Abstract
Type IV pili are bacterial extracellular filaments that can be retracted to create force and motility. The retraction is accomplished by the motor protein PilT. Crystal structures of Pseudomonas aeruginosa PilT with and without bound AMP-PCP have been solved at 2.6 and 3.1 Å resolution, respectively, revealing an interlocking hexamer formed by the action of a crystallographic 2-fold symmetry operator on three subunits in the asymmetric unit and held together with extensive ionic interactions. The roles of two invariant carboxylates, Asp Box motif Glu163 and Walker B motif Glu204, have been assigned to Mg2+ binding and catalysis, respectively. The nucleotide ligands in each of the subunits in the asymmetric unit of the AMP-PCP bound PilT are not equally well ordered. Similarly, the three subunits in the asymmetric unit of both structures exhibit differing relative conformations of the two domains. The 12° and 20° domain rotations indicate motions that occur during the ATP-coupled mechanism of disassembly of pili into membrane-localized pilin monomers. Integrating these observations, we propose a three-state Ready, Active, Release model for the action of PilT.
Keywords: Type IV pili, Motor protein, P-loop ATPase, Crystallography
Introduction
Type IV pili (Tfp) are extracellular appendages present in a wide variety of Gram-negative and some Gram-positive bacteria including pathogens of plant, fungi and animals, soil-dwelling bacteria, and extremophiles1; 2. While not necessary for viability, Tfp play an important role in the lifestyle of many bacteria by participating in biofilm formation, cell adhesion, phage uptake, DNA uptake, and a type of flagellar-independent surface motility often called twitching motility3. Thus, understanding the mechanisms that power pilus assembly and disassembly will yield significant information about how bacteria use pili for virulence and about the mechanisms of molecular motors. Gene products that control Tfp regulation, assembly, and disassembly have been identified and their roles characterized in several model organisms1; 4. The three-dimensional structure of the pilus filament has been studied using multiple biophysical techniques5 and recent work has begun to elucidate the interactions of conserved membrane proteins at the base of the pilus6.
The force generated by the retraction of a single pilus is over 100 pN7, and the retraction of a Tfp bundle leads to nN forces8. PilT, the homohexameric machinery that powers pilus retraction, is thus the strongest biological motor known. PilT belongs to a family of secretion ATPases that are conserved in the Type IV pili, Type II secretion, and Type IV secretion systems and defined by four signature sequences. The Walker A and B motifs are canonical in P-loop ATPases, a group which includes motor proteins F1-ATP synthase, myosin, RecA, and many helicases. The Asp and His boxes are motifs unique to the secretion ATPase family. As the name implies, the Asp box contains two conserved carboxylic acid residues. These are likely involved in coordinating the active site geometry. The His box contains two namesake histidine residues, and substituting one of these residues leads to loss of PilT activity in vivo9; 10.
The structures of five secretion ATPases are known9; 11; 12; 13; 14. All share a bi-lobed architecture consisting of a PAS-like N-terminal domain (NTD) joined by a flexible linker to a RecA fold C-terminal domain (CTD). The ATP binding site lies between the two domains, with all four of the signature motifs found near the nucleotide in the CTD. We have previously reported the structure of PilT from Aquifex aeolicus9, a hyperthermophile whose PilT is 51% identical to P. aeruginosa PilT. That structure highlighted another invariant feature of PilT and other secretion ATPases; a pair of clamping arginines at the tips of NTD β5 and β6, which interact with the phosphates of ATP and drive conformational change in the motor9; 14. In order to understand the detailed steps of pilus retraction we have now solved both the apo and ligand-bound structures of PilT from P. aeruginosa, an important and experimentally amenable pathogen with well-characterized Type IV pili.
Results
Tertiary and quaternary structure of AMP-PCP bound PilT
P. aeruginosa PilT (PaPilT) was crystallized in the presence of the non-hydrolyzable ATP analog β,γ-methyleneadenosine 5′-triphosphate (AMP-PCP). The PaPilT structure was solved by molecular replacement using Aquifex aeolicus PilT (AaPilT) as a model, and refined to 2.6 Å resolution (Table 1). The structures presented in this paper are the first of a retraction motor with relevance to in vivo pathogenicity. Unlike the previously solved AaPilT structures 9, the PaPilT structure has active sites that are in the correct conformation to bind and hydrolyze nucleotide as well as a sufficiently high resolution to warrant interpretation of side chain and ligand positions. The three subunits in the asymmetric unit (A, B, and C) conform to the two-domain structure (Figure 1a) and application of the crystallographic 2 fold axis forms the biologically relevant hexamer (Figure 1b). As expected each domain maps well onto the AaPilT structure, with r.m.s.d. 1.2 Å or 1.3 Å for superposition of the N- or C-terminal domain, respectively. The NTD (1–98) is a 6-stranded anti-parallel β-sheet flanked on one side by 3 α-helices. The CTD (105–344) is a curved 7-stranded β-sheet sandwiched between α-helices. Strong electron density for the extended β-hairpin between anti-parallel β–strands 12 and 13 is only observed for the C subunit. One of these is the AIRNLIRE helix, which is necessary for pilus retraction in vivo15 (Figure 1a).
Table 1.
Data collection and refinement statistics
| Data Collection | AMP-PCP bound PilT | Unliganded PilT |
|---|---|---|
| Wavelength (Å) | 0.900 | 0.979 |
| Space group | C2221 | C2221 |
| a, b, c (Å) | 108.5, 119.6, 185.5 | 108.2, 121.4, 184.4 |
| Resolution (Å) | 30.0–2.60 (2.69–2.6) | 30.00–3.10 (3.21–3.1) |
| Unique reflections (#) | 35387 (3452) | 21079 (1652) |
| Rmerge | 0.052 (0.422) | 0.098 (0.384) |
| I/σI | 32.6 (2.8) | 17.3 (3.1) |
| Completeness (%) | 94.4 (93.6) | 94.9 (76.1) |
| Redundancy | 7.4 (4.1) | 7.0 (5.6) |
| Wilson B-factor (Å2) | 68.8 | 59.8 |
| Refinement | ||
| Resolution | 29.1–2.60 (2.66–2.60) | 23.3–3.10 (3.26–3.10) |
| Reflections | 33594 (2319) | 20121 (2376) |
| Rwork/Rfree | 0.244/0.291 (0.352/0.416) | 0.229/0.282 (0.331/0.442) |
| No. atoms | 7939 | 7643 |
| Protein | 7741 | 7686 |
| Ligand/ion | 109 | 15 |
| Water | 89 | 4 |
| B-factor (Å2) | 61.0 | 63.6 |
| AMP-PCP (A/B/C) | 70.4/60.9/70.7 | n/a |
| RMS deviations | ||
| Bond lengths (Å) | 0.010 | 0.014 |
| Bond angles (°) | 1.434 | 1.477 |
| Ramachandran | ||
| Most favored | 95.8% | 86.3% |
| Outliers | 0.41% | 0.82% |
| ESU (maximum likelihood) | 0.30 Å | 0.42 Å |
Parentheses indicate highest resolution shell.
FIGURE 1. The structure of AMP-PCP bound PilT.

a) AMP-PCP (yellow carbons, blue nitrogens, red oxygens, orange phosphates) is bound at the interface of the two domains, surrounded by conserved motifs of the secretion ATPases (Walker A, α6, red; Walker B, β10, blue; Asp Box, β8, yellow; His Box, β11, orange; all shown on subunit B). AMP-PCP is distant from the AIRNLIRE helix required for retraction (cyan).
b) The PilT hexamer, looking down the crystallographic 2-fold axis with the N-terminal domains oriented towards the reader (subunit A, green; B, blue; C, purple; the second subunit C is grey to match the subunit shown in panel a; ligands colored as in panel a).
PilT forms a closed hexameric toroid in the crystal lattice with an outer diameter of 115 Å and height 60 Å (Figure 1b). The inner pore is 40 Å in diameter at the N-terminal opening and tapers to a 13.1 Å outlet constricted by Glu247, located on a loop between α9 and α10. Overall, the hexamer surface displays patches of positive, negative and neutral character including some exposed hydrophobic side chains which are not buried by crystallization (Suppl. Figure 1A). For example, NTD side chain Phe9 is on the outside of the hexameric torus while Phe66 faces into the lumen (Suppl. Fig. 1A). Extensive subunit interlocking forms the toroid, with each subunit contributing 3,000–3,100 Å2 of buried surface area. The major surface (~2,000 Å2 total per interface) is CTDn:NTDn+1 packing, including mainly ionic side-chain interactions, few hydrophobic interactions and 2 cation-π interactions (Arg176 of CTDn to Phe86 NTDn+1 and Tyr152 of CTDn to Arg35 from NTDn+1). The additional CTD:CTD interface contributes ~1,000 Å2 total per interface.
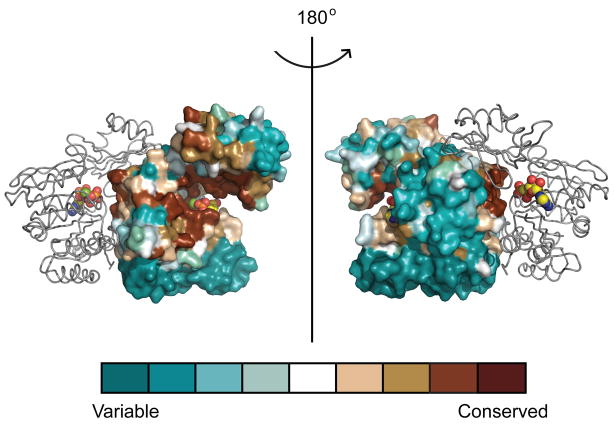
A sequence alignment of PaPilT with other secretion NTPases was mapped onto the surface of PilT in order to highlight conserved structural elements across the secretion ATPase family (Figure 2)16. The most conserved amino acids lie at or near the tripartite ATP active site formed in the cleft between the NTDN and CTDN with additional contributing residues from the back side of the adjacent CTDN−1 (Figure 2). The least conserved residues are located in the far CTD α-helices, an observation which indicates this area can be used to distinguish PilT from other assembly ATPases, such as PilB, or secretion proteins such as GspE. Only a few of the buried surface contacts are highly conserved among these 27 secretion ATPases (Figure 2).
FIGURE 2. Conservation of PilT residues among secretion ATPases.
Among 27 secretion ATPases, as mapped onto PilT, the most conserved residues (reddish-brown) are in the active site cleft or in proximity to the active site cleft of the neighboring subunit (grey ribbons). The least conserved residues (deep teal) are located on the periphery of the subunits. The active site ligands are colored as in Figure 1.
The active site configuration of the nucleotide-bound PilT
In each tripartite NTDN-CTDN:CTDN−1 nucleotide binding site, there was strong electron density for an AMP-PCP molecule and a Mg2+ ion from the beginning of the refinement process. The presence of these ligands allows us to extend current understanding of the roles of each of the four conserved secretion ATPase motifs, which are known to be essential for function 9; 10; 15. The canonical Walker A phosphate (P−) loop residue Lys136 is pointed directly at the γ-phosphate, coordinating the ligand at a distance of 2.8 – 3.6 Å (Figure 3a). The backbone nitrogens of the P-loop coordinate the β- and γ-phosphates. Walker A residue Ser137 makes a short interaction with the Mg2+ ion.
FIGURE 3. The nucleotide binding site and the His Box of PilT.
a) Wall-eyed stereo view of the active sites of liganded (blue subunit B, green subunit A, grey Mg2+, red water) and apo (light blue subunit B, light green subunit A) PilT, aligned using the conserved RecA fold of the CTD (residues 106–301). In addition to residues discussed in the text, Arg276 coordinates the ribose while Leu109 and Leu268 sandwich the adenine moiety of the AMP-PCP. The Fo−Fc omit map was calculated from the ligand bound structure without AMP-PCP or Mg2+ ion and is contoured at 3σ.
b) Thr220 and His222 (green, subunit A) and Thr132 and His229 (blue, subunit B) form a 3D His Box in the crystal structure.
In all three subunits, carboxylic acid oxygens of the Asp Box Glu163 interact directly with the well-ordered Mg2+ ion (Figure 3a). No other carboxylate side chain is in a position to fulfill this role, thus conclusively demonstrating the Mg2+ binding role of this Asp Box residue and eliminating the possibility that a side chain from the nebulous Walker B motif is directly involved in Mg2+ binding. In our earlier A. aeolicus PilT structure, the proximity of this residue to the ATP was clear, but we were unable to distinguish between a catalytic role and a Mg2+ binding role. Undoubtedly, this assignment of the Asp Box glutamate as a Mg2+ interacting residue is also appropriate for other secretion ATPases14.
The role of the long Walker B motif, which traverses the entire CTD, is not well understood for the secretion ATPases, and no results are available that suggest a role other than a structural one for the first ten amino acids of this 13 amino acid sequence. Surprisingly, weak density for the last four side chains of the Walker B motif, those closest to the nucleotide, indicates they are disordered, potentially because of solvent exposure and/or the non-biological substrate in our crystals. Nonetheless, the active site carboxylic acid closest to the γ-phosphate and in an appropriate orientation to attack and activate a water molecule close to the γ-phosphate is residue Glu204. Substitution of this side chain abolishes ATPase activity10 and we would predict this effect is largely due to an effect on kcat.
The two His Box histidines from a single subunit are not near each other in three dimensions. Although His229 approaches the γ-phosphate of the nucleotide in the binding pocket of the subunit in which it is found (3.9 Å away), His222 is closer to the nucleotide from the adjacent subunit (8.4 Å from the γ-phosphate) (Figure 3b). These two histidines are 8.1 Å from each other across the subunit interface. Indeed, a pair of ionic interactions from His229N to Thr220N−1 and from His222N−1 to Thr132N (part of the P-loop) do make a three-dimensional Histidine Box across the CTDN:CTDN−1 interface (Figure 3b).
The three ligand binding sites are not equivalent
Several conserved residues in the active site vary in conformation between subunits, with subunit B most poised to hydrolyze ATP. The greatest differences are found in the NTD clamp arginines, Arg82 and Arg97, which coordinate the γ-phosphate. Substituting either of these residues leads to an abrogation of PilT function in vivo9. In subunit A, Arg82 and Arg97 approach the γ-phosphate (5.0 and 5.2 Å respectively) (Figure 4a). In subunit B, they are locked onto the nucleotide. Arg82 makes two salt bridges to the γ-phosphate (3.1 and 3.3 Å) and Arg97 is 4.3 Å away (Figure 4b). In subunit C the clamp arginines are swung away from the nucleotide at distances of 9.0 and 4.5 Å (Figure 4c).
FIGURE 4. Ready, Active, Release conformations of the clamp arginines of the 3 PilT subunits.
a) Arginines 82 and 97 of subunit A are in a Ready conformation to bind the nucleotide. (Colors as in Fig. 1b and 3). b) The clamp arginines of subunit B are in an Active conformation, coordinating the γ-phosphate of the nucleotide. c) The clamp arginines have Released their hold on the nucleotide. d) A cartoon schematic representing the orientation of the NTDs with respect to the superimposed RecA CTDs. (Green, subunit A; blue, subunit B; purple, subunit C.)
His229 is further from the γ-phosphate in subunits A and C (4.5 or 4.4 Å) than in subunit B (3.9 Å). The close approach in subunit B could be part of a His Box mediated intersubunit communication, and is consistent with a proposed role as a phosphate sensor10. In addition to coordination across subunits, this closely-approaching His229 might contribute directly to the catalytic hydrolysis of ATP, as has been proposed for the “lynchpin Histidine” of the ABC transporter family17. Similarly, the carboxylate oxygens of Glu163 are further from the Mg2+ ion in subunits A and C (3.0 and 3.4 Å; 3.1 and 4.1 Å) than in subunit B (3.0 and 3.0 Å).
The ligands themselves have noteworthy differences among the subunits. A, B and C nucleotide and Mg2+ have accessible surface areas of 109.9, 71.7 and 91.5 Å2 and average B-values assuming full occupancy of 70.4, 60.9, 70.7 Å2, respectively. Thus, the ligands of subunit B are the most engaged inside the binding cleft. The clamp arginines in subunit C are swung away from the AMP-PCP, suggesting that they guide inorganic phosphate away from the ATP binding site towards the lumen of PilT. The hydrolyzed nucleotide can diffuse out of the highly solvent accessible active site, the same way it likely came in (Suppl. Fig. 1a).
Key differences between nucleotide bound and unliganded PilT structures
To assess the potential differences between nucleotide-bound and unliganded forms of the pilus retraction motor, PilT was purified and crystallized in the absence of nucleotide and Mg2+, yielding crystals under similar conditions and of the same space group and unit cell as the AMP-PCP form. Comparing the nucleotide-binding sites of the AMP-PCP bound B subunit and the Apo-PilT B subunit identifies key functional residues (Figure 3). The clamp residues Arg82 and Arg97 vary most between the two structures. Glu163 has also changed positions in the unliganded structure presumably due to the lack of Mg2+. The conformation of the P-loop and positioning of the catalytic Lys136 remain similar. His222 within the 3D His Box undergoes a conformational twist of 30° in A, 45° in B, and 0° in C. The β12-β13 hairpin that shelters the adenine moiety (Fig. 4a-c) is, paradoxically, less well-ordered in the nucleotide-bound than in the apo structure.
The unliganded hexamer has similar domain conformations to the AMP-PCP bound PilT toroid and the same overall outer dimensions. The calculated Rg is unchanged (34.6 Å for the Apo PaPilT hexamer and 34.5 Å for the AMP-PCP PaPilT hexamer18). To a first approximation, the openness of the PilT subunits is thus an inherent property of the hexameric structure rather than a consequence of nucleotide binding. At a more detailed level, local structural changes are manifest that could be significant for ATP hydrolysis and subunit:subunit signaling. For example, in the AMP-PCP bound subunit B, Glu336 of the far C-terminal α12 is well-ordered and forms a salt bridge to Arg123 in the CTD of neighboring subunit A. However, α12 is not visible in the apo subunit B.
A striking difference between the two structures is the conformation of the central pore. The lumen opening is more oblong in the unliganded structure, with the already limited opening decreased. The distance from subunit A Glu247 main chain nitrogen to its symmetry mate across the lumen is 15.7 Å in ligand-bound PilT vs 13.2 Å in the apo case. Glu247 side chains are also closer to the center of the hexamer in the apo structure, such that the overall constriction decreases from 13.1 to 5.8 Å. This happens as α9 and α10 are translated toward the center along their own helical axes by about 1.5 Å (refer to Figure 1b).
Direction of force generated by PilT nucleotide binding
To further investigate the conformational changes of PilT that might be important for motor function, we compared relative domain orientations among chains A, B, and C19. While the sequences of the three subunits are identical, the structures are not. Subunit A is the most open, B is intermediate, and C is the most closed (Figure 4d). Accordingly, the domain motion required to move the A subunit NTD into a position equivalent to the C subunit conformation is 19.6° (Fig. 5a). The intermediate position of B’s NTD is on the trajectory from A to C, with an 11.7° rotation from A to B and an additional 9.4° rotation from B to C. The magnitude of these motions is on par with that observed in crystal structures of other secretion NTPases, such as GspE (PDB code 2OAP) which has a 21.8° rotation14 (Fig. 5b). PaPilT does not have the 70° domain rotations observed in the AaPilT structure, which may have been a result of crystal packing forces9.
FIGURE 5. Force generation by large domain motions among hexameric ATPase proteins.
ATP binding leads to large domain movements (red arrows) within the subunit (shown in light blue cartoon). Left panels show two separate hexamer views with each subunit individually colored. The zoom in is of a single subunit (light blue) illustrating the motions of the moving domain during ATP binding, with the RecA domains fixed. Secretion ATPases (a) PilT (Chain A apo and Chain C – AMP-PCP), (b) GspE (2OAP, 2OAQ) and (c) HP0525 (1NLY, 1NLZ) exhibit domain motions that are diagonal to the central ring axis, while (d) HslU (1G3I, 1DO2) and (e) F1-ATPase (1BMF) have domain motions which are parallel and perpendicular to the ring axis, respectively.
It has been postulated that within hexameric rings of the RecA superfamily of proteins the direction of force generation can be predicted relative to the azimuthal axis of the ring based on the orientation of the central β-strands of the RecA domain. In F1-ATPase and HslU, it was noted that the force vector that connects the centers of masses of the moving domains when the RecA domains are aligned and kept constant parallels the β-strands of the RecA domain20. F1-ATPase and HslU undergo domain motions of 30° and 20°, respectively, upon binding of nucleotide21; 22; 23. The F1-ATPase β–strands are oriented nearly perpendicularly to the azimuthal ring axis, with the plane of the β sheet matching the flat plane of the hexamer, and the direction of force generation is also within this plane (Fig. 5d). The resulting motion spins the central stalk of the rotary motor in the plane of the membrane. HslU’s canonical RecA β-strands are oriented almost parallel to the ring axis, and HslU generates force parallel to the ring axis (Fig. 5d), as needed for its role in pushing polypeptide chains through its central pore and delivering them to be degraded by the HslV protease24. The RecA domain β-strands of the PilT subunits are arrayed in the third possible orthogonal orientation; the strands are again perpendicular to the ring axis but unlike F1-ATPase the β sheet climbs along the azimuthal axis (Figure 5a). This is also the case for secretion ATPases GspE and HP0525 (Fig. 5b,c). The force vector observed for these secretion ATPases when keeping superimposed RecA domains fixed and aligning the NTDs is diagonal to the ring axis. This diagonal force generation during the closing of the NTD onto the nucleotide causes the NTD to push on the CTD of the adjacent subunit (Supplementary movie S1). Although this motion does not follow the elementary predictive rule, it is indeed the sum of the first two force vectors, and the direction of this vector does match the required movements for the biology. In the simplest model for PilT motion, pilin subunits must be moved coordinately from their position in the pilus filament down the central axis toward the membrane and away from the axis into the plane of the membrane.
Discussion
Ready, Active, Release Motor
Based on the differing geometries of the three PilT subunits with respect to the ATP binding site, we propose the “Ready, Active, Release” model for PilT action (Figure 4D). Beginning with the Ready or open, unliganded state (represented by subunit A), ATP binding forces the N- and CTDs to approach each other as the clamp arginines interact with the phosphates. This is the largest conformational change during the PilT motor cycle, and results in the Active conformation for ATP hydrolysis (represented by subunit B). Subunit C represents the Release or closed, post-hydrolysis conformation. Unlike the rotary motor of the F1F0 ATPase, which depends on the sequential actions of the Binding Change Model25, we would predict the PilT subunits can act stochastically, as there is no reported rotary motor mechanism. With the simplifying assumption that PilT interacts directly with pilin subunits, one can imagine that any stabilizing interaction that prevents the pilus from slipping backwards while a second motor subunit binds to a subsequent pilin monomer and undergoes ATP-dependent domain rotation would lead to retraction. This model does not preclude sequential binding as the most efficient mode of action of the motor, possibly generating greatest forces and/or speed 26. A recent study on the dynamics of Tfp retraction by Clausen and coworkers showed that pilus retraction velocity is bimodal, and that the elementary steps of pilus retraction are less than 3 nm and therefore potentially correspond to a single pilin subunit26; 27. The net displacement of the PaPilT NTD during the domain rotation from the Ready to the Active subunit conformation is on the order of 0.8–1 nm, and the rise between pilin subunits in an assembled Type IV pilus is similarly on the order of 1 nm5. If the interaction between PilT and pilin is direct, then Clausen and coworkers’ conclusion is consistent with 1 ATP hydrolysis event by PilT leading to the disassembly of 1 pilin subunit. How might the pilus retraction motor be able to function in a low velocity and a high velocity mode? One possibility is that the high velocity mode comes from coordinated PilT activity in which all six subunits are engaged and presumably firing sequentially around the hexamer while the low velocity mode is the result of a random firing of PilT subunits. There is precedent in other ATP-driven machines, the ClpX protein translocation motor and the GroEL chaperone, to function when only a subset of the subunits have native ATP or protein binding sites, respectively28; 29.
Potential mechanism of PilT-driven pilus disassembly
How PilT couples large domain motions to type IV pilus retraction is a key question. PilT utilizes its NTD to localize to the inner membrane 30. While hydrophobic residues are typically not found on the surface of a protein, conserved Phe9 and (non-conserved) Phe66 are surface exposed residues that could be involved in interactions with lipids, an inner membrane protein dock and/or pilin itself. Pilin subunits cannot disassemble by passage through PilT, as the opening at the base is not large enough to accommodate a pilus filament nor is it logical to expect an entire pilus to retract into the cytoplasm. Rather, pilus retraction is a reversible disassembly process resulting in membrane-spanning pilin monomers.
A direct model in which PilT engages pilin α-helical tails and shuttles subunits into the inner membrane along a vector that follows the diagonal path from the base of the filament to the inner membrane is conceivable, and the relative sizes of PaPilT and full length pilin subunits are appropriate for this model. The change in ligand-bound vs. apo PilT lumen diameter suggests the lumen could change conformation to accommodate pilin tails during retraction. We hypothesize a paddle-wheel mechanism for retraction, in which the PilT lumen loops or NTD structural elements engage the exposed helical N-terminal tail of pilin (Fig. 6a), and pull on it directly as the Open subunit NTD swings toward the neighboring CTD upon ATP binding and transition to the Active state. This model is consistent with a growing appreciation that other molecular machines including SecY, HslU, and ClpX use a paddle-wheel mechanism to push or pull unfolded proteins through protein channels or across membranes31; 32; 33. There are serious disadvantages to this direct interaction model, though, including no demonstrated interaction between pilin and PilT, and the likelihood that the pilin N-terminal helix does not extend far enough across the inner membrane to interact extensively with PilT (Fig. 6a).
FIGURE 6. Schematic model for pilus retraction.
(A) If PilT were to act on pilin directly, the NTD of PilT (blue) would contact the N-terminal tail of the bottom-most pilin subunit (red, modeled with a PilT-induced kink at proline 22) in a Type IV pilus filament (2HIL27) undergoing disassembly across the inner membrane and guided through the outer membrane by the PilQ secretin (brown). (B) More likely, inner membrane proteins are also involved in the pilus retraction pathway, and the force generated upon domain closure by PilT is transferred through an inner membrane protein to the pilin subunits, thereby providing the energy needed to disrupt hydrophobic and polar pilin:pilin interactions. The association of PilT with any particular inner membrane protein remains an unproven hypothesis. Shown are one pilus filament, one PilM, a heterodimer of PilN:PilO 6 and one PilC (for simplicity; PilC is likely a dimer 45). Based on membrane topology predictions 46 for the 406 residue P. aeruginosa strain PA01 inner membrane protein PilC, we have represented PilC with three full transmembrane helices and two stubby membrane-embedded reentrant helices. This prediction should be viewed with caution, as other publicly available topology analysis algorithms 47 yield varying results.
Given the high conservation of proteins required for Tfp assembly and retraction, a model in which PilT interacts directly with a conserved inner membrane platform protein (IMPP) such as PilN, PilO, or PilC (Fig. 6b) is a likely alternative to the direct interaction model. In such a scenario, PilT’s action on the IMPP would alter the relative orientation of this protein with the lowest pilin subunits and the IMPP would act on pilin to remove it from the filament and translate it into the membrane. Recent results describing a stable PilN:PilO heterodimer with a coiled-coil region in the periplasm 6 hint at a mechanism in which a short conserved cytoplasmic region of PilN interacts directly with PilT, and force is transmitted across a long transmembrane helix and coiled-coil lever arm to the periplasm, where PilN:PilO could act directly on pilin subunits. A scissoring motion of PilN and O could be readily reversible and transmit force signals over long distances. It is also possible that the multipass inner membrane protein PilC plays a role in retraction, as its homolog in the enteropathogenic E. coli type IVb Bundle Forming Pilus pathway interacts with the two secretion ATPases in that system 34, although PilC and its homologues in Tfp-a systems behave differently than those in the Tfp-b 35. In this scenario, PilC’s large cytoplasmic N-terminal region would interact with PilT (potentially with the AIRNLIRE motif) and force would be transmitted through this domain via PilC’s inner membrane helices to pilin’s N-terminal membrane helix to act as a wrench or pliers to remove the bottom-most pilin subunit from the filament. The action of short helical hairpins within the lipid bilayer during uptake by the glutamate transporter 36 suggests there could be a function for short hydrophobic stretches not long enough to be complete transmembrane helices in the predicted membrane topology for PilC.
Materials and Methods
Protein expression and purification
The pilT gene was cloned from P. aeruginosa PA103 genomic DNA using the primers 5′-GGGAATTCCATATGGATATTACCGAGCTGCTCGCCT-3′ and 5′-CCTTTGCGGCCGCTCAGAAGTTTTCCGGGATCTTCGCCTTC-3′ engineered with NdeI and NotI sites for cloning into a pET23a expression vector (Novagen). The primers 5′-CCCGGAAAACTTCGGATCCTGGCGCCGATCCGCCGCGC-3′ and 5′-GCGCGGCGGATCGGCGCCAGGATCCGAAGTTTTCCGG-3′ were used to change the stop codon to read through the C-terminal His tag by site directed mutagenesis to produce the final expression plasmid (pET23a-PaPilT-His). The cloning was verified by automated DNA sequencing (University of Wisconsin – Madison, DNA Biotechnology Sequencing Center).
For protein expression, pET-23a-PaPilT-His was transformed into BL21(DE3)pLysS chemically competent E. coli cells (Promega). Cells were grown at 37°C with shaking at 220 rpm in Luria-Bertani growth medium supplemented with 100 μg/mL ampicillin. The culture was induced with 1 mM IPTG at an OD600 of 0.6 and the temperature was dropped to 20°C for overnight protein expression. The following morning, the cells were harvested with a 15 minute 3,000 g centrifugation and pellets were stored at −80°C until ready for use. For purification, the pellet from 1 L of bacterial culture was freeze-thawed and resuspended in 30 mL Buffer A (50 mM Tris, 1 M sodium chloride, 5% glycerol, 40 mM imidazole, pH 7.1). The cells were lysed by sonication at 30% power for 2 minutes (15 seconds on/15 seconds off) pulses. The lysate was cleared with centrifugation at 76,000 g for 30 minutes. The cleared lysate was added to 2.7 mL of Ni-NTA resin (QIAgen) and allowed to bind for 1 hour at 4°C with gentle shaking. After batch binding, the slurry was added to an empty column (Bio-Rad) and allowed to filter by gravity. The flow through was discarded, and the column was washed with 10 column volumes of Buffer A, or until no remaining protein came off the column, as assayed by A280 absorbance. The protein was eluted with Elution Buffer (50 mM Tris, 5% glycerol, 1 M sodium chloride, 250 mM imidazole, pH 7.1), and samples were concentrated using a centrifugal filter device (Millipore). Purity and homogeneity were assayed by Coomassie-stained polyacrylamide gel electrophoresis (PAGE) and by dynamic light scattering (Protein Solutions, DynaPro). Conditions for optimum solubility were identified using a solubility screen 37, following which samples were dialyzed overnight at 5°C into Buffer 5 (50 mM sodium chloride, 50 mM 2-(N-Morpholino)-ethanesulfonic acid (MES), 50 mM sodium citrate, 5% glycerol, 5 mM magnesium chloride, pH 5.5).
Crystallization
The protein was concentrated to 26 mg/mL as determined by absorbance at 280 nm and using a calculated extinction coefficient 38. AMP-PCP, a non-hydrolyzable ATP analog (Sigma), was added to a concentration of 1.4 mM such that the molar ratio of PilT subunits to nucleotide was 1:2. The protein/nucleotide complex was incubated on ice for 20 minutes prior to setting up a sparse matrix crystallization screen (JSCG - Nextal). An initial crystallization hit was obtained in drops containing 2 μL protein and 2 μL mother liquor with 10% PEG 6000 and 100 mM Hepes, pH 7.5 in the well. Crystallization conditions were refined, and crystals measuring 0.4 × 0.2 × 0.15 mm were grown in 7 days at room temperature. Crystals were cryo-preserved with a slow transfer in 6 incremental steps from cryo-protection buffer A (10% PEG 6000, 100 mM HEPES pH 7.5, 50 mM sodium citrate, 50 mM sodium chloride, 10% glycerol, 1.4 mM AMP-PCP, 5 mM magnesium chloride) to cryo-protection buffer B (15% PEG 6000, 100 mM HEPES pH 7.5, 50 mM sodium citrate, 500 mM sodium chloride, 25% glycerol, 1.4 mM AMP-PCP, 5 mM magnesium chloride) over the course of 1 hour. Crystals were flash-cooled in liquid nitrogen. Unliganded PilT crystals were obtained in a similar method, except that nucleotide and magnesium chloride were not included in the crystallization or cryoprotection solutions.
Data Collection and Processing
A 2.6 Å resolution dataset and a lower resolution shorter exposure sweep were collected on one crystal at the Advanced Photon Source (APS) beamline 14-BM-C on an ADSC Quantum 315 detector. The unliganded PaPilT dataset was collected at APS at the LS-CAT 21-ID-D beamline on a Mar CCD detector. Each dataset was integrated, scaled and merged with HKL2000 (Otwinowski and Minor, 1997) (Table 1).
Structure determination and refinement
The 2.6 Å resolution structure of full-length nucleotide bound PaPilT was determined in space group C2221 with 3 monomers per asymmetric unit. Phases were obtained by molecular replacement with PHASER 39 using a non-biological subunit, the CTDn:NTDn+1 domains of A. aeolicus PilT (PDB code 2EWV), as the search model. Manual modeling and addition of waters were conducted in XFIT and Coot 40. Restrained refinement with isotropic TLS B-factor refinement and non-crystallographic symmetry (NCS) constraints was carried out with REFMAC v5.5 41. NCS sets included NTD residues 1–93 and CTD residues 103–342 and excluded ligands and linker regions. The β-hairpin region 270–277 in chain A, 272–277 in chain B, and loop 320–326 in chain A were not modeled due to poor density in these regions. No electron density for any of the His tags was observed. Interestingly, a strong region of positive Fo−Fc was identified at the crystal packing contacts of two non-crystallographically related hexamers. A citrate ion was refined into this density due to good fit of the size and shape of the density, the positively charged residues surrounding the pocket and the presence of citrate in crystallization conditions (Supplemental Figure 1).
The 3.1 Å resolution structure of full-length unliganded PaPilT was solved in the same space group, using initial phases derived from molecular replacement using the ligand-bound PaPilT CTDN:NTDN+1 structure. Initial TLS B-factors were also obtained from the AMP-PCP structure, and Refmac was run using overall B-factor refinement. As in the AMP-PCP bound structure, density for the far C-terminus was not well defined. A weakly bound ligand, tentatively assigned as chloride ion, was identified in the nucleotide binding site of subunits A and C by inspection of the 2Fo−Fc and Fo−Fc electron density maps.
Evaluation
Buried surface areas were calculated using the web-based program PISA 42 and analyzed using the Protein Interaction Calculator43. The AMP-PCP bound PilT, subunit B, structure was processed using the web-based version of ConSurf 16. The PilT sequence was aligned using default parameters with other secretion NTPase protein sequences (26 sequences in total) with an E value cut off of 0.001.
Structure figures were made with PyMOL44.
Supplementary Material
Acknowledgments
We thank the staff at the Advanced Photon Source, Beamlines 14-BMC and 21-ID-D for assistance with data collection. H. Adam Steinberg provided assistance with Figure 5. This work was funded by the NIH (RO1GM59721 to KTF).
Footnotes
Accession Codes
The AMP-PCP and unliganded P. aeruginosa PilT structures have been deposited in the Protein Databank with codes 3JVV and 3JVU, respectively.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mattick JS. Type IV pili and twitching motility. Annu Rev Microbiol. 2002;56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- 2.Varga JJ, Nguyen V, O’Brien DK, Rodgers K, Walker RA, Melville SB. Type IV pili-dependent gliding motility in the Gram-positive pathogen Clostridium perfringens and other Clostridia. Mol Microbiol. 2006;62:680–94. doi: 10.1111/j.1365-2958.2006.05414.x. [DOI] [PubMed] [Google Scholar]
- 3.Bradley DE. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can J Microbiol. 1980;26:146–54. doi: 10.1139/m80-022. [DOI] [PubMed] [Google Scholar]
- 4.Hansen JK, Forest KT. Type IV pilin structures: insights on shared architecture, fiber assembly, receptor binding and type II secretion. J Mol Microbiol Biotechnol. 2006;11:192–207. doi: 10.1159/000094054. [DOI] [PubMed] [Google Scholar]
- 5.Craig L, Li J. Type IV pili: paradoxes in form and function. Curr Opin Struct Biol. 2008;18:267–77. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampaleanu LM, Bonanno JB, Ayers M, Koo J, Tammam S, Burley SK, Almo SC, Burrows LL, Howell PL. Periplasmic domains of Pseudomonas aeruginosa PilN and PilO form a stable heterodimeric complex. J Mol Biol. 2009;394:143–59. doi: 10.1016/j.jmb.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 7.Maier B, Potter L, So M, Long CD, Seifert HS, Sheetz MP. Single pilus motor forces exceed 100 pN. Proc Natl Acad Sci U S A. 2002;99:16012–7. doi: 10.1073/pnas.242523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biais N, Ladoux B, Higashi D, So M, Sheetz M. Cooperative retraction of bundled type IV pili enables nanonewton force generation. PLoS Biol. 2008;6:e87. doi: 10.1371/journal.pbio.0060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satyshur KA, Worzalla GA, Meyer LS, Heiniger EK, Aukema KG, Misic AM, Forest KT. Crystal structures of the pilus retraction motor PilT suggest large domain movements and subunit cooperation drive motility. Structure. 2007;15:363–76. doi: 10.1016/j.str.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang P, Sampaleanu LM, Ayers M, Pahuta M, Howell PL, Burrows LL. Functional role of conserved residues in the characteristic secretion NTPase motifs of the Pseudomonas aeruginosa type IV pilus motor proteins PilB, PilT and PilU. Microbiology. 2008;154:114–26. doi: 10.1099/mic.0.2007/011320-0. [DOI] [PubMed] [Google Scholar]
- 11.Hare S, Bayliss R, Baron C, Waksman G. A large domain swap in the VirB11 ATPase of Brucella suis leaves the hexameric assembly intact. J Mol Biol. 2006;360:56–66. doi: 10.1016/j.jmb.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 12.Robien MA, Krumm BE, Sandkvist M, Hol WG. Crystal structure of the extracellular protein secretion NTPase EpsE of Vibrio cholerae. J Mol Biol. 2003;333:657–74. doi: 10.1016/j.jmb.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Yeo HJ, Savvides SN, Herr AB, Lanka E, Waksman G. Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system. Mol Cell. 2000;6:1461–72. doi: 10.1016/s1097-2765(00)00142-8. [DOI] [PubMed] [Google Scholar]
- 14.Yamagata A, Tainer JA. Hexameric structures of the archaeal secretion ATPase GspE and implications for a universal secretion mechanism. Embo J. 2007;26:878–90. doi: 10.1038/sj.emboj.7601544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aukema KG, Kron EM, Herdendorf TJ, Forest KT. Functional dissection of a conserved motif within the pilus retraction protein PilT. J Bacteriol. 2005;187:611–8. doi: 10.1128/JB.187.2.611-618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landau M, Mayrose I, Rosenberg Y, Glaser F, Martz E, Pupko T, Ben-Tal N. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 2005;33:W299–302. doi: 10.1093/nar/gki370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaitseva J, Jenewein S, Jumpertz T, Holland IB, Schmitt L. H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J. 2005;24:1901–10. doi: 10.1038/sj.emboj.7600657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svergun D, Barberato C, Koch MHJ. CRYSOL - A program to evaluate x-ray solution scattering of biological macromolecules from atomic coordinates. J Appl Crystallogr. 1995;28:768–773. [Google Scholar]
- 19.Lee RA, Razaz M, Hayward S. The DynDom database of protein domain motions. Bioinformatics. 2003;19:1290–1. doi: 10.1093/bioinformatics/btg137. [DOI] [PubMed] [Google Scholar]
- 20.Wang J. Nucleotide-dependent domain motions within rings of the RecA/AAA(+) superfamily. J Struct Biol. 2004;148:259–67. doi: 10.1016/j.jsb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–8. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 22.Bochtler M, Hartmann C, Song HK, Bourenkov GP, Bartunik HD, Huber R. The structures of HsIU and the ATP-dependent protease HsIU-HsIV. Nature. 2000;403:800–5. doi: 10.1038/35001629. [DOI] [PubMed] [Google Scholar]
- 23.Sousa MC, Trame CB, Tsuruta H, Wilbanks SM, Reddy VS, McKay DB. Crystal and solution structures of an HslUV protease-chaperone complex. Cell. 2000;103:633–43. doi: 10.1016/s0092-8674(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 24.Rohrwild M, Coux O, Huang HC, Moerschell RP, Yoo SJ, Seol JH, Chung CH, Goldberg AL. HslV-HslU: A novel ATP-dependent protease complex in Escherichia coli related to the eukaryotic proteasome. Proc Natl Acad Sci U S A. 1996;93:5808–13. doi: 10.1073/pnas.93.12.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyer PD. Catalytic site forms and controls in ATP synthase catalysis. Biochim Biophys Acta. 2000;1458:252–62. doi: 10.1016/s0005-2728(00)00077-3. [DOI] [PubMed] [Google Scholar]
- 26.Clausen M, Koomey M, Maier B. Dynamics of type IV pili is controlled by switching between multiple states. Biophys J. 2009;96:1169–77. doi: 10.1016/j.bpj.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig L, Volkmann N, Arvai AS, Pique ME, Yeager M, Egelman EH, Tainer JA. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol Cell. 2006;23:651–62. doi: 10.1016/j.molcel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Martin A, Baker TA, Sauer RT. Rebuilt AAA + motors reveal operating principles for ATP-fuelled machines. Nature. 2005;437:1115–20. doi: 10.1038/nature04031. [DOI] [PubMed] [Google Scholar]
- 29.Farr GW, Furtak K, Rowland MB, Ranson NA, Saibil HR, Kirchhausen T, Horwich AL. Multivalent binding of nonnative substrate proteins by the chaperonin GroEL. Cell. 2000;100:561–73. doi: 10.1016/s0092-8674(00)80692-3. [DOI] [PubMed] [Google Scholar]
- 30.Chiang P, Habash M, Burrows LL. Disparate subcellular localization patterns of Pseudomonas aeruginosa Type IV pilus ATPases involved in twitching motility. J Bacteriol. 2005;187:829–39. doi: 10.1128/JB.187.3.829-839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erlandson KJ, Miller SB, Nam Y, Osborne AR, Zimmer J, Rapoport TA. A role for the two-helix finger of the SecA ATPase in protein translocation. Nature. 2008;455:984–7. doi: 10.1038/nature07439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glynn SE, Martin A, Nager AR, Baker TA, Sauer RT. Structures of asymmetric ClpX hexamers reveal nucleotide-dependent motions in a AAA+ protein-unfolding machine. Cell. 2009;139:744–56. doi: 10.1016/j.cell.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koga N, Kameda T, Okazaki K, Takada S. Paddling mechanism for the substrate translocation by AAA+ motor revealed by multiscale molecular simulations. Proc Natl Acad Sci U S A. 2009;106:18237–42. doi: 10.1073/pnas.0904756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowther LJ, Anantha RP, Donnenberg MS. The inner membrane subassembly of the enteropathogenic Escherichia coli bundle-forming pilus machine. Mol Microbiol. 2004;52:67–79. doi: 10.1111/j.1365-2958.2003.03963.x. [DOI] [PubMed] [Google Scholar]
- 35.Pelicic V. Type IV pili: e pluribus unum? Mol Microbiol. 2008;68:827–37. doi: 10.1111/j.1365-2958.2008.06197.x. [DOI] [PubMed] [Google Scholar]
- 36.Reyes N, Ginter C, Boudker O. Transport mechanism of a bacterial homologue of glutamate transporters. Nature. 2009;462:880–5. doi: 10.1038/nature08616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins BK, Tomanicek SJ, Lyamicheva N, Kaiser MW, Mueser TC. A preliminary solubility screen used to improve crystallization trials: crystallization and preliminary X-ray structure determination of Aeropyrum pernix flap endonuclease-1. Acta Crystallogr D Biol Crystallogr. 2004;60:1674–8. doi: 10.1107/S090744490401844X. [DOI] [PubMed] [Google Scholar]
- 38.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–8. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr D Biol Crystallogr. 2004;60:432–8. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 40.McRee DE. XtalView/Xfit--A versatile program for manipulating atomic coordinates and electron density. J Struct Biol. 1999;125:156–65. doi: 10.1006/jsbi.1999.4094. [DOI] [PubMed] [Google Scholar]
- 41.Murshudov GN, Vagin AA, Lebedev A, Wilson KS, Dodson EJ. Efficient anisotropic refinement of macromolecular structures using FFT. Acta Crystallogr D Biol Crystallogr. 1999;55:247–55. doi: 10.1107/S090744499801405X. [DOI] [PubMed] [Google Scholar]
- 42.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Tina KG, Bhadra R, Srinivasan N. PIC: Protein Interactions Calculator. Nucleic Acids Res. 2007;35:W473–6. doi: 10.1093/nar/gkm423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeLano WL. The PyMOL molecular graphics system. DeLano Scientific; Palo Alto, CA, USA: 2002. [Google Scholar]
- 45.Karuppiah V, Hassan D, Saleem M, Derrick JP. Structure and oligomerization of the PilC type IV pilus biogenesis protein from Thermus thermophilus. Proteins. 78:2049–57. doi: 10.1002/prot.22720. [DOI] [PubMed] [Google Scholar]
- 46.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–80. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 47.Ter Horst R, Lolkema JS. Rapid screening of membrane topology of secondary transport proteins. Biochim Biophys Acta. 1798:672–80. doi: 10.1016/j.bbamem.2009.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.