Abstract
We identified a physical complex consisting of Mtw1p, an established kinetochore protein, with Nnf1p, Nsl1p and Dsn1p and have demonstrated that Nnf1p, Nsl1p and Dsn1p localize to the Saccharomyces cerevisiae kinetochore. When challenged prior to metaphase, the temperature-sensitive mutants nsl1-16 and nsl1-42 as well as Nsl1p-depleted cells failed to establish a bipolar spindle–kinetochore interaction and executed monopolar segregation of sister chromatids. In contrast, an nsl1-16 defect could not be evoked after the establishment of bipolarity. The observed phenotype is characteristic of that of mutants with defects in the protein kinase Ipl1p or components of the Dam–Duo kinetochore complex. However nsl1 mutants did not exhibit a defect in microtubule–kinetochore untethering as the ipl1-321 mutant does. Instead, they exhibited a severe defect in the kinetochore localization of the Dam–Duo complex suggesting this to be the cause for the failure of nsl1 cells to establish bipolarity. Moreover the analysis of Nsl1p-depleted cells indicated that Nsl1p is required for the spindle checkpoint and kinetochore integrity.
Keywords: centromere/Dam–Duo complex/kinetochore/Mtw1p complex/Nsl1p
Introduction
Kinetochores facilitate high fidelity chromosome segregation (Cleveland et al., 2003). The kinetochores of sister chromatids provide dynamic chromosomal attachment sites for microtubules emanating from the opposite spindle poles. The resulting bipolar attachment of sister chromatids in metaphase is the prerequisite for the initiation of anaphase. In Saccharomyces cerevisiae this requires the cleavage of the cohesion protein Scc1p by the protease Esp1p (Nasmyth, 2002). Activities that reside with kinetochores link the achievement of bipolar spindle attachment with the initiation of anaphase by controlling the levels of Pds1p, an inhibitor of Esp1p (Musacchio and Hardwick, 2002). Kinetochores that have no bipolar attachment activate a signaling cascade, the spindle checkpoint consisting of Mad1p, Mad2p, Mad3p, Bub1p, Bub3p and Mps1p, that inactivates the anaphase-promoting complex (APC) through its regulatory subunit Cdc20p (Musacchio and Hardwick, 2002). Consequently the APC fails to initiate Pds1p degradation and Esp1p activation. It is an ongoing question whether kinetochores sense the absence of microtubule attachment, the absence of tension or both (Zhou et al., 2002). Recent experiments (Biggins and Murray, 2001; Stern and Murray, 2001) indicated that missing tension can be sensed when microtubules attach to kinetochores that lack an opposing force, as in cells that have failed to replicate their DNA or failed to establish sister chromatid cohesion. The analysis of the protein kinase Ipl1p resulted in a model that links the recognition of missing tension with the signaling of unattached kinetochores (Biggins and Murray, 2001; Tanaka et al., 2002). The ipl1-321 mutant exhibited monopolar segregation of sister chromatids predominantly with the old spindle pole body (SPB) which is positioned in the daughter cell (Pereira et al., 2001). Furthermore, in contrast to wild-type cells, the ipl1-321 mutant segregated unreplicated sister chromatids (a consequence of depleting the licensing factor Cdc6p) nearly exclusively with the old SPB, which is the one they are attached to before SPB duplication is completed. Thus wild-type cells can obviously untether the attachment of kinetochores with the old pole and allow repositioning of the kinetochore to the new pole, whereas ipl1-321 mutants cannot. This led to the conclusion that Ipl1p is involved in untethering kinetochore–microtubule interaction. Consequently Ipl1p activity is considered to resolve syntelic attachments of sister kinetochores (both with the same pole) and thus pave the way to bipolar kinetochore–spindle attachment. Furthermore, untethering kinetochore–spindle interactions provides free kinetochores that maintain an active spindle checkpoint signal. Thus Ipl1p would allow the ‘no attachment’ checkpoint to recognize the missing tension of kinetochores with syntelic attachments. A consequence of this model is the postulation that the untethering activity of Ipl1p disappears once bipolar spindle–kinetochore attachment has been achieved.
Current knowledge shows that the S.cerevisiae kinetochore is composed of four protein complexes (Cheeseman et al., 2002b; Cleveland et al., 2003). Firstly, CBF3 consisting of Ndc10p, Cep3p, Ctf13p and Skp1p nucleates the kinetochore by specifically binding to centromere DNA (Lechner and Carbon, 1991; Connelly and Hieter, 1996; Stemmann and Lechner, 1996; Espelin et al., 1997). Furthermore, Skp1p was recently shown to localize Bub1p to the kinetochore and the Bub1p–Skp1p interaction is considered to be essential for tension sensing (Kitagawa et al., 2003). Secondly, the Okp1 or Ctf19 complex (Ortiz et al., 1999; Cheeseman et al., 2002a; Measday et al., 2002), consisting of Okp1p, Ame1p, Ctf19p, Mcm16p, Mcm19p, Mcm21p, Mcm22p, Nkp1p, Nkp2p, Chl4p and Ctf3p, provides a link between the CBF3 complex and other kinetochore components. Thirdly, the Ndc80p complex, consisting of Ndc80p, Spc24p, Spc25p and Nuf2p (Janke et al., 2001; Wigge and Kilmartin, 2001). Defects in Ndc80p complex components result in a complete loss of kinetochore–microtubule interaction. Furthermore, it is essential for spindle checkpoint function. Fourthly, the Dam–Duo complex (also named DDD complex) consisting of Duo1p, Dam1p, Ask1p, Spc34p, Spc19p, Dad1p, Dad2p, Dad3p and Dad4p that localize to the kinetochore and the spindle (Cheeseman et al., 2001a; Janke et al., 2002; Li et al., 2002). Defects result in the monopolar segregation of sister chromatids with a preference to the old SPB, similar to the ipl1-321 mutant. Furthermore several proteins of the Dam–Duo complex are Ipl1p substrates and phosphorylation-site mutations resulted in kinetochore defects (Cheeseman et al., 2002a). However, it is still unclear whether the phosphorylation of Dam–Duo proteins causes microtubule–kinetochore untethering. In addition to the four protein complexes, there are several proteins that have not yet been found in complexes in substantial amounts (Cheeseman et al., 2002b). These include Cse4p that is thought to replace histone H3 in a specialized kinetochore nucleosome (Meluh et al., 1998) and Mtw1p, a protein with orthologs in Schizosaccaro myces pombe and humans (Goshima and Yanagida, 2000; Goshima et al., 2003). Three proteins referred to as Nnf1p, Nsl1p and Dsn1p, exhibit genetic interaction with each other and Mtw1p (Euskirchen, 2002). Furthermore nnf1 mutants exhibit chromosome segregation and spindle defects. Therefore they represent good candidates as additional kinetochore proteins. Here we reveal a physical complex of Mtw1p, Nnf1p, Nsl1p and Dsn1p that localizes at the kinetochore and demonstrate that Nsl1p is required for the establishment of a bipolar kinetochore–spindle interaction presumably by facilitating the Dam–Duo complex to dock onto the kinetochore. Moreover we provide evidence that Nsl1p is required for spindle checkpoint function and kinetochore integrity.
Results
Mtw1p, Nnf1p, Nsl1p and Dsn1p form a physical complex
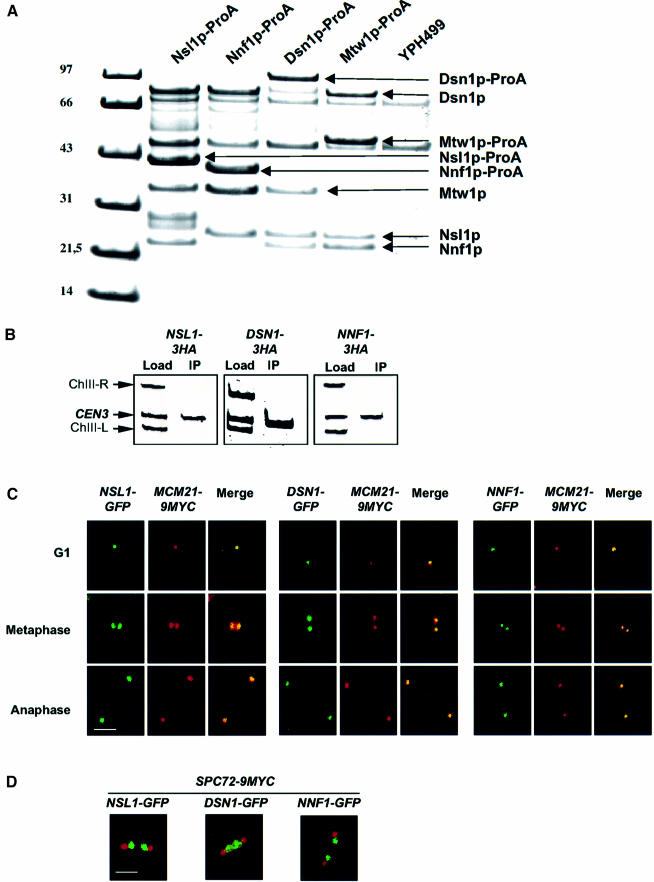
To address whether Mtw1p, Nnf1p, Nsl1p and Dsn1p form a physical complex, Protein A-tagged Mtw1p was isolated from cell extract by affinity chromatography with IgG–Sepharose. Three proteins that co-purified with the tagged Mtw1p were identified by peptide mass fingerprints (MALDI-TOF mass spectrometry) as Nnf1p, Nsl1p and Dsn1p (Figure 1A). Neither of these proteins was detected when an extract from untagged cells was analyzed (Figure 1A). Furthermore, when Protein A-tagged Nnf1p, Nsl1p or Dsn1p were isolated, Mtw1p and the corresponding two other proteins co-purified (Figure 1A). We therefore conclude that Mtw1p, Nnf1p, Nsl1p and Dsn1p form a physical complex that will be addressed as the Mtw1 complex.
Fig. 1. Mtw1p, Nnf1p, Nsl1p and Dsn1p form a physical complex that localizes to the kinetochore. (A) Protein A-tagged Mtw1p, Nnf1p, Nsl1p and Dsn1p were purified from 2000 OD578 of strains CVY10-1, CVY12-1, CVY11-1 and YJO378 respectively by affinity chromatography with IgG–Sepharose and the complete preparation was subjected to SDS PAGE. Co-purifying proteins were identified from Coomassie-stained bands by peptide mass finger printing (MALDI-TOF). Bands that are not labeled represent contaminants. (B) ChIP analysis. HA-tagged Nnf1p, Nsl1p and Dsn1p were immunoprecipitated with anti-HA antibody. The presence of CEN3 DNA and two flanking DNA regions (ChIII-R and ChIII-L) was analyzed by Triplex-PCR in the load and in the immunoprecipitate (IP). The amount of material used to analyze the load was 1% of that used for the IP. (C) Nnf1p, Nsl1p and Dsn1p co-localize with the kinetochore protein Mcm21p. GFP-tagged Nnf1p, Nsl1p and Dsn1p were detected by GFP fluorescence and Myc-tagged Mcm21p was detected by immunofluorescence microscopy. Fixed cells from G1, metaphase or anaphase were analyzed. (D) In metaphase Nnf1p, Nsl1p and Dsn1p localize as two signals in between the two SPBs. GFP-tagged Nnf1p, Nsl1p and Dsn1p were detected by GFP fluorescence and 9Myc-tagged Spc72p (as spindle pole marker) was detected by immunofluorescence microscopy with anti-Myc antibody in fixed metaphase or early anaphase cells. Bars in panels C and D: 5 µm.
Nnf1p, Nsl1p and Dsn1p localize at the kinetochore
The physical and genetic interactions with Mtw1p suggested a role for Nnf1p, Nsl1p and Dsn1p at the kinetochore. To clarify this point we performed chromatin immunoprecipitation (ChIP) with HA-tagged versions of Nnf1p, Nsl1p and Dsn1p. As shown in Figure 1B, the immunoprecipitate of Nnf1p, Nsl1p and Dsn1p contained centromere DNA enriched over control DNA fragments that flank the centromere DNA 4 kb to the left (ChIII-L) and 2 kb to the right (ChIII-R). This provides strong evidence that Nnf1p, Nsl1p and Dsn1p localize in the vicinity of the centromere DNA. Furthermore, we analyzed the cellular localization of GFP-tagged Nnf1p, Nsl1p and Dsn1p by fluorescence microscopy. Firstly, we compared the localization of these proteins with Myc-tagged Mcm21p, an established kinetochore protein, and found complete co-localization at various stages of the cell cycle (Figure 1C). Secondly, we compared the localization of GFP-tagged Nnf1p, Nsl1p and Dsn1p with Myc-tagged Spc72p, a known SPB component. It is known that kinetochores localize close to the SPB throughout most parts of the cell cycle (Jin et al., 2000). SPB and kinetochore can be optimally distinguished by fluorescence microscopy when cells have entered metaphase or early anaphase. As shown in Figure 1D for metaphase or early anaphase cells, Nnf1p, Nsl1p and Dsn1p can be visualized as two points (representing two separated clusters of sister kinetochores) that lie between the SPBs. At the end of anaphase the Nnf1p, Nsl1p and Dsn1p and Spc72p signals were in close proximity (data not shown). This localization behavior is typical for kinetochore proteins (He et al., 2001; Janke et al., 2002). Taken together, we therefore conclude that Nnf1p, Nsl1p and Dsn1p are kinetochore proteins.
Nsl1p is required for bipolar segregation of sister chromatids, is dispensable for monopolar kinetochore microtubule interaction and has a putative role in the spindle checkpoint
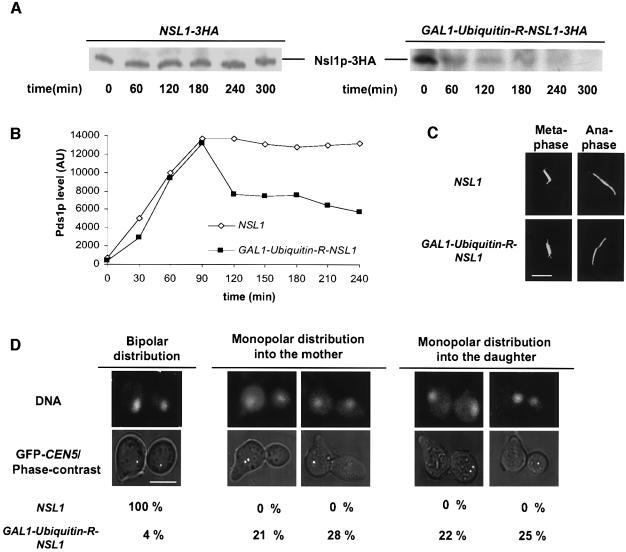
To analyze Nsl1p function we produced the temperature-sensitive (ts) mutants nsl1-16 and nsl1-42 (see below) and constructed a strain (YMS407) that contained an Ubiquitin-R-NSL1 construct (Althoefer et al., 1995) under the control of the GAL1 promoter. Three hours after a 5 h alpha factor arrest in glucose medium, the western analysis of extracts from YMS407 cells showed, in contrast to wild-type cells, no detectable amounts of Nsl1p (Figure 2A). Moreover, the amount of Nsl1p that could be detected at kinetochores after Nsl1p depletion was severely reduced (15% of wild type, Figure 6B). This was revealed by ChIP and quantification of the precipitated centromere DNA by competitive PCR. Thus YMS407 is a suitable tool to analyze kinetochore functions in the near absence of Nsl1p.
Fig. 2. Phenotype of Nsl1p-depleted cells. NSL1 and GAL1-Ubiquitin-R-NSL1 cells were synchronized by alpha factor in glucose medium for 5 h and subsequently released into glucose medium. (A) Anti-HA western analysis of extracts prepared at the indicated time points during the alpha factor arrest. (B) Compromised spindle checkpoint. PDS1-13Myc cells were released into medium containing nocodazole, extracts were prepared at the indicated time points after the release and Pds1p levels were determined by quantitative western analysis with anti-Myc antibody. AU: arbitrary units. (C) Spindles were stained by anti-Tub1 and Alexafluor 488-anti-rat antibody and visualized by fluorescence microscopy 150 min after the release. (D) Monopolar segregation of sister chromatids. DNA was visualized by Hoechst staining and sister chromatids by GFP labeling of CEN5 (Michaelis et al., 1997). Cells were analyzed by fluorescence microscopy 150 min after the release. Mother cells were identified by the alpha factor induced mating projection. For quantification (n >100) only cells with separated DNA masses were counted. These cells comprised >90% of the population. Bars in panels C and D: 5 µm.
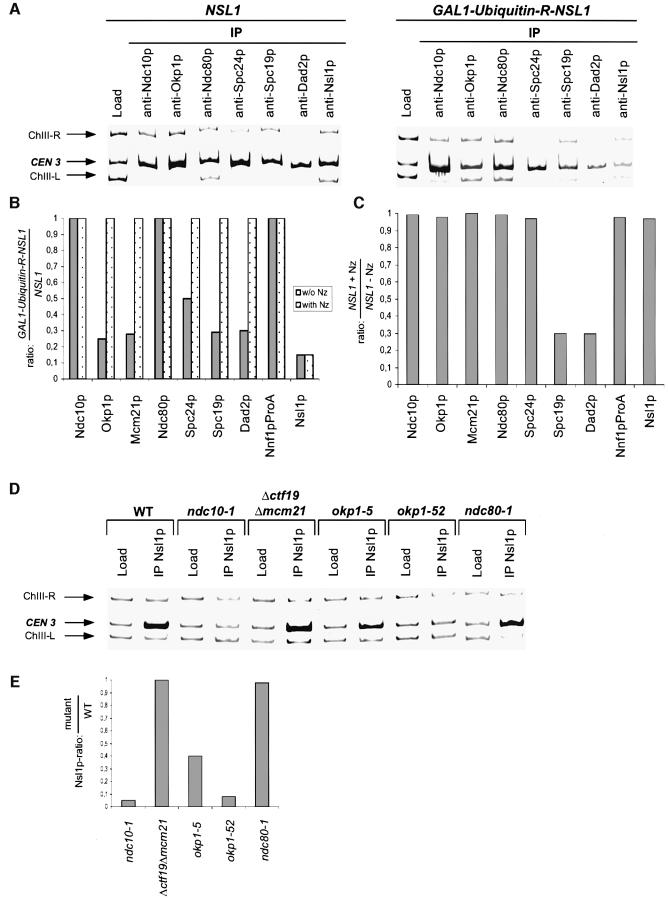
Fig. 6. Interdependency of Nsl1p and selected kinetochore proteins. The data represents the average of three independent experiments (ChIP and quantification). The standard deviation calculated from these experiments was maximal 0.1. (A and B) NSL1 and GAL1-Ubiquitin-R-NSL1 cells were synchronized by alpha factor in glucose medium for 5 h, subsequently released into glucose medium in the absence or presence of nocodazole (Nz) and analyzed by ChIP 180 min after the release. (A) Triplex-PCR. Kinetochore proteins were immunoprecipitated with the indicated antibodies. The presence of CEN3 DNA and two flanking DNA regions (ChIII-R and ChIII-L) was analyzed by Triplex-PCR in the load and in the immunoprecipitates (IP). (B and C) Quantification of CEN3 isolated by ChIP (A and not shown) was performed as in Figure 5B. ChIP of Nnf1p-ProA was performed using IgG–Sepharose. (B) Relative levels of indicated kinetochore proteins at Nsl1p-depleted kinetochores normalized to wild-type kinetochores. (C) Relative levels of indicated proteins at wild-type kinetochores in the presence of nocodazole normalized to those in the absence of nocodazole. (D and E) Cells were grown at 37°C for 3 h and then analyzed by ChIP. (D) Triplex-PCR. Nsl1p was immunoprecipitated with anti-Nsl1p antibody from extracts of the indicated kinetochore mutants. The presence of CEN3 DNA and two flanking DNA regions (ChIII-R and ChIII-L) was analyzed by Triplex-PCR in the load and in the immunoprecipitates (IP). (E) Relative levels of Nsl1p at kinetochores of selected mutants normalized to wild-type kinetochores. Quantification of precipitated CEN3 was performed as in Figure 5B.
We first asked whether the spindle checkpoint of Nsl1p-depleted cells is compromised. As shown in Figure 2B, when released from alpha factor arrest into nocodazole containing medium (which should maintain the active checkpoint state and therefore a high Pds1p level) Nsl1p-depleted cells degraded Pds1p in contrast to wild-type cells. In particular, an initial two-fold reduction of the maximal Pds1p level was observed in Nsl1p-depleted cells at time points when wild-type cells maintained the maximal Pds1p level. This observation is in agreement with the interpretation that Nsl1p is required for checkpoint function. Secondly, the analysis of spindles by fluorescence microscopy revealed that Nsl1p depletion had no detectable effect on spindle structure (at least 90% resembled wild-type spindles; Figure 2C). Thirdly, we investigated sister chromatid segregation. Nsl1p-depleted cells segregated DNA masses (Hoechst stain) to the mother and daughter cell (Figure 2D). However, they failed to segregate sister chromatids, as observed by GFP labeling of CEN5 with the tetracycline repressor/tetracycline operator method (Michaelis et al., 1997; He et al., 2000). Instead sister chromatids segregated together to one of the poles (monopolar segregation) in 96% of cells with separated DNA masses (>90% of total) (Figure 2D). No bias for mother or daughter was observed. This indicated that Nsl1p is required for bipolar spindle–kinetochore attachment. When chromosomes fail to interact with microtubules in general, as in spc24-1 (Janke et al., 2001) or ndc10-1 cells (Goh and Kilmartin, 1993), all chromatids remain within the mother cell. Therefore the observation that Nsl1p-depleted cells exhibited monopolar segregation of sister chromatids to either of the two poles strongly suggests that Nsl1p is not required for monotelic or syntelic kinetochore–microtubule interactions.
Temperature-sensitive nsl1 mutants exhibit monopolar segregation of sister chromatids and a functional spindle checkpoint
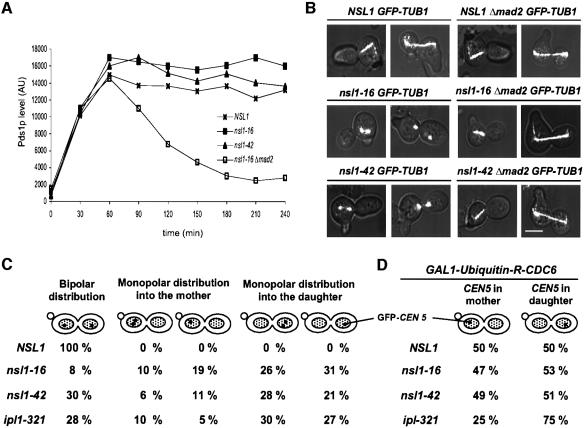
When nsl1-16 and nsl1-42 were analyzed after release from an alpha factor induced G1 arrest at 37°C their conditional phenotypes differed in several points from that of Nsl1p-depleted cells indicating that the ts alleles are hypomorphic relative to Nsl1p depletion. Firstly, the Pds1p levels of wild-type, nsl1-16 and nsl1-42 cells were indistinguishable (high) when cells were released from alpha factor arrest in the presence of nocodazole in contrast to Δmad2 cells (Figure 3A). This suggests that unattached nsl1-16 or nsl1-42 kinetochores are able to maintain an active spindle checkpoint. Furthermore nsl1-16 and nsl1-42 mutants exhibited a delay in Pds1p degradation and a G2/M delay (FACS analysis) in comparison to wild-type cells when released in the absence of nocodazole and for nsl1-16 the delay in Pds1p degradation was shown to depend on MAD2 (Supplementary figure 1, available at The EMBO Journal Online). Therefore the nsl1-16 and nsl1-42 mutations probably provoke the prolonged activation of the spindle checkpoint but they do not interfere with its function.
Fig. 3. Phenotype of conditional lethal nsl1-16 and nsl1-42 cells. (A–C) Cells were synchronized by alpha factor (t = 0). After release from the cell cycle block, cells were shifted to 37°C. (A) Pds1p levels in the presence of nocodazole. Extracts of PDS1-9Myc cells that were released in the presence of nocodazole were obtained every 30 min and subjected to quantitative western analysis with anti-Myc antibody. AU: arbitrary units. (B) Defective anaphase spindles are the result of spindle elongation in cells with an active spindle checkpoint. The spindle of GFP-TUB1 MAD2 or GFP-TUB1Δ mad2 cells was visualized by fluorescence microscopy 150 min after the release. Bar, 5 µm. (C) Monopolar segregation of sister chromatids. GFP-CEN5 labeled cells were analyzed for DNA separation and sister chromatid segregation 150 min after the release as in Figure 2D (n >100). (D) Segregation of unreplicated sister chromatids into mother and daughter is unbiased. For Cdc6p depletion, nocodazole-arrested cells were incubated in glucose medium for 1 h. After nocodazole washout, cells were synchronized by alpha factor, released into 37°C medium and distribution of GFP- labeled CEN5 into mother and daughter cells was analyzed by fluorescence microscopy 150 min after the release. Mother cells were identified by the mating projection.
Secondly, in contrast to Nsl1p-depleted cells, a severe defect in spindle structure was observed for nsl1-16 and nsl1-42 cells, 150 min after the release from G1 (Figure 3B). Seventy-three percent of the nsl1-16 spindles had a ‘broken’ morphology with strong tubulin signals at the poles and either none or a very faint signal in the middle. This phenotype could be observed for shorter spindles (1–2 µm) that resided exclusively in the mother cell (19%) and for spindles that extended over the bud neck and had the length of anaphase spindles (6–8 µm) (54%). Similar spindle defects have been described for cells that attempt spindle elongation in the absence of functional Esp1p (Uhlmann et al., 2000). Since nsl1-16 and nsl1-42 executed spindle elongation at high Pds1p levels (see above), and consequently inactive Esp1p, it appeared possible that the observed spindle defect resulted from the lack of functional Esp1p. To clarify this point, the spindles of nsl1-16 Δmad2 and nsl1-42 Δmad2 cells were examined. Deletion of MAD2 interferes with the spindle checkpoint signaling and consequently nsl1-16 Δmad2 cells did not maintain high Pds1p levels (Supplementary figure 1B). The spindles of nsl1-16 Δmad2 cells were indistinguishable from wild-type spindles, as observed by fluorescence microscopy (Figure 3B). Thus the spindle defect observed in nsl1-16 is most likely due to spindle elongation in the absence of active Esp1p although a more direct approach will be necessary to confirm this point. However, the spindle defect is not the primary cause for cell death, since nsl1-16 Δmad2 cells were not viable at 37°C and exhibited severe monopolar segregation of sister chromatids (data not shown).
Third, nsl1-16 and nsl1-42, as well as Nsl1-depleted cells, exhibited a monopolar segregation of sister chromatids (Figure 3C). When analyzed 150 min after the G1 release >90% of nsl1-16 and nsl1-42 cells had separated their DNA. Of those, 92% nsl1-16 and 70% nsl1-42 cells segregated both sister chromatids to one of the poles. This further supports that Nsl1p is required for kinetochores to obtain a stable bipolar attachment with the spindle. In contrast to Nsl1p-depleted cells, nsl1-16 and nsl1-42 cells segregated sister chromatids with a preference of ∼1:2 and 1:3 respectively to the daughter cell (Figure 3C).
Single chromatids segregate with the old and new SPBs with equal probability in nsl1-16 and nsl1-42 mutants
The phenotype of nsl1-16 and nsl1-42, in particular the preferred monopolar segregation of two sister chromatids with the old SPB into the daughter cell, is reminiscent of cells with a defect in Ipl1p (Biggins and Murray, 2001; Li et al., 2002; Tanaka et al., 2002) or in kinetochore proteins that are components of the Dam–Duo complex (Cheeseman et al., 2001a; Janke et al., 2002) and are substrates of Ipl1p (Cheeseman et al., 2002a). This phenotype is thought to arise from a defect in kinetochore–microtubule untethering, a process that could resolve a syntelic attachment of sister kinetochores with one spindle pole (see Introduction). A major support for the postulated Ipl1p function (as a regulator for kinetochore–microtubule untethering) was the observation that unreplicated chromatids segregated predominantly with the old SPB (to which they are attached before spindle pole duplication) in ipl1-321 cells whereas they segregated without bias in wild-type cells (Tanaka et al., 2002). To clarify whether the phenotype of nsl1 mutants might reflect an Ipl1p related defect, we analyzed the segregation of unreplicated chromatids in ns11 mutants. Before entering G1, nsl-16 and nsl1-42 cells with GFP-labeled CEN5 and an Ubiquitin-R-CDC6 fusion construct under the control of the GAL1 promoter were depleted of Cdc6p, a protein required for licensing of DNA replication. When released from a subsequent alpha factor arrest at non-permissive conditions, these cells failed to replicate their DNA (data not shown) and distributed single chromatids into mother or daughter cells. Quantification of the segregation events revealed that, like in wild-type cells, unreplicated chromatids segregate to the old (daughter) and new spindle pole (mother) with equal probability (Figure 3D) whereas ipl1-321 cells segregated unreplicated chromatids with a high frequency to the old spindle pole. This suggests, that the nsl1 and ipl1-321 defects, are of distinct character and that microtubule–kinetochore untethering is not affected in nsl1 mutants. Why segregation of sister chromatids in nsl1-16 and nsl1-42 cells occurred preferentially to the daughter is presently unclear.
The nsl1-16 defect correlates with the establishment of tension and is not due to the formation of functional asymmetric kinetochores
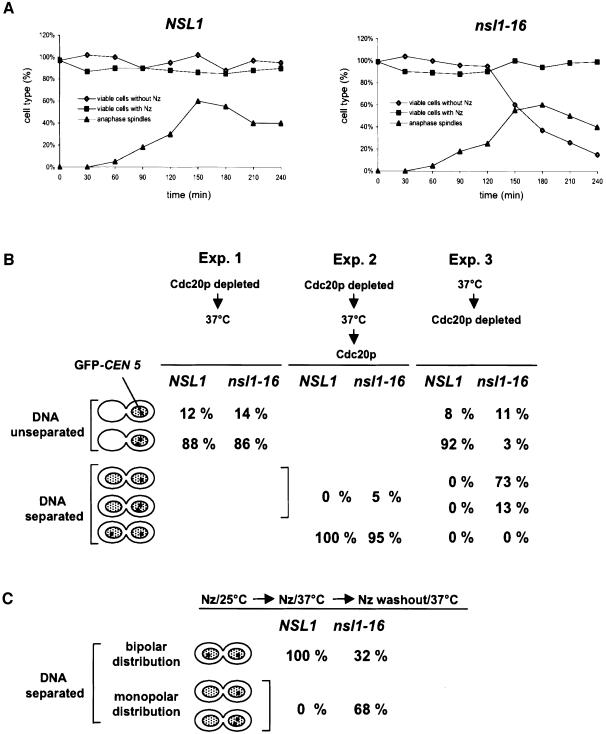
To determine at which step of mitosis the nsl1-16 mutation causes kinetochore malfunction we analyzed the survival rate of nsl1-16 cells after release from G1 at the non-permissive temperature. Cell viability dropped drastically beyond 120 min after the release (Figure 4A). This correlated with the time point when a majority of nsl1-16 cells had started monopolar segregation of sisters and had established anaphase-like broken spindles. However, if spindle formation was inhibited by nocodazole and cells were incubated at 37°C, nsl1-16 cells stayed viable after nocodazole wash out and incubation at 23°C (Figure 4A). This indicated that the functional failure that leads to irreversible monopolar segregation occurred during the time when nsl1-16 kinetochores failed to establish bipolarity. One possible explanation for this might be that nsl1-16 kinetochores cannot withhold the tension applied during bipolar attachment. If so, one might expect that nsl1-16 cells that have been arrested in metaphase at the permissive temperature by Cdc20p depletion, lose their bipolar spindle attachment after a shift to the non-permissive temperature. However, this was not the case. Bipolar spindle attachment and the consequential tension can be monitored indirectly by the number of GFP-CEN5-labeled sister kinetochores that are separated in a population of metaphase cells (Goshima and Yanagida, 2000). This number was very similar for nsl1-16 or wild-type metaphase cells that were incubated for 120 min at 37°C after the metaphase arrest (Figure 4B, experiment 1). Furthermore, when released from the metaphase arrest by Cdc20p expression (in galactose medium) at the non-permissive temperature, these cells segregated their sister chromatids to mother and daughter cells with high fidelity (Figure 4B, experiment 2). Thus the cellular defect of nsl1-16 cells cannot be induced after bipolarity has been achieved. In contrast, if the nsl1-16 cells were released from a G1 arrest at the non-permissive temperature and subsequently depleted of Cdc20p, the number of cells that arrested with one DNA mass and two separated sister kinetochores, thus revealing correct bipolar attachment, was severely reduced. Instead a high percentage of nsl1-16 cells performed monopolar segregation of sister chromatids (Figure 4B, experiment 3) with a preference of 1:2 for the daughter (data not shown). Furthermore the majority of cells formed anaphase-like broken spindles (data not shown), which is consistent with the assumption that spindle elongation in the presence of a high Pds1p level (as a result of Cdc20p depletion) results in spindle damage (see above). In conclusion, the cellular defect of nsl1-16 cells is manifested during the establishment of bipolarity and tension. The failure to achieve bipolar spindle–kinetochore attachment may be the direct cause for spindle elongation (see also Discussion).
Fig. 4. The nsl1-16 defect interferes with the establishment of bipolar kinetochore–spindle attachment but does not reflect the formation of functionally asymmetric kinetochores. (A) Cell viability. Cells were synchronized by alpha factor, released into medium with or without nocodazole (Nz) as indicated and incubated at 37°C. Samples were taken after the indicated time points and grown on plates without nocodazole at 25°C. Colonies were counted and normalized to the total cell count (hematocytometer). Spindles of cells released from an alpha factor arrest were visualized via GFP–tubulin by fluorescence microcopy. Spindles and ‘broken’ spindles with a length of >6 µm were counted as anaphase spindles. (B) Failure to achieve metaphase, but not to maintain metaphase and perform anaphase. GFP-CEN5 cells were synchronized by alpha factor and analyzed by fluorescence microscopy. CEN5 was visualized by GFP fluorescence and DNA by Hoechst staining. Cells revealing unseparated DNA with one or two observable CEN5 signals (metaphase arrest), cells revealing separated DNA with one or two CEN5 signals in the mother or the daughter cell (monopolar segregation) and cells revealing separated DNA with one CEN5 in the mother and the other in the daughter (bipolar segregation) were quantified (n >100). Experiment 1: during alpha factor arrest (3 h) and after the release GAL1-CDC20 cells were grown in glucose medium. Three hours after the release cells were shifted to 37°C and incubated for an additional 3 h before analysis. Experiment 2: as experiment 1 with the exception that after 3 h at 37°C the cells were incubated for further 20 min in galactose medium at 37°C before analysis. Experiment 3: GAL1-CDC20 cells were released from alpha factor arrest into glucose medium and incubated at 37°C for 3 h. (C) The nsl1-16 defect does not reflect the formation of functionally asymmetric kinetochores. GFP-CEN5 cells were released from alpha factor arrest into medium containing nocodazole, incubated at 25°C for 2 h and at 37°C for an additional 2 h. Subsequently the cells were transferred into medium without nocodazole, incubated at 37°C for 20 min and analyzed as in (B).
If sister chromatids contain one new kinetochore (assembled during replication) and one old kinetochore, a possible explanation for the observed monopolar segregation of sister chromatids could be the formation of functionally asymmetric kinetochores. If only kinetochores, which are assembled with an already dysfunctional Nsl1-16p (at the non-permissive temperature), are compromised or if the nsl1-16 defect interferes with kinetochore assembly, then only the new kinetochore would be defective and monopolar segregation would be a logical consequence. To investigate this possibility, we arrested nsl1-16 cells at the permissive temperature with nocodazole. In these cells both sister kinetochores have been assembled in the presence of functional Nsl1-16p. When released from the nocodazole arrest at the non-permissive temperature the nsl1-16 cells, again exhibited severe monopolar distribution of sister chromatids (Figure 4C) with no preference for mother or daughter (not shown). Thus the monopolar segregation in nsl1-16 cells is not due to the formation of functionally asymmetric kinetochores.
Localization of the Dam–Duo complex at the kinetochore is compromised in nsl1-16 cells
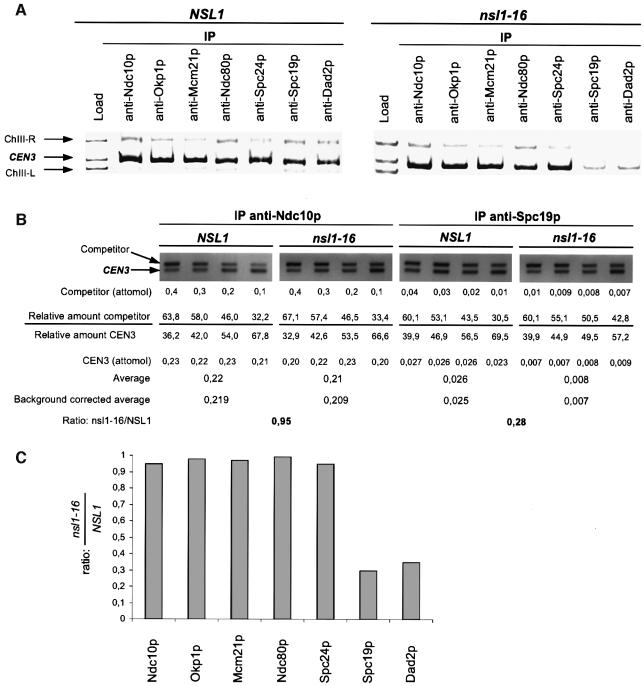
We wanted to determine how kinetochore structure is affected when cells try to establish bipolarity with compromised Nsl1p. Therefore we investigated the localization of proteins representing the known kinetochore protein complexes in nsl1-16 (Figure 5A–C) and Nsl1p-depleted cells (see below) 180 min after release from G1 arrest (when cells had completed monopolar segregation, but before cytokinesis) by ChIP in combination with competitive PCR. This revealed that nsl1-16 kinetochores, after attempting to achieve bipolarity, contained Ndc10p (CBF3 complex), Ndc80p and Spc24p (both Ndc80 complex) as well as Okp1p and Mcm21p (both Okp1p complex) in amounts indistinguishable from that of wild-type kinetochores. This suggests that the nsl1-16 defect does not result in a major breakdown of the kinetochore (or known sub-complexes). In contrast, the amounts of Spc19p and Dad2p (both Dam–Duo complex) were severely reduced (∼30% of wild type; Figure 5A–C). Both proteins are subunits of the Dam–Duo complex and, as mentioned before, compromising this complex results in monopolar segregation of sister chromatids (Cheeseman et al., 2001a; Janke et al., 2002; Li et al., 2002) as is observed for nsl1-16 cells. Thus the failure of nsl1-16 cells to dock the Dam–Duo complex onto the kinetochore may result in a failure of these cells to establish bipolarity. An alternative interpretation for the reduction of Dam–Duo proteins in nsl1-16 cells is discussed later.
Fig. 5. Kinetochore localization of Spc19p and Dad2p is compromised in nsl1-16 cells. Alpha factor synchronized cells were released into 37°C medium and analyzed after 180 min. (A) ChIP analysis. Kinetochore proteins were immunoprecipitated with the indicated antibodies. The presence of CEN3 DNA and two flanking DNA regions (ChIII-R and ChIII-L) was analyzed in the load and in the immunoprecipitates (IP) by Triplex-PCR. (B) Relative levels of Ndc10p and Spc19p at wild-type and nsl1-16 kinetochores. CEN3 DNA as obtained by ChIP (A) was quantified by competitive PCR as described in Materials and methods. Shortly, the relative amount of CEN3 and competitor was determined for four different PCR reactions with the indicated amounts of competitor. This was used to calculate and average the amount of CEN3 present in the IPs. Unspecific CEN3 precipitation (after ChIP with unspecific IgG) was quantified in the same way (not shown) and subtracted from the averaged data. As the final result, the ratio of precipitated CEN3 from nsl1-16 and wild-type cells was calculated. (C) Chart showing the relative levels of selected kinetochore proteins at nsl1-16 kinetochores normalized to wild-type kinetochores. Quantification was performed as demonstrated for Ndc10p and Spc19p (B). The data represents the average of three independent experiments (ChIP and quantification). The standard deviation calculated from these experiments was maximal 0.1.
Nsl1p is required for the integrity of kinetochores that interact with microtubules
As mentioned before, Nsl1p depletion resulted in a severe reduction of Nsl1p at the kinetochore (15% of wild type) as determined by ChIP and competitive PCR (Figure 6A and B). Despite this strong reduction, the levels of Ndc10p (CBF3 complex) and Ndc80p at kinetochores were not affected (Figure 6A and B). Surprisingly also Nnf1p, a component of the Mtw1 complex, was not affected (Figure 6B). This indicates that Nnf1p can localize to the kinetochore independently of Nsl1p. However it is presently unclear whether Nnf1p, Mtw1p and Dsn1p form a kinetochore-localized complex in the absence of Nsl1p. As had been observed for nsl1-16 cells, Dad2p and Spc19p (Dam–Duo complex) localization was severely diminished (30% of wild type). Furthermore, localization of Mcm21p, Okp1p (both Okp1 complex) and Spc24p (Ndc80 complex) were reduced to 28, 26 and 50% of the wild-type level respectively. Thus Nsl1p depletion not only interfered with the localization of the Dam–Duo complex, as was observed for nsl1-16, but also caused a more general kinetochore defect. To investigate whether this defect correlated with the establishment of microtubule–kinetochore interaction and possibly tension, we repeated the experiment with the exception that Nsl1p-depleted cells were released from alpha factor arrest into medium containing nocodazole. As expected, kinetochore localization of Nsl1p in Nsl1p-depleted cells was indistinguishable in the presence or absence of nocodazole (Figure 6B). Interestingly, Nsl1p-depleted cells and wild-type cells exhibited no difference in the kinetochore localization of all other analyzed proteins if microtubules had been depolymerized before the establishment of bipolarity (Figure 6B). This effect was not due to the nocodazole treatment per se since wild-type levels of all kinetochore proteins tested except those of Dad2p and Spc19p (see also Cheeseman et al., 2001a; Janke et al., 2002; Li et al., 2002) were not affected by nocodazole treatment (Figure 6C). Thus, only when kinetochore–microtubule interaction (and presumably tension) was permitted, kinetochore integrity was compromised in Nsl1p-depleted cells. This is in agreement with the assumption that Nsl1p function is not required before the establishment of bipolar kinetochore–spindle attachment.
Kinetochore localization of Nsl1p depends on Ndc10p and Okp1p
To investigate which kinetochore proteins are required for the localization of Nsl1p, we analyzed Nsl1p localization in kinetochore mutants by ChIP and competitive PCR. After incubation at 37°C for 3 h ndc10-1 cells, okp1-5 and okp1-52 cells exhibited 5, 40 and 8% Nsl1p respectively at the kinetochore in comparison to wild-type cells incubated at 37°C (Figure 6D and E). In contrast the kinetochore localization of Nsl1p was not altered in Δctf19 Δmcm21 and ndc80-1 cells. Thus kinetochore localization of Nsl1p depends on Okp1p and Ndc10p.
Dsn1p-depletion causes monopolar segregation of sister chromatids
Nsl1p is part of a protein complex that also consists of Mtw1p, Nnf1p and Dsn1p (see above). Mutant phenotypes of Mtw1p and Nnf1p have been described (Goshima and Yanagida, 2000; Euskirchen, 2002) and conform to the possibility that these proteins, like Nsl1p, are required to establish bipolarity. We therefore asked whether this also holds for Dsn1p. Indeed, when YST506 cells (GAL1-Ubiquitin-R-DSN1) were depleted of Dsn1p, a high percentage of cells (87%) exhibited monopolar segregation of sister chromatids. Thus it is possible that all components of the Mtw1p complex are required to establish bipolarity.
Discussion
Kinetochore function of Nsl1p
Mtw1p, Nnf1p, Nsl1p and Dsn1p form a protein complex that localizes to the kinetochore. Nsl1p has been analyzed in detail. Nsl1p is required for the establishment of a bipolar spindle–kinetochore interaction. Several lines of evidence support this notion. Firstly, nsl1-16, nsl1-42 and Nsl1p-depleted cells failed to segregate sister chromatids to the opposite poles. Separation of spindle poles occurred with monopolar attached sister chromatids resulting in the segregation of both sister chromatids to one of the poles (pseudo anaphase). Secondly, the occurrence of this pseudo anaphase correlated with a severe drop in the viability of nsl1-16 cells. However nsl1-16 cells remained viable when the (monopolar) segregation of chromatids was inhibited by the microtubule-depolymerizing drug nocodazole. Thirdly, when sister chromatid separation was inhibited by Cdc20p depletion after evoking the nsl1-16 defect, a stable metaphase arrest with separated sister kinetochores (revealing bipolar attachment) could not be observed. Whereas Nsl1p is required to establish bipolar microtubule–kinetochore interaction it is dispensable for monopolar/syntelic interactions since Nsl1p-depleted cells still exhibit monopolar sister chromatid segregation.
In Nsl1p-depleted cells the maintenance of high Pds1p levels was clearly compromised when microtubules were depolymerized by nocodazole indicating that Nsl1p is required to maintain an active spindle checkpoint. Assuming that the checkpoint function of Nsl1p is executed while associated with the kinetochore, the observation that checkpoint activity was not completely abolished could be explained by the fact that Nsl1p was still detectable at kinetochores of Nsl1p-depleted cells (∼15% of wild type). Kinetochore proteins that are required for the spindle checkpoint have been described before. These include the components of CBF3 (Gardner et al., 2001) and Spc24p (Janke et al., 2001), a component of the Ndc80p complex. In the presence of nocodazole the kinetochore localization of Ndc10p, Ndc80p and Spc24p was not affected after Nsl1p depletion. Since Nsl1p-depleted cells are compromised in recognizing unattached kinetochores in nocodazole-treated cells, the spindle checkpoint role of Nsl1p most likely goes beyond the localization of Spc24p and Ndc80p.
The Dam–Duo complex is thought to move to the kinetochore via microtubules (Li et al., 2002). Nsl1p is required for the microtubule-dependent kinetochore localization of the Dam–Duo complex components Dad2p and Spc19p. The severely diminished kinetochore localization of Dad2p and Spc19p was the only observed defect in kinetochore structure in nsl1-16 cells. Since defects in Dam–Duo complex components also result in monopolar segregation of sister chromatids (Cheeseman et al., 2001b; Janke et al., 2002; Li et al., 2002), the failure of nsl1-16 cells to establish bipolar kinetochore–spindle interaction could be due to a failure of the Dam–Duo complex to dock onto nsl1-16 kinetochores. Alternatively the failure to establish bipolarity in cells with compromised Nsl1p might occur independently of the Dam–Duo complex. In this case the reduced levels of Dad2p and Spc19p at Nsl1p-compromised kinetochores could be due to a reduction of microtubule-attached kinetochores, since Dam–Duo complex localization at the kinetochore is microtubule dependent (Li et al., 2002). However, we consider this latter possibility unsatisfactory in explaining the severity of the Dad2p and Spc19p reduction at Nsl1p-compromised kinetochores. Monopolar segregation requires that half or more (if syntelic attachments occur) of the kinetochores are attached to microtubules. In this case Dad2p and Spc19p levels should maximally be reduced to 50% of the wild-type situation. Possibly their levels would be even higher since kinetochores that were not attached to microtubules still contained 30% Dad2p and Spc19p in comparison to attached kinetochores. However the amount of Dad2p and Spc19p that was detected at Nsl1p-compromised kinetochores was considerably below the 50% value and resembled the amount (30% of wild type) found at non-attached wild-type kinetochores. Thus the reduction in microtubule-attached kinetochores is not in agreement with the reduction in kinetochore-localized Dad2p and Spc19p.
When microtubules are present, Nsl1p is required for the stable kinetochore localization of Okp1p, Mcm21p and Spc24p. However Nsl1p is not required for the localization of these proteins when microtubules have been depolymerized. We therefore speculate that Nsl1p maintains stable kinetochore localization of kinetochore components when tension is applied to the kinetochore.
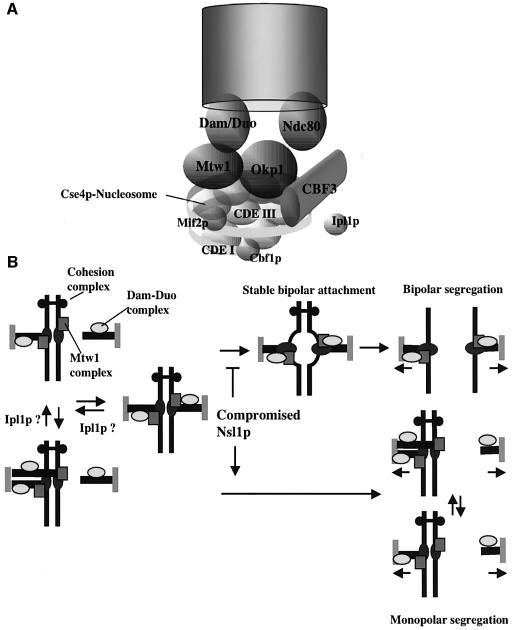
Kinetochore localization of Nsl1p depends on Ndc10p, as is the case for all known kinetochore components. Moreover it requires Okp1p. Thus the binding of Nsl1p and Okp1p at the kinetochore appears to be cooperative. Summarizing the localization data in a working model (Figure 7A) we therefore include the Okp1p complex and the Mtw1 complex at the same hierarchy level.
Fig. 7. (A) Model of the S.cerevisiae kinetochore that is based on a model described (Cleveland et al., 2003). (B) Schematic drawing focusing on the role of Nsl1p during the establishment of bipolarity. Nsl1p-compromised kinetochores fail to dock the Dam–Duo complex onto the kinetochore. Consequently these kinetochores fail to establish a bipolar spindle attachment. As a result sister chromatids that are linked by the cohesion complex cannot oppose spindle elongation. Consequently monopolar segregation of unseparated sister chromatids occurs. For further description see text.
Establishment of bipolarity
Defects in the Ipl1p protein kinase are thought to cause monopolar segregation of sister chromatids as a result of a failure to resolve syntelic attachments of sister kinetochores (Biggins and Murray, 2001; Tanaka et al., 2002). Since Dsn1p contains a consensus sequence for Ipl1p phosphorylation (Cheeseman et al., 2002a) at amino acid 62–65 it appeared reasonable to assume that the Mtw1 complex is a target for Ipl1p thus explaining the nsl1-16 phenotype. An experiment with unreplicated chromosomes however showed that this was not the case. In Δcdc6 wild-type cells as well as nsl1-16 and nsl1-42 cells, the single chromatids are attached to the old and new SPBs with equal probability. In contrast, in ipl1-321 cells they are attached preferentially to the old SPB. Therefore, if the ipl1-321 defect reflects interference with microtubule–kinetochore untethering then the nsl1-42 and nsl1-16 defects do not. The formation of functionally asymmetric kinetochores would have been another way to explain monopolar segregation in Nsl1p-compromised cells. This possibility could be excluded because when both sister kinetochores harbored compromised Nsl1p the cells still performed monopolar segregation. Consequently, the monopolar segregation of sister kinetochores in nsl1 mutants requires a different explanation.
Our model that outlines the monopolar sister chromatid segregation as a consequence of defective Nsl1p (Figure 7B) is based on published models (Cheeseman et al., 2002a; Tanaka et al., 2002). The establishment of bipolarity may involve a reversible and an irreversible step. During the reversible step, the Mtw1 complex facilitates the attachment of the Dam–Duo complex onto the kinetochore. In the irreversible step, after the establishment of bipolarity and tension, changes in kinetochore structure and/or modification occur and thus the kinetochore localization of the Dam–Duo complex and the kinetochore–microtubule interactions become persistent. A change in kinetochore structure may be supported by the observation that the nsl1-16 defect could not be evoked after the establishment of bipolarity. Compromised Nsl1p prevents the establishment of a stable Dam–Duo complex interaction with the kinetochore and this prevents the establishment of bipolarity. In wild-type cells spindle elongation is prevented due to the existence of bipolar attached sister chromatids as long as sister chromatid cohesion is maintained. If all sister kinetochores fail to establish a bipolar spindle attachment, spindle elongation and pole separation (anaphase B) can occur with sister chromatids still held together by cohesins. This results in monopolar segregation of sister chromatids.
Human homologues
Using a block search tool, hMis12 was identified as the human orthologue of Mtw1p (Goshima et al., 2003). Applying a similar search strategy no mammalian homologues of Nnf1p, Dsn1p and Nsl1p could be found if homologies within coiled-coil regions were excluded.
Materials and methods
Analysis of conditional lethal nsl1 cells
Temperature-sensitive mutants were incubated at 23°C for 3 h with alpha factor (200 ng/ml) to arrest cells in the G1 phase of the cell cycle. Alpha factor was removed by washing the cells twice with pre-warmed (37°C) YPD medium (t = 0). For the shift to galactose medium the cells were washed twice with galactose medium. To deplete Nsl1p in G1-arrested cells, a culture grown in galactose medium was shifted to glucose medium containing 200 ng/ml alpha factor and incubated for 5 h at 30°C. Alpha factor was removed by washing the cells twice with YPD medium (t = 0). When indicated, nocodazole was added at 15 µg/ml for all mutants.
ChIP and competitive PCR
ChIPs of yeast cells were performed as described (Hecht and Grunstein, 1999). Competitive PCR of the load and the immunoprecipitates was performed as described (Siebert and Larrick, 1993). For detailed information see Supplementary data.
Strains, plasmids, microscopy, MALDI-TOF and FACS
See Supplementary data.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Barbara Lang and Petra Ihrig for excellent technical assistance, Mansoureh Tabatabaei Far for help with experiments that involved the analysis of kinetochore function after Nsl1p depletion and Sue Biggins for providing the ipl1-321 strain. This work was supported by a grant from the Deutsche Forschungsgemeinschaft.
References
- Althoefer H., Schleiffer,A., Wassmann,K., Nordheim,A. and Ammerer,G. (1995) Mcm1 is required to coordinate G2-specific transcription in Saccharomyces cerevisiae. Mol. Cell. Biol., 15, 5917–5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S. and Murray,A.W. (2001) The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev., 15, 3118–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I.M. et al. (2001a) Implication of a novel multiprotein Dam1p complex in outer kinetochore function. J. Cell Biol., 155, 1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I.M., Enquist-Newman,M., Muller-Reichert,T., Drubin,D.G. and Barnes,G. (2001b) Mitotic spindle integrity and kinetochore function linked by the Duo1p/Dam1p complex. J. Cell Biol., 152, 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I.M., Anderson,S., Jwa,M., Green,E.M., Kang,J., Yates,J.R.,3rd, Chan,C.S., Drubin,D.G. and Barnes,G. (2002a) Phospho-regulation of kinetochore–microtubule attachments by the Aurora kinase Ipl1p. Cell, 111, 163–172. [DOI] [PubMed] [Google Scholar]
- Cheeseman I.M., Drubin,D.G. and Barnes,G. (2002b) Simple centromere, complex kinetochore: linking spindle microtubules and centromeric DNA in budding yeast. J. Cell Biol., 157, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D.W., Mao,Y. and Sullivan,K.F. (2003) Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell, 112, 407–421. [DOI] [PubMed] [Google Scholar]
- Connelly C. and Hieter,P. (1996) Budding yeast SKP1 encodes an evolutionarily conserved kinetochore protein required for cell cycle progression. Cell, 86, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelin C.W., Kaplan,K.B. and Sorger,P.K. (1997) Probing the architecture of a simple kinetochore using DNA–protein crosslinking. J. Cell Biol., 139, 1383–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euskirchen G.M. (2002) Nnf1p, Dsn1p, Mtw1p and Nsl1p: a new group of proteins important for chromosome segregation in Saccharomyces cerevisiae. Eukaryot. Cell, 1, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R.D., Poddar,A., Yellman,C., Tavormina,P.A., Monteagudo,M.C. and Burke,D.J. (2001) The spindle checkpoint of the yeast Saccharomyces cerevisiae requires kinetochore function and maps to the CBF3 domain. Genetics, 157, 1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh P.Y. and Kilmartin,J.V. (1993) NDC10: a gene involved in chromosome segregation in Saccharomyces cerevisiae. J. Cell Biol., 121, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G. and Yanagida,M. (2000) Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell, 100, 619–633. [DOI] [PubMed] [Google Scholar]
- Goshima G., Kiyomitsu,T., Yoda,K. and Yanagida,M. (2003) Human centromere chromatin protein hMis12, essential for equal segregation, is independent of CENP-A loading pathway. J. Cell Biol., 160, 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Asthana,S. and Sorger,P.K. (2000) Transient sister chromatid separation and elastic deformation of chromosomes during mitosis in budding yeast. Cell, 101, 763–775. [DOI] [PubMed] [Google Scholar]
- He X., Rines,D.R., Espelin,C.W. and Sorger,P.K. (2001) Molecular analysis of kinetochore–microtubule attachment in budding yeast. Cell, 106, 195–206. [DOI] [PubMed] [Google Scholar]
- Hecht A. and Grunstein,M. (1999) Mapping DNA interaction sites of chromosomal proteins using immunoprecipitation and polymerase chain reaction. Methods Enzymol., 304, 399–414. [DOI] [PubMed] [Google Scholar]
- Janke C., Ortiz,J., Lechner,J., Shevchenko,A., Magiera,M.M., Schramm,C. and Schiebel,E. (2001) The budding yeast proteins Spc24p and Spc25p interact with Ndc80p and Nuf2p at the kinetochore and are important for kinetochore clustering and checkpoint control. EMBO J., 20, 777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C., Ortiz,J., Tanaka,T.U., Lechner,J. and and Schiebel,E. (2002) Four new subunits of the Dam1–Duo1 complex reveal novel functions in sister kinetochore biorientation. EMBO J., 21, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q.W., Fuchs,J. and Loidl,J. (2000) Centromere clustering is a major determinant of yeast interphase nuclear organization. J. Cell Sci., 113, 1903–1912. [DOI] [PubMed] [Google Scholar]
- Kitagawa K., Abdulle,R., Bansal,P.K., Cagney,G., Fields,S. and Hieter,P. (2003) Requirement of skp1–bub1 interaction for kinetochore-mediated activation of the spindle checkpoint. Mol. Cell, 11, 1201–1213. [DOI] [PubMed] [Google Scholar]
- Lechner J. and Carbon,J. (1991) A 240 kd multisubunit protein complex, CBF3, is a major component of the budding yeast centromere. Cell, 64, 717–725. [DOI] [PubMed] [Google Scholar]
- Li Y., Bachant,J., Alcasabas,A.A., Wang,Y., Qin,J. and Elledge,S.J. (2002) The mitotic spindle is required for loading of the DASH complex onto the kinetochore. Genes Dev., 16, 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measday V., Hailey,D.W., Pot,I., Givan,S.A., Hyland,K.M., Cagney,G., Fields,S., Davis,T.N. and Hieter,P. (2002) Ctf3p, the Mis6 budding yeast homolog, interacts with Mcm22p and Mcm16p at the yeast outer kinetochore. Genes Dev., 16, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meluh P.B., Yang,P., Glowczewski,L., Koshland,D. and Smith,M.M. (1998) Cse4p is a component of the core centromere of Saccharomyces cerevisiae. Cell, 94, 607–613. [DOI] [PubMed] [Google Scholar]
- Michaelis C., Ciosk,R. and Nasmyth,K. (1997) Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell, 91, 35–45. [DOI] [PubMed] [Google Scholar]
- Musacchio A. and Hardwick,K.G. (2002) The spindle checkpoint: structural insights into dynamic signalling. Nat. Rev. Mol. Cell Biol., 3, 731–741. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. (2002) Segregating sister genomes: the molecular biology of chromosome separation. Science, 297, 559–565. [DOI] [PubMed] [Google Scholar]
- Ortiz J., Stemmann,O., Rank,S. and Lechner,J. (1999) A putative protein complex consisting of Ctf19, Mcm21 and Okp1 represents a missing link in the budding yeast kinetochore. Genes Dev., 13, 1140–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G., Tanaka,T.U., Nasmyth,K. and Schiebel,E. (2001) Modes of spindle pole body inheritance and segregation of the Bfa1p–Bub2p checkpoint protein complex. EMBO J., 20, 6359–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert P.D. and Larrick,J.W. (1993) PCR MIMICS: competitive DNA fragments for use as internal standards in quantitative PCR. Biotechniques, 14, 244–249. [PubMed] [Google Scholar]
- Stemmann O. and Lechner,J. (1996) The Saccharomyces cerevisiae kinetochore contains a cyclin–CDK complexing homologue, as identified by in vitro reconstitution. EMBO J., 15, 3611–3620. [PMC free article] [PubMed] [Google Scholar]
- Stern B.M. and Murray,A.W. (2001) Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr. Biol., 11, 1462–1467. [DOI] [PubMed] [Google Scholar]
- Tanaka T.U., Rachidi,N., Janke,C., Pereira,G., Galova,M., Schiebel,E., Stark,M.J. and Nasmyth,K. (2002) Evidence that the Ipl1–Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore–spindle pole connections. Cell, 108, 317–329. [DOI] [PubMed] [Google Scholar]
- Uhlmann F., Wernic,D., Poupart,M.A., Koonin,E.V. and Nasmyth,K. (2000) Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell, 103, 375–386. [DOI] [PubMed] [Google Scholar]
- Wigge P.A. and Kilmartin,J.V. (2001) The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J. Cell Biol., 152, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Yao,J. and Joshi,H.C. (2002) Attachment and tension in the spindle assembly checkpoint. J. Cell Sci., 115, 3547–3555. [DOI] [PubMed] [Google Scholar]