Abstract
Most evidence of aryl hydrocarbon receptor (AHR) signaling in prostate growth, morphogenesis, and disease stems from research using 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) to pharmacologically activate the AHR at various stages of development. This review discusses effects of TCDD on prostate morphogenesis and highlights interactions between AHR and other signaling pathways during normal and aberrant prostate growth. Although AHR signaling modulates estrogen and androgen signaling in other tissues, crosstalk between these steroid hormone receptors and AHR signaling cannot account for actions of TCDD on prostate morphogenesis. Instead, the AHR appears to act within a cooperative framework of developmental signals to regulate timing and patterning of prostate growth. Inappropriate activation of AHR signaling as a result of early life TCDD exposure disrupts the balance of these signals, impairs prostate morphogenesis, and has an imprinting effect on the developing prostate that predisposes to prostate disease in adulthood. Mechanisms of AHR signaling in prostate growth and disease are only beginning to be unraveled and recent studies have revealed its interactions with WNT5A, retinoic acid, fibroblast growth factor 10, and vascular endothelial growth factor signaling pathways.
1. Ontogeny of prostate development
Mechanisms of prostate morphogenesis are of considerable interest to prostate biologists because the molecular signals responsible for prostate development are believed to be reawakened during prostate disease. Prostate development begins before birth in most mammals. The fetal urogenital sinus (UGS) from which the prostate derives is a simple cylinder of stratified basal epithelium, surrounded by mesenchyme and positioned between the embryonic bladder and pelvic urethra. Prostate induction begins in utero by binding of fetal circulating testosterone, synthesized by fetal testes, to androgen receptors (ARs) in UGS mesenchyme (UGM) [1]. AR activation releases instructive signals from UGM that acts on UGS epithelium (UGE) to stimulate cell proliferation, form prostate ductal progenitors (prostatic buds), and regulate cell adhesion dynamics to permit prostatic bud outgrowth [2]. There are three phases of prostatic budding: (1) the specification phase, when instructive developmental cues define where buds will form in the UGS, (2) the initiation phase, when prostatic buds begin to form, and (3) the elongation phase, when proliferation, cell adhesion, and cell migration coordinate outgrowth of prostatic buds into UGM. Timing of prostatic bud formation and the quantity and pattern of buds that are formed in the UGS are strictly regulated [3, 4]. The position of prostatic buds as they emerge from the UGS in utero determines the arrangement of prostate ducts in adulthood.
Complexity is conferred on the prostate during a postnatal development phase known as branching morphogenesis. Solid cords of prostate epithelium formed in utero elongate postnatally and their tips are bifurcated into primary, secondary, and tertiary branches in a pattern that is unique for each prostate lobe. Branching morphogenesis proceeds differentially for each of the three mouse prostate lobes (ventral, dorsolateral, and anterior) and is completed by about postnatal day 20 in mice, providing each lobe with unique glandular architecture [5]. It is important to note that the developing human prostate undergoes a similar series of morphogenetic events especially during prostatic bud formation, but gives rise to a mature glandular prostate that is unique from the rodent and features peripheral, central, and transitional zones.
Concurrent with branching morphogenesis, the solid cords of epithelium formed by budding arborize and differentiate postnatally into glandular acini comprised of at least three cell layers: an innermost layer of secretory columnar luminal epithelium, a middle layer of squamous basal epithelium that also contains neuroendocrine cells, transit amplifying cells, and stem cells, and an outer layer of smooth muscle intermixed with other stromal cells[6, 7]. The basic prostate architecture is established by puberty and acquires secretory function thereafter [5].
2. Aryl Hydrocarbon Receptor (AHR) signaling restricts prostatic budding and branching morphogenesis
The AHR is an orphan receptor and member of the PAS superfamily of helix-loop-helix transcription factors. The AHR binds to a wide variety of chemicals and is most potently activated by the persistent environmental contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). TCDD elicits AHR nuclear localization, heterodimerization with the aryl hydrocarbon receptor nuclear translocator (ARNT) and subsequent binding to aryl hydrocarbon receptor enhancer elements (AHREs) located in the promoters of AHR-responsive genes.
The AHR is present in the developing fetal prostate and in the normal and diseased prostate of adult males [8, 9]. Loss of functional aryl hydrocarbon receptor (Ahr) does not grossly impair mouse prostate development but does cause minor delays in anterior and dorsolateral prostate growth [10]. This suggests the Ahr is not absolutely required for prostate development but does not exclude it from acting redundantly with other factors during this process. The first evidence for a role of AHR signaling in prostate development came from the observation that perinatal 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure retarded prostate growth in Holtzman rats [11]. This was subsequently shown in other rat strains [12, 13] and in mice [14, 15]. Further efforts to unravel the mechanism of AHR action in prostate development focused on the mouse, because of the transgenic resources available, and used the C57BL/6J strain, because it carries the Ahrb1 allele that encodes for an AHR protein with high affinity for TCDD [16]. Exposure of C57BL/6J wild type male mouse fetuses to TCDD (5 μg/kg, maternal dose) on embryonic day (E)13.5 reduced ventral prostate weight by 87%, reduced ventral prostate-selective gene expression by 99%, and significantly impaired ventral prostate epithelial cell differentiation [15]. Dorsolateral and anterior prostate development was also affected by TCDD, but to a lesser extent than ventral prostate development (Fig. 1). Impairment of prostate development by TCDD was Ahr-dependent and did not occur in Ahr null male mouse fetuses treated with TCDD.
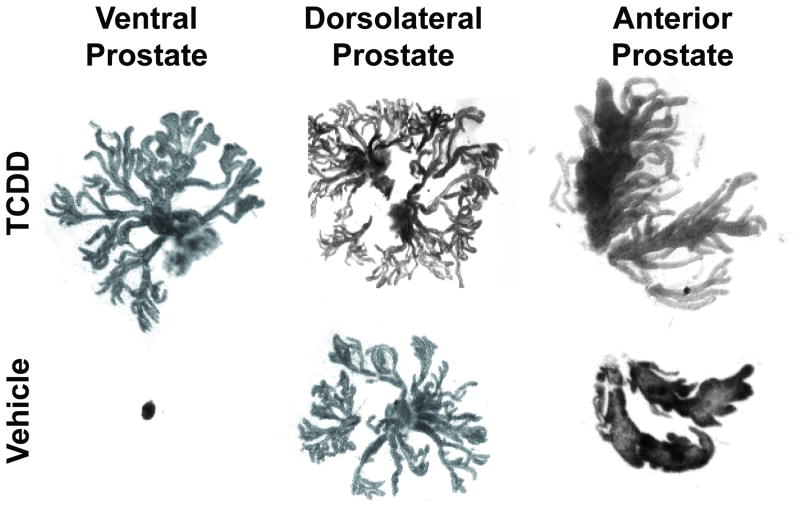
Figure 1. In utero and lactational TCDD exposure impairs mouse prostate development.
C57BL/6J mice were exposed to a maternal dose of vehicle or TCDD (5 μg/kg) on embryonic day (E) 13.5. Prostates were removed on postnatal day 90, separated into anterior, dorsolateral, and ventral lobes, and digested briefly with collagenase to expose individual ducts [18]. TCDD significantly decreased the weight of all lobes but most dramatically the ventral lobe. TCDD eliminated all main ducts in the ventral prostate causing ventral prostate agenesis, reduced the number of main ducts in the dorsolateral prostate, and reduced branching complexity of the anterior prostate.
Lin et al. [17] was the first to show that the in utero stage of mouse prostate development was more sensitive to TCDD than the postnatal stage. Mice were exposed to vehicle or TCDD (5 μg/kg, single maternal dose) on E13.5 and then fostered by dams of the same treatment group or cross-fostered to dams of the opposite treatment. Prostate growth was assessed on postnatal day 35. In utero TCDD exposure, without subsequent exposure during lactation, decreased ventral prostate weight by 84%, dorsolateral prostate weight by 26%, and anterior prostate weight by 49% compared to the control. TCDD exposure via lactation alone reduced VP weight by 41%, dorsolateral prostate weight by 20%, and anterior prostate weight by 22%. A follow-up study revealed that endpoints of postnatal prostate development were more modestly impaired by in utero and lactational (IUL) TCDD exposure [18]. That is, prostate ductal arborization and epithelial cell differentiation occurred normally in all lobes other than ventral prostate, which was developmentally arrested as a consequence of prenatal TCDD action. The number of dorsolateral prostate main ducts was decreased, which was also likely a consequence of prenatal TCDD. Moreover, anterior prostate branching was modestly decreased by IUL TCDD exposure while dorsolateral branching was unchanged (Fig. 1).
Heightened sensitivity to TCDD during fetal prostate growth suggested that AHR signaling may interfere with prostatic budding. Lin et al. [3] used scanning electron microscopy to investigate prostatic buds as they emerged from UGSs of vehicle- and TCDD-exposed mice. A single maternal dose of TCDD (5 μg/kg) on E13.5 delayed prostatic bud formation and decreased the total number of prostatic buds that formed (Fig. 2). These actions of TCDD were Ahr-dependent. Most remarkable was the fact that TCDD acted in a UGS region-selective fashion to inhibit buds: anterior budding was not affected, dorsolateral buds were reduced in number and displaced towards the anterior UGS surface, and ventral buds did not form (Fig. 2, Table 1).
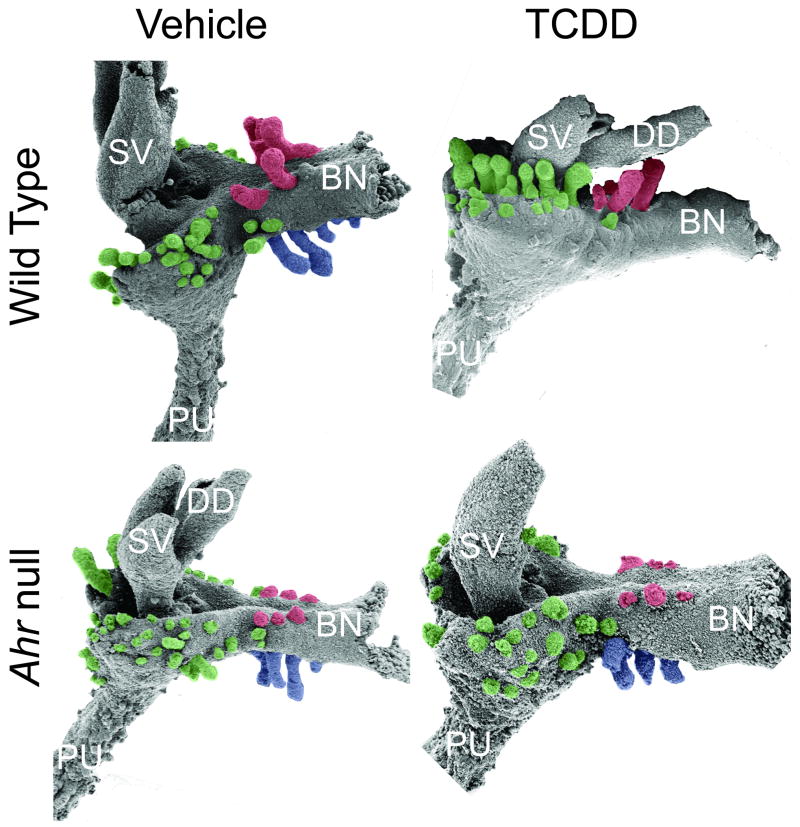
Figure 2. Activation of AHR signaling by in utero TCDD exposure interferes with normal prostatic bud patterning in the fetal male mouse.
Wild type and Ahr null mice were exposed on E13.5 to vehicle (5 ml/kg corn oil, po) or TCDD (5 μg/kg, maternal dose). UGM was removed from male UGSs on E18.5 and the underlying UGE was visualized by scanning electron microscopy (SEM). Results shown are representative SEM images of the lateral UGS surface of wild type and Ahr null mice that were exposed in utero to vehicle or TCDD. TCDD completely inhibited ventral prostatic bud formation, reduced the number of dorsolateral buds and caused them to emerge inappropriately on the anterior budding surface. Ventral prostatic buds are pseudocolored blue, dorsolateral buds are green, and anterior buds are red. Abbreviations used are: BL, bladder; DD, ductus deferens; PU, pelvic urethra; SV, seminal vesicle.
Table 1. TCDD effects on prostate development in the mouse.
| Prostate lobe | Prenatal development [4] | Postnatal development [25, 26] |
|---|---|---|
| Ventral | Complete loss of buds | Unknown effect on branching morphogenesis ↓Epithelial differentiation ↓Growth |
| Dorsolateral | ↓Bud number Abnormal bud position |
No effect on branching morphogenesis ↓Epithelial differentiation ↓Growth |
| Anterior | No effect on bud number No effect on bud position |
↓Branching morphogenesis ↓Epithelial differentiation ↓Growth |
Ventral, dorsolateral, and anterior prostatic buds are specified and initiated at different stages of UGS development [19]. It was shown recently that prostatic buds from each of these UGS regions have different windows of sensitivity to TCDD[20]. Ventral prostatic buds are inhibited when TCDD exposure occurs on or before E15.5, but dorsolateral buds are only inhibited when exposure occurs on or before E14.5. Furthermore, at no time is anterior prostatic budding sensitive to inhibition by TCDD at a 5 μg/kg dose. The significance of these results is that they have revealed the possible phases of prostate ductal development upon which TCDD acts (bud specification and initiation phases) and have exposed a potential mechanism of TCDD action: disruption of fetal prostate patterning.
Lin et al. [21] demonstrated that TCDD interferes with prostatic bud patterns by acting directly on the UGS to stimulate AHR signaling. UGSs from wild type and Ahr null mice were isolated from male fetuses on E14.5 and placed in organ culture media containing vehicle or a fixed concentration of 5α–dihydrotestosterone (DHT, 10 nM) and either vehicle or TCDD (1 nM). Prostatic buds formed in an androgen-dependent fashion and TCDD inhibited budding in the cultured wild type UGS, but not Ahr null UGS. Thus, TCDD acts directly on the UGS to impair prostatic budding.
It was subsequently shown that UGM is the site of TCDD action [22]. UGSs were removed from wild type and Ahr null E15.5 mouse fetuses and separated into ventral UGM, dorsolateral UGM, and UGE tissue components. The tissue components were then recombined and grown for 5 days in organ culture media containing 10 nM DHT and either vehicle or TCDD. The absence of functional AHR protein in UGE did not rescue prostatic budding inhibition by TCDD, but the absence of AHR protein in UGM did. Therefore, AHR in the UGM is responsible for inhibition of prostatic budding by TCDD. These results have further refined the trajectory of AHR signaling research in the developing prostate to focus on mesenchymal-epithelial interactions in the UGS.
3. Neither induction of TCDD-inducible cytochrome P450 activity nor direct interference with androgen or estrogen receptor signaling is involved in the inhibition of prostate development by TCDD
Androgens and estrogens are capable of influencing prostatic bud patterning by acting on receptors in UGM to elicit changes in epithelial proliferation [23]. The AHR signaling pathway has been shown to interface with androgen and estrogen receptor signaling[24-27], but inhibition of prostatic budding by TCDD cannot be explained simply by actions on these signaling pathways. In utero TCDD exposure in mice does not change testicular testosterone content in male fetuses, alter conversion of testosterone to DHT, or change the Ar transcript abundance or AR transcriptional activity in the UGS [28]. Furthermore, treatment of wild-type dams with excess DHT, at a concentration sufficient to masculinize female fetuses, does not protect against inhibition of prostatic budding caused by TCDD [21].
While estrogens modulate prostatic bud formation [29-31], impairment of prostatic budding by TCDD in mice is not caused by its estrogenic or anti-estrogenic properties. Prostatic budding occurs normally in mice that are deficient in either estrogen receptor α, estrogen receptor β, or both, and these mice are not protected against prostatic budding inhibition by TCDD [32]. Furthermore, exposure of wild type mice to the anti-estrogen ICI 182,790 does not impair prostatic budding or protect against prostatic budding inhibition by TCDD [32].
In some tissues, AHR-dependent transcription of cytochrome P450s (Cyp)1a1 and 1b1 mediates toxicity of TCDD and other environmental chemicals that bind to the AHR. CYP1A1 has been implicated in the mechanism of TCDD-induced wasting syndrome [33] and benzo[a]pyrene-induced hepatoxicity [34]. The metabolic activity of CYP1B1 has been associated with anti-estrogenic effects of TCDD [35], and dimethylbenz[a]anthracene-induced bone marrow toxicity [36] and lymphoma [37]. However, neither CYP1A1 nor CYP1B1 appears to be involved in the mechanism by which TCDD disrupts prostatic bud patterning. Recent studies show that Cyp1a1 transcripts and CYP1A1-mediated ethoxyresorufin-O-deethylase (EROD) activity are induced by TCDD predominantly in mouse UGE [20] excluding it from the mechanism of budding inhibition by TCDD which is mediated through activated AHR signaling in UGM [22]. Furthermore, although Cyp1b1 is induced by TCDD in the appropriate tissue (UGM) and embryonic stage to be considered as a player in the AHR mechanism of budding inhibition, Cyp1b1 null mouse fetuses undergo normal prostatic bud formation in the absence of TCDD and are not resistant to budding impairment following TCDD exposure (C. Vezina and C. Jefcoate, unpublished observations).
Prostatic budding in the ventral UGS region is not more susceptible to impairment by TCDD because there is quantitatively more AHR signaling in this region compared to the dorsolateral and anterior UGS budding regions. ARNT protein is expressed in nearly every UGS cell, Ahr transcripts have been detected in UGM and UGE and are evenly distributed in each of the prostatic budding zones, and AHR-transcriptional activity has been identified in a band of UGM tissue that circumscribes the entire UGE surface—not just the ventral region [20]. Although the distribution of AHR-responsive β-galactosidase activity in TCDD-responsive transgenic reporter mice is slightly more diffuse in ventral UGM [20], this is unlikely to account for the dramatic difference in budding inhibition by TCDD in the ventral region compared to other UGS regions. Together, these results suggest that TCDD impairs prostatic budding by interfering with the signaling pathways responsible for patterning the developing prostate.
4. The AHR interacts with multiple signaling pathways during prostate development
TCDD stimulates a paracrine signal, derived from AHR-mediated transcription in UGM, which inhibits prostatic bud formation in UGE. WNT5A, retinoid, and FGF10 signaling pathways are each necessary for prostatic bud formation in the mouse UGS [38-40] and share with TCDD an overarching mode of action that requires communication between UGM and UGE. Recent studies reveal crosstalk between WNT5A, retinoid, FGF10, and AHR signaling during early prostate development.
Genes of the Wnt superfamily have been implicated in embryonic patterning and morphogenesis [41-43] and it was discovered recently that Wnt5a, the most abundant Wnt transcript in the UGS [44], is involved in prostatic bud patterning [38]. Wnt5a mRNA is expressed in UGM in a pattern that overlaps ligand-dependent AHR activity and interferes with prostatic budding in a manner similar to TCDD. Neither WNT5A nor AHR is required for prostatic budding, but activation of either signaling pathway inhibits budding. WNT5A and TCDD are both capable of inhibiting ventral prostate development without appreciably affecting development of other prostate lobes. Moreover, inhibition of WNT5A signaling protects against prostatic budding inhibition by TCDD. These results suggest that inappropriate activation of WNT5A signaling may play a role in the mechanism of prostatic budding inhibition by TCDD.
There are multiple examples of crosstalk between AHR and retinoic acid signaling during mammalian development [45-48] and this also appears to exist during prostate development. Retinoic acid has been identified as a positive regulator of prostatic budding that acts upon receptors in UGM [40]. Retinoic acid increased prostatic budding by over 2-fold in cultured UGS tissues, TCDD completely blocked this effect, and inhibition of WNT5A signaling during TCDD exposure restored retinoic acid-induced budding [49]. TCDD did not alter the abundance of retinoic acid synthesis enzymes aldehyde dehydrogenase 1a1, 1a2, or 1a3, and did not change the abundance or transcription activity of retinoic acid receptors [50]. It therefore appears that AHR signaling functions downstream of retinoic acid receptors to restrict prostatic bud formation.
FGF10 is required for prostatic bud initiation in fetal mice and rats [39]. Fgf10 is synthesized in UGM and stimulates mitogenesis by activating fibroblast growth factor receptor type 2 (FGFR2) in UGE. AHR signaling antagonizes the actions of FGF10 during prostatic bud formation. We showed in a recent study that addition of FGF10 to UGS organ culture media increases prostatic bud number and TCDD blocks this effect [49]. However, TCDD did not alter the abundance or distribution of Fgf10 or Fgfr2 mRNA in the UGS or interfere with the ability of FGF10 to simulate ERK1/2 activation. Therefore, it appears that TCDD acts downstream of FGF10 signaling to impair prostatic bud formation.
5. Does AHR signaling play a general role in developmental patterning of the vertebrate embryo?
Stimulation of AHR signaling by TCDD produces regionalized effects on prostate development that cannot be explained by regional distribution of AHR signaling in the UGS. This suggests a basic role for the AHR in patterning the fetal prostate and we are only beginning to understand interactions between AHR and other signaling pathways involved in this process. However, it appears that molecular interactions with AHR signaling in the developing prostate may not be confined during embryogenesis exclusively to UGS tissue. The UGS is only one of the several tissues to use budding and branching as paradigms for development. Although prostate development is distinguished from these organs by its androgen-dependent growth, many of the signaling pathways in prostate morphogenesis, including those with epithelial-mesenchymal paracrine signaling components, are used redundantly in the morphogenesis of other organs including lung, molar, mammary, and salivary gland [51-55]. There is evidence that TCDD disrupts development in these tissues [56-59], raising the possibility that AHR signaling may play a general role, of which we were previously unaware, in budding and branching morphogenesis of certain organs throughout the vertebrate embryo.
6. AHR signaling during in utero development regulates prostate aging in mice
Exposure to some environmental chemicals during fetal morphogenesis imprints the prostate and predisposes it to disease later in life[60, 61]. Since benign prostate hyperplasia (BPH) and prostate cancer are diseases of aging and are more prevalent in older males [62], our laboratory investigated whether IUL TCDD exposure had latent affects on prostate physiology in aged (510 days) mice [63]. Prostate normally becomes less responsive to androgens during aging [64]. We found that IUL TCDD exposure caused prostate from senescent mice to retain its responsiveness to androgen, suggesting that perinatal TCDD exposure alters the prostate aging process [63]. We also observed that IUL TCDD exposure increased the incidence of hyperplastic lesions (cribiform structures) in 510 days old mice. Although wild type mice do not spontaneously develop prostate cancer, cribiform structures in these mice are considered by some to be pre-cancerous lesions [65, 66]. Collectively, these results indicate that inappropriate activation of AHR signaling during prostate growth in utero may permanently reprogram the prostate and increase disease susceptibly in adulthood.
The transgenic adenocarcinoma of the mouse prostate (TRAMP) model was used further to test the hypothesis that IUL TCDD exposure increases the risk of adult prostate disease. The TRAMP model was generated with a targeting vector containing a promoter element from the rat probasin gene (an androgen-responsive secretory protein expressed in mature mouse prostate) upstream of the simian virus 40 (SV40) large and small T antigens [67]. SV40 T antigens are expressed in an androgen-dependent fashion in TRAMP mouse prostate epithelium, are activated starting at about 56 days after birth, and induce prostate pathology starting at about 70 days after birth. TRAMP prostate tumors are characteristically neuroendocrine in nature [68].
The Ahr null allele was crossed onto a TRAMP mouse background to determine whether the presence of endogenous AHR signaling (in the absence of TCDD) influences prostate disease [69]. Ahr null, heterozygous, or wild type male TRAMP mice between 35 and 210 days of age were palpated for tumors at 5 weeks intervals. At 105 days of age, all mice regardless of Ahr genotype exhibited diffuse prostate epithelial hyperplasia associated with the TRAMP background. Beginning at 140 days of age; however, the percentage of mice with palpable tumors was significantly higher in mice lacking one (43%) or both (60%) copies of the functional Ahr gene compared to Ahr wild type (16%) TRAMP mice (Fig. 4). The increased incidence of tumors in Ahr null TRAMP mice was not caused indirectly by a change in prostate androgen-responsiveness or an increase in transgene expression. Experiments with TRAMP mice have indicated an inverted-U shaped relationship between the abundance of AHR signaling in the fetal UGS and incidence of tumors in adult mice: too little or too much AHR signaling during fetal prostate development increases the risk for prostate disease in adult mice.
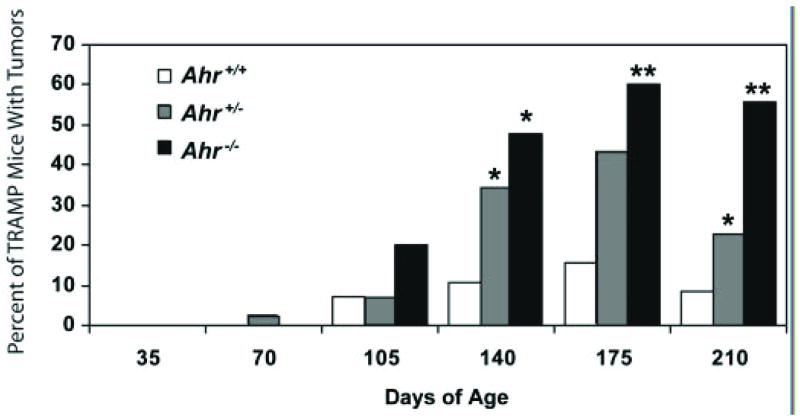
Figure 4. Ahr signaling suppresses tumor formation in the TRAMP mouse model of prostate cancer.
The percentage of mice with prostate tumors was determined at 35 days intervals in Ahr+/+, Ahr+/-, and Ahr-/- C57BL/6J TRAMP mice 35–210 days of age [40]. Starting at 140 days of age, chi-square analysis revealed a significant increase in prostate tumor incidence in TRAMP mice deficient in one or both functional Ahr alleles. A single asterisk denotes a significant difference from Ahr+/+ TRAMP mice and a double asterisk denotes a significant difference from both Ahr+/+ and Ahr+/- TRAMP mice (p < 0.05). The number of Ahr+/+, Ahr+/-, and Ahr-/- mice, respectively in each age group were: 35 days (20, 32 and 22), 70 days (20, 49 and 22), 105 days (14, 45 and 20), 140 days (19, 55 and 21), 175 days (19, 46 and 18) and 210 days (24, 43 and 18).
7. AHR signaling has different actions on the developing prostate compared to the mature prostate
AHR signaling produces different biological responses in fetal prostate cells compared to cells of the adult prostate. TCDD increases EROD activity and Cyp1a1 and Cyp1b1 transcript abundance in mouse fetal UGE[20], but not in PC3 cells derived from adult human prostate epithelium [70]. AHR signaling does not alter AR-dependent transcription [20] or cell proliferation (C. Vezina, unpublished observation) in the fetal mouse UGS, but antagonizes AR-dependent transcription and cell proliferation in mature adult prostate epithelium [71-73]. Moreover, mouse testicular testosterone levels are not affected by fetal exposure to dioxin, but circulating testosterone in the adult mouse is decreased by TCDD [74]. Fetal exposure of wild type and TRAMP mice to TCDD increases epithelial hyperplasia [63] and tumor incidence [75], respectively, in the adult, but adult exposure of TRAMP mice to TCDD protects against tumor development and increases survival (Wayne Fritz et al., unpublished results). Together, the studies indicate age-related changes in the role of AHR signaling in the prostate. The emerging theme is that activation of AHR in the fetal UGS promotes prostate disease in adult animals, while activation of AHR in the adult prostate is protective against prostate disease (Fig. 5). This relationship between age, TCDD exposure, and disease is not exclusive to prostate and appears also to occur in other hormone-responsive tissues. Using a carcinogen-induced rodent mammary tumor model, Jenkins et al. [76] showed that early life TCDD exposure increased breast cancer risk in mature female rats. However, TCDD during adulthood decreased mammary tumors in Sprague-Dawley rats [77, 78] and inhibited proliferation of adult human T47D and MCF7 breast cancer cells in vitro [79]. There is also epidemiological support for a reduction in breast cancer risk in women that were exposed to high concentrations of dioxin during adulthood [80], although, this remains controversial [81].
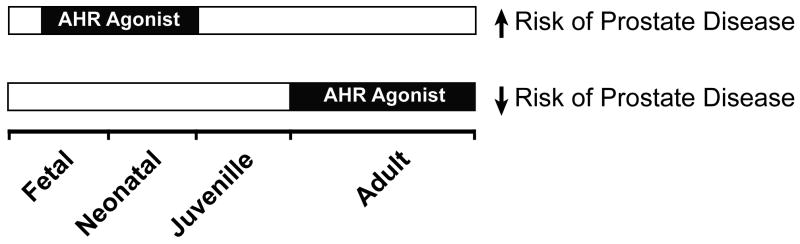
Figure 5. Perinatal and adult exposure to AHR agonists differentially affects the risk of prostate disease.
Sustained activation of AHR in the UGS by TCDD during fetal prostate development increases the risk of prostate cancer in adulthood, whereas activation of AHR signaling in the prostate during adulthood by TCDD, other full AHR agonists, and selective AHR modulators (SARHMs) are protective against prostate cancer and/or benign prostate hyperplasia.
There is experimental support for the notion that AHR activation during adulthood also protects against human prostate disease. TCDD is classified as a suspected carcinogen and is known to cause cancer in other tissues, but epidemiological studies have not identified a significant association between adult TCDD exposure and prostate cancer [82]. On the contrary, adult TCDD exposure decreases the risk of BPH. A prospective study by Gupta et al. [83] assessed the correlation between serum TCDD levels and BPH in a cohort of 1,266 Operation Ranch Hand Air Force Veterans from the Vietnam War who sprayed Agent Orange, a defoliant that was contaminated with TCDD, compared to Air Force Veterans who did not spray Agent Orange. They identified an inverse relationship between TCDD body burden and risk of BPH. Furthermore, in a follow-up study [84] the body burden of dioxin-like chemicals (using the TCDD toxic equivalents [TEQ] method) was compared in 42 men diagnosed with BPH to 99 men without clinical evidence of the disease. Men without BPH were found to have a 20.9% higher body burden of TCDD TEQs compared to men with BPH.
Adult exposure to TCDD increased tumor-free survival of TRAMP mice, indicating that AHR signaling may serve a tumor suppressor role in the prostate. Because activation of AHR signaling during adulthood has also been shown to protect against breast cancer in mouse models, less toxic alternatives to TCDD have been developed for use in cancer chemoprevention [85]. These chemicals, collectively referred to as selective AHR modulators (SARHMs), represent a diverse array of chemical structures including alternate-substituted (1,3,6,8- and 2,4,6,8-) alkyl polychlorinated dibenzofurans (PCDFs) and substituted diindolylmethanes (DIMs). SAHRMs share with TCDD the ability to activate AHR signaling, but are distinguished from TCDD by their muted toxicological activity, such as the inability to induce hepatic Cyp1a1 [86]. SAHRMs have been shown to inhibit proliferation of cultured androgen-responsive human prostate adenocarcinoma LNCaP cells. Our lab recently tested the hypothesis that dietary exposure to the SAHRM 6-methyl-1,3,8-trichlorodibenzofuran (6-MCDF) would protect against prostate carcinogenesis [87]. Eight week-old Ahr+/+ TRAMP mice were fed a diet containing 6-MCDF (0, 10, or 40 mg/kg). Tumor incidence and lymph node metastasis were determined at 140 days of age. Frequency of pelvic lymph node metastases was significantly reduced in Ahr+/+TRAMP mice fed a diet containing 40 mg/kg 6-MCDF. These results reveal that activation of AHR signaling during adulthood may protect against prostate disease in transgenic TRAMP mice.
There are multiple mechanisms by which activation of AHR signaling during adulthood may support prostate health. First, AHR signaling has been shown to act on the hypothalamic-pituitary-gonadal axis to reduce circulating androgens during adulthood [11, 88, 89], thereby downregulating the principle permissive factor for BPH and prostate cancer [88]. Second, AHR signaling intersects with and inhibits estrogen receptor signaling [90]. Estrogen receptor antagonism has been shown to be protective against prostate cancer and BPH [91, 92]. Third, AHR signaling appears to antagonize angiogenesis in the prostate, and fourth, it has been postulated that chronic inflammation may play a role in prostate carcinogenesis [93] and sustained AHR activation has been shown to be immunosuppressive [94].
Angiogenesis is initiated during prostate tumor progression by dimerization of HIF-1α with the aryl hydrocarbon nuclear receptor (ARNT), causing transcriptional activation of vascular endothelial growth factor, Vegf [95]. This occurs very early in TRAMP tumor progression and may be required for tumors to grow to a palpable size [96]. Since ARNT is a common dimerization partner of both AHR and HIF-1α, it was hypothesized by Fritz et al. [97] that AHR signaling may suppress prostate tumors by competing with HIF-1α for ARNT, thereby inhibiting Vegf transcription, angiogenesis, and tumor growth. Prostates from Ahr+/+, Ahr+/- and Ahr-/- C57BL/6J TRAMP mice were cultured in the presence of graded concentrations of sodium ortho-vanadate. Vanadate is a chemical agonist of the phosphatidylinositol 3-kinase-signaling cascade that induces HIF-1α/ARNT dimerization. Vanadate increased VEGF protein abundance in cultured TRAMP prostates that were deficient in one or more functional Ahr alleles, but not in Ahr+/+ TRAMP cultures. This occurred without appreciable differences in phosphatidylinositol 3-kinase-signaling among the genetic groups. The results of this study suggest that AHR sequesters ARNT in TRAMP prostate tissue, and suppresses prostate tumor progression by decreasing VEGF transcription.
Lin et. al [98] investigated whether AHR signaling alters the pathologic progression of inducible prostatitis in mice. Wild type and Ahr null C57Bl/6J mice were infected via intra-urethral installation with uropathogenic E. coli 1677 at 10 weeks of age. The infection induced a sub-acute inflammatory reaction in prostates of both mouse strains that proceeded to chronic inflammation before receding completely by 20 weeks. This was followed by mild epithelial hyperplasia, cellular atypia and dysplasia that persisted until the end of the 20 weeks study. The absence of functional AHR did not alter onset of dysplasia induced by inflammation, suggesting AHR signaling may function independently from inflammatory signaling in prostate. As a whole, it appears that activation of AHR signaling during prostate development predisposes to prostate diseases later in life, but AHR activation in the mature prostate protects against prostate disease.
8. Understanding the role of AHR signaling in prostate biology and disease – future opportunities
There are a number of directions for future research that need to be addressed in order to understand fully the role of AHR signaling in the prostate and in men's health. The first need is an epidemiology study that investigates the association of IUL TCDD exposure to risk of adult prostate disease. The risk assessment models that currently exist do not account for differences in susceptibility to prostate disease, based on timing of TCDD exposure during the life history of men (with early life stage TCDD exposure presenting the greatest risk). The current risk assessment models may therefore underestimate the risk of prostate disease caused by gestational TCDD exposure. Second, there is a need to compare and contrast molecular changes in the prostate induced by adult TCDD exposure to those caused by fetal TCDD exposure. Only then can we begin to understand why fetal exposure to this persistent AHR agonist promotes prostate disease while adult exposure protects against it. Third, it would be prudent to determine if the molecular changes brought on by fetal TCDD exposure, contributing to budding impairment, are the same changes that imprint the prostate and increase disease risk later in life. Fourth, the striking change in ‘prostate aging’ caused by IUL TCDD exposure implicates prostate stem cells. But we have absolutely no knowledge of how TCDD affects prostate stem cells. Does exposure to AHR agonists activate them, repress them, cause more of them to be present, or less? These are important questions. They need to be answered if we are to understand the impact of IUL AHR agonist exposure on the prostate health of aging men. Fifth, are the chemoprotective effects of adult TCDD exposure caused by AHR agonist effects occurring directly on the prostate, or are they due to effects occurring on other organs (decreased circulating testosterone, for example). The fact that SAHRMs are effective for preventing prostate cancer, coupled with epidemiology studies showing that TCDD protects against BPH, suggests that chemical activation of AHR signaling by a SARHM may be a ‘magic bullet’ for chemoprevention of BPH and prostate cancer.
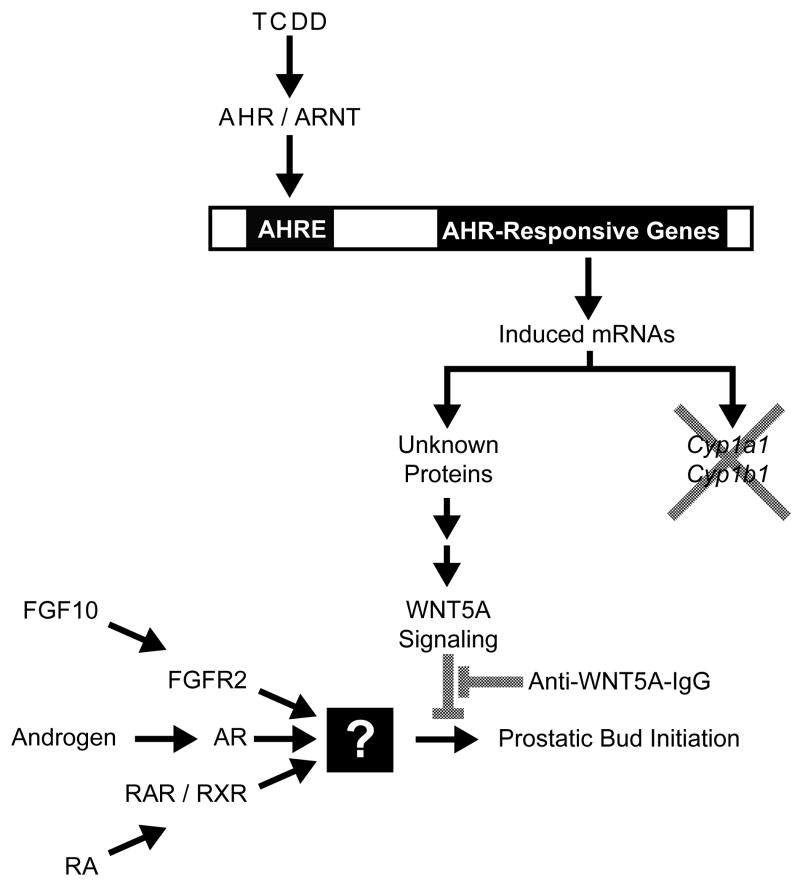
Figure 3. Interactions between the AHR signaling and WNT5A, androgen, retinoid, and FGF10 signaling during mouse prostatic bud formation.
Androgens (testosterone and 5α-dihydrotestosterone), retinoic acid (RA) and fibroblast growth factor 10 (FGF10) all increase prostatic bud formation in the mouse urogenital sinus (UGS) by activating downstream molecular targets that have not been identified (denoted by the square box). TCDD activates AHR/ARNT-mediated transcription by binding to AHR response elements (AHREs) located in the promoters of AHR-responsive genes. However, Cyp1a1 and Cyp1b1, classical AHR-responsive genes, are not involved in the mechanism of prostatic budding inhibition by TCDD. Instead, AHR/ARNT appears to trigger downstream events that lead to WNT5A signaling and repression of prostatic budding. Inhibition of WNT5A signaling with an inhibitory antibody against WNT5A (Anti-WNT5A-IgG) restores prostatic budding in the presence of TCDD.
Acknowledgments
We thank Drs. Sarah Allgeier, Wayne Fritz, Kinarm Ko, and Robert Moore for their contributions to this manuscript. This publication was supported by NIEHS grants F32ES014284 (CMV) and R37ES01332 (REP) and by NCI grant CA095751 (REP).
References
- 1.Cunha GR, Chung LW. Stromal-epithelial interactions--I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14:1317–24. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- 2.Cunha GR, Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978;205:181–93. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- 3.Lin TM, Rasmussen NT, Moore RW, Albrecht RM, Peterson RE. Region-specific inhibition of prostatic epithelial bud formation in the urogenital sinus of C57BL/6 mice exposed in utero to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2003;76:171–81. doi: 10.1093/toxsci/kfg218. [DOI] [PubMed] [Google Scholar]
- 4.Timms BG, Mohs TJ, Didio LJ. Ductal budding and branching patterns in the developing prostate. J Urol. 1994;151:1427–32. doi: 10.1016/s0022-5347(17)35273-4. [DOI] [PubMed] [Google Scholar]
- 5.Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34:961–71. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- 6.Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253:165–74. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 7.Signoretti S, Loda M. Defining cell lineages in the prostate epithelium. Cell Cycle. 2006;5:138–41. doi: 10.4161/cc.5.2.2340. [DOI] [PubMed] [Google Scholar]
- 8.Sommer RJ, Sojka KM, Pollenz RS, Cooke PS, Peterson RE. Ah receptor and ARNT protein and mRNA concentrations in rat prostate: effects of stage of development and 2,3,7, 8-tetrachlorodibenzo-p-dioxin treatment. Toxicol Appl Pharmacol. 1999;155:177–89. doi: 10.1006/taap.1998.8597. [DOI] [PubMed] [Google Scholar]
- 9.Kashani M, Steiner G, Haitel A, Schaufler K, Thalhammer T, Amann G, et al. Expression of the aryl hydrocarbon receptor (AhR) and the aryl hydrocarbon receptor nuclear translocator (ARNT) in fetal, benign hyperplastic, and malignant prostate. Prostate. 1998;37:98–108. doi: 10.1002/(sici)1097-0045(19981001)37:2<98::aid-pros6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Lin TM, Ko K, Moore RW, Buchanan DL, Cooke PS, Peterson RE. Role of the aryl hydrocarbon receptor in the development of control and 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed male mice. J Toxicol Environ Health A. 2001;64:327–42. doi: 10.1080/152873901316981312. [DOI] [PubMed] [Google Scholar]
- 11.Mably TA, Moore RW, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 1. Effects on androgenic status. Toxicol Appl Pharmacol. 1992;114:97–107. doi: 10.1016/0041-008x(92)90101-w. [DOI] [PubMed] [Google Scholar]
- 12.Gray LE, Ostby JS, Kelce WR. A dose-response analysis of the reproductive effects of a single gestational dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male Long Evans Hooded rat offspring. Toxicol Appl Pharmacol. 1997;146:11–20. doi: 10.1006/taap.1997.8223. [DOI] [PubMed] [Google Scholar]
- 13.Simanainen U, Adamsson A, Tuomisto JT, Miettinen HM, Toppari J, Tuomisto J, et al. Adult 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure and effects on male reproductive organs in three differentially TCDD-susceptible rat lines. Toxicol Sci. 2004;81:401–7. doi: 10.1093/toxsci/kfh212. [DOI] [PubMed] [Google Scholar]
- 14.Theobald HM, Peterson RE. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-rho-dioxin: effects on development of the male and female reproductive system of the mouse. Toxicol Appl Pharmacol. 1997;145:124–35. doi: 10.1006/taap.1997.8173. [DOI] [PubMed] [Google Scholar]
- 15.Lin TM, Ko K, Moore RW, Simanainen U, Oberley TD, Peterson RE. Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Toxicol Sci. 2002;68:479–87. doi: 10.1093/toxsci/68.2.479. [DOI] [PubMed] [Google Scholar]
- 16.Thomas RS, Penn SG, Holden K, Bradfield CA, Rank DR. Sequence variation and phylogenetic history of the mouse Ahr gene. Pharmacogenetics. 2002;12:151–63. doi: 10.1097/00008571-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Lin TM, Simanainen U, Moore RW, Peterson RE. Critical windows of vulnerability for effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on prostate and seminal vesicle development in C57BL/6 mice. Toxicol Sci. 2002;69:202–9. doi: 10.1093/toxsci/69.1.202. [DOI] [PubMed] [Google Scholar]
- 18.Ko K, Theobald HM, Peterson RE. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in the C57BL/6J mouse prostate: lobe-specific effects on branching morphogenesis. Toxicol Sci. 2002;70:227–37. doi: 10.1093/toxsci/70.2.227. [DOI] [PubMed] [Google Scholar]
- 19.Cunha GR. The role of androgens in the epithelio-mesenchymal interactions involved in prostatic morphogenesis in embryonic mice. Anat Rec. 1973;175:87–96. doi: 10.1002/ar.1091750108. [DOI] [PubMed] [Google Scholar]
- 20.Vezina CM, Allgeier S, Moore R, Lin TM, Bemis J, Hardin HA, et al. Dioxin Causes Ventral Prostate Agenesis by Disrupting Dorsoventral Patterning in Developing Mouse Prostate. Toxicol Sci. 2008 doi: 10.1093/toxsci/kfn183. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin TM, Rasmussen NT, Moore RW, Albrecht RM, Peterson RE. 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibits prostatic epithelial bud formation by acting directly on the urogenital sinus. J Urol. 2004;172:365–8. doi: 10.1097/01.ju.0000124989.02257.38. [DOI] [PubMed] [Google Scholar]
- 22.Ko K, Moore RW, Peterson RE. Aryl hydrocarbon receptors in urogenital sinus mesenchyme mediate the inhibition of prostatic epithelial bud formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2004;196:149–55. doi: 10.1016/j.taap.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–34. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 24.Boverhof DR, Kwekel JC, Humes DG, Burgoon LD, Zacharewski TR. Dioxin induces an estrogen-like, estrogen receptor-dependent gene expression response in the murine uterus. Mol Pharmacol. 2006;69:1599–606. doi: 10.1124/mol.105.019638. [DOI] [PubMed] [Google Scholar]
- 25.Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–64. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 26.Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, Sone H. Cross-talk between 2,3,7,8-tetrachlorodibenzo-p-dioxin and testosterone signal transduction pathways in LNCaP prostate cancer cells. Biochem Biophys Res Commun. 1999;256:462–8. doi: 10.1006/bbrc.1999.0367. [DOI] [PubMed] [Google Scholar]
- 27.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16:807–16. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 28.Ko K, Theobald HM, Moore RW, Peterson RE. Evidence that inhibited prostatic epithelial bud formation in 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed C57BL/6J fetal mice is not due to interruption of androgen signaling in the urogenital sinus. Toxicol Sci. 2004;79:360–9. doi: 10.1093/toxsci/kfh111. [DOI] [PubMed] [Google Scholar]
- 29.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci U S A. 2005;102:7014–9. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coveney D, Shaw G, Renfree MB. Effects of oestrogen treatment on testicular descent, inguinal closure and prostatic development in a male marsupial, Macropus eugenii. Reproduction. 2002;124:73–83. doi: 10.1530/rep.0.1240073. [DOI] [PubMed] [Google Scholar]
- 31.vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A. 1997;94:2056–61. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks SM, Lin TM, Mukai M, Cooke PS, Peterson RE. Estrogen receptor alpha is not required for normal prostatic bud formation or for inhibition of prostatic bud formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice. The Toxicologist (A Supplement to Toxicological Sciences) 2005;84:554. [Google Scholar]
- 33.Uno S, Dalton TP, Sinclair PR, Gorman N, Wang B, Smith AG, et al. Cyp1a1(-/-) male mice: protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol Appl Pharmacol. 2004;196:410–21. doi: 10.1016/j.taap.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Uno S, Dalton TP, Shertzer HG, Genter MB, Warshawsky D, Talaska G, et al. Benzo[a]pyrene-induced toxicity: paradoxical protection in Cyp1a1(-/-) knockout mice having increased hepatic BaP-DNA adduct levels. Biochem Biophys Res Commun. 2001;289:1049–56. doi: 10.1006/bbrc.2001.6110. [DOI] [PubMed] [Google Scholar]
- 35.Takemoto K, Nakajima M, Fujiki Y, Katoh M, Gonzalez FJ, Yokoi T. Role of the aryl hydrocarbon receptor and Cyp1b1 in the antiestrogenic activity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch Toxicol. 2004;78:309–15. doi: 10.1007/s00204-004-0550-7. [DOI] [PubMed] [Google Scholar]
- 36.Heidel SM, Holston K, Buters JT, Gonzalez FJ, Jefcoate CR, Czupyrynski CJ. Bone marrow stromal cell cytochrome P4501B1 is required for pre-B cell apoptosis induced by 7,12-dimethylbenz[a]anthracene. Mol Pharmacol. 1999;56:1317–23. doi: 10.1124/mol.56.6.1317. [DOI] [PubMed] [Google Scholar]
- 37.Buters JT, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, et al. Cytochrome P450 CYP1B1 determines susceptibility to 7, 12-dimethylbenz[a]anthracene-induced lymphomas. Proc Natl Acad Sci U S A. 1999;96:1977–82. doi: 10.1073/pnas.96.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allgeier SH, Lin TM, Vezina CM, Moore RW, Fritz WA, Chiu SY, et al. WNT5A Selectively Inhibits Mouse Ventral Prostate Development. Developmental Biology. 2008 doi: 10.1016/j.ydbio.2008.08.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donjacour AA, Thomson AA, Cunha GR. FGF-10 plays an essential role in the growth of the fetal prostate. Dev Biol. 2003;261:39–54. doi: 10.1016/s0012-1606(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 40.Vezina CM, Allgeier SH, Fritz WA, Moore RW, Strerath M, Bushman W, et al. Retinoic acid induces prostatic bud formation. Dev Dyn. 2008 doi: 10.1002/dvdy.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 42.Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313:210–24. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teuliere J, Faraldo MM, Deugnier MA, Shtutman M, Ben-Ze'ev A, Thiery JP, et al. Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development. 2005;132:267–77. doi: 10.1242/dev.01583. [DOI] [PubMed] [Google Scholar]
- 44.Zhang TJ, Hoffman BG, Ruiz de Algara T, Helgason CD. SAGE reveals expression of Wnt signalling pathway members during mouse prostate development. Gene Expr Patterns. 2006;6:310–24. doi: 10.1016/j.modgep.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Abbott BD, Birnbaum LS. Cellular alterations and enhanced induction of cleft palate after coadministration of retinoic acid and TCDD. Toxicol Appl Pharmacol. 1989;99:287–301. doi: 10.1016/0041-008x(89)90011-2. [DOI] [PubMed] [Google Scholar]
- 46.Birnbaum LS, Harris MW, Stocking LM, Clark AM, Morrissey RE. Retinoic acid and 2,3,7,8-tetrachlorodibenzo-p-dioxin selectively enhance teratogenesis in C57BL/6N mice. Toxicol Appl Pharmacol. 1989;98:487–500. doi: 10.1016/0041-008x(89)90177-4. [DOI] [PubMed] [Google Scholar]
- 47.Kohl FV, Rudiger HW. Retinoids inhibit 2,3,7,8-tetrachlorodibenzo-p-dioxine-induced activity of benzo[a]pyrene metabolizing enzymes in human diploid fibroblasts. Carcinogenesis. 1980;1:733–7. doi: 10.1093/carcin/1.9.733. [DOI] [PubMed] [Google Scholar]
- 48.Weston WM, Nugent P, Greene RM. Inhibition of retinoic-acid-induced gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem Biophys Res Commun. 1995;207:690–4. doi: 10.1006/bbrc.1995.1242. [DOI] [PubMed] [Google Scholar]
- 49.Vezina CM, Hicks SM, Moore RW, Peterson RE. TCDD modulates selected developmental signaling pathways during mouse prostate morphogenesis. Organohalogen Compounds. 2007;69:629–32. [Google Scholar]
- 50.Vezina CM, Li H, Goldberg MC, Kim KH, Peterson RE. Modulation of retinoic acid signaling may contribute to impairment of ventral prostatic bud formation by TCDD in the urogenital sinus of male fetal mice. The 45th Annual Meeting of the Society of Toxicology; San Diego, CA. 2006. p. 923. The Toxicologist (a supplement to Toxicological Sciences) [Google Scholar]
- 51.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–24. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 52.Mikkola ML, Millar SE. The mammary bud as a skin appendage: unique and shared aspects of development. J Mammary Gland Biol Neoplasia. 2006;11:187–203. doi: 10.1007/s10911-006-9029-x. [DOI] [PubMed] [Google Scholar]
- 53.Salazar-Ciudad I. Tooth morphogenesis in vivo, in vitro, and in silico. Curr Top Dev Biol. 2008;81:341–71. doi: 10.1016/S0070-2153(07)81012-X. [DOI] [PubMed] [Google Scholar]
- 54.Tucker AS. Salivary gland development. Semin Cell Dev Biol. 2007;18:237–44. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Warburton D, Bellusci S, De Langhe S, Del Moral PM, Fleury V, Mailleux A, et al. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res. 2005;57:26R–37R. doi: 10.1203/01.PDR.0000159570.01327.ED. [DOI] [PubMed] [Google Scholar]
- 56.Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2002;67:63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- 57.Kiukkonen A, Sahlberg C, Partanen AM, Alaluusua S, Pohjanvirta R, Tuomisto J, et al. Interference by 2,3,7,8-tetrachlorodibenzo-p-dioxin with cultured mouse submandibular gland branching morphogenesis involves reduced epidermal growth factor receptor signaling. Toxicol Appl Pharmacol. 2006;212:200–11. doi: 10.1016/j.taap.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Kransler KM, Tonucci DA, McGarrigle BP, Napoli JL, Olson JR. Gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin alters retinoid homeostasis in maternal and perinatal tissues of the Holtzman rat. Toxicol Appl Pharmacol. 2007;224:29–38. doi: 10.1016/j.taap.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Miettinen HM, Alaluusua S, Tuomisto J, Viluksela M. Effect of in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on rat molar development: the role of exposure time. Toxicol Appl Pharmacol. 2002;184:57–66. [PubMed] [Google Scholar]
- 60.Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol. 2007;23:374–82. doi: 10.1016/j.reprotox.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajfer J, Coffey DS. Sex steroid imprinting of the immature prostate. Long-term effects. Invest Urol. 1978;16:186–90. [PubMed] [Google Scholar]
- 62.Schulman CC. The aging male: a challenge for urologists. Curr Opin Urol. 2000;10:337–42. doi: 10.1097/00042307-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Fritz WA, Lin TM, Moore RW, Cooke PS, Peterson RE. In utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure: effects on the prostate and its response to castration in senescent C57BL/6J mice. Toxicol Sci. 2005;86:387–95. doi: 10.1093/toxsci/kfi189. [DOI] [PubMed] [Google Scholar]
- 64.Banerjee PP, Banerjee S, Lai JM, Strandberg JD, Zirkin BR, Brown TR. Age-dependent and lobe-specific spontaneous hyperplasia in the brown Norway rat prostate. Biol Reprod. 1998;59:1163–70. doi: 10.1095/biolreprod59.5.1163. [DOI] [PubMed] [Google Scholar]
- 65.Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–37. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 66.Shain SA, McCullough B, Segaloff A. Spontaneous adenocarcinomas of the ventral prostate of aged A X C rats. J Natl Cancer Inst. 1975;55:177–80. doi: 10.1093/jnci/55.1.177. [DOI] [PubMed] [Google Scholar]
- 67.Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–102. [PubMed] [Google Scholar]
- 68.Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 69.Fritz WA, Lin TM, Cardiff RD, Peterson RE. The aryl hydrocarbon receptor inhibits prostate carcinogenesis in TRAMP mice. Carcinogenesis. 2007;28:497–505. doi: 10.1093/carcin/bgl179. [DOI] [PubMed] [Google Scholar]
- 70.Loaiza-Perez AI, Trapani V, Hose C, Singh SS, Trepel JB, Stevens MF, et al. Aryl hydrocarbon receptor mediates sensitivity of MCF-7 breast cancer cells to antitumor agent 2-(4-amino-3-methylphenyl) benzothiazole. Mol Pharmacol. 2002;61:13–9. doi: 10.1124/mol.61.1.13. [DOI] [PubMed] [Google Scholar]
- 71.Endo F, Monsees TK, Akaza H, Schill WB, Pflieger-Bruss S. Effects of single non-ortho, mono-ortho, and di-ortho chlorinated biphenyls on cell functions and proliferation of the human prostatic carcinoma cell line, LNCaP. Reprod Toxicol. 2003;17:229–36. doi: 10.1016/s0890-6238(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 72.Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, Sone H. Comparative effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on MCF-7, RL95-2, and LNCaP cells: role of target steroid hormones in cellular responsiveness to CYP1A1 induction. Mol Cell Biol Res Commun. 2000;4:174–80. doi: 10.1006/mcbr.2001.0275. [DOI] [PubMed] [Google Scholar]
- 73.Kizu R, Okamura K, Toriba A, Kakishima H, Mizokami A, Burnstein KL, et al. A role of aryl hydrocarbon receptor in the antiandrogenic effects of polycyclic aromatic hydrocarbons in LNCaP human prostate carcinoma cells. Arch Toxicol. 2003;77:335–43. doi: 10.1007/s00204-003-0454-y. [DOI] [PubMed] [Google Scholar]
- 74.Fukuzawa NH, Ohsako S, Wu Q, Sakaue M, Fujii-Kuriyama Y, Baba T, et al. Testicular cytochrome P450scc and LHR as possible targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the mouse. Mol Cell Endocrinol. 2004;221:87–96. doi: 10.1016/j.mce.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Lin TM, Fritz WA, Peterson RE. Evidences that null expression of aryl-hydrocarbon receptor (AhR) and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure increase susceptibility to prostate cancer in adulthood. The fetal basis of adult disease grantee meeting; Durham, NC. 2005. [Google Scholar]
- 76.Jenkins S, Rowell C, Wang J, Lamartiniere CA. Prenatal TCDD exposure predisposes for mammary cancer in rats. Reprod Toxicol. 2007;23:391–6. doi: 10.1016/j.reprotox.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.NTP technical report on the toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (CAS No. 1746-01-6) in female Harlan Sprague-Dawley rats (Gavage Studies) Natl Toxicol Program Tech Rep Ser. 2006:4–232. [PubMed] [Google Scholar]
- 78.Kociba RJ, Keyes DG, Beyer JE, Carreon RM, Wade CE, Dittenber DA, et al. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol Appl Pharmacol. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- 79.Oenga GN, Spink DC, Carpenter DO. TCDD and PCBs inhibit breast cancer cell proliferation in vitro. Toxicol In Vitro. 2004;18:811–9. doi: 10.1016/j.tiv.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Bertazzi PA, Zocchetti C, Guercilena S, Consonni D, Tironi A, Landi MT, et al. Dioxin exposure and cancer risk: a 15-year mortality study after the “Seveso accident”. Epidemiology. 1997;8:646–52. [PubMed] [Google Scholar]
- 81.Warner M, Eskenazi B, Mocarelli P, Gerthoux PM, Samuels S, Needham L, et al. Serum dioxin concentrations and breast cancer risk in the Seveso Women's Health Study. Environ Health Perspect. 2002;110:625–8. doi: 10.1289/ehp.02110625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pavuk M, Michalek JE, Ketchum NS. Prostate cancer in US Air Force veterans of the Vietnam war. J Expo Sci Environ Epidemiol. 2006;16:184–90. doi: 10.1038/sj.jea.7500448. [DOI] [PubMed] [Google Scholar]
- 83.Gupta A, Ketchum N, Roehrborn CG, Schecter A, Aragaki CC, Michalek JE. Serum dioxin, testosterone, and subsequent risk of benign prostatic hyperplasia: a prospective cohort study of Air Force veterans. Environ Health Perspect. 2006;114:1649–54. doi: 10.1289/ehp.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta A, Schecter A, Aragaki CC, Roehrborn CG. Dioxin exposure and benign prostatic hyperplasia. J Occup Environ Med. 2006;48:708–14. doi: 10.1097/01.jom.0000205417.12621.17. [DOI] [PubMed] [Google Scholar]
- 85.Hsu EL, Yoon D, Choi HH, Wang F, Taylor RT, Chen N, et al. A proposed mechanism for the protective effect of dioxin against breast cancer. Toxicol Sci. 2007;98:436–44. doi: 10.1093/toxsci/kfm125. [DOI] [PubMed] [Google Scholar]
- 86.Safe S, Qin C, McDougal A. Development of selective aryl hydrocarbon receptor modulators for treatment of breast cancer. Expert Opin Investig Drugs. 1999;8:1385–96. doi: 10.1517/13543784.8.9.1385. [DOI] [PubMed] [Google Scholar]
- 87.Fritz WA, Lin TM, Safe S, Peterson RE. Proc Amer Assoc Cancer Res A. Anaheim, CA: 2005. Activation of aryl hydrocarbon receptor (AhR) by the selective modulator 6-methyl-1,3,8-trichlorodibenzofuran (6-MCDF) protects against prostate tumor progression in TRAMP mice. [Google Scholar]
- 88.Marks LS, Mostaghel EA, Nelson PS. Prostate tissue androgens: history and current clinical relevance. Urology. 2008;72:247–54. doi: 10.1016/j.urology.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moore RW, Jefcoate CR, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits steroidogenesis in the rat testis by inhibiting the mobilization of cholesterol to cytochrome P450scc. Toxicol Appl Pharmacol. 1991;109:85–97. doi: 10.1016/0041-008x(91)90193-i. [DOI] [PubMed] [Google Scholar]
- 90.Safe S, Wang F, Porter W, Duan R, McDougal A. Ah receptor agonists as endocrine disruptors: antiestrogenic activity and mechanisms. Toxicol Lett. 1998:102–103. 343–7. doi: 10.1016/s0378-4274(98)00331-2. [DOI] [PubMed] [Google Scholar]
- 91.Hanus M, Matouskova M. [Antiestrogens (tamoxifen) in the alternative therapy of benign prostatic hyperplasia] Rozhl Chir. 1993;72:316–8. [PubMed] [Google Scholar]
- 92.Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, Risbridger GP. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. Faseb J. 2008;22:1512–20. doi: 10.1096/fj.07-9526com. [DOI] [PubMed] [Google Scholar]
- 93.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dooley RK, Holsapple MP. Elucidation of cellular targets responsible for tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of antibody responses: I. The role of the B lymphocyte. Immunopharmacology. 1988;16:167–80. doi: 10.1016/0162-3109(88)90005-7. [DOI] [PubMed] [Google Scholar]
- 95.Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S. Constitutive activation of P I3 K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate. 2005;64:224–39. doi: 10.1002/pros.20217. [DOI] [PubMed] [Google Scholar]
- 96.Huss WJ, Hanrahan CF, Barrios RJ, Simons JW, Greenberg NM. Angiogenesis and prostate cancer: identification of a molecular progression switch. Cancer Res. 2001;61:2736–43. [PubMed] [Google Scholar]
- 97.Fritz WA, Lin TM, Peterson RE. The aryl hydrocarbon receptor (AhR) inhibits vanadate-induced vascular endothelial growth factor (VEGF) production in TRAMP prostates. Carcinogenesis. 2008;29:1077–82. doi: 10.1093/carcin/bgn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin TL, Hopkins WJ, Torrealba JR, Briggs PM, Roenneburg DA, Fritz W, et al. American Association for Cancer Research. American Association for Cancer Research; Los Angeles, CA: 2007. Development of prostate dysplasia following transient prostatitis in C57Bl/6J mice: Lack of effect of aryl hydrocarbon receptor (AhR) genotype. [Google Scholar]