Abstract
Purpose
Animal models with spontaneous epileptic seizures may be useful in the discovery of new antiepileptic drugs (AEDs). The purpose of the present study was to evaluate the efficacy of carisbamate on spontaneous motor seizures in rats with kainate-induced epilepsy.
Methods
Repeated, low-dose (5 mg/kg), intraperitoneal injections of kainate were administered every hour until each male Sprague-Dawley rat had experienced convulsive status epilepticus for at least 3 h. Five 1-month trials (n= 8–10 rats) assessed the effects of 0.3, 1, 3, 10 and 30 mg/kg carisbamate on spontaneous seizures. Each trial involved six AED-versus-vehicle tests comprised of carisbamate or 10% solutol-HS-15 treatments administered as intraperitoneal injections on alternate days with a recovery day between each treatment day.
Results
Carisbamate significantly reduced motor seizure frequency at doses of 10 and 30 mg/kg, and caused complete seizure cessation during the 6-h post-drug epoch in 7 of 8 animals at 30 mg/kg. The effects of carisbamate (0.3–30 mg/kg) on spontaneous motor seizures appeared dose dependent.
Conclusions
These data support the hypothesis that a repeated-measures, cross-over protocol in animal models with spontaneous seizures is an effective method for testing AEDs. Carisbamate reduced the frequency of spontaneous motor seizures in a dose-dependent manner, and was more effective than topiramate at reducing seizures in rats with kainate-induced epilepsy.
Keywords: Kainic acid, temporal lobe epilepsy, convulsive seizure
INTRODUCTION
About two dozen compounds are marketed as antiepileptic drugs (AEDs) worldwide, nine of which were developed since 1993. Nonetheless, 30 to 40% of patients with epilepsy remain ineffectively treated with the presently available AEDs (Bauer and Burr, 2001; Cole, 2004; Elger, 2003; Pitkanen and Sutula, 2002; Rogawski and Loscher, 2004; Sisodiya, 2003; Weiser, 2004). New methods of AED testing and development may identify treatments for this large population of pharmacoresistant patients. An important issue to consider while testing an AED is whether the animal model mimics the condition for which the drug is a potential therapy (i.e., clinical pathology and associated seizure type). Little research has been performed testing AEDs on animal models of acquired epilepsy. Rapid screens using acute seizure models such as pentylenetetrazol or maximal electroshock (i.e., chemically or electrically induced seizures in an otherwise normal animal) have been the preferred choice for animal testing and development of new AEDs (Loscher and Schmidt, 1988; White, 1997, 2003). Chronically epileptic rats with spontaneous seizures, although more labor-intensive to use, share similarities with human patients with temporal lobe epilepsy. These include: 1) the presence of spontaneous seizures, 2) histopathologic changes similar to hippocampal sclerosis (Margerison and Corsellis, 1966), and 3) molecular changes that may occur as a result of time-dependent epileptogenesis. Hypothetically, chronically epileptic rats will more successfully predict a potential AED’s clinical efficacy (Heinemann et al., 1994; Kupferberg, 2001; Leite et al., 2002; Levy et al., 2002; Loscher and Schmidt, 1988; Schmidt and Rogawski, 2002; Stables et al., 2002, 2003; White, 1997, 2003).
Carisbamate, an investigational AED with a unique anticonvulsant profile, has demonstrated broad-spectrum activity at non-toxic doses in rodent models of generalized and partial epilepsy (White et al., 2006; Novak et al., 2007; Francois et al., 2008). The main objectives of the present study were to evaluate the effect of carisbamate on the frequency of spontaneous motor seizures, and whether carisbamate completely blocks the seizures. The dose-dependent effects of carisbamate were assessed in relation to the measured concentration of carisbamate in the blood. Results for carisbamate were compared to those obtained for topiramate in a previous investigation (Grabenstatter et al., 2005). Overall, the aim of these studies was to provide “proof-of-principle” for the use of animal models of chronic epilepsy in the testing of new AEDs and to assess the efficacy of the novel AED, carisbamate, against spontaneous motor seizures.
MATERIALS AND METHODS
Kainate treatment and status epilepticus
Kainate (Ocean Produce International, Shelburne, Nova Scotia, Canada) was administered via repeated, low-dose, intraperitoneal injections (5 mg/kg) every hour until each adult Sprague-Dawley rat (180–200 g, n=8 per treatment series, 7 separate kainate treatments) experienced convulsive status epilepticus for >3 h (Hellier et al., 1998). Motor (i.e., convulsive) seizures were scored on a scale from III to V using the Racine scale (Racine, 1972). A class III seizure was characterized by forelimb clonus, an erect tail, and lordotic posturing, which could progress into a class IV seizure with continued forelimb clonus and rearing on hindlimbs. Animals that showed all of these behaviors in combination with a fall were defined as having a class V seizure, although class V seizures could include a fall without some of the other precipitating behaviors.
Following status epilepticus, rats were housed in an environmentally controlled vivarium (humidity 31–55%, temperature 21 to 22°C, 12-h light-dark cycle, lights on at 6:00 AM, food and water ad-libitum). All animals were directly observed for the occurrence of spontaneous seizures for 6 h/week, and animals with low seizure rates were not used in the experiments. The animals were used for the doses of 0.3, 1, 3, 10, and 30 mg/kg carisbamate at 212.6 ± 42.1, 176.6 ± 42.1, 297.9 ± 64.0, 315 ± 38.3, and 321.9 ± 42.8 days respectively after kainate-induced status epilepticus. The mean pre-study seizure rates for the animals included in the 0.3, 1, 3, 10, and 30 mg/kg carisbamate trials were 3.79 ± 0.74, 2.54 ± 0.58, 2.29 ± 0.73, 2.67 ± 0.80, and 2.59 ± 0.32 seizures per 6-h direct monitoring period, respectively, with hourly seizure frequencies ranging between 0.16667 and 2.66667 seizures per hour.
Repeated-measures, cross-over protocol and carisbamate administration
Five 1-month trials (n=8–10 rats) were conducted to assess the effects of 0.3, 1, 3, 10, and 30 mg/kg carisbamate on spontaneous convulsive seizures. Each trial involved six pairs of carisbamate and vehicle treatments administered as intraperitoneal injections on alternate days with a recovery day between each treatment day (Fig. 1). This paradigm controlled for changes in seizure frequency with time, and each animal served as its own control. Carisbamate was dissolved in 10% solutol-HS-15 (BASF, Ludwigshafen, Germany) with vigorous stirring in a warm water bath (40–60°C).
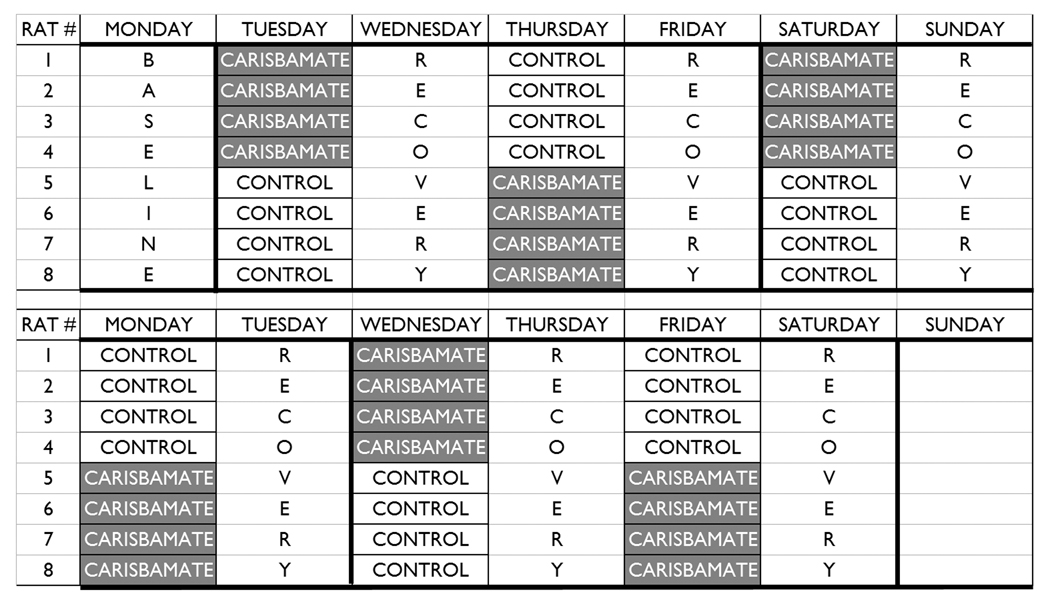
Fig. 1. Experimental protocol.
Carisbamate or 10% HS-solutol-15 was administered every other day for 24 days (e.g., Tuesday, Thursday, Saturday, Monday, Wednesday, and Friday). The protocol shown above was doubled in length to include six AED-versus-vehicle tests. Daily 24-h video recordings were made for the duration of the protocol. The tapes that contained the actual injection began 1 h before and ended 7 h after the injection (i.e., start tape at 8:00, inject at 9:00 A.M.) For the dose-response data, 6 h of data from each tape were analyzed to determine the maximal effect of each dose, starting 1 h after injection (i.e., 10:00 A.M – 4:00 P.M).
Continuous video-monitoring of spontaneous seizures
Each rat was continuously video-monitored for the entire experiment on 8-h videotapes by a Panasonic WV-BP334 black-and-white camera (G&G Technologies, Secaucus, NJ, USA). A trained technician, blinded to the treatments and dates, analyzed videotapes for any possible seizures. The Racine scale (Racine, 1972) was implemented to score seizure occurrence and severity during the first 6-h epoch (i.e., 10:00 a.m. to 4:00 p.m.) and a second 8-h epoch 15–23 h after drug administration (12:00 a.m. to 8:00 a.m.). Seizure frequency and severity were not analyzed for the first hour after intraperitoneal injection of AED to eliminate possible seizure-inducing effects of animal handling from the analysis.
Blood concentration analysis
In a separate series of experiments, the blood concentration of carisbamate was measured 4 h after injections of vehicle (i.e., 10% solutol-HS-15), 3 mg/kg carisbamate, 10 mg/kg carisbamate, and 30 mg/kg of carisbamate in a saline-control group (n=8) and in a group of rats with kainate-induced epilepsy (n=8). Therefore, each rat in the saline group and in the kainate group was treated accordingly (i.e., one of the four treatments was administered), and the blood was collected 4 h later. In a second series of experiments, the blood concentration of carisbamate was measured 24 h after injections of vehicle (10% Solutol-HS-15) and 30 mg/kg of carisbamate in saline-treated control rats (n=8) and in rats with kainate-induced epilepsy (n=8). In separator tubes with lithium heparin (Becton Dickinson and Company, Franklin Lakes, NJ, USA), 400 µl of whole blood was collected via tail vein. The tubes were inverted several times and iced immediately. From these samples, plasma (200 µl) was collected into Eppendorf tubes after centrifugation (3000 rpm at 4°C for 5 min). The plasma samples were frozen and stored at −80°C until shipment on dry ice for mass spectral analysis.
Statistical analyses
Due to asymmetrical (i.e., non-Gaussian) distribution and heterogeneous variance, seizure frequency data were analyzed in the log10 (y+0.1) scale. The inter- and intra-animal variability in seizure frequencies is likely explained by the occurrence of both seizure clusters and seizure rates equivalent to zero (detailed explanation of repeated-measures crossover protocol and statistical analysis has been published previously in Grabenstatter et al., 2005). To perform a log transformation and compare AED and vehicle treatments using a parametric statistical analysis (i.e., repeated-measures ANOVA), the addition of 0.1 to all seizure frequency values was required to account for the high occurrence of zeros in the data set. However, an artificial “floor effect” was created by the addition of 0.1 to all values, thus masking the effects of highly potent AED treatments (i.e., nearly complete seizure cessation). Therefore, the data were reanalyzed on the linear scale (i.e., sum of all seizures after individual treatments per total number of AED tests conducted) using a non-parametric test (i.e., the Wilcoxon signed-rank test), which does not assume a Gaussian distribution of the data (Grabenstatter et al., 2007). Potential significant differences between similar doses of AEDs were tested using an unpaired t-test with a Welch correction.
To evaluate possible alterations in seizure severity, an analysis of mean severity scores (Racine, 1972) was conducted during 6-h epochs following AED and vehicle treatments. The severity score was calculated as the ratio of the sum of severity scores per number of seizures, excluding epochs with no seizures. Significant differences in these severity scores were assessed using a Student’s t-test. In another analysis, the frequency of seizures in each severity class (Racine, 1972) during AED treatments was compared to the frequency of that severity class during the vehicle treatments (i.e., the frequency of class III seizures after a particular dose of carisbamate was compared to the frequency of class III seizures after vehicle). Significant differences in the frequency of class III, IV, or V seizures during AED treatment compared to vehicle treatment were determined using a Student’s t-test with an a priori Bonferroni adjustment for multiple comparisons. For blood analyses, the concentration of carisbamate was measured at 4 h after injections of 3 mg/kg, 10 mg/kg, and 30 mg/kg of carisbamate in a saline-control group and in a group of rats with kainate-induced epilepsy, and the differences were assessed with a Student’s t-test using an a priori Bonferroni adjustment. For a dose of 30 mg/kg carisbamate, a similar Student’s t-test was used to compare blood concentrations of vehicle- and kainate-treated animals at 4 h and 24 h.
RESULTS
Seizure frequency
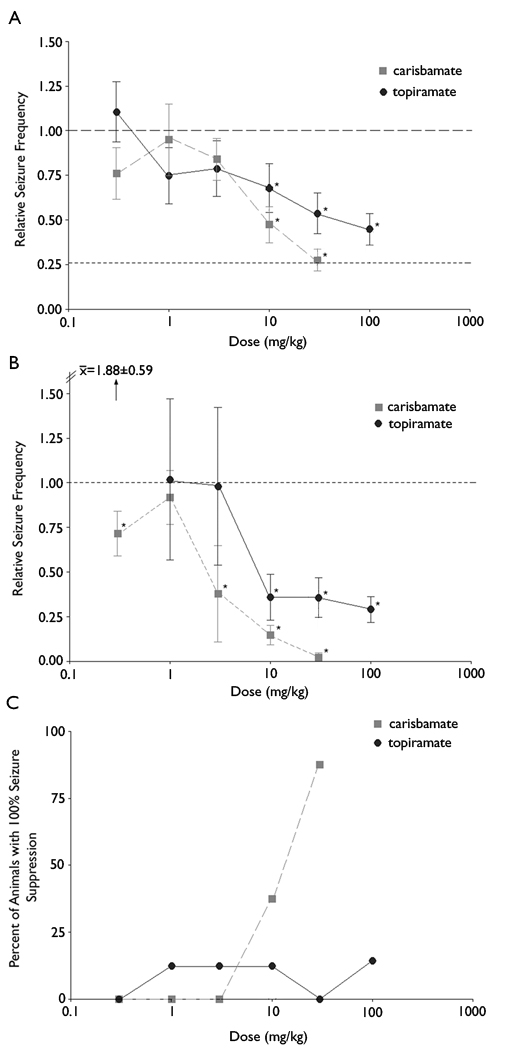
The first issue addressed in these experiments was the effect of carisbamate on the frequency of spontaneous convulsive seizures. Figure 2 shows the effect of different doses of carisbamate on the number of spontaneous seizures during the 6-h epoch after drug injection. For comparison, Figure 2 also shows similar results obtained previously for topiramate (Grabenstatter et al., 2005). Single intraperitoneal injections of 30 mg/kg carisbamate reduced relative seizure frequency by 74% during the first 6-h epoch, producing a relative seizure frequency of 0.26 (±0.06, p<0.0001). Using the same method and dosage, topiramate only reduced convulsive seizures by about half (i.e., relative seizure frequency = 0.51 ±0.20, p<0.0001). While both drugs significantly reduced relative seizure frequency when compared to vehicle at high doses, carisbamate exerted a more robust effect at 10 mg/kg (p = 0.0300) and 30 mg/kg (p = 0.0375) than topiramate. At 30 mg/kg, carisbamate was more effective at suppressing convulsive seizures than 100 mg/kg topiramate (p = 0.0230) following the parametric, repeated-measures ANOVA (Fig. 2A). Using the non-parametric statistical analysis on non-transformed data (i.e., the Wilcoxon signed-rank test), carisbamate (3, 10, and 30 mg/kg) also reduced seizure frequency in a dose-dependent manner. Using either statistical analysis, carisbamate was more effective than topiramate at 10 mg/kg and 30 mg/kg (Fig. 2B). However, interpretations of the results directly comparing potency of carisbamate and topiramate must be made with caution due to differing molecular weights of the two AEDs.
Fig. 2. Carisbamate reduced seizures more effectively than topiramate.
(A) Seizure frequencies were log-transformed [i.e., log10(y+0.1)] because the data set had a non-Gaussian distribution and was bound below by zeros. The transformation allowed the use of a parametric test (i.e., a repeated-measures ANOVA). Relative seizure frequencies (i.e., a ratio of seizure frequency following AED injection per seizure frequency following vehicle injection) in the log scale were back-transformed to the original linear scale for presentation of results. Carisbamate and topiramate both reduced relative seizure frequency. Because the log transformation necessarily involves the addition of 0.1 to all seizure frequencies, “relative seizure frequency” would have a discrete value even with a complete block of all seizures (Grabenstatter et al., 2007). The lower dashed horizontal line represents the estimated mean “floor effect” when absolute seizure block was simulated. In this and subsequent figures, the upper dashed horizontal line shows the baseline (i.e., no effect). (B) Because of the non-Gaussian distribution, the Wilcoxon signed-rank test (a non-parametric test) was used and also showed that carisbamate and topiramate significantly reduced seizure frequency at these doses. (C) The percent-of-animals showing complete seizure cessation during the first 6-h epoch following a single IP injection of AED were compared for carisbamate and topiramate. Vertical bars, ±SEM. Asterisks show significant differences (p<0.05).
Complete cessation of seizures
Another measure that may be predictive of clinical success for a potential new AED is the percentage of chronically epileptic animals with complete seizure cessation (i.e., 100% seizure suppression). Carisbamate completely blocked seizures in 3 of 8 animals at 10 mg/kg and 7 of 8 rats at 30 mg/kg. Topiramate only suppressed all spontaneous seizures in 1 of 8 animals tested in the 10 mg/kg trial and the 100 mg/kg trial, but did not completely block seizures in any of the animals at 30 mg/kg (Fig. 2C). Therefore, carisbamate was much more effective than topiramate at completely suppressing spontaneous convulsive seizures in rats with kainate-induced epilepsy.
Seizure severity
An important question is whether carisbamate reduced the severity of the remaining seizures (e.g., did the AED cause a shift from more-severe to less-severe seizures or did it preferentially block the more severe seizures?). Our previous analysis of the possible effect of topiramate on seizure severity (Grabenstatter et al., 2005) utilized intensity-weighted seizure number (i.e., the sum of severity scores) as a measure, and yielded apparent dose-dependent reductions in seizure severity. The use of intensity-weighted seizure number for analysis of possible drug effects on seizure severity, however, included drug-induced alterations in seizure frequency, which may have dominated the analysis and led to an apparent reduction in seizure severity when the primary effect was actually on seizure frequency. Using mean seizure severity (i.e., the ratio of the sum of severity scores per number of seizures, excluding epochs with no seizures) as a measure, carisbamate only reduced seizure severity at a dose of 10 mg/kg (p=0.0205). A possible effect of 30 mg/kg carisbamate on seizure severity could not be determined because this dose had such a large effect on seizure frequency (i.e., 7 of 8 animals had no seizures during the 6-h epoch after 30 mg/kg carisbamate). A re-analysis of the previous topiramate data (Grabenstatter et al., 2005) using mean severity score showed no effect on seizure severity at doses of 10 mg/kg (p=0.1056), 30 mg/kg (p=0.5860) and 100 mg/kg (p=0.2368). Therefore, although a small but statistically significant effect of seizure severity was found with 10 mg/kg carisbamate, topiramate and carisbamate at other doses had no detectable effect on the severity of behavioral seizures (i.e., independent of the overall drug effects on seizure frequency).
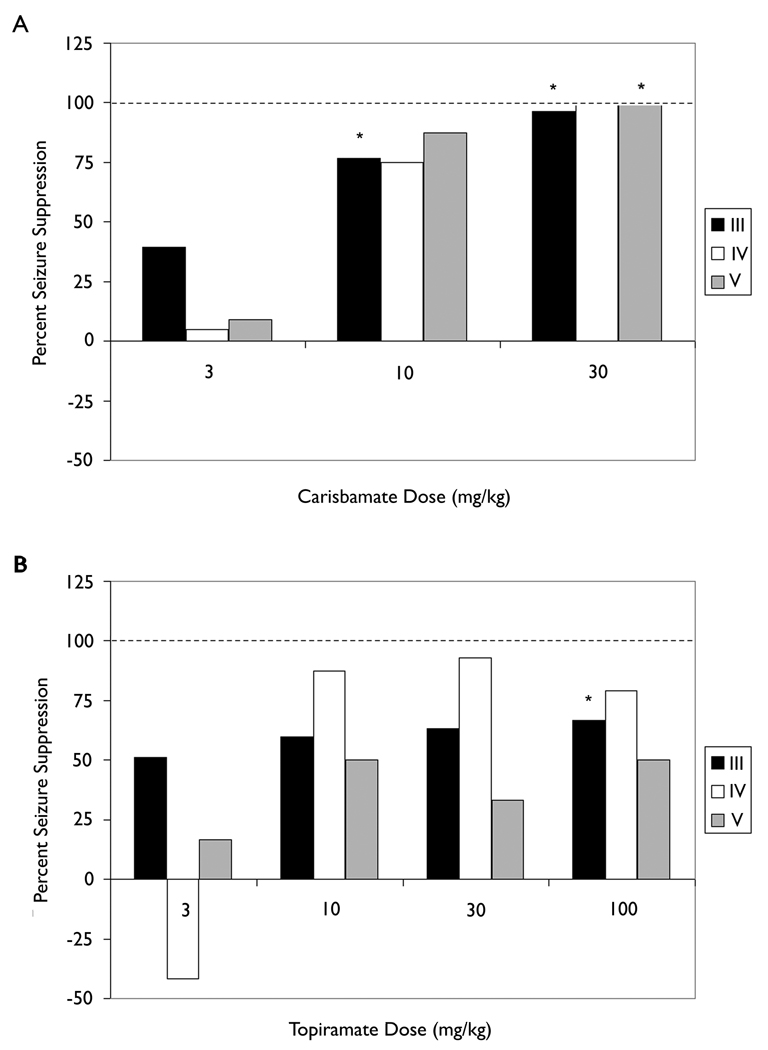
To assess further possible drug effects on seizure severity, the frequency of seizures of each severity class (Racine, 1972) during drug treatment (relative to vehicle treatment) was compared across doses. Figure 3A shows that carisbamate significantly reduced class III seizures at 10 mg/kg (p<0.05) and class III and class V seizures at 30 mg/kg (p<0.05), blocking all seizures of the latter type (i.e., tonic-clonic seizures). Topiramate significantly reduced class III seizures at 100 mg/kg (p<0.05), but did not reduce class IV and class V seizures at 30 mg/kg or 100 mg/kg (Fig. 3B). Although the two drugs did appear to block some severity classes, the main effect appeared to be at high doses when the drugs had large effects on seizure frequency.
Fig. 3. Effect of carisbamate and topiramate on seizures of different Racine classes.
A categorical analysis showing the distribution of seizure occurrence according to seizure severity (Racine, 1972). The magnitude of suppression for different classes of seizures is shown using black bars to represent reduction in Class III seizures, white bars to show the percent reduction of Class IV seizures, and grey bars to show the effects of the two AEDs on Class V seizures. A) Carisbamate significantly (p<0.05) reduced the frequency of class III seizures at a dose of 10 mg/kg, and class III and class V seizures were significantly reduced at 30 mg/kg carisbamate. B) Topiramate (100 mg/kg) significantly (p<0.05) decreased class III seizures, and was not significantly effective in reducing class IV or V seizures. The dashed horizontal line marks the 100% suppression level.
Blood concentration
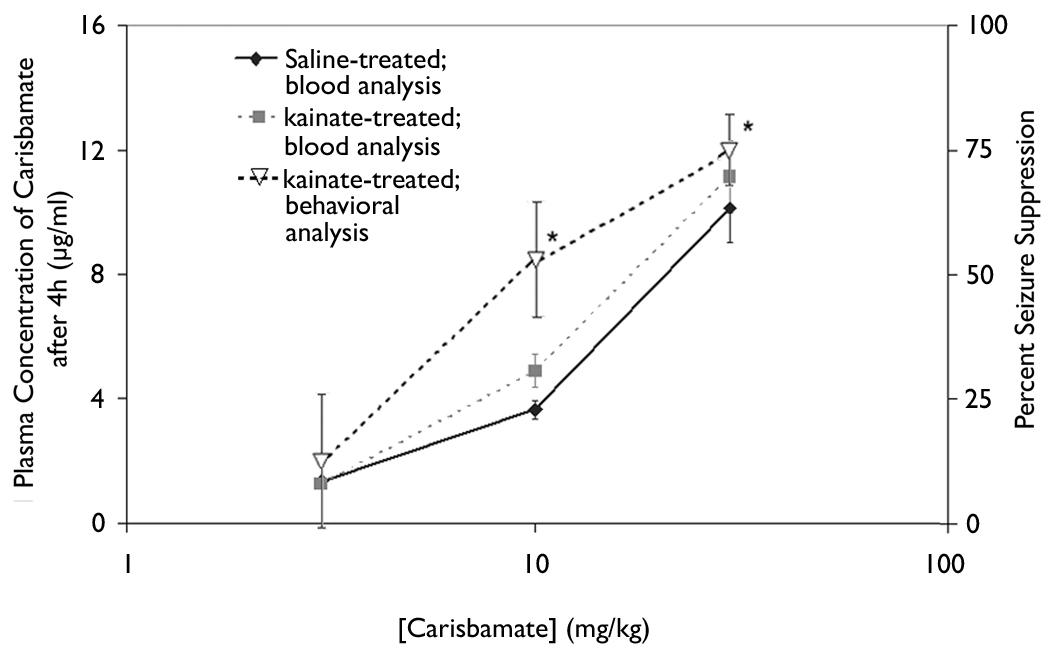
The corresponding blood concentration of three doses of carisbamate was measured in order to analyze the relationship between blood concentration and anticonvulsant activity. Blood concentration was measured in both control rats and in rats with kainate-induced epilepsy. The effect of the dose of carisbamate on blood concentration was determined in the middle of the first 6-h time epoch after drug administration (i.e., 4 h after the injection of carisbamate, or at 1:00 p.m.). The doses of 3 mg/kg, 10 mg/kg, and 30 mg/kg of carisbamate were chosen for blood-level determinations, because they ranged from ineffective (3 mg/kg) to highly effective (30 mg/kg) at suppressing seizures (Fig. 4). At 4 h after a single intraperitoneal injection, the measured blood concentrations for the doses of 3 mg/kg, 10 mg/kg, and 30 mg/kg paralleled the effect of carisbamate on percent seizure suppression. No significant difference was observed in the concentration of carisbamate between animals with kainate-induced epilepsy and aged-matched control animals.
Fig. 4. Effect of dose of carisbamate on plasma concentration of carisbamate 4 h after drug injection.
The blood concentration for each dose (3, 10, and 30 mg/kg) was measured 4 h after systemic injection of carisbamate. Each dose is compared using black diamonds and solid lines to represent blood concentration results for saline-treated animals; grey squares with grey dashed lines correspond to plasma concentrations of carisbamate for kainate-treated animals; and open triangles with black dashed lines display seizure data for each concentration of carisbamate measured. No significant difference was found between epileptic and control animals. Vertical bars, ±SEM.
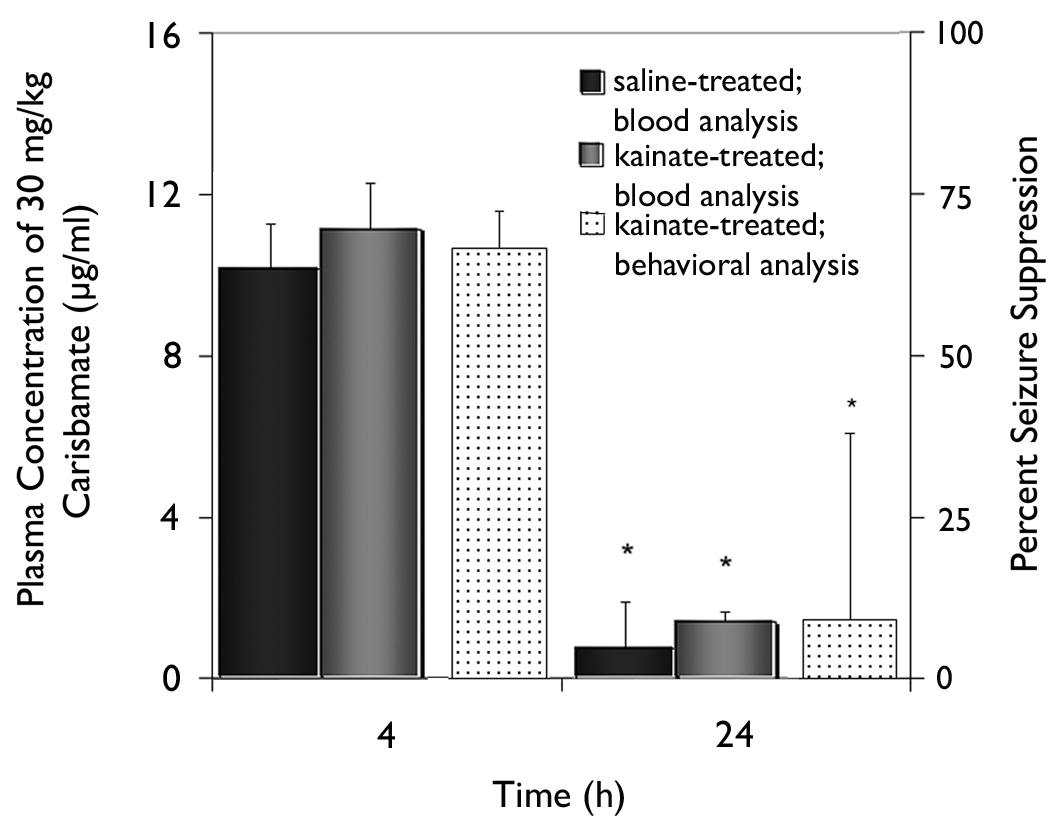
Using blood concentration measurements of carisbamate, the issues of time-dependent recovery from single intraperitoneal injections and the potential for drug accumulation at the highest dose were addressed. Seizure frequency was not significantly different from control in the 8-h epoch between 15 h and 23 h after administration of 30 mg/kg carisbamate (Fig. 5); therefore, the concentration of carisbamate was measured at 24 h after treatment for comparison. Measurement of blood levels of carisbamate indicated virtually complete drug clearance had occurred by 24 h in the repeated-measures, cross-over protocol (i.e., before the subsequent vehicle-control injection at 48 h). A significant difference (p<0.05) in blood concentration of carisbamate was detected between the two time points (4 h versus 24 h). No significant difference in blood concentration of carisbamate was observed between animals with kainate-induced epilepsy and control rats. Therefore, the availability of the drug in the blood after a high-dose injection (as measured by blood concentration values) was directly linked to seizure suppression by carisbamate.
Fig. 5. Effect of 30 mg/kg carisbamate plasma concentration at 4 and 24 h after drug injection.
Blood concentrations of AED at 4 h and 24 h after IP injection of 30 mg/kg carisbamate were significantly different (p<0.0125). Results were compared for saline-treated animals (black bars) and rats with kainate-induced epilepsy (grey bars). Behavioral data analyzed to determine percent seizure suppression is displayed for kainate-treated animals (dot-filled bars) for both time points. The high concentration of carisbamate at 4 h was associated with high seizure suppression, which was not present at 24 h when carisbamate was at low levels in the blood. No significant difference was detected in the concentration of carisbamate in control and epileptic animals. Vertical bars, ±SEM.
DISCUSSION
Summary of comparative analysis of carisbamate and topiramate
The repeated-measures, cross-over protocol in rats with kainate-induced epilepsy previously showed that topiramate significantly reduced the frequency of spontaneous seizures, but did not completely block the seizures (Grabenstatter et al., 2005). The same protocol was used in the present study to assess the effects of carisbamate, a new AED. Carisbamate was more effective than topiramate at reducing seizure frequency and the percentage of animals with complete seizure cessation. Blood concentrations of carisbamate 1) increased with administration of higher doses of drug, which correspondingly reduced seizure frequency, and 2) decreased over time, as the frequency of convulsive seizures returned to control values. These results suggest that carisbamate may potentially be an improved AED for the treatment of refractory temporal lobe epilepsy.
A high percentage of animals had complete suppression of seizures (i.e., seizure frequencies equivalent to zero) during the first 6-h epoch after single IP injections of 30 mg/kg carisbamate, thus demonstrating the potential “floor effect” that occurs when results are analyzed on the log10(y+0.1) scale. When these data were reanalyzed on the linear scale with a non-parametric statistical analysis, it was possible to ascertain the differences between drugs at doses where seizures were completely blocked or nearly completely blocked (Fig 2). Interpreting relative frequency measures using a parametric test of log-transformed data (i.e., a repeated-measures ANOVA) is a rigorous method to detect low levels of AED effects. However, a non-parametric statistical analysis of linear data (i.e., the Wilcoxon signed-rank test) is the most appropriate method for assessing highly effective AEDs that block nearly all spontaneous seizures (Grabenstatter et al., 2007).
Measures of drug efficacy
Relative seizure frequency versus percent seizure suppression
The effect of a drug on the frequency of seizures is typically normalized to seizure frequency without drug. Our previous experiments on topiramate (Grabenstatter et al., 2005) used relative seizure frequency, which is the ratio of the number of seizures observed in a defined time period (i.e., seizure frequency during a 6-h epoch) after an AED injection relative to the seizure frequency after a control injection. Relative seizure frequency essentially yields an expression of the reduction in seizure frequency induced by the drug. Percent seizure suppression (100 × [1- relative seizure frequency]) is a measure of the degree to which a drug blocks seizures during a specific time period; therefore, percent seizure suppression and relative seizure frequency are similar but inverted measures. For the purposes of this study, percent seizure suppression is a useful measure of efficacy because it provides a direct comparison to the percentage of animals with total seizure cessation (i.e., 100% seizure suppression, see below) and to the blood concentration of carisbamate.
Percentage of rats with complete seizure suppression
An ideal AED would suppress all seizures, and the percentage of animals that experience complete seizure cessation after AED treatment is another measure of efficacy. Complete seizure suppression in any particular animal was defined as occurring when no seizures were observed during the first 6-h epoch after any of the AED treatments. Although inclusion of data from animals that were not tested in all six AED-versus-vehicle tests per dose is a potential confound in the interpretation of the data on complete seizure cessation, the likelihood of an animal being eliminated from the study was equal for carisbamate and topiramate. Carisbamate completely suppressed seizures in nearly all rats at 30 mg/kg during the first 6-h epoch, but additional work is needed to test whether prolonged treatment with carisbamate (e.g., chronic administration of 30 mg/kg) completely eliminates seizures in rats with kainate-induced epilepsy.
Seizure severity
The level of behavioral involvement is an important measure of seizure severity. Two interrelated analyses did not consistently detect an effect of either carisbamate or topiramate on seizure severity, when performed in a manner to account for alterations in seizure frequency. This lack of effect could be due at least in part to a lack of statistical power, because our analyses essentially divided all seizures into three categories (i.e., class III, IV, and V). Another possibility is that the distinction between class III, IV, and V seizures is artificial in this context. Future studies concerning the effect of carisbamate on seizure severity need to investigate non-convulsive electrographic seizures and seizure duration.
Blood concentration analysis
These experiments showed that higher doses of carisbamate led to increased blood concentrations, which caused greater seizure suppression. The repeated-measures, cross-over protocol requires complete recovery from drug within 48 h before the subsequent vehicle treatment, and the present studies showed that recovery from a single IP injection of carisbamate occurs within approximately 24 h. Therefore, the effect of carisbamate on spontaneous seizures was dose-dependent.
Use of rats with chronic epilepsy in AED discovery
The repeated-measures, cross-over protocol for testing potential AEDs has the advantage that one can use an animal as its own control, and the design has a relatively high level of statistical power, considering the use of a comparatively small number of animals. At a significance level of α=0.05, 8 animals and six AED-versus-vehicle tests yields a power of 0.9 (i.e., a total of 48 AED-versus-vehicle tests per trial). In its present form, approximately 1 month was required to complete the analysis of each dose, but fewer cross-overs (i.e., AED-versus-vehicle tests) and less time would be needed if more animals were used. Thus, the same power would be achieved with each dose if an investigator performed the same total number of tests using more animals (e.g., 24 animals and two cross-overs). The kainate-treated rats were selected to ensure that they had high seizure rates, but animal models of epilepsy that have less frequently occurring spontaneous seizures would probably be amenable to a modified repeated-measures, crossover protocol (e.g., longer AED-vehicle administration periods). Finally, the present studies only analyzed behavioral seizures, and so non-convulsive electrographic seizure activity was not assessed. Therefore, the approach of using chronically epileptic animals has several possible variants for future study, and it has the advantage of potentially allowing detection of AEDs that preferentially act on “epileptic” versus normal brain.
Acknowledgements
These experiments were supported by ‘Johnson & Johnson’ PRD LLC, and the National Institutes of Health (NS049620). We thank Drs. B. Klein, R.E. Twyman and H.S. White for comments and suggestions; Dr. S. Clark for technical assistance; and Drs. Boyu Zhao, Norman Huebert, Bill Hageman, Daksha Desai-Krieger and Mr. Edward Kaczynsk for assistance with the mass spectroscopy analysis. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Disclosure of conflicts of interest: F. E. Dudek acknowledges that he has served as a paid consultant to Johnson & Johnson PRD.
Reference List
- Bauer J, Burr W. Course of chronic focal epilepsy resistant to anticonvulsant treatment. Seizure. 2001;10:239–246. doi: 10.1053/seiz.2000.0499. [DOI] [PubMed] [Google Scholar]
- Cole AJ. Guidelines for new epilepsy drugs. New epilepsy drugs are safer and cause fewer adverse effects than their predecessors. But they're not more effective. Health News. 2004;10:6–7. [PubMed] [Google Scholar]
- Elger CE. Pharmacoresistance: modern concept and basic data derived from human brain tissue. Epilepsia. 2003;44 Suppl 5:9–15. doi: 10.1046/j.1528-1157.44.s.5.3.x. [DOI] [PubMed] [Google Scholar]
- Francois J, Boehrer A, Nehlig A. Effects of carisbamate (RWJ-333369) in two models of genetically determined generalized epilepsy, the GAERS and the Audiogenic Wistar. Epilepsia. 2008;49:393–399. doi: 10.1111/j.1528-1167.2007.01278.x. [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Ferraro DJ, Williams PA, Chapman PL, Dudek FE. Use of chronic epilepsy models in antiepileptic drug discovery: the effect of topiramate on spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia. 2005;46:8–14. doi: 10.1111/j.0013-9580.2005.13404.x. [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Clark S, Dudek FE. Anticonvulsant Effects of carbamazepine on spontaneous seizures in rats with kainate-induced epilepsy: comparison of intraperitoneal injections with drug-in-food protocols. Epilepsia. 2007 doi: 10.1111/j.1528-1167.2007.01263.x. (in press) [DOI] [PubMed] [Google Scholar]
- Heinemann U, Draguhn A, Ficker E, Stabel J, Zhang CL. Strategies for the development of drugs for pharmacoresistant epilepsies. Epilepsia. (Suppl 5) 1994;35:S10–S21. doi: 10.1111/j.1528-1157.1994.tb05959.x. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Kupferberg H. Animal models used in the screening of antiepileptic drugs. Epilepsia. 2001;42 Suppl 4:7–12. [PubMed] [Google Scholar]
- Leite JP, Garcia-Cairasco N, Cavalheiro EA. New insights from the use of pilocarpine and kainate models. Epilepsy Res. 2002;50:93–103. doi: 10.1016/s0920-1211(02)00072-4. [DOI] [PubMed] [Google Scholar]
- Levy RH, Mattson RH, Meldrum BS, Perucca E. Antiepileptic Drugs. In: White HS, Woodhead JH, Wilcox KS, Stables JP, Kupferberg HJ, Wolf HH, editors. Discovery and Preclinical Development of Antiepileptic Drugs. Fifth Edition. Philadelphia, Lippincott: Williams, & Wilkins; 2002. pp. 36–48. [Google Scholar]
- Loscher W, Schmidt D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988;2:145–181. doi: 10.1016/0920-1211(88)90054-x. [DOI] [PubMed] [Google Scholar]
- Margerison JH, Corsellis JA. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- Novak GP, Kelley M, Sannikos P, Klein B. Carisbamate (RWJ-333369) Neurotherapeutics. 2007;4:106–109. doi: 10.1016/j.nurt.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr.Clin.Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat.Rev.Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Rogawski MA. New strategies for the identification of drugs to prevent the development or progression of epilepsy. Epilepsy Res. 2002;50:71–78. doi: 10.1016/s0920-1211(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Sisodiya SM. Mechanisms of antiepileptic drug resistance. Curr.Opin.Neurol. 2003;16:197–201. doi: 10.1097/01.wco.0000063771.81810.6c. [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram EH, White HS, Coulter DA, Dichter MA, Jacobs MP, Loscher W, Lowenstein DH, Moshe SL, Noebels JL, Davis M. Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia. 2002;43:1410–1420. doi: 10.1046/j.1528-1157.2002.06702.x. [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram E, Dudek FE, Holmes G, Mathern G, Pitkanen A, White HS. Therapy discovery for pharmacoresistant epilepsy and for disease-modifying therapeutics: summary of the NIH/NINDS/AES models II workshop. Epilepsia. 2003;44:1472–1478. doi: 10.1111/j.0013-9580.2003.32803.x. [DOI] [PubMed] [Google Scholar]
- White HS, Srivastava A, Klein B, Zhao B, Choi YM, Gordon R, Lee SJ. The novel investigational neuromodulator RWJ 333369 displays a broad-spectrum anticonvulsant profile in rodent seizure and epilepsy models. Epilepsia. 2006;47 Suppl 4:200. Abst. [Google Scholar]
- White HS. Clinical significance of animal seizure models and mechanism of action studies of potential antiepileptic drugs. Epilepsia. 1997;38 Suppl 1:S9–S17. doi: 10.1111/j.1528-1157.1997.tb04523.x. [DOI] [PubMed] [Google Scholar]
- White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia. 2003;44 Suppl 7:2–8. doi: 10.1046/j.1528-1157.44.s7.10.x. [DOI] [PubMed] [Google Scholar]
- Wieser HG. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]