Abstract
We describe a novel signaling mechanism mediated by the G-protein-coupled receptor (GPCR) angiotensin II (Ang II) type 2 receptor (AT2). Yeast two-hybrid studies and affinity column binding assay show that the isolated AT2 C-terminus binds to the transcription factor promyelocytic zinc finger protein (PLZF). Cellular studies employing confocal microscopy show that Ang II stimulation induces cytosolic PLZF to co-localize with AT2 at the plasma membrane, then drives AT2 and PLZF to internalize. PLZF slowly emerges in the nucleus whereas AT2 accumulates in the perinuclear region. Nuclear PLZF binds to a consensus sequence of the phosphatidylinositol-3 kinase p85α subunit (p85α PI3K) gene. AT2 enhances expression of p85α PI3K followed by enhanced p70S6 kinase, essential to protein synthesis. An inactive mutant of PLZF abolishes this effect. PLZF is expressed robustly in the heart in contrast to many other tissues. This cardiac selective pathway involving AT2, PLZF and p85α PI3K may explain the absence of a cardiac hypertrophic response in AT2 gene-deleted mice.
Keywords: angiotensin II/AT2 receptor/cardiac hypertrophy/PLZF/transcription factor
Introduction
Angiotensin II (Ang II) plays important roles in the development of cardiovascular diseases including hypertension, cardiac hypertrophy and ischemic heart disease. Two major subtypes (AT1 and AT2) of the Ang II receptor have been identified in mammals by subtype-specific receptor blockers and cloning of their respective cDNAs (Sasaki et al., 1991; Murphy et al., 1991; Kambayashi et al., 1993; Mukoyama et al., 1993). Both are G-protein-coupled receptors (GPCRs) with the structural features of seven-transmembrane domains (Rockman et al., 2002). AT1 and AT2 share very limited sequence homology (∼34% amino acid sequence identity) (Inagami et al., 1992). The pathophysiological roles and signaling mechanisms of AT2 are less clear and have little resemblance to AT1 (Matsubara, 1998; de Gasparo et al., 2000). Ang II interaction with AT2 does not elicit typical second messenger responses. There is no rapid desensitization or downregulation in response to Ang II. The expression of AT2 is developmentally regulated, with widespread distribution in fetal tissues and expression limited to a few tissues in the adult, but it increases in tissues undergoing remodeling such as cardiac hypertrophy and ischemic heart disease (Matsubara, 1998). Overexpression of AT2 in certain cell lines leads to apoptosis (Miura and Karnick, 2000). AT1 shows strong cell growth and proliferating activity, whereas AT2 often is growth inhibitory to various cultured cells via the activation of protein tyrosine or serine/threonine phosphatases such as mitogen-activated protein kinase phosphatase (MKP-1), or the protein tyrosine phosphatase SHP-1 (Lu et al., 1996; Bedecs et al., 1997; Horiuchi et al., 1997). However, recent in vivo and in vitro studies on vascular smooth muscle cells (VSMCs) also report observations that the AT2 stimulation results in growth promotion. The AT2-specific antagonist PD123319 prevents rat aortic remodeling and fibrosis involving collagen synthesis, and the partial AT2 agonist CGP24112 stimulates collagen synthesis in AT2-transfected VSMCs (Levy et al., 1996; Mifune et al., 2000). In the rat heart, Ang II-induced apoptosis is mediated by AT1 but not by AT2 (Diep et al., 2002).
The present study began with our finding that the AT2 gene-deleted mice lost the ability to develop cardiac hypertrophy in response to pressure overload or to chronic Ang II infusion, whereas wild-type animals developed hypertrophy (Senbonmatsu et al., 2000; Ichihara et al., 2001). These mice in their response to pressure overload also failed to upregulate p70s6K, a kinase essential to protein synthesis. These findings suggest that AT2 promotes cardiac hypertrophy. The present study investigates molecular aspects of AT2 signaling that may participate in the cardiac hypertrophic response.
Using the yeast two-hybrid system, we identified a binding protein that binds to the C-terminal peptide of AT2. Sequence analysis identified it as the transcription factor PLZF, which is highly expressed in the heart. Ang II stimulation provoked internalization of AT2 and PLZF and translocation of PLZF to the nucleus. Nuclear PLZF activates the p85α PI3K gene leading to subsequent activation of protein synthesis. The activation by the receptor of a transcription factor and subsequent activation of p85α PI3K gene expression is a novel GPCR signaling mechanism. This mechanism may contribute to cardiac hypertrophy elicited by pressure overload or chronic Ang II infusion.
Results
PLZF binds to the AT2 C-terminus
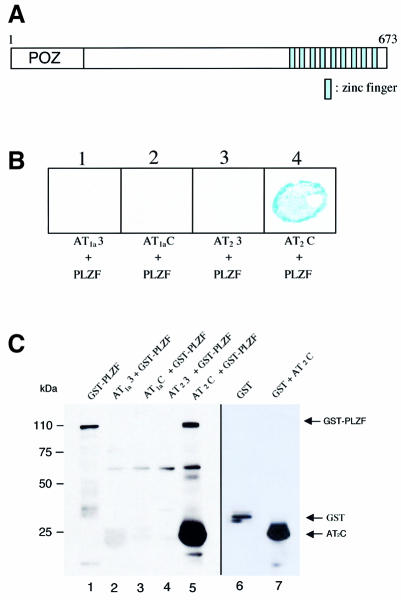
Using a cDNA fragment encoding the C-terminal intracellular domain of rat AT2 as a bait, we screened a human heart cDNA library. Sixteen positive clones were isolated by β-galactosidase assay as candidates. The clones were found to be various fragments of a single protein. To confirm that the isolated clones encode a specific AT2-binding protein, recapitulation of binding and the absence of interaction with non-specific baits were examined. In seven out of 16 positive clones, sequence analysis of the AT2-binding protein identified it as PLZF belonging to the POZ/zinc finger family with a 74 kDa molecular mass containing 673 amino acid residues (Figure 1A) (Chen et al., 1993). It has nine C2H2 zinc finger domains, which bind directly to DNA. At the N-terminus, this protein contains a BTB/POZ domain that mediates homodimerization and transcriptional repression through recruitment of nuclear co-repressors (Reid et al., 1995; Chang et al., 1996). To confirm the specific binding of AT2 and PLZF, additional yeast two-hybrid assays and in vitro affinity column binding assays were performed. Only the AT2 C-terminus bound to PLZF (Figure 1B and C).
Fig. 1. The association of the C-terminal intracellular domain of the AT2 receptor with PLZF. (A) Schematic representation of the PLZF domain structure. (B) Testing the interaction of PLZF and Ang II receptor peptides in the yeast two-hybrid system. Lane 1, AT1a third intracellular loop + PLZF; lane 2, AT1a C-terminus + PLZF; lane 3, AT2 third intracellular loop + PLZF; lane 4, AT2 C-terminus + PLZF. Binding was visualized by the β-galactosidase assay. (C) Interaction of the GST–PLZF and Ang II receptor peptides tested by affinity columns. Lane 1, recombinant GST–PLZF; lane 2, GST–PLZF + His6-tagged AT1a third intracellular loop; lane 3, GST–PLZF + His6-tagged AT1a C-terminus; lane 4, GST–PLZF + His6-tagged AT2 third intracellular loop; lane 5, GST–PLZF + His6-tagged AT2 C-terminus; lane 6, recombinant GST; lane 7, GST + His6-tagged AT2 C-terminus. Each band was visualized by western blot analysis using anti-GST and AT2 C-terminus antibodies.
Localization of PLZF
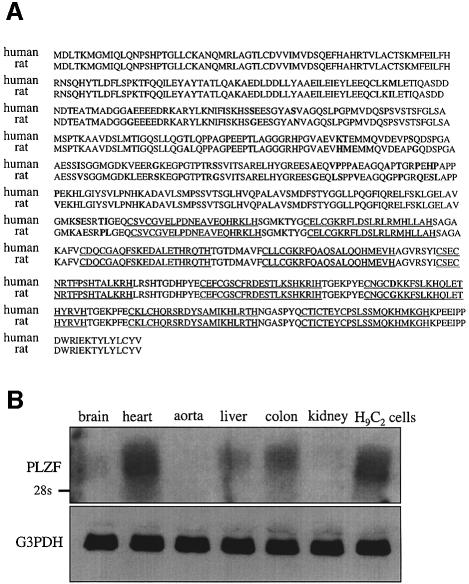
Using human PLZF cDNA as probe, we screened a rat heart cDNA library and cloned rat PLZF cDNA and determined its nucleotide sequence. The rat PLZF amino acid sequence was 96% identical to the human counterpart (Figure 2A). To identify tissues expressing PLZF in an adult rat, northern blotting was performed using the rat PLZF cDNA as a probe. A high level expression of PLZF was observed only in heart, with a low level in liver and colon, and none detectable in brain, aorta and kidney. The immortalized rat cardiomyocyte cell line H9C2 expressed PLZF at a detectable level (Figure 2B). Previous studies of adult mouse tissues reported high PLZF expression in the heart and its absence or low expression in other tissues (Cook et al., 1995).
Fig. 2. Rat PLZF.(A) Amino acid sequences of human and rat PLZF. Bold characters indicate amino acid residues different between human and rat. Amino acid sequence identity between human and rat is 96%. Underlining indicates a zinc finger domain. (B) Localization of rat PLZF. Northern blotting was performed using the rat PLZF cDNA as a probe. A 20 µg aliquot of total RNA from various tissues was used.
AT2 internalization and translocation to the nucleus of PLZF by Ang II stimulation
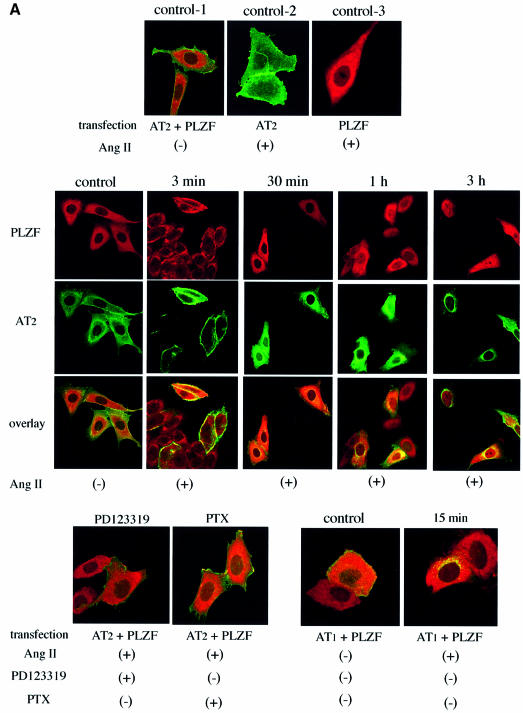
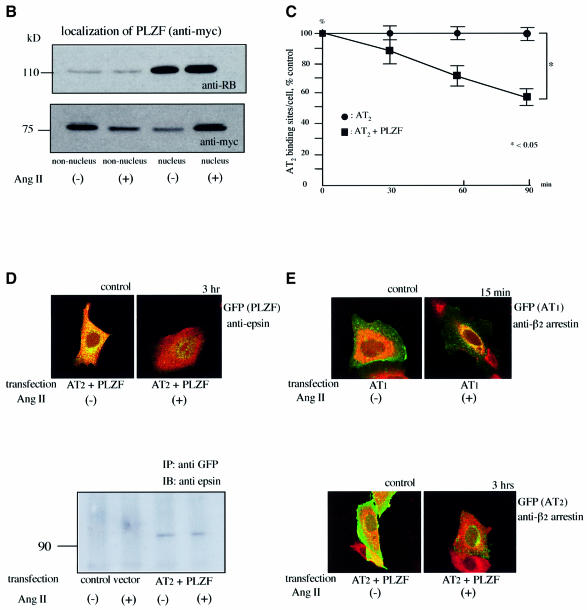
We performed immunohistochemical analysis of AT2 and PLZF localization. After the dual transfection of enhanced green fluorescent protein (EGFP)-tagged AT2 and myc-tagged PLZF to CHO-K1 cells, which did not express either AT2 or PLZF endogenously, Ang II stimulation induced co-localization of PLZF with AT2 at the plasma membrane at 3 min and then translocation of PLZF to the nucleus from 1 up to 3 h after Ang II stimulation (Figure 3A). Pre-treatment with the AT2-specific antagonist PD123319 or the Gi/o inhibitor pertussis toxin (PTX) blocked the nuclear translocation of PLZF following Ang II stimulation. In control experiments, Ang II failed to induce the nuclear translocation of PLZF in cells transfected with myc-tagged PLZF alone or myc-tagged PLZF and EGFP-tagged AT1a. After a single transfection of EGFP-tagged PLZF to early stage R3T3 cells, which expressed AT2 endogenously, Ang II stimulation also induced translocation of PLZF to the nucleus (data not shown). A western blotting was performed using the cytosol and nuclear extracts from early stage R3T3 cells transfected with myc-tagged PLZF. PLZF localized in the cytosol without stimulation. Upon Ang II stimulation, the cytosolic PLZF level decreased and its nuclear level was increased (Figure 3B).
Fig. 3. (A) Translocation of AT2 and PLZF by Ang II stimulation in CHO-K1 cells transfected with AT2 and PLZF. Green fluorescence (EGFP) indicates AT2 or AT1, red fluorescence (rhodamine Red-X) indicates anti-myc (PLZF). CHO-K1 cells were transfected and treated as indicated. Control means without Ang II treatment, and indicated time refers to the duration of Ang II exposure. The green and red channels were used separately for the first row and the second row to visualize AT2 and PLZF separately in the same cells. The third row is the overlay. (B) The nuclear localization of PLZF (anti-myc) in early stage R3T3 cells transfected with PLZF and stimulated with Ang II for 60 min. RB is used as the nuclear marker. (C) Percentage decrease of AT2-binding sites on the cell surface using [125I]Ang II in early stage R3T3 cells transfected with PLZF. Indicated time refers to the duration of Ang II exposure. Data are representative of three independent experiments with nearly identical results. (D) PLZF is associated with epsin 1 before Ang II stimulation. CHO-K1 cells were transfected with AT2 and PLZF. Green fluorescence (EGFP) indicates PLZF, red fluorescence (rhodamine Red-X) indicates anti-epsin. Control is without Ang II treatment, and indicated time refers to the duration of Ang II exposure. Immunoprecipitation was performed with anti-GFP and then western blot was performed with anti-epsin. (E) AT2–PLZF complex did not involve β2-arrestin. CHO-K1 cells were transfected with AT1 alone, or AT2 and PLZF. Green fluorescence (EGFP) indicates AT1 or AT2, red fluorescence (rhodamine Red-X) indicates anti-β2-arrestin. Control is without Ang II treatment, and indicated time refers to the duration of Ang II exposure.
Concomitantly, Ang II stimulation induced internalization and translocation of AT2 from the plasma membrane to the perinuclear region but not into the nucleus (Figure 3A). Internalization of AT2 was observed from 1 to 3 h after Ang II stimulation. Ang II-dependent internalization of AT2 was not observed in the cells lacking PLZF expression. The AT2-specific antagonist PD123319 or the Gi/o inhibitor PTX inhibited the internalization of AT2 (Figure 3A). A single transfection with EGFP-tagged PLZF to early stage R3T3 cells, which expressed AT2 endogenously, also showed internalization of AT2 following Ang II stimulation when examined by histochemical analysis with anti-AT2 antibody (data not shown). There was also a marked decrease in cell surface AT2-binding sites as determined using [125I]Ang II in comparison with early stage R3T3 cells not transfected with PLZF (Figure 3C) (Dudley and Summerfelt, 1993). This was consistent with the observed AT2 internalization.
Recently, Hyman et al. (2000) reported that PLZF is bound to epsin 1 via the epsin N-terminal homology domain (ENTH domain). Epsin 1 is a cytosolic protein involved in clathrin-mediated endocytosis via its direct interaction with clathrin. We performed double staining and western blotting using pairs of antibodies against EGFP and epsin 1 in CHO-K1 cells dually transfected with EGFP-tagged PLZF and AT2. We clearly observed by merged fluorescence that PLZF associated with epsin 1 in the cytosol before Ang II stimulation. Ang II stimulation induced translocation of PLZF and epsin 1 to the nucleus without merged fluorescence (Figure 3D). This translocation did not include β-arrestin, a protein that mediates internalization of AT1 (Ferguson, 2001) (Figure 3E).
Inactivation of PLZF
After dual transfection with EGFP-tagged AT2 and myc-tagged PLZF to CHO-K1 cells, immunoblot analysis indicated that Ang II stimulation induced tyrosine phosphorylation of PLZF that peaks at 5 min (Figure 4A). Mutation of PLZF by changing Tyr669 (Y669F) abolished tyrosine phosphorylation of PLZF (Figure 4A), co-localizing of PLZF with AT2 and translocation of PLZF to the nucleus (Figure 4B). The tyrosine kinase-specific inhibitor genistein also inhibited nuclear translocation of PLZF (Figure 4B). All other tyrosine mutants of PLZF translocated to the nucleus following Ang II stimulation (data not shown).
Fig. 4. (A) Tyrosine phosphorylation of PLZF and Y669F PLZF at various times after Ang II stimulation in CHO-K1 cells. (B) Y669F PLZF lost the ability to co-localize with AT2 and translocate to the nucleus. Green fluorescence (EGFP) indicates AT2, red fluorescence (rhodamine Red-X) indicates anti-myc (PLZF or Y669F PLZF). CHO-K1 cells were transfected and treated as indicated. Indicated time refers to the duration of Ang II exposure. (C) EMSA using nuclear extracts of rat cardiomyocyte-derived H9C2 cells that express PLZF. The probes are: W, [α-32P]dATP-labeled wild-type probe; m1 or m2, [α-32P]dATP-labeled mutant probes. Cold 10, etc is the excess of cold wild-type or mutant probe added. Nuclear extracts of Cos7 cells that do not express PLZF were used as control. Data are representative of three sets of independent experiments with nearly identical results. (D) Supershift using H9C2 cell nuclear extracts and anti-PLZF antibodies. Lane 1, [α-32P]dATP-labeled wild-type probe; lane 2, [α-32P]dATP-labeled wild type probe + 200-fold excess of cold wild-type probe; lane 3, [α-32P]dATP-labeled wild-type probe + 3 µl of anti-PLZF antibody. Data are representative of three independent experiments with nearly identical results. (E) Luciferase assay using Cos7 cells transfected with AT2 and PLZF permanently. The filled square is a PLZF-binding element. Lane 1, 1.6 kbp of p85αPI3K promoter region that contains the PLZF-binding element; lane 2, 0.8 kbp of p85αPI3K promoter region that contains the PLZF-binding element; lane 3, 0.6 kbp of p85αPI3K promoter region that does not contains the PLZF-binding element; lane 4, 1.6 kbp of p85αPI3K promoter region that contains a mutated PLZF-binding element (T→G: mutant 1); lane 5, 1.6 kbp of p85αPI3K promoter region that contains a mutated PLZF-binding element (T→C: mutant 2). Data are representative of five independent experiments with nearly identical results.
Zinc finger domain of PLZF binds to the upstream flanking region of the p85α PI3K gene
According to Li et al. (1997), a core consensus sequence for the specific binding of the PLZF zinc finger domain is A(T/G)(G/C)T(A/C)(A/C)AGT. p85α PI3K is a regulatory subunit of several isoforms of PI3K (Vanhaesebroeck et al., 1997). An ‘ATGTACTAGTGT’ sequence was found in the upstream flanking region of the p85α PI3K gene. We performed an electrophoretic mobility shift assay (EMSA) using the labeled probe ‘ATGTACTAGTGT’ and nuclear extract of H9C2 cells that expressed PLZF endogenously. The probe showed a positive band that was abolished by the cold probe, but neither of the mutant probes ‘ATGGACTAGTGT’ (m1) or ‘ATGCACTAGTGT’ (m2) was bound or competed with the wild-type probe (Figure 4C). These results suggested that the upstream flanking region of the p85α PI3K gene might be the target of PLZF. We also performed a supershift assay using the nuclear extract from H9C2 cells and anti-PLZF antibody. The ‘ATGTACTAGTGT’ probe bound to PLZF and anti-PLZF antibody produced a supershifted band (Figure 4D). To investigate further whether binding of PLZF to p85α PI3K activates p85α PI3K expression, we performed a luciferase-based receptor assay using the promoter region of p85α PI3K that fuses to the phRL-null vector. As shown in Figure 4E, the activity of p85α PI3K long 1 and 2 that contain ‘ATGTACTAGTGT’ was ∼3-fold greater than p85α PI3K short that does not contain it. Neither mutant 1 nor 2 was activated (Figure 4E).
Increased expression of p85α PI3K requires both AT2 and PLZF
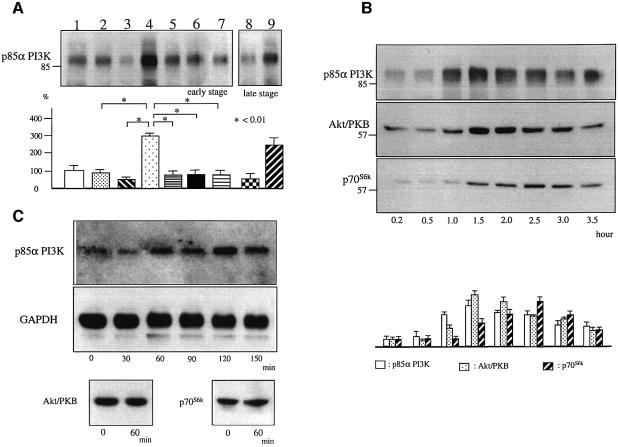
Myc-tagged PLZF was transfected into early stage R3T3 cells, which expressed endogenous AT2. The R3T3 cells employed did not express endogenous AT1 (Dudley and Summerfelt, 1993). The protein level of p85α PI3K significantly increased at 1 h following Ang II stimulation (Figure 5A, lane 4). However, it did not increase in early stage R3T3 cells transfected with Y669F PLZF (Figure 5A, lane 7). The AT2-specific antagonist PD123319 inhibited the increase of p85α PI3K (Figure 5A, lane 5). In late stage R3T3 cells, which no longer expressed AT2, the dual transfection with EGFP-tagged AT2 and myc-tagged PLZF also showed an increase in p85α PI3K (Figure 5A, lane 9). In contrast, the dual transfection with EGFP-tagged AT1a and myc-tagged PLZF (Figure 5A, lane 8) to the late stage R3T3 cells did not increase P85α PI3K.
Fig. 5. (A) Expression level of p85α PI3K in PLZF-transfected R3T3 cells 1 h after Ang II stimulation. Lanes 1–7, early stage R3T3 cells, which expressed AT2 endogenously. Lane 1, transfection with control vector; lane 2, transfection with control vector with Ang II stimulation; lane 3, transfection with PLZF; lane 4, transfection with PLZF with Ang II stimulation; lane 5, transfection with PLZF and treated with Ang II and PD123319; lane 6, transfection with PLZF and treated with Ang II and PTX; lane 7, transfection with Y669F PLZF with Ang II stimulation; lanes 8 and 9 employed late stage R3T3 cells, which no longer expressed AT2 and were transfected with AT1 and PLZF (lane 8) or AT2 and PLZF (lane 9) as indicated. Each band was visualized by western blot analysis using anti-p85αPI3K. Data are representative of three independent experiments with nearly identical results. ∗P < 0.01. (B) Protein level of p85α PI3K, Akt/PKB and p70S6k in PLZF-transfected R3T3 cells at various times after Ang II stimulation. Early stage R3T3 cells, which expressed AT2 endogenously, were transfected with PLZF in all samples. Each band was visualized by western blot analysis using specific antibodies. Data are representative of three independent experiments with nearly identical results. (C) mRNA of p85α PI3K in PLZF-transfected R3T3 cells at various times after Ang II stimulation. mRNA of Akt/PKB and p70S6k in PLZF-transfected R3T3 cells at 1 h of Ang II stimulation. Each band was visualized by northern blot analysis using a specific probe.
We followed the time course of the p85α PI3K protein level following Ang II stimulation. The increase in p85α PI3K was maximum from 1 to 1.5 h after Ang II stimulation. Furthermore, the downstream enzyme activities Akt/PKB and p70s6k were also increased in PLZF transfected cells. The time course of Akt/PKB and p70s6k expression was delayed from that of p85α PI3K. The increase in Akt/PKB peaked at 1.5 h after Ang II stimulation, and p70s6k reached a maximum at 2.5 h (Figure 5B). We also performed the time course of the p85α PI3K gene expression level following Ang II stimulation by northern blot analysis of mRNA. The p85α PI3K gene was increased from 1 h after Ang II stimulation (Figure 5C). However, neither the Akt/PKB nor the p70s6k mRNA level was increased at 1 h after Ang II stimulation.
Ang II treatment in vivo stimulates p85α PI3K in the mouse heart
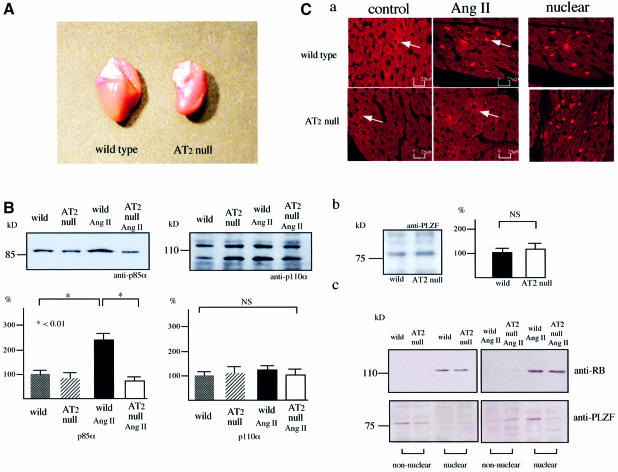
We reported that cardiac hypertrophy was not induced in the AT2-null mouse by pressure overload or chronic Ang II infusion, and this was probably due to the significantly lower expression of p70s6k (Figure 6A) (Senbonmatsu et al., 2000; Ichihara et al., 2001). In the present study, Ang II treatment in vivo provoked the nuclear translocation of PLZF in the wild-type mouse heart but not in the AT2-null mouse heart (Figure 6C). After Ang II treatment in vivo, the regulatory subunit p85α PI3K was stimulated in the wild-type mouse heart but not in the AT2-null mouse heart (Figure 6B). The catalytic p110 PI3K subunit remained unchanged in both groups. These observations suggest that the markedly attenuated hypertrophic response of the AT2-null mouse is due, in large part, to suppression of the AT2–PLZF–p85α PI3K pathways in mice lacking the AT2 receptor. No difference was observed in the expression of MAP kinases (extracellular signal-regulated kinase, p38 mitogen-activated kinase and Jun N-terminal kinase), calcineurin or calcineurin activity between AT2-null and wild-type mice (data not shown).
Fig. 6. (A) The heart of an AT2-null mouse treated with 4.2 ng/kg/min Ang II for 3 weeks. (B) p85α and p110 PI3K in the wild-type and AT2-null mouse heart. Data are representative of three independent experiments with nearly identical results. ∗P < 0.01. (C) PLZF expression in the Ang II-treated wild-type and AT2-null mouse heart. (a) Cellular localization of PLZF in the mouse heart. The nuclear PLZF is detected in the Ang II-treated-wild type mouse heart. (b) Total PLZF expression in the Ang II-treated wild-type and AT2-null mouse heart. Data are representative of three independent experiments with nearly identical results. (c) Cytosolic or nuclear localization of PLZF in the Ang II-treated wild-type and AT2-null mouse heart as indicated. Non-nuclear indicates cytosol. RB is used as the nuclear marker. Data are representative of three independent experiments with nearly identical results.
AT2 stimulates protein synthesis in cardiomyocytes
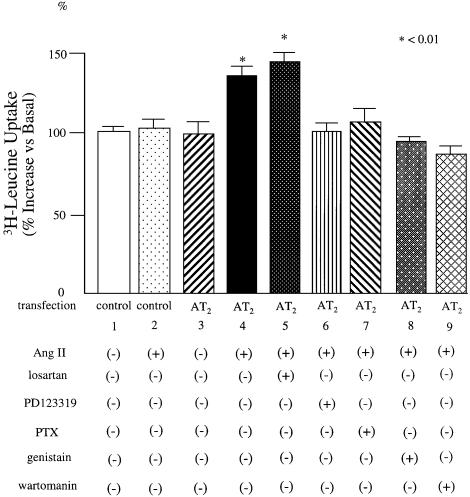
To ascertain that increased expression of p85α PI3K affects downstream protein synthesis, we measured the incorporation of [3H]leucine into H9C2 cells, which are derived from immortalized rat cardiomyocytes. H9C2 cells express PLZF but have lost AT2 expression. In H9C2 cells transfected with AT2 by retrovirus transfection, Ang II induced a 35% increase of [3H]leucine uptake in comparison with control (Figure 7). Upon pre-treatment with the AT1-specific blocker losartan, Ang II induced a 43% increase in [3H]leucine uptake compared with the control. PD123319, genistein and wortmannin inhibited the increase in [3H]leucine uptake.
Fig. 7. [3H]Leucine incorporation into AT2-transfected H9C2 cells. H9C2 cells were transfected with control vector or AT2 and stimulated with Ang II and treated with inhibitors as indicated. Data are representative of four independent experiments with nearly identical results. ∗P < 0.01.
Gi/o protein mediates AT2-mediated translocation of PLZF
On the basis of characteristic amino acid sequence, AT2 belongs to the family of serpentine receptors or GPCRs, which relay signals from extracellular stimuli to heterotrimeric G proteins. AT2 has been found to interact with Giα2 and Giα3 (Zhang and Pratt., 1996; Hansen et al., 2000). Pre-treatment of cells with 25 µM PTX inhibited the simultaneous internalization of AT2 and nuclear translocation of PLZF, and also blocked the increased expression of p85α PI3K following Ang II stimulation (Figures 3, 5 and 7). These results indicated that AT2 interaction with Gi is involved in its interaction with PLZF and in the nuclear translocation of PLZF.
Discussion
AT2–PLZF translocation
The present studies describe a novel signaling pathway of a GPCR. This pathway was discovered initially when yeast two-hybrid studies with the C-terminal intracellular domain of rat AT2 as bait indicated that a specific protein was bound to the AT2 C-terminus. Sequence analysis identified this protein as the transcription factor PLZF. Affinity binding assay further confirmed the specificity of this interaction.
Confocal microscopy studies define the initial element of the signaling pathway leading to translocation of PLZF to the nucleus. In the CHO cells dually transfected with AT2 and PLZF, confocal microscopy in unstimulated cells showed AT2 localized to the plasma membrane and PLZF in the cytosol. Following Ang II stimulation, within 3 min there is PLZF co-localized with AT2 in the plasma membrane. At 30 min, both are found in the cytosol. At 1 h, AT2 is densely perinuclear and a considerable amount of PLZF is in the nucleus. At 3 h, the PLZF appears to be entirely nuclear and AT2 appears to be strongly perinuclear. Measurement of cell surface binding sites indicates that upon Ang II stimulation, PLZF-transfected cells lose a large fraction of the surface AT2 receptor. The AT2 signaling mechanism involving PLZF and resulting in receptor internalization and direct nuclear translocation of the transcription factor has not been reported previously to the best of our knowledge (Pucell et al., 1991; Hunyady et al., 1994; Hein et al., 1997). Cells transfected with AT2 or PLZF only do not respond to Ang II in the fashion described above. Transfection with PLZF and AT1 instead of AT2 followed by Ang II stimulation results in rapid perinuclear localization of AT1 within 15 min.
Genistein, a tyrosine kinase inhibitor, prevents the AT2–PLZF co-localization and PLZF translocation to the nucleus. Ang II stimulation induced a PLZF tyrosine phosphorylation with a peak at 5 min following Ang II stimulation. Tyrosine groups of PLZF were mutated individually to phenylalanine and the cells were transfected with mutants. Only Y669F abolished the Ang II-induced tyrosine phosphorylation and the nuclear translocation of PLZF. We postulate that the tyrosine phosphorylation of PLZF is required for the translocation of PLZF to the plasma membrane to initiate the signaling pathway. AT2 has been shown to interact with Giα2 and Giα3. PD123319, an AT2 receptor blocker, prevents the AT2–PLZF co-localization and PLZF translocation to the nucleus. PTX, a Gi/o inhibitor, also prevents the response. However, it is not clear whether the Gi has an active role such as activating an unspecified tyrosine kinase to phosphorylate PLZF at Y669 as required for the nuclear translocation of PLZF.
The binding of isolated C-terminal sequence of AT2 to PLZF has not been established for the intact AT2 in these experiments. The possible binding of AT2 and PLZF does not withstand the detergent necessary for the co-immunoprecipitation. Detailed studies of the specific amino acids involved in the AT2 C-tail–PLZF interaction are underway in another study.
The cytosolic C-termini of various GPCRs have been shown previously to regulate receptor endocytosis and parameters of the endocytotic cycle that strongly modulate receptor function through phosphorylation and β-arrestin binding (Ferguson, 2001). Ang II rapidly induced translocation of AT1 to the perinuclear region in the absence of PLZF. This occurred in association with β-arrestin as reported in other studies (Ferguson, 2001). The much slower internalization of AT2 did not involve β-arrestin. Epsin 1 is a cytosolic protein previously reported to be bound to cytosolic PLZF (Hyman et al., 2000). It is associated in the cytosol in the present study before Ang II stimulation.
PI3K–p70s6k pathway
We found that in the nucleus, PLZF binds to the 5′-flanking region of the p85α PI3K gene. PLZF binding to the promoter region was active in a luciferase assay (Figure 4E). In R3T3 cells, both AT2 and functional PLZF were required for p85α PI3K gene activation. The inactive PLZF mutant (Y669F) abolished the gene activation (Figure 5A). PLZF has been found previously to act as a cell growth repressor due to the POZ repressor domain at the N-terminus (Shaknovich et al., 1998; Albagli et al., 1999). PLZF has an obligatory role in the regulation of limb and axial skeleton pattern and HOX gene expression (Barna et al., 2000). PLZF also activates gene expression, specifically binding to the thrombopoietin receptor and inducing its expression in megakaryocytes (Guerriero et al., 1995; Labbaye et al., 2002). These indicate that PLZF is a multifunctional transcription factor. The consensus DNA sequence for PLZF binding is present in the p85α PI3K regulatory subunit but is absent from p110 PI3K catalytic subunits. The PI3K regulatory subunit p85α plays a critical role in growth promotion. Enhanced PI3K activation led to an increase of p70s6k, which stimulates protein synthesis. Kang et al. (2002) reported that the p85α PI3K subunit activates gene transcription by nuclear factor of activated T cells (NFAT) independently of p85α binding to the p110 subunit of PI3K. NFAT-3 has been implicated in earlier studies as a potential activator of cardiac hypertrophy (Molkentin et al., 1998). Gonzalez-Garcia et al. (2002) also reported that the p85α PI3K may play an important role in p70s6k activation mediated by the formation of a ternary complex with p70s6k and FKBP 12–rapamycin-associated protein (FRAP). Akt/PKB, downstream of PI3K, inhibits glycogen synthase kinase 3β (GSK3β). Since GSK3β inhibits translation initiation factor, cyclin D1 and NFAT, the action of Akt/PKB upregulates their activity and contributes to the hypertrophic response (Takano et al., 2002).
PLZF and cardiac hypertrophy
PLZF is perhaps uniquely highly expressed in the heart (Cook et al., 1995). Earlier, we reported that cardiac hypertrophy was not induced in the AT2 gene-deleted mouse heart due to decreased p70s6k (Senbonmatsu et al., 2000), whereas it did induce cardiac hypertrophy in the AT1 gene-deleted mouse (Harada et al., 1998). In the present study, we found that the Ang II-treated AT2-gene deleted mouse heart shows significantly lower expression of the p85α PI3K regulatory subunit. In the model system using R3T3 cells, Ang II stimulation elevated p85α PI3K, and coordinately, at a later time in sequence downstream, Akt/PKB and p70s6k. The p85α PI3K promoter had a PLZF consensus sequence, but this is absent from the Akt/PKB or the p70s6k promoter as implied by the later onset of its enhanced expression. In the Ang II-treated AT2-null mouse hearts, neither cardiac hypertrophy nor nuclear translocation of PLZF occurred, whereas similarly treated wild-type mouse hearts showed the hypertrophy and clearly visible nuclear translocation of PLZF. These results indicate that nuclear translocation of PLZF is regulated specifically by AT2. Ang II stimulation increased [3H]leucine incorporation in the AT2-transfected cardiac myocyte-derived H9C2 cells. This increase was unaffected in cells pre-treated with the AT1 antagonist losartan. These observations are in agreement with the mechanism proposed by Shioi et al. (2000) who showed that the cross-sectional size of cardiomyocytes is determined by PI3K activity and its downstream kinases using heart-specific dominant-active and negative PI3K transgenic mice. Crackower et al. (2002) employing the tumor suppressor PTEN-deficient mice reported that PI3Kα mediates cardiomyocyte size increase whereas PI3Kγ, which does not interact with p85α, is a negative regulator of cardiac contractility. Multiple growth factors and downstream signaling pathways such as Ras and calcineurin are also implicated in the cardiac hypertrophic response (Clerk and Sugden, 2000; Frey et al., 2000; Molkentin and Dorn, 2001). The regulatory subunit p85α that is upregulated by AT2 depends for its activation on growth factor receptors such as those for FGF or IGF. p85α PI3K enhancement may facilitate the growth factor-mediated cardiac hypertrophic pathway.
Materials and methods
Yeast two-hybrid system
A cDNA fragment encoding amino acids 322–364 of the rat AT2 receptor was used as a bait for the yeast two-hybrid system. It was fused in-frame with the LexA DNA-binding domain. BamHI and PstI restriction enzyme sites were attached to each end of the AT2 C-terminus and inserted into the PBTM 116 yeast expression vector. The PBTM AT2 C-terminus was co-transformed into L40 yeast with the human heart cDNA library (BD Biosciences Clontech, Palo Alto, CA) that contained the GAL4 activation domain in the pACT2 vector. Positive clones were isolated by β-galactosidase assay. To examine whether isolated clones bind to AT2 specifically, recapitulation of the binding of AT2 and other baits (AT1a C-terminus, AT1a intracellular third intracellular loop, AT2 third intracellular loop and PBTM 116 control vector) and of the positive clones were performed.
In vitro binding assay
The His6-tagged AT1 third intracellular loop (1.2 nmol), His6-tagged AT1 C-terminus (1.2 nmol), His6-tagged AT2 third intracellular loop (1.2 nmol) and His6-tagged AT2 C-terminus (1.2 nmol) were immobilized on the Ni-NTA resin column (150 µl) (QIAGEN Santa Clarita, CA) to make affinity columns. GST–PLZF (1.2 nmol) was applied to each affinity column that is pre-equilibrated with 20 mM Tris–HCl pH 7.5, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol (DTT), 1 µg/ml leupeptin, 1 mM p-aminophenylmethanesulfonyl fluoride and 1% (w/v) NP-40 and followed by intensive washing. The proteins on the beads were eluted by incubating with 1× Laemmli’s buffer (50 µl). The eluate was subjected to SDS–PAGE followed by western blotting analysis using anti-GST (Amersham Pharmacia Biotech, Piscataway, NJ) and anti-AT2, which recognizes the C-terminus (Santa Cruz Biotechnology, Santa Cruz, CA).
Cloning of rat PLZF and northern blot analysis
We screened an adult rat heart cDNA library in the Uni-ZAP XR library (Stratagene, La Jolla, CA) with a human PLZF probe. The probe was labeled with [α-32P]dATP using a Prime-It II random priming kit (Stratagene). Northern blot analysis of rat PLZF was performed as previously reported (Thomas, 1980).
Cells
We used the cells shown in Table I as the model system.
Table I. Cells used as the model system.
| AT1 | AT2 | PLZF | |
|---|---|---|---|
| R3T3 cells | – | + | – |
| CHO cells | – | – | – |
| Cos7 cells | – | – | – |
| H9C2 cells | + | – | + |
Anti-AT2 antibody and immunofluorescent staining
A monoclonal anti-AT2 antibody was produced as described previously (Frei et al., 2001). Expression vectors for AT1, AT2 and PLZF were constructed in pEGFP-N2 (BD Biosciences Clontech) or pCDNA4 (Invitrogen, Carlsbad, CA) by standard methods. CHO-K1 or R3T3 cells were transfected with pCDNA4-PLZF and/or pEGFP-N2-AT2, pcDNA4-PLZF and pEGFP-N2-AT1. The cells were serum starved for 24 h, stimulated by 100 nM Ang II for various periods, fixed with 4.0% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min, permeabilized in ice-cold acetone for 5 min and then blocked with 1% bovine serum albumin (BSA) in PBS for 5 min. The cells were stained by incubation for 1 h at room temperature with antibody against c-myc, epsin 1, β2-arrestin (Santa Cruz Biotechnology) or AT2 at 1:50 dilution in 1% BSA in PBS. Then the cells were washed with PBS twice, and incubated for an additional 30 min at room temperature with rhodamine Red-X-conjugated anti-rabbit, goat or mouse antibodies (Jackson Immuno Research, West Grove, PA) at 1:100 dilution in 1% BSA in PBS. Images were acquired with a Zeiss LSM 410 confocal laser-scanning microscope under a 63× oil immersion lens (Carl Zeiss, Germany). For double labeling experiments, the 488 and 568 nm filters were used for the detection of EGFP and rhodamine Red-X respectively.
AT2 expression in R3T3 cells
To measure the density of the AT2-binding sites of R3T3cells or PLZF-transfected R3T3 cells, the radioligand receptor binding assay was performed using [125I]Ang II as described previously (Dudley and Summerfelt, 1993).
Transfection of PLZF and/or AT2 receptor to R3T3 or CHO-K1 cells, immunoprecipitation and western or northern blot analysis
Cells were transfected with pCDNA4-PLZF, pCDNA4-PLZF and pCDNA4-AT2, or pCDNA4-PLZF and pCDNA4-AT1 vectors. The cells were serum starved for 24 h and stimulated with 100 nM Ang II for various periods, washed with cold PBS three times and then lysed with 20 mM Tris–HCl pH 7.5, 150 mM NaCl, 2 mM EDTA, 1 mM sodium orthovanadate, 5 mM 2-mercaptoethanol, 50 mM NaF, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 1 mM p-aminophenylmethanesulfonyl fluoride and 1% (w/v) NP-40 buffer. Lysed R3T3 cells were collected and stirred for 1 h at 4°C followed by centrifugation at 15 000 g for 1 h at 4°C. The supernatant was collected (1 mg protein/ml) and 1 ml was stirred with 20 µl of protein A/G–Sepharose (Santa Cruz Biotechnology) overnight at 4°C. After removal of beads, 10 µg of anti-p85α PI3K, Akt/PKB, p70S6k or myc antibodies (New England Biolabs, Beverly, MA/Santa Cruz Biotechnology), and 20 µl of protein A/G–Sepharose were added to the supernatant and incubated overnight at 4°C. Protein A/G–Sepharose beads were washed intensively, and eluted by 1× Lammili’s buffer (50 µl). The eluted sample was subjected to SDS–PAGE followed by western blot analysis. The outer borders of the bands were traced and the areas and densities were determined with the NIH image system. Northern blot analysis of p85α PI3K, Akt/PKB or p70S6k was performed as previously reported (Thomas, 1980).
Nuclear fraction
Lysed R3T3 cells were homogenized in a Dounce homogenizer. The homogenized material was centrifuged at 1500 g for 10 min to sediment nuclei. The supernatant was resedimented at 15 000 g for 10 min and then its supernatant was termed the non-nuclear fraction. The nuclear pellet was washed with lysis buffer and then resuspended with lysis buffer containing 0.5 M NaCl to extract nuclear proteins. The extracted material was centrifuged at 15 000 g for 15 min and then its supernatant was termed the nuclear fraction. Using each sample, western blot analysis was performed using anti-myc, anti-RB antibody.
AT2 inhibition
Transfected cells were pre-treated with 10 µM PD123319 for 1 h at 37°C prior to stimulation by 100 mM Ang II. Alternatively, cells were pre-treated with 200 ng/ml PTX for 24 h at 37°C prior to stimulation by 100 mM Ang II.
Inhibition of tyrosine phosphorylation and PI3K
Transfected cells were pre-treated with 0.7 µg/ml genistein for 15 min at 37°C prior to stimulation by 100 mM Ang II. Alternatively, cells were pre-treated with 1 µM wortmannin for 10 min at 37°C prior to stimulation by 100 mM Ang II.
Tyrosine-mutated PLZF
Each tyrosine in pcDNA4-PLZF was changed to a phenylalanine using a site-directed mutageneis kit (Stratagene, La Jolla, CA). Early stage R3T3 cells were transfected with pCDNA4 tyrosine-mutated PLZF or PLZF. Immnoblot analysis was performed using anti-PY20 or myc antibody. The confocal microscopy was performed with CHO-K1 cells dually transfected with pCDNA4 tyrosine-mutated PLZF and pEGFP-N2-AT2.
Electrophoretic mobility shift assay
Nuclear extracts were prepared from H9C2 cells according to the method described previously (Dignam et al., 1983). Protein concentrations were estimated using a Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA). For EMSA, DNA probes 5′-ACATGTACTAGTGTTGT-3′ (wild-type: w), 5′-ACAGGTAGTAGTGTTGT-3′ (mutant 1: m1) and 5′-ACACGGACTAGTGTTGT-3′ (mutant 2: m2) were prepared. Each probe was labeled with [α-32P]dATP using a Priming Kit II (Stratagene). Nuclear extracts were incubated with 1.0 × 104 c.p.m. of labeled probes for 30 min at room temperature in a binding buffer containing 10 mM HPES-KOH pH 7.8, 50 mM KCl, 1 mM EDTA, pH 8.0, 5 mM MgCl2, 10% glycerol, 5 mM DTT, 0.75 mM p-aminophenylmethanesulfonyl fluoride, 2 µg/ml aprotinin, 2 µg/ml pepstain, 2 µg/ml leupeptin, 1 mM sodium orthovanadate and 50 µg/ml poly(dI–dC) (dI–dC) (Amersham Pharmacia Biotech). For competition experiments, a 10- to 200-fold molar excess of unlabeled wild-type, m1 and m2 probes was added to nuclear extracts. For supershift assay, reaction mixtures of the nuclear extracts from H9C2 cells and the labeled wild-type probe were pre-incubated with 3 µl of antibody against PLZF (Santa Cruz Biotechnology) for 1 h at 4°C. All reaction mixtures were subjected to 4.5% PAGE under non-denaturing conditions. The gel was dried and exposed to an X-ray film at –80°C.
Permanently transfected Cos7 cells of AT2 and PLZF
The AT2 receptor and PLZF cDNA were fused into pIRES vector (BD Biosciences Clontech) (pIRES-AT2-PLZF). The pIRES-AT2-PLZF was transfected into Cos7 cells followed by G418 selection. Cloned Cos7 cells transfected with AT2 and PLZF were used.
Luciferase assay
The promoter region of the human p85αPI3K gene was amplified by PCR using primers 5′-GATGTTGTTGCGTTCCTCACTGC-3′/5′-CAGAGC AGTCTGATTTTACG-3′ (long 1),5′-ACTGCCTGTTCTAGCTCTTC TCA-3′/5′-CAGAGCAGTCTGATTTTACG-3′ (long 2) and 5′-CTA AGACATTGCCTCATGCCG-3′/5′-CAGAGCAGTCTGATTTTACG-3′ (short). Long 1 and 2 contain a PLZF-binding element but this is not present in short. Each PCR product was fused into the phRL-null vector (Promega Corporation, Madison, WI). Mutant 1 or 2, which was the same as the mutant probe in the EMSA, was prepared from phRL long 1. Cos7 cells transfected with AT2 and PLZF were transfected with each vector and then luciferase-based reporter assays were performed using the Dual-Glo luciferase assay system (Promega). The data were normalized by Renilla luciferase activity, and similar results were obtained from at least five independent experiments.
AT2 gene-deleted mouse
We used 20-week-old male, AT2 null (n = 12) and wild-type mice (n = 10) weighing 22–24 g. We produced the AT2-null mice as described previously (Ichiki et al., 1995). AT2-null mice from the tenth generation of the backcross to C57/BL6 mice were used. The wild-type mice were generated as littermates by the same mating procedure. All animal protocols were approved by the Vanderbilt Institutional Animal Care Committee.
Ang II treatment and western blot analysis
We treated mice using an Ang II pellet (Innovative Research of America, Sarasota, FL) as described previously (Ichihara et al., 2001). The pellets were prepared to release Ang II at a rate of 4.2 ng/kg/min for 3 weeks. We performed western blot analysis with ventricular extracts of control AT2-null and wild-type mice and Ang II-treated AT2-null and wild-type mice as previously reported (Towbin et al., 1979). We used rabbit polyclonal phospho-specific antibodies to ERK, p38 MAPK, JNK (New England Biolabs), rabbit polyclonal antibodies to calcineurin (New England Biolabs), p85αPI3K, p110αPI3K and goat polyclonal antibodies to PLZF (Santa Cruz Biotechnology). The outer borders of the bands were traced and the areas and densities were determined with the NIH image system. The nuclear fraction of AT2-null or wild-type mouse heart was extracted as described above, and the western blot analysis was performed. Histological analysis was performed on AT2-null or wild-type mouse heart. The hearts were isolated after perfusion with 40 mM KCl, fixed in 4% paraformaldehyde with PBS, dehydrated in graded ethanol solution, and transferred to xylene and then to paraffin. The paraffin-embedded hearts were sectioned at 4 µm and stained by antibody against PLZF or RB using the same method as above. Images were acquired with a Zeiss LSM 410 confocal laser-scanning microscope under a 63× oil immersion lens (Carl Zeiss, Germany).
Retroviral transfection and measurement of [3H]leucine incorporation into H9C2 cells
The AT2 receptor cDNA was fused into the retroviral vector pLXRN (Stratagene) (pLXRN-AT2). The pLXRN-AT2 and pVSV-G vector were transfected into the GP2-293 cell lines (BD Biosciences Clontech). The supernatant from AT2-producing cells was used to transfect H9C2 cells. The AT2 receptor-transfected H9C2 cells were serum starved for 24 h and stimulated by 100 nM Ang II and radiolabeled with [3H]leucine (1 µCi/ml) for 24 h. Cells were washed twice with ice-cold PBS and incubated with ice-cold 5% trichloroacetic acid for 1 h. After washing with ice-cold PBS, cells were lysed with 1 M NaOH, neutralized with 1 M HCl and subjected to liquid scintillation counting.
Acknowledgments
Acknowledgements
We thank the Vanderbilt Animal Physiology core laboratory for assistance with animal experiments, the Vanderbilt University Medical Center Cell Imaging Core Resource for assistance with microscope experiments, Smriti Bardhan for experimental assistance, and Tina Stack for secretarial assistance. This study was supported in part by research grants HL-58205, DK-20593, CA-68485 from the NIH and AHA grant 0255563B.
References
- Albagli O. et al. (1999) Overexpressed BCL6 (LAZ3) oncoprotein triggers apoptosis, delays S phase progression and associates with replication foci. Oncogene. 18, 5063–5075. [DOI] [PubMed] [Google Scholar]
- Barna M., Hawe,N., Niswander,L. and Pandolfi,P.P. (2000) Plzf regulates limb and axial skeletal patterning. Nat. Genet., 25, 166–172. [DOI] [PubMed] [Google Scholar]
- Bedecs K., Elbaz,N., Sutren,M., Masson,M., Susini,C., Strosberg,A.D. and Nahmias,C. (1997) Angiotensin II type 2 receptors mediate inhibition of mitogen-activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem. J., 325, 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.C., Ye,B.H., Chaganti,R.S. and Dalla-Favera,R. (1996) BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc. Natl Acad. Sci. USA, 93, 6947–6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Brand,N.J., Chen,A., Chen,S.J., Tong,J.H., Wang,Z.Y., Waxman,S. and Zelent,A. (1993) Fusion between a novel Kruppel-like zinc finger gene and the retinoic acid receptor-α locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J., 12, 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerk A. and Sugden,P.H. (2000) Small guanine nucleotide-binding proteins and myocardial hypertrophy. Circ. Res., 86, 1019–1023. [DOI] [PubMed] [Google Scholar]
- Cook M. et al. (1995) Expression of the zinc-finger gene PLZF at rhombomere boundaries in the vertebrate hindbrain. Proc. Natl Acad. Sci. USA, 92, 2249–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crackower M.A. et al. (2002) Regulation of myocardial contractility and cell size by distinct PI3K–PTEN signaling pathways. Cell, 110, 737–749. [DOI] [PubMed] [Google Scholar]
- de Gasparo M., Catt,K.J., Inagami,T., Wright,J.W. and Unger,T. (2000) International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev., 52, 415–472. [PubMed] [Google Scholar]
- Diep Q.N., El Mabrouk,M., Yue,P. and Schiffrin,E.L. (2002) Effect of AT(1) receptor blockade on cardiac apoptosis in angiotensin II-induced hypertension. Am. J. Physiol., 282, H1635–H1641. [DOI] [PubMed] [Google Scholar]
- Dignam J.D., Lebovitz,R.M. and Roeder,R.G. (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res., 11, 1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley D.T. and Summerfelt,R.M. (1993) Regulated expression of angiotensin II (AT2) binding sites in R3T3 cells. Regul. Pept., 44, 199–206. [DOI] [PubMed] [Google Scholar]
- Ferguson S.S. (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev., 53, 1–24. [PubMed] [Google Scholar]
- Frei N., Weissenberger,J., Beck-Sickinger,A.G., Hofliger,M., Weis,J. and Imboden,H. (2001) Immunocytochemical localization of angiotensin II receptor subtypes and angiotensin II with monoclonal antibodies in the rat adrenal gland. Regul. Pept., 101, 149–155. [DOI] [PubMed] [Google Scholar]
- Frey N., McKinsey,T.A. and Olson,E.N. (2000) Decoding calcium signals involved in cardiac growth and function. Nat. Med., 6, 1221–1227. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia A., Garrido,E., Hernandez,C., Alvarez,B., Jimenez,C., Cantrell,D.A., Pullen,N. and Carrera,A.C. (2002) A new role for the p85-phosphatidylinositol 3-kinase regulatory subunit linking FRAP to p70 S6 kinase activation. J. Biol. Chem., 277, 1500–1508. [DOI] [PubMed] [Google Scholar]
- Guerriero R. et al. (1995) Unilineage megakaryocytic proliferation and differentiation of purified hematopoietic progenitors in serum-free liquid culture. Blood, 86, 3725–3736. [PubMed] [Google Scholar]
- Hansen J.L., Servant,G., Baranski,T.J., Fujita,T., Iiri,T. and Sheikh,S.P. (2000) Functional reconstitution of the angiotensin II type 2 receptor and G(i) activation. Circ. Res., 87, 753–759. [DOI] [PubMed] [Google Scholar]
- Harada K. et al. (1998) Pressure overload induces cardiac hypertrophy in angiotensin II type 1A receptor knockout mice. Circulation, 97, 1952–1959. [DOI] [PubMed] [Google Scholar]
- Hein L., Meinel,L., Pratt,R.E., Dzau,V.J. and Kobilka,B.K. (1997) Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol. Endocrinol., 11, 1266–1277. [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Hayashida,W., Kambe,T., Yamada,T. and Dzau,V.J. (1997) Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J. Biol. Chem., 272, 19022–19026. [DOI] [PubMed] [Google Scholar]
- Hunyady L., Bor,M., Balla,T. and Catt,K.J. (1994) Identification of a cytoplasmic Ser–Thr–Leu motif that determines agonist-induced internalization of the AT1 angiotensin receptor. J. Biol. Chem., 269, 31378–31382. [PubMed] [Google Scholar]
- Hyman J., Chen,H., Di Fiore,P.P., De Camilli,P. and Brunger,A.T. (2000) Epsin 1 undergoes nucleocytosolic shuttling and its eps15 interactor NH(2)-terminal homology (ENTH) domain, structurally similar to Armadillo and HEAT repeats, interacts with the transcription factor promyelocytic leukemia Zn(2)+ finger protein (PLZF). J. Cell Biol., 149, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara S., Senbonmatsu,T., Price,E.,Jr, Ichiki,T., Gaffney,F.A. and Inagami,T. (2001) Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II-induced hypertension. Circulation, 104, 346–351. [DOI] [PubMed] [Google Scholar]
- Ichiki T. et al. (1995) Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature, 377, 748–750. [DOI] [PubMed] [Google Scholar]
- Inagami T., Iwai,N., Sasaki,K., Yamamo,Y., Bardhan,S., Chaki,S., Guo,D.F. and Furuta,H. (1992) Cloning, expression and regulation of angiotensin II receptors. J. Hypertens., 10, 713–716. [PubMed] [Google Scholar]
- Kambayashi Y., Bardhan,S., Takahashi,K., Tsuzuki,S., Inui,H., Hamakubo,T. and Inagami,T. (1993) Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J. Biol. Chem., 268, 24543–24546. [PubMed] [Google Scholar]
- Kang H., Schneider,H., Rudd,C.E. (2002) Phosphatidylinositol 3-kinase p85 adaptor function in T-cells. Co-stimulation and regulation of cytokine transcription independent of associated p110. J. Biol. Chem., 277, 912–921. [DOI] [PubMed] [Google Scholar]
- Labbaye C., Quaranta,M.T., Pagliuca,A., Militi,S., Licht,J.D., Testa,U. and Peschle,C. (2002) PLZF induces megakaryocytic development, activates Tpo receptor expression and interacts with GATA1 protein. Oncogene, 21, 6669–6679. [DOI] [PubMed] [Google Scholar]
- Levy B.I., Benessiano,J., Henrion,D., Caputo,L., Heymes,C., Duriez,M., Poitevin,P. and Samuel,J.L. (1996) Chronic blockade of AT2-subtype receptors prevents the effect of angiotensin II on the rat vascular structure. J. Clin. Invest., 98, 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.Y., English,M.A., Ball,H.J., Yeyati,P.L., Waxman,S. and Licht,J.D. (1997) Sequence-specific DNA binding and transcriptional regulation by the promyelocytic leukemia zinc finger protein. J. Biol. Chem., 272, 22447–22455. [DOI] [PubMed] [Google Scholar]
- Lu D., Yang,H. and Raizada,M.K. (1996) Angiotensin II regulation of neuromodulation: downstream signaling mechanism from activation of mitogen-activated protein kinase. J. Cell Biol., 135, 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara H. (1998) Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ. Res., 83, 1182–1191. [DOI] [PubMed] [Google Scholar]
- Mifune M., Sasamura,H., Shimizu-Hirota,R., Miyazaki,H. and Saruta,T. (2000) Angiotensin II type 2 receptors stimulate collagen synthesis in cultured vascular smooth muscle cells. Hypertension, 36, 845–850. [DOI] [PubMed] [Google Scholar]
- Miura S. and Karnik,S.S. (2000) Ligand-independent signals from angiotensin II type 2 receptor induce apoptosis. EMBO J., 19, 4026–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin J.D. and Dorn,G.W.,II (2001) Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol., 63, 391–426. [DOI] [PubMed] [Google Scholar]
- Molkentin J.D., Lu,J.R., Antos,C.L., Markham,B., Richardson,J., Robbins,J., Grant,S.R. and Olson,E.N. (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell, 93, 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukoyama M., Nakajima,M., Horiuchi,M., Sasamura,H., Pratt,R.E. and Dzau,V.J. (1993) Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J. Biol. Chem., 268, 24539–24542. [PubMed] [Google Scholar]
- Murphy T.J., Alexander,R.W., Griendling,K.K., Runge,M.S. and Bernstein,K.E. (1991) Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature, 351, 233–236. [DOI] [PubMed] [Google Scholar]
- Pucell A.G., Hodges,J.C., Sen,I., Bumpus,F.M. and Husain,A. (1991) Biochemical properties of the ovarian granulosa cell type 2-angiotensin II receptor. Endocrinology, 128, 1947–1959. [DOI] [PubMed] [Google Scholar]
- Reid A. et al. (1995) Leukemia translocation gene, PLZF, is expressed with a speckled nuclear pattern in early hematopoietic progenitors. Blood, 86, 4544–4552. [PubMed] [Google Scholar]
- Rockman H.A., Koch,W.J. and Lefkowitz,R.J. (2002) Seven-transmembrane-spanning receptors and heart function. Nature, 415, 206–212. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Yamano,Y., Bardhan,S., Iwai,N., Murray,J.J., Hasegawa,M., Matsuda,Y. and Inagami,T. (1991) Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type-1 receptor. Nature, 351, 230–233. [DOI] [PubMed] [Google Scholar]
- Senbonmatsu T., Ichihara,S., Price,E.,Jr, Gaffney,F.A. and Inagami,T. (2000) Evidence for angiotensin II type 2 receptor-mediated cardiac myocyte enlargement during in vivo pressure overload. J. Clin. Invest., 106, R25–R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaknovich R., Yeyati,P.L., Ivins,S., Melnick,A., Lempert,C., Waxman,S., Zelent,A. and Licht,J.D. (1998) The promyelocytic leukemia zinc finger protein affects myeloid cell growth, differentiation and apoptosis. Mol. Cell. Biol., 18, 5533–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi T., Kang,P.M., Douglas,P.S., Hampe,J., Yballe,C.M., Lawitts,J., Cantley,L.C. and Izumo,S. (2000) The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J., 19, 2537–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano H. et al. (2002) Inhibitory molecules in signal transduction pathways of cardiac hypertrophy. Hypertens. Res., 25, 491–498. [DOI] [PubMed] [Google Scholar]
- Thomas P.S. (1980) Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc. Natl Acad. Sci. USA, 77, 5201–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin,T. and Gordon,J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA, 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Leevers,S.J., Panayotou,G. and Waterfield,M.D. (1997) Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem. Sci., 22, 267–272. [DOI] [PubMed] [Google Scholar]
- Zhang J. and Pratt,R.E. (1996) The AT2 receptor selectively associates with Giα2 and Giα3 in the rat fetus. J. Biol. Chem., 271, 15026–15033. [PubMed] [Google Scholar]