Abstract
OBJECTIVES
To determine whether the mean leukocyte telomere length (LTL) serves as a biomarker of disability assessed by Activities of Daily Living (ADL) and what factors may modify this relationship.
DESIGN
Retrospective cross-sectional study.
SETTING
A subset of the National Long Term Care Survey (NTLCS), a Medicare-based U.S. population longitudinal study focused on trends of overall health and functional status in the elderly.
PARTICIPANTS
Six hundred and twenty four individuals from the 1999 wave of the NTLCS cohort.
MEASUREMENTS
Relative LTL determined by quantitative PCR. LTL has previously been shown to correlate with common age-related disorders and mortality, as well as with socioeconomical status.
RESULTS
We observed gender difference of LTL, but not age-dependent shortening or association with socio-economical status. Importantly, LTL was associated with disability and functional status assessed by ADL. The association between ADL and LTL was more significant among non-diabetic subjects, while associations were not seen when diabetic subjects only were analyzed. Associations of LTL with cardiovascular diseases and cancer were also present in the non-diabetic group, but not in the diabetic group.
CONCLUSION
Our findings support the concept that LTL is a biomarker of overall well-being that is predictive of disability of older individuals in the US population. Diabetes plays an important role as a modifier of the association of LTL with disability, cardiovascular diseases, and cancer. These associations have obvious clinical implications due to the potential predictive value of LTL and deserve further investigation.
Keywords: disability, telomere, aging, disease, human
INTRODUCTION
Telomeres are the TTAGGG repeat sequences at the ends of chromosomes that prevent their end-to-end fusion. They become progressively shorter at each cell division due to the end replication problem 1. Telomere shortening occurs in vivo during human aging, and the rates of the shortening are determined by genetic, epigenetic, and environmental factors 2, 3. Oxidative damage is thought to be the major environmental factor that accelerates telomere shortening in vitro 4 as well as in vivo 5. Recently, leukocyte telomere length (LTL) has been the subject of intense study to examine relationships to the physiological and pathological changes associated with aging of human populations 6.
Correlations have been observed between LTL and common age-related disorders, including atherosclerosis 7, myocardial infarction 8, 9, chronic heart failure 10, vascular dementia 11, Alzheimer’s disease 12, osteoporosis 13, cancer 14, 15, diabetes Type 2 9, 16, 17 and insulin resistance 5, 9, 18, 19. Shorter telomeres were also shown to correlate with increased levels of inflammation 9, 19. Increased oxidative damage and low antioxidative capacity have been postulated as one of the underlying causes of accelerated telomere shortening that leads to the increased incidences of these disorders 4, 5. Telomere shortening has also been observed in some premature aging syndromes, such as dyskeratosis congenita 20 and aplastic anemia 21, supporting the notion that accelerated telomere attrition with age predisposes to disease.
Some of the most important risk factors for aging-related diseases, such as smoking, obesity, and lack of physical activity, appear to shorten telomeres 18, 22. In addition, low socio-economic status has also been related to shorter telomeres, independently of the effects of smoking, obesity, and lack of exercise 23. However, the relation between social status and telomere length is controversial, as a second study failed to find this association 24. Interestingly, life stress, both self-assessed and objectively scored, correlates with higher oxidative stress, determined by F2-isoprostanes, and shorter telomeres 25. These findings are consistent with a number of studies that have linked chronic stress and poor health to shorter telomeres, and point to LTL as an overall indicator of well being 26.
Moreover, shorter leukocyte telomeres have also been associated with mortality in persons of age 60 and older 27 that is attributed to heart disease and infectious diseases. However, these results were not reproduced in subsequent studies, arguably due to the fact that they were performed in older populations 28, 29, 30. Recently, a retrospective study of elderly twins demonstrated that LTL predicts mortality, suggesting that it might be not only a bioindicator of aging, but also a determinant of lifespan 31. In addition, LTL has been associated with mortality in specific groups of patients, such as stroke survivors 11, patients with Alzheimer’s disease 12, and patients with stable coronary artery disease 32. The fact that females live longer than males and also have significantly longer telomeres 33 suggests a relation between longevity and telomere length but, as indicated above, there are many additional factors likely to drive this relationship.
In this study, we aim to explore the role of LTL as a biomarker of successful aging by analyzing its relation with measurements of disability (overall health and functional status) collected at the National Long Term Care Survey (NLTCS). The NLTCS is a cross sectional and longitudinal study representative of U.S. Medicare recipients and it is considered to be one the best designed surveys to assess chronic (90+ days) disability 34-36. We examined whether LTL predicts disability indicated by Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL), and if so, what factors may modify this relationship. To our knowledge, this is the first study to demonstrate the correlation between disability and LTL.
METHODS
Study population
The NLTCS is a nationally representative survey which provides a large set of self- and proxy-reported data on health and functioning of the US elderly (65+) over 18 years. It had five waves (1982, 1984, 1989, 1994, and 1999; data from the sixth wave completed in 2004/2005 were not available for this study), each linked to Medicare service use and vital statistics files. The NLTCS is considered to be one of the best designed surveys to assess chronic (90+ days) disability 34. A two-stage-selection interviewing process was used. First, a screening interview assessing chronic disability (activity limitations due to disability or health problems which require either active help, standby help, or equipment use and last 90+ days) was given to all participants randomly selected from the Medicare enrollees. Second, a detailed interview was given to (i) those who reported at least one chronic disability in activities of daily living (ADL) or instrumental activities of daily living (IADL, see below), (ii) institutionalized individuals, and (iii) those who received a detailed interview in a previous survey. The details of this protocol have been described previously (for further details see 34, 37, 38). The 1994 and 1999 surveys also explicitly included samples of individuals who were designated for detailed interviews even if initially “screened out” as non-institutional and unimpaired during the screening interview (“healthy supplements”). Thus, by the survey design, disabled persons were over-sampled in detailed questionnaires, which provided a unique opportunity to focus on such vulnerable portion of the older population.
Disability measures
The NLTCS assesses chronic disability at two levels: more serious disabilities, which include the limitation to perform ADL 39, and less serious disabilities associated with limitations to perform IADL 40. Disabilities in ADL include: eating, getting in/out of bed, getting around inside, getting to the bathroom/using the toilet, dressing, and bathing. Disabilities in IADL include: doing heavy work, doing light work, doing laundry, cooking, shopping for groceries, getting around outside, going places outside of walking distance, managing money, making telephone calls, and taking medicines. Thus, in this study we considered any disability (including both ADL and IADL), and ADL disability. Since the ADLs reflect more serious disabilities, by choosing these two groups we cover disabilities with distinct severity levels.
The 1999 NLTCS subset
Blood samples were obtained from a subset of 638 individuals of the Medicare-linked NLTCS population during the 1999 survey 34-36. Demographic information including age, gender, race, education, mortality, BMI, and smoking was obtained from the 1999 NLTCS interview. Mortality data (as of 09/30/2006) were calculated from the Medicare Vital Statistics files linked to the NLTCS data. Body mass index (BMI) was calculated as weight (Kg)/height2 (m2). Smoking indicates the smoking status at the time of the 1999 NLTCS interview. Occurrence of infectious disease, cardiovascular diseases and diabetes mellitus were obtained from the Medicare service use files linked to the NLTCS data. The Medicare claims data records are classified by types of services (Part A benefits covering inpatient care in short- and long-stay hospitals, skilled nursing facilities, home health, and hospice care, or Part B benefits covering physician services, outpatient care, durable medical equipment, and home health agency in some cases) and contain information on dates and costs of services, ICD-9-CM (International Classification of Diseases – 9th Revision – Clinical Modification) diagnoses responsible for the services, and auxiliary diagnostic codes and procedure codes. We used information from both Part A and Part B claims for available time period (since January 1, 1991) to define occurrence of the diseases in that time interval based on the respective ICD-9-CM codes and dates of claims. If an individual had any Medicare claim (either Part A or Part B) with date of service earlier than the date of blood draw and associated with respective ICD-9-CM codes (001-139 for infectious diseases; 401-405, 410-414, 428-429, and 440-442 for cardiovascular diseases; and 250 and 357.2 for diabetes; all 4- and 5-digit codes within the respective codes were used), then the disease status was set as “prevalent” for that individual. Note that as the claims data are available since January 1, 1991, the term “prevalent” reflects the occurrence of respective disease since that time point until the date of blood draw (during about 10 years) and generally it may not correspond to the history of the disease during the entire life span in case of non-chronic diseases. Lung diseases (pneumonia, bronchitis, emphysema, and asthma), musculoskeletal diseases (rheumatism or arthritis, and broken hip or other broken bone), and cancer are based on the NLCTS interview.
Genomic DNA was isolated with QIAamp blood isolation kits and Qiagen BioRobot2000 system (Qiagen Inc. Valencia, CA) according to manufacturer’s instructions. Genomic DNA samples were kept in 96 well microtiter plates in duplicates, and stored at −80 °C. Fourteen cases had insufficient DNA available for telomere length measurements, thus the final number of cases included in this study was 624. ApoE genotyping had been previously performed in the same DNA samples 37, 38. This study has been approved by the Institutional Review Board of the University of Washington.
Telomere quantitative PCR
LTL was measured by Quantitative PCR (Q-PCR), as previously described 15, 41. For each sample, two PCR reactions were performed: one to amplify the telomeric DNA and the other to amplify a single-copy control gene (36B4, acidic ribosomal phosphoprotein PO), The latter serves as an internal standard to normalize the starting amount of DNA. A four-point standard curve using 10, 5, 2.5 and 1.25 ng of control DNA was used to transform cycle threshold into nanograms of DNA. All samples were run in triplicate and the median was used for subsequent calculations. A relative measurement of the telomere lengths, T/S ratio, was calculated by the amount of telomeric DNA (T) divided by the amount of single-copy internal control gene DNA (S). Two control DNA samples were included in each run to allow for normalization between experiments and periodic reproducibility experiments were performed to guarantee accurate measurements. The intra- and inter-assay variability (coefficient of variation) for Q-PCR was 6 and 7%, respectively.
Statistical analysis
As the distribution of LTL as measured by T/S ratio was confirmed to be normal, comparison of means between groups was performed with nonpairwise two-sided t test, and the p-values were adjusted for multiple comparisons. Logistic regression was used to evaluate the odd ratios (OR) and 95% confidence intervals (95% CI) of LTL, using two indicator variables that compare lower tertile vs. upper tertile (referent category) and middle vs. upper tertiles of the LTL distribution, for risk of any disability or ADL disability. We estimated three types of models with different adjustments on covariates: a) unadjusted; b) adjusted by age and gender; c) adjusted by age, gender, race, BMI, ApoE allele 4, smoking status and education. All analyses were performed using MATLAB’s Statistics Toolbox.
RESULTS
The cohort used for this study included a subset of 624 participants from the 1999 wave of NLTCS that provided blood samples. The characteristics of the cohort are summarized in Table 1. It comprised 43.9% males and 56.1% females. There were no significant differences in the age distribution, race, and education between males and females. Disability appeared more prevalent among females, but did not reach statistical significance (p=0.053). Males had significantly higher frequency of smoking (p=0.009) and higher BMI (p<0.001). The frequencies of ApoE genotypes were not different between males and females 37, 38.
TABLE 1.
Characteristics of 624 participants from the National Long Term Care Survey used of this study
| Total (n=624) | Male (n=275) | Female (n=349) | ||||
|---|---|---|---|---|---|---|
| Variable | n | %* | n | %* | n | %* |
| Age | ||||||
| 65-69 | 158 | 25.3 | 80 | 29.1 | 78 | 22.3 |
| 70-74 | 178 | 28.5 | 75 | 27.3 | 103 | 29.5 |
| 75-79 | 206 | 33.0 | 91 | 33.1 | 115 | 33.0 |
| 80-84 | 63 | 10.1 | 24 | 8.7 | 39 | 11.2 |
| 85-89 | 19 | 3.0 | 5 | 1.8 | 14 | 4.0 |
| Race | ||||||
| Caucasian | 575 | 92.1 | 254 | 92.4 | 321 | 92.0 |
| African-American | 37 | 5.9 | 15 | 5.5 | 22 | 6.3 |
| Others | 11 | 1.8 | 5 | 1.8 | 6 | 1.7 |
| Education | ||||||
| Less than | ||||||
| high school | 175 | 28.0 | 84 | 30.5 | 91 | 26.1 |
| High school | ||||||
| graduate or more | 440 | 70.5 | 186 | 67.6 | 254 | 72.8 |
| Smoker | ||||||
| current | 51 | 8.2 | 31 | 11.3 | 20 | 5.7 † |
| not current | 556 | 89.1 | 238 | 86.5 | 318 | 91.1 |
| BMI (Kg/m2) | ||||||
| ≤25 | 227 | 36.4 | 87 | 31.6 | 140 | 40.1 ‡ |
| 25-30 | 229 | 36.7 | 127 | 46.2 | 102 | 29.2 |
| >30 | 133 | 21.3 | 49 | 17.8 | 84 | 24.1 |
| Functional Status | ||||||
| Non-disabled | 388 | 62.2 | 184 | 66.9 | 204 | 58.5 |
| IADL only | 53 | 8.5 | 25 | 9.1 | 28 | 8.0 |
| 1-2 ADL | 107 | 17.1 | 43 | 15.6 | 64 | 18.3 |
| 3-4 ADL | 51 | 8.2 | 14 | 5.1 | 37 | 10.6 |
| 5-6 ADL | 15 | 2.4 | 7 | 2.5 | 8 | 2.3 |
| institutionalized | 7 | 1.1 | 1 | 0.4 | 6 | 1.7 |
| Apo E genotype | ||||||
| E 2/2 | 3 | 0.5 | 2 | 0.7 | 1 | 0.3 |
| E 2/3 | 91 | 14.6 | 36 | 13.1 | 55 | 15.8 |
| E 2/4 | 17 | 2.7 | 8 | 2.9 | 9 | 2.6 |
| E 3/3 | 382 | 61.2 | 176 | 64.0 | 206 | 59.0 |
| E 3/4 | 120 | 19.2 | 49 | 17.8 | 71 | 20.3 |
| E 4/4 | 11 | 1.8 | 4 | 1.5 | 7 | 2.0 |
| Diabetes | ||||||
| Yes | 204 | 32.7 | 92 | 33.5 | 112 | 32.1 |
| No | 420 | 67.3 | 183 | 66.5 | 237 | 67.9 |
Percentages for some variables do not add to 100 due to missing cases.
p<0.05 for Chi-square comparison of males vs. females.
p<0.001 for Chi-square comparison of males vs. females.
The average age of the individuals in this study was 74 years (range: 65-88). The average LTL determined by Q-PCR was 0.658±0.13 (min: 0.309, max: 1.053), expressed on the T/S scale. LTL was not significantly associated with age in a simple linear regression (R2=0.0009, p=0.45), or when the linear regression model was adjusted by gender, BMI, smoking, and diabetes (R2=0.012, p=0.8). Nor did we observe age-dependent telomere shortening in specific groups of individuals based on demographic and clinical variables (data not shown).
LTL of females was significantly longer than that of males (p=0.012) (Table 2 and Figure 1A). Age (p=0.474), race (p=0.32), smoking (p=0.45), BMI (p=0.99), and ApoE genotype (p=0.50) did not show significant association with telomere length in this sample population (Table 2). Education levels higher than high school graduate showed a trend of association with longer telomeres, but it was not significant (p=0.11).
TABLE 2.
Associations of telomere length with demographic and genetic variables
| Variable | n | Mean LTL | SD* | p-value (original) | p-value (multiple comparison |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 275 | 0.643 | 0.13 | 0.01 | 0.14 |
| Female | 349 | 0.670 | 0.13 | ||
| Age | |||||
| 65-69 | 158 | 0.647 | 0.14 | 0.47 | 0.60 |
| 70-74 | 178 | 0.661 | 0.13 | ||
| 75-79 | 206 | 0.667 | 0.13 | ||
| 80-84 | 63 | 0.641 | 0.14 | ||
| 85-89 | 19 | 0.676 | 0.12 | ||
| Race | |||||
| Caucasian | 575 | 0.656 | 0.13 | 0.32 | 0.55 |
| African-American | 37 | 0.690 | 0.16 | ||
| Others | 11 | 0.662 | 0.10 | ||
| Education | |||||
| Less than high school | 175 | 0.644 | 0.14 | 0.11 | 0.29 |
| High school graduate or more | 440 | 0.663 | 0.13 | ||
| Smoker | |||||
| never | 51 | 0.643 | 0.15 | 0.45 | 0.60 |
| ever | 556 | 0.658 | 0.13 | ||
| BMI (Kg/m2) | |||||
| ≤25 | 227 | 0.657 | 0.14 | 0.99 | 0.99 |
| 25-30 | 229 | 0.657 | 0.13 | ||
| >30 | 133 | 0.656 | 0.14 | ||
| Functional Status | |||||
| Non-disabled | 388 | 0.665 | 0.13 | 0.12 | 0.29 |
| IADL only | 53 | 0.666 | 0.13 | ||
| 1-2 ADL | 107 | 0.628 | 0.14 | ||
| 3-4 ADL | 51 | 0.651 | 0.14 | ||
| 5-6 ADL | 15 | 0.639 | 0.13 | ||
| institutionalized | 7 | 0.720 | 0.21 | ||
| Non-disabled | 388 | 0.665 | 0.13 | 0.07 | 0.27 |
| Disabled | 233 | 0.645 | 0.14 | ||
| no ADL | 441 | 0.665 | 0.13 | 0.03 | 0.15 |
| ADL | 180 | 0.639 | 0.14 | ||
| Apo E genotype | |||||
| E 2/2 | 3 | 0.734 | 0.13 | 0.50 | 0.60 |
| E 2/3 | 91 | 0.656 | 0.13 | ||
| E 2/4 | 17 | 0.608 | 0.13 | ||
| E 3/3 | 382 | 0.656 | 0.14 | ||
| E 3/4 | 120 | 0.667 | 0.13 | ||
| E 4/4 | 11 | 0.683 | 0.10 | ||
| no allele 4 | 476 | 0.657 | 0.13 | 0.72 | 0.79 |
| allele 4 | 148 | 0.661 | 0.13 |
SD, standard deviation.
Figure 1.
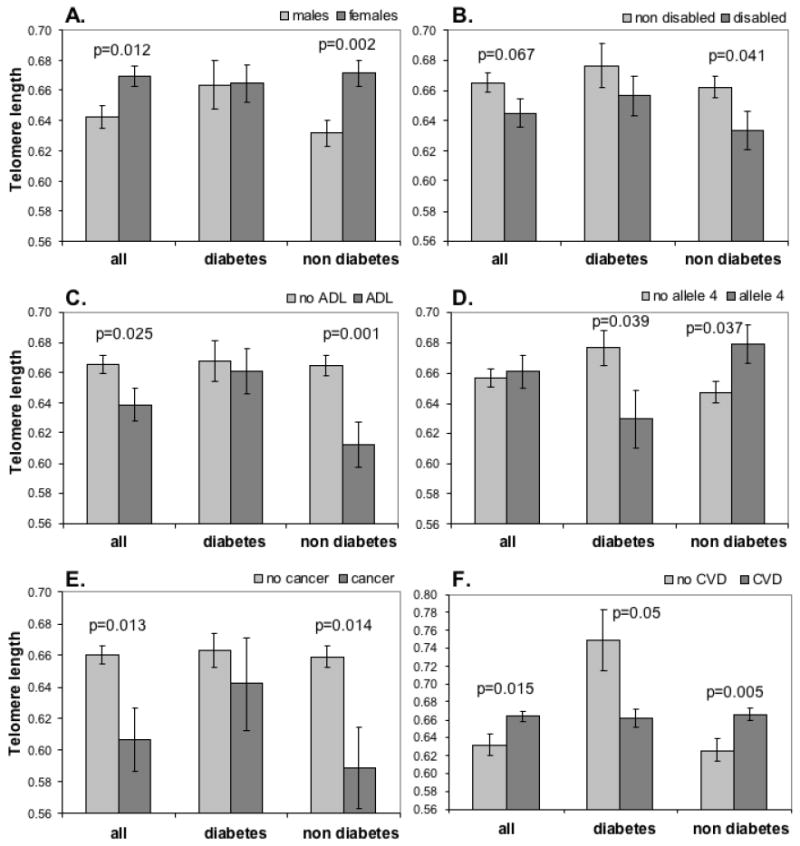
Effect of diabetes mellitus on the correlation of leukocyte telomere lengths (LTLs, arbitrary scale) and modifying factors. LTLs of males and females (A), non-disabled and disabled groups (B), no ADL and ADL groups (C), groups with and without one or two ApoE4 alleles (D), cancer and no cancer groups (E), and CVD and no CVD groups (F) were compared in all cases, diabetic cases, and non diabetic cases. P-values are unadjusted.
Functional status assessed by ADL and IADL were available in 621 individuals (Table 1). The association between telomere length and functional status categorized in 6 groups (non-disabled, IADL, 1-2 ADL, 3-4 ADL, 5-6 ADL and institutionalized) was not significant (p = 0.12; Table 2). However, when individuals with any disability (IADL and any ADL) were grouped together and compared to non-disabled individuals, the former showed a reduction of telomere length that was near significant (p=0.07, Table 2 and Figure 1B). Moreover, the individuals that had disabilities in ADL had significantly shorter telomeres compared to individuals that did not have any serious disabilities (that is, non-disabled or disabled only for IADL) (p=0.03, Table 2 and Figure 1C).
Next, we assessed the correlation between common age-related disorders and LTL in this population. The common disorders examined were infectious diseases, cardiovascular diseases, cancer, diabetes mellitus, lung diseases, and musculoskeletal diseases (Table 3). After adjusting for multiple comparisons, we observed a significant decrease of telomere length in patients with cancer compared to those cancer-free (p=0.048, Table 3 and Figure 1E). We also observed significantly longer telomeres in patients with CVD compared to patients without (p=0.048, Table 3 and Figure 1F). All the other diseases did not show a significant association with LTL after adjusting for multiple comparisons. We also calculated linear regression models controlling for age and sex. The observed associations between LTL and the diseases were similar to that presented in Table 3. Respective regression parameters for the diseases (coded as 1 – prevalent, 0 – not prevalent) were: -0.051 for cancer (95% confidence interval: (-0.094, -0.008)); 0.015 (-0.011, 0.041) for lung diseases; -0.025 (-0.047, -0.002) for musculoskeletal diseases; 0.016 (-0.006, 0.037) for infectious diseases; 0.03 (0.004, 0.057) for CVD; and 0.01 (-0.013, 0.032) for diabetes. So, individuals with cancer still tend to have shorter LTL, whereas those with CVD tend to have longer LTL, after controlling for age and sex. Addition of cancer as a covariate (for diseases other than cancer) did not substantially change the estimates (data not shown).
TABLE 3.
Associations of telomere length with common age-related diseases
| Disease | Prevalent | N | Mean LTL | SD | p-value (original) | p-value (multiple comparison) |
|---|---|---|---|---|---|---|
| cancer (1999 NLTCS) | yes | 39 | 0.607 | 0.13 | 0.01 | 0.048 |
| no | 571 | 0.660 | 0.13 | |||
| lung diseases (1999 NLTCS) | yes | 129 | 0.671 | 0.14 | 0.19 | 0.30 |
| no | 481 | 0.653 | 0.13 | |||
| musculoskeletal diseases (1999 NLTCS) | yes | 383 | 0.650 | 0.13 | 0.10 | 0.19 |
| no | 227 | 0.669 | 0.14 | |||
| infectious diseases (Medicare) | yes | 336 | 0.667 | 0.14 | 0.046 | 0.11 |
| no | 288 | 0.646 | 0.13 | |||
| CVD (Medicare) | yes | 499 | 0.664 | 0.13 | 0.01 | 0.048 |
| no | 125 | 0.632 | 0.13 | |||
| diabetes (Medicare) | yes | 204 | 0.664 | 0.14 | 0.41 | 0.47 |
| no | 420 | 0.655 | 0.13 |
Given the high level of interaction between different age-related factors, it is conceivable that the associations of telomere length with disability and aging might be evident only for subsets of individuals with or without certain demographic or clinical parameters. To test this hypothesis, we stratified our population by the categories defined by all the variables of study, and investigated possible associations of LTL with disability and age-related diseases in each of the subgroups. We found striking differences in the LTL associations of diabetic and non-diabetic groups that were not observed after stratifying by other aging diseases (data not shown). While the telomere length differences between genders and disability groups were not seen in diabetic patients, in non-diabetic patients they were significant and even more pronounced than in the whole population (Figure 1A, 1B and 1C). Moreover, we found an intriguing opposite association of telomere length with ApoE allele 4 depending on diabetes (Figure 1D). While the allele 4 was associated with significantly shorter telomeres in diabetic patients (p=0.039), it was related to significantly longer telomeres in non-diabetic individuals (p=0.037). The inverse correlation of telomere length with cancer was also significant for non-diabetic patients (p=0.014, Figure 1E), but was lost in diabetic patients. In addition, the association of CVD diseases with telomere length was positive for non-diabetic patients (p=0.005), but negative for diabetic patients (p=0.05, Figure 1F).
Lastly, we used logistic regression models to determine the risk of disability due to shorter telomeres and to explore if this risk was still significant after adjusting by variables known to be related to telomere length. Shorter telomeres were significantly associated to higher risk of ADL disability in the unadjusted model and both adjusted models. The highest OR (for lower vs. upper tertiles of the LTL distribution) corresponded to the model adjusted by age, gender, race, BMI, ApoE allele 4, smoking and education (OR=1.82, 95% CI: 1.14-2.92). For the risk of any disability, the model with multiple adjustments also showed a significant increase of risk associated with shorter telomeres. Individuals with telomeres in the lower tertile (LTL 0.31-0.59) had 1.57 (95% CI: 1.02-2.43) times higher risk of any disability (ADL or IADL) than individuals with telomeres in the referent category of upper tertile (LTL 0.71-1.05), independently of age, gender, race, BMI, ApoE allele 4, smoking or education. None of the models showed significant results for lower vs. middle tertiles of the LTL distribution. Interestingly, when stratifying by diabetes, we observed an even higher risk of ADL disability associated to shorter telomeres. Non-diabetic subjects with telomeres in the lower tertile had 2.94 (95% CI: 1.52-5.72) times higher risk of ADL disability than individuals with telomeres in the upper tertile, after multiple adjustments. However, the risk for any disability in non-diabetics was smaller (OR=1.64) and non significant. The same effects were observed when quartiles of telomere length were used for the same analysis of risk, the only difference being slightly higher OR and larger p-values, due to smaller number of individuals in each group (data not shown).
DISCUSSION
We have demonstrated that LTL is associated with disability of older individuals in the US population. While many studies have shown that LTL is related to diseases and risk factors of aging, to our knowledge, this is the first study that reports a comprehensive analysis of the potential of LTL to predict disability. This has been possible thanks to the NLTCS, a valuable resource for aging studies, as it was specifically designed to assess health and functioning in the elderly. By classifying disability into ADL and IADL, we were able to demonstrate that individuals with shorter telomeres were more likely to have disabilities, especially severe disabilities, than individuals with longer telomeres. These included disabilities in any of the following activities of daily living: eating, getting in/out of bed, getting around inside, getting to the bathroom/using the toilet, dressing, and bathing. While these disabilities might be the consequence of diseases of aging, their quantification as ADL or IADL disabilities constitutes a better cumulative index of physical and cognitive health/well-being than a specific list of age-related diseases. Aging is defined as the progressive loss of biological functions, and thus, it is highly relevant that a comprehensive measurement of major biological disabilities correlates with LTL 42. Our results are in agreement with a recent study that reported a positive association between LTL and years of healthy life 30.
An interesting aspect of our results is the modulating effect of diabetes in the associations between telomere length and disability. Surprisingly, shorter telomeres were related to disability only in non-diabetic subjects. Diabetes seemed to modify not only the association between telomere length and disability, but also the association with gender, cancer, CVD, and ApoE allele 4. Interestingly, ApoE allele 4 had opposite effects on telomere length depending on this disease status: in diabetic patients the presence of the allele 4 was associated with shorter telomeres, while in non-diabetic patients was associated with longer telomeres. ApoE plays an important role in the transport of cholesterol and other lipids and it has been associated with CVD, Alzheimer’s, and longevity 43. We have recently demonstrated that ApoE polymorphisms are also related to disability, but these relationships are not simple and involve differences in genotype, gender, and severity of disability 37. Thus, the complex relationships that exist between all these factors 44 might explain the modifying effect of diabetes observed here and also the discrepancies between studies. For instance, while diabetes has been associated with shorter telomeres in some studies 9, 16, 17, that was not the case in this study and others 8, 10. It should be pointed out, however, that the caveat of this analysis is the fact that a number of analyses were performed prior to the test of diabetes as a modifier. Because of this, there is a need to replicate these results in order to confirm our findings.
Regarding associations with diseases of aging, we found that shorter telomeres are related to cancer, in agreement with previous studies by us and others 14, 15. However, we found positive correlation between longer telomeres and CVD, which was opposite from our expectation. Interestingly, this association is reversed in the case of diabetic patients, in which CVD are associated with shorter telomeres, as it has been previously reported in the literature 8-10. It should be pointed out, however, that we saw only 6 cases that had diabetes and no CVD. One possible explanation to why those who have CVD had longer LTL in our study is that CVD patients with relatively short telomeres might have deceased earlier, which could skew the remaining participants toward the CVD patients with relatively longer telomeres. A second explanation for these unexpected results might be in part due to the bias of the physicians who made the diagnosis for Medicare, as the Medicare records suffer from the lack of standardization 45. The potential bias includes the under-diagnosis of the disorders due to the lack of Medicare claims data for ages less than 65 years old and for years before 1991. A third explanation is based on sample selection and the complex interactions between CVD related factors. While the literature has clearly demonstrated that shorter LTL are associated with CVD in general, it is important to point out that there are multiple discrepancies between studies depending on the specific parameter to be tested. For example, shorter leukocyte telomeres have been associated with hypertension 5; however, several other studies have found associations with other clinical manifestations related to CVD, but not with hypertension 8-10. These discrepancies might be related to differences in the study population, including age and gender distribution, as well as other telomere length confounding factors such as smoking or obesity. A strength of our study is that it is representative of the US elderly population, so there is no bias towards specific subsets of individuals that might favor finding specific associations. Independent larger studies, however, are needed to clarify these discrepancies.
Regarding associations with aging risk factors, we have found shorter telomeres in males than in females, as expected 33, but not a decrease of telomere length with age. This lack of association has previously been reported in some populations enriched in older subjects 28, 29. The association of shorter telomeres with smoking and obesity has been reported in some studies 18, but it is not significant in others 9, 46, including this report. Many factors could account for these differences, amongst them the age of the population of study. For instance, the association of telomere shortening with insulin resistance and inflammation was reported to be absent in menopausal women 19, which underscores a possible hormonal influence on LTL and indicates that these associations might not be found when studying older groups of individuals.
There is the potential for bias due to the fact that subset of the 1999 NLTCS used in this sample is less disabled (37%) than the sample from which it was selected. The 1999 NLTCS community interview respondents, consisting of “screened in” disabled patients and non-disabled “healthy supplement” (see section “Material and Methods”), contained about 60% disabled individuals. In addition, those greater than age 90 and those who were in serious conditions were excluded from LTL analysis because of the potential risk of blood drawing in these subjects, leading to a sampling bias toward less disabled people in this LTL analysis.
In our study, as in all but one 47 published study of health correlates of peripheral blood telomere length, we have measured telomere length only in unfractionated peripheral blood. Peripheral blood is, however, a complex mixture of cell types and it is possible that one or more cell subsets are responsible for the strength of association with health related parameters. This might be particularly true for associations of telomere length with age, as cell subsets (especially naive and memory T cells) are known to change with age 48, 49. However, we believe that this explanation is unlikely in our study, as 1) age was not a significant covariate with telomere length; 2) the health conditions and stratifiers that were associated with telomere length (cancer, CVD, disability and diabetes) are not known to be associated with any consistent change in proportions of peripheral blood cell subsets. Nevertheless, in this study, as in others, study of telomere lengths within cell subsets would be a useful future direction.
In conclusion, we have reported a novel association between LTL and disability, which supports the role of LTL as a biomarker of overall well-being, as suggested by others 30. Interestingly, it has been reported that physical activity in leisure time is associated with longer telomeres 22. Shorter telomeres among sedentary subjects could not be explained by the prevalence of chronic diseases that can lead to the reduced activity. Similarly, longer leukocyte telomeres have been associated with diets high in vitamin D 50. These findings support the notion that a healthier life style might delay telomere shortening and thus, the effects of aging. Follow up studies with independent populations are needed to confirm the role of LTL as a biomarker of overall well-being of the elderly.
TABLE 4.
Association between tertiles of LTL and disability
| ALL |
DIABETICS |
NON DIABETICS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ADL disability | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Lower vs. upper tertiles of telomere length | |||||||||
| Unadjusted | 1.56 | [1.02; 2.40] | 0.04 | 0.91 | [0.46; 1.80] | 0.78 | 2.91 | [1.54; 5.50] | 0.001 |
| Adjusted by age and gender | 1.69 | [1.09; 2.62] | 0.02 | 0.92 | [0.45; 1.85] | 0.80 | 3.08 | [1.61; 5.90] | 0.001 |
| Multiple adjustments* | 1.82 | [1.14; 2.92] | 0.01 | 1.02 | [0.46; 2.26] | 0.97 | 2.94 | [1.52; 5.72] | 0.001 |
| Middle vs. upper tertiles of telomere length | |||||||||
| Unadjusted | 1.22 | [0.79; 1.89] | 0.37 | 0.89 | [0.46; 1.73] | 0.74 | 1.77 | [0.90; 3.48] | 0.10 |
| Adjusted by age and gender | 1.27 | [0.82; 1.98] | 0.29 | 0.83 | [0.42; 1.66] | 0.60 | 1.78 | [0.90; 3.55] | 0.10 |
| Multiple adjustments* | 1.37 | [0.85; 2.21] | 0.19 | 0.85 | [0.40; 1.82] | 0.67 | 1.75 | [0.87; 3.54] | 0.12 |
| Any disability | |||||||||
| Lower vs. upper tertiles of telomere length | |||||||||
| Unadjusted | 1.38 | [0.93; 2.06] | 0.11 | 1.30 | [0.65; 2.62] | 0.46 | 1.63 | [0.97; 2.74] | 0.07 |
| Adjusted by age and gender | 1.47 | [0.98; 2.20] | 0.06 | 1.33 | [0.65; 2.72] | 0.43 | 1.69 | [0.99; 2.88] | 0.05 |
| Multiple adjustments* | 1.57 | [1.02; 2.43] | 0.04 | 1.60 | [0.72; 3.55] | 0.25 | 1.64 | [0.94; 2.86] | 0.08 |
| Middle vs. upper tertiles of telomere length | |||||||||
| Unadjusted | 0.96 | [0.64; 1.44] | 0.84 | 0.94 | [0.48; 1.84] | 0.86 | 0.96 | [0.55; 1.68] | 0.89 |
| Adjusted by age and gender | 0.98 | [0.65; 1.48] | 0.93 | 0.89 | [0.45; 1.77] | 0.74 | 0.95 | [0.54; 1.68] | 0.86 |
| Multiple adjustments* | 0.99 | [0.64; 1.54] | 0.97 | 0.94 | [0.45; 2.00] | 0.88 | 0.91 | [0.50; 1.65] | 0.75 |
age, gender, race, BMI, Apo E allele 4, smoking and education
OR, odd ratio. CI, confidence interval.
Acknowledgments
We thank Jasmine L. Gallaher for technical support and Christine Y. Park for editorial assistance.
This work was supported by NIH grant, P30AG13280, P01AG008761, R01AG028259, R01AG030612 and R24CA78088.
Sponsor’s Role: The sponsor did not play a role in the study design, conduct, management, data analysis, review, or authorization for submission.
Footnotes
Author Contributions: Dr. Risques conducted the LTL measurements and was involved in the statistical analysis and interpretation of the data. Drs. Arbeev, Yashin and Ukraintseva were responsible for the sorting of demographical and clinical data, statistical analysis, and the interpretation of the statistical results. Drs. Martin and Rabinovitch contributed in the design of the study and the final draft of the manuscript. Dr. Oshima was responsible for the overall design and execution of the project.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
References
- 1.Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 2.Aviv A. Telomeres and human aging: Facts and fibs. Sci Aging Knowledge Environ. 2004;2004:pe43. doi: 10.1126/sageke.2004.51.pe43. [DOI] [PubMed] [Google Scholar]
- 3.Wong JM, Collins K. Telomere maintenance and disease. Lancet. 2003;362:983–988. doi: 10.1016/S0140-6736(03)14369-3. [DOI] [PubMed] [Google Scholar]
- 4.Tchirkov A, Lansdorp PM. Role of oxidative stress in telomere shortening in cultured fibroblasts from normal individuals and patients with ataxia-telangiectasia. Hum Mol Genet. 2003;12:227–232. doi: 10.1093/hmg/ddg023. [DOI] [PubMed] [Google Scholar]
- 5.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 6.Aviv A, Valdes AM, Spector TD. Human telomere biology: Pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006;35:1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- 7.Benetos A, Gardner JP, Zureik M, et al. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 8.Brouilette S, Singh RK, Thompson JR, et al. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2006;165:14–21. doi: 10.1093/aje/kwj346. Epub 2006 Oct 16. [DOI] [PubMed] [Google Scholar]
- 10.van der Harst P, van der Steege G, de Boer RA, et al. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Ruiz C, Dickinson HO, Keys B, et al. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60:174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- 12.Honig LS, Schupf N, Lee JH, et al. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann Neurol. 2006;60:181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- 13.Valdes AM, Richards JB, Gardner JP, et al. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos Int. 2007 doi: 10.1007/s00198-007-0357-5. [DOI] [PubMed] [Google Scholar]
- 14.McGrath M, Wong JY, Michaud D, et al. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16:815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 15.Risques RA, Vaughan TL, Li X, et al. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2649–2655. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- 16.Adaikalakoteswari A, Balasubramanyam M, Mohan V. Telomere shortening occurs in Asian Indian Type 2 diabetic patients. Diabet Med. 2005;22:1151–1156. doi: 10.1111/j.1464-5491.2005.01574.x. [DOI] [PubMed] [Google Scholar]
- 17.Sampson MJ, Winterbone MS, Hughes JC, et al. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29:283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- 18.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 19.Aviv A, Valdes A, Gardner JP, et al. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab. 2006;91:635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- 20.Vulliamy T, Marrone A, Szydlo R, et al. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004;36:447–449. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi H, Calado RT, Ly H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 22.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 23.Cherkas LF, Aviv A, Valdes AM, et al. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 24.Adams J, Martin-Ruiz C, Pearce MS, et al. No association between socio-economic status and white blood cell telomere length. Aging Cell. 2007;6:125–128. doi: 10.1111/j.1474-9726.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- 25.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houben JM, Moonen HJ, van Schooten FJ, et al. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44:235–246. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Cawthon RM, Smith KR, O’Brien E, et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Ruiz CM, Gussekloo J, van Heemst D, et al. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: A population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 29.Bischoff C, Petersen HC, Graakjaer J, et al. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006;17:190–194. doi: 10.1097/01.ede.0000199436.55248.10. [DOI] [PubMed] [Google Scholar]
- 30.Njajou OT, Hsueh WC, Blackburn EH, et al. Association Between Telomere Length, Specific Causes of Death, and Years of Healthy Life in Health, Aging, and Body Composition, a Population-Based Cohort Study. J Gerontol A Biol Sci Med Sci. 2009;64:860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura M, Hjelmborg JV, Gardner JP, et al. Telomere length and mortality: A study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farzaneh-Far R, Cawthon RM, Na B, et al. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: Data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008;28:1379–1384. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benetos A, Okuda K, Lajemi M, et al. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 34.Manton KG, Corder L, Stallard E. Chronic disability trends in elderly United States populations: 1982-1994. Proc Natl Acad Sci U S A. 1997;94:2593–2598. doi: 10.1073/pnas.94.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sloan FA, Picone G, Brown DS, et al. Longitudinal analysis of the relationship between regular eye examinations and changes in visual and functional status. J Am Geriatr Soc. 2005;53:1867–1874. doi: 10.1111/j.1532-5415.2005.53560.x. [DOI] [PubMed] [Google Scholar]
- 36.Kulminski A, Yashin A, Ukraintseva S, et al. Accumulation of health disorders as a systemic measure of aging: Findings from the NLTCS data. Mech Ageing Dev. 2006;127:840–848. doi: 10.1016/j.mad.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulminski A, Ukraintseva SV, Arbeev KG, et al. Association between APOE epsilon 2/epsilon 3/epsilon 4 polymorphism and disability severity in a national long-term care survey sample. Age Ageing. 2008;37:288–293. doi: 10.1093/ageing/afn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulminski AM, Ukraintseva SV, Arbeev KG, et al. Health-protective and adverse effects of the apolipoprotein E epsilon2 allele in older men. J Am Geriatr Soc. 2008;56:478–483. doi: 10.1111/j.1532-5415.2007.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz S, Akpom CA. 12. Index of ADL. Med Care. 1976;14:116–118. doi: 10.1097/00005650-197605001-00018. [DOI] [PubMed] [Google Scholar]
- 40.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 41.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aviv A. Telomeres and human somatic fitness. J Gerontol A Biol Sci Med Sci. 2006;61:871–873. doi: 10.1093/gerona/61.8.871. [DOI] [PubMed] [Google Scholar]
- 43.Ang LS, Cruz RP, Hendel A, et al. Apolipoprotein E, an important player in longevity and age-related diseases. Exp Gerontol. 2008;43:615–22. doi: 10.1016/j.exger.2008.03.010. Epub 2008 Apr 4. [DOI] [PubMed] [Google Scholar]
- 44.Martins IJ, Hone E, Foster JK, et al. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–736. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 45.Ukraintseva S, Sloan F, Arbeev K, et al. Increasing rates of dementia at time of declining mortality from stroke. Stroke. 2006;37:1155–1159. doi: 10.1161/01.STR.0000217971.88034.e9. [DOI] [PubMed] [Google Scholar]
- 46.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: A nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 47.Damjanovic AK, Yang Y, Glaser R, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iancu EM, Speiser DE, Rufer N. Assessing ageing of individual T lymphocytes: mission impossible? Mech Ageing Dev. 2008;129:67–78. doi: 10.1016/j.mad.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Alter BP, Baerlocher GM, Savage SA, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richards JB, Valdes AM, Gardner JP, et al. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clin Nutr. 2007;86:1420–1425. doi: 10.1093/ajcn/86.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]


