Abstract
Venous injury and attendant venous stenosis are major contributors to the failure of hemodialysis vascular accesses. This report describes the presence of neoangiogenesis in the intima and adventitia of the venous limb of an arteriovenous (AV) fistula in the rat, the latter induced by creating an aortocaval fistula. Immunohistochemistry of the venous limb demonstrated the presence of c-Kit-positive cells lining new microvessels with lumen formation and that these c-Kit-positive cells exhibited either a smooth muscle phenotype as reflected by concomitant expression of calponin, or an endothelial phenotype as reflected by expression of endothelial nitric oxide synthase (eNOS). Western analysis confirmed upregulation of eNOS in the venous limb of the AV fistula. Measurement of systemic concentrations of angiogenic cytokines, namely, monocyte chemotactic protein-1, stromal cell-derived factor-1 (SDF-1), cytokine-induced neutrophil chemoattractant, and VEGF, failed to reveal an increase in these cytokines either at 3 or 10 wk after creation of the AV fistula. The angiogenic cytokines VEGF and SDF-1 were not upregulated in the venous limb of the AV fistula either at 2 or 16 wk. We conclude that in this model of an AV fistula in the rat, neoangiogenesis occurs and is constituted, at least in part, by bone marrow-derived cells, the latter differentiating to exhibit either an endothelial or smooth muscle phenotype. In view of these findings, we suggest that this model may offer an experimental approach by which to explore the evolution and significance of neoangiogenesis in the formation and pathobiology of vascular plaques, and the mechanisms that promote dysfunction of hemodialysis AV fistulas.
Keywords: venous stenosis, intimal hyperplasia, vascular access, hemodialysis
Dysfunction and failure of hemodialysis vascular accesses critically contribute to the morbidity and mortality of patients maintained on chronic hemodialysis (1, 2, 9, 12, 26, 27, 34–38). The favored vascular access for maintenance hemodialysis is the arteriovenous (AV) fistula: AV fistulas achieve higher patency rates compared with accesses utilizing synthetic grafts, and compared with central venous catheters, they provide more effective dialysis while incurring a lesser risk of local and systemic infections (1, 2, 9, 12, 26, 27, 34–38).
A fundamental lesion contributing to dysfunction and failure of an AV fistula is intimal hyperplasia occurring in the venous limb of the AV fistula (1, 9, 18, 34–38). Such lesions cause venous stenosis, predispose to thrombosis, and ultimately compromise the ability of the AV fistula to achieve the desired blood flow rates. Intimal hyperplasia also predisposes to the dysfunction and/or failure of vascular accesses based on synthetic grafts. While the adverse consequences of intimal hyperplasia are clearly recognized, the pathogenesis of this lesion is poorly understood and has received, until quite recently, relatively little attention. Understanding the pathogenesis of venous intimal hyperplasia in either type of vascular access may suggest strategies that can either facilitate the regression of such lesions once present or reduce the likelihood that such lesions would develop in the venous limb of these accesses.
The pathogenesis of intimal hyperplasia in other vasculopathic states, such as primary atherosclerosis, vein graft and cardiac allograft vasculopathy, and restenosis after percutaneous intervention atherosclerosis, is much more extensively studied and better understood (5, 19, 24, 28, 29, 42, 43). Such studies have increasingly probed the pathobiological significance of neoangiogenesis, the latter often accompanying intimal hyperplasia within the diseased vasculature (10, 19, 29, 43). New microvessel formation may emanate from the vasa vasorum of the adventitia, and this may be instigated by hypoxic and/or inflammatory stimuli occurring in the hyperplastic vessel; in turn, neoangiogenesis may promote intimal hyperplasia, the growth of atherosclerotic and fibroproliferative plaques, and the rupture of plaques (10, 19, 29, 43). Intimal hyperplasia and neoangiogenesis thus may not only coexist but may be codependent processes, serving to sustain each other, and ultimately, the progression of vascular disease.
The present report provides a novel description and characterization of neoangiogenesis occurring in the venous limb of an AV fistula in the rat, a model which, as we previously demonstrated, exhibits intimal hyperplasia and proinflammatory changes (31).
MATERIALS AND METHODS
Rat aortocaval fistula mode
This model is described in detail in our prior studies (20, 21, 31). In brief, the abdominal aorta and inferior vena cava were cross-clamped, and a fistula was created by puncturing the aorta approximately at a point above the aortic bifurcation that was one-third the length of the infrarenal abdominal aorta; the needle was then advanced into the vena cava. Following the removal of the needle, the puncture site on the aorta was sealed by cyanoacrylate glue. Sham-operated rats underwent laparotomy, cross-clamping of the aorta and vena cava without puncturing of the vessels, but with the placement of a drop of cyanoacrylate glue on the aorta. Our studies were approved by our Institutional Animal Care and Use Committee and were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Immunohistochemical analysis
Immunohistochemical analysis was performed as described previously on deparaffinized and rehydrated sections (4, 8, 40). Primary antibodies against c-Kit (catalog no. sc-5535, Santa Cruz Biotechnology, Santa Cruz, CA), calponin (catalog no. M3556, Dako, Carpinteria, CA), and endothelial nitric oxide synthase (eNOS; catalog no. 610297, BD Transduction Laboratories, San Diego, CA) were employed along with Cy3-conjugated goat anti-rabbit IgG, goat anti-mouse IgG (catalog nos. 21082328 and 21071069, Chemicon, Temecula, CA) or Alexa Fluor 488-conjugated goat anti-rabbit IgG (catalog no. A11008, Molecular Probes/Invitrogen, Carlsbad, CA) as secondary antibodies. Dual labeling was performed in certain cases to identify the smooth muscle or endothelial lineage of neovascularization.
Western blot analysis
Western blot analysis was performed as described previously (33). Blots were incubated with a primary antibody to eNOS (catalog no. 610297, BD Transduction Laboratories) followed by a horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody and visualization using a chemiluminescence method (Amersham Pharmacia, Piscataway, NJ). Equal protein loading was confirmed by immunoblotting for β-actin (catalog no. 612657, BD Transduction Laboratories).
mRNA expression by quantitative real-time RT-PCR
Total RNA from rat veins was extracted and purified using the TRIzol method (Invitrogen) and a RNeasy Mini Kit (Qiagen, Valencia, CA), and used in reverse transcription reactions (Transcriptor First Strand cDNA Synthesis Kit, Roche Applied Science, Indianapolis, IN) according to each manufacturer’s protocol (33). Quantitative real-time PCR reactions were performed as previously described, and analyzed on an ABI Prism 7900HT (Applied Biosystems, Foster City, CA). Probe and primer sets were designed with Primer Express software (Applied Biosystems) and are detailed in Table 1. A probe and primer set for the analysis of VEGF gene expression (TaqMan Gene Expression Assay, stock no. Rn00582935, Applied Biosystems) was also utilized in our standard procedure.
Table 1.
Primers and probes used for quantitative real-time RT-PCR
| Gene | Primer/Probe | Sequence |
|---|---|---|
| SDF-1 | Forward | 5′-TCCTTGCCGAGAGTCACTCA-3′ |
| Reverse | 5′-CCCCTGACACTGAACTGGAAA-3′ | |
| TaqMan | 5′-(FAM)CTCAAGGTTGGGCAGAGGCTCC(TAMRA)-3′ | |
| 18S | Forward | 5′-CACGGCCGGTACAGTGAAA-3′ |
| Reverse | 5′-AGAGGAGCGAGCGACCAA-3′ | |
| TaqMan | 5′-(FAM)TGCGAATGGCTCATTAAATCAGTTATGGTTCC(TAMRA)-3′ |
SDF-1, stromal cell-derived factor-1.
Measurement of systemic concentrations of cytokines
ELISA kits for stromal cell-derived factor-1 (SDF-1; Quantikine MCX 120, R&D Systems, Minneapolis, MN), cytokine-induced neutrophil chemoattractant (CINC-1; Quantikine RCN 100, R&D Systems), and monocyte chemotactic protein-1 (MCP-1; catalog no. 555130, BD Biosciences Pharmingen, San Diego, CA) were used to determine serum cytokine levels. An ELISA kit (Quantikine RRV00, R&D Systems) was used to determine the VEGF levels in the rat plasma.
Statistics
Data are expressed as means ± SE. For comparison of two groups, statistical analysis employed the Student’s t-test for parametric data and the Mann-Whitney U-test for nonparametric data. Results are considered significant for P < 0.05
RESULTS
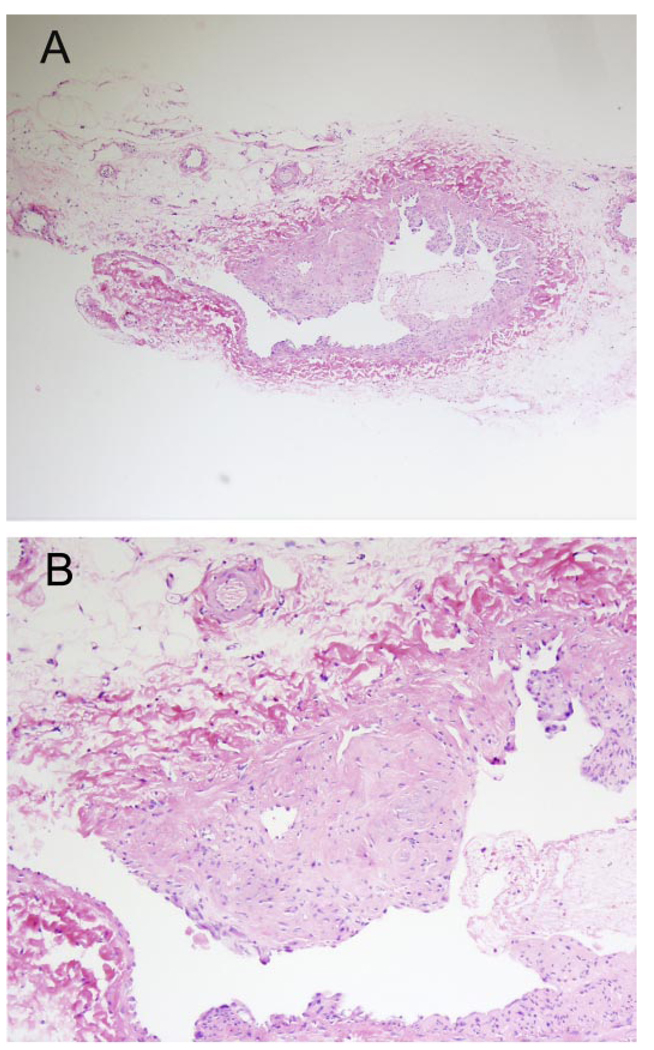
Shown in Fig. 1 are intimal hyperplasia in the venous limb of the AV fistula at 16 wk and the presence of neoangiogenesis at the base of the hyperplastic plaque.
Fig. 1.
Histology of the venous limb of an arteriovenous (AV) fistula. A: low-power light microscopy demonstrating intimal hyperplasia and intimal plaque formation. B: high-power light microscopy demonstrating angiogenesis at the base of the plaque in the venous limb of the AV fistula. Sections are stained with hematoxylin and eosin.
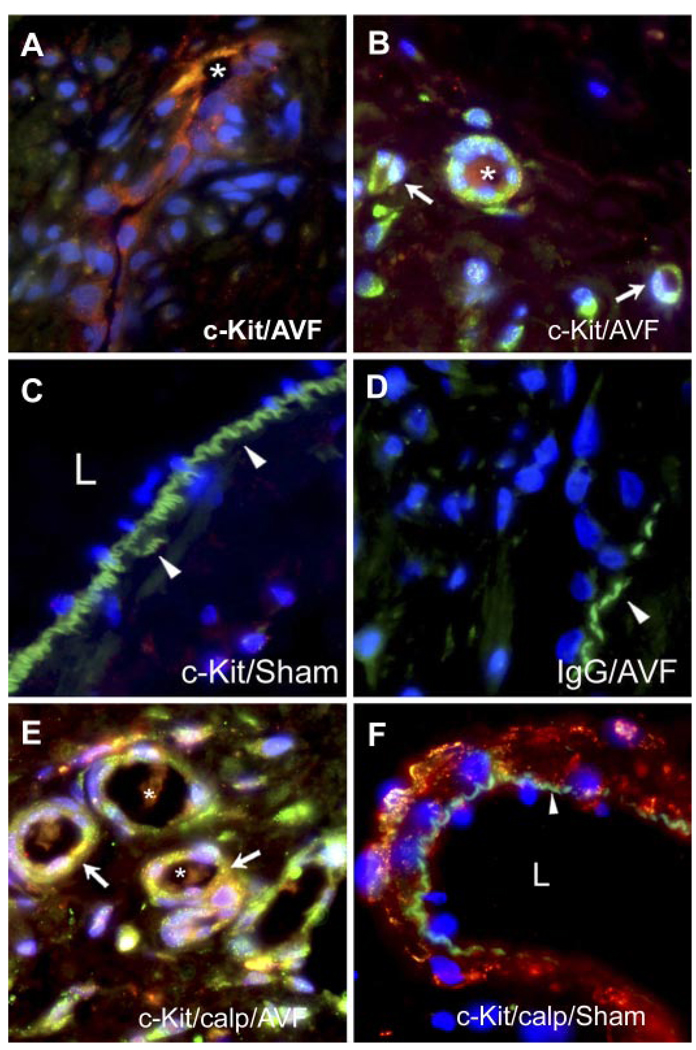
Using c-Kit as a marker for bone marrow-derived cells, immunohistochemistry was undertaken to determine if bone marrow-derived cells populated the plaque. In the intimal plaque, c-Kit-positive cells were detected lining the walls of microvessels, the latter exhibiting patent lumina (Fig. 2A). c-Kit-positive cells were also observed in microvessels in the adventitia of the venous limb of the AV fistula (Fig. 2B). The venous wall in the sham-operated rats did not stain for c-Kit (Fig. 2C), and there was no staining for c-Kit in the IgG isotype-matched controls in the AV fistula (Fig. 2D).
Fig. 2.
Immunohistochemistry for c-Kit in the venous limb of the AV fistula and sham-operated rats. A: c-Kit-positive cells within a longitudinal section of a microvessel within the intimal plaque of the AV fistula; the lumen of the microvessel is marked by an asterisk. B: c-Kit-positive cells within the adventitia of the venous limb of the AV fistula. c-Kit-positive cells forming signet ring structures are marked by white arrows. Also displayed is a microvessel formed from multiple c-Kit-positive cells that exhibits a patent lumen marked by an asterisk. C: absence of c-Kit-positive immunoreactivity in the venous limb in sham-operated rats. Arrowheads indicate the internal elastic lamina, and L indicates the lumen of the vein. D: absence of staining with IgG isotype-matched control in the venous limb of the AV fistula. E: neovascularized zone in the adventitia of the venous limb of the AV fistula showing dual staining for c-Kit (green) and calponin (calp; red) to reveal a merged image (yellow) and that is marked by white arrows; patent lumina are marked by asterisks. F: combined staining for c-Kit and calponin showing only calponin immunoreactivity in the venous limb in the sham-operated rat and an absence of c-Kit immunoreactivity. The arrowhead indicates the internal elastic lamina, and L indicates the lumen.
c-Kit-positive cells assumed at least one of two phenotypes, as demonstrated by dual labeling studies undertaken with a marker for smooth muscle cells (calponin) or endothelial cells (eNOS). As shown in Fig. 2E, a certain subset of c-Kit-positive cells stained concomitantly with calponin, thereby exhibiting a smooth muscle phenotype. The venous limb of the sham-operated rat showed calponin staining only, with an absence of c-Kit staining (Fig. 2F).
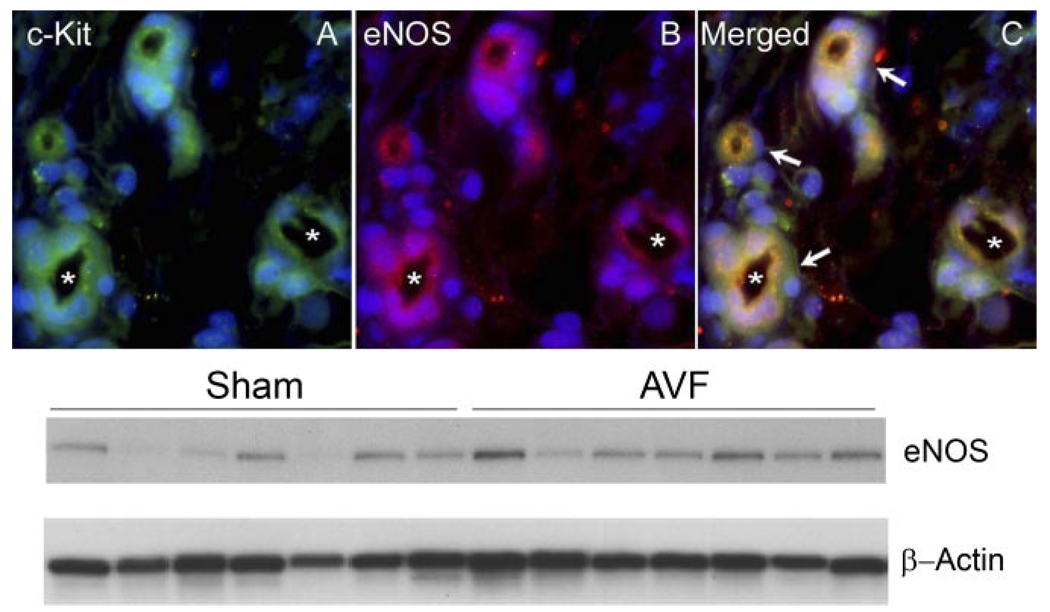
c-Kit-positive cells also stained for an endothelial marker, namely, eNOS, as shown in Fig. 3; these findings indicate that these progenitor cells may also exhibit an endothelial phenotype. We confirmed that eNOS is induced in the AV fistula by Western blot analysis, the latter demonstrating a twofold increase for eNOS (0.58 ± 0.15 vs. 1.21 ± 0.21, standardized densitometric units, P < 0.05, Fig. 3). We also assessed the expression of HO-1 in the venous limb of the AV fistula since HO-1, like eNOS, may be angiogenic and anti-inflammatory. Few vessels in the adventitia and only occasional endothelial cells were positive for HO-1 (data not shown).
Fig. 3.
Immunohistochemistry for c-Kit and endothelial nitric oxide synthase (eNOS) in the adventitia of the venous limb of the AV fistula. A: c-Kit-positive immunoreactivity (green) in adventitial microvessels, exhibiting lumina, some of which are marked by asterisks. B: eNOS-positive immunoreactivity (red) in the same adventitial microvessels. C: merged image showing combined immunostaining (yellow) in all 4 adventitial microvessels, as indicated by white arrows. Bottom: Western blot analysis demonstrating expression of eNOS in the venous limb of the AV fistula and sham-operated rats; equivalency of loading of the gel was assessed by immunostaining for β-actin.
To determine whether systemic levels of angiogenic substances were altered in this model, we measured the serum/plasma levels of MCP-1, SDF-1, CINC-1, and VEGF. As shown in Table 2, systemic levels of angiogenic cytokines were not elevated in the AV fistula model at a relatively early (3 wk) or a delayed time point (10 wk).
Table 2.
Serum concentrations of SDF-1, MCP-1, and CINC-1 and plasma concentration of VEGF in sham-operated rats, and rats with an AV fistula
| 3 Wk | 10 Wk | |||
|---|---|---|---|---|
| Sham (n = 6) | AVF (n = 6) | Sham (n = 7) | AVF (n = 7) | |
| SDF-1, ng/ml | 2.9±0.1 | 2.5±0.1 | 3.3±0.6 | 2.6±0.2 |
| MCP-1, ng/ml | 196.6±36.2 | 147.9±9.3 | 58.8±5.7 | 52.3±4.6 |
| CINC-1, pg/ml | 106.4±13.5 | 131.6±10.5 | 104.2±3.3 | 89.8±10.9 |
| VEGF | ND | ND | ND | ND |
Values are means ± SE. n, No. of rats; Sham, sham-operated; AVF, arteriovenous fistula; MCP-1, monocyte chemotactic protein-1; CINC-1, cytokine-induced neutrophil chemoattractant; ND, not detectable. Measurements were obtained at 3 and 10 wk.
We also examined whether expression of VEGF and SDF-1 was increased in the venous limb of the AV fistula. VEGF mRNA was not induced in the AV fistula either at 2 wk [0.51 ± 0.09 vs. 0.52 ± 0.19, standardized units, P = not significant (NS)] or 16 wk (0.51 ± 0.06 vs. 0.32 ± 0.08, standardized units, P = NS); SDF-1 mRNA was also not induced in the AV fistula either at 2 wk (1.08 ± 0.12 vs. 1.84 ± 0.54, standardized units, P = NS) or 16 wk (1.33 ± 0.18 vs. 1.76 ± 0.61, standardized units, P = NS).
DISCUSSION
Exposing the venous vasculature to the arterial circulation, as occurs in an AV fistula, subjects veins to a degree of hemodynamic stress that the venous system was never designed to bear. The creation of an AV fistula subjects the venous system with its relatively low vascular resistance to the comparatively high pressures present in the arterial system, and this leads to markedly increased venous blood flow and shear stress; in areas of the fistula, turbulent blood flow may also occur (32). Such hemodynamic stress in the venous circulation following the fashioning of an AV fistula may be attended by structural alterations in the venous vasculature. For example, dilatation of veins, by Poiseuille’s law, mitigates the increase in shear stress imposed by increased blood flow; and increased thickness of the venous wall, by Laplace’s law, lessens the increase in wall tension that would otherwise occur in the venous limb of the AV fistula. These adaptive changes may also be accompanied by maladaptive ones. For example, the venous limb of the AV fistula, as shown in our prior studies of this model, can exhibit exuberant intimal hyperplasia and upregulation of genes that are proinflammatory, procoagulant, and profibrotic (31).
Our present studies uncover another alteration in the venous vasculature, namely, neoangiogenesis. Neoangiogenesis is well recognized in models of vascular injury (5, 10, 15, 19, 28, 29, 42, 43) and may represent an alteration designed to maintain oxygenation and the supply of nutrients to the thickened vessel wall. Healthy vessels are normally supplied by intraluminal blood or vasa vasorum in the adventitia. Diffusion of oxygen and nutrients from either source is hampered in hypertrophic/hyperplastic vessels, and this impediment to the delivery of oxygen and nutrients may be circumvented by neoangiogenesis within the wall of the diseased vessel. Such neoangiogenesis, however, may be a maladaptive response as it may promote the progression of atherosclerotic plaques and plaque rupture. Thus neoangiogenesis is considered a pathogenetically significant lesion, and much attention is currently directed to understanding the origin and basis for such new vessel growth (10, 19, 29, 43).
To determine the basis for neoangiogensis in the venous limb, we determined the origin of these cells, specifically questioning whether cells that constitute these microvessels were derived from the bone marrow. Using the marker, c-Kit, a stem cell receptor protein expressed by bone marrow-derived cells, we noted that the nascent microvessels were lined by c-Kit-positive cells. To determine the phenotype of these c-Kit-positive cells, we employed a colabeling technique that also probed for the presence of a marker for smooth muscle cells (calponin) or endothelial cells (eNOS). These studies demonstrate that these bone marrow-derived cells assume the phenotype of either endothelial or smooth muscle cells.
In an attempt to determine the mechanism accounting for the recruitment of such bone marrow-derived cells, we explored the expression of several potential candidates. Our prior studies demonstrated that MCP-1, a chemokine and recruiter of bone marrow-derived endothelial progenitors, is markedly upregulated in the AV fistula at an early time point (31). Other potential candidates include VEGF and SDF-1 (5, 15). Indeed, recent studies demonstrate that VEGF and SDF-1 act in concert to induce angiogenesis: VEGF, derived from tissues exhibiting angiogenesis, recruits bone marrow-derived cells to these tissues; angiogenic precursors within these recruited cells are then retained within these tissues by SDF-1, the latter, itself, upregulated by VEGF (14, 39). In this regard, we measured VEGF and SDF-1 in the circulation. However, at the time points studied, namely, 3 and 10 wk, we were unable to see upregulation of either of these angiogenic species, or of another angiogenic species, CINC-1. It is possible that changes in the level of these angiogenic substances may exist at time points other than those at which measurements were made.
We also examined whether expression of VEGF and SDF-1 was induced within the venous wall of the AV fistula. Neither cytokine was upregulated in the AV fistula. In this regard, it is notable that in our prior studies a 15-fold increase in MCP-1 expression was observed in the venous limb of this AV fistula. MCP-1 is recognized not only as a potent chemoattractant but also as an angiogenic species (5, 13, 15, 16), and this chemokine may thus contribute to the angiogenesis observed in the present study.
In the current study, upregulation of eNOS by both immunofluorescence and Western blot analysis was observed in the venous limb of the AV fistula. In models characterized by increased arterial flow, studies by us and others have demonstrated that adaptive increments in eNOS in the arterial circuit allow such increased flow to occur (20–22). The present studies extend these observations by demonstrating that up-regulation of eNOS also occurs in the venous circulation subjected to increased blood flow.
Clinical observations attest to the fact that proinflammatory as well as profibrogenic cytokines promote the dysfunction and/or failure of hemodialysis AV fistulas (6, 7, 17, 34–38, 41). It is also possible that expression of anti-inflammatory molecules may be relevant to the patency of AV fistulas. In this regard, there is interest in the expression of heme oxygenase-1 (HO-1), the latter representing an anti-inflammatory molecule which can decrease expression of MCP-1 and other proinflammatory species (30). For example, polymorphisms in the HO-1 gene that lead to less HO activity are associated with decreased patency rates for AV fistulas (23); additionally, expression of HO-1 may stabilize atherosclerotic plaques in carotid arteries (3, 11). In the current studies, expression of HO-1 was observed in some new vessels in the adventitia and occasional endothelial cells in the AV fistula.
In summary, we demonstrate that neoangiogenesis occurs in the venous limb of the AV fistula in the rat, and that the composition of these nascent microvessels involves, at least in part, bone marrow-derived cells. In view of these findings, we suggest that this model affords an approach to explore the evolution and pathobiological significance of neoangiogenesis in the pathogenesis of vascular plaques and their complications. We also suggest that these findings are clinically relevant to hemodialysis AV accesses. Neoangiogenesis is well documented in failed AV grafts (18, 36) and in dysfunctional AV fistulas (44), and a recent presentation called attention to the finding that stenosis in dysfunctional/failed fistulas is accompanied by a relative lack of an angiogenic response (25). The current model thus offers an experimental approach in analyzing the pathobiological significance of new vessel formation in structural and functional alterations in an AV fistula.
ACKNOWLEDGMENTS
We gratefully acknowledge the secretarial expertise of Sharon Heppelmann in the preparation of this manuscript.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-70124.
REFERENCES
- 1.Agarwal A, Segal MS. Intimal exuberance: veins in jeopardy. Am J Pathol. 2003;162:1759–1761. doi: 10.1016/S0002-9440(10)64310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002;62:1109–1124. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- 3.Ameriso SF, Villamil AR, Zedda C, Parodi JC, Garrido S, Sarchi MI, Schultz M, Boczkowski J, Sevlever GE. Heme oxygenase-1 is expressed in carotid atherosclerotic plaques infected by Helicobacter pylori and is more prevalent in asymptomatic subjects. Stroke. 2005;36:1896–1900. doi: 10.1161/01.STR.0000177494.43587.9e. [DOI] [PubMed] [Google Scholar]
- 4.Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci USA. 2003;100:4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caplice NM, Doyle B. Vascular progenitor cells: origin and mechanisms of mobilization, differentiation, integration, and vasculogenesis. Stem Cells Dev. 2005;14:122–139. doi: 10.1089/scd.2005.14.122. [DOI] [PubMed] [Google Scholar]
- 6.Chang CJ, Ko YS, Ko PJ, Hsu LA, Chen CF, Yang CW, Hsu TS, Pang JH. Thrombosed arteriovenous fistula for hemodialysis access is characterized by a marked inflammatory activity. Kidney Int. 2005;68:1312–1319. doi: 10.1111/j.1523-1755.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 7.Chou CY, Kuo HL, Yung YF, Liu YL, Huang CC. C-reactive protein predicts vascular access thrombosis in hemodialysis patients. Blood Purif. 2006;24:342–346. doi: 10.1159/000092558. [DOI] [PubMed] [Google Scholar]
- 8.Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: a study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–1249. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- 9.Dixon BS. Why don’t fistulas mature? Kidney Int. 2006;70:1413–1422. doi: 10.1038/sj.ki.5001747. [DOI] [PubMed] [Google Scholar]
- 10.Doyle B, Caplice NM. Plaque neovascularization and anti-angiogenic therapy for atherosclerosis. J Am Coll Cardiol. doi: 10.1016/j.jacc.2007.01.089. In press. [DOI] [PubMed] [Google Scholar]
- 11.Espinola-Klein C, Blankenberg S, Munzel T. Is heme oxygenase-1 a causal player for plaque stability? Stroke. 2005;36:1901–1903. [PubMed] [Google Scholar]
- 12.Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7:523–535. doi: 10.1681/ASN.V74523. [DOI] [PubMed] [Google Scholar]
- 13.Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, Imada T, Iwasaka T, Matsubara H. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93:980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- 14.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 15.Heil M, Schaper W. Arteriogenic growth factors, chemokines and proteases as a prerequisite for arteriogenesis. Drug News Perspect. 2005;18:317–322. doi: 10.1358/dnp.2005.18.5.917327. [DOI] [PubMed] [Google Scholar]
- 16.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 17.Heine GH, Ulrich C, Sester U, Sester M, Kohler H, Girndt M. Transforming growth factor beta1 genotype polymorphisms determine AV fistula patency in hemodialysis patients. Kidney Int. 2003;64:1101–1107. doi: 10.1046/j.1523-1755.2003.00176.x. [DOI] [PubMed] [Google Scholar]
- 18.Kelly BS, Heffelfinger SC, Whiting JF, Miller MA, Reaves A, Armstrong J, Narayana A, Roy-Chaudhury P. Aggressive venous neointimal hyperplasia in a pig model of arteriovenous graft stenosis. Kidney Int. 2002;62:2272–2280. doi: 10.1046/j.1523-1755.2002.00684.x. [DOI] [PubMed] [Google Scholar]
- 19.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 20.Lam CF, Peterson TE, Croatt AJ, Nath KA, Katusic ZS. Functional adaptation and remodeling of pulmonary artery in flow-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2005;289:H2334–H2341. doi: 10.1152/ajpheart.00375.2005. [DOI] [PubMed] [Google Scholar]
- 21.Lam CF, Peterson TE, Richardson DM, Croatt AJ, d’Uscio LV, Nath KA, Katusic ZS. Increased blood flow causes coordinated upregulation of arterial eNOS and biosynthesis of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2006;290:H786–H793. doi: 10.1152/ajpheart.00759.2005. [DOI] [PubMed] [Google Scholar]
- 22.Lehoux S, Tronc F, Tedgui A. Mechanisms of blood flow-induced vascular enlargement. Biorheology. 2002;39:319–324. [PubMed] [Google Scholar]
- 23.Lin CC, Yang WC, Lin SJ, Chen TW, Lee WS, Chang CF, Lee PC, Lee SD, Su TS, Fann CS, Chung MY. Length polymorphism in heme oxygenase-1 is associated with arteriovenous fistula patency in hemodialysis patients. Kidney Int. 2006;69:165–172. doi: 10.1038/sj.ki.5000019. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Nath KA, Katusic ZS, Caplice NM. Smooth muscle progenitor cells in vascular disease. Trends Cardiovasc Med. 2004;14:288–293. doi: 10.1016/j.tcm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Loverre A, Simone S, Porreca S, Capobianco C, Schena FP, Pertosa G, Grandaliano G. Reduced angiogenesis is associated with stenosis of arteriovenous fistula (AVF) in hemodialysis (HD) patients (Abstract) J Am Soc Nephrol. 2006;17:74A. [Google Scholar]
- 26.Maya ID, Oser R, Saddekni S, Barker J, Allon M. Vascular access stenosis: comparison of arteriovenous grafts and fistulas. Am J Kidney Dis. 2004;44:859–865. [PubMed] [Google Scholar]
- 27.Miller PE, Carlton D, Deierhoi MH, Redden DT, Allon M. Natural history of arteriovenous grafts in hemodialysis patients. Am J Kidney Dis. 2000;36:68–74. doi: 10.1053/ajkd.2000.8269. [DOI] [PubMed] [Google Scholar]
- 28.Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006;84:115–124. doi: 10.1111/j.1440-1711.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- 29.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O’Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 30.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70:432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 31.Nath KA, Kanakiriya SK, Grande JP, Croatt AJ, Katusic ZS. Increased venous proinflammatory gene expression and intimal hyperplasia in an aorto-caval fistula model in the rat. Am J Pathol. 2003;162:2079–2090. doi: 10.1016/S0002-9440(10)64339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulson WD, Jones SA. Hemodynamics of the hemodialysis access: implications for clinical management. Contrib Nephrol. 2004;142:238–253. doi: 10.1159/000074846. [DOI] [PubMed] [Google Scholar]
- 33.Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int. 2005;68:611–622. doi: 10.1111/j.1523-1755.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 34.Roy-Chaudhury P. Endothelial progenitor cells, neointimal hyperplasia, and hemodialysis vascular access dysfunction: novel therapies for a recalcitrant clinical problem. Circulation. 2005;112:3–5. doi: 10.1161/CIRCULATIONAHA.105.548651. [DOI] [PubMed] [Google Scholar]
- 35.Roy-Chaudhury P, Kelly BS, Melhem M, Zhang J, Li J, Desai P, Munda R, Heffelfinger SC. Vascular access in hemodialysis: issues, management, and emerging concepts. Cardiol Clin. 2005;23:249–273. doi: 10.1016/j.ccl.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, Heffelfinger SC. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 37.Roy-Chaudhury P, Kelly BS, Zhang J, Narayana A, Desai P, Melham M, Duncan H, Heffelfinger SC. Hemodialysis vascular access dysfunction: from pathophysiology to novel therapies. Blood Purif. 2003;21:99–110. doi: 10.1159/000067863. [DOI] [PubMed] [Google Scholar]
- 38.Roy-Chaudhury P, Melhem M, Husted T, Kelly BS. Solutions for hemodialysis vascular access dysfunction: thinking out of the box!! J Vasc Access. 2005;6:3–8. doi: 10.1177/112972980500600102. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz de Almodovar C, Luttun A, Carmeliet P. An SDF-1 trap for myeloid cells stimulates angiogenesis. Cell. 2006;124:18–21. doi: 10.1016/j.cell.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Simper D, Wang S, Deb A, Holmes D, McGregor C, Frantz R, Kushwaha SS, Caplice NM. Endothelial progenitor cells are decreased in blood of cardiac allograft patients with vasculopathy and endothelial cells of noncardiac origin are enriched in transplant atherosclerosis. Circulation. 2003;108:143–149. doi: 10.1161/01.CIR.0000081703.34526.5D. [DOI] [PubMed] [Google Scholar]
- 41.Stracke S, Konner K, Kostlin I, Friedl R, Jehle PM, Hombach V, Keller F, Waltenberger J. Increased expression of TGF-beta1 and IGF-I in inflammatory stenotic lesions of hemodialysis fistulas. Kidney Int. 2002;61:1011–1019. doi: 10.1046/j.1523-1755.2002.00191.x. [DOI] [PubMed] [Google Scholar]
- 42.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 43.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 44.Weiss MF, Scivittaro V, Anderson JM. Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis. 2001;37:970–980. doi: 10.1016/s0272-6386(05)80013-7. [DOI] [PubMed] [Google Scholar]