Abstract
In spite of tremendous growth in recent years in our knowledge of the molecular basis of Parkinson's disease and the molecular pathways of cell injury and death, we remain without therapies that forestall disease progression. While there are many possible explanations for this lack of success, one is that experimental therapeutics to date have not adequately focused on an important component of the disease process, that of axon degeneration. It remains unknown what neuronal compartment, either the soma or the axon, is involved at disease onset, although some have proposed that it is the axons and their terminals that take the initial brunt of injury. Nevertheless, this concept has not been formally incorporated into many of the current theories of disease pathogenesis, and it has not achieved a wide consensus. More importantly, in view of growing evidence that the molecular mechanisms of axon degeneration are separate and distinct from the canonical pathways of programmed cell death that mediate soma destruction, the possibility of early involvement of axons in PD has not been adequately emphasized as a rationale to explore the neurobiology of axons for novel therapeutic targets. We propose that it is ongoing degeneration of axons, not cell bodies, that is the primary determinant of clinically apparent progression of disease, and that future experimental therapeutics intended to forestall disease progression will benefit from a new focus on the distinct mechanisms of axon degeneration.
Parkinson's disease (PD) has served as the prototypic adult-onset neurodegenerative disorder for which breakthroughs in experimental therapeutics have provided lasting, clinically significant improvements in the quality of life. Such was the case for the discovery of levodopa, and more recently for the discovery that deep brain stimulation is an effective adjunctive treatment1,2. In spite of these important advances we remain unable to offer therapies that halt the progression of the disease, and in this crucial respect therapies for PD are as limited as those for other degenerative neurological disorders. Towards the close of The Decade of the Brain (1990-2000), hope was expressed that an ability to forestall the progression of these devastating diseases was not far off3. Yet 10 years later, we seem no closer to our goal despite a multitude of important advances in our understanding of the molecular and genetic basis of PD and neuron death. Why has this therapeutic goal been so resistant to our best efforts? There are many possible reasons why this goal has remained elusive4. For instance, suboptimal animal models of PD and the complexities of clinical trial design may deter our ability to identify disease-modifying agents.
At the molecular level, the discovery that neurodegeneration is a highly regulated cell-autonomous process of programmed cell death (PCD)5 has fostered hope that true protective therapies may be within our grasp. It is reasoned that if neurodegeneration is an ordered process of PCD, then it should be possible to intervene even if the primary insult is unknown. Indeed, there have been numerous dramatic examples in animal studies of the prevention of neuron death due even to the most destructive neurotoxins by experimental blockade of PCD. The striking discordance between these dramatic neuroprotective effects and the complete failure of anti-apoptotic approaches in human clinical trials4,6 has been frustrating and baffling. Yet in this discordance there may be clues for a better approach.
In numerous animal studies it has been observed that remarkable protection of cell bodies achieved by blocking PCD is often not accompanied by protection at the axon level7-10. This discrepancy was not unexpected, because there is substantial evidence that the canonical pathways of PCD seem to play a minor role in axon degeneration11,12. The concept that destruction of the neuron cell body, which is brought about by these pathways, is a separate and distinct process from the destruction of axons by a process now sometimes called “programmed axonal death”13 has fairly broad recognition among investigators in cell death. However, this concept is not widely acknowledged in discussions about experimental therapeutics. This is somewhat surprising given that many investigators believe that at the onset of PD the brunt of the pathology is at the level of the axon terminal. Our purpose here therefore is to propose that mechanisms of axon degeneration merit greater attention in thinking about neuroprotection in PD.
How Does the Disease Process of PD Propagate within Neurons?
In the course of PD, eventually both axons and cell bodies of neurons degenerate. But in what cell compartment does this process begin? Does dysfunction begin at the cell soma and result in a secondary, anterograde degeneration of the axon? Or does dysfunction begin at the nerve terminal, or within the axon, when the cell soma is healthy, and then later, by a retrograde process, result in its degeneration? The sequence of events is critical in designing an approach to neuroprotection, because the earliest possible interventions will prevent the greatest number of secondary effects, including possibly the degenerative process itself. The question posed here, asking where the disease begins at the cellular level, is very different from the question of where it begins in the brain at the regional level. It has been proposed that PD begins in the regions of the dorsal motor nucleus of the vagus and the olfactory bulb14. However, this proposal has been controversial15-17 and does not relate directly to the question of the initial events at the cellular level. In the assessment of evidence relevant to the cellular compartment where PD begins, we turn mainly to data for the nigro-striatal dopaminergic system, because it has been most extensively studied in PD. We do not mean to imply by this focus on dopaminergic systems, however, that they are exclusively involved in this disease; on the contrary, it has been well known for many years that non-dopaminergic, non-catecholaminergic and non-motor systems are involved18. However, conclusions reached about the cellular sequence of events in the nigro-striatal dopamine system are likely to be relevant to the involvement of other, non-motor systems.
Estimates of SN Neuron Loss at Disease Onset
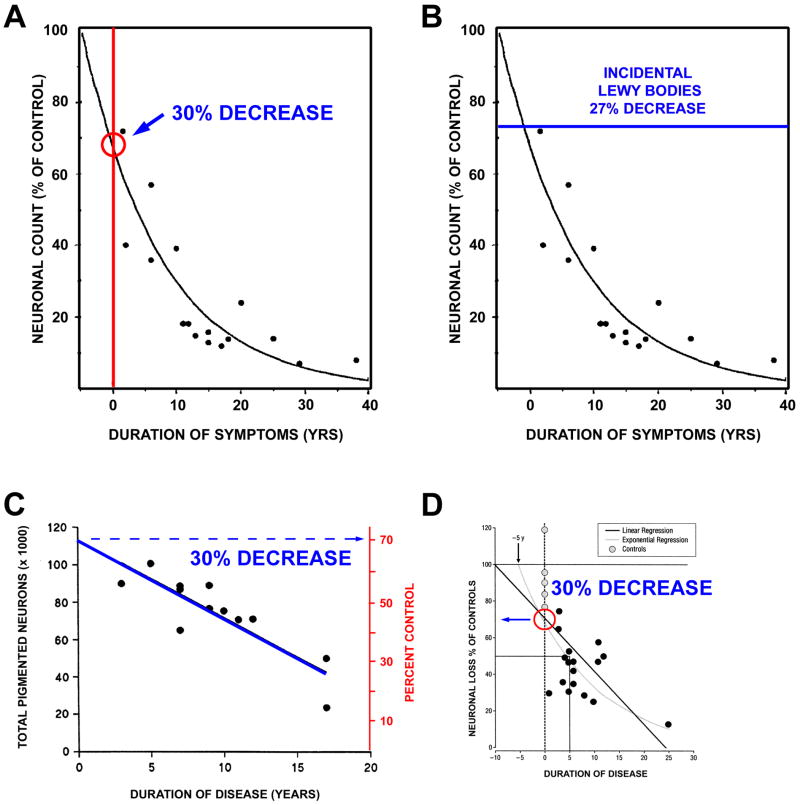
In most reviews of PD, it is stated that motor signs first appear when about 50% of substantia nigra (SN) dopamine neurons are lost19,20, although estimates of up to 60 to 70% have also been proposed21,22. The work most frequently cited for estimates of SN neuron loss is that of Fearnley and Lees23. Their regression analysis of neuron counts versus duration of PD indicated that the number of neurons lost at time of symptom onset is 31%, adjusted for age (Fig 1A). This estimate is compatible with their observation that individuals with incidental Lewy bodies (ILB), who may represent pre-clinical cases of PD, as discussed further below, showed a mean 27% age-adjusted loss of SN pigmented neurons without manifest motor signs (Fig 1B).
Fig. 1.
Estimates of loss of SN dopamine neurons at the time of PD symptom onset. (A) Fearnley and Lees23 examined the number of pigmented neurons in the SN in relation to duration of symptoms, and performed a regression analysis based on an exponential decline in their number. They estimated about a 30% total loss, adjusted for age. (B) In keeping with this estimate, they also found a sub-threshold loss of 27% of these neurons among individuals with incidental Lewy bodies. (C) A similar estimate can be derived from the data of Ma and colleagues, based on their dissector counts of pigmented SN neurons24. A linear regression analysis of their data with extrapolation to time of disease onset yields an estimate of about a 30% loss. (D) Greffard and colleagues performed counts of neurons in the SNpc, and determined density per unit volume. By either a linear or a negative exponential best fit analysis, they estimated a 30% loss at the time of symptom onset25.
Subsequent quantitative morphologic studies have supported an estimate of about a 30% loss of SN neurons at the time of onset of motor signs24,25. Using a dissector approach, Ma and colleagues examined the relationship between total number of pigmented neurons in SN and duration of disease (Fig 1C). Extrapolation of their linear regression analysis to Time 0 reveals an intercept at about 70% of control values. Performing a density per volume analysis of SN neurons, Greffard and colleagues likewise found that by either a linear or exponential regression, there is about a 30% loss at the time of the appearance of motor signs25. Thus, there is a good consistency in the available data to suggest that the motor signs of PD appear when there is about a 30% loss of total SN neurons in comparison to age-matched controls.
Estimates of Striatal Dopamine Terminal Loss at Disease Onset
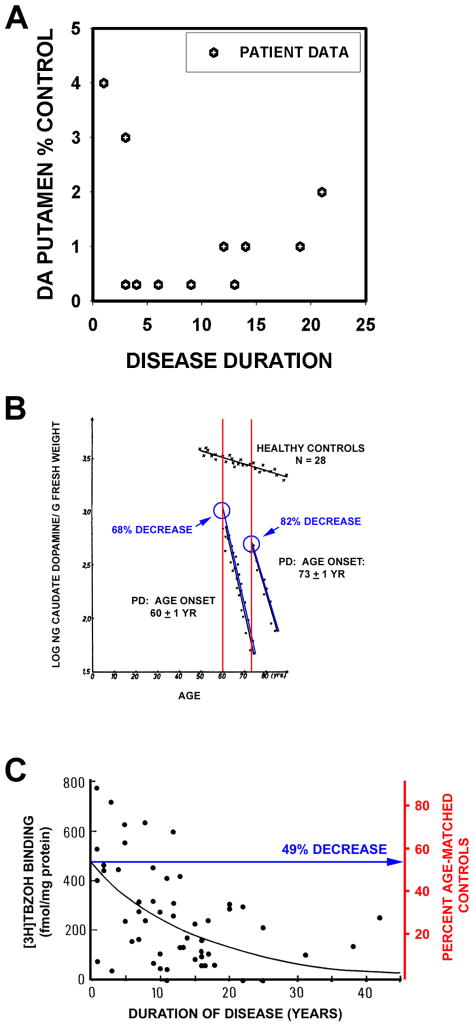
The work of Bernheimer and colleagues26 is often cited in support of the statement that Parkinson motor signs first appear when about 80% of striatal or putaminal dopamine is lost19,22. However, the Bernheimer study does not provide useful information for this estimate. Only 39 of the 64 patients studied had PD, and among them only 13 had dopamine measured in brain. There was no analysis of dopamine content as a function of disease duration, or a regression analysis. The data provided are too few and variable for this purpose (Fig 2A). Riederer and Wuketich analyzed postmortem data for caudate dopamine in relation to disease onset27. They studied two cohorts: one consisting of 27 patients with disease onset at 60 ± 1 years, and a second consisting of 12 patients with onset at 73 ± 1 years. As shown in Figure 2B, extrapolation to time of onset reveals a 68% and 82% decrease, respectively, in caudate dopamine for these two groups in relation to age-matched controls.
Fig. 2.
Estimates of loss of striatal dopamine terminals markers at time of symptom onset. (A) A graphical representation of the data presented in the oft-cited Bernheimer study to support the statement that there is an 80% reduction at the time of disease onset26. In the Bernheimer study, only 13 brains from patients with a diagnosis of PD were subjected to biochemical analysis. No regression analysis was performed. (B) Reiderer and Wuketich27 measured caudate dopamine content in two PD cohorts, one with an age of onset at 60 ± 1 years, and a second at 73 ± 1 years. Back extrapolation indicates a 68% and an 82% decrease, respectively, in caudate dopamine at the time of onset of disease in the two groups. (C) Scherman and colleagues analyzed [3H]TBZOH binding to the vesicular monoamine transporter in postmortem caudate nucleus of 54 PD patients. Polynomial regression analysis indicated a loss of 49% of binding sites at time of disease onset.
Postmortem studies of dopamine are subject to concerns about the effects of postmortem delay. Measurements of vesicular monoamine transporter (VMAT2) binding sites with tritiated α-dihydrotetrabenazine ([3H]TBZOH) help to address these issues28. Scherman and colleagues analyzed [3H]TBZOH binding in postmortem caudate in 57 PD patients and 49 controls, and concluded that motor signs become apparent when there is about a 50% decrease relative to the levels at the average age of disease onset (Fig 2C). In both studies depicted in Figures 2B, C the data is for the caudate. In the Scherman study, there was a substantially greater degree of loss in the putamen. Based on the analysis of Kish and colleagues29 it would be expected that there would be a greater degree of dopamine loss in putamen, at time of disease onset, than that reported for caudate. Thus, these estimates of loss of dopaminergic markers in caudate are likely to underestimate total striatal loss.
Additional information about the extent of striatal damage at the time of symptom onset can be derived from studies of ILB. The concept that patients with ILB, i.e. Lewy bodies in brain in the absence of clinical parkinsonism or dementia, may represent pre-clinical PD is supported by the aforementioned study by Fearnley and Lees23. Similar findings were reported by Ross and colleagues20 who observed a significant 17% decrease in SN neuron density in ILB brains. In an assessment of ILB brains, Beach and colleagues determined that the putamen had a 50% reduction in tyrosine hydroxylase (TH) protein30.
While counts of remaining neurons in the SN are not likely to be altered by factors affecting the quality of postmortem tissue preservation, biochemical assessments are. Concern may therefore be raised that the greater apparent losses of striatal dopaminergic markers than SN neurons in these studies may be an artifact due to the analysis of postmortem tissues. It is therefore worthwhile to consider radioligand imaging analysis of striatal dopaminergic markers obtained in vivo. Numerous imaging studies have examined the relationship between striatal dopaminergic marker loss and onset of motor signs. We will consider only those studies that have used either a regression analysis with extrapolation to time of disease onset, or, alternatively, have studied patients with unilateral PD (i.e., Hoehn and Yahr stage I (HY I)) and have compared the degree of loss for the unaffected side to that of the affected side (Table). Among these studies, three types of radioligand have been used: [18F] dopa to assess levodopa metabolism, ligands for the dopamine transporter, or ligands for the vesicular monoamine transporter (see Nandhagopal et al for review31). Estimates of dopamine terminal loss at time of disease onset are less for those obtained with [18F] dopa (20-50% in putamen) than for these obtained with the other ligands (50-70% in putamen). It has been suggested that compensatory upregulation of aromatic acid decarboxylase may result in an underestimate of terminal losses by use of [18F] dopa PET31,32. If we therefore restrict our attention to losses assessed by the other ligands, the estimates of 50 to 70% correspond fairly well to the estimates of about 50% loss based on the postmortem studies of [3H]TBZOH (see Fig 2)28, and on measurement of TH protein in patients with ILB30.
TABLE. PD Imaging Studies of Striatal or Putaminal Dopaminergic Deficits at Time of Symptom Onset.
| AUTHOR | YEAR | N | MODALITY | LIGAND | STRIATUM (% LOSS) | PUTAMEN (% LOSS) | ANALYSIS |
|---|---|---|---|---|---|---|---|
| LEVODOPA METABOLISM | |||||||
| Morrish et al | 1995 | 11 | PET | [18F]DOPA | --- | 20-43 | HY I (IPSI vs CONTRA) |
| Morrish et al | 1998 | 32 | PET | [18F]DOPA | --- | 25 | REGRESSION |
| Lee et al | 2000 | 13 (HY I) | PET | [18F]DOPA | --- | 38-52 | HY I (IPSI vs CONTRA) |
| Hilker et al | 2005 | 31 | PET | [18F]DOPA | --- | 31 | REGRESSION |
| DOPAMINE TRANSPORTER BINDING | |||||||
| Tissingh et al | 1998 | 8 (HY I) | SPECT | [123I]β-CIT | 39-51 | 51-64 | HY I (IPSI vs CONTRA) |
| Lee et al | 2000 | 13 (HY I) | PET | [11C]MP | --- | 56-71 | HY I (IPSI vs CONTRA) |
| Schwartz et al | 2004 | 6 | SPECT | [123I]IPT | 43 | 56 | REGRESSION |
| VESICULAR MONOAMINE TRANSPORTER BINDING | |||||||
| Lee et al | 2000 | 13 (HY I) | PET | [11C]DTBZ | 51-62 | HY I (IPSI vs CONTRA) | |
In each study, the degree of dopaminergic terminal loss in whole striatum or putamen was determined either by regression analysis with back extrapolation to Time = 0, or by determination of the loss on the side ipsilateral (IPSI) to symptoms in comparison to the side contralateral (CONTRA) to symptoms in patients with unilateral PD (Hoehn and Yahr Stage I (HY I)). β-CIT: 2β-carbomethoxy-3 β -(4-iodophenyl); MP: methylphenidate; IPT: N-(3-iodopropene-2-yl)-2β - carbomethoxy-3β -(chlorophenyl); DTBZ: dihydrotetrabenazine.
In conclusion, assessment of available data suggests that at the time of motor symptom onset the extent of loss of striatal or putaminal dopaminergic markers exceeds that of SN dopamine neurons. This conclusion is consistent with observations that, at the time of death, depending on disease duration, while there has been 60-80% loss of SN dopamine neurons23,33, there has been a much more profound loss of striatal or putaminal dopaminergic markers26,28,29. It may still be argued that it is difficult to make comparisons in this data, due to differences in technique, subregions examined, and consideration of the effect of age. There is clearly a need to attempt to directly compare extent of axonal pathology to neuron loss in postmortem PD brain material. A preliminary report suggests that such a comparison does reveal that striatal dopaminergic axon loss is indeed an early and predominant feature34. However, based on the limitations of available postmortem data, it is worthwhile to consider independent evidence of early axon involvement in PD, based on genetic causes.
Early Axon Involvement in PD: Evidence from Genetic Causes
The discovery of disease-causing mutations in the gene for α-synuclein has had a major impact on our understanding of the pathogenesis of PD (reviewed in35). The impact was further amplified when it was discovered that α-synuclein protein is a major component of Lewy bodies (LBs)36. With the development of sensitive antibodies to α-synuclein, it has become easier to detect LBs in tissue sections, and they have become a great focus of attention. The ease of detection of LBs is due not only to the availability of sensitive anti-α-synuclein antibodies, but also their prominent and distinct intracellular appearance, and their tendency to cluster in vulnerable nuclear groups. However, the ease of detection of LBs should not be equated with pathogenic significance. When effort is made to seek α-synuclein pathology in axons, by use of refined immunohistochemical procedures37 or unique immunoreagents38,39, it is readily observed. Strikingly, by use of a novel paraffin-embedded tissue blot technique, in which tissue sections are subjected to pre-digestion, the greatest abundance of synuclein aggregates is found not in cell bodies, but in the neuropil40 in dementia with Lewy Body (DLB) brains. Furthermore, these studies show that the preponderance of α-synuclein small aggregates in DLB brains are entrapped within pre-synaptic terminals40. Thus, there is evidence that α-synuclein pathology is abundant in axons and pre-synaptic terminals, consistent with the observation that its normal localization is predominantly in pre-synaptic terminals.
There have been few studies attempting to determine the sequence of development of synuclein pathology at the cellular level in neurons. However, Oriomo and colleagues have exploited the known propensity of PD to affect peripheral autonomic neurons and their axons to explore the timing of events41. Based on patterns of α-synuclein pathology and TH immunostaining in cardiac sympathetic axons and ganglia in patients with ILB, PD and controls, they conclude that the disease process begins in the distal axon and proceeds retrograde.
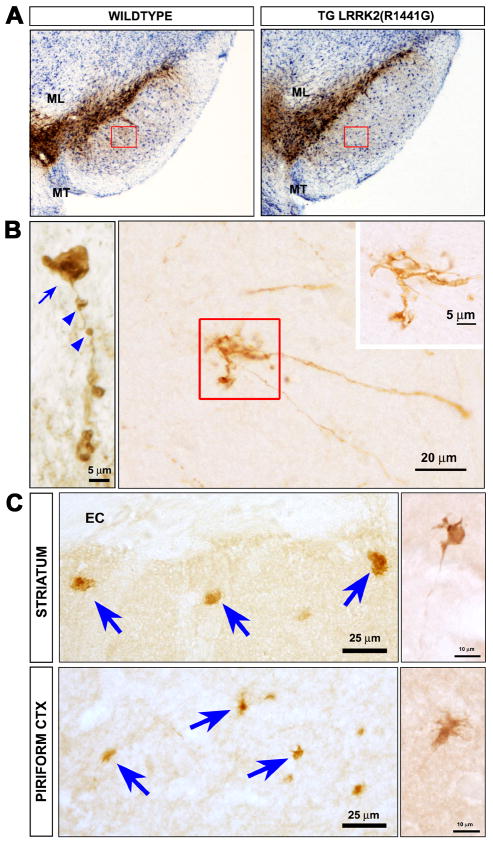
While the discovery of synuclein mutations has had great impact, they are rare. The most common genetic cause of typical PD are mutations in the gene for leucine rich repeat kinase 2 (LRRK2)42. The recent description of a new BAC transgenic mouse model, created with a disease-causing human mutant (hLRRK2 (R1441G)) has shed some light on possible early disease events43. These mice show the development of an age-related hypokinesia by 9-10 months that is reversible by treatment with levodopa. There is no loss of mesencephalic dopamine neurons (Fig 3A), but pathology is observed in dopaminergic axons. On TH immunostaining, the axons are fragmented, they are associated with axonal spheroids, and they form dystrophic neurites43 (Fig 3B). These abnormal axonal features are also observed by staining for abnormally phosphorylated tau (Fig 3C). Additional evidence supports the possibility that LRRK2 plays an important role in the regulation of neurite growth and integrity. MacLeod and colleagues have reported that mutant forms of LRRK2 induce decreases of neurite length in primary neuron culture 44. Similar observations were made for the LRRK2(G2019S) mutant in neuronally differentiated neuroblastoma cells 45 and in primary neurons derived from transgenic mice 46. The molecular basis of these effects is not known, but of potential interest in this regard is the identification of moesin, and the closely allied proteins ezrin and radixin, as possible LRRK2 substrates 47. These proteins have been implicated in the regulation of neurite outgrowth 48. The ability of LRRK2 to regulate the phosphorylation status of these proteins, and the closely correlated degree of neurite growth, has been observed in primary cultures 46.
Fig. 3.
Axonopathy in hLRRK2(R1441G) BAC transgenic mice. (A) Immunoperoxidase staining for TH reveals no loss of SN dopamine neurons in the hLRRK2(R1441G) transgenics. (B) At the single axon level, staining for tyrosine hydroxylase (TH) reveals fragmentation (blue arrowheads), axonal spheroids (blue arrow), and dystrophic neurites at axon terminals (red square and inset). (C) Axonal abnormalities in the striatum and piriform cortex of the transgenic mice are also revealed by immunostaining for phosphorylated tau. Spheroids (blue arrows) and dystrophic neurites (side panels) similar to those visualized by TH staining, are observed. (Images adapted from Li et al43).
Thus, based on analysis of the predominant site of pathology in PD at its onset, and evidence from autosomal dominant genetic forms of the disease, it is reasonable to hypothesize that axon dysfunction may be an early feature of PD.
The Molecular Mechanisms of Neuron Soma and Axon Degeneration are Distinct
The concept that axons may be involved early in the course of PD is not new; it was proposed by Hornykiewicz years ago when he suggested that in PD neurodegeneration may be a dying-back process that begins in the striatal terminals49. Our purpose in re-assessing the evidence in support of this idea is that only more recently has it become clear that this concept, most importantly, also implies that different molecular mechanisms may underlie the onset and motor progression of PD than those that are involved in the destruction of SN neurons.
Neuron soma degeneration in the absence of axon degeneration: The Wallerian Degeneration Slow (WldS) Mouse
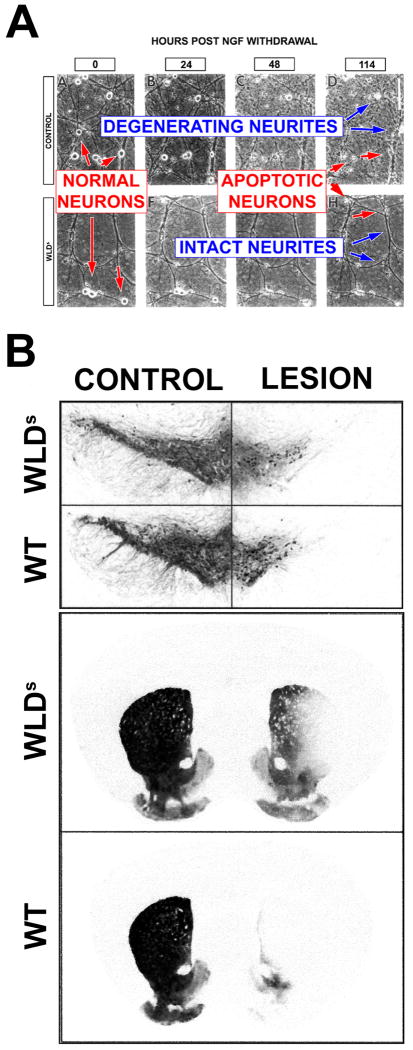
The most striking evidence that axons can survive even in the presence of destruction of the neuronal soma derives from observations made in the WldS mouse50. This mutation arose spontaneously in C57Bl/6 mice, and it was demonstrated to cause delayed Wallerian degeneration in peripheral nerve after axotomy51. The mutation was identified as an 85-kb tandem triplication that results in a novel chimeric mRNA that encodes for the N-terminal 70 amino acids of ubiquitination factor E4B (Ube4B), followed by the complete coding sequence for the nicotinamide adenine dinucleotide (NAD) synthesizing enzyme nicotinamide mononucleotide adenylyltransferase (NMNAT)52,53. It was shown by Deckwerth and Johnson54 that axons of sympathetic ganglion neurons derived from WldS mice survive following withdrawal of nerve growth factor in spite of induction of apoptosis in the cell soma (Fig 4A).
Fig. 4.
Studies of the WldS mutant mouse demonstrate that degeneration of neuron cell bodies and axons is mediated by distinct mechanisms. (A) Deckwerth and Johnson54 demonstrated that both the cell bodies and the neurites of wild type sympathetic ganglion neurons degenerate in culture after NGF withdrawal, but only cell bodies of WldS mice degenerate. (B) In a living mouse model Sajadi et al58 demonstrated that, remarkably, after medial forebrain bundle 6OHDA lesion, while there has been a substantial loss of neurons in mice of both genotypes, there is a substantial preservation of axons in WldS. The top panels show loss of dopamine neurons by immunostaining for TH, and the bottom panels show striatal dopaminergic innervation by immunostaining for the dopamine transporter.
The WldS mutation protects axons of many different types of neurons, in diverse species, from a wide variety of injuries, including toxic peripheral neuropathies55 and genetic neuropathies56 (and see Coleman13 and Luo and O'Leary57 for reviews). In neurotoxin models of parkinsonism, the WldS mutation protects dopaminergic axons, but not cell bodies, from medial forebrain bundle injection of 6-hydroxydopamine (6OHDA)58 (Fig 4B) and injection of MPTP59.
These observations suggest that with a deeper understanding of the mechanisms underlying the WldS phenotype, it may be possible to target the molecular pathways of axon degeneration with therapeutic benefit. It is now clear that enzymatic activity of NMNAT is necessary, but not sufficient, for axon protection60-63. In addition to its enzymatic activity, NMNAT appears to require correct cellular targeting. Interestingly, the full protection phenotype can be observed in experiments with NMNAT3, a mitochondrially-targeted isoform64-66.
Canonical Pathways of PCD Play a Restricted Role in Axon Degeneration
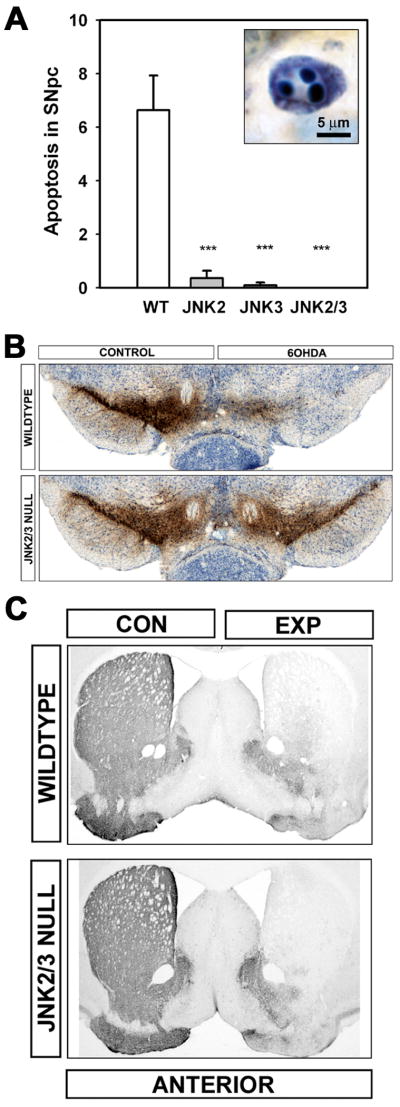
The concept that important mediators of PCD, such as the caspases, do not play a role in axon degeneration received considerable support from investigations reported by Finn and colleagues who noted that caspase-3 is not activated in a variety of models of axon degeneration11. These negative results are not universal, however; in other contexts a role for caspases has been identified67-71. Nevertheless, it remains true that experimental measures intended to block apoptosis commonly prevent cell body, but not axonal, degeneration. This discordance has been observed with a variety of anti-apoptotic approaches in a variety of neurotoxin models of parkinsonism in adult rodents7-10,72 and in a genetic model of motor neuron disease73. Recent work from our laboratory provides a particularly striking example of this discordance10. Our studies were undertaken to determine whether the c-jun N-terminal kinases (JNKs) play an essential role in apoptotic death of SN dopamine neurons. The JNKs play a central role in mitogen-activated protein kinase (MAPK) apoptotic pathways. These pathways have been widely implicated as mediators of PCD in neurons (reviewed in Silva74). To assess the role of the JNKs, we studied mice null for the JNK2 or –3 isoforms, or both, by injection of 6OHDA in the striatum. Mice homozygous null for both JNK2 and JNK3 were entirely resistant to the induction of apoptosis in this highly destructive model, and there was a virtually complete protection of dopamine neuron cell bodies in the SNpc (Fig 5A,B). However, there was no protection at all of axons in this model of retrograde axonal degeneration (Fig 5C).
Fig. 5.
Resistance of neuron cell bodies, but not axons, to degeneration in JNK null mice. (A) The intrastriatal 6OHDA neurotoxin model induces apoptosis in SN dopamine neurons in wildtype mice. A typical example of an apoptotic profile, with characteristic chromatin clumps, is demonstrated by thionin counterstain in the inset. The homozygous jnk2 and jnk3 single null mutations suppressed apoptosis by 95% and 98%, respectively, and the homozygous jnk2/3 double null mutations completely abrogated apoptosis. (B) The homozygous jnk2/3 double null mutations provided virtually complete protection of SN dopamine neurons. Among wildtype (WT) mice, there was a 63% loss of dopamine neurons, typical for this model, whereas among jnk2/3 nulls, there was only a 4% decrease. Low power photomicrographs of representative TH-immunostained SN sections from wildtype (top) and jnk2/3 nulls (bottom) following unilateral 6OHDA injection. (C) Homozygous jnk2/3 double null mice are not resistant to retrograde degeneration of nigrostriatal dopaminergic axons induced by intrastriatal 6OHDA. Following 6OHDA, there is a virtually complete loss of TH-positive fiber staining in homozygous jnk2/3 null mice, as in wildtype, throughout the striatum.
In conclusion, there is now abundant evidence, based on diverse experimental approaches, that the molecular mechanisms of axon degeneration are distinct from those of PCD, and therefore they should be considered as separate and distinct candidates for a role in pathogenesis, and as targets for therapeutics.
Axon Degeneration and PD: Implications for Neuroprotection
While our principal focus here has been on the evidence that the earliest cellular locus of abnormality in PD may be the axons and their terminals, it is also well known that after the disease has run its course, the brunt of the pathology continues to be at the level of the axons and their terminals. These observations therefore suggest that throughout the course of the disease the axons and their terminals are the principal site of pathology. Given also that the terminals are, of course, the principal site of dopamine release and the essential mediators of this primary function of SN dopamine neurons, it would follow that it is the progressive degeneration of axons and their terminals, and not neuron loss, that is the primary determinant of clinical progression. If such is the case, then the concept that the mechanisms of axon and soma degeneration are separate and distinct has implications for both the early treatment and diagnosis of PD.
Although there is much evidence for a role for PCD in PD75, and although preservation of neuron cell bodies is essential for the function and prolonged survival of axons, the protection of cell bodies alone will not be sufficient to prevent clinical deterioration. This point can perhaps be illustrated by consideration of the failure of the PRECEPT neuroprotection trial in PD76. This trial examined the ability of a mixed lineage kinase inhibitor, CEP-1347, to forestall disease progression in early PD. The rationale for the trial was that blockade of the MAPK signaling pathway by a variety of means, including administration of CEP-1347, had been shown to block apoptosis and provide neuroprotection in a variety of PD models (reviewed in Silva74). While there are of course many possible reasons why the trial failed, as previously reviewed77, an important possibility is that although blockade of MAPK signaling blocks apoptosis, it does not protect axons in the mature brain 9,10.
A new emphasis on protecting axons may provide new therapeutic approaches. For example, we have recently shown, as a proof of principle, that signaling through the Akt-Rheb-mTor pathway can forestall retrograde axon degeneration in the dopaminergic nigro-striatal pathway following either neurotoxin or axotomy lesion78 (and submitted).
The emphasis on the neurobiology of axons presented here also has implications for neurorestorative approaches to the treatment of PD. To date, the approaches that have received the most emphasis are based on cell replacement. However, these approaches face many daunting challenges related to cell survival, malignant growth, loss of phenotype, absence of normal anatomic connectivity and regulation. If the clinical progression of PD is due first and foremost to axon degeneration, then the wisdom of cell replacement approaches needs reassessment. At the onset of the disease, an estimated 70% of dopamine neurons remain, and it is not their number that is limiting, but rather their axonal projections. Restoration of these projections, by the induction of axon sprouting from the neurons that remain in situ, rather than implanting new exogenous cells in ectopic locations, would seem a more practical and effective therapeutic goal. While classically the central nervous system has been thought to be incapable of axonal re-growth, this not the case. Axon regeneration can be promoted in the adult central nervous system by PTEN/Akt/mTor signaling79, and there is preliminary evidence that the same can be achieved in the dopaminergic nigro-striatal system as well80.
Conclusions
Approaches to experimental therapeutics in PD would benefit from a greater emphasis on the neurobiology of axons of the central nervous system. This new emphasis will provide realistic grounds for optimism in efforts to develop neuroprotective approaches. Our perspective would suggest that at the time of first diagnosis of PD, only 30% or so of dopamine neurons of the SN have been lost and only 50-60% of their axon terminals. There is therefore a substantial ‘window of opportunity’ to preserve what remains. Furthermore, restoration of axon growth in the mature nervous system should not be considered as unrealistic. We are at very early stages in our understanding of both the mechanisms of axon degeneration and the potential for axon regrowth in the mature central nervous system. There will undoubtedly be found many opportunities for future therapeutic targets.
Acknowledgments
This work was supported by NIH NINIDS NS26836, NS38370, the Parkinson's Disease Foundation and the RJG Foundation.
Footnotes
Statement of Conflict: The authors have no financial interests to disclose
References
- 1.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 2.Limousin P, Pollak P, Benazzouz A, et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 3.Shoulson I. Experimental therapeutics of neurodegenerative disorders: unmet needs. Science. 1998;282:1072–1074. doi: 10.1126/science.282.5391.1072. [DOI] [PubMed] [Google Scholar]
- 4.Olanow CW, Kieburtz K, Schapira AH. Why have we failed to achieve neuroprotection in Parkinson's disease? Ann Neurol. 2008;64 2:S101–S110. doi: 10.1002/ana.21461. [DOI] [PubMed] [Google Scholar]
- 5.Ellis RE, Yuan J, Horvitz HR. Mechanisms and functions of cell death. Annual Review of Cell Biology. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, Pearce LA, Ravina BM, et al. Neuroprotection trials in Parkinson's disease: systematic review. Mov Disord. 2009;24:647–654. doi: 10.1002/mds.22432. [DOI] [PubMed] [Google Scholar]
- 7.Eberhardt O, Coelln RV, Kugler S, et al. Protection by synergistic effects of adenovirus-mediated X-chromosome-linked inhibitor of apoptosis and glial cell line-derived neurotrophic factor gene transfer in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J Neurosci. 2000;20:9126–9134. doi: 10.1523/JNEUROSCI.20-24-09126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayley S, Crocker SJ, Smith PD, et al. Regulation of dopaminergic loss by Fas in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J Neurosci. 2004;24:2045–2053. doi: 10.1523/JNEUROSCI.4564-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Rzhetskaya M, Kareva T, et al. Antiapoptotic and trophic effects of dominant-negative forms of dual leucine zipper kinase in dopamine neurons of the substantia nigra in vivo. J Neurosci. 2008;28:672–680. doi: 10.1523/JNEUROSCI.2132-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ries V, Silva RM, Oo TF, et al. JNK2 and JNK3 combined are essential for apoptosis in dopamine neurons of the substantia nigra, but are not required for axon degeneration. J Neurochem. 2008;107:1578–1588. doi: 10.1111/j.1471-4159.2008.05713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn JT, Weil M, Archer F, et al. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J Neurosci. 2000;20:1333–1341. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 13.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 15.Burke RE, Dauer WT, Vonsattel JP. A critical evaluation of the Braak staging scheme for Parkinson's disease. Ann Neurol. 2008;64:485–491. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jellinger KA. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta. 2009;1792:730–740. doi: 10.1016/j.bbadis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Lees AJ. The Parkinson chimera. Neurology. 2009;72:S2–11. doi: 10.1212/WNL.0b013e318198daec. [DOI] [PubMed] [Google Scholar]
- 18.Agid Y, Agid F, Ruberg M. Biochemistry of neurotransmitters in Parkinson's disease. In: Marsden CD, Fahn S, editors. Movement Disorders 2. London: Butterworths; 1987. pp. 166–230. [Google Scholar]
- 19.Marsden CD. Parkinson's disease. Lancet. 1990;335:948–952. doi: 10.1016/0140-6736(90)91006-v. [DOI] [PubMed] [Google Scholar]
- 20.Ross GW, Petrovitch H, Abbott RD, et al. Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol. 2004;56:532–539. doi: 10.1002/ana.20226. [DOI] [PubMed] [Google Scholar]
- 21.Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 22.Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 23.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 24.Ma SY, Roytta M, Rinne JO, et al. Correlation between neuromorphometry in the substantia nigra and clinical features in Parkinson's disease using disector counts. J Neurol Sci. 1997;151:83–87. doi: 10.1016/s0022-510x(97)00100-7. [DOI] [PubMed] [Google Scholar]
- 25.Greffard S, Verny M, Bonnet AM, et al. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol. 2006;63:584–588. doi: 10.1001/archneur.63.4.584. [DOI] [PubMed] [Google Scholar]
- 26.Bernheimer H, Birkmayer W, Hornykiewicz O, et al. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 27.Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson's disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm. 1976;38:277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- 28.Scherman D, Desnos C, Darchen F, et al. Striatal dopamine deficiency in Parkinson's disease: Role of aging. Ann Neurol. 1989;26:551–557. doi: 10.1002/ana.410260409. [DOI] [PubMed] [Google Scholar]
- 29.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 30.Beach TG, Adler CH, Sue LI, et al. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol. 2008;115:445–451. doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nandhagopal R, McKeown MJ, Stoessl AJ. Functional imaging in Parkinson disease. Neurology. 2008;70:1478–1488. doi: 10.1212/01.wnl.0000310432.92489.90. [DOI] [PubMed] [Google Scholar]
- 32.Lee CS, Samii A, Sossi V, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson's disease. Ann Neurol. 2000;47:493–503. [PubMed] [Google Scholar]
- 33.Pakkenberg B, Moller A, Gundersen HJ, et al. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodiya HB, Chu Y, Beach TG, et al. The status of dopaminergic putaminal innervation in Parkinson's disease as a function of disease duration: Relevance to trophic factor therapy. Abstr Soc Neurosci. 2009 [Google Scholar]
- 35.Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 36.Spillantini MG, Schmidt ML, Lee VMY, et al. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 37.Braak H, Sandmann-Keil D, Gai W, et al. Extensive axonal Lewy neurites in Parkinson's disease: a novel pathological feature revealed by alpha-synuclein immunocytochemistry. Neurosci Lett. 1999;265:67–69. doi: 10.1016/s0304-3940(99)00208-6. [DOI] [PubMed] [Google Scholar]
- 38.Galvin JE, Uryu K, Lee VM, et al. Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc Natl Acad Sci U S A. 1999;96:13450–13455. doi: 10.1073/pnas.96.23.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duda JE, Giasson BI, Mabon ME, et al. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52:205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 40.Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orimo S, Uchihara T, Nakamura A, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson's disease. Brain. 2008;131:642–650. doi: 10.1093/brain/awm302. [DOI] [PubMed] [Google Scholar]
- 42.Greggio E, Cookson MR. Leucine-rich repeat kinase 2 mutations and Parkinson's disease: three questions. ASN Neuro. 2009;1 doi: 10.1042/AN20090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Liu W, Oo TF, et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLeod D, Dowman J, Hammond R, et al. The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Plowey ED, Cherra SJ, III, Liu YJ, et al. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parisiadou L, Xie C, Cho HJ, et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J Neurosci. 2009;29:13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaleel M, Nichols RJ, Deak M, et al. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson's disease mutants affect kinase activity. Biochem J. 2007;405:307–317. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paglini G, Kunda P, Quiroga S, et al. Suppression of radixin and moesin alters growth cone morphology, motility, and process formation in primary cultured neurons. J Cell Biol. 1998;143:443–455. doi: 10.1083/jcb.143.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hornykiewicz O. Biochemical aspects of Parkinson's disease. Neurology. 1998;51:S2–S9. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 50.Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- 51.Lunn ER, Perry VH, Brown MC, et al. Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 52.Conforti L, Tarlton A, Mack TG, et al. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc Natl Acad Sci U S A. 2000;97:11377–11382. doi: 10.1073/pnas.97.21.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mack TG, Reiner M, Beirowski B, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 54.Deckwerth TL, Johnson EM., Jr Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis) Dev Biol. 1994;165:63–72. doi: 10.1006/dbio.1994.1234. [DOI] [PubMed] [Google Scholar]
- 55.Wang MS, Fang G, Culver DG, et al. The WldS protein protects against axonal degeneration: a model of gene therapy for peripheral neuropathy. Ann Neurol. 2001;50:773–779. doi: 10.1002/ana.10039. [DOI] [PubMed] [Google Scholar]
- 56.Mi W, Beirowski B, Gillingwater TH, et al. The slow Wallerian degeneration gene, WldS, inhibits axonal spheroid pathology in gracile axonal dystrophy mice. Brain. 2005;128:405–416. doi: 10.1093/brain/awh368. [DOI] [PubMed] [Google Scholar]
- 57.Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 58.Sajadi A, Schneider BL, Aebischer P. Wlds-mediated protection of dopaminergic fibers in an animal model of Parkinson disease. Curr Biol. 2004;14:326–330. doi: 10.1016/j.cub.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 59.Hasbani DM, O'Malley KL. Wld(S) mice are protected against the Parkinsonian mimetic MPTP. Exp Neurol. 2006;202:93–99. doi: 10.1016/j.expneurol.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 60.Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 61.MacDonald JM, Beach MG, Porpiglia E, et al. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 62.Conforti L, Wilbrey A, Morreale G, et al. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J Cell Biol. 2009;184:491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sasaki Y, Vohra BP, Lund FE, et al. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci. 2009;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sasaki Y, Araki T, Milbrandt J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Avery MA, Sheehan AE, Kerr KS, et al. Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J Cell Biol. 2009;184:501–513. doi: 10.1083/jcb.200808042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yahata N, Yuasa S, Araki T. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J Neurosci. 2009;29:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srinivasan A, Roth KA, Sayers RO, et al. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death and Differentiation. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- 68.Buki A, Okonkwo DO, Wang KK, et al. Cytochrome c release and caspase activation in traumatic axonal injury. J Neurosci. 2000;20:2825–2834. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cowan CM, Thai J, Krajewski S, et al. Caspases 3 and 9 send a pro-apoptotic signal from synapse to cell body in olfactory receptor neurons. J Neurosci. 2001;21:7099–7109. doi: 10.1523/JNEUROSCI.21-18-07099.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Khodor BF, Burke RE. Medial forebrain bundle axotomy during development induces apoptosis in dopamine neurons of the substantia nigra and activation of caspases in their degenerating axons. J Comp Neurol. 2002;452:65–79. doi: 10.1002/cne.10367. [DOI] [PubMed] [Google Scholar]
- 71.Nikolaev A, McLaughlin T, O'Leary DD, et al. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Silva RM, Ries V, Oo TF, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem. 2005;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sagot Y, Dubois-Dauphin M, Tan SA, et al. Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. J Neurosci. 1995;15:7727–7733. doi: 10.1523/JNEUROSCI.15-11-07727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silva RM, Kuan CY, Rakic P, et al. Mixed lineage kinase-c-jun N-terminal kinase signaling pathway: A new therapeutic target in Parkinson's disease. Mov Disord. 2005;20:653–664. doi: 10.1002/mds.20390. [DOI] [PubMed] [Google Scholar]
- 75.Burke RE. Programmed cell death and new discoveries in the genetics of parkinsonism. J Neurochem. 2008;104:875–890. doi: 10.1111/j.1471-4159.2007.05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.The Parkinson Study Group PRECEPT Investigators. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007;69:1480–1490. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- 77.Waldmeier P, Bozyczko-Coyne D, Williams M, et al. Recent clinical failures in Parkinson's disease with apoptosis inhibitors underline the need for a paradigm shift in drug discovery for neurodegenerative diseases. Biochem Pharmacol. 2006 doi: 10.1016/j.bcp.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 78.Cheng HC, Oo TF, Kareva T, et al. Akt/PKB inhibits retrograde axonal degeneration in the nigro-striatal dopaminergic pathway in vivo. Abstr Soc Neurosci. 2008 [Google Scholar]
- 79.Park KK, Liu K, Hu Y, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen X, Kareva T, Rzhetskaya M, et al. Restoration of the nigrostriatal dopaminergic pathway in mouse models of Parkinson's disease: effects of a constitutively active form of AKT. Abstr Soc Neurosci. 2008 [Google Scholar]