SUMMARY
Earlier, we reported that S6K1−/− mice have reduced body fat mass, elevated rates of lipolysis, severely decreased adipocyte size, and are resistant to high-fat-diet (HFD)-induced obesity. Here we report that adipocytes of S6K1−/− mice on a HFD, have the capacity to increase in size to a degree comparable to that of wild-type (WT) mice, but not in number, indicating an unexpected lesion in adipogenesis. Tracing this lesion revealed that S6K1 is dispensable for terminal adipocyte differentiation, but is involved in the commitment of embryonic stem cells (ESCs) to early adipocyte progenitors. We further show that absence of S6K1 attenuates the upregulation of transcription factors critical for commitment to adipogenesis. These results led to the conclusion that a lack of S6K1 impairs the generation of de novo adipocytes when mice are challenged with a HFD, consistent with a reduction in early adipocyte progenitors.
INTRODUCTION
Obesity is a worldwide epidemic that until recently was largely confined to industrialized countries of the Western world, but is now also on the ascent in urban centers of underdeveloped nations (James et al., 2006). The key factor in promoting the obese state is nutrient overload (Um et al., 2006) combined with the innate drive to acquire and store fat in adipose depots (Schwartz et al., 2003). The obese phenotype is further accentuated by the body’s tendency to protect newly acquired adipose depots in the face of deliberate reductions in food intake (Schwartz et al., 2003). A consequence of this response is the increased probability of initiating a number of pathologies including insulin resistance, cardiovascular disease (Morgan et al., 2004), and cancer (Calle and Kaaks, 2004). Given the obesity epidemic and associated pathologies, it is critical to understand the underlying mechanisms responsible for the development and maintenance of normal adipose tissue.
Adipose depots evolved to serve as the major energy reserves when glucose becomes limiting. Although adipocyte number can increase throughout life, adult-onset obesity is generally characterized by an increase in adipocyte size, whereas obesity earlier in life involves both an increase in the size and number of adipocytes (Kissebah and Krakower, 1994). The development of adipose depots can be broadly divided into two stages (i) commitment, which involves differentiation of ESCs into multipotent mesodermal stem cells, followed by the generation of preadipocytes and (ii) terminal differentiation, which gives rise to adipocytes from preadipocytes and is regulated by a network of transcription factors, including the master regulators PPARγ and C/EBPα (Gesta et al., 2007; Rosen et al., 2002). In terminal differentiation, the identification of the molecular components involved has been particularly assisted by studies in genetically modified mice. In contrast, such studies have yet to define the molecular components involved in the commitment step (Rosen and MacDougald, 2006). However, the recent identification of early adipocyte progenitors within the vascular niche of adipose tissue (Tang et al., 2008) (Rodeheffer et al., 2008), opens an opportunity to analyze adipocyte progenitors with respect to their molecular makeup and role in adipocyte development.
An emerging component in adipogenesis is the mammalian target of rapamycin Complex 1 (mTORC1) and its effectors, ribosomal protein S6 kinase 1 (S6K1) and the 4E binding proteins (4E-BP) 1 and 2. These mTORC1 effectors appear to act as critical mediators of a nutrient/hormonal signaling network that is involved in the development of obesity (Le Bacquer et al., 2007; Um et al., 2004). Earlier studies showed that S6K1−/− mice have reduced adipose tissue mass, increased energy expenditure, and are resistant to diet-induced obesity (Um et al., 2004). The reduction in fat mass on a normal-chow diet (NCD) was attributed to a striking decrease in adipocyte cell size due to an increase in lipolysis (Um et al., 2004). In contrast, 4E-BP1−/−;4E-BP2−/− mice displayed increased sensitivity to diet-induced obesity due to an acceleration in adipogenesis (Le Bacquer et al., 2007). These mice also display hyperactivation of S6K1 (Le Bacquer et al., 2007), which may in part explain the accelerated gain in adiposity. Consistent with these observations, the mTOR inhibitor rapamycin inhibits terminal adipocyte differentiation in 3T3-L1 preadipocytes (El-Chaar et al., 2004; Yeh et al., 1995) and protects mice from HFD-induced obesity (Chang et al., 2009). Moreover, the TSC1/TSC2 tumor suppressor, which blunts mTORC1 signaling, inhibits MEF differentiation into adipocytes (Zhang et al., 2009). The inhibition by rapamycin is reversed by expression of rapamycin-resistant mTOR, but not by the expression of rapamycin-resistant S6K1 (Kim and Chen, 2004), despite the lean phenotype observed in S6K1-deficient mice (Um et al., 2004). These studies have underscored the importance of elucidating the role of S6K1 in adipogenesis.
Here, we set out to define the role of S6K1 in adipocyte cell size regulation following an extended HFD challenge. Unexpectedly, we observed that, in S6K1-deficient mice, adipocyte size increased to almost the same extent as in WT mice, but cell number did not, indicating a lesion adipogenesis. To resolve whether S6K1 plays a role in the adipogenic developmental processes of commitment, terminal differentiation, or both, we took advantage of the distinct steps of adipogenesis, which can be recapitulated in vitro by using 3T3L1 preadipocytes, primary MEFs, adipose-derived stem cells (ADSCs) from adult adipose depots, or ESCs. These studies revealed that S6K1 function is dispensable for terminal adipocyte differentiation, but plays a critical role in early adipocyte commitment. Consistent with these findings, ADSCs isolated from the stromal vascular fraction (SVF) of S6K1-deficient mice maintained on a HFD, as compared to WT mice, were more severely impaired in their ability to generate new adipocytes. This finding was supported by fluorescence-activated cell sorter (FACS) analysis of the isolated SVF from adult adipose depots of S6K1-deficient mice, which revealed a significant reduction in early adipocyte progenitors.
RESULTS
Adipocyte hyperplasia is reduced in S6K1−/− mice on a HFD
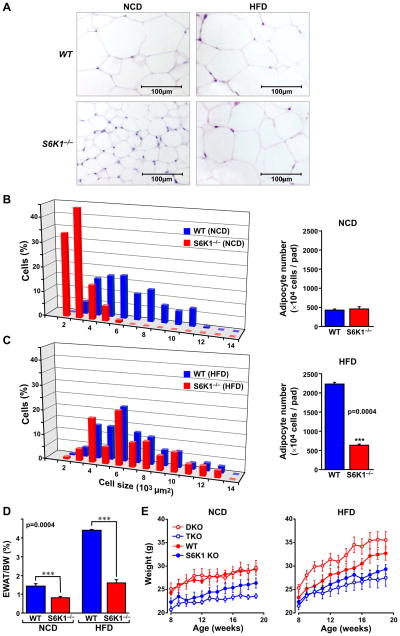
At birth, S6K1−/− mice are 10% to 15% smaller in size, with all their tissues reduced proportionally to the same extent, except for those that respond to insulin, including skeletal muscle, β-cells, and fat depots (Um et al., 2006). In the latter cases the effect on tissue mass has been attributed to a reduction in cell size, not cell number (Um et al., 2004). We reasoned that the impaired ability of S6K1-deficient mice to accumulate fat when on a HFD was due to a lesion in their ability to increase in cell size. To test this possibility we analyzed hematoxylin/eosin (H&E)-stained histological sections from the epididymal fat pads of WT and S6K1−/− mice maintained on either a NCD or HFD for six months. As noted previously (Um et al., 2004), adipocytes from S6K1−/− mice maintained on a NCD were dramatically reduced in size as compared to those of WT mice (Figure 1A). Morphometric analysis showed an ~ 70% reduction in size, whereas there was no measurable difference in cell number (Figure 1B). In contrast, we found an unexpected increase in adipocyte cell size in S6K1−/− mice on a HFD, such that they increased to approximately the same size as those from WT mice (Figures 1A and C). This increase in cell size, unlike that of WT mice, was associated with only a limited increase in cell number (Figures 1B and C). Consistent with this result, the mass of the S6K1−/− epididymal fat pad, even when normalized for the smaller mouse size, increased less than 2-fold on a HFD, whereas that of WT mice increased more than 3-fold (Figure 1D). Thus, the adipose tissue of S6K1−/− mice on a HFD contains fewer adipocytes (Figures 1B and C). These observations demonstrate an apparent lesion in de novo adipogenesis in S6K1−/− mice when challenged with a HFD. These results also raised the possibility that elevated levels of S6K1 activity observed in 4E-BP1−/−;4E-BP2−/− double knock-out (DKO) mice contributes to their exhibited diet-induced obesity (Le Bacquer et al., 2007). This was tested by crossing 4E-BP1−/−;4E-BP2−/− DKO mice with S6K1−/− mice to generate triple knock-out (TKO) mice. The body weight of 4E-BP1−/−;4E-BP2−/− DKO mice maintained on a NCD was similar to WT mice, whereas on a HFD they had a propensity to become obese (Figure 1E and (Le Bacquer et al., 2007)). This latter effect was totally blunted in TKO mice, whose body weight was comparable to that of S6K1−/− mice (Figure 1E). These findings indicate that in addition to its metabolic effects on adipose tissue, S6K1 plays a role in de novo adipogenesis.
Figure 1. Adipose tissue of S6K1−/− mice on a HFD shows adipocyte hypertrophy but not hyperplasia.
(A) Histological analysis of adipose tissue sections of S6K1−/− and WT mice fed on a NCD or HFD. (B) Morphometric analysis of adipocyte cell size from NCD epididymal white adipose tissue (EWAT) based on a total of 200 cells (left), and total cell number of adipocytes per fat pad (right) from WT and S6K1−/− mice (n=3 for each genotype). (C) Morphometric analysis of adipocyte cell size from HFD EWAT based on a total of 200 cells (left) and total cell number of adipocytes per fat pad (right) in WT and S6K1−/− mice (n=3 for each genotype). (D) EWAT total mass was measured from WT or S6K1−/− mice maintained on a NCD or HFD. Values were normalized on the basis of total body weight. (E) Body weight analysis of S6K1−/− (S6K1), 4E-BP1/2−/− (DKO), and S6K1−/−/4EBP1/2−/− triple knockouts (TKO) maintained on a NCD (left) or HFD (right) for 6 weeks (n=6 for each genotype). p values are listed in Supplemental Data, Table S1.
Adipogenesis is impaired in S6K1−/− MEFs and ADSCs
To examine the role of S6K1 in adipocyte terminal differentiation, we took advantage of two models: the adipogenic induction of MEFs and ADSCs (Rosen and MacDougald, 2006), the latter representing the adherent cells from SVF of adipose tissue (Noel et al., 2008). S6K1−/− MEFs and ADSCs showed reduced adipocyte differentiation, as visualized by a decrease in Oil Red O staining (Figures 2A and B). Quantification revealed an approximate 50% reduction in adipocyte number when compared with either WT MEFs or WT ADSCs (Figures 2C and D), consistent with the in vivo data from S6K1−/− mice (Figures 1B and C). To determine whether the effects of loss of S6K1 were cell autonomous, Oil Red O staining was measured in S6K1−/− and WT MEFs infected with lentiviruses expressing either S6K1 or green fluorescent protein (GFP), as a control. The results show that increased expression of S6K1 had no effect on the differentiation of either cell type (Figures 2A and C). Higher levels of S6K1 expression did not interfere with kinase activation, as measured by S6K1 T389 phosphorylation (Figure 2E). In parallel, we used shRNAs delivered by lentiviruses to examine the effect of S6K1 depletion on adipogenesis in WT ADSCs, which showed no effect on adipogenic potential, as assessed by adipocyte aP2 protein expression or Oil Red O staining (Figures 2F and G). These findings suggested that S6K1 is not required for terminal differentiation. To test this further, we turned to mouse 3T3-L1 preadipocytes, an extensively employed model for the differentiation of preadipocytes into mature adipocytes (Gesta et al., 2007; Rosen and MacDougald, 2006). Depletion of S6K1 in these cells with either of two S6K1-shRNA lentiviruses (Supplemental Data, Figure S1A), had no effect on terminal adipocyte differentiation, as assessed by Oil Red O staining, or by expression of PPARγ or Glut4 mRNAs (Supplemental Data, Figures S1B-D). These data show that S6K1-deficient MEFs and ADSCs are impaired in adipogenesis, but that the kinase is dispensable for terminal adipocyte differentiation.
Figure 2. Reduced adipogenesis in S6K1−/− MEFs and ADSCs.
(A–B) Oil Red O staining of adipocytes derived from S6K1−/− MEFs that are (A) non-infected (-LV), infected with lentivirus overexpressing GFP (LV-GFP), or with lentivirus overexpressing S6K1 (LV-S6K1); or (B) from ADSCs of WT or S6K1−/− mouse adipose tissue. (C–D) Quantification of Oil Red O-stained adipocytes derived from either (C) differentiated MEFs or (D) WT and S6K1−/− ADSCs. (E) S6K1 was ectopically re-expressed in S6K1−/− MEFs by infection with a lentivirus carrying S6K1 cDNA (LV-S6K1). Total S6K1, T389 S6K1 phosphorylation (P-T389 S6K1), and β-actin levels were determined by western blot analysis. (F) S6K1 was depleted in WT ADSCs by infection with lentiviruses carrying either non-silencing shRNAs (shNS) or S6K1-specific shRNAs (shS6K1). Cells were differentiated into adipocytes (day 9) and analyzed by western blot to determine expression levels of total S6K1, S240/S244 S6 phosphorylation (P-S240/244 S6), aP2, and β-actin. (G) Terminal adipocyte differentiation of WT and S6K1−/− ADSCs was assessed by quantification of Oil Red O staining. Values in C, D, and G are given as mean ± SEM. (ns, not statistically significant). See also Figure S1.
Rapamycin treatment or S6K1 depletion suppresses ESC adipocyte differentiation
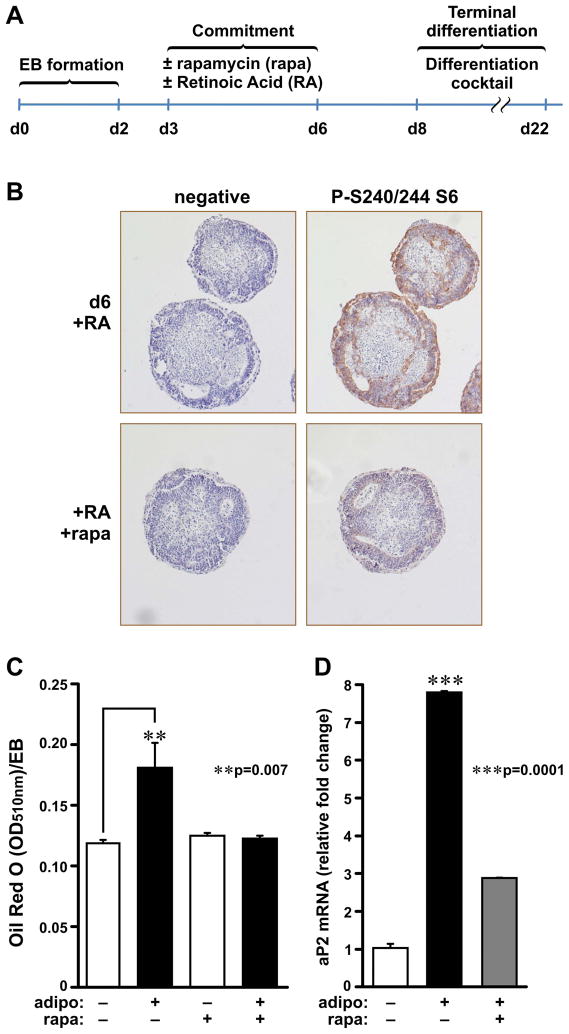
The findings above suggest that S6K1 exerts its pro-adipogenic effects at a stage of adipogenesis earlier than terminal differentiation. Therefore, we turned to pluripotent ESCs and embryoid bodies (EBs) (Murry and Keller, 2008). EB adipogenesis can be divided into two stages: (i) commitment into early adipocyte progenitors, which is dependent on all-trans-retinoic acid (RA) treatment (Bost et al., 2002); and (ii) terminal adipocyte differentiation, which can be induced by treating early adipocyte EB progenitors with an adipogenic cocktail (Phillips et al., 2003). To determine if mTORC1 effectors are involved in the commitment stage, the effect of RA treatment on ribosomal protein S6 phosphorylation, a downstream target of mTORC1/S6K1 (Dann et al., 2007), was measured in the absence or presence of rapamycin during this phase of the adipocyte development, day 3 (d3) to d6 (Figure 3A). During RA-induced commitment, there was an increase in S6 S240/S244 phosphorylation, as measured by immunohistochemistry (IHC), which was attenuated by rapamycin (Figure 3B). To measure the effect of rapamycin on terminal differentiation, d6 EBs, which were incubated with rapamycin from d3 to d6, were subjected to an adipogenic cocktail treatment (Bost et al. 2002 ). The results show that EB adipocyte terminal differentiation, as assessed by Oil Red O staining and aP2 mRNA expression, was also strongly attenuated by rapamycin treatment during d3 to d6 (Figures 3C and D), indicating that mTORC1 is critical during the commitment phase of adipogenesis.
Figure 3. Rapamycin and S6K1 depletion affect determination step of EB-to-adipocyte differentiation.
(A) Schematic representation of the differentiation of WT ESCs into adipocytes upon rapamycin treatment. EB formation (d0-d2), EBs in suspension under RA treatment (d3-d6), adaptation to culture on gelatin-coated plates (d6-8), and terminal differentiation of EBs into adipocytes induced by adipogenic cocktail (d6-d22). (B) H&E staining (left) and P-S240/244 S6 IHC with hematoxylin counterstain of RA-treated EBs with or without rapamycin (rapa). (C) Quantification of differentiated adipocytes derived from WT EBs (d22) with or without 20 nM rapamycin (rapa) treatment in the presence of 1 μM RA (d3-d6) with or without adipogenic differentiation inducers (d8-d22) (adipo). Oil Red O-stained lipids were extracted, the absorbance at 510 nm (OD510) was measured, and the value was normalized for EB number and size. (D) Adipocyte marker aP2 mRNA levels were analyzed by qPCR and normalized to 18S rRNA. Values in C and D are given as mean ± SEM.
To assess the role of S6K1 in the commitment phase, we depleted its levels in ESCs by using an shRNA lentiviral vector (Figure 4A). Reduction of S6K1 levels resulted in the formation of EBs of approximately 50% the size of control EBs (Figure 4B), which was accounted for by an estimated 50% reduction in cell number (Figure 4C) and not due to increased apoptosis (data not shown). Following induction of adipocyte differentiation, and after normalization for EB size, S6K1-shRNA-expressing EBs showed a reduction in Oil Red O-stained adipocytes and aP2 protein levels as compared to the non-silencing (NS) shRNA control (Figures 4D, 4E and Supplemental Data Figure S2B). Re-introduction of S6K1 into S6K1-depleted ESCs (Supplemental Data, Figure S2A) rescued EB cell number and adipocyte differentiation (Figures 4F and G). Consistent with these findings, S6K1−/− ESCs also display impaired commitment and a reduced ability to terminally differentiate into adipocytes (Supplemental Data, Figure S2C). These results indicate that S6K1 cooperates with other pathways to control the commitment stage of adipogenesis.
Figure 4. S6K1 is required for commitment of ESCs to the adipocyte lineage.
(A) ESCs were infected with lentivirus carrying shNS or shS6K1 and stable cell lines were generated. Levels of S6K1 and β-actin protein were determined by western blot analysis. (B) Size measurements of EBs (d2) derived from shNS and shS6K1 lentivirus-infected ESC lines as obtained by seeding either 2×103 or 5×103 cells in suspension; micrograph of d2 EBs (right panel). (C) Cell numbers during EB formation by shNS and shS6K1 ESC lines at 0hrs (d0), 24hrs (d1), and 48hrs (d2). (D) Oil Red O staining of differentiated adipocytes derived from shNS and shS6K1 EBs. (E) Adipocyte differentiation was assessed by spectrophotometric detection at 510 nm (OD510) of Oil Red O in extracts from EBs infected with shNS- or shS6K1-containing lentiviruses. Values were normalized to EB size and number. (F) S6K1 was ectopically re-expressed in shS6K1 ESCs (shS6K1+LVS6K1) and cell number was determined during EB formation at the 24hr time point. (G) Adipocyte differentiation assessed, as in E, by measuring Oil Red O stain in cell extracts, and normalized according to EB size and number. Values in B, C, E, F, and G are given as mean ± SEM. See also Figure S2.
S6K1 mediates RA induction of transcription factors required for adipogenic commitment
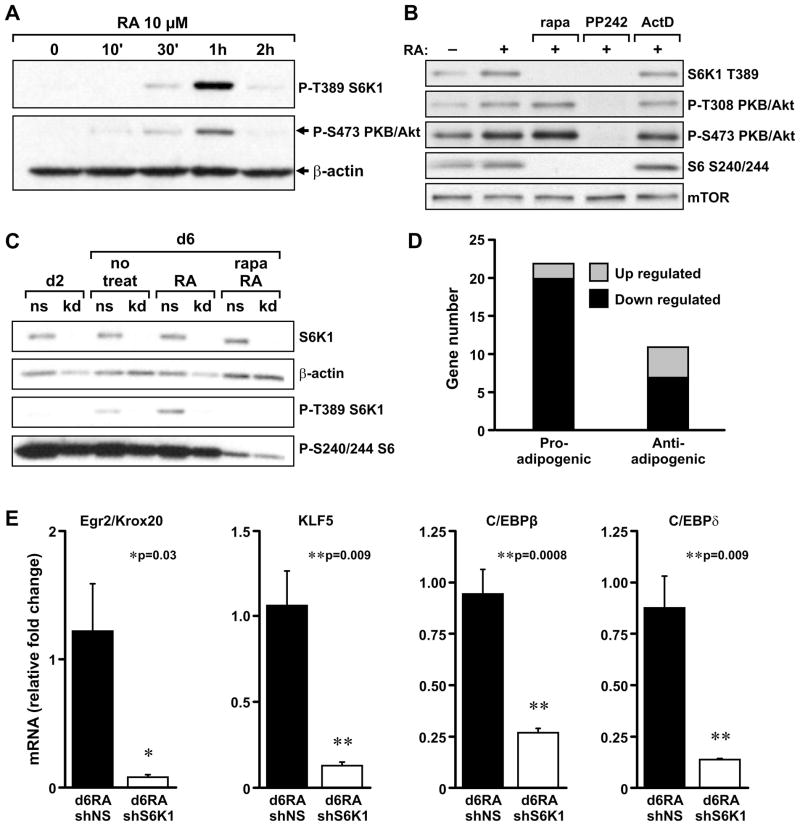
RA treatment results in the increased expression of a number of transcription factors required for early commitment to the adipocytic lineage. As this step is repressed by S6K1 depletion, we investigated whether RA induces mTORC1 signaling in ESCs and EBs, and determined the effect of depleting S6K1 on the induction of specific transcription factors. In growing ESCs, RA induced a transient but robust activation of S6K1 T389 phosphorylation starting at 30min and peaking at 1hr, which closely paralleled PKB/Akt S473 phosphorylation (Figure 5A). RA-induced activation of S6K1 T389 phosphorylation was inhibited by rapamycin, arguing that this is an mTORC1-dependent event (Figure 5B). In contrast, rapamycin potentiated the activation of PKB/Akt S473 phosphorylation, as well as T308 phosphorylation (Figure 5B), most likely by inhibiting the negative feedback loop from S6K1 to PKB/Akt (Um et al., 2006). To confirm that this response was mTOR specific, we employed the mTOR ATP-competitive inhibitor PP242 (Feldman et al., 2009), which abolished S6K1 T389 as well as PKB/Akt T308 and S473 phosphorylation (Figure 5B). Moreover, none of these RA-activated responses was inhibited by actinomycin D (Figure 5B), arguing that RA does not act through transcription to activate mTORC1 signaling. Analysis of EBs treated with RA from d3 to d6 also revealed elevated levels of S6K1 T389 phosphorylation, which were abolished by either S6K1-shRNA knockdown (kd) or by treatment with rapamycin (Figure 5C). To determine the effect of S6K1 depletion on transcription factors required for early commitment, we used cDNA microarray analysis to compare the gene expression patterns of RA-treated, S6K1-depleted, and NS-shRNA–treated EBs. Analysis of the microarray results, cross-referenced with the Entrez Gene database, showed that a large number of pro-adipogenic mRNAs were proportionally decreased following RA induction in EBs depleted of S6K1 (Figure 5D), including KLF4 (Birsoy et al., 2008), Lipin1 (Koh et al., 2008), and Igfbp1 (Nueda et al., 2008)(Supplemental Data Table 2). The downregulation of a number of transcription factors involved in early commitment to the adipocytic lineage, including Egr2/Krox20 (Wang et al., 2009), KLF5 (Oishi et al., 2005), C/EBPβ (Chen et al., 2005), and C/EBPδ (Hishida et al., 2009) were confirmed by qRT-PCR (Figure 5E). These data indicate that S6K1 plays a critical role in mediating RA-induced expression of early transcription factors during adipogenic commitment.
Figure 5. Retinoic Acid (RA) treatment activates S6K1 phosphorylation in ESCs and EBs.
(A) Left panel: ESCs were treated with 10μM RA for 2 hours. S6K1 T389 and PKB/Akt S473 phosphorylation were measured by western blot analysis and β-actin was used as a protein-loading control. (B) ESCs were pre-treated for 15min with 20nM Rapamycin (rapa), 8μM PP242, or 5μg/ml Actinomycin D (ActD), and then stimulated with 10μM RA for an additional 45 min. Levels of P-T389 S6K1, P-S473 PKB/Akt, P-T308 PKB/Akt, and P-S240/244 S6 were detected by western blot analysis. mTOR was used as protein-loading control. (C) EBs formed from the shNS ESC line (ns) or shS6K1ESC line (kd) were treated with 1μM RA for 3 days (d3-d6). Total S6K1, P-T389 S6K1, and P-S240/244 S6 were determined by western blot analysis in d6 EBs, with β-actin used as a protein-loading control. (D) Number of adipogenesis-related genes upregulated or downregulated in microarray analyses of RA-treated shNS and shS6K1 EBs (see Experimental Procedures). (E) Expression levels of early adipogenic marker genes, Krox20/Egr2, KLF5, C/EBPβ, and C/EBPδ. Total RNA was prepared from RA-treated shNS EBs (d6RA shNS) or shS6K1 EBs (d6RA shS6K1) and analyzed by qPCR. The expression values were normalized to 18s rRNA levels. Values in D are given as mean ± SEM. See also Table S2.
S6K1 deletion affects adipocyte differentiation of precursor cells from mice fed a high-fat diet
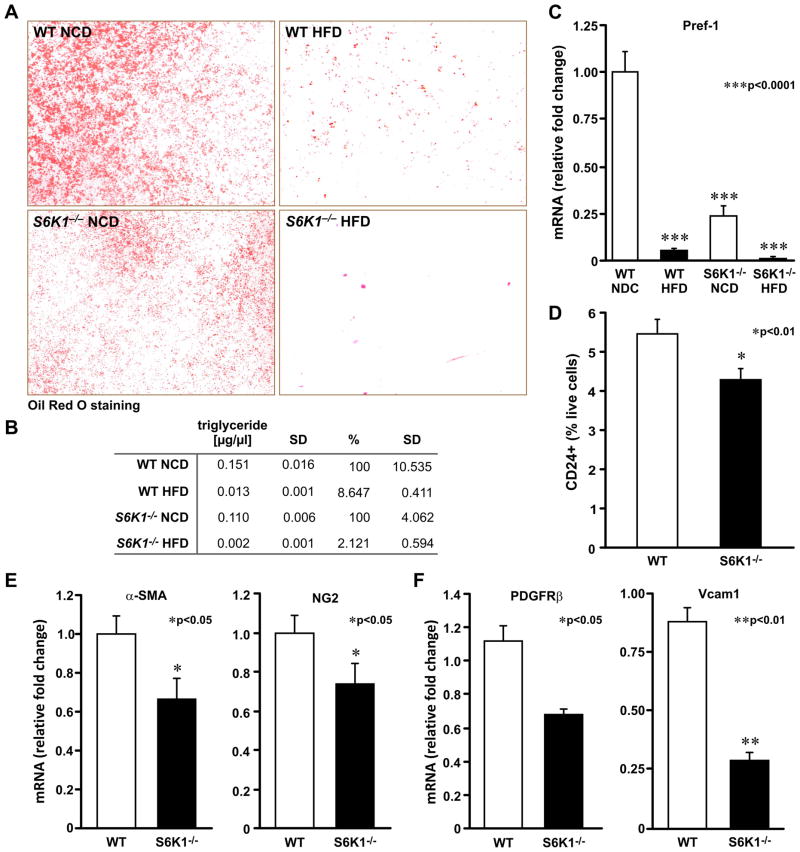
As S6K1−/− ESs and ADSCs are impaired in their ability to generate preadipocytes in vitro, we asked whether this impairment contributes to their resistance to HFD-induced obesity. To test this possibility, ADSCs from mice maintained on a NCD or HFD for 4 months were submitted to a differentiation protocol (Experimental Procedures). The results show that de novo adipocyte generation and the accumulation of triglycerides was impaired in WT mice maintained on a HFD versus a NCD (Figures 6A and B). ADSCs from S6K1−/− mice maintained on a HFD were also impaired in their ability to generate new adipocytes; however, as compared to S6K1−/− mice maintained on a NCD, the effect was more severe than observed for WT mice (Figures 6A and B). In neither case was there an indication of enhanced cell death (data not shown). Consistent with the reduction in differentiated adipocytes, mRNA levels of the preadipocyte marker Pref-1 were proportionally reduced in isolated ADSCs from WT and S6K1−/− mice (Figure 6C). Thus, the lesion in adipocyte stem cell commitment appears to contribute to resistance to diet-induced obesity in S6K1−/− mice.
Figure 6. S6K1 is required for the generation and differentiation of adipocyte precursor cells in adipose tissue of mice exposed to a high-fat diet.
(A) Oil Red O staining of differentiated adipocytes from WT or S6K1−/− ADSC cells derived from adipose tissue of 6-month-old mice maintained either on a NCD or a HFD (n=3 mice of each genotype). (B) Quantification of total triglyceride content from differentiated cells, normalized to total DNA content. Values in B are given as mean ± SD. (C) Expression levels of Pref-1 marker in ADSCs. (D) Sorting of Lin−/CD29+/Sca1+/CD34+/CD24+ cells (CD24+) from SVFs of WT and S6K1−/− mice. (E) Expression levels of pericyte markers α-Sma and NG2 in total fat depots. (F) Expression levels of PDGFRβ and Vcam1 in SVFs. (E–F) Data obtained by qPCR measurements and normalized to 18S rRNA levels (Values given in mean ± SEM). See also figure S3.
Early adipocyte progenitor cells are reduced in S6K1−/− mice
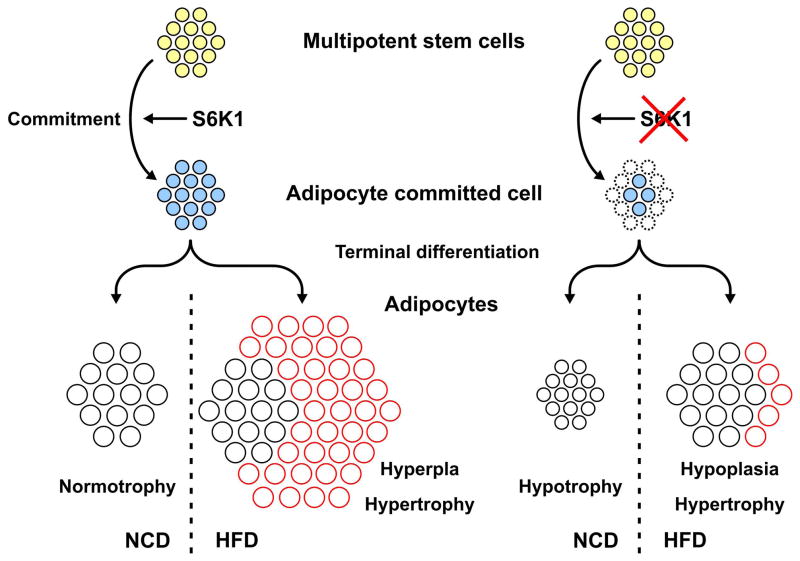
To address whether the early adipose stem cell population is reduced in S6K1−/− mice, we used FACS analysis to measure the adipocyte progenitor population from SVF, as characterized by the Lin−/CD29+/CD34+/Sca+/CD24+ markers (Rodeheffer et al., 2008). This cell population, was reduced by 20% in S6K1−/− mice, as compared with WT mice (Figure 6D and Supplemental Data, Figure 3), consistent with the deficits observed in the adipogenic potential of ADSC derived from S6K1−/− mice (Figures 2B, 6A, and Supplemental Data, Figure 3). To further characterize the reduction in the putative adipose stem cell population, we used qRT-PCR to examine the expression of pericyte markers (Garmy-Susini et al., 2005; Tang et al., 2008) in total fat depots (α-Sma and NG2), or in the SVF (PDGFRβ and Vcam1). In all four cases there was a significant reduction in the expression of each transcript in S6K1−/− versus WT mice (Figures 6E and F). These results indicate that the loss of S6K1 leads to a reduction in the number of early adipocyte progenitors, which plays a critical role in diet-induced obesity (Figure 7).
Figure 7.
Model depicting the effect of S6K1 loss on adipogenesis in mice maintained on a NCD or when challenged with a HFD (see text for details).
DISCUSSION
S6K1 and fat accumulation
Given the epidemic in obesity and its associated morbidities, a basic understanding of adipose development would greatly enhance the development of novel therapeutics. An increase in both adipocyte cell size and number are required for adipose tissue growth. However, whereas adipocyte hypertrophy is strongly influenced by diet, adipocyte hyperplasia is thought to be more dependent on genetic background (Jo et al., 2009). Moreover, there is continuous adipocyte turnover in humans, with approximately 10% cell renewal per year (Spalding et al., 2008). Therefore, adipocyte precursors, resident in fat tissue, are poised to contribute to the expansion of adipose tissue when faced with a nutrient challenge. However, little is known about the precursor cells or the signaling pathways that control their development. Earlier studies (Um et al., 2004), combined with those presented here, support the argument that the S6K1−/− lean phenotype can be attributed to two distinct phenotypes. First, on a NCD, S6K1−/− mice, as compared to WT mice, have elevated basal rates of lipolysis and energy expenditure, which together contribute to a reduction in adipocyte cell size and fat mass (Um et al., 2004). Recent studies have attributed this effect to an increase in AMPK signaling and elevated rates of β oxidation (Aguilar et al., 2007). Second, as shown here, adipocytes derived from S6K1−/− mice challenged with a HFD, increase in size to a level comparable to WT adipocytes, but not in total fat mass, due to a lesion in adipogenesis. This lesion is due to a reduction in early adipocyte progenitors (Figure 7), which, combined with the metabolic defects, account for the resistance to diet-induced obesity exhibited by S6K1−/− mice.
S6K1 and adipose tissue growth and differentiation
That S6K1 is not critical for terminal differentiation is consistent with the failure of rapamycin-resistant S6K1 to protect 3T3L1 adipocytes from inhibition of this response by rapamycin (Kim and Chen, 2004). With respect to adipogenesis, it is of interest that, in contrast to S6K1−/− mice, 4E-BP1−/−;4E-BP2−/− mice have increased adiposity and exhibit enhanced adipogenesis in vitro, which is paralleled by increased levels of C/EBPδ mRNA and S6K1 hyperactivation (Le Bacquer et al., 2007). S6K1 hyperactivation is most likely due to increased levels of available mTORC1, suggesting that the 4E-BPs not only suppress initiation factor eIF4E, but indirectly suppress S6K1 activation by competing for available mTORC1 via raptor binding to their TOS motifs (Schalm and Blenis, 2002). This hypothesis is consistent with our earlier findings that overexpression of dominant-interfering S6K1 not only blocked reporter S6K1 activation, but also suppressed reporter 4E-BP1 phosphorylation (von Manteuffel et al., 1997). Moreover, these findings suggest a dominant role for S6K1 versus 4E-BP1 and 4E-BP2 in adipogenesis as S6K1−/−/4E-BP1−/−/2−/− mice have an adipogenic phenotype equivalent to S6K1-deficient mice (Figure 1E). This model also fits with recent data demonstrating that mice harboring an adipose-specific deletion of raptor are lean and have increased mitochondrial respiration (Polak et al., 2008) and that rapamycin treatment protects mice from HFD-induced obesity by reducing lipid accumulation (Chang et al., 2009). Taken together, these data suggest that 4E-BP1 and 4E-BP2 play a central role in terminal adipocyte differentiation, whereas S6K1 plays a critical role in precursor cell commitment.
In humans, the ability of ADSCs to differentiate into mature adipocytes is negatively correlated with both body mass index and adipocyte cell size (Isakson et al., 2009). Our results in WT mice maintained on a HFD are consistent with this finding, revealing a reduction in ADSCs and the marker Pref-1. Recently, two groups pointed to the perivascular adipose tissue as a niche for ADSC precursors, which appear to be pericytes or mural cells (da Silva Meirelles et al., 2008; Rodeheffer et al., 2008; Tang et al., 2008). Consistent with this finding, we observed a reduction in the pericyte markers α-Sma, NG2, Vcam1, and PDGFRβ in S6K1−/− adipose tissue. Taken together, our studies suggest that S6K1 is a modulator of cell fate at very early stages of development, and that absence of the gene in vivo acts to suppress diet-induced obesity by preventing the increase of white adipose tissue mass (Figure 7). Interestingly, similar to ADSCs, rapamycin inhibits the differentiation of primary mouse bone marrow stromal cells into osteoblasts (Singha et al., 2008). However, although S6K1−/− mice remain lean as a function of age, they display an attenuation of age-dependent loss of cancellous bone (Selman et al., 2009). This raises the possibility that inhibitors of the S6K1 branch of mTORC1 signaling, may be useful in the treatment of obesity and associated diseases, as well as protective against age-related pathologies such as osteoporosis.
S6K1 and stem cell commitment to the adipocyte lineage
Given the central role of stem cell biology in tissue replacement, it has become important to understand the underlying molecular mechanisms that coordinate the distinct cell differentiation programs involved in tissue development and remodeling (Xu et al., 2008). Here we show that absence of S6K1 plays a critical role in modulating the lineage specificity of early adipocyte progenitors by limiting the number of preadipocytes during development and throughout adult life. Consistent with this finding, the two upstream activating S6K1 kinases, mTORC1 and the phosphoinositide-dependent protein kinase (PDK1), are known to regulate ESC growth, stem cell self-renewal, embryo development, and germ layer differentiation (Bone and Welham, 2007; Gangloff et al., 2004; Takahashi et al., 2005; Zhou et al., 2009). The data presented here demonstrate that S6K1 is involved in the control of proliferation and differentiation of stem cells. In agreement with this, we (Gangloff et al., 2004) and others (Murakami et al., 2004) demonstrated that trophoblasts and ESCs, the latter isolated from the blastocyst inner cell mass (ICM), fail to proliferate when derived from mTOR−/− embryos. That these effects are elicited through mTORC1 is supported by recent studies demonstrating that rapamycin treatment of ESCs inhibits EB cell proliferation, resulting in reduced EB size and cellularity (Sampath et al., 2008). However, these effects were largely attributed to the 4E-BPs, whereas our studies suggest they are due to S6K1. This conclusion is consistent with the decreased cell proliferation and total EB size seen in ESCs deficient for PDK1, an upstream effector of S6K1, but not of the 4E-BPs (Bone and Welham, 2007). The effects of PDK1 were initially attributed to its role as a downstream effector of class IA PI3K (Bone and Welham, 2007; Riley et al., 2006). However, it should be noted that PDK1 phosphorylation and activation of S6K1 does not require increased phosphatidylinositide-3,4,5-P3 (Pullen et al., 1998). Instead, it requires only mTORC1 phosphorylation at S6K1 T389, which acts as a docking site for PDK1 (Biondi et al., 2001). Moreover, mTORC1 activation and early embryo development are driven by nutrients, such as amino acids (Gangloff et al., 2004), which can act independently of growth factors and class IA PI3K to drive full mTORC1 activation (Hara et al., 1998). Indeed, stimulation of mTORC1, by amino acids alone, drives the expression of PPARγ, a master regulator of adipogenesis (Kim and Chen, 2004). These findings raise the possibility that the effects of S6K1 on EB formation may not only rely on class 1A PI3K (see below), but also on nutrient input to mTORC1.
A potential mediator of S6K1 activation during early adipocyte development is RA (Dani et al., 1997). It has been shown that embryonic stem cell commitment to the adipocyte lineage is dependent on RA activation of ERK and GSK3 (Bost et al., 2002; Monteiro et al., 2009). Upon RA treatment, S6K1 T389 phosphorylation is increased in control EBs and this activation is suppressed by rapamycin (Fig. 6B), arguing that this is an mTORC1-dependent event. Moreover, we demonstrate that the RA-induced expression of early adipogenic transcription factors, including Krox20, KLF5, C/EBPβ, and C/EBPδ, is reduced in S6K1-depleted EBs, as compared with WT. The two isoforms of S6K1, which are generated from the same transcript through alternative translational initiation start sites (Montagne and Thomas, 2004), can localize to the nucleus (Panasyuk et al., 2006) and have been implicated in the control of mRNA transcription and processing at multiple levels. S6K1 has been shown to directly phosphorylate and regulate a number of transcription factors including cAMP responsive element modulator τ (CREMτ) (de Groot et al., 1994) and estrogen receptor α, (ERα) (Yamnik et al., 2009), as well as the RNA Polymerase 1 upstream binding factor (UBF) (Hannan et al., 2003). However, S6K1 can also act indirectly on transcription, as we have recently demonstrated in the case of stabilization of c-Myc through the phosphorylation and inactivation of GSK3β (Gulati et al., 2008), and as others have shown through the regulation of the exon junction complex (Ma et al., 2008). Present studies are aimed at elucidating the level at which S6K1 exerts an effect on the early adipogenic transcription factors.
In summary, we show that, in addition to the metabolic effects of S6K1 depletion on adipose tissue development, S6K1 and mTORC1 signaling pathway play a major role in early stages of adipogenesis by affecting the ability of ESCs to commit to the adipocytic lineage. These findings suggest that pharmacological intervention in the nutrient-sensing mTORC1 pathway is a relevant and promising target in tackling the epidemic in obesity, as recently suggested by others (Chang et al., 2009). Our results also suggest that the mTORC1 signaling pathway is critical in stem cell biology and in modulating differentiation programs, such that augmenting the activity of this pathway could be efficacious in regenerative medicine.
EXPERIMENTAL PROCEDURES
Mice
S6-kinase–deficient mice, created previously by homologous recombination, were genotyped as described (Shima et al., 1998) and kept in a hybrid 129/svXBalb/C or inbred C57Bl/6 background. Eif4ebp1 and Eif4ebp2 mutant mice were generated as previously described (Banko et al., 2005; Tsukiyama-Kohara K, 2001). For the generation of triple knockout (TKO) mice, S6K1−/− (129/svXBalb/C) were crossed with 4E-BP1/2 DKO (C57BL/6XDBA/2J). Mice were housed in plastic cages and maintained at 22°C with a 12-hour dark, 12-hour light schedule. Eight-week-old animals were fed either a control NCD (D12450B; 20% protein, 70% carbohydrate, and 10% fat) or a HFD (D12492; 20% protein, 20% carbohydrate, and 60% fat) for 16 weeks (Research Diets Inc.). All studies were IACUC approved, and performed according to the ethical guidelines of the University of Cincinnati and the McGill University Animal Resource Center.
Histology and morphometric analysis of tissues
Adipose tissue was analyzed by examining H&E-stained histological sections as previously described (Picard et al., 2002). Morphometric analysis of epididymal WAT was performed by measuring 200 or more cells from at least three H&E sections per mouse and genotype (n=3 mice) with the aid of ImageJ software (NIH) and AdiposeTissueAnalyzer v2.1 (Tiago Ferreira). To quantify cell number, adipocytes were isolated from 100mg of adipose tissue by using 2mg/ml collagenase type II-S (Sigma C-1764) digestion buffer, and counted using a hemacytometer. Results were normalized to the fat-pad weight of each mouse (n=3).
Western blot analysis and antibodies
Cells were harvested in lysis buffer. Proteins were resolved by SDS-PAGE and transferred to ECL-Hybond nitrocellulose membranes (GE healthcare). Antibodies against total S6K1 were purchased from BD Biosciences, and antibodies against β-actin, mTOR, Thr389-phosphorylated S6K1, Ser473 and Thr-308 phospho-PKB/Akt, S6, and Ser240/244-phosphorylated S6 were purchased from Cell Signaling. The aP2 antibody was purchased from Cayman Chemical. See protocol details in Supplemental Experimental Procedures
Adipocyte differentiation of primary MEFs and ADSCs
Primary MEFs (passage 0 or 1) from 13.5- to 14.5-day-old embryos were grown to confluence (day 0) in maintenance medium (DMEM containing 10% FBS). Adipocyte differentiation was induced for 2 days by treating confluent cells for 48 hours in differentiation medium supplemented with 500 μM 3-Isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich), 1μM dexamethasone (DEX) (Sigma-Aldrich), and insulin 10μg/ml (Sigma-Aldrich). After the induction period, the medium was replaced with differentiation medium supplemented with 1μM ciglitazone (Sigma-Aldrich), which was then changed every second day. After 8 additional days in differentiation medium (day 10), the cells exhibited a fully differentiated phenotype with massive accumulation of multilocular fat droplets. For ADSC differentiation, adipocytes and their precursor cells were isolated from WT and S6K1−/− epididymal and inguinal white adipose tissue by collagenase digestion, as previously described (Prunet-Marcassus et al., 2006). After centrifugation, cells from the pellet were cultured on tissue-culture plates. Cells attaching to the plates constitute ADSCs, which were trypsinized and seeded at the same concentration. Confluent cells were differentiated by adding 10μg/ml insulin, 1 nM 3,3′,5-triiodo- L-thyronine (T3, Sigma-Aldrich), and 125 μM indomethacin (Sigma-Aldrich). The medium was changed every 2 days and fully differentiated cells were analyzed after 9 days. Three to four mice of each genotype were used per experiment. Experiments were carried out in duplicate.
ESC growth and differentiation
C57BL/6J ESCs were a gift from Dr. Jon Neuman, and CCE cells, a mouse ESC line derived from the 129/Sv mouse strain, were donated by STEMCELL Technologies. ESCs were grown in DMEM supplemented with 15% ES FCS (GIBCO), 1x non-essential amino acids (Sigma-Aldrich), 50 μM β-mercaptoethanol (Sigma-Aldrich), 0.1% sodium bicarbonate (Sigma-Aldrich), 100 IU/ml penicillin, 100μg/ml streptomycin (Hyclone), and 1000 units/ml murine leukemia inhibitory factor (LIF) (ESGRO; Chemicon) (ES cell Medium). Adipocyte differentiation was performed as described in Dani et al., 2001. Briefly, 1 to 5 ×103 cells were seeded (day0) in hanging drops on bacteriological plate lids and allowed to form EBs for two days in ES cell medium without LIF. EBs were then transferred to plates and kept in suspension. After 24 hrs (day 3), the EBs were incubated with 1μM all-trans-retinoic acid (RA) (Sigma-Aldrich) for 3 days (till day6), with or without 20nM rapamycin. After RA treatment, the spherical EBs were further cultured on gelatin-coated tissue-culture plates for the induction of adipogenic differentiation. After 48 hours of adaptation in maintenance medium (day 8 from start), 0.5μg/ml insulin and 2nM triiodothyronine (T3) was added to the medium to initiate adipocyte differentiation. Cells were kept in differentiation medium for 2 additional weeks, with fresh medium added every 2 days.
Oil Red O staining and triglyceride content measurement are described in Supplemental Experimental Procedures
Image Analysis
Axion Vision 4.0 software was used to count cells, to quantify adipocyte number by Oil Red O staining, to determine EB size by calculating area and diameter, and to measure Oil Red O staining intensity.
Isolation of RNA, Quantitative RT-PCR, and Microarray Analyses
RNA extraction, Quantitative RT-PCR and Microarray analyses of gene expression [deposited in the Gene Expression Omnibus (GEO) under accession number GSE20349] were performed as described in Supplemental Experimental Procedures.
Statistical Analysis
Data are presented as mean ± SEM. The effects were analyzed by one-way ANOVA or two-tailed unpaired t test. Analyses were performed using GraphPad Prism software (San Diego, CA). Differences were considered to be statistically significant at P<0.05 versus WT.
Supplementary Material
Acknowledgments
The authors want to thank V. Zimmerman for technical assistance, J.C. Neumann for providing mESCs, C. Gallo and D. Plas for assistance with the FACS analysis, and M. Medvedovic, J. Chen and B. Aronow for microarray analysis. The microarray data analysis and management is funded by NIEHS grant P30-ES006096. We would also like to thank G. M. Keller, O. MacDougald and C. R. Kahn for fruitful discussions as well as Y. Zheng, M. Daston, and G. Doerman for their critical reading of the manuscript, editing, and drawing of figures, respectively. These studies were supported by a fellowship on adipogenesis from Procter and Gamble Pharmaceuticals to LC; an NIH grant 5R01DK073802 to G.T., who is the John and Gladys Strauss Professor of Cancer Biology; and a grant from the CIHR (MOP-37968) to N.S. who is a Howard Hughes Medical Institute International Research Scholar and a James McGill Professor. IT is a Special Fellow of the Leukemia and Lymphoma Society (USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, et al. S6 Kinase Deletion Suppresses Muscle Growth Adaptations to Nutrient Availability by Activating AMP Kinase. Cell Metab. 2007;5:476–487. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi RM, Kieloch A, Currie RA, Deak M, Alessi DR. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. Embo J. 2001;20:4380–4390. doi: 10.1093/emboj/20.16.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy K, Chen Z, Friedman J. Transcriptional regulation of adipogenesis by KLF4. Cell Metab. 2008;7:339–347. doi: 10.1016/j.cmet.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone HK, Welham MJ. Phosphoinositide 3-kinase signalling regulates early development and developmental haemopoiesis. Journal of cell science. 2007;120:1752–1762. doi: 10.1242/jcs.003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost F, Caron L, Marchetti I, Dani C, Le Marchand-Brustel Y, Binetruy B. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem J. 2002;361:621–627. doi: 10.1042/0264-6021:3610621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Chang GR, Chiu YS, Wu YY, Chen WY, Liao JW, Chao TH, Mao FC. Rapamycin Protects Against High Fat Diet-Induced Obesity in C57BL/6J Mice. J Pharmacol Sci. 2009;109:496–503. doi: 10.1254/jphs.08215fp. [DOI] [PubMed] [Google Scholar]
- Chen Z, Torrens JI, Anand A, Spiegelman BM, Friedman JM. Krox20 stimulates adipogenesis via C/EBPbeta-dependent and -independent mechanisms. Cell Metab. 2005;1:93–106. doi: 10.1016/j.cmet.2004.12.009. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- Dani C, Smith AG, Dessolin S, Leroy P, Staccini L, Villageois P, Darimont C, Ailhaud G. Differentiation of embryonic stem cells into adipocytes in vitro. Journal of cell science. 1997;110(Pt 11):1279–1285. doi: 10.1242/jcs.110.11.1279. [DOI] [PubMed] [Google Scholar]
- Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007 doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- de Groot RP, Ballou LM, Sassone-Corsi P. Positive regulation of the cAMP-responsive activator CREM by the p70 S6 kinase: An alternative route to mitogen-induced gene expression. Cell. 1994;79:81–91. doi: 10.1016/0092-8674(94)90402-2. [DOI] [PubMed] [Google Scholar]
- El-Chaar D, Gagnon A, Sorisky A. Inhibition of insulin signaling and adipogenesis by rapamycin: effect on phosphorylation of p70 S6 kinase vs eIF4E-BP1. Int J Obes Relat Metab Disord. 2004;28:191–198. doi: 10.1038/sj.ijo.0802554. [DOI] [PubMed] [Google Scholar]
- Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-Site Inhibitors of mTOR Target Rapamycin-Resistant Outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmy-Susini B, Jin H, Zhu Y, Sung RJ, Hwang R, Varner J. Integrin alpha4beta1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J Clin Invest. 2005;115:1542–1551. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan KM, Brandenburger Y, Jenkins A, Sharkey K, Cavanaugh A, Rothblum L, Moss T, Poortinga G, McArthur GA, Pearson RB, et al. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol. 2003;23:8862–8877. doi: 10.1128/MCB.23.23.8862-8877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- Hishida T, Nishizuka M, Osada S, Imagawa M. The role of C/EBPdelta in the early stages of adipogenesis. Biochimie. 2009;91:654–657. doi: 10.1016/j.biochi.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58:1550–1557. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James WP, Rigby N, Leach R. Obesity and the metabolic syndrome: the stress on society. Annals of the New York Academy of Sciences. 2006;1083:1–10. doi: 10.1196/annals.1367.002. [DOI] [PubMed] [Google Scholar]
- Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Chen J. regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes. 2004;53:2748–2756. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- Koh YK, Lee MY, Kim JW, Kim M, Moon JS, Lee YJ, Ahn YH, Kim KS. Lipin1 is a key factor for the maturation and maintenance of adipocytes in the regulatory network with CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma 2. J Biol Chem. 2008;283:34896–34906. doi: 10.1074/jbc.M804007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest. 2007;117:387–396. doi: 10.1172/JCI29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Yoon SO, Richardson CJ, Julich K, Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell. 2008;133:303–313. doi: 10.1016/j.cell.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Montagne J, Thomas G. S6K Integrates Nutrient anf Mitogenic Signals to Control Cell Growth. Cold Spring Laboratory Press; 2004. [Google Scholar]
- Monteiro MC, Wdziekonski B, Villageois P, Vernochet C, Iehle C, Billon N, Dani C. Commitment of mouse embryonic stem cells to the adipocyte lineage requires retinoic acid receptor beta and active GSK3. Stem cells and development. 2009;18:457–463. doi: 10.1089/scd.2008.0154. [DOI] [PubMed] [Google Scholar]
- Morgan KP, Kapur A, Beatt KJ. Anatomy of coronary disease in diabetic patients: an explanation for poorer outcomes after percutaneous coronary intervention and potential target for intervention. Heart. 2004;90:732–738. doi: 10.1136/hrt.2003.021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–6718. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Noel D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, Jorgensen C, Cousin B. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res. 2008;314:1575–1584. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Nueda ML, Garcia-Ramirez JJ, Laborda J, Baladron V. dlk1 specifically interacts with insulin-like growth factor binding protein 1 to modulate adipogenesis of 3T3-L1 cells. J Mol Biol. 2008;379:428–442. doi: 10.1016/j.jmb.2008.03.070. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, Nishimura G, Maemura K, Yamauchi T, Kubota N, et al. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Panasyuk G, Nemazanyy I, Zhyvoloup A, Bretner M, Litchfield DW, Filonenko V, Gout IT. Nuclear export of S6K1 II is regulated by protein kinase CK2 phosphorylation at Ser-17. J Biol Chem. 2006;281:31188–31201. doi: 10.1074/jbc.M602618200. [DOI] [PubMed] [Google Scholar]
- Phillips BW, Vernochet C, Dani C. Differentiation of embryonic stem cells for pharmacological studies on adipose cells. Pharmacol Res. 2003;47:263–268. doi: 10.1016/s1043-6618(03)00035-5. [DOI] [PubMed] [Google Scholar]
- Picard F, Gehin M, Annicotte J, Rocchi S, Champy MF, O’Malley BW, Chambon P, Auwerx J. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111:931–941. doi: 10.1016/s0092-8674(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Prunet-Marcassus B, Cousin B, Caton D, Andre M, Penicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res. 2006;312:727–736. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- Riley JK, Carayannopoulos MO, Wyman AH, Chi M, Moley KH. Phosphatidylinositol 3-kinase activity is critical for glucose metabolism and embryo survival in murine blastocysts. J Biol Chem. 2006;281:6010–6019. doi: 10.1074/jbc.M506982200. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Sampath P, Pritchard DK, Pabon L, Reinecke H, Schwartz SM, Morris DR, Murry CE. A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell. 2008;2:448–460. doi: 10.1016/j.stem.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Curr Biol. 2002;12:632–639. doi: 10.1016/s0960-9822(02)00762-5. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003;52:232–238. doi: 10.2337/diabetes.52.2.232. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70s6k/p85s6k gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17:6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singha UK, Jiang Y, Yu S, Luo M, Lu Y, Zhang J, Xiao G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. Journal of cellular biochemistry. 2008;103:434–446. doi: 10.1002/jcb.21411. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Murakami M, Yamanaka S. Role of the phosphoinositide 3-kinase pathway in mouse embryonic stem (ES) cells. Biochem Soc Trans. 2005;33:1522–1525. doi: 10.1042/BST0331522. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama-Kohara K PF, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, Harper ME, Tremblay ML, Sonenberg N. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med. 2001;7:1128–1132. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- von Manteuffel SR, Dennis PB, Pullen N, Gingras AC, Sonenberg N, Thomas G. The insulin-induced signalling pathway leading to S6 and initiation factor 4E binding protein 1 phosphorylation bifurcates at a rapamycin-sensitive point immediately upstream of p70s6k. Mol Cell Biol. 1997;17:5426–5436. doi: 10.1128/mcb.17.9.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Huang L, Huang Y, Yin JW, Berk AJ, Friedman JM, Wang G. Mediator MED23 links insulin signaling to the adipogenesis transcription cascade. Dev Cell. 2009;16:764–771. doi: 10.1016/j.devcel.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453:338–344. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]
- Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem. 2009;284:6361–6369. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Bierer BE, McKnight SL. Rapamycin inhibits clonal expansion and adipogenic differentiation of 3T3-L1 cells. Proc Natl Acad Sci U S A. 1995;92:11086–11090. doi: 10.1073/pnas.92.24.11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HH, Huang J, Duvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One. 2009;4:e6189. doi: 10.1371/journal.pone.0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Su P, Wang L, Chen J, Zimmermann M, Genbacev O, Afonja O, Horne MC, Tanaka T, Duan E, et al. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci U S A. 2009;106:7840–7845. doi: 10.1073/pnas.0901854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.