Abstract
Osteoclasts (OCs) undergo rapid apoptosis without trophic factors, such as macrophage colony-stimulating factor (M-CSF). Their apoptosis was associated with a rapid and sustained increase in the pro-apoptotic BH3-only Bcl-2 family member Bim. This was caused by the reduced ubiquitylation and proteasomal degradation of Bim that is mediated by c-Cbl. Although the number of OCs was increased in the skeletal tissues of bim–/– mice, the mice exhibited mild osteosclerosis due to reduced bone resorption. OCs differentiated from bone marrow cells of bim–/– animals showed a marked prolongation of survival in the absence of M-CSF, compared with bim+/+ OCs, but the bone-resorbing activity of bim–/– OCs was significantly reduced. Overexpression of a degradation-resistant lysine-free Bim mutant in bim–/– cells abrogated the anti-apoptotic effect of M-CSF, while wild-type Bim did not. These results demonstrate that ubiquitylation-dependent regulation of Bim levels is critical for controlling apoptosis and activation of OCs.
Keywords: apoptosis/bim/M-CSF/osteoclast/ubiquitylation
Introduction
Apoptosis is a genetically programmed process for killing unwanted cells (Kerr et al., 1972). Abnormalities in apoptosis regulation can promote cancer, autoimmune disease or degenerative disorders (Thompson, 1995). Mammals have two distinct apoptosis signaling pathways that converge upon activation of aspartate-specific cysteine proteases (caspases), which mediate cell demolition (Strasser et al., 1995). One pathway is initiated by death receptors, members of the tumor necrosis factor receptor (TNF-R) family with an intracellular death domain, and propagated through FADD adaptor protein-mediated activation of caspase-8. The other pathway is regulated by pro- and anti-apoptotic Bcl-2 family members and involves mitochondrial release of cytochrome c, which causes Apaf-1 adaptor-mediated activation of caspase-9 (Gross et al., 1999; Strasser et al., 2000). The anti-apoptotic Bcl-2 family members include mammalian Bcl-2, Bcl-xL, Bcl-w, Mcl-1, A1 and Boo/Diva, and Caenorhabditis elegans CED-9, and they share similarity within three or four Bcl-2 homology (BH) domains. So far, >20 pro-apoptotic Bcl-2 family proteins have been identified in mammals. They can be divided further into two groups: multidomain members possess homology in two or three BH regions (mammalian Bax, Bak, Bok/Mtd, Bcl-xS and Bfk), whereas the BH3 domain-only proteins (mammalian Bik/Nbk, Bad, Bid, Hrk/DP5, Bim/Bod, Noxa, Bmf and Puma/Bbc3, and C.elegans EGL-1) share only the short BH3 region (Huang and Strasser, 2000). Genetic studies and experiments with transfected cells have shown that BH3-only proteins are essential for initiation of apoptosis (Huang and Strasser, 2000), whereas Bax/Bak-like proteins play an essential role further downstream (Cheng et al., 2001; Zong et al., 2001). Bax/Bak-like proteins are ubiquitously expressed, whereas BH3-only family members have a more tissue-specific distribution, indicating that the latter may play a tissue/cell-specific and death stimulus-specific role in apoptosis. The pro-apoptotic activity of BH3-only proteins is strictly regulated at both the transcriptional and post-translational level to prevent inappropriate cell killing (Huang and Strasser, 2000).
The BH3-only protein Bim was first identified as a Bcl-2-interacting protein by screening a λ phage expression library constructed from a mouse thymic lymphoma (O’Connor et al., 1998). Bim is expressed in hematopoietic, epithelial, neuronal and germ cells (O’Reilly et al., 2000), and alternative splicing generates various Bim isoforms, including BimS, BimL and BimEL. Experiments with knock-out mice have shown that Bim is essential for apoptosis of T lymphocytes, B lymphocytes, myeloid cells and neurons (Bouillet et al., 1999, 2002; Putcha et al., 2001; Whitfield et al., 2001; Villunger et al., 2003). Pro-apoptotic activity of Bim is regulated both transcriptionally and post-transcriptionally (Huang and Strasser, 2000). Osteoclasts (OCs) are multinucleated giant cells primarily responsible for bone resorption (Baron, 1989; Suda et al., 1992; Tanaka et al., 2003). They are terminally differentiated cells, and undergo rapid apoptosis in the absence of trophic factors such as macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) (Hughes et al., 1995). We demonstrate here that Bim is critical for normal OC survival and function.
Results
Rapid induction of Bim protein in cytokine-deprived OCs
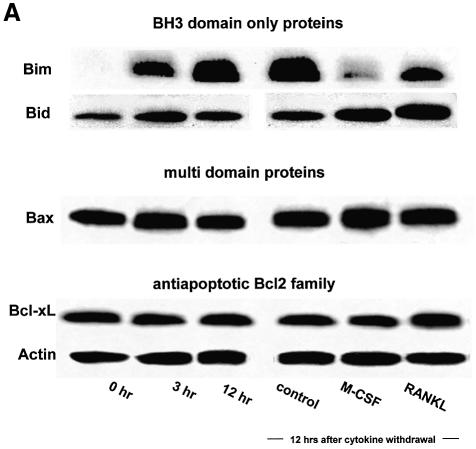
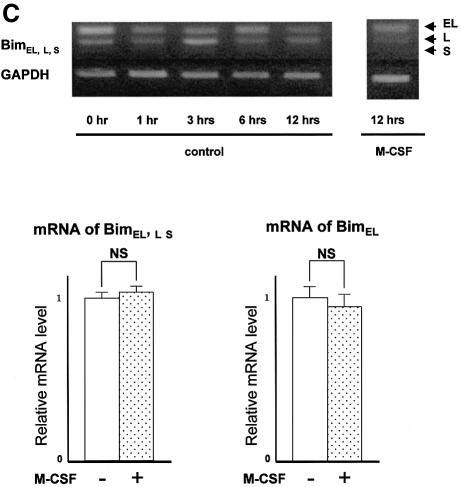
To obtain murine OCs, we used a co-culture system of osteoblastic cells and bone marrow cells in the presence of 1α, 25-dihydroxyvitamin D3 [1α, 25(OH)2D3] and prostaglandin E2 (PGE2), as previously reported (Takahashi et al., 1988). OCs purified from co-cultures by removing osteoblastic cells by collagenase and dispase treatment were then maintained in the presence of M-CSF (10 ng/ml) for an additional 12 h. When M-CSF was removed from the culture, OCs underwent rapid cell death. Within 24 h, 70% of OCs had died and virtually all cells died out after 48 h, as previously reported (Miyazaki et al., 2000). We examined whether cytokine withdrawal caused changes in the expression levels of pro- or anti-apoptotic Bcl-2 family members. Immunoblot analysis revealed a marked increase in Bim expression levels within 3 h of M-CSF removal and it remained high for at least 12 h (Figure 1A), while expression levels of Bid, Bax or Bcl-xL were not affected. Induction of Bim was reversed by M-CSF or, albeit less efficiently, by RANKL treatment for 12 h (Figure 1A). A rapid increase in Bim levels was also observed in cytokine-deprived OC precursors (data not shown). However, no significant difference in bim mRNA level was observed between OCs cultured in the presence or absence of M-CSF either by RT–PCR or by real-time PCR (Figure 1C), demonstrating that the changes in Bim protein levels are due to post-tranlational mechanisms.
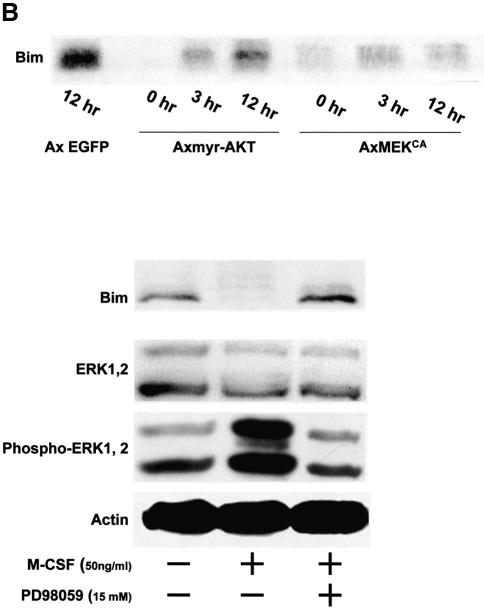
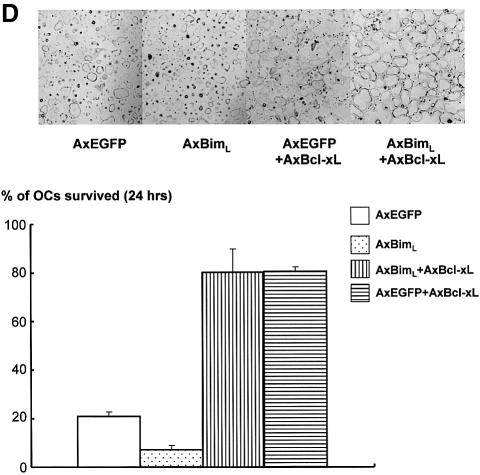
Fig. 1. Regulation of Bim expression in OCs. (A) Cytokine withdrawal caused rapid upregulation of Bim levels in OCs. OCs purified from co-cultures by removing osteoblastic cells by collagenase and dispase treatment were then maintained in the presence of M-CSF (10 ng/ml) for an additional 12 h. The expression levels of Bim and other apoptosis-regulatory proteins in OCs after M-CSF removal were analyzed by western blotting using specific antibodies. Bim levels increased within 3 h, and the upregulation was sustained at least for 12 h. This upregulation of Bim level was strongly suppressed by M-CSF, and to a lesser extent by sRANKL treatment for 12 h. (B) Intracellular signaling pathways leading to Bim downregulation. Upper panel: introduction of MEKCA strongly suppressed the upregulation of Bim after M-CSF removal. Overexpression of myr-Akt had less effect on Bim expression in OCs. Lower panel: treating the cells with a specific inhibitor of MEK/ERK pathways, PD98059, completely abolished the suppressive effect of M-CSF on Bim expression. (C) Transcriptional regulation of bim in OCs. No significant change in the mRNA level of three isoforms of bim, i.e. bimEL, L and S (upper and lower left), or the bimEL specific mRNA level (lower right) was detected in OCs in the presence or absence of M-CSF as determined by RT–PCR (upper) or real-time PCR (lower). The y-axis indicates the relative mRNA levels. NS = not significantly different. (D) Effect of adenovirus vector-mediated overexpression of BimL and/or Bcl-xL on OC survival. Upper panel: TRAP staining. Lower panel: percentage of OCs surviving. Overexpression of BimL promoted apoptosis of OCs (AxBimL). Not only did Bcl-xL overexpression suppress apoptosis of OCs (AxEGFP + AxBcl-xL), but co-expression of Bcl-xL together with BimL completely abrogated the pro-apoptotic effect of BimL (AxBimL + AxBcl-xL).
We previously reported that the Ras/extracellular signal-regulated kinase (ERK) pathway promotes survival of OCs (Miyazaki et al., 2000), while others found that the phosphatidylinositol 3-kinase (PI3-kinase)/Akt pathway has an effect on this process (Wong et al., 1999; Glantschnig et al., 2003). To analyze whether these pathways are involved in downregulation of Bim by M-CSF, we employed adenovirus vectors encoding constitutively active MEK1 (MEKCA) or Akt (myr-Akt), which contains a Src myristoylation signal that promotes association with the plasma membrane, causing constitutive activation. As shown in Figure 1B, enforced expression of MEKCA reversed the induction of Bim after M-CSF removal, while myr-Akt had less effect. Treating the cells with a specific inhibitor of MEK/ERK pathways, PD98059, completely abolished the effect of M-CSF on Bim expression (Figure 1B). These results indicate that Ras/ERK signaling is a major pathway for downregulation of Bim by M-CSF in OCs.
We next examined the effects of Bim and Bcl-xL overexpression in OC apoptosis caused by cytokine withdrawal using adenovirus vectors. Less than 5% of OCs overexpressing Bim survived 12 h after M-CSF removal, whereas 20% of control virus-infected cells remained alive at this time (Figure 1D). Co-expression of Bcl-xL abrogated the pro-apoptotic effects of the BimL adenovirus, indicating that the balance between Bim and Bcl-xL may determine the fate of OCs (Figure 1D).
Expression of Bim in skeletal tissues
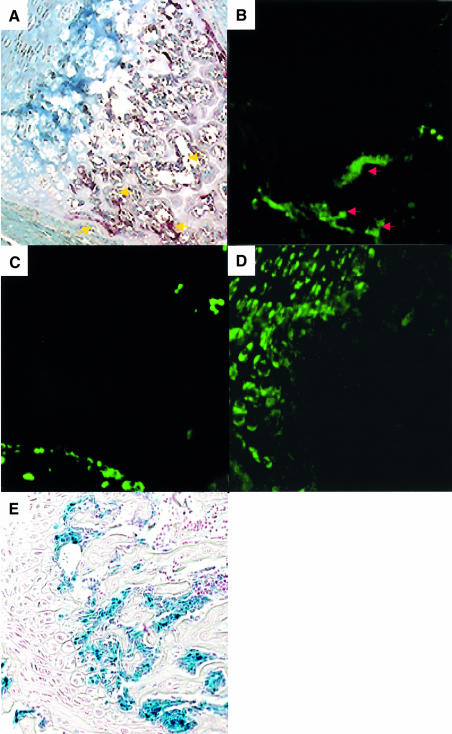
To determine the expression of bim in skeletal tissues, we performed in situ hybridization analysis using a bimL antisense probe which detects bimS, bimL and bimEL. Strong expression of bim mRNA was observed in the bone trabeculae of 5-week-old male mouse metatarsal bone, which was co-localized with tartrate-resistant acid phosphatase (TRAP) enzymatic staining, i.e. with OCs (Figure 2A and B). On the other hand, bim transcripts were hardly detectable in osteoblasts or chondrocytes, whose localization was determined by procollagen type IA and type IIA expression, respectively (Figure 2B–D). The expression pattern of bim in the skeletal tissues was confirmed further by X-gal staining of mutant mice, in which a lacZ reporter gene was knocked into the bim locus by homologous recombination (Figure 2E).
Fig. 2. In situ hybridization of the section of the metatarsal bones from a 5-week-old male wild-type mouse using digoxigenin-labeled mouse bimL (B), procollagen type IA (C) and type II A (D) riboprobes, and TRAP enzymatic staining (A). The labeling was detected by anti-digoxigenin antibody and Alexa 488-labeled anti-rabbit IgG antibody. Note the co-localization of bim transcripts with TRAP staining (OCs) (A and B). No positive bimL staining was co-localized with procollagen type IA staining (osteoblasts) or type IIB staining (chondrocytes). X-gal staining of the tibia from 5-week-old transgenic mice in which lacZ gene was introduced into the bim locus by homologous recombination also showed the clear positive staining in OCs but not in chondrocytes or osteoblasts (E).
Mild osteosclerosis in Bim-deficient mice is due to reduced bone turnover
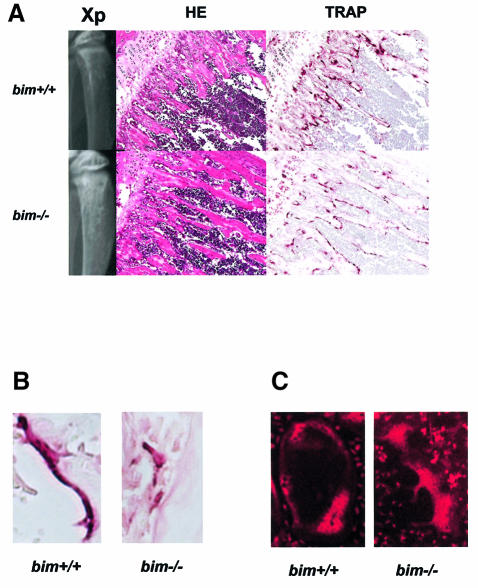
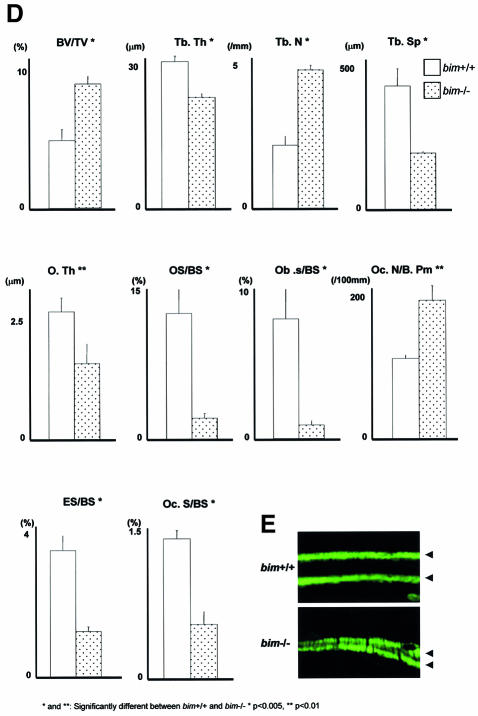
We next studied the skeletal tissues of bim–/– mice. Histological examination and X-ray analysis revealed that bim–/– mice have mild osteosclerosis (Figure 3A). Histomorphometry demonstrated that bim–/– mice had abnormally low levels of new bone formation and turnover of the skeletal tissues (Figure 3D and E). The osteoid surface (OS/BS) and osteoblast surface (Ob.S/BS) were lower in bim–/– mice compared with wild-type animals (Figure 3D), and calcein double labeling revealed a marked decrease in the mineral apposition rate (MAR) in the bim–/– mice (Figure 3E). Although the OC number per bone perimeter (Oc.N/B.Pm) was increased in bim–/– mice, the percentage of the eroded surface (ES/BS) and OC surface (Oc.S/BS) was reduced (Figure 3D). It appears likely that this defect is a consequence of the abnormally small size and impaired actin ring formation of the bim–/– OCs (Figure 3B and C).
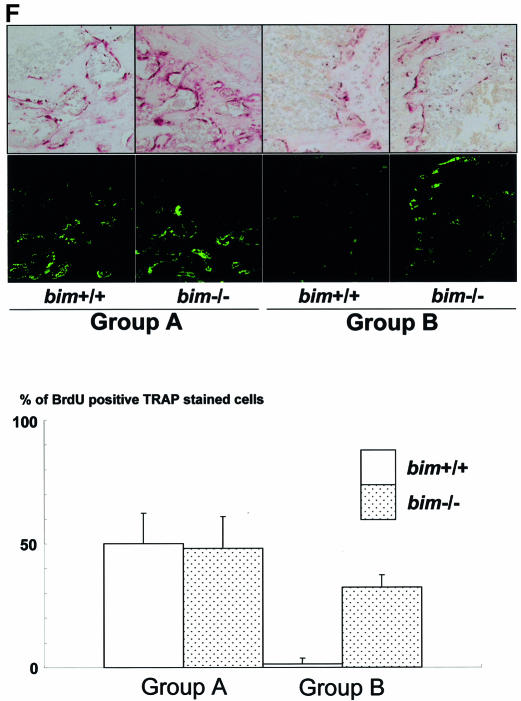
Fig. 3. Bim deficiency causes mild osteosclerosis due to impaired OC activation. (A) Radiological and histological analysis of tibia from 5-week-old bim+/+ and bim–/– male mice (H&E and TRAP staining). bim–/– tibia showed expanded secondary spongiosa, exhibiting mild osteosclerosis. (B) bim–/– OCs showed smaller and shrunken morphological features compared with bim+/+ OCs in vivo as shown by TRAP staining. (C) Impaired actin ring formation in bim–/– OCs. More than 90% of bim+/+ OCs formed actin rings (left), whereas only 30% of bim–/– cells were able to do this (right). (D) Histomorphometric analysis: parameters are measured in the proximal tibia of bim+/+ and bim–/– mice. Data are expressed as means ± SD from five mice of each genotype. BV/TV, trabecular bone volume expressed as a percentage of tibia tissue volume; Tb.Th, trabecular bone thickness; Tb.N, trabecular bone number per mm; Tb.Sp, average space between neighboring trabecular bones; O.Th, osteoid thickness; OS/BS, percentage of bone surface covered by osteoid; Ob.S/BS, percentage of bone surface covered by cuboidal osteoblast; ES/BS, percentage of eroded surface; Oc.N/B.Pm, number of mature OCs per 10 mm of bone perimeter; Oc.S/BS, percentage of bone surface covered by mature OCs. bim–/– bones showed reduction of both bone formation markers (O.Th, OS/BS and Ob.S/BS) and bone resorption markers (ES/BS and Oc.S/BS), and increased Oc.N/B/Pm. *&** = significantly different. *P < 0.005, **P < 0.01. (E) Calcein double labeling of tibia trabecular bone from 13-week-old bim+/+ (left) and bim–/– mice (right). Labeling is visualized by fluorescent microscopy. (F) bim–/– OCs have a longer life span in vivo. Five-week-old bim+/+ and bim–/– mice (n = 4) were fed with water containing 1 mg/ml BrdU for 1 week (labeling period). Mice were then sacrificed either on the next day (group A) or after 6 weeks (group B) of the labeling period, and their tibias were examined by anti-BrdU immunohistochemistry. More than 100 OCs were examined by BrdU immunostaining in the serial sections of the tibia, and the number of BrdU-positive OCs was counted. Fifty percent of bim+/+ OCs and 48% of bim–/– OCs in group A were positively stained by BrdU. However, the proportion of BrdU-positive OCs was markedly reduced to <5% in group B bim+/+ mice, due to the apoptotic cell death, while >30% of group B bim–/– OCs still exhibited BrdU labeling.
Elongated life span of bim–/– OCs in vivo
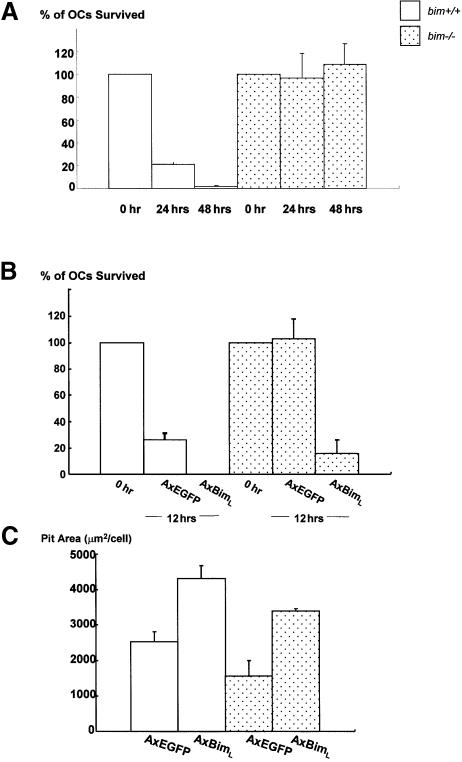
We next examined whether bim–/– OCs have a longer life span in vivo (Figure 3F). Five-week-old bim+/+ and bim–/– mice were fed with water containing 1 mg/ml of 5′-bromo-2′-deoxyuridine (BrdU) for 1 week (labeling period). Mice were then sacrificed either on the next day (group A) or after 6 weeks (group B) of the labeling period. Immunostaining with anti-BrdU antibody demonstrated that almost similar proportions of OCs were positively stained in group A bim+/+ and bim–/– mice (50 and 48%, respectively). However, the proportion of BrdU-positive OCs was markedly decreased to <5% in group B bim+/+ mice, while that in group B bim–/– mice was maintained at 33%. This suggests that bim–/– OCs have a longer life span than bim+/+ OCs in vivo.
bim–/– OCs are resistant to cytokine withdrawal-induced apoptosis but have abnormally low bone resorption activity in vitro
Bone marrow cells obtained from bim–/– and bim+/+ mice were subjected to OC formation assay by co-culturing with osteoblastic cells in the presence of 1α,25(OH) 2D3 and PGE2. The number of TRAP-positive multinucleated cells formed from bim–/– bone marrow cells was comparable with that from bim+/+ cells. The bim–/– OCs were, however, remarkably resistant to cytokine withdrawal. Within 48 h of M-CSF depletion, all the wild-type OCs had undergone apoptosis, whereas >90% of bim–/– OCs remained alive (Figure 4A).
Fig. 4. Effects of Bim on survival and activity of OCs. (A) Survival of bim+/+ and bim–/– OCs. More than 90% of bim–/– OCs survived 48 h after M-CSF removal, while all the bim+/+ OCs had died. (B) Adenovirus vector-mediated BimL expression-promoted apoptosis in bim–/– OCs as well as in bim+/+ OCs after 12 h of M-CSF deprivation. (C) Pit-forming activity. The resorption pit area formed by bim–/– OCs was significantly less than that formed by bim+/+ OCs. Overexpression of BimL promoted bone-resorbing activity in both bim+/+ and bim–/– OCs.
Interestingly, despite their prolonged survival, bim–/– OCs had less bone-resorbing activity than bim+/+ cells, as determined by the pit formation assay (Figure 4C). Adeno virus vector-mediated BimL expression in bim–/– OCs not only reduced their survival, but also recovered bone-resorbing activity of the cells (Figure 4B and C). These results indicate that the skeletal abnormalities in bim–/– mice result from impaired activity of OCs.
M-CSF stimulation promotes ubiquitylation-dependent degradation of Bim in OCs
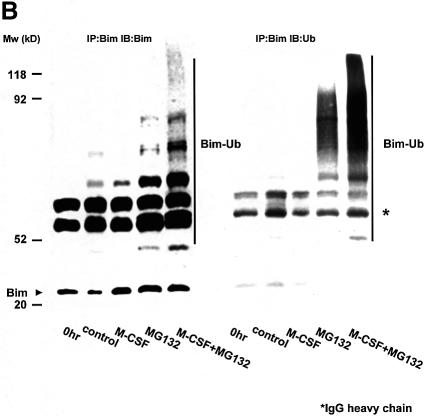
Induction of Bim protein by M-CSF withdrawal (Figure 1A) without changes in mRNA expression levels (Figure 1C) suggested that Bim was regulated post-translationally. Interestingly, treatment of OCs with proteasome inhibitors, such as lactacystin or MG132, greatly enhanced the expression of Bim in OCs even in the presence of M-CSF (Figure 5A). In contrast, MG132 did not affect the expression of other Bcl-2 family members (Figure 5A). This indicates that the ubiquitin–proteasome degradation system is involved in the regulation of Bim expression in these cells. Immunoprecipitation of Bim followed by immunoblot analysis using anti-ubiquitin antibody demonstrated a high level of Bim polyubiquitylation in OCs cultured in MG132, which was greatly enhanced by M-CSF (Figure 5B).
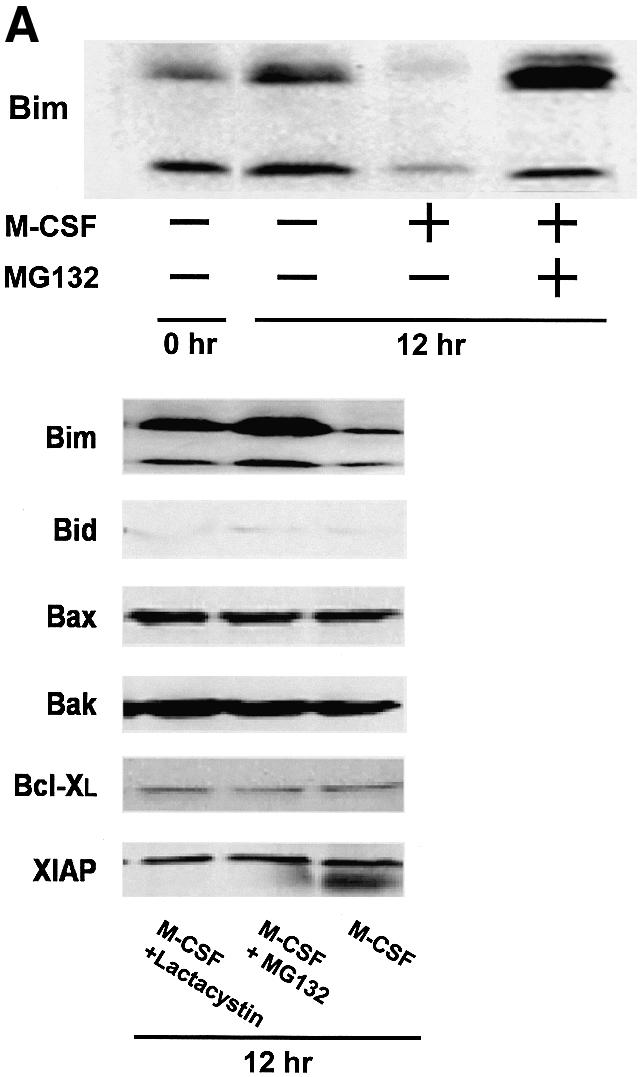
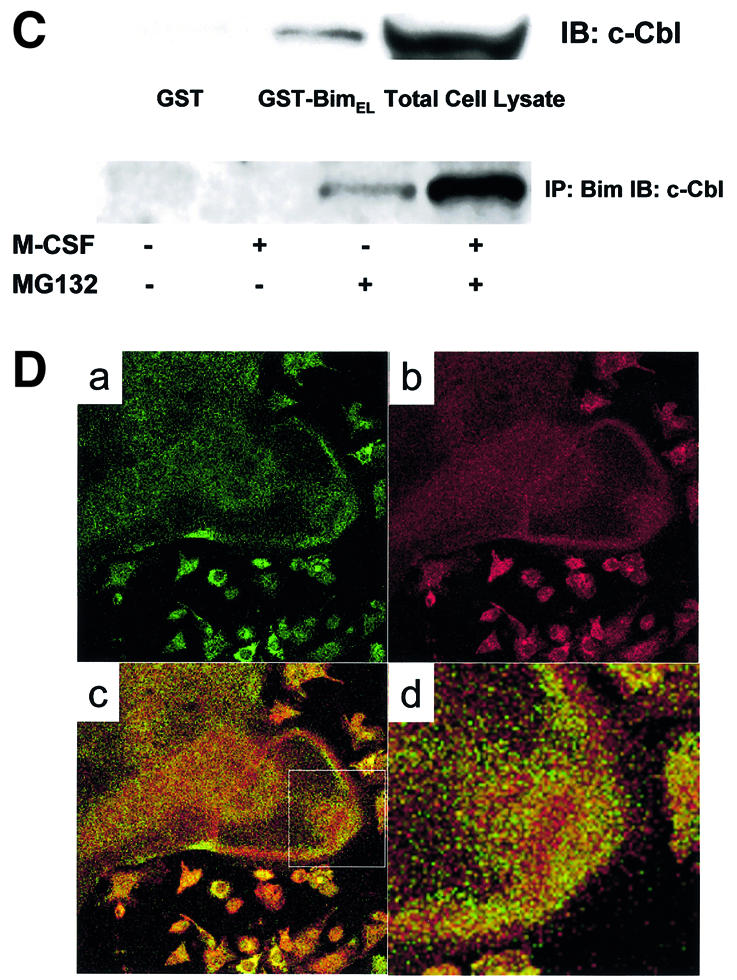
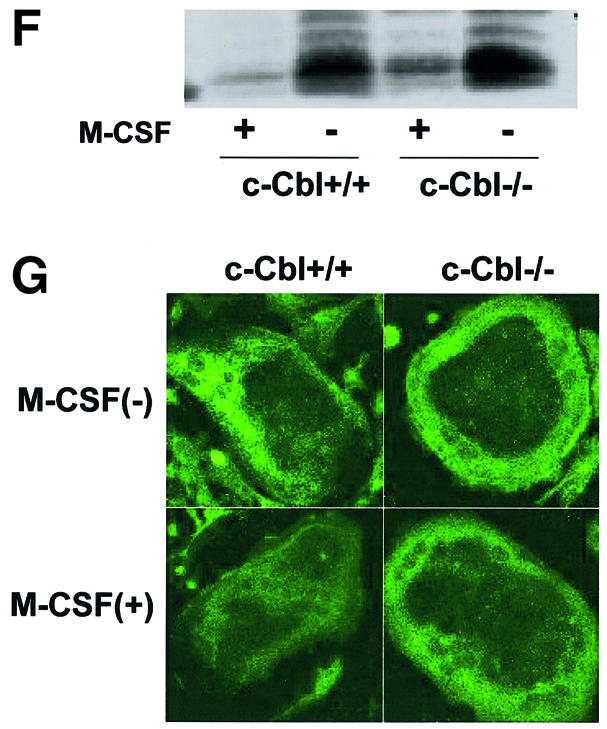
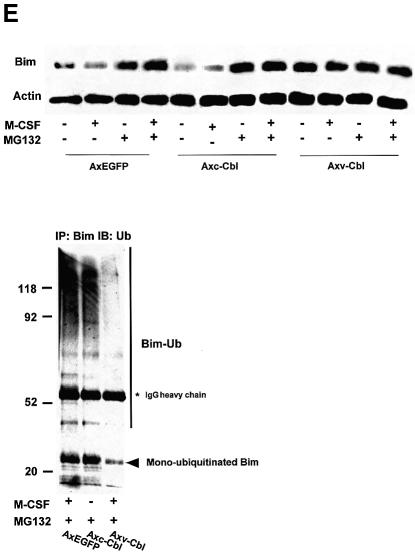
Fig. 5. Ubiquitylation-dependent degradation of Bim in OCs. (A) Western blotting. Upper panel: Bim protein level increased after 12 h of osteoblast removal, which was suppressed in the presence of M-CSF. MG132 treatment strongly upregulated Bim expression even in the presence of M-CSF. Lower panel: no obvious upregulation or downregulation of Bid, Bax, Bak, Bcl-xL and XIAP level was induced by MG132 or lactacystin. (B) Ubiquitylation of Bim in OCs. Proteins immunoprecipitated with anti-Bim antibody were immunoblotted with an anti-Bim antibody (left) or an anti-ubiquitin antibody (right). Ubiquitylated Bim was detected as upper-shifted bands in both anti-Bim and anti-ubiquitin blotting (Bim-Ub). Ubiquitylated Bim was detected when cells were treated with MG132, and marked enhancement of its ubiquitylation was induced by M-CSF treatment. (C) Association of c-Cbl with Bim in OCs. Upper panel: cell lysates of M-CSF-treated OCs were incubated with bacterially expressed GST–BimEL fusion protein, and association with c-Cbl was determined by western blotting. c-Cbl was associated with GST–BimEL but not with GST. Lower panel: Bim was immunoprecipitated from OC cell lysates, and its association with c-Cbl was examined by western blotting. Co-immunoprecipitation of c-Cbl with Bim was observed in MG132- treated OCs, which was enhanced by M-CSF treatment. (D) Double immunofluorescence of (a) Bim (green) and (b) c-Cbl (red) in authentic OCs isolated from normal mice. Co-localization of these two molecules (yellow in c and d) was observed in the presence of MG132 and M-CSF. (d) An enlargement of the rectangular area in (c). (E) Involvement of c-Cbl in the ubiquitylation of Bim in OCs. Upper panel: adenovirus vector-mediated overexpression of c-Cbl (Axc-Cbl) decreased the protein level of Bim in OCs in the absence of M-CSF (compare lanes 1 and 5). MG132 treatment increased Bim expression in c-Cbl-overexpressing cells with or without M-CSF (lanes 7 and 8). On the other hand, adenovirus vector-induced overexpression of v-Cbl (Axv-Cbl) increased Bim expression even in the absence of MG132, which was not affected by M-CSF treatment (lanes 9–12). Lower panel: Bim was immunoprecipitated and immunoblotted with anti- ubiquitin antibody. c-Cbl-overexpressing OCs showed Bim ubiquitylation in the absence of M-CSF (lane 2) to a similar extent as in EGFP virus-infected OCs treated with M-CSF (lane 1). v-Cbl overexpression suppressed Bim ubiquitylation even in the presence of MG132 and M-CSF (lane 3). (F) M-CSF-induced downregulation of Bim was suppressed in c-Cbl–/– OCs. OCs generated from bone marrow cells of c-Cbl–/– mice (c-Cbl–/–) or their normal littermates (c-Cbl+/+) were treated or not with M-CSF. Western blotting with anti-Bim antibody demonstrates that although no difference in Bim level was observed between c-Cbl+/+ and c-Cbl–/– OCs in the absence of M-CSF (lanes 2 and 4, respectively), M-CSF-induced downregulation of Bim was suppressed in c-Cbl–/– OCs (lane 3). (G) Immunofluorescence of primary OCs with anti-Bim antibody. Primary OCs isolated from c-Cbl+/+ and c-Cbl–/– mice were cultured in the presence or absence of M-CSF (10 ng/ml) for 12 h, and immunostained with anti-Bim antibody. Although no apparent difference in fluorescence intensity was observed between c-Cbl+/+ and c-Cbl–/– OCs in the absence of M-CSF, M-CSF-induced downregulation was scarcely detected in c-Cbl–/– OCs.
Possible involvement of c-Cbl in ubiquitylation of Bim
The Cbl family proteins are evolutionarily conserved negative regulators of activated tyrosine kinase-coupled receptors, act as E3 ubiquitin ligases, and are involved in OC function (Tanaka et al., 1996; Joazeiro and Weissman, 2000; Sanjay et al., 2001). To determine the role of c-Cbl in the ubiquitylation and degradation of Bim in OCs, we first examined the association of Bim and c-Cbl. GST pull-down experiments showed that recombinant GST–BimEL fusion protein specifically associated with c-Cbl (Figure 5C, upper panel). c-Cbl was co-immunoprecipitated with Bim in OC lysates in the presence of proteasome inhibitor MG132, and this association was enhanced by M-CSF treatment (Figure 5C, lower panel). Immuno fluorescence analysis showed clear co-localization (yellow in Figure 5D, c and d) of Bim and c-Cbl in OCs treated with MG132 and M-CSF. We constructed adenovirus vectors carrying c-Cbl or v-Cbl, which lacks the ubiquitin ligase RING finger domain and acts in a dominant-negative fashion (Taher et al., 2002), and examined their effects on the expression level of Bim in OCs. The upper panel of Figure 5E shows by western blotting with anti-Bim antibody that c-Cbl overexpression (without MG132) reduced Bim expression by increasing Bim ubiquitylation (compare lanes 1 and 5). MG132 treatment increased Bim expression in c-Cbl-overexpressing cells by suppressing proteasomal degradation with or without M-CSF (lanes 7 and 8). In contrast, overexpression of v-Cbl increased the expression of Bim to the maximal level even in the absence of MG132, and the expression was not affected by M-CSF (lanes 9–12). As shown in the lower panel of Figure 5E, overexpression of c-Cbl enhanced Bim ubiquitylation in the absence of M-CSF, and v-Cbl expression suppressed it even in the presence of MG132 and M-CSF.
The role of c-Cbl in M-CSF-dependent ubiquitylation of Bim was confirmed further using OCs generated from c-Cbl–/– mouse bone marrow cells. M-CSF-induced downregulation of Bim was suppressed in c-Cbl–/– OCs as shown by western blotting in Figure 5F. Immuno staining with anti-Bim antibody demonstrated that although no obvious difference in fluorescence intensity was observed between authentic OCs isolated from c-Cbl+/+ and c-Cbl–/– mice, downregulation of Bim by M-CSF treatment was suppressed in c-Cbl–/– OCs (Figure 5G). These results suggest that c-Cbl plays an important role in the ubiquitylation and proteasome-dependent degradation of Bim in OCs.
Stabilization of Bim by lysine mutation reduced the ability of M-CSF to inhibit OC apoptosis
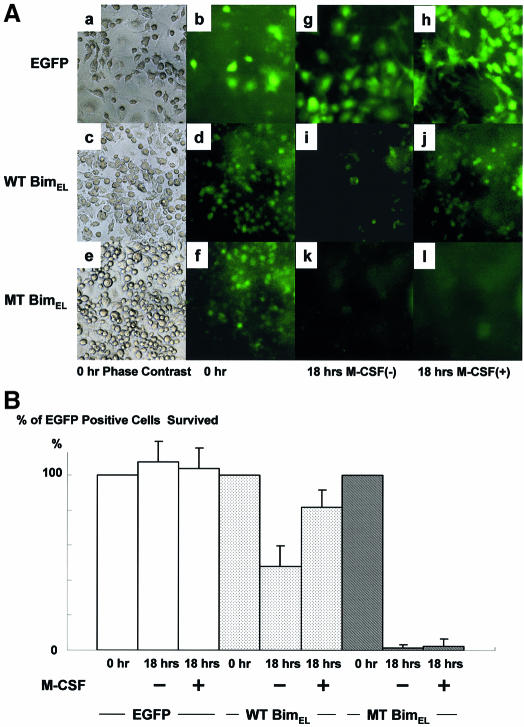
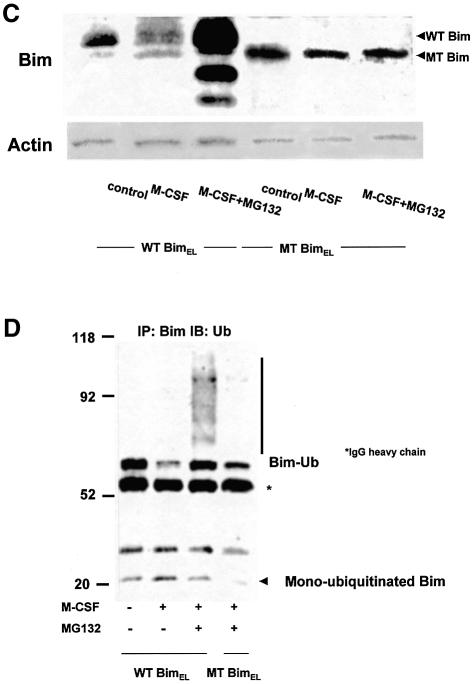
One of the first steps in the ubiquitin–proteasome degradation system includes selective modification of ε-NH2 groups of lysine residues by ubiquitylation. We constructed retroviral vectors encoding wild-type (wt) or mutated (mt) BimEL, in which all the ubiquitin acceptor lysine residues (Lys3 and Lys108) are mutated to arginine. These vectors also contained an internal ribosomal entry site (IRES) and enhanced green fluorescent protein (EGFP) to allow identification of infected cells. bim–/– OC precursors were infected with either control virus (pMx-IRES-EGFP), pMxBimEL-IRES-EGFP or pMxmtBimEL-IRES-EGFP in the presence of M-CSF and a pan-caspase inhibitor zVAD-FMK. Removal of zVAD-FMK induced rapid apoptosis in the cells expressing mtBimEL within 18 h even in the presence of M-CSF, while almost all of control virus- or 77% of pMx-IRES-BimEL-infected cells survived for at least 24 h. MG132 treatment clearly upregulated the expression of wtBimEL, while it did not affect mtBim expression (Figure 6C). Immunoprecipitated mtBim was not ubiquitylated even in the presence of M-CSF and MG132, compared with the wtBim (Figure 6D). These results demonstrate that ubiquitylation and proteasome-mediated degradation are critical regulators of the pro-apoptotic activity of Bim, at least in OCs and their precursors.
Fig. 6. Effect of mutations in Bim that prevent ubiquitylation. (A) Phase contrast (a, c and e) and immunofluorescence microscopy: bim–/– bone marrow cells cultured in the presence of M-CSF (100 ng/ml) and zVAD-FMK (100 µM) were infected with either pMx-IRES-EGFP, pMxBimEL-IRES-EGFP or pMxmtBimEL-IRES-EGFP. After 7 days of the retrovirus infection, when gene expression was confirmed by EGFP fluorescence (b, d and f), cultures were deprived of zVAD-FMK. At 18 h after zVAD-FMK removal, most of pMx-IRES-EGFP- and pMxBimEL-IRES-EGFP-infected cells survived as identified by EGFP fluorescence (h and j), compared with the survival rate of 5% in pMxmtBimEL-IRES-EGFP virus-infected cells (l). Both pMxBimEL-IRES-EGFP- and pMxmtBimEL-IRES-EGFP-infected cells died when M-CSF was removed from the cultures (i and k). (B) The survival ratio of EGFP-positive cells. Almost 100% of the control cells survived even 18 h after z-VAD-FMK removal in the presence of M-CSF. At 18 h after zVAD-FMK removal, almost 100% of pMx-IRES-EGFP- and >70% of pMxBimEL-IRES-EGFP-infected cells survived as identified by EGFP fluorescence (EGFP and WTBimEL), compared with the survival rate of 5% in pMxmtBimEL-IRES-EGFP virus-infected cells (MTBimEL). (C) The proteasome inhibitor MG132 enhanced the expression level of wtBim in pMxBimEL-IRES-EGFP-infected OC precursors (WTBim) even in the presence of M-CSF, while no obvious upregulation of MTBim was observed in pMxmtBimEL-IRES-EGFP infected cells (MTBim). (D) WTBim or MTBim was immunoprecipitated from cell lysates of pMxBimEL-IRES-EGFP-infected (WTBimEL) or pMxmtBimEL-IRES-EGFP-infected cells (MTBimEL) using anti-Bim polyclonal antibody, and the immunoprecipitates were immunoblotted with anti-ubiquitin antibody. Treating the cells with the proteasome inhibitor MG132 strongly increased the ubiquitylation of Bim, while no ubiquitylation of mtBim was observed.
Discussion
OCs are terminally differentiated cells with a short life span and, in the absence of trophic factors, such as M-CSF or RANKL, they undergo rapid apoptosis. In the present study, we found that Bim is essential for cytokine withdrawal-induced apoptosis of OCs. Interestingly, although the number of OCs per bone surface was increased and the life span of OCs was elongated in bim–/– mice, these animals had mild osteosclerosis due to the low turnover of the skeletal tissues. This phenotype appears to be due to abnormalities in OCs because they are the only relevant cell type in skeletal tissues that express Bim. Moreover, experiments in tissue culture demonstrated that in spite of the retarded apoptosis of bim–/– OCs, bone-resorbing activity was significantly reduced compared with bim+/+ OCs. Adenovirus vector-mediated restoration of BimL expression in bim–/– OCs not only promoted cytokine withdrawal-induced apoptosis but also upregulated their bone-resorbing activity. Our results are reminiscent of previous studies by Roodman and co-workers, which showed that OC-specific overexpression of Bcl-xL and SV40 large T antigen under the control of TRAP promoter in transgenic mice induced osteosclerosis although OC apoptosis was markedly reduced in the animals (Hentunen et al., 1998). These results suggest that the balance between Bim and anti-apoptotic Bcl-2 family members, such as Bcl-2 and Bcl-xL, is critical for normal skeletal homeostasis. The molecular mechanism of reduced bone-resorbing activity in bim–/– OCs still remains unclear. Several previous studies demonstrated that although M-CSF promotes the survival of OCs, it rather suppresses their bone-resorbing activity (Hattersley et al., 1988; Fuller et al., 1993; Miyazaki et al., 2000). M-CSF induces cytoskeletal reorganization of OCs and enhances their migration and chemotaxis, and motile OCs are non-polarized cells with low bone-resorbing activity. Because M-CSF strongly suppressed Bim expression in OCs, we speculate that bim deficiency somehow mimics M-CSF treatment, which may explain the reduced bone-resorbing activity of the cells. Alternatively, aged OCs that have already engorged a lot of bone become exhausted, making it difficult to resorb more bone, and the presence of aged OCs suppresses the beginning of the new bone remodeling cycle. Further studies are required to clarify further the molecular mechanism of the reduced bone resorption of bim–/– OCs and the osteosclerosis in bim–/– mice.
Recently, the role of ubiquitylation in regulating apoptosis has been widely recognized and attracted a great deal of interest (Jesenberger and Jentsch, 2002). For example, p53, which is essential for DNA damage-induced apoptosis in many cell types, is degraded by the ubiquitin–proteasome pathways (Haupt et al., 1997; Kubbutat et al., 1997). Moreover, inhibitor of apoptosis proteins (IAPs) (e.g. XIAP, cIAP1 and cIAP2), which can bind and neutralize initiator and/or effecter caspases, contain RING finger domains, and promote their auto-ubiquitylation and proteasome-mediated degradation when cells are exposed to apoptotic stimuli (Yang et al., 2000). Dephosphoryl ation and subsequent ubiquitylation-dependent degradation of Bcl-2 was reported to be involved in TNF-α-induced apoptosis of endothelial cells (Dimmeler et al., 1999). Finally, proteasome-dependent degradation of the BH3-only proteins Bid (tBid) and Bik has been reported in HeLa cells and leukemia-derived cells, respectively (Breitschopf et al., 2000; Marshansky et al., 2001). Previous studies have revealed that Bim can be regulated at the transcriptional level in hemopoietic progenitors and neurons (Putcha et al., 2001; Shinjyo et al., 2001; Whitfield et al., 2001; Dijkers et al., 2002), and the regulation of Bim activity by phosphorylation by ERK and/or JNK has been reported (Biswas and Greene, 2002; Lei and Davis, 2003; Weston et al., 2003). Very recently, the ubiquitylation of Bim and its upregulation by proteasome inhibitors were reported (Ley et al., 2003). However, the physiological function of such a modification, especially in primary cells, has not been established. We demonstrate here that Bim ubiquitylation is regulated by M-CSF in OCs, and this modification plays a critical role in their survival. In M-CSF-stimulated cells, Bim is ubiquitylated and its expression kept at low levels by proteasomal degradation. After cytokine withdrawal, Bim protein levels are rapidly increased due to the reduced ubiquitylation and degradation. Bim is associated with c-Cbl in OCs, and overexpression of c-Cbl suppressed the expression of Bim in OCs by promoting its ubiquitylation, while that of v-Cbl, which acts in a dominant-negative fashion, increased the Bim level even in the presence of M-CSF, suggesting the important role of c-Cbl in Bim ubiquitylation. This was confirmed further using OCs from c-Cbl–/– mice (Figure 5F and G).
The critical role of ubiquitylation and proteasome-mediated degradation in regulating the pro-apoptotic activity of Bim was proven by showing that a mutant of BimEL that cannot be ubiquitylated can kill OCs even in the presence of M-CSF, whereas wtBimEL can be kept in check by cytokine receptor signaling. We found that MEK1CA introduction downregulates Bim expression in OCs, and a specific inhibitor of MEK/ERK pathways, PD98059, abolished the effect of M-CSF (Figure 5B), indicating that ERK signaling mainly promotes Bim ubiquitylation. This suggests that the state of phosphorylation of Bim may be important for its ubiquitylation, or that E3 ubiquitin ligation to Bim is regulated mainly by ERK pathways. The detailed mechanism of Bim ubiquitylation still remains elusive.
Targeted disruption of bim induced the accumulation of myeloid and lymphoid cells, and perturbation of T-cell development, and caused autoimmune disorders (Bouillet et al., 1999, 2002; Villunger et al., 2003). Bim is also involved in the apoptosis of neurons (Putcha et al., 2001; Whitfield et al., 2001), and we found that it plays an essential role in regulating the survival and bone-resorbing activity of OCs. There is substantial evidence that abnormalities in the ubiquitylation/proteasome degradation machinery can cause diseases, such as neurodegenerative diseases, cancers and autoimmune diseases (Glickman and Ciechanover, 2002). Therefore, the failure in the ubiquitin–proteasome-dependent degradation of Bim may cause various abnormalities in the skeletal homeostasis, the immune systems and neuronal systems. Further studies are required to elucidate the mechanism of action and the regulation of Bim, and the role of Bim in skeletal disorders.
Materials and methods
Antibodies and chemicals
Antibodies to Bid and Bax were purchased from Cell Signaling Technology (Beverly, MA). Antibodies to Bim (M-20 for immunoprecipitation), Bcl-xL, ubiquitin and c-Cbl were from Santa Cruz Technology (Santa Cruz, CA), and antibodies to Bim (for western blotting) were from BD Biosciences Pharmingen (San Jose, CA). Antibody to Bim (for immunocytochemistry) was from Oncogene Research Products (Cambridge, MA). Recombinant mouse M-CSF was bought from TECHNE Co. (Minneapolis, MN) and soluble RANKL was from Wako Pure Chemical Co. (Osaka, Japan). α-Modified minimum essential medium (αMEM) was purchased from Gibco-BRL, Life Technologies Inc. (Rockville, MD), and fetal bovine serum (FBS) was from Sigma (St Louis, MO). Bacterial collagenase was purchased from Wako Pure Chemical Co. (Osaka, Japan), 1α,25(OH)2D3 from Calbiochem (La Jolla, CA) and dispase from Godoshusei (Tokyo, Japan). The broad-spectrum caspase inhibitor zVAD-FMK was from Calbiochem. Other chemicals and reagents used in this study were of analytical grade.
Expression constructs and gene transduction
The recombinant adenovirus vectors were constructed as previously described (Miyazaki et al., 2000; Mochizuki et al., 2002; Yamaguchi et al., 2003). Adenovirus infection of OCs was performed as previously reported (Tanaka et al., 1998; Miyazaki et al., 2003). Retroviral vectors, pMx-BimEL-IRES-EGFP and pMx-mtBimEL-IRES-EGFP, were constructed by inserting full-length mouse cDNA of bimEL and mutated bimEL, in which the two ubiquitin acceptor lysine residues (Lys3 and Lys108) were mutated to arginine, into pMx-IRES-EGFP vector. Retrovirus packaging was performed by transfection of the pMx vectors into BOSC cells. Retrovirus infection of OC precursors was carried out as previously described (Kobayashi et al., 2001).
Animals, cells and cultures
Newborn and 8-week-old male ddY mice were purchased from Shizuoka Laboratories Animal Center (Shizuoka, Japan). The breeding and genotyping of bim–/– mice (on a C57BL/6 N>12 genetic background) was performed as previously described (Bouillet et al., 1999). The transgenic mice in which a reporter lacZ gene was introduced into the bim locus was generated by homologous recombination. c-Cbl–/– mice were generated and identified as previously reported (Naramura et al., 1998). Authentic OCs were obtained from the long bones of 2- to 4-day-old neonatal mice as reported (Chiusaroli et al., 2003). To obtain large numbers of cells for biochemical analyses, we utilized the co-culture system established by Takahashi et al. (1988).
Immunoblotting and immunoprecipitation
Immunoblotting and immunoprecipitation were performed as previously reported (Tanaka et al., 1998).
Immunofluorescence
Cells cultured on glass coverslips were fixed in 3.7% (v/v) formaldehyde in phosphate-buffered saline (PBS) for 10 min, and then washed three times in PBS. Cells were permeabilized in 0.05% saponin for 30 min, blocked in 5% normal goat serum (Boehringer) for 30 min, incubated in appropriate primary antibodies, washed in PBS, incubated with fluorescein-conjugated secondary antibody, and finally washed in PBS and mounted in FluorSave. Cells were examined using a confocal imaging system (MRC-600; Bio-Rad Laboratories).
GST fusion proteins
A pGEX plasmid containing the GST–BimEL was used to transform Escherichia coli B21 cells (Life Technologies, Inc.). After induction of protein expression with 0.1 µM isopropyl-1-thio-β-d-galactopyranoside (Sigma) for 2–4 h, the bacteria were resuspended in a lysis buffer containing 50 mM Tris–HCl, pH 8.0, 100 µM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1% Triton X-100 and were disrupted further by sonication. Following centrifugation at 10 000 g for 20 min, the induced proteins were adsorbed to bead-immobilized glutathione. Soluble GST fusion proteins were obtained by elution with 2 mM reduced glutathione in 50 mM Tris–HCl pH 8.0. For in vitro binding assays, bead-adsorbed GST or GST fusion proteins (5 µg/sample) were incubated with OC cell lysates at 4°C on a rotator for 3 h. The beads were then washed three times with lysis buffer, and bound proteins were subjected to SDS–PAGE under reducing conditions followed by immunoblotting.
RT–PCR and real-time PCR
mRNAs was isolated from OCs, and reverse-transcribed by the Super Script First-Strand Synthesis system for RT–PCR (Invitrogen), according to the manufacturer’s protocol. The primers we utilized to detect bim and gapdh were as follows: bim, 5′-ATGGCCAAGCAACCTTCTGA and 3′-TCAATGCCTTCTCCATACCAG; gapdh, 5′-GTATGTCGTGGAGTCTACTGGTGT and 3′-TACTCCTTGGAGGCCATGTAGGCC. The primers utilized to detect bax and bcl-xL were as previously reported by Okahashi et al. (1998). Reverse-transcribed mRNAs were analyzed by the ABI Prism® 7000 Sequence Detection System (Applied Biosystems, CA). The primers we utilized in real-time PCR to detect the common form of all bim splice variants and the bimEL-specific form were as follows: bim common form, 5′-CTTCCATACGACAGTCTC and 3′-AACCATTTGAGGGTGGTCTTC; bimEL-specific form, 5′-GTCCTCCAGTGGGTATTTCT and 3′-CAGATCTTCAGGTTCCTCCT.
In situ hybridization
In situ hybridization was performed as described previously (Lee et al., 1995) by using complementary digoxigenin-labeled riboprobes for mouse bimL, procollagen type IA and procollagen type IIA.
Histomorphometry
Histomorphometric analysis of proximal tibia from bim+/+ and bim–/– littermates was performed as previously described (Akune et al., 2002).
Survival of OCs
The survival of OCs was measured as previously reported (Miyazaki et al., 2000). Cell survival is expressed as the percentage of morphologically intact TRAP-positive multinucleated cells. Other cultures were incubated further for the indicated times, and then the number of living OCs was counted. The number of viable cells remaining at the different time points is shown as a percentage of the cells at the start of the experiment.
Pit formation assay
The pit formation assay was performed as previously described (Tanaka et al., 1998). The resorbed area was measured using an image analysis system (SYSTEM SUPPLY, Nagano, Japan) linked to a light microscope (Nikon, Tokyo, Japan).
Statistical analysis
Each series of experiments was repeated at least three times. The results obtained from a typical experiment were expressed as the means ± SD. Significant differences were determined using factorial analysis of variance.
Acknowledgments
Acknowledgements
The authors thank R.Yamaguchi and M.Ikeuchi (Department of Orthopaedic Surgery, The University of Tokyo), L.Neff (Yale University) and M.Robati (WEHI) for expert technical assistance, Y.Azuma (Teijin Institute for Biomedical Research) for histomorphometric analysis, A.Asai and T.Kirino (Department of Neurosurgery, The University of Tokyo) for providing Bcl-xL adenovirus, H.Katagiri (Tohoku University) and T.Asano (The University of Tokyo) for MEKCA and myrAKT adenoviruses, T.Kitamura (Institute of Medial Science, The University of Tokyo) for pMx vectors, and J.Adams and S.Cory (WEHI) for bim–/– mice. This work was supported by fellowships and Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan, the Health Science Research Grants from the Ministry of Health and Welfare of Japan, Uehara Memorial Award, Nakatomi Health Science Foundation Award, Grants-in-Aid from the Research Society for Metabolic Bone Diseases to S.T., and by the NHMRC (Canberra), the Leukemia and Lymphoma Society of America and the Dr Josef Steiner Cancer Research Foundation (Bern).
References
- Akune T. et al. (2002) Insulin receptor substrate-2 maintains predominance of anabolic function over catabolic function of osteoblasts. J. Cell Biol., 159, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. (1989) Molecular mechanisms of bone resorption by the osteoclast. Anat. Rec., 224, 317–324. [DOI] [PubMed] [Google Scholar]
- Biswas S.C. and Greene,L.A. (2002) Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J. Biol. Chem., 277, 49511–49516. [DOI] [PubMed] [Google Scholar]
- Bouillet P., Metcalf,D., Huang,D.C., Tarlinton,D.M., Kay,T.W., Kontgen,F., Adams,J.M. and Strasser,A. (1999) Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis and to preclude autoimmunity. Science, 286, 1735–1738. [DOI] [PubMed] [Google Scholar]
- Bouillet P. et al. (2002) BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature, 415, 922–926. [DOI] [PubMed] [Google Scholar]
- Breitschopf K., Zeiher,A.M. and Dimmeler,S. (2000) Ubiquitin-mediated degradation of the proapoptotic active form of bid. A functional consequence on apoptosis induction. J. Biol. Chem., 275, 21648–21652. [DOI] [PubMed] [Google Scholar]
- Cheng E.H., Wei,M.C., Weiler,S., Flavell,R.A., Mak,T.W., Lindsten,T. and Korsmeyer,S.J. (2001) BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell, 8, 705–711. [DOI] [PubMed] [Google Scholar]
- Chiusaroli R., Sanjay,A., Henriksen,K., Engsig,M.T., Horne,W.C., Gu,H. and Baron,R. (2003) Deletion of the gene encoding c-Cbl alters the ability of osteoclasts to migrate, delaying resorption and ossification of cartilage during the development of long bones. Dev. Biol., 261, 537–547. [DOI] [PubMed] [Google Scholar]
- Dijkers P.F., Birkenkamp,K.U., Lam,E.W., Thomas,N.S., Lammers,J.W., Koenderman,L. and Coffer,P.J. (2002) FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J. Cell Biol., 156, 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S., Breitschopf,K., Haendeler,J. and Zeiher,A.M. (1999) Dephosphorylation targets Bcl-2 for ubiquitin-dependent degradation: a link between the apoptosome and the proteasome pathway. J. Exp. Med., 189, 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller K., Owens,J.M., Jagger,C.J., Wilson,A., Moss,R. and Chambers,T.J. (1993) Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J. Exp. Med., 178, 1733–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantschnig H., Fisher,J.E., Wesolowski,G., Rodan,G.A. and Reszka,A.A. (2003) M-CSF, TNFα and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ., 10, 1165–1177. [DOI] [PubMed] [Google Scholar]
- Glickman M.H. and Ciechanover,A. (2002) The ubiquitin–proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev., 82, 373–428. [DOI] [PubMed] [Google Scholar]
- Gross A., McDonnell,J.M. and Korsmeyer,S.J. (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev., 13, 1899–1911. [DOI] [PubMed] [Google Scholar]
- Hattersley G., Dorey,E., Horton,M.A. and Chambers,T.J. (1988) Human macrophage colony-stimulating factor inhibits bone resorption by osteoclasts disaggregated from rat bone. J. Cell. Physiol., 137, 199–203. [DOI] [PubMed] [Google Scholar]
- Haupt Y., Maya,R., Kazaz,A. and Oren,M. (1997) Mdm2 promotes the rapid degradation of p53. Nature, 387, 296–299. [DOI] [PubMed] [Google Scholar]
- Hentunen T.A. et al. (1998) Immortalization of osteoclast precursors by targeting Bcl-XL and simian virus 40 large T antigen to the osteoclast lineage in transgenic mice. J. Clin. Invest., 102, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D.C. and Strasser,A. (2000) BH3-only proteins—essential initiators of apoptotic cell death. Cell, 103, 839–842. [DOI] [PubMed] [Google Scholar]
- Hughes D.E., Wright,K.R., Uy,H.L., Sasaki,A., Yoneda,T., Roodman,G.D., Mundy,G.R. and Boyce,B.F. (1995) Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res., 10, 1478–1487. [DOI] [PubMed] [Google Scholar]
- Jesenberger V. and Jentsch,S. (2002) Deadly encounter: ubiquitin meets apoptosis. Nature Rev. Mol. Cell. Biol., 3, 112–121. [DOI] [PubMed] [Google Scholar]
- Joazeiro C.A. and Weissman,A.M. (2000) RING finger proteins: mediators of ubiquitin ligase activity. Cell, 102, 549–552. [DOI] [PubMed] [Google Scholar]
- Kerr J.F., Wyllie,A.H. and Currie,A.R. (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer, 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N., Kadono,Y., Naito,A., Matsumoto,K., Yamamoto,T., Tanaka,S. and Inoue,J. (2001) Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J., 20, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbutat M.H., Jones,S.N. and Vousden,K.H. (1997) Regulation of p53 stability by Mdm2. Nature, 387, 299–303. [DOI] [PubMed] [Google Scholar]
- Lee K., Deeds,J.D. and Segre,G.V. (1995) Expression of parathyroid hormone-related peptide and its receptor messenger ribonucleic acids during fetal development of rats. Endocrinology, 136, 453–463. [DOI] [PubMed] [Google Scholar]
- Lei K. and Davis,R.J. (2003) JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl Acad. Sci. USA, 100, 2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R., Balmanno,K., Hadfield,K., Weston,C.R. and Cook,S.J. (2003) Activation of the ERK1/2 signalling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein Bim. J. Biol. Chem., 278, 18811–18816. [DOI] [PubMed] [Google Scholar]
- Marshansky V., Wang,X., Bertrand,R., Luo,H., Duguid,W., Chinnadurai,G., Kanaan,N., Vu,M.D. and Wu,J. (2001) Proteasomes modulate balance among proapoptotic and antiapoptotic Bcl-2 family members and compromise functioning of the electron transport chain in leukemic cells. J. Immunol., 166, 3130–3142. [DOI] [PubMed] [Google Scholar]
- Miyazaki T. et al. (2000) Reciprocal role of ERK and NF-κB pathways in survival and activation of osteoclasts. J. Cell Biol., 148, 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., Neff,L., Tanaka,S., Horne,W.C. and Baron,R. (2003) Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J. Cell Biol., 160, 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T. et al. (2002) Akt protein kinase inhibits non-apoptotic programmed cell death induced by ceramide. J. Biol. Chem., 277, 2790–2797. [DOI] [PubMed] [Google Scholar]
- Naramura M., Kole,H.K., Hu,R.J. and Gu,H. (1998) Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc. Natl Acad. Sci. USA, 95, 15547–15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor L., Strasser,A., O’Reilly,L.A., Hausmann,G., Adams,J.M., Cory,S. and Huang,D.C. (1998) Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J., 17, 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahashi N., Koide,M., Jimi,E., Suda,T. and Nishihara,T. (1998) Caspases (interleukin-1β-converting enzyme family proteases) are involved in the regulation of the survival of osteoclasts. Bone, 23, 33–41. [DOI] [PubMed] [Google Scholar]
- O’Reilly L.A., Cullen,L., Visvader,J., Lindeman,G.J., Print,C., Bath,M.L., Huang,D.C. and Strasser,A. (2000) The proapoptotic BH3-only protein bim is expressed in hematopoietic, epithelial, neuronal and germ cells. Am. J. Pathol., 157, 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha G.V., Moulder,K.L., Golden,J.P., Bouillet,P., Adams,J.A., Strasser,A. and Johnson,E.M. (2001) Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron, 29, 615–628. [DOI] [PubMed] [Google Scholar]
- Sanjay A. et al. (2001) Cbl associates with Pyk2 and Src to regulate Src kinase activity, α(v)β(3) integrin-mediated signaling, cell adhesion and osteoclast motility. J. Cell Biol., 152, 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinjyo T. et al. (2001) Downregulation of Bim, a proapoptotic relative of Bcl-2, is a pivotal step in cytokine-initiated survival signaling in murine hematopoietic progenitors. Mol. Cell. Biol., 21, 854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris,A.W., Huang,D.C., Krammer,P.H. and Cory,S. (1995) Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J., 14, 6136–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., O’Connor,L. and Dixit,V.M. (2000) Apoptosis signaling. Annu. Rev. Biochem., 69, 217–245. [DOI] [PubMed] [Google Scholar]
- Suda T., Takahashi,N. and Martin,T.J. (1992) Modulation of osteoclast differentiation. Endocr. Rev., 13, 66–80. [DOI] [PubMed] [Google Scholar]
- Taher T.E., Tjin,E.P., Beuling,E.A., Borst,J., Spaargaren,M. and Pals,S.T. (2002) c-Cbl is involved in Met signaling in B cells and mediates hepatocyte growth factor-induced receptor ubiquitination. J. Immunol., 169, 3793–3800. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Akatsu,T., Udagawa,N., Sasaki,T., Yamaguchi,A., Moseley,J.M., Martin,T.J. and Suda,T. (1988) Osteoblastic cells are involved in osteoclast formation. Endocrinology, 123, 2600–2602. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Amling,M., Neff,L., Peyman,A., Uhlmann,E., Levy,J.B. and Baron,R. (1996) c-Cbl is downstream of c-Src in a signalling pathway necessary for bone resorption. Nature, 383, 528–531. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Takahashi,T., Takayanagi,H., Miyazaki,T., Oda,H., Nakamura,K., Hirai,H. and Kurokawa,T. (1998) Modulation of osteoclast function by adenovirus vector-induced epidermal growth factor receptor. J. Bone Miner. Res., 13, 1714–1720. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Nakamura,I., Inoue,J., Oda,H. and Nakamura,K. (2003) Signal transduction pathways regulating osteoclast differentiation and function. J. Bone Miner. Metab., 21, 123–133. [DOI] [PubMed] [Google Scholar]
- Thompson C.B. (1995) Apoptosis in the pathogenesis and treatment of disease. Science, 267, 1456–1462. [DOI] [PubMed] [Google Scholar]
- Villunger A., Scott,C., Bouillet,P. and Strasser,A. (2003) Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2 and Bcl-w in the control of granulocyte survival. Blood, 101, 2393–2400. [DOI] [PubMed] [Google Scholar]
- Weston C.R., Balmanno,K., Chalmers,C., Hadfield,K., Molton,S.A., Ley,R., Wagner,E.F. and Cook,S.J. (2003) Activation of ERK1/2 by ΔRaf-1: ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene, 22, 1281–1293. [DOI] [PubMed] [Google Scholar]
- Whitfield J., Neame,S.J., Paquet,L., Bernard,O. and Ham,J. (2001) Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron, 29, 629–643. [DOI] [PubMed] [Google Scholar]
- Wong B.R., Besser,D., Kim,N., Arron,J.R., Vologodskaia,M., Hanafusa,H. and Choi,Y. (1999) TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell, 4, 1041–1049. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Okada,T., Takeuchi,K., Tonda,T., Ohtaki,M., Shinoda,S., Masuzawa,T., Ozawa,K. and Inaba,T. (2003) Enhancement of thymidine kinase-mediated killing of malignant glioma by BimS, a BH3-only cell death activator. Gene Ther., 10, 375–385. [DOI] [PubMed] [Google Scholar]
- Yang Y., Fang,S., Jensen,J.P., Weissman,A.M. and Ashwell,J.D. (2000) Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science, 288, 874–877. [DOI] [PubMed] [Google Scholar]
- Zong W.X., Lindsten,T., Ross,A.J., MacGregor,G.R. and Thompson,C.B. (2001) BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev., 15, 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]