To the editor:
Blood coagulation is initiated in vivo by formation of a tissue factor (TF)/factor VIIa (FVIIa) complex.1 Under physiologic conditions, TF is sequestered from blood and coagulation is initiated only after vascular injury. This paradigm has been challenged by studies suggesting that platelets express active TF after de novo synthesis,2,3 transfer from monocytes,4 or release from α-granules.5 In other studies,6,7 however, the existence of platelet TF could not be demonstrated.
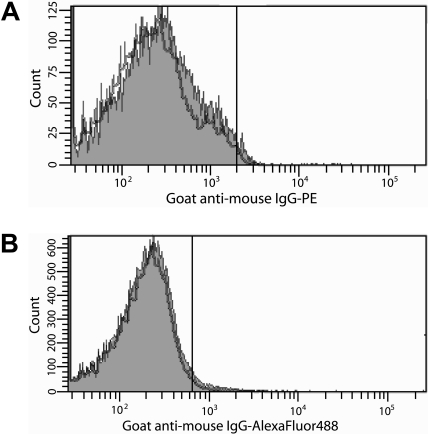
To resolve this ongoing controversy, experiments were performed with specific, inhibitory anti-TF antibodies8 using well-defined TF antigen and activity assays.6,8 Flow cytometric analyses indicated that no TF was expressed by protease-activated receptor (PAR)1- and PAR4-activated platelets (1.6% ± 1.2%) compared with unactivated platelets (1.5% ± 0.6%; Figure 1A) despite maximal α-granule release (> 98% P-selectin–positive platelets), consistent with previous studies demonstrating a lack of platelet TF expression after A23187-induced platelet activation and α-granule release.6 Consistent with studies performed in whole blood,6,7 no TF antigen (< 0.2pM) was detected by immunoassay8 after prolonged stimulation of platelets with lipopolysaccharide (LPS). In contrast, LPS-stimulated THP-1 cells expressed 6.6 plus or minus 2.0pmol/L TF/106 cells. These observations were confirmed in 2 TF-dependent activity-based assays.6 No factor Xa (FXa) (< 0.1pmol/L FXa/second) was generated by extrinsic FXase using unstimulated or stimulated platelets as a possible TF source. Similarly, no clot was formed in a plasma-based clotting assay (> 999 seconds). In contrast, FXa generation (1.6pmol/L FXa/second) and clot formation (∼ 71 seconds) were observed using LPS-stimulated THP-1 cells, but not unstimulated cells. Both were TF-dependent as an inhibitory anti-TF antibody8 decreased the rate of FXa generation to approximately 0.5pmol/L FXa/second and prolonged the clot time to 255 seconds, which corresponds to approximately 90% inhibition of activity. These observations contradict studies suggesting that platelets synthesize and express TF de novo in a time-dependent manner in response to platelet activation2,3 and preclude the notion that TF is stored in and released from platelet α-granules.5
Figure 1.
Search for TF on platelets by flow cytometry. (A) Washed platelets were activated with PAR1 (100μM) and PAR4 (500μM) agonist peptides (2 hours, 37°C). (B) Platelet-rich plasma was incubated with THP-1 cells in the presence or absence of 250 ng/mL LPS (4 hours, 37°C). For all experiments, TF expression on platelets was determined by immunostaining with an anti-TF antibody8 (0.5μM) in 20mM Hepes, 0.15 M NaCl (pH 7.4) containing 10 μg/mL human Fc, followed by either (A) goat anti–mouse IgG-PE (1:10 dilution) or (B) goat anti–mouse IgG–Alexa Fluor 488 (1:400 dilution) in 10% goat serum. Platelets (10 000) were analyzed by flow cytometry using a Becton Dickinson LSR II flow cytometer. The positive analyses regions were defined such that approximately 2% of the platelets stained with secondary antibodies alone were positive. The gray histograms depict anti-TF antibody staining of platelets activated with PAR peptides (A) or incubated with THP-1 cells and LPS (B). The black histograms depict immunostaining of unactivated platelets (A) or platelets incubated with THP-1 cells alone (B). The data shown are representative of 2 experiments performed in triplicate.
Other experiments tested the hypothesis that TF is transferred to platelets from monocytes in a P-selectin/P-selectin glycoprotein ligand-1 (PSGL-1)–dependent manner.4 Platelet-rich plasma isolated from contact pathway suppressed blood9 was incubated with THP-1 cells in the presence or absence of LPS. Hirudin and a FXa inhibitor, C921-78,10 were included to prevent thrombin generation without inhibiting a P-selectin/PSGL-1 interaction. Expression of cell surface– and microparticle-associated TF by LPS-stimulated THP-1 cells was confirmed by flow cytometry (data not shown). The percentage P-selectin–positive platelets that stained positively for TF when incubated in the presence of THP-1 cells and LPS (1.2% ± 0.6%) was virtually identical to that observed in the absence of LPS (2.4% ± 1.1%; Figure 1B), confirming previous observations made in whole blood6,7 and suggesting that TF on monocytes or monocyte-derived microparticles is not transferred to platelets.
Based on these observations, we conclude that platelets do not express detectable TF antigen or activity. Discrepancies between our data and those published by others may be a result of the assays used to quantify TF antigen and activity in different laboratories.8,11 Our assays use specific and highly sensitive anti-TF monoclonal antibodies and physiologically relevant standards and controls, whereas other reported assays use combinations of monoclonal and polyclonal antibodies, which may recognize TF fragments or cross-react with other proteins.8 Furthermore, the majority of the studies reporting the presence of TF in platelets used commercial, poorly validated assays, whereas our assays were developed and validated in-house.6,8 Indeed, it was recently reported that a commercially available TF activity assay sometimes leads to an assignment of TF-independent activity to TF.11
Authorship
Acknowledgments: The authors thank Matthew Gissel and Aimee Paradis for their technical assistance and Dr U. Sinha (Portola Pharmaceuticals Inc, South San Francisco, CA) for the gift of factor Xa inhibitor, C921-78. This work was supported by National Institutes of Health grant HL46703 (Project 2; S.B.).
Contribution: B.A.B. designed experiments, collected, analyzed and interpreted data, and wrote the manuscript; and S.B. and K.G.M. conceived of research, designed experiments, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Saulius Butenas, University of Vermont, Department of Biochemistry, 208 S Park Dr, Suite 2, Rm T227B, Colchester, VT 05446; e-mail: sbutenas@uvm.edu.
References
- 1.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues: implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134(5):1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 2.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109(12):5242–5250. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 3.Schwertz H, Tolley ND, Foulks JM, et al. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203(11):2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falati S, Liu Q, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197(11):1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqui FA, Desai H, Amirkhosravi A, Amaya M, Francis JL. The presence and release of tissue factor from human platelets. Platelets. 2002;13(4):247–253. doi: 10.1080/09537100220146398. [DOI] [PubMed] [Google Scholar]
- 6.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105(7):2764–2770. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 7.Osterud B, Olsen JO, Bjorklid E. What is blood borne tissue factor? Thromb Res. 2009;124(5):640–641. doi: 10.1016/j.thromres.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Parhami-Seren B, Butenas S, Krudysz-Amblo J, Mann KG. Immunologic quantitation of tissue factors. J Thromb Haemost. 2006;4(8):1747–1755. doi: 10.1111/j.1538-7836.2006.02000.x. [DOI] [PubMed] [Google Scholar]
- 9.Cawthern KM, van 't Veer C, Lock JB, DiLorenzo ME, Branda RF, Mann KG. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91(12):4581–4592. [PubMed] [Google Scholar]
- 10.Betz A, Wong PW, Sinha U. Inhibition of factor Xa by a peptidyl-alpha-ketothiazole involves two steps: evidence for a stabilizing conformational change. Biochemistry. 1999;38(44):14582–14591. doi: 10.1021/bi990958a. [DOI] [PubMed] [Google Scholar]
- 11.Bogdanov VY, Cimmino G, Tardos JG, Tunstead JR, Badimon JJ. Assessment of plasma tissue factor activity in patients presenting with coronary artery disease: limitations of a commercial assay. J Thromb Haemost. 2009;7(5):894–897. doi: 10.1111/j.1538-7836.2009.03315.x. [DOI] [PubMed] [Google Scholar]