Abstract
Despite implementation of CDC recommendations and bundled interventions for preventing catheter-associated blood stream infection, ventilator-associated pneumonia, or urinary catheter–associated infections, nosocomial infections and sepsis remain a significant cause of morbidity and mortality in critically ill children. Recent studies suggest that acquired critical illness stress-induced immune suppression (CRISIS) plays a role in the development of nosocomial infection and sepsis. This condition can be related to inadequate zinc, selenium, and glutamine levels, as well as hypoprolactinemia, leading to stress-induced lymphopenia, a predominant TH2 monocyte/macrophage state, and subsequent immune suppression. Prolonged immune dysfunction increases the likelihood of nosocomial infections associated with invasive devices. Although strategies to prevent common complications of critical illness are routinely employed (eg, prophylaxis for gastrointestinal bleeding, thrombophlebitis), no prophylactic strategy is used to prevent stress-induced immune suppression. This is the authors’ rationale for the pediatric CRISIS prevention trial (NCT00395161), designed as a randomized, double-blind, controlled clinical investigation to determine if daily enteral supplementation with zinc, selenium, and glutamine as well as parenteral metoclopramide (a dopamine 2 receptor antagonist that reverses hypoprolactinemia) prolongs the time until onset of nosocomial infection or sepsis in critically ill children compared to enteral supplementation with whey protein. If effective, this combined nutritional and pharmacologic approach may lessen the excess morbidity and mortality as well as resource utilization associated with nosocomial infections and sepsis in this population. The authors present the design and analytic plan for the CRISIS prevention trial.
Keywords: critical care, nosocomial infection, prolactin, zinc, selenium, lymphocyte function
Nosocomial infections and sepsis occur more commonly in patients with neutropenia caused by chemotherapeutic agents, acquired immunodeficiency, lymphopenia, or other primary immune deficiency disorders that result in critical reductions in both T-cells and B-cells. An absolute lymphocyte count (ALC) ≤1200/mm3 is associated with infection.1–3 Similarly, lymphopenia acquired with critical illness may also result in nosocomial sepsis and death. Critically ill patients who die from nosocomial sepsis have depletions of T-cells and B-cells from thymus, lymph nodes, and spleen.4–6 Treatment with caspase inhibitors prevents stress-induced lymphocyte apoptosis, reduces bacterial cell counts, and improves survival in experimental sepsis.6 In a model of hemorrhagic shock, treatment with the dopamine 2 receptor antagonist, metoclopramide, maintains prolactin levels, prevents lymphocyte apoptosis, and improves survival with experimentally induced “nosocomial” sepsis.7–9
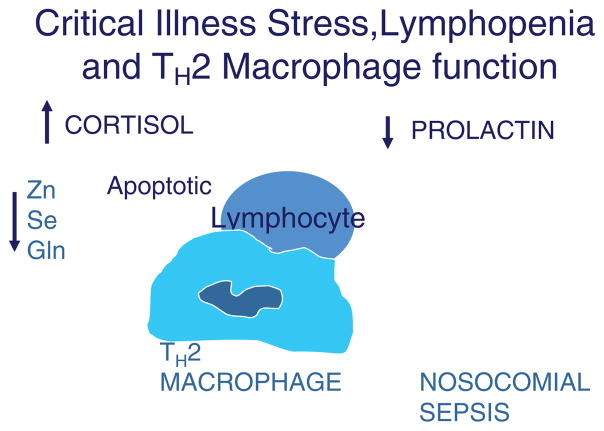
A recent study10 investigating critical illness–associated nosocomial sepsis in children observed an association between prolonged lymphopenia (ALC ≤ 1000/mm3 for ≥ 7 days) and the development of nosocomial sepsis (multivariate odds ratio [OR] = 5.5; 95% confidence interval [CI] 1.7–17), lymphoid depletion (multivariate OR = 42.2; 95% CI 3.2–473), and death (multivariate OR = 6.8; 95% CI 1.3–34). Increased apoptosis of B-cells, T-cells, and dendritic cells was found in the thymus, lymph nodes, and spleen in patients who died with lymphopenia and nosocomial sepsis. Lymphocyte apoptosis was also associated with a predominant TH2 monocyte/macrophage state, partly due to phagocytosis of these apoptotic cells. Prolonged hypoprolactinemia increased the risk of developing prolonged lymphopenia (multivariate OR = 8.3; 95% CI 2.1–33) and lymphoid depletion (multivariate OR = 12.2; 95% CI 2.2–65). This same study reported, in contrast, that patients who died without lymphopenia had normal numbers of B-cells, T-cells, and dendritic cells in the thymus, lymph nodes, and spleen, and did not develop nosocomial sepsis prior to death. Thus, prevention of stress-induced lymphocyte apoptosis could reduce nosocomial infection and sepsis in critically ill children (Figure 1).
Figure 1.
Biological mechanism of critical illness stress-induced immune suppression. Increased cortisol/adrenocorticotropic hormone levels in the presence of hypoprolactinemia and/or glutamine (Gln), zinc (Zn), and selenium (Se) deficiency causes lymphocyte apoptosis. Subsequent phagocytosis of apoptotic lymphocytes leads to a monocyte/macrophage TH2 state and immunoparalysis with increased susceptibility to nosocomial sepsis.
The dosage of metoclopramide commonly used for gastrointestinal (GI) prokinesis maintains prolactin levels in the high normal range in children. Metoclopramide delayed the time to onset of nosocomial pneumonia by 50%, but it had no effect on the incidence of nosocomial pneumonia or mortality in a randomized controlled trial in adults.11 Zinc, selenium, and glutamine may complement prolactin’s ability to prevent stress-induced lymphopenia and a TH2 monocyte/macrophage state. Randomized controlled trials of zinc supplementation have demonstrated reduced morbidity and/or mortality in children with severe pneumonia12,13 or diarrhea.14–16 The World Health Organization now recommends zinc supplementation for all children with severe pneumonia or diarrhea, the 2 leading worldwide causes of pediatric sepsis. Zinc supplementation also reduced infectious disease mortality in infants small for gestational age (OR = 0.32; 95% CI 0.12–0.89).17 Randomized controlled trials have demonstrated that selenium supplementation (relative risk [RR] = 0.73; 95% CI 0.57–0.93)18 or glutamine-enriched enteral nutrition (OR = 0.32; 95% CI 0.14–0.74)19 reduced the risk of nosocomial sepsis in preterm neonates. Furthermore, enteral glutamine safely maintains TH1 function for bacterial killing.20,21
Study Design
Hypothesis
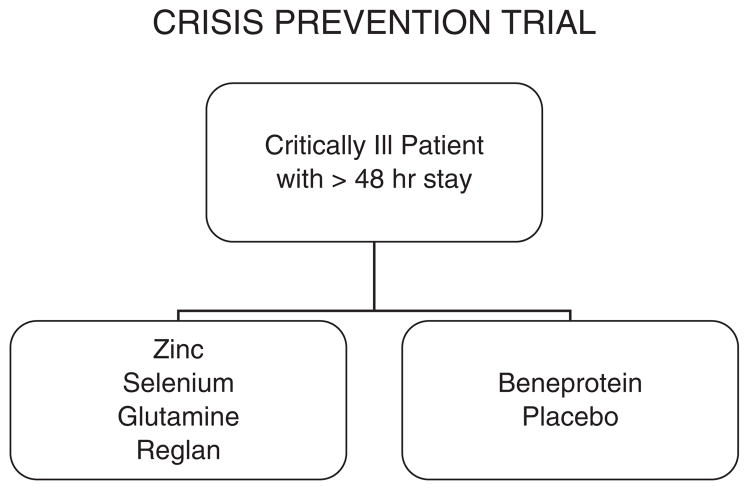
We hypothesize that daily intravenous (IV) metoclopramide, and enteral zinc, selenium, and specific glutamine nutrition supplementation will prolong the time to developing nosocomial infection and sepsis 1.5-fold compared to daily enteral supplementation with whey protein (Beneprotein™; Nestle Nutrition, Vevey, Switzerland) in critically ill children (Figure 2).
Figure 2.
Patient flow diagram. Patients are randomized in a blind fashion within 48 hours and receive 1 of 2 treatments within 72 hours; either (a) enteral glutamine, zinc, and selenium plus intravenous metoclopramide or (b) enteral Beneprotein™ powder (a whey protein supplement; Nestle Nutrition, Vevey, Switzerland) plus intravenous normal saline. Treatment is continued for whichever occurs first, discharge from the pediatric intensive care unit, loss of enteral tubes and intravenous access, or 28 days.
Primary Outcomes
The primary study endpoint is the time between admission to the pediatric intensive care unit (PICU) and occurrence of nosocomial infection or clinical sepsis in PICU patients requiring an endotracheal tube, central venous access, or a urinary catheter.
Secondary Outcomes
Secondary endpoints of this study are (1) the rate of nosocomial infection or clinical sepsis per 100 PICU days, (2) the number of antibiotic-free days, (3) the incidence of prolonged lymphopenia, and (4) all-cause 28-day mortality. Other outcomes to be noted and analyzed include absolute lymphocyte count and incidence of moderate lymphopenia (absolute lymphocyte count ≤1000/mm3 for ≥3 days). To corroborate the biological mechanisms underlying the hypothesized effect of active study drugs on our primary and secondary outcomes, we will also measure serum prolactin, zinc, and selenium levels before treatment and after 7 days of treatment (or at study exit, whichever occurs earlier).
Explanatory variables, measured in a subset of enrolled patients, include glutathione peroxidase levels (a selenoenzyme antioxidant, which is a functional marker of selenium levels), γ globulin levels (hypogammaglobulinemia is a functional marker of B-cell lymphopenia), and the whole blood tumor necrosis factor response to lipopolysaccharide (a level <200 pg/mL is a marker of a predominant TH2 monocyte function). These variables will be measured on the day of enrollment and at weekly intervals thereafter. Process outcomes will be analyzed to corroborate the beneficial effects of reducing nosocomial sepsis in patients receiving the active study drugs, including antibiotic-resistant nosocomial infections, Pediatric Logistic Organ Dysfunction (PELOD scores),22,23 and Organ Failure Index (OFI scores)24 recorded on days 1, 7, 14, 21, and 28 after enrollment, as well as ventilator-free days, and mortality in the PICU.
Centers for Disease Control and Prevention (CDC) Definitions of Nosocomial Infection/Sepsis
Nosocomial refers to a clinical event occurring more than 48 hours after PICU admission and up until 5 days after discharge from the PICU. Clinical sepsis occurs when patients older than 1 year develop fever (≥38°C), hypotension (≤90 mm Hg), or oliguria (≤20 mL/h), and the clinician initiates antibiotic therapy with no positive microbiological evidence and no other recognized cause for the symptoms. In patients younger than 1 year, clinical sepsis occurs with fever (≥ 38°C) or hypothermia (≤ 37°C), apnea, or bradycardia, and a physician institutes antibiotic therapy with no positive microbiological evidence. Nosocomial infection occurs when microbiologically (culture, antigen, polymerase chain reaction [PCR], or antibody) proven infection is observed in a patient with fever, hypothermia, chills, or hypotension.
Inclusion Criteria
Patients will be eligible for enrollment if they (1) are more than 1 year and less than 18 years of age; (2) are within the first 48 hours of PICU admission; (3) have an endotracheal tube, central venous catheter (new or old, tunneled or not tunneled), or Foley catheter; (4) are anticipated to require PICU care and have an indwelling arterial or venous catheter for blood sampling during the first 3 days of study enrollment; and (5) are anticipated to have venous access and an enteral feeding tube for the administration of study drugs.
Infants younger than 1 year will not be enrolled until after the first interim analysis (200 patients), following review by the Data Safety Monitoring Board (DSMB), whose composition and proposed meeting schedule are discussed below. Based on the observed safety of the study drug in the first 200 children enrolled, the DSMB will make a recommendation concerning expanding enrollment to infants between 40 weeks gestational age and 1 year of age. This facet of the study design enhances the safety of the proposed interventions in young infants at greater risk for nutrition and immune deficiencies.
Exclusion Criteria
Patients will be ineligible for enrollment if they meet any of the following exclusion criteria: (1) are ≥ 18 years of age; (2) have a known allergy to metoclopramide; (3) will undergo planned removal of endotracheal tube, central venous, and Foley catheters, within 72 hours after study enrollment; (4) have suspected intestinal obstruction, intestinal surgery, or bowel disruption, or have other contraindications to the enteral administration of drugs or nutrients; (5) receive chronic metoclopramide therapy prior to enrollment; (6) have a known allergy to whey (milk) or soy-based products; (7) were discharged from the PICU in the previous 28 days; (8) were previously enrolled in this study; (9) have a positive pregnancy test; or (10) their parents have indicated a lack of commitment to aggressive intensive care therapies.
Enrollment Plan
Children will be screened, enrolled with parental permission, and randomized to (active group) treatment with enteral zinc, selenium, and glutamine with parenteral metoclopramide or to (control group) enteral Beneprotein™ with parenteral placebo (saline) within the first 48 hours of PICU admission. Randomization, in a 1:1 ratio between treatment arms, will be stratified by clinical center and by immune status (immune compromised vs immune competent). Treatment assignments will be balanced within strata, using randomized blocks of varying length.
Study Drug and Enteral Supplements
All study drug and enteral supplements are approved for use in this study under a Food and Drug Administration (FDA) Investigational New Drug application, and subject to the standard regulations of the FDA in addition to the local institutional review board (IRB) approvals. The metoclopramide and saline supplements are FDA-approved parenteral formulations. The zinc and selenium supplements are FDA-approved parenteral formulations used enterally, and the glutamine and beneprotein supplements are food grade formulations.
Children allocated to the metoclopramide/zinc/selenium/glutamine arm will receive IV metoclopramide 0.2 mg/kg/dose every 12 hours (the dose often selected for facilitation of enteral feeding), and children allocated to the placebo arm will receive an equivalent volume of IV saline. For children > 50 kg, the maximum dose of metoclopramide should be 10 mg. The study drug will be administered intravenously over 30 minutes or according to institutional protocol, which can be no faster than over 3–5 minutes. If IV administration is not available, then metoclopramide/placebo will be discontinued.
IV preparations of zinc and selenium have been chosen to facilitate proper dosing, but these drugs will be administered enterally. Children allocated to the metoclopramide/zinc/selenium/glutamine arm will receive 1 enteral dose daily of zinc chloride (10 mg/day elemental zinc for infants ≤ 1 year of age, and 20 mg/day elemental zinc for patients > 1 year of age). They will also receive 1 enteral dose daily of selenium (40 μg for infants < 8 months of age, 60 μg for infants 8–12 months of age, 90 μg for children 1–3 years, 150 μg for children 4–8 years, 280 μg for children 9–13 years, and 400 μg for children >13 years).
Zinc absorption is reduced when given with foods or drugs containing high amounts of calcium or phosphate. Therefore, antacids should not be administered within 1 hour (prior or after) of study drug administration.
Children allocated to the metoclopramide/zinc/selenium/glutamine arm will also receive 1 enteral dose daily of L-glutamine powder (0.3 gm/kg/day). Children allocated to the placebo group will receive 1 enteral dose daily of Beneprotein™ whey protein supplement (0.3 gm/kg/day). The study supplement will be administered via an enteric feeding tube (nasogastric, NG; nasoduodenal, ND; nasojejunal, NJ; gastrostomy, GT), clamping the feeding tube for 1 hour after study drug administration. The morning dose of zinc, selenium, and glutamine will be administered after administration of IV study drug (metoclopramide or placebo). In the event that the child has multiple enteral tubes, the study drug should be administered via the most distal site. For example, if a child has an NJ and NG tube, the drug should be administered through the NJ tube. If the child does not have an enteral feeding tube, administration of all enteral supplements will be discontinued. All other nutrition, zinc, selenium, and/or glutamine supplementation or metoclopramide received will be recorded on a daily basis.
The study drug/placebo/supplements will be administered within 72 hours of admission to the PICU and continued until one of the following occurs: discharge from the PICU, loss of inclusion criteria, development of exclusion criteria, or 28 days. Patients will continue to be followed and data collected for 5 days past discharge from the PICU and again at 28 days if not in the PICU at 28 days.
Adverse Event Monitoring
Adverse events and serious adverse events are monitored and recorded daily according to FDA and local IRB guidelines. Expected drug-related adverse events associated with metoclopramide are listed in the micromedex. The most common is dystonia, which is treated with diphenhydramine and discontinuation of metoclopramide. There are no expected supplement-related adverse events at the dosages given. The decision to stop drug/placebo/supplements due to an adverse event will be at the discretion of the informed non-research-related primary physician. Parents also have the option to request discontinuation of drug/placebo/supplements at any time.
Statistical Analysis
This prospective, double-blind randomized trial will be analyzed as an intention-to-treat study, and the sample size determination is based on the combined events of clinical sepsis (not culture, PCR, or antigen positive) plus clinical infection (culture, PCR, or antigen positive).
A logrank test with a 2-sided α = .05 will be used to compare the primary study outcome, freedom from nosocomial infection, or sepsis (from the time of PICU admission until up to 5 days following PICU discharge) between treatment arms. Outcome rates over time will be presented using Kaplan-Meier freedom from event curves, and median time to the first nosocomial infection or sepsis event will be reported by treatment arm. Treatment effect will be quantified using relative risks derived from the Cox proportional hazard model. Rates of nosocomial infection or sepsis events between the 2 treatment arms, and the cumulative number of events per patient over time, will also be analyzed using Poisson regression analyses that allow counting of multiple events on a single patient. We will also examine event rates over time calculated using the competing risks method.25
Differences in antibiotic free days will be analyzed over 28 days as time until first use of antibiotics for a nosocomial event, using Kaplan Meier analysis and the logrank test. Patients discharged without antibiotics will be considered antibiotic-free from the day of discharge through day 28. Differences in incidence of prolonged lymphopenia and in all-cause hospital mortality will be analyzed using the χ2 test or exact analogues when numbers of events are small.
Adverse event occurrences, severity, and their possible relationship to study drug are recorded and, subsequently during the analytic phase, will be compared between the 2 study arms.
The study has been designed to test the primary hypothesis in the entire study population. Additionally, subgroup analyses will be performed for the following prespecified subgroups: (1) immune compromise status at study entry (immune compromised or immune competent), (2) postsurgical status (admitted to PICU after surgical procedure or not), (3) gender, (4) race/ethnicity, and (5) clinical center/site. Reporting of subgroup analysis results will adjust for multiple comparisons. It is expected that any observed subgroup differences would lead to future confirmatory studies in the appropriate subpopulation(s).
Power and Sample Size
Power for the primary outcome is affected by the hypothesized beneficial effect of the study intervention. For study planning, our a priori estimate was that median time to infection would be 1.5 times longer in the active drug arm than in the control arm (technically, an inverse hazard rate of 1.5). For comparison of survival curves using the logrank test, statistical power is directly dependent on the number of events observed. For 90% power to detect an inverse hazard rate as small as 1.5, using a 2-sided test with Type I error of 0.05, a total of 263 patients with events are required.25,26 Assuming that the DSMB will use conservative O’Brien-Fleming monitoring boundaries when considering early study stopping due to observed treatment efficacy, a small increase in sample size is required to maintain 90% power accounting for 2 interim looks at the data.27 Thus, the Critical Illness Stress-induced Immune Suppression (CRISIS) study plans to enroll patients until a total of 268 patients with events have been observed, defining the first analytic point.
The number of patients that must be enrolled to observe this number of events depends on various parameters, including median time to infection and median stay in the ICU. For example, if median time to infection in the placebo arm is 6 days and median ICU length of stay (LOS) is 7 days, then under a true inverse hazard of 1.5, we estimate that 530 total patients must be enrolled to achieve 90% power. This estimate increases to 636 if median LOS is only 5 days, and to 762 if median LOS is 5 days and median time to infection in the placebo arm is 8 days. It is anticipated that reasonably accurate estimates of needed enrollment numbers will be available after review of data from the first 200 patients. Thus far, 140 patients have been enrolled in 14 months. Enrollment rate is expected to increase if and when the DSMB allows expansion to infants <1 year of age as 40% of admissions to the PICU are in this age range.
Study Organization
Data Safety Monitoring Board
This study will be monitored by a DSMB appointed by the National Institute of Child Health and Human Development (NICHD). The CRISIS DSMB is comprised of 4 members not affiliated with the study, including experts in the fields of pediatrics, critical care medicine, and biostatistics. The DSMB will have final jurisdiction regarding frequency of meetings, appropriate formal monitoring boundaries for study stopping in terms of superiority, and other study monitoring issues.
The proposed DSMB monitoring plan for this study includes 2 interim analyses, the first after approximately 200 patients have been enrolled, and the second after approximately 180 primary study events have been observed in the enrolled patients. At the time of the first interim analysis, the DSMB will also make recommendations regarding expanding study eligibility to infants between 40 weeks gestational age and 12 months.
The Collaborative Pediatric Critical Care Research Network
The NICHD Collaborative Pediatric Critical Care Research Network (CPCCRN) will perform the study. The CPCCRN Data Coordinating Center (DCC) is located at the University of Utah, and the 7 Clinical Centers in the network include Children’s Hospital of Los Angeles, Mattel Children’s Hospital, Seattle Children’s Hospital, Children’s Hospital of Michigan, Arkansas Children’s Hospital, Children’s National Medical Center, and Children’s Hospital of Pittsburgh.
Discussion
The most fundamental rationale for this study is the common use of prophylactic strategies to prevent morbid events in critically ill patients. For example, treatments to reduce gastric acidity and prevent catastrophic GI bleeding are considered as standards of care. The CRISIS prevention study utilizes the nutrients zinc, selenium, and glutamine and the dopamine-2 antagonist metoclopramide to prevent nosocomial sepsis and infection. These agents are also inexpensive, relatively safe, and easily titratable, similar to GI prophylaxis regimens.
Maki and colleagues28 showed that glove and gown isolation reduced secondary episodes of infection/sepsis from 10/100 patient days to 7/100 days, and Slota and colleagues29 showed a tendency toward a decrease in infection/sepsis from 5/100 days to 3/100 days with similar interventions. The sample size calculation for the CRISIS prevention trial was based on the incidence of infection observed in these 2 studies. Similarly, the inclusion criteria chosen for the CRISIS prevention trial are based on the work of Maki et al and Slota et al. Specifically, children with invasive catheters or endotracheal tubes for greater than 72 hours are the population of interest. Nosocomial sepsis/infection by definition occurs after the first 48 hours in the PICU. Risk increases with PICU LOS, and in particular, with each increased invasive device day, as well as illness severity at admission to the PICU, and with depressed immune status.30
Of note, the CRISIS prevention trial evaluates the effect of a multimodal therapy rather than monotherapy. This design is based on the observation that previous “monotherapy trials” of selenium, glutamine, zinc, or metoclopramide for nosocomial infection/sepsis prophylaxis showed small or inconsistent treatment effects. Because the immune system requires normal levels of zinc, selenium, glutamine, and prolactin to function properly, we hypothesize that prevention of CRISIS must use all 4 strategies to maximize benefit.
The enteral route was chosen for administration of zinc, selenium, and glutamine because the predominant site of the developing immune system is the intestinal tract. The parenteral route was chosen for the administration of metoclopramide because it is not a nutrient and is widely administered intravenously in the PICU for gastric dysmotility. The doses chosen were all within the safe limits of recommended dosing to ensure optimum safety.
Traditionally, 2 endpoints are used to measure nosocomial infection/sepsis. These are (1) the time to infection/sepsis and (2) the number of infection/sepsis episodes (“rate”) per 100 PICU days. Although a valid argument may be made for using either of these, time until infection/sepsis was chosen as the primary outcome variable because of potential difficulties in determining new versus recurrent infections in the same subject. However, these endpoints do measure 2 different outcomes, namely, the susceptibility to first infection and susceptibility to multiple infections, respectively. Evidence of both will be assessed in the analytic plan.
Sepsis is a clinical syndrome with no consensus definition. We chose to use the published CDC criteria that depend in part on the clinical intention of the primary caregiver to treat a new proven infection in the ICU (nosocomial infection) or a new unproven infection in the ICU (nosocomial sepsis) in the presence of associated signs and symptoms. Endpoint adjudication is performed for each patient by consensus among CPCCRN Steering Committee members.
Several limitations are important to recognize in the rationale and design of this study. First, this is not a placebo-controlled trial in the strict sense in which the term is usually understood. Investigators with special interest in critical care nutrition thought it was important to ensure that any effect that might be demonstrated with prophylaxis with zinc, selenium, glutamine, and metoclopramide was not simply a result of better protein nutrition. Thus, Beneprotein™ powder was chosen as the protein supplement in the control arm of this study to ensure protein supplementation in the active and the control arms. Beneprotein™ is a whey protein supplement advertised by the manufacturer as an immune nutrition supplement. Although the manufacturer does not claim that zinc or selenium is present in this supplement, there is some glutamine at approximately 1/10 the amount given in the active treatment arm. Hence the CRISIS prevention trial compares the effect of 2 different immune nutrition strategies on time to development of nosocomial infection/sepsis. This approach without a true placebo presents a conundrum; however, it is consistent with Helsinki Convention guidelines in human experimentation. Prior studies of glutamine supplementation have used various single amino acids for comparison as placebos; however, these single amino acids also have effects on the immune system.31 A second limitation of this study is that it does not test the effects of other immunonutrients including arginine, vitamin A, ω-3 fatty acids, or simply protein supplementation alone. These other nutrient strategies remain to be studied after the present trial is completed.
Coincidently, the pediatric CRISIS prevention trial is paralleling 2 new and exciting adult trials that are testing somewhat similar pharmaconutrient strategies. Daren Heyland and colleagues are performing a multiple center North American randomized controlled trial of selenium, glutamine, and other antioxidants administered both enterally and parenterally in 1200 critically ill adults with organ failure with a primary outcome variable of 28-day mortality (The REDOXS study).32 Eric Milbrandt is simultaneously performing a single-center randomized controlled trial to test the hypothesis that haloperidol (a drug with dopamine-2 receptor antagonist activity similar to that of metoclopramide) reduces 28-day mortality in mechanically ventilated critically ill adults (NCT00300391). All 3 studies should reach completion in a similar time frame, giving the nutrition community an opportunity to better understand the potential role of lymphocyte-targeted nutriceuticals in the prevention of nosocomial sepsis.
Acknowledgments
Members of the CPCCRN participating in this study: Children’s Hospital of Pittsburgh, Pittsburgh, PA (Joseph Carcillo, MD, Michael Bell, MD, Alan Abraham, BA); University of Utah (Data Coordinating Center), Salt Lake City, UT (J. Michael Dean, MD, Jeri Burr, BS, RNC, CCRC, Amy Donaldson, MS, Richard Holubkov, PhD, Tracy Hellem, RN, Brynna Morrison, BS, Rene Enriquez, BS, Jeff Yearley, BS); Children’s National Medical Center, Washington, DC (John Berger, MD, Angela Ratney, MD, Jean Reardon, BSN, RN); Children’s Hospital of Michigan, Detroit, MI (Kathleen L. Meert, MD, Sabrina Heidemann, MD, Maureen Frey, PhD, RN); Arkansas Children’s Hospital, Little Rock, AR (KJS Anand, MBBS, DPhil, Parthak Prodhan, MD, Glenda Hefley, MNSc, RN); Seattle Children’s Hospital, Seattle, WA (Jerry Zimmerman, MD, PhD, David Jardine, MD, Ruth Barker, RRT); Children’s Hospital Los Angeles, Los Angeles, CA (Christopher J. L. Newth, MB, ChB, J. Francisco Fajardo, CLS [ASCP], RN, MD); Mattel Children’s Hospital at University of California Los Angeles, Los Angeles, CA (Rick Harrison, MD); University of Virginia Children’s Hospital, Charlottesville, VA (Douglas F. Willson, MD); National Institute of Child Health and Human Development, Bethesda, MD (Carol Nicholson, MD, Tammara Jenkins, MSN, RN).
Footnotes
Financial disclosure: This work was supported by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Department of Health and Human Services (DHHS): U10HD050096, U10HD049981, U10HD500009, U10HD049945, U10HD049983, U10HD050012, and U01HD049934.
References
- 1.Dallman PR. White blood cells: developmental changes in numbers. In: Rudolph CD, Rudolph AM, Hoffman JIE, editors. Pediatrics. 19. Norwalk, CT: Appleton and Lange; 1987. p. 1061. [Google Scholar]
- 2.Anonymous. Immunization in special clinical circumstances. In: Pickering LK, editor. Redbook: Report of the Committee of Infectious Diseases. 25. Elk Grove Village, IL: Academy of Pediatrics; 2000. p. 59. [Google Scholar]
- 3.Stiehm ER. Immunologic Disorders in Infants and Children. Philadelphia, PA: W. B. Saunders; 1989. [Google Scholar]
- 4.Hotchkiss RS, Tinsley KW, Swanson PE, Jr, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166(11):6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 5.Gurevich P, Ben-Hur H, Czernobilsky B, Nyska A, Zuckerman A, Zusman I. Pathology of lymphoid organs in low birth weight infants subjected to antigen-related diseases: a morphological and morphometric study. Pathology. 1995;27(2):121–126. doi: 10.1080/00313029500169702. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Chang KC, Swanson PE, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1(6):496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 7.Zellweger R, Wichmann MW, Ayala A, Chaudry IH. Metoclopramide: a novel and safe immunomodulating agent for restoring the depressed macrophage immune function after hemorrhage. J Trauma. 1998;44(1):70–77. doi: 10.1097/00005373-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Zellweger R, Zhu XH, Wichmann MW, Ayala A, DeMaso CM, Chaudry IH. Prolactin administration following hemorrhagic shock improves macrophage cytokine release capacity and decreases mortality from subsequent sepsis. J Immunol. 1996;157(12):5748–5754. [PubMed] [Google Scholar]
- 9.Knoferl MW, Angele MK, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Insight into the mechanism by which metoclopramide improves immune functions after trauma-hemorrhage. Am J Physiol Cell Physiol. 2000;279(1):C72–C80. doi: 10.1152/ajpcell.2000.279.1.C72. [DOI] [PubMed] [Google Scholar]
- 10.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174(6):3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 11.Yavagal DR, Karnad DR, Oak JL. Metoclopramide for preventing pneumonia in critically ill patients receiving enteral tube feeding: a randomized controlled trial. Crit Care Med. 2000;28(5):1408–1411. doi: 10.1097/00003246-200005000-00025. [DOI] [PubMed] [Google Scholar]
- 12.Brooks WA, Yunus M, Santosham M, et al. Zinc for severe pneumonia in very young children: double-blind placebo-controlled trial. Lancet. 2004;363(9422):1683–1688. doi: 10.1016/S0140-6736(04)16252-1. [DOI] [PubMed] [Google Scholar]
- 13.Walker CF, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24:255–275. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- 14.Baqui AH, Black RE, El Arifeen S, et al. Zinc therapy for diarrhoea increased the use of oral rehydration therapy and reduced the use of antibiotics in Bangladeshi children. J Health Popul Nutr. 2004;22(4):440–442. [PubMed] [Google Scholar]
- 15.Raqib R, Roy SK, Rahman MJ, et al. Effect of zinc supplementation on immune and inflammatory responses in pediatric patients with shigellosis. Am J Clin Nutr. 2004;79(3):444–450. doi: 10.1093/ajcn/79.3.444. [DOI] [PubMed] [Google Scholar]
- 16.Bhatnagar S, Bahl R, Sharma PK, Kumar GT, Saxena SK, Bhan MK. Zinc with oral rehydration therapy reduces stool output and duration of diarrhea in hospitalized children: A randomized controlled trial. J Pediatr Gastroenterol Nutr. 2004;38(1):34–40. doi: 10.1097/00005176-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Sazawal S, Black RE, Menon VP, et al. Zinc supplementation in infants born small for gestational age reduces mortality: a prospective, randomized, controlled trial. Pediatrics. 2001;108(6):1280–1286. doi: 10.1542/peds.108.6.1280. [DOI] [PubMed] [Google Scholar]
- 18.Darlow BA, Austin NC. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst Rev. 2003;4:CD003312. doi: 10.1002/14651858.CD003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Berg A, van Elburg RM, Westerbeek EA, Twisk JW, Fetter WP. Glutamine-enriched enteral nutrition in very-low-birth-weight infants and effects on feeding tolerance and infectious morbidity: a randomized controlled trial. Am J Clin Nutr. 2005;81(6):1397–1404. doi: 10.1093/ajcn/81.6.1397. [DOI] [PubMed] [Google Scholar]
- 20.Boelens PG, Houdijk AP, Fonk JC, et al. Glutamine-enriched enteral nutrition increases in vitro interferon-gamma production but does not influence the in vivo specific antibody response to KLH after severe trauma. A prospective, double blind, randomized clinical study. Clin Nutr. 2004;23(3):391–400. doi: 10.1016/j.clnu.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Yalcin SS, Yurdakok K, Tezcan I, Oner L. Effect of glutamine supplementation on diarrhea, interleukin-8 and secretory immunoglobulin A in children with acute diarrhea. J Pediatr Gastroenterol Nutr. 2004;38(5):494–501. doi: 10.1097/00005176-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Leteurtre S, Martinot A, Duhamel A, et al. Development of a pediatric multiple organ dysfunction score: use of two strategies. Med Decis Making. 1999;19(4):399–410. doi: 10.1177/0272989X9901900408. [DOI] [PubMed] [Google Scholar]
- 23.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362(9379):192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 24.Doughty LA, Kaplan SS, Carcillo JA. Inflammatory cytokine and nitric oxide responses in pediatric sepsis and organ failure. Crit Care Med. 1996;24(7):1137–1143. doi: 10.1097/00003246-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Kim WR, Therneau TM, Benson JT, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology. 2006;43:345–351. doi: 10.1002/hep.21025. [DOI] [PubMed] [Google Scholar]
- 26.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1:121–129. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- 27.Jennison C, Turnbull BW. Group Sequential Methods With Applications to Clinical Trials. Boca Raton, FL: Chapman & Hall; 2000. [Google Scholar]
- 28.Klein BS, Perloff WH, Maki DG. Reduction of nosocomial infection during pediatric intensive care by protective isolation. N Engl J Med. 1989;320(26):1714–1721. doi: 10.1056/NEJM198906293202603. [DOI] [PubMed] [Google Scholar]
- 29.Slota M, Green M, Farley A, Janosky J, Carcillo J. The role of gown and glove isolation and strict handwashing in the reduction of nosocomial infection in children with solid organ transplantation. Crit Care Med. 2001;29(2):405–412. doi: 10.1097/00003246-200102000-00034. [DOI] [PubMed] [Google Scholar]
- 30.Leclerc F, Leteurtre S, Duhamel A, et al. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Respir Crit Care Med. 2005;171(4):348–353. doi: 10.1164/rccm.200405-630OC. [DOI] [PubMed] [Google Scholar]
- 31.Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98(2):237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 32.Heyland DK, Dhaliwal R, Day AG, et al. the Canadian Critical Care Trials Group. REducing Deaths due to OXidative Stress (The REDOXS Study): rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically ill patients. Proc Nutr Soc. 2006;65:250–263. doi: 10.1079/pns2006505. [DOI] [PubMed] [Google Scholar]