Summary
Nuclear magnetic resonance (NMR) spectroscopy enables determination of membrane protein structures in lipid environments, such as micelles and bilayers. This chapter outlines the steps for membrane-protein structure determination using solution NMR with micelle samples, and solid-state NMR with oriented lipid-bilayer samples. The methods for protein expression and purification, sample preparation, and NMR experiments are described and illustrated with examples from γ and CHIF, two membrane proteins that function as regulatory subunits of the Na+- and K+-ATPase.
Keywords: Bilayer membrane, expression, FXYD, lipid, micelle, NMR, protein, structure
1. Introduction
Integral membrane proteins constitute approx 30% of all expressed genes, and are the major regulators of the most basic cellular functions as well as the major targets for drug discovery initiatives. Despite membrane protein prevalence and importance, the Protein Data Bank (www.rcsb.org/pdb) contains only hundreds of membrane-protein structures, compared with the tens of thousands deposited for globular proteins to-date. This disparity is because of the lipophilic character of membrane proteins, which makes them difficult to overexpress and purify, and complicates their crystallization for X-ray analysis. The examples of membrane proteins whose structures have been determined with atomic resolution are exceptional, and highlight the importance of developing new methods for experimental structure determination.
Because the physical interactions of the lipid bilayer with membrane proteins are more important in determining protein stability and fold than specific lipid-binding interactions, it is desirable to determine protein structures within the lipid-bilayer environment. Nuclear magnetic resonance (NMR) spectroscopy has the potential to accomplish this goal because it can be applied to molecules in all physical states, including the liquid-crystalline bilayer and the micelle environments provided by the lipids that associate with membrane proteins. Solution NMR methods can be used on samples of proteins in lipid micelles, while solid-state NMR methods can be applied to samples of membrane proteins in lipid bilayers, enabling structures to be determined in a native-like environment. The two approaches are complementary, and can be used in combination as a unified method to membrane-protein structure determination.
High-quality solution NMR spectra can be obtained for some fairly large-membrane proteins in micelles, however, for helical proteins it is very difficult to measure and assign a sufficient number of long-range nuclear overhauser effect (NOE) restraints to determine protein folds. This limitation can be overcome by preparing weakly aligned micelle samples for the measurement of residual dipolar couplings (RDCs) and residual chemical shift anisotropies. High-resolution solid-state NMR spectra can be obtained for membrane proteins that are expressed, isotopically labeled, and reconstituted in uniaxially oriented planar lipid bilayers. The spectra have characteristic resonance patterns that directly reflect protein structure and topology, and this direct relationship between spectrum and structure provides the basis for methods that enable the simultaneous sequential assignment of resonances and the measurement of orientation restraints for protein structure determination.
Recent developments in sample preparation, recombinant bacterial expression systems for the preparation of isotopically labeled membrane proteins, pulse sequences for high-resolution spectroscopy, and structural indices that guide the structure assembly process, have greatly extended the capabilities of these NMR techniques. Thus, structures of a variety of membrane proteins have been determined by NMR in both micelles and bilayers (1–9). In this chapter, the methods are illustrated with examples from γ (FXYD2) and CHIF (FXYD4, channel-inducing factor, corticosteroid hormone-induced factor), two homologous membrane proteins that function as regulatory subunits of the Na+-, K+-ATPase, the primary enzyme responsible for maintaining the distribution of Na+ and K+ concentrations across animal cell membranes (10–12).
FXYD2 and CHIF belong to the FXYD family of Na+-, K+-ATPase regulatory membrane proteins, and are each expressed in distinct, specialized segments of the kidney, with unique expression patterns that help explain the physiological differences in Na+-, K+-ATPase activity among the nephron segments (13–17). The FXYD protein sequences are highly conserved through evolution, and characterized by a 35-amino acid FXYD homology domain, which includes the short signature motif of the family (Pro, Phe, X, Tyr, and Asp) and a single transmembrane domain. Conserved basic residues flank the transmembrane domain, the extracellular N-termini are acidic, and the cytoplasmic C-termini are basic. Despite their relatively small sizes ranging from about 60 to 160 amino acids, all FXYD proteins are encoded by genes with six to nine small exons, and NMR has shown that the protein structures reflect the structures of their corresponding genes, suggesting that they were assembled from modules through exon shuffling (18).
2. Materials
The specialized materials used for the experiments described in this chapter, and their sources, are listed in Table 1. They include lipids for protein reconstitution, and Escherichia coli cells, isotopically labeled salts, sugars, and amino acids, used to produce 15N-, 13C-, and 2H-proteins by bacterial expression. The pBCL plasmids for protein expression (Fig. 1) were developed in our laboratory and are available on request. The sources of the free programs (NMRPipe, TALOS, XPLOR-NIH, Sparky, and REDCAT) used to process and analyze the NMR data are listed in Table 1.
Table 1.
Specialized Materials Used for the Experiments Described in This Chapter, and Their Sources
| Material | Source |
|---|---|
| Reagents | |
| DHPC | Avanti Polar Lipids (www.avantilipids.com) |
| DOPC (di-oleoyl-phosphatidyl-choline) | Avanti Polar Lipids (www.avantilipids.com) |
| DOPG (di-oleoyl-phosphatidyl-glycerol) | Avanti Polar Lipids (www.avantilipids.com) |
| LPPG | Avanti Polar Lipids (www.avantilipids.com) |
| OG | Fluka (www.sigmaaldrich.com) |
| 2H-SDS (2H-sodiumdodecylsulfate) | Cambridge Isotopes laboratories (www.isotope.com) |
| 2H-DPC (2H-dodecyl-phosphocholine) | Cambridge Isotopes laboratories (www.isotope.com) |
| 2H2O | Cambridge Isotopes laboratories (www.isotope.com) |
| (15NH4)2SO4 | Cambridge Isotopes laboratories (www.isotope.com) |
| 13C-glucose | Cambridge Isotopes laboratories (www.isotope.com) |
| E. coli C41(DE3) cells | Avidis (www.overexpress.com) |
| FF-S ion-exchange chromatography column | Amersham (www.amershambiosciences.com) |
| Delta-Pak C4 reverse-phase chromatography column |
Waters (www.waters.com) |
| Programs | |
| NMRPipe | (spin.niddk.nih.gov/bax/software) |
| TALOS | (spin.niddk.nih.gov/bax/software) |
| Sparky | www.cgl.ucsf.edu/home/sparky/ |
| XPLOR-NIH | (nmr.cit.nih.gov) |
| REDCAT | (tesla.ccrc.uga.edu/software) |
Fig. 1.
(A) Construction of the pBCL173 and pBCL99 fusion protein expression plasmids. The target sequence with N-terminal Met is inserted between the Afl II and Xho I cloning sites. BCL173 has a cleavable Met after the His tag, whereas BCL99 does not. (B) Amino acid sequence of the Bcl-XL fusion protein. Both the BCL173 and BCL99 fusion proteins lack the Bcl-XL hydrophobic C-terminus (highlighted in the gray box). BCL173 (solid underline) also lacks the flexible loop of Bcl-XL, while BCL99 (dotted underline) lacks the first 116 residues. (C) Amino acid sequences of the FXYD proteins γ-b and CHIF. The transmembrane domains are in the gray box.
3. Methods
3.1. Protein Expression and Purification
The FXYD proteins γ and CHIF, are expressed using the pBCL plasmid vector, which we have developed for the large-scale expression of membrane proteins (19). This plasmid directs the expression of a target polypeptide fused to the C-terminus of a mutant form of the antiapoptotic protein Bcl-XL, where the hydrophobic C-terminus has been deleted, to be replaced with a hydrophobic polypeptide gene of interest by insertion at an engineered cloning site (Afl II/Xho I), and Met residues have been mutated to Leu to facilitate CNBr cleavage after a single Met inserted at the beginning of the target sequence (Fig. 1A). In cases where the target protein contains Met residues that cannot be mutated, separation from BCL can be obtained by introducing amino acid sequences specific for cleavage by other chemical means, such as hydroxylamine (Asn-Gly), or for cleavage by one of the commonly used proteases: thrombin, factor Xa, enterokinase, and tobacco-etch virus protease. Chemical cleavage is an attractive option because it eliminates the difficulties—poor specificity and enzyme inactivation— often encountered with protease treatment of hydrophobic proteins in detergents. The plasmid utilizes a T7 expression system (20), and the fusion tag has an N-terminal (His)6 sequence for protein purification by Ni-affinity chromatography.
For the FXYD proteins, the expression levels obtained using pBCL are greater than those obtained using the TrpΔLE (pTLE) (21–23), or the ketosteroid isomerase (pKSI) (24) fusion protein expression systems. Thus, we could obtain milligram quantities of pure, isotopically labeled protein easily and quickly. After cleavage from the fusion partner, highly pure FXYD proteins were obtained using a combination of Ni-affinity, size exclusion, and reverse-phase chromatography, with yields in the range of 10 mg of purified protein per liter of culture in M9 minimal medium.
For protein expression, 5–10 µL of transformed C41(DE3) cells from a frozen glycerol stock were used to inoculate 10 mL of LB media, and grown for 5 h at 37°C with vigorous shaking. Then, 1 mL of this starter culture was added to 100 mL of minimal M9 media and grown overnight. All media contained 100 µg/mL of ampicillin. In the morning, 1 L of fresh M9 media was inoculated with the overnight culture, and the cells were grown to a cell density of OD600 = 0.7. Protein expression was induced by the addition of 1 mM IPTG for 4–5 h at 37°C. The cells were subsequently harvested by centrifugation and stored at −20°C overnight. For uniformly 15N-labeled proteins, (15NH4)2SO4 was supplied to the M9 salts as the sole nitrogen source.
Frozen cells from 1 L of culture were lysed by French press in 30 mL of buffer A (50 mM Tris-HCl, pH 8.0, 15% glycerol). The soluble fraction was removed by centrifugation (48,000g, 4°C, 30 min), and the pellet was washed twice by resuspension in 30 mL of buffer A, followed by centrifugation (48,000g, 4°C, 30 min) to remove the soluble fraction. The resulting pellet was dissolved in 30 mL of 6 M GdnHCl, and again centrifuged (48,000g, 4°C, 2 h) to remove any insoluble materials. The 6 M GdnHCl protein solution was adjusted to 0.1 N HCl (pH 0.2), a 100-fold molar excess of solid CNBr was added, and the mixture was allowed to react overnight, in the dark, at room temperature.
CNBr is extremely toxic by inhalation and must be weighed and handled in the fume hood. In the morning, the reaction mixture was dialyzed against water until the pH reached about 5.0 (6 h with several changes of 4 L of water, in a dialysis membrane with a molecular weight cutoff of 1 kD), lyophilized to powder, and dissolved in buffer B (20 mM Tris-HCl, pH 7.0, 8 M urea). Proteins were purified by ion-exchange chromatography with a NaCl gradient (FF-S column), or by size-exclusion chromatography in buffer C (20 mM Tris-HCl, pH 7.0, 4 mM sodium dodecyl-sulfate [SDS]), followed by preparative reverse-phase high-pressure liquid chromatography (C4 column) with a gradient of acetonitrile in water and 0.1% trifluoroacetic acid. Purified proteins were stored as lyophilized powder at −20°C. Alternatively, the lyophilized cleavage mixture could be dissolved directly in buffer C, and the protein purified with reverse-phase high-pressure liquid chromatography.
3.2. Structural Studies in Micelles
3.2.1. Solution NMR Experiments
The NMR experiments were performed on a Bruker AVANCE 600 MHz spectrometer (Billerica, MA) using a triple-resonance 1H/13C/15N-probe equipped with three-axis pulsed field gradients (www.bruker-biospin.com). All NMR experiments were performed at 40°C using a 1-s recycle delay. The chemical shifts were referenced to the 1H2O resonance, set to its expected position of 4.5999 ppm at 40°C (25). The NMR data were processed using NMRPipe (26), and the spectra were assigned and analyzed using Sparky (27). These programs are free and available for a variety of platforms.
The standard fast heteronuclear single-quantum correlation (fHSQC) experiment was used for isotropic samples with 1024 points in t2 and 256 in t1 (28). Backbone resonance assignments were made using a standard HNCA experiment with constant time evolution for 15N, and solvent suppression was accomplished with a water flip-back pulse after the original 1H–15N magnetization transfer (29–31). The spectra from selectively 15N-labeled protein samples were necessary to resolve assignment ambiguities because of the extensive overlap among the Cα-resonances that is typical of helical membrane proteins in micellees. 1H–15N heteronuclear NOE measurements were made using difference experiments with and without 3 s of saturation of the 1H resonances between scans (32).
The 1H–15N RDCs were measured using a sensitivity-enhanced 1H–15N IPAP (in-phase–antiphase) experiment modified for suppression of the NH2 signals from the acrylamide in the gel (33–35). The contribution to the RDC splitting from the isotropic scalar coupling was determined by performing the same experiment on an isotropic micelle sample, and subtracting the value of the isotropic J-coupling obtained from that measured for the weakly aligned gel sample.
3.2.2. Choosing the Right Detergent
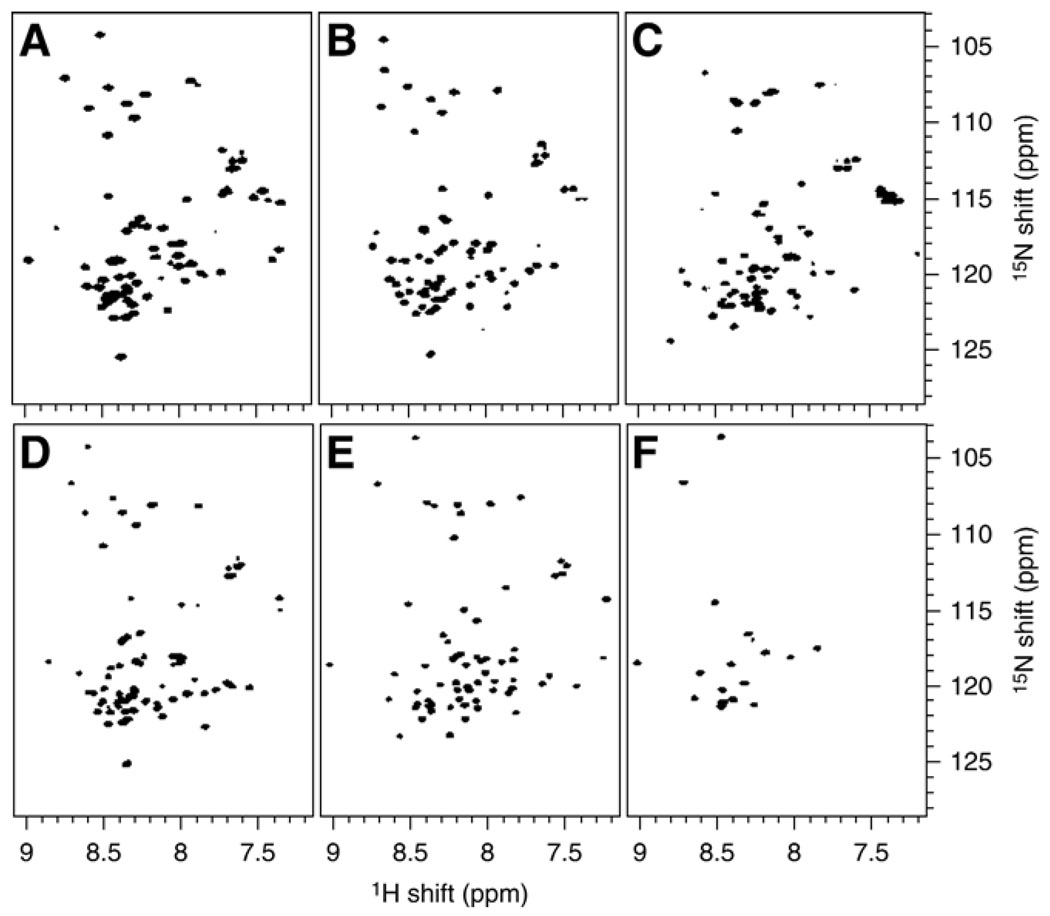
The first step in our structural studies of the FXYD family proteins was to find a detergent that would be suitable for high-resolution NMR spectroscopy. We examined the 1H/15N HSQC spectra of the proteins in the best-characterized micelle-forming detergents: dodecylphosphocholine (DPC), diheptanoyl-phosphocholine (DHPC), lyso-palmitoyl-phosphoglycerol (LPPG), octyl-glucopyranoside (OG), and SDS, at various conditions (protein, detergent, salt concentration, pH, and temperature) (Fig. 2). The highest quality spectra were obtained in SDS micelles, at 40°C (Fig. 2E,F).
Fig. 2.
2D 1H/15N HSQC spectra of uniformly 15N-labeled γ-b in detergent micelles. The samples were prepared by dissolving each protein in buffer (20 mM sodium citrate, pH 5.0, 10 mM DTT, and 1 mM sodium azide, in 90% H2O, 10% D2O) plus detergent. (A) 500 mM DPC; (B) 180 mM DHPC; (C) 100 mM LPPG; (D) 200 mM OG; and (E) 500 mM SDS. In (F) the spectrum of γ-b in 500 mM SDS was acquired 3 h after the addition of 100% D2O.
Although SDS is widely assumed to be an universal protein-denaturing detergent because of its common use in protein electrophoresis, many hydrophobic membrane proteins actually retain their structures in SDS micelles (36). Furthermore, since all functional studies of Na+, K+-ATPase have been done on enzymes purified in the presence of SDS, and the noncovalent associations of the α, β and FXYD subunits are maintained through the SDS purification process (37–39), we reasoned that this detergent would also be a good choice for FXYD structural studies.
3.2.3. Protein Structure Determination
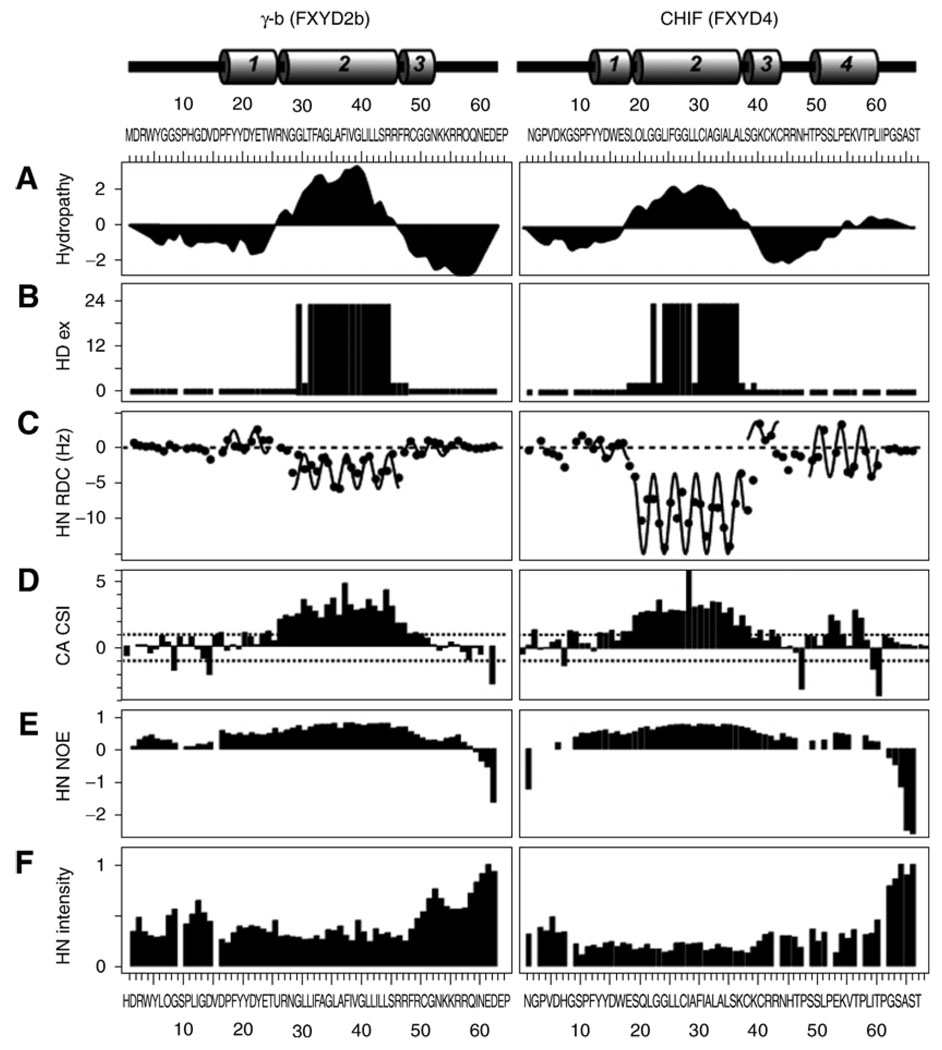
The HSQC spectra show that γ and CHIF adopt unique folded structures in SDS. The 1.5 ppm dispersion of the amide 1H chemical shifts is typical of native helical membrane proteins in micelles, and the HSQC spectra obtained in D2O revealed a core region in each protein, with amide protons that exchange very slowly with the surrounding aqueous solvent (Figs. 2F and 3B). These regions match the hydrophobic transmembrane segments of the proteins identified by the hydropathy plots (Fig. 3A), and reflect the strong intramolecular hydrogen bonds present in transmembrane helices in the low dielectric environment of the membrane, or, in this case, the micelle interior. Hydrogen exchange experiments were performed by dissolving the lyophilized protein in SDS buffer with 100% D2O, and then acquiring HSQC spectra at 0.5-h time intervals.
Fig. 3.
Summary of NMR parameters for γ-b and CHIF, plotted as a function of residue number. The protein secondary structures are shown at the top of the figure (helices 1–4 are numbered). The residue numbers for the three proteins begin after the signal sequences. (A) Kyte-Doolittle hydropathy plot (60); (B) amide hydrogen/deuterium exchange profiles, with exchange rates classified as rapid (<1 h), short (<3 h), medium (<12 h), or long (>24 h); (C) Values of 1H–15N RDCs with the characteristic periodicity in the α-helical regions of the proteins fitted to sinusoids; (D) 13Cα-chemical shift index; (E) 1H/15N heteronuclear NOEs; and (F) Normalized 1H/15N HSQC peak intensities. Positions that are left blank correspond to prolines or overlapped resonances.
To determine the protein secondary structures we relied primarily on 1H–15N RDCs, measured from weakly aligned samples and analyzed in terms of dipolar waves (40) (Fig. 3C). These were supplemented with chemical shifts, analyzed in terms of chemical shift indices (41) (Fig. 3D), and with the program TALOS (42). For the measurement of RDCs, the protein-SDS micelles were weakly aligned in 7% polyacrylamide gels, using either vertical compression (34,43) or expansion (44). These samples were prepared by soaking the protein micelle solution into a column of acrylamide that had been cast inside an NMR tube, and then rinsed and dried. After soaking, the acrylamide column was inserted inside an NMR tube with smaller diameter for stretched gels, as described by Bax and coworkers (44). The apparatus for preparing stretched gels is available from New Era Enterprises (www.newera-spectro.com). Alternatively, for compressed gels, the soaked acrylamide was inserted in a NMR tube with larger diameter and compressed with a glass plunger (www.shigeminmr.com).
The RDCs were analyzed using MATLAB scripts as described in refs. 40, 45, and 46. Helical regions were identified by applying a sliding window algorithm to fit the experimental RDCs. The RDCs within a five-residue window were fit to a sinusoid of periodicity 3.6, and the RMSD between the sinusoid and the data were plotted as a function of residue number. Continuous stretches of amino acids with low RMSD (less than the experimental error of 1.5 Hz) were identified as helices and fitted to a single sinusoid. Higher RMSDs were generally interpreted as deviations from ideality, including kinks, curvature, and loops. This analysis relating the orientation of the helix to the amplitude, average value, and phase of the sinusoid is an initial step toward structure determination, and can determine the relative orientations of helices to within four degenerate solutions (45,47).
The protein backbone dynamics are characterized with measurements of the 1H–15N heteronuclear NOE, and resonance intensities. Within the core-helical regions of the three proteins, all residues have similar positive values of 1H–15N NOE, reflecting similar rotational correlation times, and indicating that the three helices are rigidly connected (Fig. 3E). Lower negative values of the 1H–15N NOE, reflecting additional backbone motions, are present in residues near the N- and C-termini, and at the helix boundaries that are also marked by exon junctions. Furthermore, all of the resonances from amino acids within the central helical regions of the three proteins have similar peak intensities that plateau at minimum values (Fig. 3F), indicating that the helices are rigidly connected. In contrast, residues at the core module boundaries and at the terminal regions of the proteins have greater intensities, reflecting narrower linewidths that result from the increased dynamics in these regions.
An important finding of these structural studies is that the helical secondary structures of the FXYD family proteins reflect the structures of their corresponding genes (18). The coincidence of intron–exon junctions with helical structures and flexible connecting segments, support the hypothesis that the FXYD proteins may have been assembled from discrete structural modules through exon shuffling. Despite their relatively small sizes (60–160 residues), the FXYD family proteins are all encoded by genes with six to nine small exons, and this has been previously suggested to reflect modular gene assembly (10). The presence of conserved modules in different FXYD family members suggests that they are derived from a common ancestor gene, from which FXYD5 appears to have been the first to diverge (12). The multiple exon organization of the FXYD genes could serve to confer high structural and functional diversity among the family members.
3.4. Structural Studies in Lipid Bilayers
3.4.1. Solid-State NMR Experiments
The solid-state NMR spectra of membrane proteins in oriented lipid bilayers have frequencies that reflect the orientation of their respective sites relative to the direction of the magnetic field. Since the lipid-bilayer plane is perpendicular to the magnetic field direction, each resonance frequency reflects the orientation of its corresponding protein site in the membrane (48). The spectra were obtained at 23°C on a Bruker AVANCE 500 (Billerica) spectrometer with a wide-bore 500/89 Magnex magnet (Yarnton, UK). The double-resonance (1H/15N or 1H/31P) probes had square radiofrequency (rf) coils wrapped directly around the samples. The NMR data were processed using the programs NMRPipe (26). The 15N spectra were obtained with single contact 1-ms cross polarization with mismatch-optimized IS polarization transfer (CPMOIST) (49,50), and the 31P spectra with a single pulse. Both types of spectra were acquired with continuous 1H irradiation (rf field strength 63 kHz) to decouple the 1H–15N and 1H–31P dipolar interactions. The 15N and 31P chemical shifts were referenced to 0 ppm for liquid ammonia and phosphoric acid.
The polarization inversion with exchange at the magic angle (PISEMA) experiment (51) gives high-resolution, two-dimensional (2D), 1H–15N dipolar coupling and 15N chemical shift correlation spectra of oriented membrane proteins where the individual resonances contain orientation restraints for structure determination (4,52). The spectra were obtained with a cross polarization contact time of 1 ms, a 1H 90° pulse width of 5 µs, and continuous 1H decoupling of 63 kHz rf field strength. The 2D data were acquired with 512 accumulated transients and 256 complex data points, for each of 64 real t1 values incremented by 32.7 µs. The recycle delay was 6 s.
3.4.2. Sample Preparation
The samples were prepared by first dissolving 2 mg of 15N-labeled FXYD protein in 0.5 mL of trifluoroethanol with 50 µL of β-mercaptoethanol, and then adding 100 mg of lipid, di-oleoyl-phosphatidyl-choline/di-oleoyl-phosphatidyl-glycerol (8/2 molar ratio), in 1 mL of chloroform. After spreading this solution on the surface of 35 glass slides (dimensions 11 × 20 × 0.06 mm3) (Paul Marienfeld GmbH, Germany), the solvents were removed under vacuum overnight, and the slides were stacked. Oriented-lipid bilayers were formed by equilibrating the stacked slides for 24 h, at 40°C, in a chamber containing a saturated solution of ammonium phosphate, which provides an atmosphere of 93% relative humidity. The samples were wrapped in parafilm and then sealed in thin polyethylene film before insertion in the NMR probe. Hydrogen exchanged samples were prepared by exposing the stacked-oriented bilayer samples to an atmosphere saturated with 2H2O. This was achieved by placing the sample in a closed chamber containing 2H2O and incubating at 40°C for 24 h. Protein purity is crucial for obtaining highly oriented lipid-bilayer samples that give high-resolution NMR spectra.
3.4.3. 1D Solid-State 15N NMR Spectra
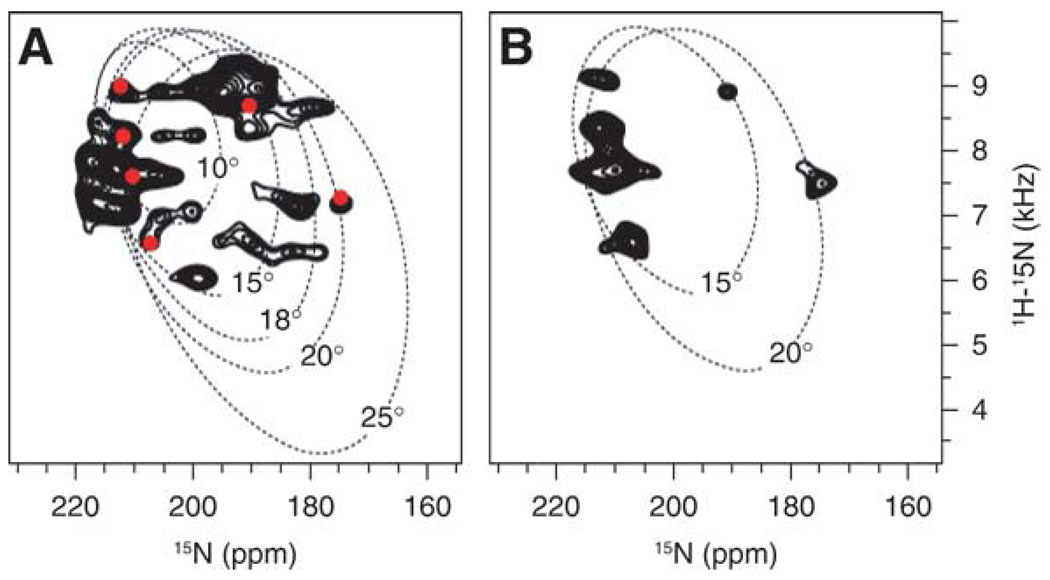
The spectrum of 15N-labeled CHIF in oriented lipid bilayers (Fig. 4B) reflects the presence of transmembrane and C-terminal helical segments similar to those found in micelles (53). It displays significant resolution throughout the frequency range of the 15N amide chemical shift. The resonance intensity near 200 ppm is from backbone-amide sites in the transmembrane helix, with the amide-NH bonds being located nearly perpendicular to the plane of the membrane. On the other hand, the intensity near 80 ppm is from sites in the N- and C-termini of the protein, with the NH bonds being located nearly parallel to the membrane surface. The peak near 35 ppm is from the amino groups of the lysine sidechains and the N-terminus. The narrow chemical shift dispersion in the frequency range near 200 ppm associated with transmembrane helices indicates that the protein crosses the membrane with only a very small tilt angle, which is estimated to be around 15° from the 2D 1H/15N PISEMA spectrum (Fig. 5).
Fig. 4.
Solid-state NMR 15N and 31P chemical shift spectra of uniformly 15N-labeled CHIF in unoriented (A,D) and oriented (B,C,E) lipid bilayers. Resonances near 200 ppm are from amino acid residues in the CHIF transmembrane helix; (C) amide hydrogens in the transmembrane helix of CHIF are resistant to hydrogen exchange, and their resonances remain after exposure to D2O while the resonances from exchangeable hydrogens disappear. The15N and 31P chemical shifts are referenced to 0 ppm for liquid ammonia and phosphoric acid.
Fig. 5.
1H/15N solid-state NMR PISEMA spectra of uniformly 15N-labeled (A) and Leu 15N-labeled (B) CHIF in oriented lipid bilayers. In (A) the spectrum is superimposed on the Pisa wheels calculated for ideal α-helices with different tilts in the lipid bilayer, and on the spectrum of 15N-Leu labeled CHIF, which is represented as red dots. 15N chemical shifts are referenced to 0 ppm for liquid ammonia.
The spectrum of the oriented sample is strikingly different from that of the unoriented sample, which provides no resolution among resonances (Fig. 4A). Most of the backbone sites are structured and immobile on the time-scale of the 15N chemical shift interaction (10 kHz), contributing to the characteristic amide powder pattern between about 220 and 60 ppm. Some of the backbone sites, in the loop and terminal regions, are mobile, giving rise to the resonance band centered near 120 ppm (the isotropic frequency). Some resonances near 120 ppm, however, may reflect specific orientations of their corresponding sites.
Amide hydrogen exchange rates are useful for identifying residues that are involved in hydrogen-bonding and exposed to water. Although lipid bilayers are permeable to water and other small polar molecules, the amide hydrogens in transmembrane helices can have very slow exchange rates because of strong hydrogen bonds in the low dielectric of the lipid-bilayer environment, and their 15N chemical shift NMR signals persist for days after exposure to D2O. Faster exchange rates are observed for transmembrane helices that are not tightly hydrogen-bonded and are exposed to bulk water because they participate in channel pore formation (54). Faster exchange rates are also observed for other water-exposed helical regions of proteins with weaker hydrogen-bonded networks. Many of the amide hydrogens exchange when the CHIF sample is exposed to D2O. Since the signals are generated by cross polarization from 1H, the amide resonances, thus, disappear from the spectrum (Fig. 4C). However, the amide hydrogens in the transmembrane helix did not exchange, and their signals persisted in the spectrum, indicating that the CHIF transmembrane helix forms a tight hydrogen bonding network that is resistant to hydrogen exchange.
3.4.4. 31P NMR Spectra of the Membrane Lipids
The phospholipid phase and the degree of phospholipid-bilayer alignment can be assessed with 31P NMR spectroscopy of the lipid phosphate headgroup. The 31P NMR spectrum obtained for unoriented bilayer vesicles containing CHIF (Fig. 4D) is characteristic of lipids in a bilayer arrangement, while the spectrum for oriented lipids with CHIF has a single resonance near 30 ppm that is characteristic of oriented lipid-bilayer membranes (Fig. 4E). The presence of a single peak demonstrates that the samples are highly oriented, as required for NMR structure determination. Thus, taken together, the 15N and 31P spectra provide evidence that the FXYD proteins insert in membranes without disruption of the membrane structure.
3.4.5. 2D Solid-State 1H/15N Correlation Spectra: PISEMA
The PISEMA spectra of membrane proteins in oriented lipid bilayers provide sensitive indices of protein secondary structure and topology because they exhibit characteristic wheel-like patterns of resonances, called Pisa wheels, that reflect helical wheel projections of residues in both α-helices and β-sheets (55–57). When a Pisa wheel is observed, no assignments are needed to determine the tilt of a helix, and a single resonance assignment can be sufficient to determine the helix rotation in the membrane. This information is extremely useful for determining the supramolecular architectures of membrane proteins and their assemblies. The shape and position of the Pisa wheel in the spectrum depends on the protein secondary structure and its orientation relative to the lipid-bilayer surface, as well as the amide N–H bond length and the magnitudes and orientations of the principal elements of the amide 15N chemical shift tensor. This direct relationship between spectrum and structure makes it possible to calculate solid-state NMR spectra for specific structural models of proteins, and provides the basis for a method for backbone structure determination from a limited set of uniformly and selectively 15N-labeled samples (4,58).
The 2D 1H/15N PISEMA spectra of uniformly and selectively Leu 15N-labeled CHIF in lipid bilayers are shown in Fig. 5. The Pisa wheel that is observed in the region from 6 to 10 kHz and 180 to 220 ppm in the spectrum, provides definitive evidence that the protein associates with the lipid bilayer as a transmembrane helix. To estimate the tilt of the CHIF transmembrane helix we compared the experimental spectrum with those calculated for an ideal α-helix, with 3.6 residues per turn and identical backbone dihedral angles for all residues (ϕ, φ = 57°, −47°), tilted at 10°, 15°, and 20° relative to the lipid-bilayer normal. This comparative analysis demonstrates that the CHIF helix is tilted by about 15° in the membrane (or 75° from the membrane surface). According to the solution NMR data in micelles, the peaks in the spectrum of 15N-Leu-labeled CHIF should account for Leu 17 and 19 in helix 1 preceding the transmembrane helix (helix 2), and Leu 22, 27, 28, 35, and 37, in the transmembrane helix. The data suggest that the peaks in the PISEMA spectrum will have to be fitted with Pisa wheels of different tilts, in agreement with the results obtained in micelles showing that the CHIF helices 1 and 2 have different orientations.
4. Conclusions
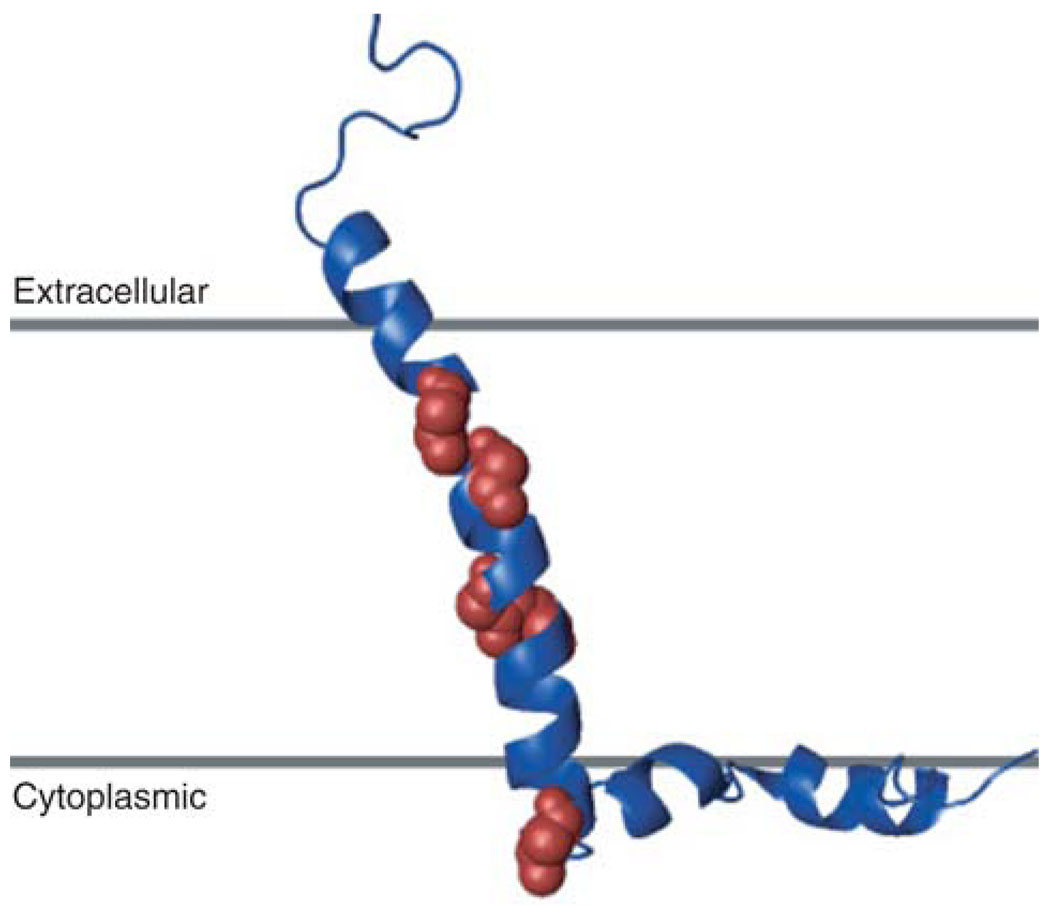
The structure of CHIF (Fig. 6) was determined by combining the restraints from measurements of RDCs, chemical shift, and H/D exchange, obtained by solution NMR in micelles, with the 15° tilt of the transmembrane helix obtained from solid-state NMR experiments in oriented lipid bilayers. Protein structures were calculated from the experimental data using a basic simulated annealing protocol in the program X-PLOR-NIH (9,59). Because helices 1, 2, 3, and 4 are rather rigidly connected, their relative orientations could be obtained from the combined measurements of RDCs and chemical shifts, and their analysis using the programs REDCAT (47) and TALOS (42). Additional measurement of RDCs from a sample with a different alignment would yield the helix orientations unambiguously.
Fig. 6.
Structure of CHIF determined by combining the NMR restraints obtained in micelles with those obtained in lipid bilayers. Leucine residues are shown as spheres.
The combination of solution NMR with lipid micelle samples, and solid-state NMR with lipid-bilayer samples, is a powerful approach for determining the structure of a membrane protein in an environment that closely resembles the biological membrane. New and ongoing developments in protein-expression systems, methods for reconstitution, NMR experiments and equipment, and computational methods, are bringing ever more complex membrane proteins into the range of molecules whose structures can be determined by NMR.
Acknowledgments
This research was supported by grants from the National Institutes of Health (CA082864, GM065374). The NMR studies utilized the Burnham Institute NMR Facility and the UCSD Resource for Molecular Imaging of Proteins, each supported by grants from the National Institutes of Health (P30CA030199, P41EB002031).
References
- 1.Ketchem RR, Hu W, Cross TA. High-resolution conformation of gramicidin A in a lipid bilayer by solid-state NMR. Science. 1993;261:1457–1460. doi: 10.1126/science.7690158. [DOI] [PubMed] [Google Scholar]
- 2.Opella SJ, Marassi FM, Gesell JJ, et al. Structures of the M2 channel-lining segments from nicotinic acetylcholine and NMDA receptors by NMR spectroscopy. Nat. Struct. Biol. 1999;6:374–379. doi: 10.1038/7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Kim S, Kovacs F, Cross TA. Structure of the transmembrane region of the M2 protein H(+) channel. Protein Sci. 2001;10:2241–2250. doi: 10.1110/ps.17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marassi FM, Opella SJ. Simultaneous assignment and structure determination of a membrane protein from NMR orientational restraints. Protein Sci. 2003;12:403–411. doi: 10.1110/ps.0211503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SH, Mrse AA, Nevzorov AA, et al. Three-dimensional structure of the channel-forming trans-membrane domain of virus protein “u” (Vpu) from HIV-1. J. Mol. Biol. 2003;333:409–424. doi: 10.1016/j.jmb.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 6.Oxenoid K, Chou JJ. The structure of phospholamban pentamer reveals a channel-like architecture in membranes. Proc. Natl. Acad. Sci. USA. 2005;102:10,870–10,875. doi: 10.1073/pnas.0504920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamoon J, Mascioni A, Thomas DD, Veglia G. NMR solution structure and topological orientation of monomeric phospholamban in dodecylphosphocholine micelles. Biophys.J. 2003;85:2589–2598. doi: 10.1016/s0006-3495(03)74681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorgen PL, Cahill SM, Krueger-Koplin RD, Krueger-Koplin ST, Schenck CC, Girvin ME. Structure of the Rhodobacter sphaeroides light-harvesting 1 beta subunit in detergent micelles. Biochemistry. 2002;41:31–41. doi: 10.1021/bi011576j. [DOI] [PubMed] [Google Scholar]
- 9.Howell SC, Mesleh MF, Opella SJ. NMR structure determination of a membrane protein with two transmembrane helices in micelles: MerF of the bacterial mercury detoxification system. Biochemistry. 2005;44:5196–5206. doi: 10.1021/bi048095v. [DOI] [PubMed] [Google Scholar]
- 10.Sweadner KJ, Rael E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics. 2000;68:41–56. doi: 10.1006/geno.2000.6274. [DOI] [PubMed] [Google Scholar]
- 11.Crambert G, Geering K. FXYD proteins: new tissue-specific regulators of the ubiquitous Na,K-ATPase. Sci. STKE. 2003;2003:RE1. doi: 10.1126/stke.2003.166.re1. [DOI] [PubMed] [Google Scholar]
- 12.Garty H, Karlish SJ. Role of FXYD Proteins in Ion Transport. Annu. Rev. Physiol. 2005 doi: 10.1146/annurev.physiol.68.040104.131852. [DOI] [PubMed] [Google Scholar]
- 13.Mercer RW, Biemesderfer D, Bliss DP, Jr, Collins JH, Forbush B., 3rd Molecular cloning and immunological characterization of the gamma polypeptide, a small protein associated with the Na,K-ATPase. J. Cell Biol. 1993;121:579–586. doi: 10.1083/jcb.121.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attali B, Latter H, Rachamim N, Garty H. A corticosteroid-induced gene expressing an “IsK-like” K+ channel activity in Xenopus oocytes. Proc. Natl. Acad. Sci. USA. 1995;92:6092–6096. doi: 10.1073/pnas.92.13.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arystarkhova E, Wetzel RK, Asinovski NK, Sweadner KJ. The gamma subunit modulates Na(+) and K(+) affinity of the renal Na, K-ATPase. J. Biol. Chem. 1999;274:33,183–33,185. doi: 10.1074/jbc.274.47.33183. [DOI] [PubMed] [Google Scholar]
- 16.Shi H, Levy-Holzman R, Cluzeaud F, Farman N, Garty H. Membrane topology and immunolocalization of CHIF in kidney and intestine. Am. J. Physiol. Renal Physiol. 2001;280:F505–F512. doi: 10.1152/ajprenal.2001.280.3.F505. [DOI] [PubMed] [Google Scholar]
- 17.Wetzel RK, Sweadner KJ. Immunocytochemical localization of Na-K-ATPase alpha- and gamma-subunits in rat kidney. Am. J. Physiol. Renal Physiol. 2001;281:F531–F545. doi: 10.1152/ajprenal.2001.281.3.F531. [DOI] [PubMed] [Google Scholar]
- 18.Franzin CM, Yu J, Thai K, Choi J, Marassi FM. Correlation of Gene and Protein Structures in the FXYD Family Proteins. J. Mol. Biol. 2005 doi: 10.1016/j.jmb.2005.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thai K, Choi J, Franzin CM, Marassi FM. Bcl-XL as a fusion protein for the high-level expression of membrane-associated proteins. Protein Sci. 2005;14:948–955. doi: 10.1110/ps.041244305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 21.Miozzari GF, Yanofsky C. Translation of the leader region of the Escherichia coli tryptophan operon. J. Bacteriol. 1978;133:1457–1466. doi: 10.1128/jb.133.3.1457-1466.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleid DG, Yansura D, Small B, et al. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981;214:1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- 23.Staley JP, Kim PS. Formation of a native-like subdomain in a partially folded intermediate of bovine pancreatic trypsin inhibitor. Protein Sci. 1994;3:1822–1832. doi: 10.1002/pro.5560031021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuliopulos A, Nelson NP, Yamada M, et al. Localization of the affinity peptide-substrate inactivator site on recombinant vitamin K-dependent carboxylase. J. Biol. Chem. 1994;269:21,364–21,370. [PubMed] [Google Scholar]
- 25.Cavanagh J. Protein NMR spectroscopy: principles and practice. San Diego: Academic Press; 1996. [Google Scholar]
- 26.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 27.Goddard TD, Kneller DG. 2004 [Google Scholar]
- 28.Mori S, Abeygunawardana C, Johnson MO, Vanzijl PCM. Improved Sensitivity of HSQC Spectra of Exchanging Protons at Short Interscan Delays Using a New Fast HSQC (FHSQC) Detection Scheme That Avoids Water Saturation. J. Magn. Reson. B. 1995;108:94–98. doi: 10.1006/jmrb.1995.1109. [DOI] [PubMed] [Google Scholar]
- 29.Ikura M, Kay LE, Bax A. A novel approach for sequential assignment of 1H, 13C, and 15N spectra of proteins: heteronuclear triple-resonance three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry. 1990;29:4659–4667. doi: 10.1021/bi00471a022. [DOI] [PubMed] [Google Scholar]
- 30.Sattler M, Schleucher J, Griesinger C. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog. Nucl. Magn. Reson. Spectrosc. 1999;34:93–158. [Google Scholar]
- 31.Grzesiek S, Bax A. Correlating backbone amide and side chain resonances in larger proteins by multiple relayed triple resonance NMR. J. Am. Chem. Soc. 1992;114:6291–6293. [Google Scholar]
- 32.Farrow NA, Zhang O, Forman-Kay JD, Kay LE. A heteronuclear correlation experiment for simultaneous determination of 15N longitudinal decay and chemical exchange rates of systems in slow equilibrium. J. Biomol. NMR. 1994;4:727–734. doi: 10.1007/BF00404280. [DOI] [PubMed] [Google Scholar]
- 33.Ottiger M, Delaglio F, Bax A. Measurement of J and dipolar couplings from simplified two-dimensional NMR spectra. J. Magn. Reson. 1998;131:373–378. doi: 10.1006/jmre.1998.1361. [DOI] [PubMed] [Google Scholar]
- 34.Ishii Y, Markus MA, Tycko R. Controlling residual dipolar couplings in high-resolution NMR of proteins by strain induced alignment in a gel. J. Biomol. NMR. 2001;21:141–151. doi: 10.1023/a:1012417721455. [DOI] [PubMed] [Google Scholar]
- 35.Ding K, Gronenborn AM. Sensitivity-enhanced 2D IPAP, TROSY-anti-TROSY, and E.COSY experiments: alternatives for measuring dipolar 15N-1HN couplings. J. Magn. Reson. 2003;163:208–214. doi: 10.1016/s1090-7807(03)00081-8. [DOI] [PubMed] [Google Scholar]
- 36.Tanford C, Reynolds JA. Characterization of membrane proteins in detergent solutions. Biochim. Biophys. Acta. 1976;457:133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- 37.Jorgensen PL. Purification of Na+, K+-ATPase: enzyme sources, preparative problems, and preparation from mammalian kidney. Methods Enzymol. 1988;156:29–43. doi: 10.1016/0076-6879(88)56005-6. [DOI] [PubMed] [Google Scholar]
- 38.Maunsbach AB, Skriver E, Jorgensen PL. Analysis of Na+,K+-ATPase by electron microscopy. Methods Enzymol. 1988;156:430–441. doi: 10.1016/0076-6879(88)56041-x. [DOI] [PubMed] [Google Scholar]
- 39.Ivanov AV, Gable ME, Askari A. Interaction of SDS with Na+/K+-ATPase: SDS-solubilized enzyme retains partial structure and function. J. Biol. Chem. 2004;279:29,832–29,840. doi: 10.1074/jbc.M401986200. [DOI] [PubMed] [Google Scholar]
- 40.Mesleh MF, Lee S, Veglia G, Thiriot DS, Marassi FM, Opella SJ. Dipolar waves map the structure and topology of helices in membrane proteins. J. Am. Chem. Soc. 2003;125:8928–8935. doi: 10.1021/ja034211q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wishart DS, Sykes BD. Chemical shifts as a tool for structure determination. Methods Enzymol. 1994;239:363–392. doi: 10.1016/s0076-6879(94)39014-2. [DOI] [PubMed] [Google Scholar]
- 42.Cornilescu G, Delaglio F, Bax A. Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J. Biomol. NMR. 1999;13:289–302. doi: 10.1023/a:1008392405740. [DOI] [PubMed] [Google Scholar]
- 43.Sass HJ, Musco G, Stahl SJ, Wingfield PT, Grzesiek S. Solution NMR of proteins within polyacrylamide gels: diffusional properties and residual alignment by mechanical stress or embedding of oriented purple membranes. J. Biomol. NMR. 2000;18:303–309. doi: 10.1023/a:1026703605147. [DOI] [PubMed] [Google Scholar]
- 44.Chou JJ, Gaemers S, Howder B, Louis JM, Bax A. A simple apparatus for generating stretched polyacrylamide gels, yielding uniform alignment of proteins and detergent micelles. J. Biomol. NMR. 2001;21:377–382. doi: 10.1023/a:1013336502594. [DOI] [PubMed] [Google Scholar]
- 45.Mesleh MF, Veglia G, DeSilva TM, Marassi FM, Opella SJ. Dipolar waves as NMR maps of protein structure. J. Am. Chem. Soc. 2002;124:4206–4207. doi: 10.1021/ja0178665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mesleh MF, Opella SJ. Dipolar Waves as NMR maps of helices in proteins. J. Magn. Reson. 2003;163:288–299. doi: 10.1016/s1090-7807(03)00119-8. [DOI] [PubMed] [Google Scholar]
- 47.Valafar H, Prestegard JH. REDCAT: a residual dipolar coupling analysis tool. J. Magn. Reson. 2004;167:228–241. doi: 10.1016/j.jmr.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Marassi FM. NMR of peptides and proteins in membranes. Concepts Magn. Reson. 2002;14:212–224. [Google Scholar]
- 49.Pines A, Gibby MG, Waugh JS. Proton-enhanced NMR of dilute spins in solids. J. Chem. Phys. 1973;59:569–590. [Google Scholar]
- 50.Levitt MH, Suter D, Ernst RR. Spin Dynamics and Thermodynamics in Solid-State NMR Cross-Polarization. J. Chem. Phys. 1986;84:4243–4255. [Google Scholar]
- 51.Wu CH, Ramamoorthy A, Opella SJ. High-resolution heteronuclear dipolar solid-state NMR spectroscopy. J. Magn. Reson. A. 1994;109:270–272. [Google Scholar]
- 52.Marassi FM, Ramamoorthy A, Opella SJ. Complete resolution of the solid-state NMR spectrum of a uniformly 15N-labeled membrane protein in phospholipid bilayers. Proc. Natl. Acad. Sci. USA. 1997;94:8551–8556. doi: 10.1073/pnas.94.16.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franzin CM, Yu J, Marassi FM. Solid-state NMR of the FXYD family membrane proteins in lipid bilayers. In: Ramamoorthy A, editor. NMR Spectroscopy of Biological Solids. Marcel Dekker; 2005. pp. 187–210. [Google Scholar]
- 54.Tian C, Gao PF, Pinto LH, Lamb RA, Cross TA. Initial structural and dynamic characterization of the M2 protein transmembrane and amphipathic helices in lipid bilayers. Protein Sci. 2003;12:2597–2605. doi: 10.1110/ps.03168503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marassi FM, Opella SJ. A solid-state NMR index of helical membrane protein structure and topology. J. Magn. Reson. 2000;144:150–155. doi: 10.1006/jmre.2000.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Denny J, Tian C, et al. Imaging membrane protein helical wheels. J. Magn. Reson. 2000;144:162–167. doi: 10.1006/jmre.2000.2037. [DOI] [PubMed] [Google Scholar]
- 57.Marassi FM. A simple approach to membrane protein secondary structure and topology based on NMR spectroscopy. Biophys. J. 2001;80:994–1003. doi: 10.1016/S0006-3495(01)76078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marassi FM, Opella SJ. Using pisa pies to resolve ambiguities in angular constraints from PISEMA spectra of aligned proteins. J. Biomol. NMR. 2002;23:239–242. doi: 10.1023/a:1019887612018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwieters CD, Kuszewski JJ, Tjandra N, Marius Clore G. The Xplor-NIH NMR molecular structure determination package. J. Magn. Reson. 2003;160:65–73. doi: 10.1016/s1090-7807(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 60.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]