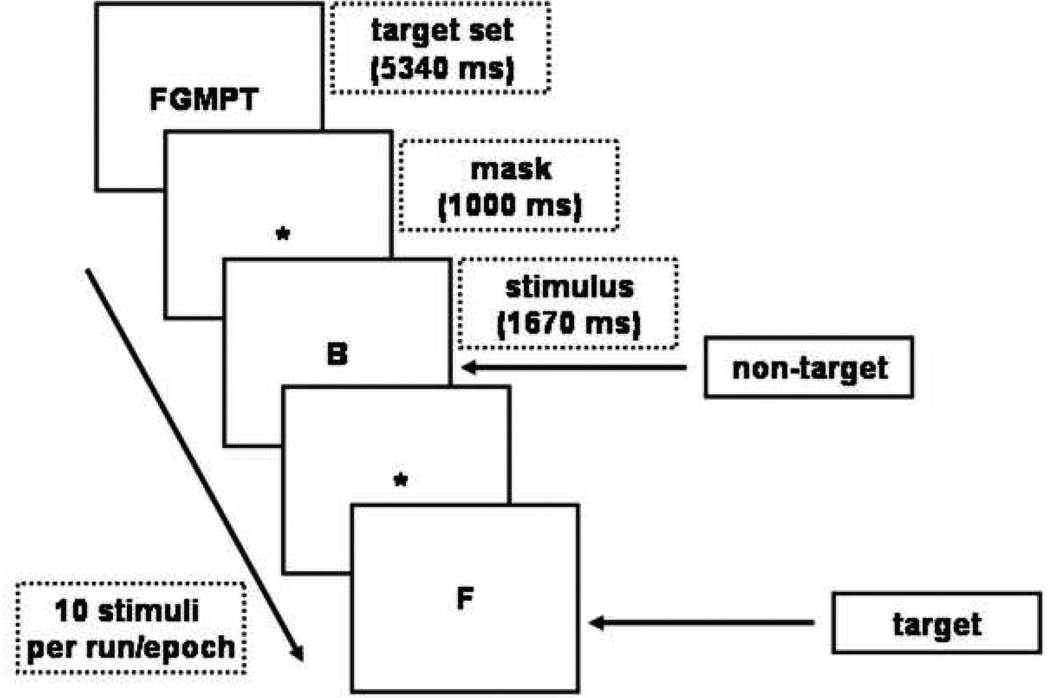
Two fMRI tasks were administered: a verbal WM task (Figure 1) based on Sternberg’s item-recognition paradigm (denoted STERN), and a pictorial AM task (denoted PMT; Figure 2). Both tasks are described in detail in previous papers 13,14. STERN assesses the WM system before and following practice (automatization). Subjects were instructed to memorize a set of five letters (memory set) and subsequently respond to single letters (probes) by pressing a button if the probe was in the memory set (target). A novel (NT) and a practiced task (PT) were administered. In PT a fixed memory set was used repeatedly, on which subjects were trained before scanning to induce automatization. In the NT the composition of the memory set was changed after every epoch. An additional reaction time control task (CT) was included during which subjects made a button press when the symbol ‘< >’ appeared. In the scanner, each task (CT, PT and NT) was presented in 6 epochs (duration 29 seconds) of 10 stimuli each, as well as 6 rest periods of equal epoch duration.
Figure 1.
The temporal sequence of events for the working memory task. Each epoch starts with presentation of the memory-set (a set of five consonants, for example ‘FGMPT’), and is followed by ten trials showing a single consonant. Subjects have to press a button as fast as possible, if the letter belongs to the memory-set.
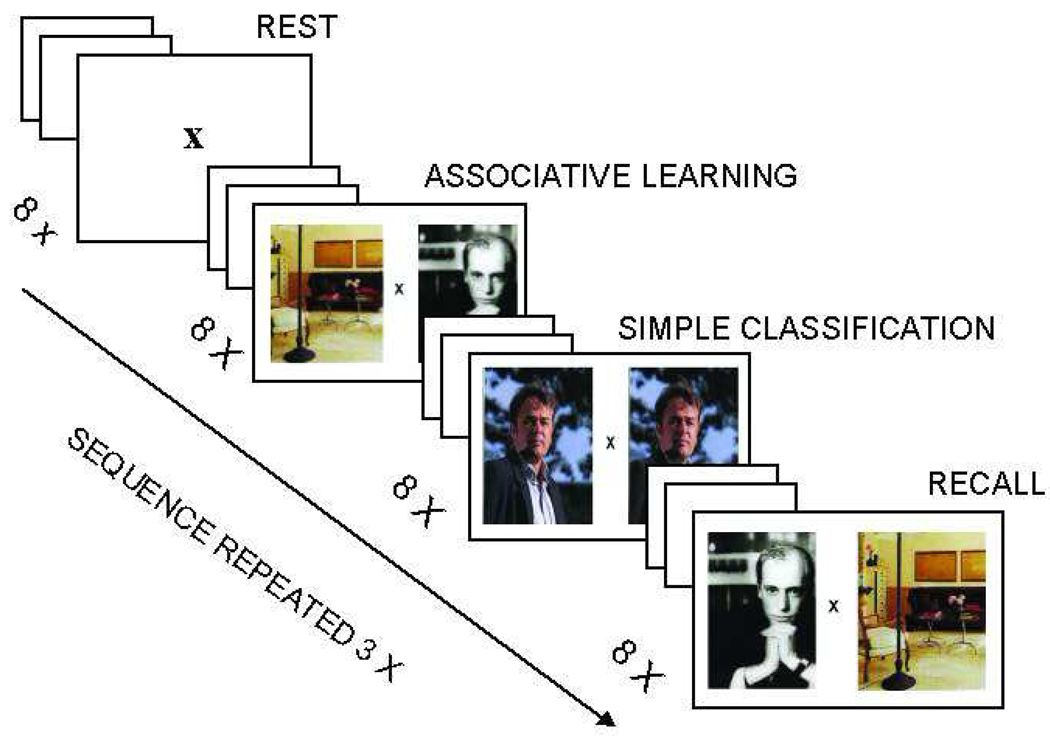
Figure 2.
The temporal sequence of events the associative memory task. Each epoch starts with an instruction slide (5 sec) followed by a fixation cross (2.5 sec). This is followed by 8 trials of 7.5 sec each (picture pair 5 sec, fixation cross 2.5 sec). Subjects respond by pressing one out of two buttons, according to the instruction in each task condition.
PMT assesses (para)hippocampal-dependent AM and involves three tasks. First, an associative learning task (AL) which requires subjects to establish a meaningful connection between two pictures and to memorize the combination. Next, single pictures have to be classified (SC), which serves as a control task, i.e. compared to AL it requires the same amount of perceptual processing and a motor response, but it lacks the associative learning component. Finally, the retrieval task asks (RE) subjects to recognize specific combinations previously presented during AL, and provides a performance measure. Each task was presented in 3 epochs (duration 65 seconds) of 8 stimuli each, as well as 3 rest periods of equal duration.
FMRI acquisition
Imaging was performed using a clinical 3 T MRI scanner (Philips Achieva (NL) and Siemens Magneton Trio (US), both with an 8-channel head coil. Pilot data on various T1 and EPI scan sequences from three subjects (i.e., researchers GJ and NFR and a research assistant (JZ)) were tested for homogeneity of signal-to-noise (SNR) and temporal signal-to-noise (tSNR) ratios across sites and vendors. The scan parameters yielding highest similarity between SNR and tSNR maps across scanners were used for both tasks: TE/TR 35/2000 ms, flip angle 70°, FOV 256 × 256 mm, acquisition matrix 64 × 64, slice thickness 3.6 (plus a 0.4 mm gap), voxelsize 4.0 mm isotropic, 26 slices, scan orientation transaxial for STERN and parallel to the long axis of the hippocampus for PMT. Details on the issue of multi-site studies and scanner compatibility are presented in Figure S1, available online. For STERN a single run of 312 scans was acquired over a period of 10.4 minutes. For PMT a single run of 324 scans lasted 10.8 minutes. In addition, a volumetric T1-weighted MR anatomical scan was acquired for spatial localization (TR 25 ms, flip angle 30°, FOV read 256 mm, voxelsize 1.0 mm isotropic, 176 slices, scan duration 7.8 minutes).
Statistical analysis
Sample characteristics and drug use
Cannabis parameters were: age of onset, cumulative use lifetime (estimated number of joints), current or recent use (number of joints last year), frequency and duration of use, and abstinence (weeks since last use). Additional drug use data included last year use of alcohol (average number of drinks/week), tobacco (average number of cigarettes/week) and lifetime use of other illegal substances (number of occasions lifetime). Because distribution of the drug variables was skewed, scores were log-transformed, except for age of onset of cannabis which was normally distributed. Other variables included age, estimated IQ, country (US versus NL) and a diagnosis of conduct disorder (yes/no). Group differences were tested using t-tests and non-parametric Kolmogorow-Smirnov Z tests.
Task performance
Outcome measures included reaction times (STERN only) and accuracy. General linear model (GLM) repeated measures analysis was applied, with task condition (CT, PT, and NT for STERN; AL, SC, and RE for PMT) and outcome measure (reaction time and accuracy) as within-subject factor, and group (user versus control) as between-subject factor. To adjust for normal developmental effects, i.e. younger boys performing relatively worse than older ones, age was included as a covariate.
FMRI
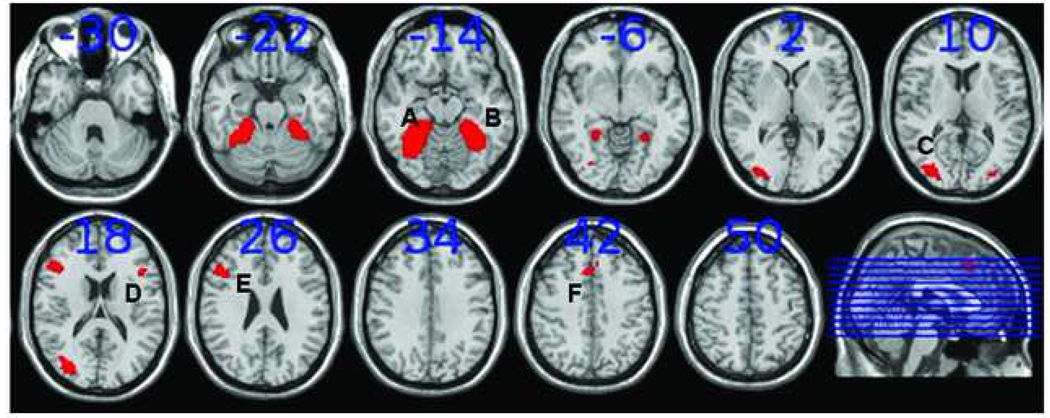
Imaging data were analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Pre-processing included realignment (motion correction) and unwarping, co-registration, normalization and smoothing with an 8 mm (FWHM) Gaussian kernel. First, statistical activity maps were generated for each subject for all task conditions (CT, PT, and NT for STERN; AL, SC, and RE for PMT) by analyzing time series data with multiple regression analyses using a vector representing the design of a task and including cosine basis functions to remove low frequency drifts in the signal (for details on preprocessing and 1st level statistic analysis see Figures S1, available online. Next, for STERN individual contrast maps were created for NT and PT, corrected for individual offset activity levels (i.e. subtracting activity levels during the control task (CT), representing baseline activity unrelated to WM processing, from activity levels during NT and PT respectively). Similarly, for PMT we created individual activity maps for AL and RE, corrected for individual offset activity levels during the control condition, contrasting both AL and RE with SC. Contrast maps were then used in a second-level whole brain analysis to test for effects of cannabis use on brain activity. Age and a dichotomized variable for country (0=NL; 1=US) were added as covariates, to take into account potential systematic effects of age and/or differences in MRI-scanners across sites. In addition to whole brain analysis, region-of-interest (ROI) analyses were performed. To check whether users and non-users activated similar networks of brain regions during WM (as expected, as there is no a priori reason to assume that cannabis users show significant functional reorganization of the brain), we defined ROIs for both groups separately (group contrast maps; NT-CT for STERN and AL-SC for PMT, p<0.05, FEW corrected). Visual inspection of the group activation maps indicated no differential activation patterns between users and controls. Therefore, ROIs used for further analyses (listed in Table 2) were derived from group contrast maps (NT-CT for STERN and AL-SC for PMT) of all subjects combined (p < 0.05, FWE corrected). For STERN (see Figure 3), this yielded activated regions in the left superior parietal cortex (l-SPC), the left inferior frontal gyrus (l-IFG), the left precentral and dorsolateral prefrontal cortex (l-PCC/DLPFC) and the anterior cingulate cortex (ACC). For PMT activated regions included bilateral regions in the parahippocampal gyrus, the middle occipital gyrus and prefrontal areas (see Figure 4)). ROIs for both tasks involved regions known from previous studies from our lab, using the same task paradigms13,14,16, and hence, met a priori expectations. ROIs were marked and activity values for all subjects were obtained per ROI by averaging beta-values across all contained voxels for NT and PT, and AL and RE (corrected for individual offset activity levels), using the Marsbar toolbox in SPM5.17 Finally, these variables were entered into GLM repeated measures analyses with task conditions (NT and PT for STERN, AL and RE for PMT) and ROIs as within-subject factors, and group as between-subject factor. Similar to the whole-brain analyses, country and age were entered as covariates in all ROI analyses.
Table 2.
Regions of interest during working memory and associative memory
| Task | Region | Brodmann area |
Number of voxels |
X | Y | Z | T-max |
|---|---|---|---|---|---|---|---|
| STERN | ACC | 6/24 | 77 | −4 | 12 | 48 | 9.55 |
| l-PCC/DLPFC | 9/46 | 48 | −40 | 0 | 36 | 6.21 | |
| l-IFG | 47 | 35 | −36 | 24 | 0 | 6.13 | |
| l-SPC | 39 | 6 | −28 | −60 | 36 | 4.73 | |
| PMT | r-PHG/MOG | 36/37/19 | 310 | 36 | −52 | −20 | 13.49 |
| l-PHG | 36/37 | 139 | −36 | −48 | −20 | 12.80 | |
| r-DLPFC | 9/46 | 43 | 48 | 28 | 20 | 8.74 | |
| ACC | 6/24 | 20 | 4 | 20 | 44 | 7.68 | |
| l-DLPFC | 46 | 17 | −44 | 20 | 20 | 7.18 | |
| l-MOG | 19 | 17 | −24 | −92 | 4 | 7.60 |
Montréal Neurological Institute (MNI) coordinates for the regions of interest involved in working memory (Sternberg item-recognition task) and associative memory (pictorial memory task). The coordinates X, Y, and Z represent location of the voxels with the highest t-value in the group map. ‘l-‘ and ‘r-‘ stands for left and right, ACC = anterior cingulate cortex, PCC/DLPFC = precentral/dorsolateral prefrontal cortex, IFG = inferior frontal gyrus, SPC = superior parietal cortex, PHG = parahippocampal gyrus, MOG = middle occipital gyrus.
Figure 3.
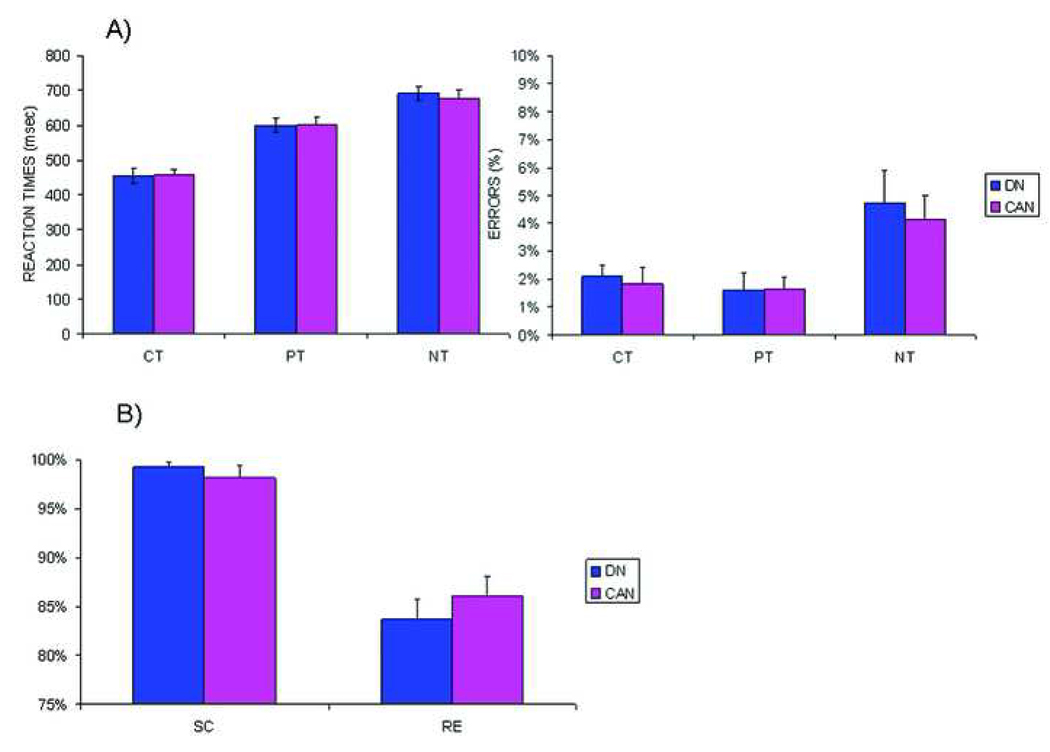
Behavioral data
Graphs A) Working memory: Mean reaction time ± standard error of mean (SEM) of correct responses on targets for both groups and mean percentage of errors as percent of all trials (± SEM) during the control task (CT), following (PT) and before practice (NT). B) Associative memory: Accuracy during simple classification (SC) and recognition (RE) (± SEM) for both groups. DN = drug naive controls, CAN = users.
Figure 4.
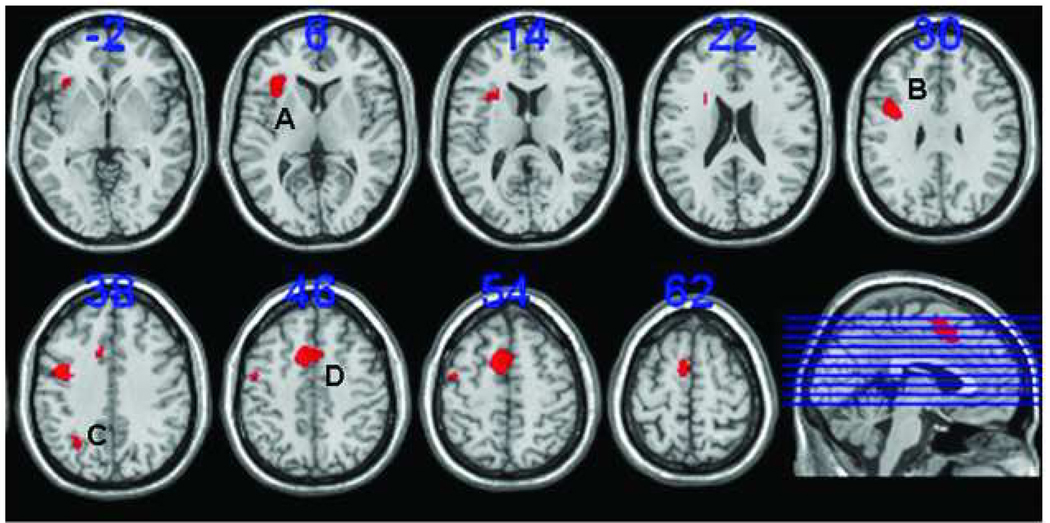
Regions of interest for the working memory task: A) the left inferior frontal gyrus (l-IFG); B) the left precentral/dorsolateral prefrontal cortex (l-PCC/DLPFC); C)the left superior parietal cortex (l-SPC); and D) the anterior cingulate cortex (ACC). Regions of interest are based on the contrast between novel and control task (NT-CT) (p < 0.001). The numbers above the slices indicate the z-coordinates of the Montreal Neurological Institute (MNI) system. Slices are in neurological orientation (left side is left hemisphere).
Potential confounders
Nine out of twelve US cannabis users met criteria for a diagnosis of conduct disorder, which may act as a confounding factor. However, as the co-occurrence of conduct disorder was restricted to the US users, it was strongly correlated to the factor country (which was included as a covariate in all analysis together with age) the effects of conduct disorder could not be disentangled from those of country. Cannabis use in both US and Dutch users correlated significantly with lower IQ, use of alcohol and tobacco. Any significant group difference found on output parameters (task performance, brain activity) resulting from the main analyses (see previous paragraph) was, therefore, considered rather an effect of a ‘cannabis using lifestyle’ than an effect of cannabis use alone. Yet, to explore the relative strength of the effect of cannabis alone, output parameters were re-computed after controlling for confounding effects of IQ, use of alcohol and tobacco by means of multiple regressions, i.e. saving the standardized residuals. In a subsequent analysis, we then re-entered these standardized residuals into group analyses (ANOVA, GLM repeated measures). This constitutes a conservative approach where interactions between effects of cannabis and other effects are prevented from leaking into the ‘cannabis’ effect.
Results
Two users were excluded based on positive urine drug tests, and STERN fMRI data were lost for one control due to technical malfunction. Results are reported for N = 45 (21 users, 24 controls) for sample characteristics, drug use and PMT data, and for N = 44 (21 users, 23 controls) for STERN.
Sample characteristics and drug use
Table 3 summarizes sample characteristics and drug use for users (US and Dutch) and non-users (US and Dutch). Users were on average abstinent for 5.1 weeks (± 4.2). Groups did not differ in age, but users had significantly lower IQ-scores than controls (p<0.01), and reported significantly higher last year consumption of alcohol (mean alcoholic drinks per week 13.3 (± 13.6) for users compared to 3.4 (± 5.8) for controls) and tobacco (mean number of cigarettes smoked per week 63.8 (±53.4) for users compared to 6.1 (± 14.9) for controls). Additionally, demographic and cannabis use parameters were compared for the user and non-user group separately, comparing US subjects with Dutch subjects, to identify potentially site related biases. Dutch users were older than US users (mean age 17.9 (SD 0.9) and 16.7 (SD 0.8) respectively, p<0.05), but no significant differences were found on IQ scores or cannabis use parameters. Nine US users had a C-DISC diagnosis of conduct disorder, whereas none of the Dutch users or the controls (both US and Dutch) did.
Table 3.
Demographics and drug use (mean (± SD, range)
| N = 45 | Users (N =21) | Controls (n =24) | p-valuea |
|---|---|---|---|
| Age | 17.2 (1.0, 15 – 19) | 16.8 (1.3, 13 – 19) | ns |
| IQ | 101 (10.7, 82 – 116) | 111 (11.6, 94 – 138) | < 0.01 |
| Cannabis | |||
| Lifetime (nr of joints) | 4006 (7555, 224 – 32,850) | 1.8 (4.0, 0 – 15) | < 0.001 |
| Last year (nr of joints) | 741 (772, 208 – 3528) | 1.0 (2.9, 0 – 12) | < 0.001 |
| Age of onset (years) | 13.2 (2.3, 8 – 16) | 15.0 (1.6, 12 – 17) | ns |
| Abstinence (weeks since last use) | 5.1 (4.2, 1 – 16) | ||
| Other substances (use last year) | |||
| Alcohol (drinks/week last year) | 13.3 (13.6, 0 – 46) | 3.4 (5.8, 0 – 24) | < 0.01 |
| Tobacco (cigarettes/week last year) | 63.8 (53.4, 0 – 144) | 6.1 (14.9, 0 – 53) | < 0.001 |
| Ecstasy (episodes lifetime) | 0.3 (0.9, 0 – 4) | 0.1 (0.4, 0 – 2) | ns |
| Amphetamines (episodes lifetime) | 1.3 (2.4, 0 – 7) | -- | |
| Cocaine (episodes lifetime) | 0.4 (0.9, 0 – 4) | -- | |
| Psilocybin (magic mushrooms) (episodes lifetime) |
0.4 (0.6, 0 – 2) | 0.1 (0.4, 0 – 2) | ns |
| LSD (episodes lifetime) | 0.1 (0.2, 0 – 1) | -- | |
| Laughing gas (episodes lifetime) | 0.7 (2.1, 0 – 9) | 0.1 (0.4, 0 – 2) | ns |
| Benzodiazepines (episodes lifetime) | 2.86 (8.8, 0 – 40) | 0.2 (0.8, 0 – 4) | ns |
| C-DISC | |||
| Conduct disorder (yes/no) | N=9 (yes), N=12 (no) | N=24 (no) | < 0.001 |
Significance of differences calculated using independent samples T-tests (age, IQ, age of onset) and non-parametric Kolmogorov-Smirnow Z tests, two-tailed. Values between brackets denote standard deviation and range. C-DISC = Children’s Diagnostic Interview Schedule.
Task performance
Figure 3 shows performance data.
WM (STERN)
Users did not differ from controls on reaction times and accuracy during the STERN task. On average, reaction times and accuracy were similar to adult performance levels as observed in our previous study 13, indicating normal behavioral WM capacity and efficiency. A main effect of age was observed (F(1,40)=11.91, p=0.001), indicating older boys performed faster and more accurately than younger boys but this was independent of cannabis use.
AM (PMT)
Users performed equally accurate as controls and accuracy levels were comparable to those observed in adults 14, indicating AM performance was unaffected by cannabis use. No effects of age were found.
fMRI data
Scanner compatibility (for details see Figure S1, available online) was quantified by a direct comparison of temporal signal-to-noise maps (tSNR) maps for both STERN and PMT imaging data between the two sites, using non-parametric statistics in SPM5 (SnPM5-toolbox, see also www.sph.umich.edu/~nichols/SnPM/). The results revealed some areas with differential tSNR between sites, predominantly in the orbito- and ventromedial prefrontal regions. However, there was no overlap between regions showing scanner-related differences in tSNR and regions-of-interest (see below) for the WM or AM task.
WM (STERN)
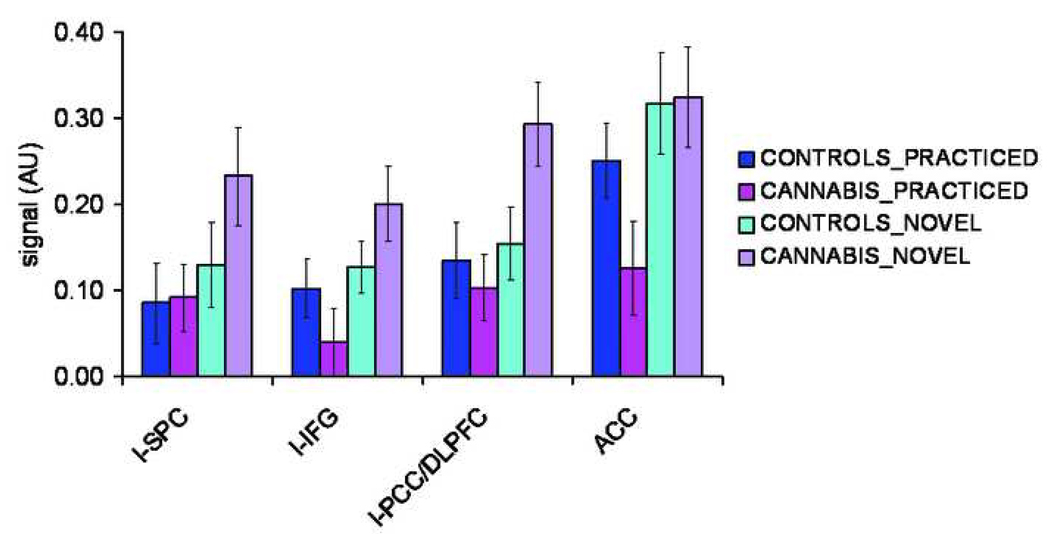
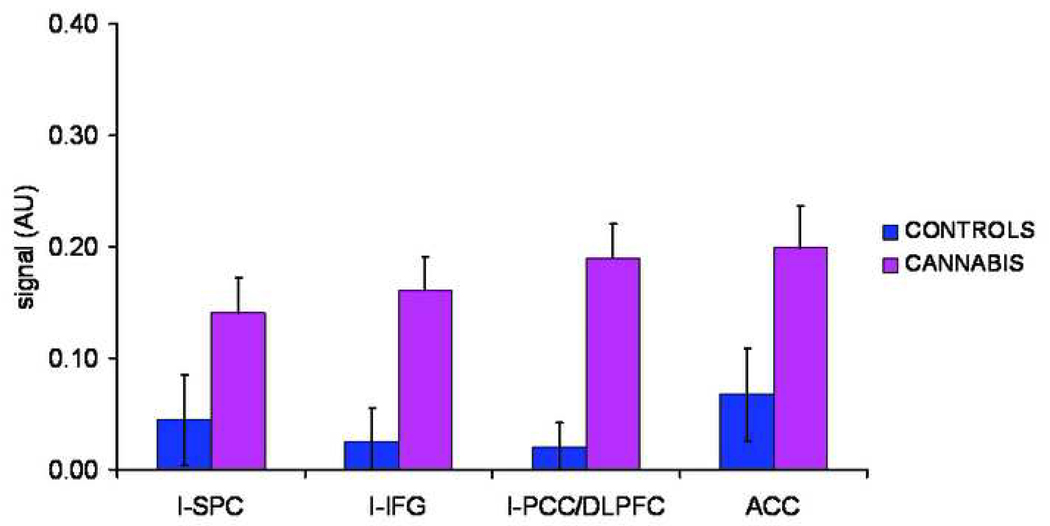
A second level whole brain analysis in SPM5 (p < 0.05, FWE corrected) revealed no significant differences between users and controls in working-memory related brain activity before or following practice (NT-CT and PT-CT contrast respectively). A subsequent ROI- GLM repeated measures analysis (see Table 2 and Figure 4 for ROIs), with group as between-subjects factor, task (NT, PT) and ROI (l-IFG, l-SPC, l-PCC/DLPFC, ACC) as within-subjects factors, and age and country as covariates, yielded a significant task*group interaction (F(1,40) = 12.85, p = 0.001). Separate GLM analyses for NT and PT showed that the WM system tended to be overactive before automatization (NT) in users (F(1,40)=2.77, p=0.10) (see Figure 5). They showed significantly larger differences in activity before and following practice, e.g. NT-PT contrast values in the l-IFG (F(1,40)=12.04, p=0.001), the l-PCC/DLPFC (F(1,40)=22.37, p<0.001) and ACC (F(1,40)=5.42, p=0.03). As learning (PT) reduced activity in the WM system to the same level in both groups in most areas, this indicates overall excessive effort in users to achieve normal performance when a task is novel (see Figure 6).
Figure 5.
Activity levels per Region-of-interest (ROI) for the working memory task (in arbitrary units(AU)) following practice (PT) and before (NT) for both groups. Activity levels were marginally higher in users compared to controls during NT (F(1,40)=2.77, p=0.10), but no significant group effects were found during PT. l-SPC = left superior parietal cortex; l-IFG = left inferior frontal gyrus; l-PCC/DLPFC = left precentral/dorsolateral prefrontal cortex; ACC = anterior cingulate cortex .
Figure 6.
Contrast values per Region-of-Interest (ROI) for the working memory task (in arbitrary units(AU)), i.e. the difference in activity before (NT) and following practice (PT). l-SPC = left superior parietal cortex; l-IFG = left inferior frontal gyrus; l-PCC/DLPFC = left precentral/dorsolateral prefrontal cortex; ACC = anterior cingulate cortex . * difference between groups at p<0.05.
AM (PMT)
A second level whole brain analysis in SPM5 (p < 0.05, FWE corrected) yielded no significant group differences in associative learning or recognition-related brain activity (AL-SC and RE-SC contrasts respectively). Neither did a subsequent ROI-analysis (all p-values > 0.20; see Table 3 and Figure 7 for details on ROIs).
Figure 7.
Regions of interest for the associative memory task: A + B) left and right (para)hippocampal gyrus (PHG); C) the left middle occipital gyrus (l-MOG); D + E) right and left dorsolateral prefrontal cortex (DLPFC); and F) the anterior cingulate cortex (ACC). ROIs are based on the contrast associative learning versus simple classification (AL-SC) (p < 0.001). The numbers above the slices indicate z-coordinates of the Montreal Neurological Institute (MNI) system. Slices are in neurological orientation (left side is left hemisphere).
Potential confounders
To explore the relative strength of the effect of cannabis alone on region-of-interest between-group differences in WM brain activity, average beta values across all contained voxels per ROI were recomputed, by running multiple regression analyses, including IQ, alcohol and tobacco use as regressors, and saving the standardized residuals. Standardized residuals, which were now devoid of effects of IQ, alcohol and tobacco, were entered into GLM repeated measures analyses. The task*group interaction remained significant (F(1,40)=6.81, p=0.01. Posthoc ANCOVA with and age and country as covariates revealed that even after adjustment for effects of IQ or alcohol and tobacco use, the group differences in NT-PT contrast remained significant in l-IFG and l-PCC/DLPFC (F(1,40) = 5.99, p=0.02 and F(1,40) = 12.05, p=0.001 respectively) but became marginally significant in ACC (F(1,40) = 2.56, p=0.10).
In users (N=21) we assessed whether cannabis use parameters (number of joints, age of onset) predicted ROI-specific difference in activity before and after automatization (NT-PT contrast). In the l-IFG, number of joints last year was significantly correlated to the difference in activity before and after automatization (Spearman’s rho = 0.52, p<0.05) whereas for number of joints lifetime there was a trend (rho = 0.42, p=0.07), tentatively indicating that part of the effects found was selectively associated with cannabis use.
Discussion
This study examined the effects of regular cannabis use on adolescent WM and AM brain function. No evidence was found for effects of cannabis use on AM, both at the behavioral and at the neurophysiological level, but the WM system was overactive in users during a novel task, whereas automatization reduced overall activity to the same level in users and controls. Our findings extend those of previous studies in adolescents with cannabis and alcohol use disorders reporting increased dorsolateral prefrontal activation during a spatial WM task. 18 Increased activity levels in hippocampal and parietal regions have also been observed in abstinent cannabis using teens during verbal WM19, all with normal performance levels.
We have previously observed subtle alterations during WM in the superior parietal cortex in adult cannabis users, but not in prefrontal regions.13 The present results, therefore, support our hypothesis of age-specific effects of adolescent cannabis use on still developing brain function and seem to confirm the vulnerability of developing frontal lobe functioning.
Animal studies on the neural consequences cannabis exposure during adolescence suggest greater, more persistent memory deficits and hippocampal abnormalities in adolescent than in adult animals.20 Contrary to what we expected, our results yielded no proof of impaired AM performance or temporal lobe dysfunction. It is, however, in line with neuropsychological data in cannabis-using teenagers indicating significant impact of cannabis on spatial WM and verbal learning but not on associative learning.21 In adult cannabis users we have found hypo-activity compared to controls in (para)hippocampal regions and the right dorsolateral prefrontal cortex during AM.14. A recent study by Nestor and colleagues22, however, reports parahippocampal hyperactivity and frontocortical hypo-activity in adult users during an associative face-name learning task. The current study fails to replicate either of these results. One possible explanation for the discrepancies between effects of frequent cannabis use on AM brain function between adolescents and adults may be different abstinence periods, as they vary between short intervals (mean 15 hours; range 2 – 45)22, at least one week, but on average not much longer14, and on average five weeks (range 1 week – four months) in the present study. It has been argued repeatedly that the non-acute effects of cannabis use on brain functioning might vary according to the duration of abstinence23 and this may even be different in adolescents.8
This study has several limitations. Groups differed on a number of key variables, with users displaying lower estimated IQ scores, greater alcohol and tobacco smoking histories. Although the main findings remained unchanged after controlling for these factors and cannabis use parameters were linked to the excessive activation in the left inferior frontal gyrus during the novel task, use of alcohol and tobacco pose the possibility of synergistic effects. Together, these factors constitute a ‘cannabis-using lifestyle’ which may be predictive for detrimental effects on development of cognition and brain function. The high prevalence of conduct disorder in the US users (9 out of 12 subjects) may be related to site differences in recruitment strategies. In the Netherlands, subjects were recruited using a variety of strategies, including advertisements on the internet and with help of schools. In the US, however, recruitment was restricted to local substance abuse councils and adolescent health and resource centers offering education and treatment programs to minors that got involved with the legal system because of possession of cannabis or other drugs. Boys ending up in these programs may display more externalizing behavior problems and more often meet criteria for conduct disorder. Banich and colleagues24 compared brain activation patterns during a color-word Stroop interference task between adolescents with severe substance and conduct problems and controls. Similar to our results, they found that patients needed to engage prefrontal brain regions to a greater extent than controls during the interference condition to obtain the same level of performance. However, the question remains whether these differences in brain activation are a predisposing factor in patients with severe, co-morbid conduct and substance problems, or whether they result from the prior ingestion of illicit substances. The design of our study did not permit conclusions on the nature of the potential confounding effect of conduct disorder, as presence of conduct disorder was country-specific and could not be disentangled from other site-related differences. Still, as any effects related to country-differences were regressed out, we feel justified in arguing that the confounding influence of conduct disorder on the main findings is limited.
Besides the advantage of increased power, the multi-center design also poses a challenge in terms of scanner compatibility. We assessed scanner compatibility in the preparation stage of the study, opted for scan sequences that showed highest similarities in scans across sites, and after study completion used several statistical methods to further minimize and/or quantify systematic effects due to site-related differences in scanner equipment. Nonetheless, we cannot completely rule out the influence of scanner differences on between subjects variability, predominantly in the orbitoen ventromedial regions. These areas are involved in inhibitory processes and decision-making, and altered brain function in these areas has been reported in relation to chronic cannabis (ab)use and/or use of other drugs25,26. We acknowledge limited sensitivity to detect cannabis-related effects in orbito- and ventromedial regions in the present study. However, the experimental paradigms applied in the present study are unlikely to elicit activations in those areas. Hence, we feel confident the results of our ROI-analyses have not been compromised by the multicenter approach.
A third limitation is the cross-sectional design, and hence, the possibility that the observed differences in WM related brain function predated the onset of regular cannabis use. There is ample literature on genetic, neurobehavioral and personality profiles increasing the liability to substance use and abuse (for review see 27,28). Such pre-existent factors may both increase the tendency of adolescents to get involved in risky behaviors like drug (ab)use, and may affect neurocognitive development.
Finally, the inclusion of cannabis using boys only, excludes the possibility to explore gender-specific differences in the impact of adolescent cannabis use on cognition and brain function, for which there is tentative evidence from animal studies. 13
Future neuroimaging studies should attempt to understand the structural and neurochemical correlates that underlie the observed alterations in brain activation in cannabis users during a cognitive challenge, because the clinical significance of these alterations is far from clear. 23 Findings of increased brain activation combined with normal performance are commonly interpreted as functional compensation to maintain normal task performance. Yet, a warning against simplistic or mechanistic interpretation of increased versus decreased brain activation in the absence of differences in task performance seems appropriate, especially with regard to fMRI studies in adolescents. During adolescence, both brain maturation and learning and experience shape the neural circuitry that underlies cognitive brain function, resulting in highly dynamical changes in brain function over time.2 Hence, alterations in brain activity patterns as observed in the present study may signify persistent dysfunction, but, alternatively, may also reflect a shift or delay in normal neurodevelopmental changes in cognitive brain function. In this context, it is interesting to note that our current findings of excessive activation in several prefrontal areas during the most demanding task condition show similarities with a study on automatization and WM capacity in schizophrenia by Van Raalten and colleagues.16 This study reports that patients with schizophrenia, who often display deficits in executive functioning, displayed similar levels of brain activity following automatization compared to controls, but higher levels of brain activity during the novel, more demanding task, indicated inefficient WM function and a failure to properly engage the WM brain system when task demands increase.16 The present results resemble these findings. It has been suggested, based on overlap in cognitive dysfunction associated with long-term cannabis use and schizophrenia, that with regard to the neurobiology underlying the dysregulation of higher-order cognitive processes, the endocannabinoid (eCB) system may be implicated.29 The eCB system could be involved, either directly or through its interactions with other neurotransmitter systems, notably dopamine, in the development of similar cognitive deficits associated with both cannabis abuse and schizophrenia. 30 This notion is consistent with the increasing number of studies detecting cognitive dysfunction in adolescent cannabis users7–9 as well as a greater incidence of juvenile psychotic symptoms and other mental health problems. 31 During critical periods of neurodevelopment, in particular adolescence, cannabis use may have more impact, especially in otherwise genetically predisposed individuals, and may precipitate the onset of psychosis.32 This is not to say that cannabis use itself causes psychosis in young people, but a complex association between cannabis use and schizophrenia may be due to dysfunction of the eCB system29, which may be reflected in similarities in abnormal neurophysiology underlying cognitive brain functions.
In conclusion, teenage cannabis use may reduce the ability to process information requiring frequent updating. The present study indicates that predominantly prefrontal brain regions are prone to adverse consequences of cannabis use during this stage of life. Whether the effects of adolescent cannabis use on WM-related brain activation persist over longer periods of abstinence, as well as their clinical relevance in terms cognitive dysfunction, remains to be determined.
Supplementary Material
Acknowledgments
This work was supported by grants of The Netherlands Organization for Health Research and Development (ZonMW 31100003) and The National Institute of Drug Abuse (NIDA 5R01DA019338) as part of their bi-national Addiction Program.
The authors gratefully acknowledge Jordan Zuccarelli, Megan Becker, Catherine Fruehling-Wall for US data acquisition; Vincent Magnotta, and Daniel O’Leary for support at the Iowa Imaging Center and the Iowa Imaging Processing Laboratory; all Dutch and US institutes that assisted with recruitment of the participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
References
- 1.Sundram S. Cannabis and neurodevelopment: implications for psychiatric disorders. Hum Psychopharmacol. 2006;21:245–254. doi: 10.1002/hup.762. [DOI] [PubMed] [Google Scholar]
- 2.Bunge SA, Wright SB. Neurodevelopmental changes in WM and cognitive control. Curr Opin Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 4.Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial WM. Psychiatry Res. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapert SF, Schweinsburg AD, Drummond SP, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009;92:559–565. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Reviews. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: An overview of animal and human research. Current Drug Abuse Reviews. 2008;1:114–123. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- 10.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Giedd JN, Clasen LS, Lenroot R, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;25:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez de FF, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Jager G, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Long-term effects of frequent cannabis use on WM and attention: an fMRI study. Psychopharmacology (Berl) 2006;185:358–368. doi: 10.1007/s00213-005-0298-7. [DOI] [PubMed] [Google Scholar]
- 14.Jager G, Van Hell HH, De Win MM, et al. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol. 2007;17:289–297. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin RS, Darwin WD, Chiang CN, Shih M, Li SH, Huestis MA. Urinary elimination of 11-nor-9-carboxy-delta9-tetrahydrocannnabinol in cannabis users during continuously monitored abstinence. J Anal Toxicol. 2008;32:562–569. doi: 10.1093/jat/32.8.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Raalten TR, Ramsey NF, Jansma JM, Jager G, Kahn RS. Automatization and WM capacity in schizophrenia. Schizophr Res. 2008;100:161–171. doi: 10.1016/j.schres.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 17.Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- 18.Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial WM in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Quinn HR, Matsumoto I, Callaghan PD, et al. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- 21.Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26:309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- 22.Nestor L, Roberts G, Garavan H, Hester R. Deficits in learning and memory: parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage. 2008;40:1328–1339. doi: 10.1016/j.neuroimage.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol Rev. 2007;17:347–361. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- 24.Banich MT, Crowley TJ, Laetitia L, et al. Brain activation during the Stroop task in adolescents with severe substance and conduct problems: A pilot study. Drug Alcohol Depend. 2007;90:175–182. doi: 10.1016/j.drugalcdep.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov I, Schulz KP, London ED, Newcorn JH. Inhibitory control deficits in childhood and risk for substance use disorders: a review. Am J Drug Alcohol Abuse. 2008;34:239–258. doi: 10.1080/00952990802013334. [DOI] [PubMed] [Google Scholar]
- 28.Schepis TS, Adinoff B, Rao U. Neurobiological processes in adolescent addictive disorders. Am J Addict. 2008;17:6–23. doi: 10.1080/10550490701756146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solowij N, Michie PT. Cannabis and cognitive dysfunction: parallels with endophenotypes of schizophrenia? J Psychiatry Neurosci. 2007;32:30–52. [PMC free article] [PubMed] [Google Scholar]
- 30.D'Souza DC, bi-Saab WM, Madonick S, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Rey JM, Martin A, Krabman P. Is the party over? Cannabis and juvenile psychiatric disorder: the past 10 years. J Am Acad Child Adolesc Psychiatry. 2004;43:1194–1205. doi: 10.1097/01.chi.0000135623.12843.60. [DOI] [PubMed] [Google Scholar]
- 32.Weiser M, Davidson M, Noy S. Comments on risk for schizophrenia. Schizophr Res. 2005;79:15–21. doi: 10.1016/j.schres.2005.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.