Abstract
Inflammation and cardiovascular disease are associated with elevated serum levels of C-Reactive Protein (CRP) and homocysteine. The presence of both molecules in saliva provides an opportunity for development of non-invasive assessments of disease risk. However, salivary CRP and homocysteine reference ranges and their correlation with serum levels are unknown. This study investigated if CRP and homocysteine could be routinely detected in the saliva of healthy adults and the relationship between salivary and blood levels. CRP and homocysteine concentrations were determined using ELISA and enzymatic assays respectively. Homocysteine was detected in only two saliva samples (n = 55). CRP was measurable in all saliva samples (range: 0.05 to 64.3 μg/L; median = 1.2 μg/L) and plasma samples (range: 0.14 to 31.1 mg/L; median = 2.0 mg/L). Regression analysis demonstrated no relationship between CRP concentration in saliva and plasma (R2 = 0.001). Generalized linear models including variables such as saliva flow rate and time since eating or drinking also did not pass lack of fit testing. Therefore, a relationship between CRP concentration in saliva and blood could not be established in this group of subjects. More sensitive detection methods are needed to determine if a correlation between salivary and serum homocysteine levels exists.
Keywords: salivary biomarker, C-reactive protein, homocysteine
Introduction
The serum biomarkers homocysteine and C-reactive protein (CRP) are often used to assess cardiovascular disease risk.1 Homocysteine is a product of the methionine metabolic pathway that can accumulate as a result of folate, vitamin B12 or vitamin B6 deficiency, kidney disease, or polymorphisms in enzymes regulating its metabolism. Synthesis of the acute phase protein CRP increases dramatically in response to infection, but mildly elevated levels are also associated with chronic inflammation. The development of high sensitivity CRP assays has enabled reliable detection at these levels and several studies have demonstrated that both CRP and homocysteine are useful biomarkers for predicting cardiovascular events and mortality in certain populations.2–5
Elevated serum levels of CRP and homocysteine have also been linked to obesity and related disorders including polycystic ovary syndrome, type II diabetes, and metabolic syndrome.6–8 These findings have generated interest in the potential use of these biomarkers to identify patients at the greatest risk of developing not just cardiovascular disease, but also other complications of obesity. Such screening efforts would be simplified by the development of non-invasive methods for measuring these molecules. Both CRP and homocysteine have been detected in human saliva suggesting that novel approaches to assessing disease risk and monitoring the response to treatment may be possible.9,10 However, reference ranges for salivary concentrations of these molecules have not been established. In addition, although a correlation between serum and salivary CRP concentration has been observed in pigs,11 this relationship has not been investigated in human subjects. The goal of the study reported here was to determine if homocysteine and CRP can be routinely detected in the saliva of healthy adults and to investigate the relationship between salivary and blood levels of these molecules.
Methods
Subjects and sample collection
Study participants (n = 69) were recruited from the second-year class at the West Virginia School of Osteopathic Medicine. The study was approved by the WVSOM Institutional Review Board and written informed consent was obtained from each participant. All subjects well enough to attend class on the day of the study were eligible to participate. The subjects were asked to fast for at least one hour prior to giving their blood and saliva samples. Data regarding time since eating and drinking were obtained and failure to meet the fasting requirements resulted in exclusion from the study. Unstimulated whole saliva was obtained using the passive drool method and flow rates were determined.12 The saliva samples were immediately placed on ice following collection and stored at −20 °C. Blood samples were collected by venipuncture into EDTA tubes and were immediately centrifuged to separate blood components. The plasma supernatants were transferred to cryotubes for storage and frozen at −20 °C.
Homocysteine and C-reactive protein measurement
Prior to performing homocysteine and CRP assays, saliva and plasma aliquots were thawed at 4 °C and saliva samples were centrifuged for 15 minutes at 1500 × g at 4 °C in order to pellet the mucins. Saliva supernatants were transferred to fresh tubes and total homocysteine assays were performed immediately. The concentration of homocysteine in saliva and plasma was determined using the Axis-Shield liquid stable two-reagent enzymatic assay kit (Dundee, Scotland). Because some subjects did not produce large amounts of saliva, the protocol was slightly modified for measurement in small volumes using a 96-well format and full standard curve. The modified salivary homocysteine assay had intra- and inter-assay variability (%CV) of less than 10% and less than 15% respectively. Average recovery from saliva was 103% and the lower limit of detection (LLOD) for homocysteine in saliva was 0.8 μmol/L. Agreement with the original protocol was verified using reference materials provided by Axis-Shield. The LLOD for homocysteine in plasma using the standard kit protocol is 0.4 μmol/L with intra and inter-assay variability both less than 10%. The concentration of CRP in saliva was determined using a salivary CRP ELISA kit from Salimetrics, Inc (State College, PA). Intra- and inter-assay variability for this assay were less than 10% and recovery was 98%. CRP levels in plasma were determined using an AssayMax ELISA kit from Asssaypro (St. Charles, MO) Intra- and interassay variability for this assay were also less than 10% and recovery was 93%.
Statistical analysis
All statistical analyses were completed using SPSS version 16 software. Subjects with missing data points were excluded. Because levels of CRP in serum and saliva lacked normal distribution, values were logarithmically transformed. Shapiro-Wilk testing confirmed that the transformed data did not deviate from normality. Generalized linear modeling was used to determine the relationship between saliva and blood levels of the biomarkers. Variables including time since eating or drinking and salivary flow rate were also incorporated into the models. Pearson productmoment correlation coefficients were calculated to investigate the relationship between these parameters and biomarker concentrations.
Results and Discussion
Sixty-nine subjects were recruited from a class of second-year medical students at WVSOM to participate in this study. Because students are a protected population, potentially identifying information was not linked to samples. The average age of the population from which subjects were recruited was 26 with a range of 24–48 and 48% were female. Following exclusion of subjects for failure to meeting fasting requirements or problems with sample collection, biomarker assays were performed with samples from 55 subjects.
CRP was detected in all saliva samples and ranged from 0.05 to 64.3 μg/L with a median value of 1.2 μg/L (Table 1). Plasma CRP concentration ranged from 0.14 to 31.1 mg/L (median = 2.0 mg/L) (Table 1). Box and whisker plots were constructed and two extreme outliers for salivary CRP (>3 times the interquartile range) were identified. One of these was also an extreme outlier for plasma CRP. Homocysteine could only be detected in the saliva of two subjects (LLOD = 0.8 μmol/L). One of these subjects was an extreme outlier for salivary CRP but both had plasma homocysteine levels within the normal range (4–14 μmol/L).13
Table 1.
Crp levels in saliva and plasma of healthy adult subjects (n = 55). Standard deviations are shown with mean values.
| Salivary CRP (μg/L) | Plasma CRP (mg/L) | |
|---|---|---|
| Range | 0.05 – 64.3 | 0.14 – 31.1 |
| Median | 1.2 | 2 |
| Mean | 5.0 + 10.4 | 4.3 ± 5.9 |
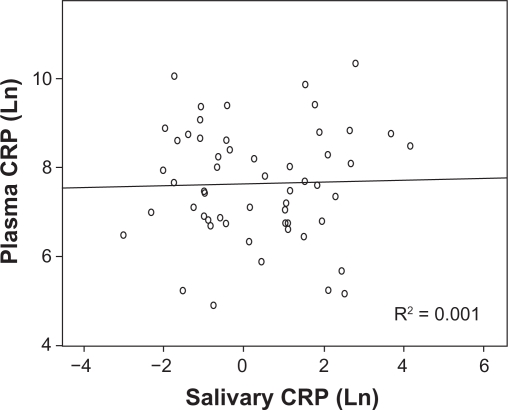
Because data for salivary and plasma CRP levels were not normally distributed, the values were logarithmically transformed prior to regression analysis. Shapiro-Wilk testing confirmed that the transformed data did not deviate from normality. Linear regression analysis showed no correlation between salivary and plasma levels of CRP (Fig. 1). Removal of extreme outliers from the analysis did not result in a better fit. No significant relationship was seen even when values were corrected for salivary flow rate. Generalized linear models including flow rate and time since eating and drinking in the analysis did not pass lack of fit testing and no correlation between flow rate (r = 0.078), time since eating (r = 0.039) or drinking (r = 0.055) and salivary CRP was found. These results are not consistent with findings from a study in pigs which demonstrated a significant linear relationship between salivary and serum CRP levels.11 However, the majority of the animals used in this analysis had experimentally induced inflammation and serum CRP levels that were more than 5-fold greater than baseline.
Figure 1.
Relationship between salivary and plasma CRP levels. Data for CRP concentration were not normally distributed. Therefore, values for both salivary and plasma concentration were logarithmically transformed prior to analysis.
Five of the subjects in our study had blood CRP concentrations higher than 10 mg/L, levels typically associated with infection, myocardial infarction or non-infectious inflammatory pathologies such as arthritis.1 In these five subjects, a trend suggesting a linear relationship with salivary levels could be seen but was not statistically significant (R = 0.705, P = 0.12). These results suggest that while salivary CRP measurement may be a potential surrogate for blood measurement in conditions such as infection or myocardial infarction, it may not be useful for determining cardiovascular disease risk in otherwise healthy adults. One study using chip-based sensors concluded that measuring salivary CRP along with several other molecules could aid in the diagnosis of acute myocardial infarction.14 Therefore, additional studies investigating the diagnostic uses of salivary CRP would be of interest.
A limitation of this study is that information regarding oral health status was not collected. Some studies have suggested that elevated salivary CRP levels are associated with periodontal disease.9,15 Therefore, salivary CRP concentration may be more indicative of oral health than of systemic inflammation. However, elevated serum CRP levels have also been reported in periodontal disease and little information on the source of salivary CRP is available.16 Although some molecules diffuse or are actively transported into saliva from the blood, others are synthesized by the salivary glands. CRP is primarily synthesized in the liver but one study has demonstrated increased CRP mRNA expression in the submandibular glands of rats with experimentally induced inflammation.17 If CRP is synthesized by salivary glands, a correlation with serum levels would not necessarily be expected.
Another limitation of this study is that the homocysteine detection methods used were not sensitive enough to measure the low levels found in saliva. A previous study investigating salivary homocysteine using liquid chromatography reported a mean concentration of 1.34 μmol/L but only eight subjects were used.10 Our results confirm that homocysteine can be detected in saliva but the LLOD for the enzymatic assay used here was 0.8 μmol/L. This is close to the mean value reported previously and the development of more sensitive assay methods will be needed in order to investigate the use of salivary homocysteine as a diagnostic tool.10
Acknowledgments
We would like to thank the WVSOM Class of 2012, Dr. David Leech, Dr. Timothy Leonard, Ken Moon and Lance Ridpath for their support and assistance. This project was funded by a WVSOM intramural grant. H. Cornwell was supported by a Project SEED internship from the American Chemical Society.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Pearson GA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, Pencina M, Jacques P, Selhub J, D’Agostino R, O’Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circulation Cardiovascular Quality and Outcomes. 2008;1:92–7. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2009;7:496–507. doi: 10.7326/0003-4819-151-7-200910060-00010. [DOI] [PubMed] [Google Scholar]
- 4.de Ruijter W, Westendorp RG, Assendelft WJ, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ (Clinical Research ed.) 2009;338:a3083. doi: 10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and metaanalysis. Mayo Clin Proc. 2008;83:1203–12. doi: 10.4065/83.11.1203. [DOI] [PubMed] [Google Scholar]
- 6.Kirilmaz B, Asgun F, Alioglu E, et al. High inflammatory activity related to the number of metabolic syndrome components. Journal of Clinical Hypertension. 2010;12:136–44. doi: 10.1111/j.1751-7176.2009.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González AS, Guerrero DB, Soto MB, Díaz SP, Martinez-Olmos M, Vidal O. Metabolic syndrome, insulin resistance and the inflammation markers C-reactive protein and ferritin. European Journal of Clinical Nutrition. 2006;60:802–9. doi: 10.1038/sj.ejcn.1602384. [DOI] [PubMed] [Google Scholar]
- 8.Guzelmeric K, Alkan N, Pirimoglu M, Unal O, Turan C. Chronic inflammation and elevated homocysteine levels are associated with increased body mass index in women with polycystic ovary syndrome. Gynecological Endocrinology. 2007;23:505–10. doi: 10.1080/09513590701554306. [DOI] [PubMed] [Google Scholar]
- 9.Christodoulides N, Mohanty S, Miller CS, et al. Application of microchip assay system for the measurement of C-reactive protein in human saliva. Lab on a Chip. 2005;3:261–9. doi: 10.1039/b414194f. [DOI] [PubMed] [Google Scholar]
- 10.Bald E, Głowacki R. Analysis of saliva for glutathione and metabolically related thiols by liquid chromatography with ultraviolet detection. Amino Acids. 2005;28:431–3. doi: 10.1007/s00726-005-0195-8. [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez AM, Martínez-Subiela S, Eckersall PD, Cerón JJ. C-reactive protein quantification in porcine saliva: a minimally invasive test for pig health monitoring. Veterinary Journal. 2009;181:261–5. doi: 10.1016/j.tvjl.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Poll E-M, Kreitschmann-Andermahr I, Langejuergen Y, et al. Saliva collection method affects predictability of serum cortisol. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2007;382:15–9. doi: 10.1016/j.cca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Pagana KD, Pagana TJ. Mosby’s Manual of Diagnostic and Laboratory Tests. 3rd ed. St. Louis: Oxford University Press; 2006. [Google Scholar]
- 14.Floriano PN, Christodoulides N, Miller CS, et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clinical Chemistry. 2009;55:1530–8. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pederson ED, Stanke SR, Whitener SJ, Sebastiani PT, Lamberts BL, Turner DW. Salivary levels of alpha 2-macroglobulin, alpha 1-antitrypsin, C-reactive protein, cathepsin G and elastase in humans with or without destructive periodontal disease. Archives of Oral Biology. 1995;40:1151–5. doi: 10.1016/0003-9969(95)00089-5. [DOI] [PubMed] [Google Scholar]
- 16.Yoshii S, Tsuboi S, Morita I, et al. Temporal association of elevated C-reactive protein and periodontal disease in men. Journal of Periodontology. 2009;80:734–9. doi: 10.1902/jop.2009.080537. [DOI] [PubMed] [Google Scholar]
- 17.Wei W, Parvin N, Tsumura K, et al. Induction of C-reactive protein, serum amyloid P component, and kininogens in the submandibular and lacrimal glands of rats with experimentally induced inflammation. Life Sci. 2001;69:359–68. doi: 10.1016/s0024-3205(01)01129-8. [DOI] [PubMed] [Google Scholar]