Abstract
Tumor neovascularization and growth may be promoted by recruitment of bone marrow-derived cells (BMDCs), which include endothelial precursor cells (EPCs) and “vascular modulatory” myelomonocytic (CD11b+) cells. BMDCs may also drive tumor re-growth after certain chemotherapeutic and vascular disruption treatments. In this study, we evaluated the role of BMDC recruitment in breast and lung carcinoma xenograft models after local irradiation (LI). We depleted the bone marrow by including whole body irradiation (WBI) of 6Gy as part of a total tumor dose of 21Gy, and compared the growth delay with the one achieved after LI of 21Gy. In both models, including WBI induced longer tumor growth delays. Moreover, including WBI increased lung tumor control probability by LI. Exogenous delivery of BMDCs from radiation-naïve donors partially abrogated the WBI effect. Myeloid BMDCs, primarily macrophages, rapidly accumulated in tumors after LI. Intratumoral expression of SDF-1α, a chemokine that promotes tissue retention of BMDCs, was noted 2 days after LI. Conversely, treatment with an inhibitor of SDF-1α receptor CXCR4 (AMD3100) with LI significantly delayed tumor re-growth. However, when administered starting from 5 days post-LI, AMD3100 treatment was ineffective. Lastly, with restorative bone marrow transplantation of Tie2-GFP-labeled BMDC population we observed an increased number of monocytes but not EPCs in tumors that recurred following LI. Our results suggest that an increase in intratumoral SDF-1α triggered by local irradiation recruits myelomonocyte/macrophage which promote tumor re-growth.
Keywords: SDF1alpha, irradiation, tumor, relapse, CXCR4, BMDCs
Introduction
The recruitment of various blood-borne bone marrow-derived cells (BMDCs) may be important for tumor neovascularization and growth, but their roles are often difficult to differentiate (1, 2). Endothelial precursor cells (EPCs) may directly incorporate in 0-50% of the newly formed tumor vessels, depending on tumor type, organ site and mouse strain (3). In addition, other BMDCs, referred to as “vascular modulatory/accessory” cells, may support angiogenesis in a paracrine manner. Among them, arguably the most important for angiogenesis are cells of myeloid/monocyte lineage (CD11b+), in particular macrophages and Tie2-expressing monocytes (TEMs)(4, 5). The trafficking and tissue retention of BMDCs may depend, at least in part, on stromal-derived factor 1 alpha (SDF1α)-CXCR4 receptor pathway activation (6, 7).
EPCs may be increasingly attracted to tumor sites as a result of certain therapies and influence their outcome (e.g., vascular-disruptive and certain chemotherapeutic treatments (8, 9)). But it remains unknown if EPCs contribute significantly after local irradiation (LI) of tumors, whose neovascularization is presumed to be deficient (10, 11). Infiltration by other BMDCs—e.g., myelomonocytes/macrophages—has been previously documented in irradiated tumors (12-16). However, their role as modulators of tumor radiation response remains largely uncharacterized. Here, we evaluated the role of various BMDCs in tumor re-growth after LI in lung and breast tumor models.
Materials and Methods
Animals and tumors
54A human lung tumors were xenografted in male athymic NCr/Sed nude (nu/nu) mice, and MCa8 mouse mammary carcinomas were implanted in female syngeneic FVB mice, both subcutaneously in the hindlimb (3, 11). To detect specific BMDC populations in tumors (i.e., Tie2+CD11b− EPCs and Tie2+CD11b+ TEMs), we irradiated wild-type (WT) FVB mice with 9Gy of WBI followed by restorative bone marrow transplantation (BMT) from Tie2-GFP-FVB donors to create chimeric WT/Tie2-GFP-BMT mice (3). MCa8 tumors were implanted in these mice 6 weeks post-BMT.
Treatments and response evaluation
Tumor size was measured with a caliper at least thrice a week. When a tumor reached 5.5mm in mean diameter, the mouse was randomized to a treatment group. Tumors were γ-irradiated using the same dose delivered either locally alone or locally plus a sublethal whole-body irradiation (WBI) dose of 6Gy. (For detailed treatment procedures, see Supplementary Material.) Unsorted BMDCs or FACS-sorted Sca1−CD11b+ or Sca1+CD11b+ BMDCs were intravenously infused in mice receiving LI plus WBI. CXCR4 inhibition, alone or after LI, was achieved using AMD3100 (5mg/kg/day, Sigma-Aldrich, St. Louis, MO) delivered by ALZET micro-osmotic pumps (DURECT Corporation, Cupertino, CA) over 2 weeks. Therapeutic efficacy was measured as the time taken for tumors to grow to 7.5mm in diameter (i.e., ∼2.5-fold increase compared to the pretreatment volume).
Immunohistochemistry and image analysis
Tumors were excised, cut in half, fixed for 2h in 4% formaldehyde in PBS, incubated in 30% sucrose in PBS overnight at 4°C and frozen in OCT (Tissue-Tek, Midland, ON). Transverse tumors sections, 10μm-thick, were immunostained with antibodies to endothelial marker CD31, pan-myeloid marker CD11b or SDF-1α, and counterstained by mounting with DAPI-containing medium (Vectashield, Vector Labs, Burlingame, CA). Images were captured with a stack step of 5 μm using an Olympus confocal microscope, and the stained areas were quantified using in-house segmentation algorithms coded on a Matlab platform (Mathworks, Natick, MA). The antibodies used and details of image analysis are described in the Supplementary Material.
Flow cytometric analysis
Flow cytometry was performed on single-cell suspensions prepared from whole tumors after digestion with collagenase type II (Worthington, Lakewood, NJ) and immunostaining for CD11b, Gr1, F4/80 (using fluorescence-labeled monoclonal antibodies from BD-Pharmingen), as described (3), using an LSR-II flow cytometer (BD-Biosciences).
Statistical analysis
Differences between mean values in each group were evaluated by t test for independent samples and considered significant when p<0.05. Data are presented as mean±SEM. The comparison between collections of groups was carried out using t test for linear contrasts in one-way analysis of variance. Tumor control probabilities were compared using Chi-square test.
Results and Discussion
WBI delays tumor re-growth after irradiation
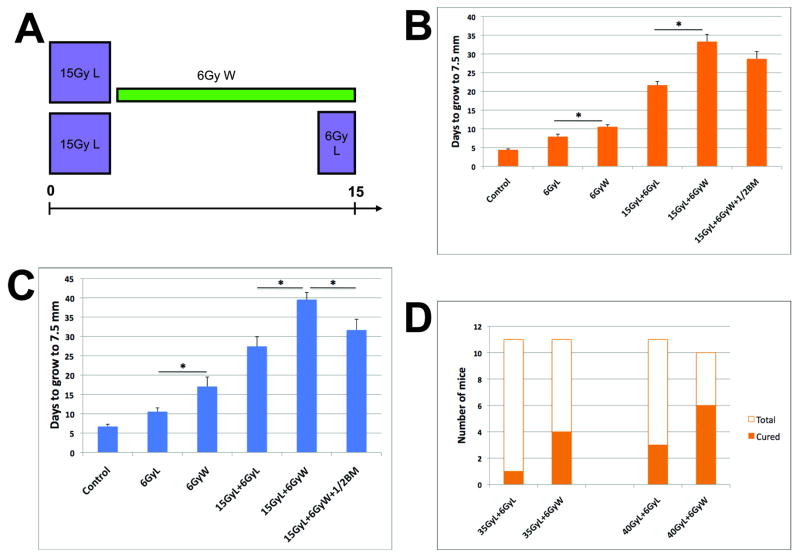
To diminish the potential involvement in tumors of host-derived cells following LI, we treated tumor-bearing mice with a sub-lethal dose of 6Gy WBI. This dose is known to damage the bone marrow and deplete leukocytes temporarily (for 1.5-2 weeks) from the blood circulation (17, 18). Using this approach, we compared the efficacy of the same radiation dose (21Gy) given to tumors either as two LI fractions or LI plus WBI; a flow chart of these treatments is presented in Fig. 1A, note the identical duration of irradiation. The growth of 54A tumors in nude mice was arrested in both groups, but tumor re-growth was significantly delayed when radiotherapy included WBI (Supplementary Fig. S1A). In the case of MCa8 carcinomas grown in immunocompetent FVB mice, tumors shrank by 1.5-2 mm post-treatment in both groups and WBI significantly delayed their re-growth (Supplementary Fig. S1B). In both tumors, the growth inhibition by 6Gy of WBI alone was also longer than that by 6Gy of LI. As summarized in Fig. 1B,C, WBI provided additional tumor growth delay in both models despite the difference in tumor post-radiation dynamics and distinct immune profiles of the hosts. Finally, WBI significantly enhanced tumor curability following LI with higher doses compared to LI alone in 54A xenografts (Fig. 1D). Of note, WBI did not change weight, appearance or behavior of mice (data not shown).
Figure 1. Anti-tumor effect of local (L) irradiation with or without whole-body (W) irradiation.
A, Experimental design: Tumors received the same total radiation dose (21Gy) over the same period of time, either by L and W irradiation or by two fractions of L irradiation. B,C, W irradiation significantly delayed tumor re-growth after radiation therapy in both 54A (B) and MCa8 (C) models, and this effect was abrogated by infusion of bone marrow cells from non-irradiated donors (n=7-9, *denotes p<0.05). D, At higher L irradiation doses, addition of W irradiation (n=21) significantly enhanced the local control probability (>90 days) of 54A tumor compared to L irradiation alone (n=22, p<0.05).
BMDC infusion promotes tumor re-growth after LI plus WBI
Infusion of unsorted BMDCs in mice treated with 15Gy of LI plus 6Gy of WBI abrogated the significant re-growth delay achieved by WBI in both tumor models (Fig. 1B,C). The “tumor-rescuing” effect was greater for irradiated MCa8 tumors (p<0.05, Fig. 1C). Moreover, a similar effect was seen after infusion of myeloid progenitor BMDCs (Sca1+CD11b+) or more mature myeloid BMDCs (Sca1−CD11b+) in mice with 54A tumors treated with 15Gy of LI plus 6Gy of WBI (data not shown). These results indicate that recruitment of radiation-naïve BMDCs can facilitate tumor re-growth after LI. As SDF-1α is a critical cytokine for BMDC recruitment (7), we then compared its intratumoral levels before and after LI.
LI up-regulates SDF-1α expression in tumors
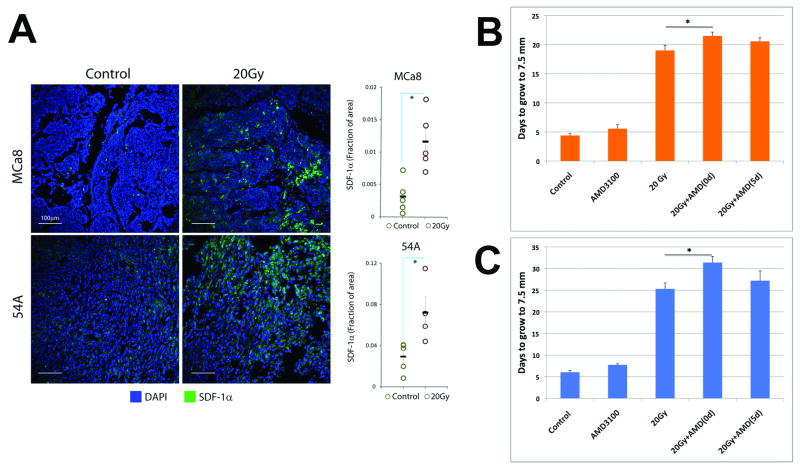
In both 54A and MCa8 models, 20Gy of LI significantly increased SDF-1α protein expression in tumor tissues, as measured 2 days later (Fig. 2A). In addition, we found a trend for increased SDF1α expression even after irradiation at a dose of 4Gy (Supplementary Fig. S2). This rapid upregulation of SDF-1α is likely induced directly by radiation, and is consistent with previous reports of SDF-1α up-regulation shortly after irradiation of normal tissues or cancer cells in vitro (19, 20). SDF1α might also be upregulated at later time-points if tumors become hypoxic (16). As SDF-1α is thought to exert its effects on BMDCs via the CXCR4 receptor (6, 21), we tested next if CXCR4 blockade can delay tumor re-growth after LI.
Figure 2. The role of SDF-1α-CXCR4 pathway in tumor re-growth after local irradiation.
A, Representative fluorescence confocal microscopy images of tumor immunostaining for SDF-1α and quantification of SDF-1α expression before and 2 days after 20Gy of radiation. Irradiation induced a significant increase in SDF-1α. B,C, Effect of CXCR4 blockade using AMD3100 on tumor re-growth after irradiation of 54A (B) and MCa8 (C) tumors. CXCR4 blockade delayed tumor growth when commenced immediately after but not 5 days after irradiation (n=6-8). (*denotes p<0.05).
CXCR4 blockade delays tumor re-growth only when administered immediately after LI
Inhibition of SDF-1α/CXCR4 signaling for 2 weeks using AMD3100-containing osmotic pumps did not affect tumor growth but significantly inhibited tumor re-growth of both 54A and MCa8 tumors when commenced immediately after 20Gy of LI (Fig. 2B,C and Supplementary Fig. S3). This is consistent with recent data from glioma xenografts (16). However, this effect was abrogated when AMD3100 treatment was initiated 5 days after 20Gy of LI. On the other hand, combination of AMD3100 with 6Gy of WBI immediately after 15Gy of LI provided no additional growth delay in MCa8 tumors (Supplementary Fig. S4). This indicates that WBI and AMD3100 treatment have overlapping effects post-irradiation. Collectively, these results suggest a critical role for the rapid, radiation-induced recruitment of BMDCs in tumors, mediated by SDF-1α-CXCR4 signaling. Therefore, we then studied the early BMDC infiltration in tumors after the treatment regimens shown in Fig. 1A.
Rapid accumulation of myeloid BMDCs post-LI may facilitate tumor relapse
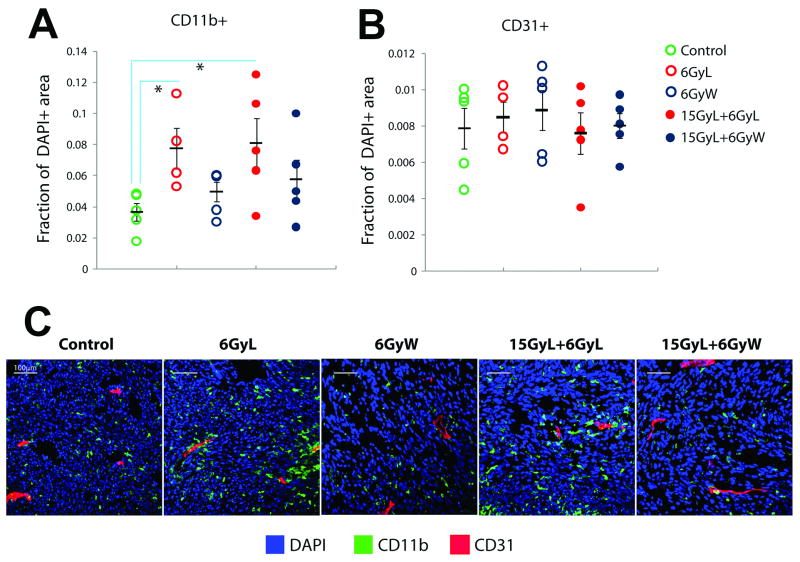
We measured tumor accumulation of myeloid BMDCs at 3 days after radiation treatments. Immunostaining for CD11b showed a significant increase in myeloid BMDC infiltration after LI alone in 54A tumors, while the regimens containing WBI abrogated this effect (Fig. 3A). Of interest, immunostaining for CD31 demonstrated no significant change in tumor vessel density at day 3 in any group after radiation compared to non-irradiated tumors (Fig. 3A). Flow cytometric analysis of whole tumor lysates confirmed the significant increase in number of CD11b+ cells after LI and its reduction by WBI, both in 54A and MCa8 tumors (Fig. 3B,C). Further phenotypic analyses showed that the vast majority of these cells were F4/80+ macrophages. The number of F4/80+ macrophages was also significantly decreased in tumors by WBI (p<0.05, using the linear contrast test for groups with versus without WBI). CD11b+Gr1+ immune-suppressive myeloid cells were elevated in tumors after 21Gy of LI, but were not reduced by WBI. These results further support the importance of rapid, myeloid cell accumulation for tumor resistance to LI. This LI-induced macrophage infiltration may associate with HIF-1α activation as well as iNOS and VEGF over-expression and lead to better survival and further proliferation of irradiated endothelial cells (14).
Figure 3. BMDC infiltration into tumors 3 days post-irradiation.
A, Immunohistochemistry for CD11b and CD31 expression in 54A tumors. Local (L) irradiation significantly increased the number of CD11b+ myeloid BMDCs compared to control tumors, whereas inclusion of whole-body (W) irradiation abrogated this effect. No significant difference was seen in microvascular density measured by CD31 expression. B,C, Flow cytometry analysis of whole tumor lysates confirmed the changes in myeloid BMDCs after L and W irradiation (n=5). The majority of BMDCs were F4/80+ macrophages in both 54A (B) and MCa8 (C) tumors. (*denotes p<0.05).
TEM infiltration but not EPC vessel incorporation is increased in recurring tumors
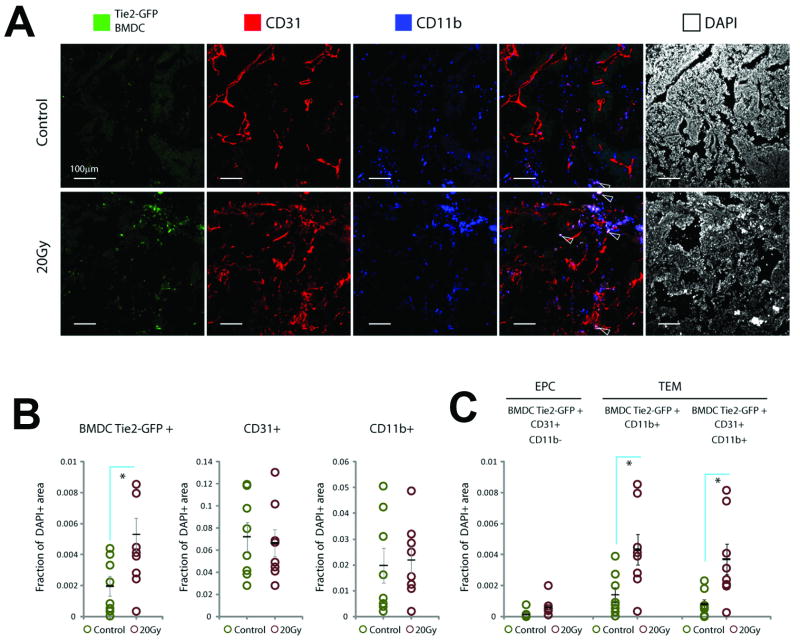
Finally, we tested the contribution of various BMDCs to tumors recurring after LI. Although incompletely understood, tumor angiogenesis post-LI may be deficient compared to non-irradiated tumors (11, 15). In this context, vasculogenesis by non-irradiated EPCs could play a more substantial role than in the case of radiation-naïve tumor growth. To test this, we used MCa8 tumor growing in chimeric WT/Tie2-GFP-BMT mice, a tumor model in which EPC recruitment is negligible in the absence of treatment (3). We detected no significant effect of the prior BMT on the tumor growth rate and the growth delay after 20-25 Gy of LI, which suggested an efficient bone marrow reconstitution and a lack of apparent local “tumor bed effect” beyond 6 weeks after BMT in FVB mice. Since new vessel formation is mandatory only to support the increasing tumor mass post-irradiation (10), we analyzed EPC incorporation in the vasculature of relapsed tumors (i.e., when they reached a diameter of 7.5mm). Tumor tissue analysis by confocal fluorescence microscopy showed no difference in CD31+ microvascular density or total myeloid CD11b+ BMDC infiltration between LI-treated tumors and size-matched non-irradiated tumors (Fig. 4). However, the accumulation of Tie2-GFP+ BMDCs was significantly increased in tumors recurring after LI. The vast majority of Tie2-GFP+ BMDCs were CD11b+CD31+ myeloid cells localized in tumor interstitium but not incorporated into the vessel wall. Thus, recurring tumors contained significantly more vascular-modulatory TEMs. In contrast, the number of EPCs incorporated in tumor vessels was substantially lower than TEM accumulation in tumor tissues and not significantly different in recurring versus non-irradiated tumors. Collectively, these data support the potential role of TEM paracrine influence but not EPC-based vasculogenesis in recurring tumors after LI. The latter is consistent with the negligible incorporation of EPCs in the vasculature of tumors growing in pre-irradiated normal tissues (15).
Figure 4. Analysis of BMDCs in tumors recurring after local irradiation in WT/Tie2-GFP-BMT mice.
A, Representative confocal microscopy images of fluorescence immunohistochemistry in tumors recurring after 20Gy of radiation versus non-irradiated size-matched (control) tumors. Note localization of GFP expression (green) in CD11b+ cells outside vessels (blue, see arrows) but not in CD31+CD11b− vascular endothelial cells (red). B,C, Quantification of BMDCs: Overall myeloid cell infiltration (CD11b+) and CD31+ microvascular density were not significantly changed, but the total number of Tie2+ BMDCs increased in irradiated tumors (B). These represented mostly Tie2+CD11b+ TEMs, the majority of which were also CD31+ but had peri-vascular location; in contrast the number of vessel-incorporated Tie2+CD31+CD11b− EPCs was negligible and not different in tumors grwoing after irradiation (C). (*denotes p<0.05).
Conclusions and implications
This study provides compelling evidence that host-derived BMDC infiltration in tumors is stimulated by LI and facilitates tumor recurrence through paracrine effects on irradiated tumor vasculature, inside and adjacent to the regressing/stabilized tumors. The rapid recruitment of non-irradiated myeloid BMDCs—primarily macrophages—is mediated at least in part by SDF1α and may create a microenvironment that promotes survival of tumor and endothelial cells and re-growth Once tumors recur, an increased TEM but not EPC infiltration may promote tumor growth. These results suggest that targeting the SDF-1α-CXCR4 pathway in a specific “therapeutic window” or TEM accumulation may delay tumor recurrence after radiotherapy.
Supplementary Material
Acknowledgments
We thank A. Tyrrell and N. Kirkpatrick for help with data analysis, and C. Koppel, E.J.A. Steller and C. Smith for expert technical assistance with flow cytometry and immunohistochemistry.
This study was supported by NIH grant R01-CA115767 (to RKJ) and partially by NIH grants P01-CA80124 and R01-CA126642 (to RKJ) and R21-CA139168 (to DGD).
References
- 1.Coffelt SB, Lewis CE, Naldini L, Brown JM, Ferrara N, De Palma M. Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol. 2010;176:1564–76. doi: 10.2353/ajpath.2010.090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–35. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 3.Duda DG, Cohen KS, Kozin SV, et al. Evidence for incorporation of bone marrow-derived endothelial cells into perfused blood vessels in tumors. Blood. 2006;107:2774–6. doi: 10.1182/blood-2005-08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Loges S, Schmidt T, Carmeliet P. “Antimyeloangiogenic” therapy for cancer by inhibiting PlGF. Clin Cancer Res. 2009;15:3648–53. doi: 10.1158/1078-0432.CCR-08-2276. [DOI] [PubMed] [Google Scholar]
- 6.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 8.Shaked Y, Ciarrocchi A, Franco M, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–7. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 9.Shaked Y, Henke E, Roodhart JM, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–73. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denekamp J. Limited role of vasculature-mediated injury in tumor response to radiotherapy. J Natl Cancer Inst. 1993;85:935–7. doi: 10.1093/jnci/85.12.935. [DOI] [PubMed] [Google Scholar]
- 11.Kozin SV, Winkler F, Garkavtsev I, Hicklin DJ, Jain RK, Boucher Y. Human tumor xenografts recurring after radiotherapy are more sensitive to anti-vascular endothelial growth factor receptor-2 treatment than treatment-naive tumors. Cancer Res. 2007;67:5076–82. doi: 10.1158/0008-5472.CAN-06-3664. [DOI] [PubMed] [Google Scholar]
- 12.Stephens TC, Currie GA, Peacock JH. Repopulation of gamma-irradiated Lewis lung carcinoma by malignant cells and host macrophage progenitors. Br J Cancer. 1978;38:573–82. doi: 10.1038/bjc.1978.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung H, Kruger HJ, Brammer I, Zywietz F, Beck-Bornholdt HP. Cell population kinetics of the rhabdomyosarcoma R1H of the rat after single doses of X-rays. Int J Radiat Biol. 1990;57:567–89. doi: 10.1080/09553009014552701. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Sonveaux P, Rabbani ZN, et al. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heissig B, Rafii S, Akiyama H, et al. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005;202:739–50. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seung LP, Weichselbaum RR, Toledano A, Schreiber K, Schreiber H. Radiation can inhibit tumor growth indirectly while depleting circulating leukocytes. Radiat Res. 1996;146:612–8. [PubMed] [Google Scholar]
- 19.Zong ZW, Cheng TM, Su YP, et al. Recruitment of transplanted dermal multipotent stem cells to sites of injury in rats with combined radiation and wound injury by interaction of SDF-1 and CXCR4. Radiat Res. 2008;170:444–50. doi: 10.1667/rr0744.1. [DOI] [PubMed] [Google Scholar]
- 20.Tabatabai G, Frank B, Mohle R, Weller M, Wick W. Irradiation and hypoxia promote homing of haematopoietic progenitor cells towards gliomas by TGF-beta-dependent HIF-1alpha-mediated induction of CXCL12. Brain. 2006;129:2426–35. doi: 10.1093/brain/awl173. [DOI] [PubMed] [Google Scholar]
- 21.Jin DK, Shido K, Kopp HG, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–67. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.