Abstract
Central pain is an enigmatic, intractable condition, related to destruction of thalamic areas, resulting in likely loss of inhibitory synaptic transmission mediated by GABA. It is proposed that treatment of central pain, a localized process, may be treated by GABA supplementation, like Parkinson’s disease and depression. At physiologic pH, GABA exists as a zwitterion that is poorly permeable to the blood brain barrier (BBB). Because the pH of the cerebral spinal fluid (CSF) is acidic relative to the plasma, ion trapping may allow a GABA ester prodrug to accumulate and be hydrolyzed within the CSF. Previous investigations with ester local anesthetics may be applicable to some GABA esters since they are weak bases, hydrolyzed by esterases and cross the BBB. Potential non-toxic GABA esters are discussed. Many GABA esters were investigated in the 1980s and it is hoped that this paper may spark renewed interest in their development.
Keywords: central pain, GABA, GABA esters
Definitions
Central pain is defined as pain caused by a lesion of the central nervous system (CNS). With broadening of the term “lesion”, other clinical pain conditions such as deafferentation, phantom, and delusional pain conditions can be considered central pain, but for the purposes of this paper, central pain will refer to the enigmatic intractable pain condition primarily caused by destruction of areas of the thalamus or projections from the thalamus to the cortex (thalamo-cortical tracts) and other closely related areas of neural destruction including supportive glial cells, shell wide dynamic range neurons of the thalamus and the zona incerta often injured after stroke.1 Central pain from trauma to the spinal cord or spinal-thalamic tract is not considered at this time.
Is Central Pain Caused by Depletion of GABA?
For over 100 years, the etiology of central pain has puzzled scientists and clinicians because there is not a uniform and consistent anatomic lesion that can explain the symptoms even though thalamic or thalamic cortical tracts are frequently injured. Since the anatomy is variable and the biochemistry is obscure, the pharmacological therapy as discussed in this paper is based on the following information:
1) Clinical reports of central pain relief after the administration of intrathecal baclofen, a GABA-B agonist.2–6 2) Clinical trials in which tricyclic antidepressant therapy produce analgesia presumably through reversal of GABA inhibition.7 3) Laboratory reports in animals in which application of GABA in the cerebral cortex produced analgesia and intrathecal GABA antagonists produced allodynia and hyperalgesia.8
Based on clinical observations, one hypothesis also supports the role of GABA deficiency in the etiology of central pain and is as follows: In the three neuron network of nocioceptive transmission, a destructive procedure, including cordotomy, rhizotomy, neurectomy, and neurolysis of first order neurons produces an initial analgesic response because it is likely that the net neuronal excitation and glutamate:GABA ratio has been reduced. Also, the analgesia produced by cervical cordotomy, ablation of the spinal thalamic tracts, reliably produces analgesia.9 However, over time, with regeneration and recruitment, the analgesia from these destructive lesions may be transformed into pain. Since destruction of the first or second order excitatory neurons produces analgesia, then it seems plausible that destruction of the third order neurons produces central pain because these neurons are net inhibitory and there is a deficiency of GABA or increase in the glutamate: GABA ratio. Neural destruction may cause central pain by depleting GABA via a number of mechanisms including inhibition of glutamate decarboxylase (GAD), restricting glutamate substrate, paralyzing reuptake and increasing the percentage of GABA uptake by glial cells. However the paracrine and autocrine effects of GABA potentially confuse the link between central pain and GABA especially when one tries to define GABA effects as exclusively related to synaptic transmission.10,11
This paper is not the first publication to link central pain and GABA deficiency and addresses a basic hypothesis without taking into account possible other neurotransmitters and collateral pathways.12,13 My hope is to present a potential therapy for the treatment of central pain, an intractable devastating condition, with selected GABA esters including special consideration to parallel pharmacologic properties with local ester anesthetics that may speed drug development.
Baclofen and Central Pain
The most commonly prescribed GABA agonist, baclofen, binds to the metabotropic heterodimeric GABA-B receptor and is not antagonized by bicuculline, a GABA-A agonist.14 Because oral dosing poorly permeates the blood brain barrier (BBB), intrathecal baclofen therapy is indicated for the treatment of spasticity from pyramidal tract disease or multiple sclerosis and has been reported to relieve central pain.2–5 Intrathecal administration of any medication has many potential problems, including infection, bleeding, catheter migration, obstruction of tubing, replacement of generators, granuloma formation, refilling mishaps and expense.15 This author believes that spinal infusion of a GABAergic medication is not a sustainable long-term therapy for the large number of patients who suffer from central pain.
Can GABA Replacement Treat Central Pain Instead of Specific Synthetic Agonists?
In present medical therapy, localized deficiencies of a neurotransmitter or naturally occurring substrate are treated by direct or indirect replacement even though the replacement therapy has significant side effects. 1) In Parkinson’s disease, the local deficiency in dopamine within the substantia nigra is treated with L-dopa which is converted to dopamine, a ubiquitous neurotransmitter. Systemic effects like hypotension and constipation are tolerated for the treatment of the CNS dopamine deficiency. 2) Monoamine and serotonin therapy for the treatment of depression may produce unwanted systemic effects like hypotension, tachycardia, and dry mouth, but the benefits of therapy outweigh the undesirable systemic side effects. 3) Numerous examples exist in the field of endocrinology (panpituitary, adrenal, and thyroid deficiencies) where estimating hormone replacement is lifesaving and the side effects are tolerated.
Because the molecular structure is not restrained, GABA freely rotates around all three carbon atoms and there exists a myriad of conformations that makes synthesis of active agonists challenging. There are many subtypes of GABA receptors, evolving through permutations of the subunits that may explain why GABA deficiency is associated with many disease states.16 Matching a specific GABA receptor subtype agonist to the variable lesions of central pain and introducing a subtype specific GABA agonist into the delicate CNS would be a significant undertaking. For example, vigabatrin, a GABA agonist with good bioavailability and analgesic action, produced visual field defects in 1/3 of patients during therapy and tiagabine, a selective inhibitor of GABA transport (GAT1), took twenty years to bring to market.17,18
Although designing and developing a central pain-specific GABA prodrug, with known effects inside and outside the CNS would be ideal, it would not be easy. From what we know about the possible toxicity of GABA, it would seem unlikely that central nervous system replacement to physiologic levels of 2 nmoles/ml would be detrimental. The following supports this proposition: 1) Systemically injected doses of GABA of up to 1 g/kg (9.7 mmol/kg) have little effect on motor activity in rats possibly because there is rapid metabolism by GABA-alphaoxoglutarate aminotransferase (GABA-T) that prevents accumulation and excessive serum levels.19 2) There are no reports of major side effects from large doses of GABA supplements ingested by athletes. 3) In humans, the LD50 for GABA has not been determined. 4) Because of hydrolysis, effects of elevated serum levels of prodrug would likely be muted, as known to occur when comparing CNS toxicities of ester local anesthetics to direct cardiac toxicities of amide local anesthetics. Thus, direct replacement therapy with GABA, may be more achievable even with known GABA effects outside the CNS such as activity on the peripheral nervous system, pancreas, smooth muscle, female reproductive organs and cerebral blood vessels.
After Degradation by Plasma Esterases, Would Enough GABA Ester Exist to Cross the BBB?
This is an unknown in humans, but the patented work of Victor E. Shashoua, “GABA esters and GABA analog esters” along with the well-developed pharmacology of ester type local anesthetics would suggest that some ester will be available to act on the CNS.19 Procaine and 2-chloroprocaine, two ester local anesthetics administered intravenously, intramuscularly, or subcutaneously, will be hydrolyzed by plasma butyrylcholinesterase (pseudocholinesterase), but sufficient ester will penetrate the BBB to induce significant CNS effects and in some cases temporary relief of pain.20 Therefore, even with plasma esterase concentrations 20–100 fold greater than CSF levels, it is still possible to safely introduce an ester compound into the central nervous system.21
Can GABA Esters Penetrate the BBB?
To date, discovering GABA analogs that penetrate the BBB by designing GABA conjugates to known transporter systems, increasing lipophilic GABA analogs, neutralizing one or both charges on GABA by cyclicization, or developing molecular “Trojan horses” has had limited success.22,23 Physical methods to disrupt the integrity of the BBB, focusing intracranial ultrasound waves or infusing osmotically active substances such as mannitol into the carotid artery have opened the endothelial tight junctions of the BBB, but have not become acceptable clinical therapies.23,24 Although many calculations and measurements of the in vitro octanol/water partition coefficient, (logP), reflecting BBB permeability, can be determined, a better measurement of BBB penetration is the Brain Penetration Index (BPI) which is the amount of compound present per gram of brain tissue as a percent of the amount per gram of liver tissue. In an animal model, the BPI (with GABA standardized as 1) of the following GABA esters showed that selected GABA esters can cross the BBB:19
| BPI | Kow | |
|---|---|---|
| GABA | 1 | 0.004 |
| 3-Gluosyl-GABA | 104 | 0.21 |
| Dexamethasone-GABA | 81 | 0.9 |
| Butyl-GABA | 74 | |
| Cholesteryl-GABA | 25 | 110 |
| Inosital-GABA | 11 |
Can the Difference Between CSF and Plasma pH Enhance Ion Trapping of GABA Esters?
Dr. Povl Krogsgaard-Larsen has discussed the potential development of amino acid analogs of GABA that would penetrate the BBB in the non-ionized form and increase drug CSF levels through ion trapping. 25 The decrease in buffer proteins, particularly hemoglobin, the increase in PCO2 from inequality of diffusion, and increased lactic acid production from glycolysis, produce a Gibbs Donnan equilibrium and pH gradient where the pH of CSF is approximately 0.09–0.10 pH units more acidic than the pH of the plasma.26 As a consequence of this pH gradient, ion trapping of selected GABA esters within the CSF likely occurs and is similar to the pH dependent ion trapping of local anesthetics across the placenta of a distressed acidotic fetus (a phenomena well recognized by obstetrical anesthesiologists).27 Also, ion trapping was an important consideration for the development of the proton pump inhibitors, since the highly acidic environment of the gastric parietal cells permitted intracellular trapping of medications like omeprazole.28 Because this trapping effect within the CSF is not well appreciated in the literature and is important for understanding the pharmacokinetics of GABA esters, an example follows: If the pKa value of a hypothetical GABA ester is 10, the pH of the CSF is 7.3 and the pH of plasma 7.4 then this effect may be approximated for comparison between the concentrations of protonated plasma ester [HB+plasma] and protonated CSF ester [HB+csf] as:
| [HB+plasma] = [H+plasma] + [Bplasma] ∼ {BBB} ∼ [Bcsf] + [H+csf] = [HB+csf] | ||
| pKa –pHplasma = log([HB+plasma]/[Bplasma]) | pKa – pHcsf = log ([HB+csf]/[Bcsf]) | |
| 2.6 = log ([HB+plasma]/[Bplasma]) | 2.7 = log([HB+csf]/[Bcsf]) | |
| Antilog of equations | ||
| 398 = [HB+plasma]/[Bplasma] | 501 = [HB+csf]/[Bcsf] | |
| [Bplasma] ∼ [Bcsf] | ||
| [HB+plasma]/398 = [Bplasma] | [Bcsf] = [HB+csf]/501 | |
| [HB+plasma]/398 = [HB+csf]/501 | ||
| [HB+csf]/[HB+plasma] = 501/398 = 1.26 | ||
Therefore the CSF concentration of protonated GABA ester is approximately 1.26 × the plasma concentration from ion trapping effects secondary to pH differences between plasma and CSF.
Why are Esters of GABA Ideal Prodrugs?
-
Easy to synthesize
Protecting the amine group with t-butoxycarbonyl derivatives (t-BOC), numerous GABA esters have been synthesized through condensation of the desired alcohol in acid milieu with GABA.19 Synthesis of more complex GABA esters, such as glyceryl and inositol derivatives, has required protection of various OH groups that are not to be esterified.19 Overall, with reliable protection groups many GABA esters can be synthesized.
-
Inductive effects of ester groups
Although difficult to estimate, the electron inductive effects of the ester group preferentially favors the free base form and penetration through the BBB.
-
Ester vs. amide prodrug
Although other conjugates of GABA such as amides may be more stable in vivo, it is unlikely that they would be hydrolyzed by an amidase within the CNS. Lidocaine and bupivacaine are amide local anesthetics commonly administered intrathecally and their action is terminated by redistribution rather than enzymatic degradation within the CSF.29 Also, GABA amides of nortriptyline and fluoxetine were found to be active, but GABA was not found to be a metabolite in the plasma.30 Picamilon, the niacin amide of GABA, available as a dietary supplement in the United States and widely investigated in Russia, has not been shown to be hydrolyzed by an amidase in the CSF.31 A further advantage of ester conjugates of GABA vs. amide conjugates is that, in general, amide conjugates of GABA are more difficult to synthesize.
-
Esterases exist in the CSF and plasma32
Esterases, acetylcholinesterase, butyrylcholinesterase and perhaps others which could hydrolyze the GABA ester exist in the plasma, blood, liver, kidney, and cerebral spinal fluid. The concentration of CSF butyrylcholinesterase is approximately 1/20– 1/100 of the plasma concentration.33 Much work has been done on the genetics of cholinesterase and butyrylcholinesterase because the neuromuscular blocking drug succinylcholine, commonly used in anesthesiology, undergoes ester hydrolysis. Prolonging the actions of local ester anesthetics and succinylcholine, a small percentage of the populace has an autosomal recessive trait in which they express an atypical butyrylcholinesterase. Others may have metabolic conditions associated with liver disease, uremia, malnutrition, pregnancy and medications that result in decreased amounts of this enzyme. Many laboratories provide assays of cholinesterases, both acetylcholinesterse and butyrylcholinesterse levels, that may need to be measured prior to treatment with a GABA ester.34
-
Comparison to the ester local anesthetic model
Since ester local anesthetics have been in use for over 100 years there is a wealth of clinical data regarding the bioavailability, redistribution, metabolism, and excretion of ester local anesthetics that could be very useful for the development of a GABA ester medication. Selected GABA esters and ester local anesthetics are weak bases with a lipophilic terminus and may have similar pKa values and similar pharmacokinetic profiles. Methodologies used to establish efficacy and safety for ester local anesthetics may translate to GABA ester development.
-
Safety
Compared with other GABA conjugates and agonists, introducing a GABA ester into the CNS, metabolized by esterases, may be the least dangerous therapy aimed to replenish GABA deficiency in patients suffering from central pain. This author feels it is essential that the prodrug be metabolized to GABA and a known non-toxic metabolite. This excludes many of the GABA esters described in the literature. Even with these considerations, the potential toxicity of the prodrug, itself, including the plasma levels of prodrug required to compete with degradation from plasma esterases could preclude development of a safe drug.
Literature Review of Animal Pharmacology of Selected GABA Esters
GABA esters of perphenazine and fluphenazine were synthesized and investigated by Nudelman et al. The perphenazine conjugate was found to be rapidly orally absorbed, cross the BBB and produce neuroleptic activity with reduced extrapyramidal symptoms and sedation. Autoradiography of rat brain slices did not differentiate whether GABA had been hydrolyzed from the compound.35
Jacob et al synthesized two lipid esters of GABA and showed that these compounds could penetrate the BBB 75 and 127 fold greater than that of free GABA after systemic administration, and the compounds were hydrolyzed into GABA and lipid by brain homogenates. Dose response changes in activity were described, but no toxicity data were reported.35
Carelli et al synthesized a GABA benzyl ester linked to a tetrahydrodinicotinamide salt that was systemically injected and produced GABA mimetic effects, but no toxicity data were reported.37
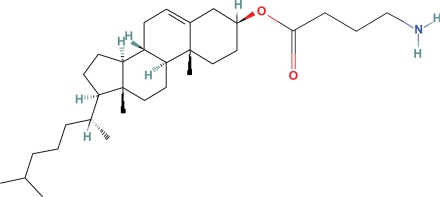
Shashoua synthesized the n-butyl, linolenoyl, cholesteryl and dexamethasone esters of GABA that crossed the BBB as evidenced by BPI data (see earlier table). Only the cholestryl ester was active in reducing the general activity test in mice. He subsequently synthesized glyceryl esters of GABA and showed that they penetrated the BBB. This led to his conclusion that “in fact there seems to be no blood-barrier for the lipid derivatives”. Systemic administration of these compounds produced modest effects on general locomotor and bicucullineinduced seizure activity in mice. Also synthesized were a wide variety of GABA glucose-3-esters and gamma-vinyl esters.19
Discussion of Potential Preferred GABA Esters
Unless a suitable enteric coating were developed, it is unlikely that any of the proposed GABA esters would be absorbed by the oral route because of inactivation in the stomach. However, it is likely that some of these medications could be absorbed through the mucosa, skin, or muscle similar to esters of local anesthetics and testosterone. The hydrolysis of the prodrug should yield GABA and non-toxic metabolites within the CSF preferably of substances already familiar to the central nervous system. A few examples of GABA esters and cleavage products that may meet these criteria will be discussed. These esters are illustrated in neutral form but are most likely to be stored and administered as hydrochloride salts to avoid transamidation: 1) ethyl ester GABA 2) glucose ester GABA 3) diethylamino ethanol (DEAE), a metabolite of intrathecal local anesthetics procaine and tetracaine, ester conjugated to GABA 4) dehydroascorbic acid ester GABA 5) cholesterol ester GABA 6) inositol ester GABA.
-
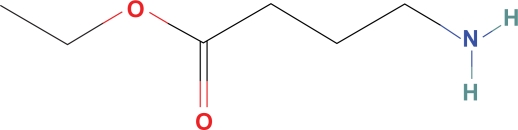
Ethyl ester GABA (ethyl 4 aminobutyrate) (Fig. 1) is commercially available, soluble in water, may cross the BBB since it is probably more permeable into the CNS than the double charged GABA zwitterion and, in addition, related n-butanol ester GABA has been shown to cross the BBB. Preliminary work has shown that esterase in plasma and CSF hydrolyze ethyl 4 aminobutyrate into GABA and ethyl alcohol.38 Calculation of the ethanol concentration to replace estimated physiologic levels of GABA is far below physiologic effects or detection levels (0.01%) in most ethanol assays and is calculated as:
Calculation of 0.01% ethanol level in mmoles per ml mw ethanol 46 mg/mmole
0.01% ethanol = 0.01 mg/100 mg ∼0.1 mg/ml × 1 mmole/46 mg
2.1 × 10−3 moles of ethanol/ml csf vs. 2.0 × 10−9 moles GABA/ml csf (physiologic replacement).
Therefore, intrathecal hydrolysis of ethyl ester GABA probably produces an acceptable quantity of ethanol, oxidized by catalase to acetaldehyde, and probably not detectable by motor or behavioral deficits. However, systemic effects of ethanol from plasma hydrolysis of ethyl ester GABA could be significant.
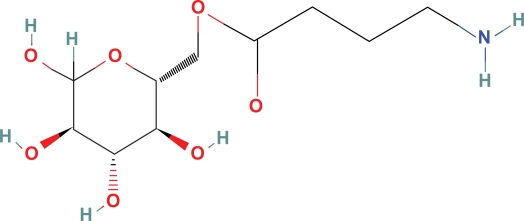
Glucose ester GABA (Fig. 2) has a BPI of 104 in mice and Kow = 0.21 so it likely crosses the BBB in humans.19 Transport into the CSF may be from active glucose transport via GLUT-1, passive diffusion, or both. This compound may be hydrolyzed to glucose and GABA. One would expect with supplementation of GABA to 2 nmole/ml, the small addition of glucose to the CSF and plasma as a consequence of the ester hydrolysis of glucose ester GABA would likely produce insignificant physiologic effects. Average CSF glucose concentration of 60 mg/dl converts to 3,000 nmoles/ml
-
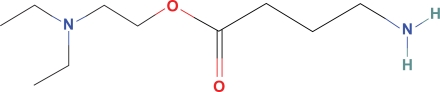
Diethylamino ethyl ester GABA (Fig. 3), a derivative of the local ester anesthetics, 2-chloroprocaine and tetracaine, may be a possible choice for replenishing GABA within the CNS. Procaine and tetracaine, commonly administered ester intrathecal anesthetics, have been in clinical use for decades with rare reports of neurotoxicity. Although, the majority (97%) of intrathecal procaine and tetracaine exits the CSF through redistribution, some must be degraded to diethylamino ethanol (DEAE) and benzoic acid because esterases are present in the CSF.39 Assuming 3% of the commonly administered 10 mg intrathecal dose of tetracaine is hydrolyzed, this would yield an intrathecal concentration of DEAE much higher than would be anticipated from the hydrolysis of DEAE ester GABA:
Ten (10 mg) tetracaine HCl × 1 mmole/301 mg × 0.03/150 ml csf = 6.6 × 10−6 mmoles/ml = 6.6 × 10−9 moles/ml compared to 2 × 10−9 moles of GABA/ml. For 100 mg procaine × 1 mmole/272 mg × 0.03/150 ml csf = 7.35 × 10−5 mmoles/ml = 7.35 × 10−8 moles/ml compared to 2 × 10−9 moles of GABA/ml.
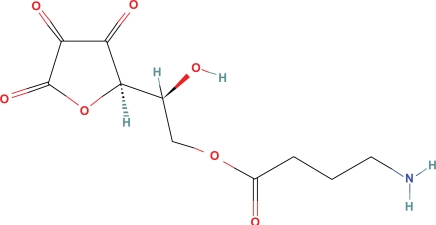
Dehydroascorbic acid, the oxidized form of vitamin C, is actively carried into the CNS by GLUT-1 transporter, producing vitamin C levels in the brain that are 10 fold greater than other tissues. It is not known whether the transporter would recognize dehydroascorbic acid ester GABA, (Fig. 4) but additional vitamin C introduced in the CNS by hydrolysis of this GABA would probably have low toxicity.
Cholesteryl-GABA (Fig. 5) easily crosses the BBB in mice (Kow = 110), and probably in humans, in amounts needed for GABA replacement, and it may not be toxic.19 Free and esterified cholesterol concentrations range between 3.1–5.4 microgm/ml in the CSF or approximately 2.5 mmole/ml, more than 1000 times greater than the 2 nmole/ml concentration of GABA, and therefore would probably be acceptable additional cholesterol.40
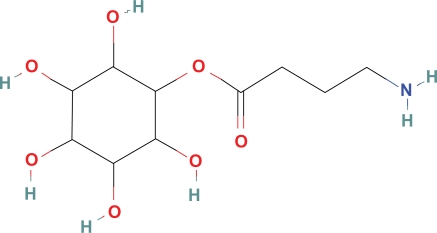
Inositol ester GABA (Fig. 6) with a BPI of 11 may have fair penetration of the BBB but is included in this list of GABA esters because inositol is naturally found in significant quantities within the CSF.
Figure 1.
Ethyl ester GABA.
Figure 2.
Glucose ester GABA.
Figure 3.
Diethylamino ethyl GABA.
Figure 4.
Dehydroascorbic acid ester GABA.
Figure 5.
Cholesteryl GABA.
Figure 6.
Inositol ester GABA.
GABA Esters and Patent Protections
After filing on May 7, 1990, on September 24, 1991, the United States Patent Office issued a patent protecting numerous GABA esters to McClean Hospital, a Harvard University affiliate.19 For many classes of claimed GABA esters this patent describes synthesis and biological activity including radio-labeled brain uptake, locomotor and anticonvulsant activity. It is unlikely that this patent had any significant impact on the further development of GABA esters especially since the recent license was not renewed.41 Dr. Victor E. Shashoua, (1929–2008) is to be given credit for much of the initial development of these potential prodrugs and this author does not know why they were not further tested. If a successful GABA ester were brought to market exclusive rights could be available from the FDA.
Conclusion
Based on neurophysiology and neuropharmacology, devastating central pain caused by lesions of the inhibitory neurons of the thalamus and thalamocortical tracts probably produces GABA deficiency. If post synaptic receptors are functional after brain injury, replenishing GABA may provide relief of symptoms. At physiologic pH, GABA exists as a zwitterion impermeable to the blood brain barrier, but selected GABA esters of ethanol, glucose, diethamino ethanol, dehydroascorbic acid, cholesterol, and inositol may cross the BBB and replace GABA in the CNS provided that central nervous system esterases can hydrolyze the prodrug. GABA esters possess chemical similarities to local ester anesthetics for which there is extensive laboratory and clinical experience, some of which may aid in the development of these prodrugs. Ion trapping and ester electron inductive effects may favor increasing CSF levels of the prodrug. This author feels that GABA esters with the greatest safety within the CNS should be hydrolyzed by an esterase into GABA as well as a compound with a known exposure and toxicity to the human CNS. Even with these considerations, the prodrug or the plasma level of prodrug required to offset degradation by hydrolysis could limit the effectiveness of this strategy. This paper hopes to spark interest in development of GABA ester analogs as novel analgesics for central pain and perhaps other conditions of the CNS where GABA deficiency is thought to exist.
Acknowledgments
The author would like to thank Julie Rosato of Duke University for assistance in preparation of this manuscript and Matthew Dickson, David Lindsay, Thomas van de Ven and Billy Huh from Duke University School of Medicine for proofreading.
Footnotes
Disclosures
The author and peer reviewers of this paper have no conflicts of interests to report
This manuscript has been read and approved by the author. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The author confirms that no copyrighted material is reproduced.
References
- 1.Yokota T. Thalamic mechanism of pain: shell theory of thalamic nociception. Jpn J Physiol. 1989;39(3):335–48. doi: 10.2170/jjphysiol.39.335. Review. [DOI] [PubMed] [Google Scholar]
- 2.Brennan PM, Whittle IR. Intrathecal baclofen therapy for neurological disorders: a sound knowledge base but many challenges remain. Br J Neurosurg. 2008 Aug;22(4):508–19. doi: 10.1080/02688690802233364. Review. [DOI] [PubMed] [Google Scholar]
- 3.Slonimski M, Abram SE, Zuniga RE. Intrathecal baclofen in pain management. Reg Anesth Pain Med. 2004 May–Jun;29(3):269–76. doi: 10.1016/j.rapm.2004.01.005. Review. [DOI] [PubMed] [Google Scholar]
- 4.Taira T, Ochiai T, Goto S, Hori T. Fifteen year experience of intrathecal baclofen treatment in Japan. Acta Neurochir Suppl. 2006;99:61–3. doi: 10.1007/978-3-211-35205-2_12. [DOI] [PubMed] [Google Scholar]
- 5.Taira T. Intrathecal administration of GABA agonists in the vegetative state. Prog Brain Res. 2009;177:317–28. doi: 10.1016/S0079-6123(09)17721-X. [DOI] [PubMed] [Google Scholar]
- 6.Taira T, Hori T. Intrathecal baclofen in the treatment of post-stroke central pain, dystonia, and persistent vegetative state. Acta Neurochir Suppl. 2007;97(Pt 1):227–9. doi: 10.1007/978-3-211-33079-1_31. [DOI] [PubMed] [Google Scholar]
- 7.Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/s1054-3589(06)54001-3. Review. [DOI] [PubMed] [Google Scholar]
- 8.Hao JX, Xu XJ, Wiesenfeld-Hallin Z. Intrathecal gamma-aminobutyric acidB (GABAB) receptor antagonist CGP 35348 induces hypersensitivity to mechanical stimuli in the rat. Neurosci Lett. 1994 Dec 5;182(2):299–302. doi: 10.1016/0304-3940(94)90821-4. [DOI] [PubMed] [Google Scholar]
- 9.Meyerson BA. Neurosurgical approaches to pain treatment. Acta Anaesthesiol Scand. 2001 Oct;45(9):1108–13. doi: 10.1034/j.1399-6576.2001.450910.x. Review. [DOI] [PubMed] [Google Scholar]
- 10.Gamel-Didelon K, Corsi C, Pepeu G, Jung H, Gratzl M, Mayerhofer A. Neuroendocrinology. An autocrine role for pituitary GABA: activation of GABA-B receptors and regulation of growth hormone levels. 2002 Sep;76(3):170–7. doi: 10.1159/000064523. [DOI] [PubMed] [Google Scholar]
- 11.Lux-Lantos VA, Bianchi MS, Catalano PN, Libertun C. GABA(B) receptors in neuroendocrine regulation. Cell Mol Neurobiol. 2008 Sep;28(6):803–17. doi: 10.1007/s10571-008-9263-4. Epub 2008 Feb 9. Review. [DOI] [PubMed] [Google Scholar]
- 12.Canavero S, Bonicalzi V. The neurochemistry of central pain: evidence from clinical studies, hypothesis and therapeutic implications. Pain. 1998 Feb;(2–3):74. 109–14. doi: 10.1016/s0304-3959(97)00089-4. Review. [DOI] [PubMed] [Google Scholar]
- 13.Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/s1054-3589(06)54001-3. Review. [DOI] [PubMed] [Google Scholar]
- 14.Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RW, 4th, Pin JP. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev. 2009 Apr;60(1):43–56. doi: 10.1016/j.brainresrev.2008.12.007. Epub 2008 Dec 25. Review. [DOI] [PubMed] [Google Scholar]
- 15.Ghafoor VL, Epshteyn M, Carlson GH, Terhaar DM, Charry O, Phelps PK. Intrathecal drug therapy for long-term pain management. Am J Health Syst Pharm. 2007 Dec 1;64(23):2447–61. doi: 10.2146/ajhp060204. [DOI] [PubMed] [Google Scholar]
- 16.Johnston GA. Medicinal chemistry and molecular pharmacology of GABA(C) receptors. Curr Top Med Chem. 2002 Aug;2(8):903–13. doi: 10.2174/1568026023393453. Review. [DOI] [PubMed] [Google Scholar]
- 17.Krogsgaard-Larsen P, Frølund B, Frydenvang K. GABA uptake inhibitors. Design, molecular pharmacology and therapeutic aspects. Curr Pharm Des. 2000 Aug;6(12):1193–209. doi: 10.2174/1381612003399608. Review. [DOI] [PubMed] [Google Scholar]
- 18.Jasmin L, Wu MV, Ohara PT. GABA puts a stop to pain. Curr Drug Targets CNS Neurol Disord. 2004 Dec;3(6):487–505. doi: 10.2174/1568007043336716. Review. [DOI] [PubMed] [Google Scholar]
- 19.Shashoua Victor E, United States Patent 5,051,448, GABA esters and GABA analog esters, 1991 Sep 24.
- 20.Schnapp M, Mays KS, North WC. Intravenous 2-chloroprocaine in treatment of chronic pain. Anesth Analg. 1981 Nov;60(11):844–5. [PubMed] [Google Scholar]
- 21.Atack JR, Perry EK, Bonham JR, Perry RH. Molecular forms of acetylcholinesterase and butyrylcholinesterase in human plasma and cerebrospinal fluid. J Neurochem. 1987 Jun;48(6):1845–50. doi: 10.1111/j.1471-4159.1987.tb05746.x. [DOI] [PubMed] [Google Scholar]
- 22.Egleton RD, Davis TP. Development of neuropeptide drugs that cross the blood-brain barrier. NeuroRx. 2005 Jan;2(1):44–53. doi: 10.1602/neurorx.2.1.44. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodor N. Targeting drugs to the brain by sequential metabolism. NIDA Res Monogr. 1995;147:1–32. Review. [PubMed] [Google Scholar]
- 24.Patel MM, Goyal BR, Bhadada SV, Bhatt JS, Amin AF. Getting into the brain: approaches to enhance brain drug delivery. CNS Drugs. 2009;23(1):35–58. doi: 10.2165/0023210-200923010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Krogsgaard-Larsen P, Frølund B, Frydenvang K. GABA uptake inhibitors. Design, molecular pharmacology and therapeutic aspects. Curr Pharm Des. 2000 Aug;6(12):1193–209. doi: 10.2174/1381612003399608. Review. [DOI] [PubMed] [Google Scholar]
- 26.van Heijst a, Visser BF, Maas AH. A micromethod for the determination or pH and PCO2 in human cerebrospinal fluid. Clin Chim Acta. 1961 Jul;:6, 589–90. doi: 10.1016/0009-8981(61)90156-5. [DOI] [PubMed] [Google Scholar]
- 27.Biehl D, Shnider SM, Levinson G, Callender K. Placental transfer of lidocaine: effects of fetal acidosis. Anesthesiology. 1978 Jun;48(6):409–12. doi: 10.1097/00000542-197806000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Larson GM, Sullivan HW., Jr Omeprazole, a long-lasting inhibitor of gastric secretion. J Surg Res. 1984 May;36(5):503–7. doi: 10.1016/0022-4804(84)90133-1. [DOI] [PubMed] [Google Scholar]
- 29.de Jong Ruldoph E.Local Anesthetics, Chapter 9 Uptake, Distribution, and Elimination, St Louis, Mosby-Year Book1994 [Google Scholar]
- 30.Rephaeli A, Gil-Ad I, Aharoni A, et al. Gamma-aminobutyric acid amides of nortriptyline and fluoxetine display improved pain suppressing activity. J Med Chem. 2009 May 14;52(9):3010–7. doi: 10.1021/jm900143u. [DOI] [PubMed] [Google Scholar]
- 31.Matsuyama K, Yamashita C, Noda A, et al. Evaluation of isonicotinoyl-gamma-aminobutyric acid (GABA) and nicotinoyl-GABA as pro-drugs of GABA. Chem Pharm Bull (Tokyo) 1984 Oct;32(10):4089–95. doi: 10.1248/cpb.32.4089. [DOI] [PubMed] [Google Scholar]
- 32.Sirviö J, Rakonczay Z, Hartikainen P, Kasa P, Riekkinen PJ. The molecular forms of acetylcholinesterase in cerebrospinal fluid of normal subjects—effect of aging. J Neural Transm Gen Sect. 1991;86(2):147–50. doi: 10.1007/BF01250575. [DOI] [PubMed] [Google Scholar]
- 33.Kambam JR, Horton B, Parris WC, Hyman SA, Berman ML, Sastry BV. Pseudocholinesterase activity in human cerebrospinal fluid. Anesth Analg. 1989 Apr;68(4):486–8. [PubMed] [Google Scholar]
- 34.Evans RT. Cholinesterase phenotyping: clinical aspects and laboratory applications. Crit Rev Clin Lab Sci. 1986;23(1):35–64. doi: 10.3109/10408368609165794. Review. [DOI] [PubMed] [Google Scholar]
- 35.Nudelman A, Gil-Ad I, Shpaisman N, et al. A mutual prodrug ester of GABA and perphenazine exhibits antischizophrenic efficacy with diminished extrapyramidal effects. J Med Chem. 2008 May 8;51(9):2858–62. doi: 10.1021/jm7012453. [DOI] [PubMed] [Google Scholar]
- 36.Jacob JN, Hesse GW, Shashoua VE. gamma-Aminobutyric acid esters. 3. Synthesis, brain uptake, and pharmacological properties of C-18 glyceryl lipid esters of GABA with varying degree of unsaturation. J Med Chem. 1987 Sep;30(9):1573–6. doi: 10.1021/jm00392a008. [DOI] [PubMed] [Google Scholar]
- 37.Carelli V, Liberatore F, Scipione L, et al. Synthesis and biological evaluation of GABA derivatives able to cross the blood-brain barrier in rats. Bioorg Med Chem Lett. 2003 Nov 3;13(21):3765–9. doi: 10.1016/j.bmcl.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Private communication.
- 39.de Jong Ruldoph E. Local Anesthetics, Chapter 10 Biotransformation, St Louis, Mosby-Year Book1994
- 40.Illingworth DR, Glover J. The composition of lipids in cerebrospinal fluid of children and adults. J Neurochem. 1971 May;18(5):769–76. doi: 10.1111/j.1471-4159.1971.tb12006.x. [DOI] [PubMed] [Google Scholar]
- 41.Private communication.