Abstract
Behavioural learning depends on the brain's capacity to respond to instructive experience and is often enhanced during a juvenile sensitive period. How instructive experience acts on the juvenile brain to trigger behavioural learning remains unknown. In vitro studies show that forms of synaptic strengthening thought to underlie learning are accompanied by increased stability, number and size of dendritic spines, the major site of excitatory synaptic transmission in the vertebrate brain1–7. In vivo imaging studies in sensory cortical regions reveal that these structural features can be affected by disrupting sensory experience and that spine turnover is elevated during sensitive periods for sensory map formation8–12. These observations support two hypotheses: 1) the increased capacity for behavioural learning during a sensitive period is associated with enhanced spine dynamics on sensorimotor neurons important to the learned behaviour; 2) instructive experience rapidly stabilizes and strengthens these dynamic spines. Here we tested these hypotheses using two-photon in vivo imaging to measure spine dynamics in zebra finches, which learn to sing by imitating a tutor song during a juvenile sensitive period13,14. Spine dynamics were measured in the forebrain nucleus HVC, the proximal site where auditory information merges with an explicit song motor representation15–19, immediately before and after juvenile finches first experienced tutor song20. Higher levels of spine turnover prior to tutoring correlated with a greater capacity for subsequent song imitation. In juveniles with high levels of spine turnover, hearing a tutor song led to the rapid (~24h) stabilization, accumulation and enlargement of dendritic spines in HVC. Moreover, in vivo intracellular recordings made immediately before and after the first day of tutoring revealed robust enhancement of synaptic activity in HVC. These findings suggest behavioural learning results when instructive experience is able to rapidly stabilize and strengthen synapses on sensorimotor neurons important to the control of the learned behaviour.
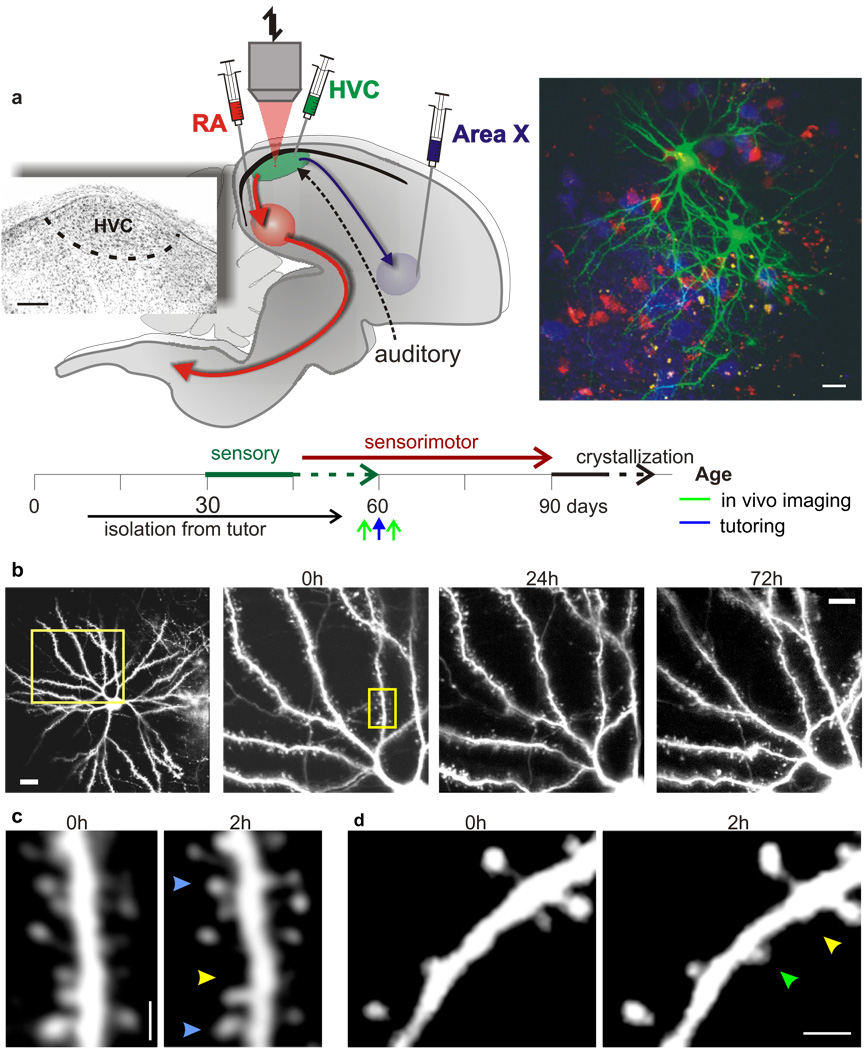
Investigating structural correlates of song learning requires repeated imaging of dendritic structure as a juvenile bird learns to sing. We used lentivirus-GFP constructs to fluorescently label neurons21,22, retrograde tracers to localize the boundaries of HVC, and two-photon microscopy to image dendritic spines on individual HVC neurons through a surgically implanted cranial window in male zebra finches (Fig. 1a). We focused on spinous HVC neurons, which are projection neurons (PNs) important to singing and song learning 23,24. To minimize interference with the bird’s behaviour, images were collected during its subjective nighttime. Initial experiments in which neurons in either juveniles (60–90d, N = 2) or adults (120–130d, N = 3) raised with normal access to a tutor were repeatedly imaged revealed that the dendritic arbours of HVC PNs remained stable over the course of several nights (Fig. 1b). Importantly, repeated (Δt=2h) imaging within a single night also detected a subset of dendritic spines that underwent turnover (Fig. 1 c, d; N = 9 birds, 6 (60–90d) and 3 (130d) birds). Imaging on consecutive nights revealed that 91.75 ± 2.19% of the spines maintained over 2h also were maintained over 24h (Supplementary Fig. 1a). The observed 24h survival fraction was significantly higher than estimates based on the 2h turnover measurements (P = 3.8 * 10−5; Supplementary Fig. 1a), indicating HVC neurons possess two populations of dendritic spines25: a larger (>90%) stable population and a smaller (<10%) transient population, the dynamics of which can be captured in a 2h time window. Therefore, viral labelling of HVC neurons with GFP combined with two-photon imaging offers a means to examine rapid learning-related structural changes to sensorimotor neurons.
Figure 1. Tutor song experience affects spine turnover in juvenile zebra finches.
a, Top: schematic of the zebra finch song system, experimental protocol and timeline of the experiments. Inset: Nissl stained image of HVC in parasagittal section showing its location on the floor of the lateral telencephalic ventricle, ~100µm below the pial surface. Scale bar, 200µm. Right: low magnification in vivo two-photon image of GFP labelled spinous HVC neurons amidst retrogradely labelled HVCRA (red) and HVCX (blue) projection neurons. Scale bar, 20µm. b, Repeated in vivo imaging of dendritic branches from an HVC neuron of a 130d finch over 4 days (yellow box, left image; scale bar, 20 µm on left image and 10 µm on right). c, High magnification view of dendritic segment shown in b (yellow box, scale bar, 2 µm), imaged two hours apart. Arrows point to stable (blue), lost (yellow) and gained (green) spines. d, High magnification view of another dendritic segment of an HVC neuron showing the gain and loss of dendritic spines across a two hour imaging interval (scale bar, 2 µm).
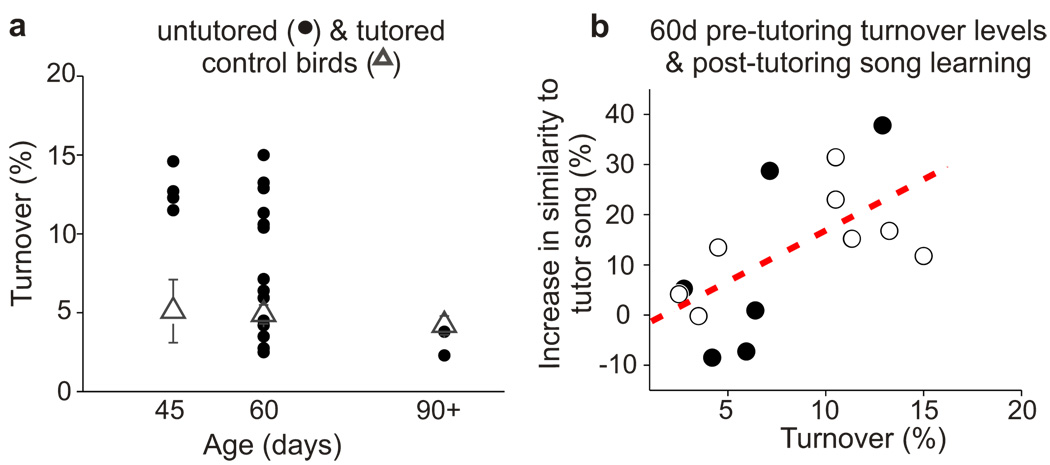
Songbirds learn to sing during a juvenile sensitive period by memorizing a tutor song (i.e., sensory learning) and using auditory feedback to match their own song to this memorized model (i.e., sensorimotor learning). In juvenile male zebra finches, sensory learning spans from ~30–60d after hatching and sensorimotor learning extends from ~45–90d13,14. To examine whether experience with a tutor affects spine dynamics in HVC, spine turnover levels were quantified in juveniles raised without access to a tutor. Initial experiments focused on untutored 60d juveniles, an age when zebra finches raised with a tutor become refractory to copying new song models13. Imaging HVC neurons in untutored 60d birds revealed a wide range of spine turnover levels, with some birds (6/14) exhibiting levels substantially higher (>2 SD) than in age-matched juveniles raised with a tutor, and others (8/14) showing levels similar (±1SD) to the control animals (Fig. 2a). One possibility is that this variation reflects individual differences in when the sensitive period for sensory learning closes. To test this idea, we quantified spine turnover in 45 and 90d untutored birds as well as age-matched birds raised with a tutor. Spine turnover in all untutored 45d birds was significantly higher than in age-matched control birds (P < 0.01) and overlapped with the upper end of the turnover distribution in untutored 60d birds. Conversely, spine turnover in all untutored 90d birds was in the range of age-matched control birds (P = 0.3) and overlapped with the lower end of the turnover distribution in untutored 60d birds. These observations support the idea that tutor experience acts during early stages of normal development to diminish spine turnover in HVC while also showing that spine turnover can eventually decrease even in the absence of tutor experience. Because untutored birds use auditory feedback to learn and stabilize their “isolate” songs, this transition to lower levels of turnover may reflect a commitment to a vocal behaviour learned in reference to an innate model.
Figure 2. Levels of HVC dendritic spine turnover correlate with song imitation.
a, Mean two-hour HVC spine turnover levels are elevated in 45d (P = 0.009) and 60d (P = 0.04), but not 90d, untutored birds (circles) compared to age matched control birds (triangles)(untutored 45d: 180 spines from 6 cells in 4 birds; 45d control: 621 spines from 11 cells in 3 birds; untutored 60d: 1,419 spines from 17 cells in 14 birds; 60d control: 1,579 spines from 23 cells in 16 normally reared birds; untutored 90d: 188 spines from 4 cells in 2 birds; 90d control: 1,544 spines from 23 cells in 11 birds). b, Levels of turnover in HVC prior to tutoring correlate with subsequent song imitation. Scatter plot showing the relationship between levels of HVC dendritic spine turnover (2hr) measured the night prior to the first exposure to a song model, and the total increase in similarity to the tutor song over song development (P = 0.02, r = 0.63, 1,419 spines from 17 cells in 14 birds). Each circle represents a single bird. The eight open circles correspond to birds in which post-tutoring dendritic spine turnover measurements were taken.
In this light, the transition to low levels of spine turnover in untutored birds could reflect a diminished ability to learn from a tutor. If this view is correct, then the variable levels of turnover in untutored 60d birds would be expected to correlate with the ability to copy a tutor song. To test this idea, previously untutored 60d juveniles (N = 14) were exposed to either a live tutor or operant tutoring methods for three consecutive days and their song development was tracked into adulthood. Consistent with prior observations26, delayed tutoring resulted in a wide range of song learning outcomes, with some birds copying elements from the tutor song (up to 37.8% increase in similarity to the tutor song by adulthood) and other birds copying little or not at all (Fig. 2b, Supplementary Fig. 2). Spine turnover levels measured in HVC prior to tutoring correlated positively with the subsequent increase in similarity of the pupil’s song to the tutor (P = 0.02, r = 0.63). Therefore, the capacity for learning a new behaviour is associated with enhanced spine dynamics in sensorimotor circuits important to that behaviour.
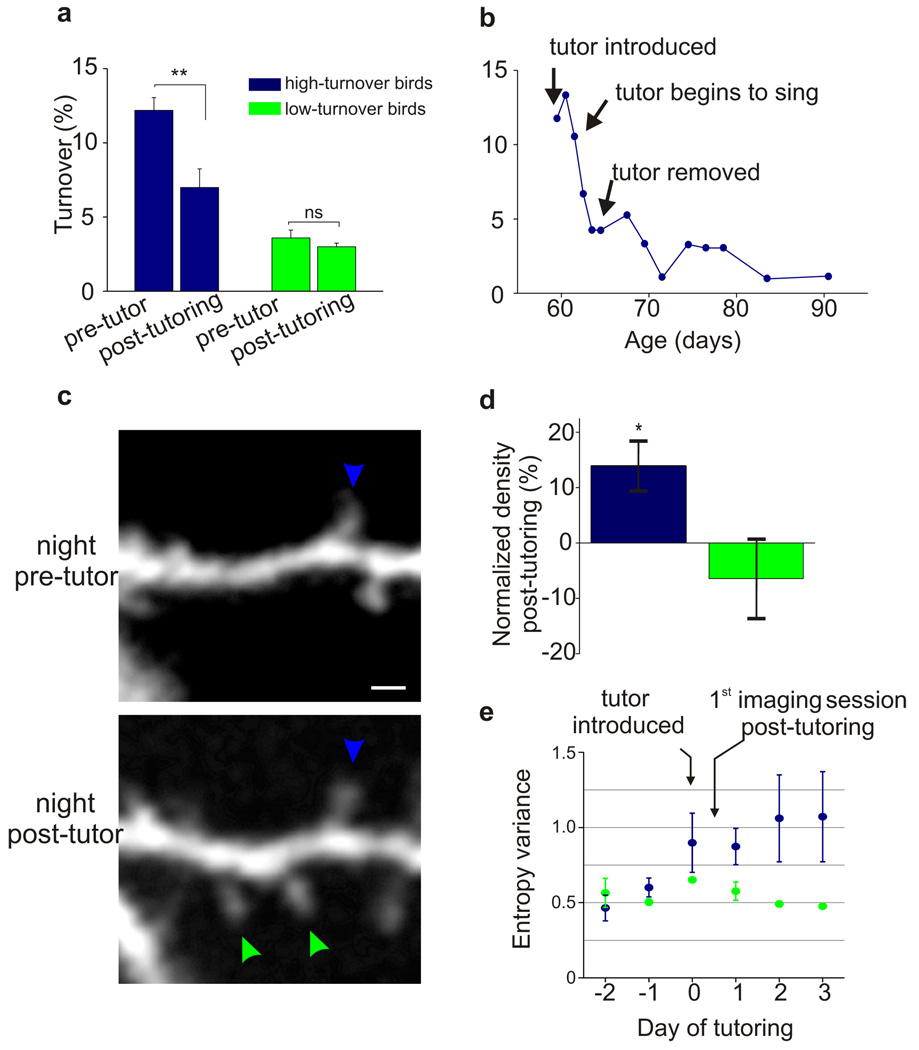
The observations that the capacity for song learning is associated with enhanced spine dynamics in HVC and that spine turnover in all untutored 45d birds was significantly higher than in age-matched controls suggest that tutor song experience resulting in learning may stabilize dendritic spines in HVC. To test this idea, we repeatedly imaged the same dendritic regions in HVC at least one night before and one night after the first day of exposure to tutor song (Fig. 2b; N = 8 birds; a subset of the 14 untutored 60d birds described previously). Five of these birds, termed high-turnover (HT) birds, displayed pre-tutoring levels of spine turnover >2SD higher than in age-matched controls, while the other three, termed low-turnover (LT) birds, displayed pre-tutoring levels of spine turnover commensurate to controls.
In all five HT birds, spine turnover in HVC decreased significantly by the night following the first day of either live (N = 3) or operant (N = 2) tutoring (Fig. 3a; P < 0.01). The finding that decreased spine turnover could occur with operant tutoring indicates that these structural effects did not depend on social interactions with the tutor, and instead are related to hearing a tutor song. Furthermore, in one live tutoring experiment, the tutor failed to sing until the third day after it was housed with the juvenile. Notably, spine turnover only decreased after the tutor began to sing (Fig. 3b), further underscoring the importance of auditory rather than social experience in triggering structural changes in HVC. Moreover, in this live-tutored juvenile, it was possible to repeatedly image the same dendritic regions for 30d following tutor exposure, a period encompassing the remainder of sensorimotor learning. In this case, the decrease in turnover over the first 48h after tutoring accounted for the bulk (67%) of the decrease that occurred over sensorimotor learning (Fig. 3b). In addition to displaying rapid spine stabilization, 4 of 5 HT birds displayed increased spine density the night following the first day of tutoring (Fig. 3c, d, Supplementary Fig. 3; P = 0.03). In contrast, LT birds showed no changes in either spine turnover or spine density following live or operant tutoring (Fig. 3a, d). Finally, increased syllable entropy variance, an early indicator of song imitation, was detected in HT but not LT birds by the end of the first day of tutoring (Fig. 3d; Post-hoc Tukey test, α = 0.05, 3 of 4 HT birds) and, as adults, HT birds had copied more from their tutors than LT birds (HT birds: 19.6 ± 3.5% similarity increase to the tutor over song development; LT birds: 6.2 ± 4.0% increase). These findings support the idea that instructive experience leads to the stabilization and accumulation of dendritic spines on sensorimotor neurons important to the learned behaviour.
Figure 3. Tutoring can trigger rapid stabilization and accumulation of dendritic spines on HVC neurons.
a, Tutoring triggers a rapid decrease in the level of HVC dendritic spine turnover in HT (P < 0.01), but not LT (P = 0.3) birds. Levels of HVC dendritic spine turnover measured the night before and the night after the first day of either live (N = 5) or operant (N = 3) tutoring in HT and LT birds (HT birds: P < 0.01, 12.2 ± 0.8% → 7.0 ± 1.2% pre and post-tutoring turnover, respectively, 468 spines from 5 birds; LT birds: P = 0.3, spine turnover = 3.6 ± 0.5% → 3.0 ± 0.2% pre and post-tutoring, respectively, 449 spines from 3 birds). Further, spine turnover levels measured in HVC prior to tutoring correlated positively with the post-tutoring decrease in spine turnover (P = 0.02, r = 0.79, N = 8 birds, not shown). b, The bulk of the decrease in turnover levels (67%) observed during sensorimotor learning occur during the first 48hs following hearing a tutor for the first time. Levels of HVC dendritic spine turnover in a “high turnover” bird measured over 30 days following initial exposure to a tutor. The tutor did not sing during the first two days with the HT juvenile, at which point turnover levels markedly and persistently decreased. c, Example of stable spines (green arrows) that formed during the first day of tutoring in a bird with high pre-tutoring levels of spine turnover (scale bar, 2 µm). d, Tutoring triggers a rapid increase in HVC dendritic spine density in HT (P = 0.03), but not LT (P = 0.5) birds. Percent change in dendritic spine density by the first night following tutoring (post-tutoring density / pre-tutoring density; HT birds: P = 0.03, 14.0 ± 4.4% increase in spine density; LT birds: P = 0.5, −6.4 ± 7.0% increase in spine density; from 4 HT and 3 LT birds, 1,198 spines). Further, spine turnover levels measured in HVC prior to tutoring correlated positively with the post-tutoring increase in spine density (P = 0.04, r = 0.71, N = 8 birds, not shown). e, Mean afternoon entropy variance scores increase by the afternoon of the first day of tutoring in HT birds (blue), but not LT birds (Post-hoc Tukey test, α = 0.05 in 3 of 4 HT birds, data shows mean afternoon EV value for 4 HT birds and 3 LT birds). Error bars denote s.e.m.
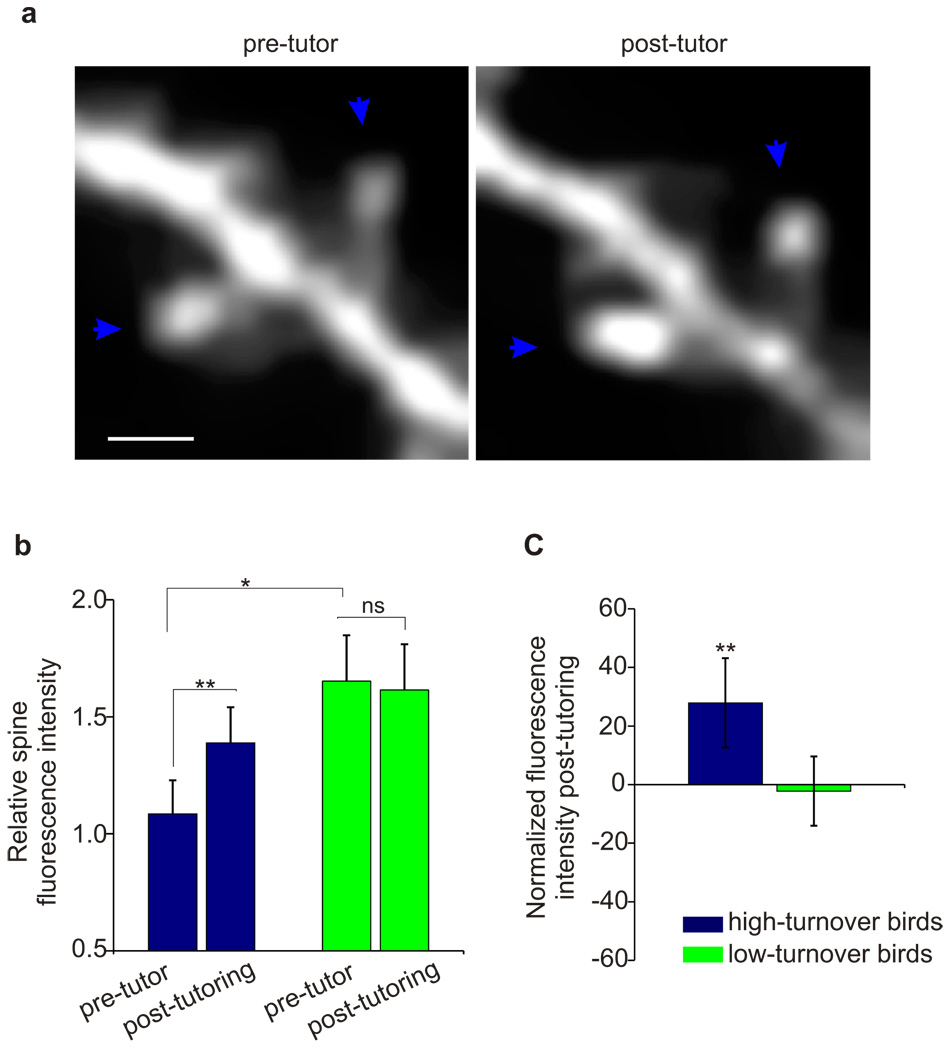
In other systems, increased spine stability and accumulation of new spines have been linked to synaptic strengthening2,4,6,7. Another structural hallmark of synaptic strengthening is a dynamic increase in the volume of pre-existing (i.e., stable) spines1,8,27. We tracked individual HVC dendritic spines maintained across pre- and post-tutoring imaging sessions and measured changes in their fluorescence intensity, a feature monotonically related to spine volume28 (81 spines, 8 birds). The night following the first tutoring session, the size of stable dendritic spines increased 28% in HT birds, but remained unchanged in LT birds (Fig. 4, Supplementary Fig. 3; HT bird, P = 0.001; LT birds, P = 0.4). Additionally, prior to tutoring, stable dendritic spines in HT birds were 52% smaller than in LT birds (Fig. 4b, P = 0.02), a difference erased by the night following the first tutoring session (P = 0.3). The smaller stable spines and higher levels of turnover in HT birds observed prior to tutoring may reflect functionally weaker excitatory synaptic connections onto HVC neurons1,27. Thus, instructive experience can act on more dynamic and presumably weaker dendritic spines to increase their size, number and stability, all hallmarks of functional enhancement of synaptic transmission.
Figure 4. Tutoring triggers enlargement of stable dendritic spines in HVC.
a, Example of two stable spines that exhibited increased fluorescence intensity following tutoring, indicating an increase in dendritic spine volume28. Scale bar, 2 µm. b, The size of stable spines increased in HT but not LT birds by the first night following tutoring, as revealed by measurements of relative integrated fluorescence intensity (81 spines, 8 birds; HT bird: P = 0.001, 1.08 ± 0.14 → 1.39 ± 0.16 (relative fluorescence intensity ± s.e.m.), n = 47 spines, Wilcoxon-Signed rank test for paired samples; LT birds: P = 0.4, 1.68 ± 0.20 → 1.61 ± 0.20, n = 34 spines, Wilcoxon-Signed rank test for paired samples). Moreover, HT birds had smaller stable dendritic spines prior to tutor exposure (P = 0.02; HT spines: 1.08 ± 0.14, n = 47 spines; LT birds: 1.67 ± 0.20, n = 34 spines). c, The first night following tutor exposure, the mean size of stable spines increased by 28% in HT birds, but did not change in LT birds (HT birds, P = 0.001; LT birds, P = 0.4).
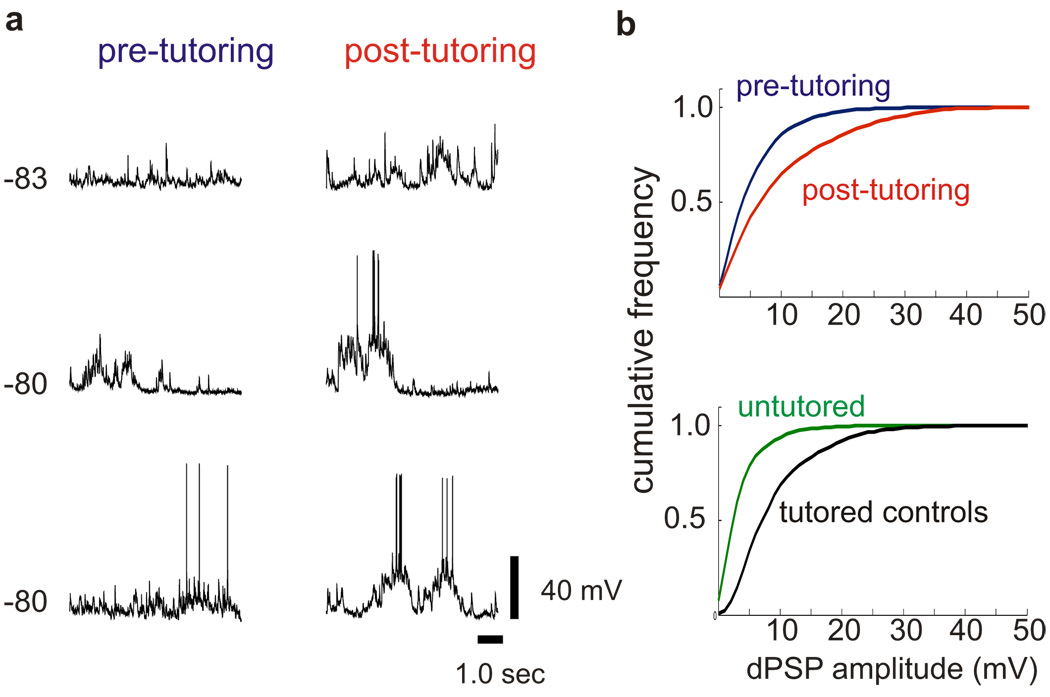
Indeed, tutoring rapidly enhanced synaptic activity in HVC, consistent with the idea that structural changes to spines elicited by tutoring are associated with functional changes to synapses. By adapting the windowing methods used for in vivo imaging, we were able to obtain sharp intracellular recordings from electrophysiologically identified PNs23 in the same small region of HVC one night before and one night after a juvenile bird’s initial exposure to a tutor (Fig. 5). To maximize the likelihood that HVC dendritic spines of all birds were in a high turnover state, experiments were conducted in ~45d juveniles previously raised without a tutor. Comparing spontaneous synaptic activity recorded before and after the first day of tutoring revealed a marked increase in the amplitude of depolarizing synaptic activity (Fig. 5a, b and Supplementary Fig. 4a; 24 cells in 3 birds, P < 0.00001) and the emergence of prolonged (~1sec) bursts of synaptic activity. These functional changes were not paralleled by changes in resting membrane potential (P = 0.6) or action potential firing rate (P = 0.3). Therefore, the synaptic enhancement was unlikely to arise from increased driving force on synaptic currents or increased firing of HVC PNs, which are a major source of synaptic input onto other HVC PNs. Rather, the functional and structural changes observed in HVC following tutoring are suggestive of rapid synaptic strengthening, although another mechanism that cannot be excluded is increased excitability of neurons afferent to HVC. The rapid enhancement of synaptic activity was even detected in a juvenile that failed to sing during its first tutoring session (P < 0.00001, Supplementary Fig. 4a), suggesting that it could occur in the absence of vocal practice and its associated auditory feedback. Lastly, the cumulative distributions of spontaneous synaptic events recorded in HVC the night following the first tutoring session were similar to those from age-matched juveniles raised with access to a tutor (Supplementary Fig. 4b; P = 0.1), consistent with the idea that tutoring results in a rapid physiological strengthening of initially weaker synaptic inputs onto HVC PNs.
Figure 5. Tutoring triggers enhancement of spontaneous synaptic activity in HVC.
a, In vivo intracellular membrane potential recordings made from six different HVC neurons in a juvenile bird one night before (left) and one night after (right) initial tutor exposure show that tutoring drives a rapid increase in the amplitude of spontaneous depolarizing synaptic activity. b, (top) Cumulative frequency distribution of spontaneous synaptic activity showing the amplitude of depolarizing synaptic events recorded intracellularly in HVC immediately before (blue) and after (red) tutoring (P < 0.00001). Data collected from 24 cells in 3 birds. (bottom) Cumulative frequency distribution of spontaneous synaptic activity showing the amplitude of depolarizing synaptic events recorded intracellularly in HVC on two consecutive days from a 45d untutored bird (green, 5 cells on each night) and a normally reared 45d bird (black, 2 cells on each night).
The current findings show that behavioural learning is favoured when instructive experience rapidly stabilizes structurally dynamic dendritic spines and enhances synaptic activity of sensorimotor neurons, thereby forging a link between experience, structural and functional properties of synapses, and behavioural learning. Prior studies in juvenile mammals established that the quality of sensory experience can affect the structural dynamics of dendritic spines in the corresponding regions of sensory cortex9,11. Our findings show that the consequences of a single instructive signal – a tutor song – are rapidly manifested as structural changes to dendritic spines, as well as functional changes to synapses in HVC, a sensorimotor region important to the control of learned vocalizations 18. A variety of evidence implicates HVC as the source of precise timing signals for song patterning19, the source of corollary discharge signals harnessed to enable song imitation15, and as a primary site where auditory signals merge with these song motor representations15,16,19. The location of HVC at the sensorimotor interface suggests that the large scale structural changes to dendritic spines we observed following tutoring can have direct consequences for how the HVC network translates auditory and motor-related activity into song. Consistent with this view, tutoring rapidly enhanced spontaneous synaptic activity in HVC and also led to rapid changes in vocal behaviour. Moreover, spontaneous bursting activity in song premotor neurons immediately downstream of HVC has been found to increase dramatically during the initial stages of tutor song imitation29, a functional change that is likely to be the consequence of the structural and functional changes occurring in HVC. Overall, our findings suggest experience can act in the juvenile brain to rapidly stabilize and strengthen a structurally dynamic sensorimotor network, providing a foundation for learning new behaviours.
Methods Summary
Juvenile male zebra finches were raised either in isolation from an adult song model (isolates) or with normal access to adult male song (controls). We used repeated in vivo two-photon optical imaging in the sensorimotor song nucleus HVC to assess structural plasticity of dendritic spines before and after the onset of song learning. Lentivirus coding for eGFP injected 14–20 days prior to cranial windowing was combined with retrograde labelling of HVC projection neurons to genetically label and identify HVC neurons for in vivo two-photon laser scanning microscopy (Zeiss LSM 510, Tsunami Ti:Sapphare laser (Spectra-Physics) at 910 nm, ×40 infrared Zeiss Archoplan objective). Changes in turnover, density and fluorescence intensity of dendritic spines on HVC projections neurons were calculated (see Methods) from two-hour imaging sessions conducted the night before and the night after initial exposure to a song model (total of 7,048 spines from 103 cells in 61 birds). Sharp intracellular recordings from electrophysiologically identified HVC projection neurons made the night before and the night after initial exposure to a song model were used to analyze associated changes to synaptic activity (see Methods). To quantify song learning we measured how much the pupil’s song gained in similarity to the tutor over song development using % similarity score in Sound Analysis Pro (adult % similarity – pre-tutoring % similarity). To measure the onset of changes in song with tutoring, we calculated the entropy variance of identified proto-syllable clusters from afternoon recording sessions, starting two days prior to tutoring and during the three days of tutoring (see Methods). Entropy variance is a measure of song complexity and an early indicator of tutor song imitation. Standard non-parametric and parametric statistical methods were used to detect significant differences (α = 0.05).
Methods
Finches were raised in our breeding colony or in isolation from an adult tutor and imaged beginning at 45, 60, 90 or 130 days posthatch, in accordance with a protocol approved by the Duke Institutional Animal Care and Use Committee. All birds were placed on reverse day-night cycles (14:10::L:D) ≥15 days prior to imaging. Control birds were raised in our breeding colony and given full access to adult tutors until ≥43d posthatch. Experimental juvenile zebra finches were isolated from adult male tutors by 10d posthatch and then housed in nesting groups and cared for by two adult female zebra finches or female Bengalese finches. Beginning at ~45d (range 40 – 50 days posthatch) juvenile males from the nesting groups were separated and housed individually in visual isolation from each other until adulthood (115 – 130d). Some of these juvenile birds were then tutored beginning on day 60 (59–62 days post-hatching). For tutoring, juvenile birds were allowed to trigger playback of an adult zebra finch song or were introduced to an adult male zebra finch starting the morning following the initial night of imaging. Birds were given access to keypeck-triggered tutoring or a live singing tutor for 3 consecutive days. Vocalizations of juvenile birds subjected to delayed tutoring were recorded continuously using automated methods (Sound Analysis Pro). Songs were recorded daily beginning 2–7 days before and ≥3 days following tutoring, then recorded either continuously or semi-continuously (every 3–10 days) into adulthood.
Viral and tracer injections
Male zebra finches were anesthetized with Isoflurane inhalation (2%) and placed in a stereotaxic apparatus. Injection target sites were located using stereotaxic coordinates and multi-unit neural recordings. A glass pipette attached to a pressure injection unit (Drummond Nanoject II) was used to deliver either the lentivirus, expressing enhanced green fluorescent protein under the control of the Ruos Sarcoma Virus LTR (FRGW)21, to HVC, or the neuronal retrograde tracers Fast Blue and/or Alexa-Fluor 594 conjugated dextran amines to Area X and RA, respectively (lentivirus: 32.2 nL/injection × ~30 injections for total ~1µL; tracers: 32.2 nl/injection × 2–5 injections). Lentiviral injections were made 15–20 days before imaging and retrograde tracer injections were made 5–7 days before imaging to optimize labelling of HVC neurons.
In vivo two-photon imaging
We longitudinally imaged dendritic spines on GFP-expressing HVC neurons in male zebra finches (7,041 spines, 61 birds; see Supplementary Table) aged 41–130 days. Birds were injected with Mannitol (10µL/g, i.m.), anesthetized with Isoflorane inhalation (2%) and positioned in a stereotaxic apparatus. The scalp overlying HVC was removed and the scalp margins were sealed to the surface of the skull using Vetbond (n-butyl cyanoacrylate). Bilateral craniotomies (~1 – 1.5mm2) were made in the skull overlying HVC. The dura mater was excised, leaving intact the pia mater, the 60–150µm thick layer of neural tissue and the lateral telencephalic ventricle overlying HVC. A custom cut coverslip (no. 1 thickness) was placed directly on the pial surface or on a thin layer of agarose covering the brain, then sealed to the skull with dental acrylic. A headpost was also affixed to the skull with dental acrylic. Birds were placed onto a custom stage under a Zeiss Laser Scanning Two-Photon Microscope 510. Only GFP-labeled neurons located within a field of retrogradely labeled HVCRA and/or HVCX neurons were classified as HVC neurons and imaged. Dendritic segments of HVC neurons were imaged at high resolution during the bird’s subjective nighttime (1024 ×1024 pixels, 76 × 76µm2, 3.2 µs/pixel, averaging 2 samples per pixel with 1 µm Z-steps, focused through 4×/0.8NA Zeiss IR-Archoplan immersion objective). Birds were returned to a darkened holding cage and allowed to sleep until reimaged two hours later. Two-hour imaging sessions were repeated during the same period each night for 1–5 nights.
Image Analysis
Three-dimensional image stacks were auto-aligned and smoothed using a Gaussian filter (NIH ImageJ) and the same dendritic segment, imaged 2 or 24 hours apart, was selected. Images exhibiting changes in fluorescence or rotational artifacts were excluded from further analysis. All sets of selected three-dimensional image stacks were coded and scored by researchers blind to the experimental condition. To assess spine growth and retraction, individual dendritic spines were compared across two hour time intervals and spine stability [Stability = 100(Nstable / (Ntotal)], spine elimination [100(Nlost / Ntotal)], spine addition [100(Ngained / Ntotal)] and spine turnover [100(Ngained + Nlost / 2Ntotal)] were calculated. Changes in spine density [ Ntotal / dendritic length (µm) ] and spine fluorescence intensity were measured from the same dendritic segments used to assess spine turnover. Spine fluorescence intensity (integrated GFP intensity) was measured by summing all pixels for a dendritic spine, subtraction-corrected for the mean background fluorescence measured from a non-labelled region of equal area located adjacent to the dendritic spine. This value was normalized by dividing by the mean dendritic fluorescence measured on a segment of dendrite at the base of the spine, subtraction-corrected for the background fluorescence. Standard non-parametric and parametric statistical methods were used to detect significant differences (α = 0.05).
Repeated Intracellular Recording
Sharp intracellular recordings were made on two consecutive nights from the same small location in HVC using repeated electrode penetrations through the same opening in the dura. During recording sessions birds were lightly anesthetized with Diazepam (50µl, 2.5mg/ml). Electrode impedances were 100–150 MΩ when filled with 2M KAc. Between nightly recording sessions the craniotomy overlying HVC was filled with silicone oil (Advance Weight Systems), and covered with a glass coverslip and a silicone adhesive (Kwik-Sil, WPI). Intracellular electrophysiological data was captured as previously described23. Briefly, recordings were amplified via an AxoClamp 2B amplifier (Axon Instruments, Foster City, CA) and low-pass filtered at 3 kHz and digitized at 10 kHz. Analysis of depolarizing PSP amplitudes from spontaneous electrical activity was conducted on median filtered traces using custom event detection software (Matlab, written by K. Hamaguchi).
Behavioral Analysis
We quantified the amount that juvenile birds copied from their tutor using % similarity score in Sound Analysis Pro. Acoustic similarity is calculated by measuring “pitch”, amplitude modulation, frequency modulation, Weiner entropy and goodness of “pitch”. This aggregate score reflects the percentage of elements in the pupil’s song that are similar to those in the tutor’s song. We first calculated how much the pupil copied from the tutor by analyzing similarity scores from recordings of the pupils’ adult song (115 – 130d). Next, we measured how similar the pupil song was to the tutor immediately prior to tutor exposure (58 – 59d) by retrospectively identifying syllable clusters in the juvenile song that were proto-syllables for the adult song motif (n ≈ 45 comparisons per bird per time point). From these measurements we calculated how much the pupil gained in similarity to the tutor over song development (adult % similarity – pre-tutoring % similarity). This approach was used instead of absolute similarity because some juvenile songs prior to tutor exposure (58–60d) were sufficiently structured to return spuriously high similarities to a tutor song. To measure the onset of changes in song with tutoring we calculated the entropy variance, which is a measure of song complexity and an early indicator of tutor song imitation20,26,29, of identified proto-syllable clusters from afternoon recording sessions, starting two days prior to tutoring and during the three days of tutoring.
Supplementary Material
Acknowledgements
We thank M. Ehlers and L. Katz for access to the two-photon microscope and support in making lentivirus, Kosuke Hamaguchi for peak detection and analysis software and D. Kloetzer for animal husbandry and laboratory support. D. Fitzpatrick, D. Purves and M. Ehlers provided helpful comments on the manuscript. This work was supported by grants from the NSF and NIH (R.M.). T.F.R. was supported by an NRSA from NIH, K.A.T. was supported by a pre-doctoral award from NSF and M.E.K. was supported by HHMI (M. Ehlers).
Footnotes
Supplementary Information accompanies the paper
Author Contributions
T.F.R. and R.M. designed the study and wrote the manuscript. T.F.R. and K.A.T. collected and analyzed the imaging and behavioural data. T.F.R. and M.E.K. designed the lentiviral construct and M.E.K. made the lentivirus. T.F.R and R.M. collected the electrophysiological data.
References
- 1.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Roo M, Klauser P, Muller D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol. 2008;6(9):e219. doi: 10.1371/journal.pbio.0060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431(7010):782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- 4.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399(6731):66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 6.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283(5409):1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 7.Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61(2):247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457(7227):313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majewska A, Sur M. Motility of dendritic spines in visual cortex in vivo: changes during the critical period and effects of visual deprivation. Proc Natl Acad Sci U S A. 2003;100(26):16024–16029. doi: 10.1073/pnas.2636949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46(2):181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436(7048):261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
- 12.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441(7096):979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 13.Eales LA. Song learning in zebra finches: some effects of song model availability on what is learnt and when. Anim Behav. 1985;33:1293–1300. [Google Scholar]
- 14.Immelmann K. Song development in zebra finch and other Estrildid finches. In: Hinde RA, editor. Bird Vocalisations. London: Cambridge University Press; 1969. pp. 61–74. [Google Scholar]
- 15.Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451(7176):305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- 16.Bauer EE, et al. A synaptic basis for auditory-vocal integration in the songbird. J Neurosci. 2008;28(6):1509–1522. doi: 10.1523/JNEUROSCI.3838-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCasland JS, Konishi M. Interaction between auditory and motor activities in an avian song control nucleus. Proc Natl Acad Sci USA. 1981;78(12):7815–7819. doi: 10.1073/pnas.78.12.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165(4):457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 19.Hahnloser R, Kozhevnikov A, Fee M. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- 20.Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291(5513):2564–2569. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- 21.Roberts TF, Klein ME, Kubke MF, Wild JM, Mooney R. Telencephalic neurons monosynaptically link brainstem and forebrain premotor networks necessary for song. J Neurosci. 2008;28(13):3479–3489. doi: 10.1523/JNEUROSCI.0177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dittgen T, et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci U S A. 2004;101(52):18206–18211. doi: 10.1073/pnas.0407976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mooney R. Different subthreshold mechanisms underlie song-selectivity in identified HVc neurons of the zebra finch. Journal of Neruoscience. 2000;20(14):5420–5436. doi: 10.1523/JNEUROSCI.20-14-05420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron. 2000;25(2):481–492. doi: 10.1016/s0896-6273(00)80910-1. [DOI] [PubMed] [Google Scholar]
- 25.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420(6917):788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 26.Deregnaucourt S, Mitra PP, Feher O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433(7027):710–716. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- 27.Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26(7):2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtmaat AJ, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45(2):279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458(7234):73–77. doi: 10.1038/nature07615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.