Abstract
New endomorphin-2 (EM-2) analogues incorporating (Z)-α,β-didehydro-phenylalanine (ΔZPhe) in place of the native phenylalanine in EM-2 are reported. Tyr-Pro-ΔZPhe-Phe-NH2 {[ΔZPhe3]EM-2} (1), Tyr-Pro-Phe-ΔZPhe-NH2 {[ΔZPhe4]EM-2} (2) and Tyr-Pro-ΔZPhe-ΔZPhe-NH2 {[ΔZPhe3,4]EM-2}(3) have been synthesized, their opioid receptor binding affinities and tissue bioassay activities were determined, and their conformational properties were examined. Compound 2 shows high µ opioid receptor selectivity and µ agonist activity comparable to that of the native peptide. The conformation adopted in solution and in the crystal by N-Boc-Tyr-Pro-ΔZPhe-Phe-NH2 (8) is reported.
Introduction
The alteration of the backbone sequence of native bioactive peptides through incorporation of unnatural amino acids or exogenous fragments of a different structure, is a common strategy adopted in medicinal chemistry in order to study their structure activity relationships and to obtain compounds with improved potency, selectivity, and potential therapeutic value. Recent and significant examples of this approach can be found in the field of endomorphins, two endogenous neuropeptides [endomorphin-1: Tyr-Pro-Trp-Phe-NH2 (EM-1a) and endomorphin-2: Tyr-Pro-Phe-Phe-NH2 (EM-2)], whose main proposed biological function is pain control through activation, with high affinity and selectivity, of µ-opioid receptors.1,2 A systematic evaluation of a variety of synthetic endomorphin analogues have provided insights into various structural and conformational features which are critical for the bioactivity of this family of neuropeptides.3 The role exerted by the proline residue at position 2 of the tetrapeptide backbone, as well as the spatial orientation adopted by the two Phe aromatic side chains at positions 3 and 4, appear to be particularly relevant.4 Several papers, dealing with the incorporation at position 2 of EM with higher and lower–homologues of proline,5 and cyclic β-residues6 document the attention dedicated to this first point. Also clearly documented is the influence that the mutual spatial orientation of the aromatic rings exerts on the interaction between EMs and their receptors.7 The interest focused on this specific effect is based on the well recognized influence of the non bonded aromatic/aromatic and backbone/aromatic interactions on the conformational stability of the peptide.
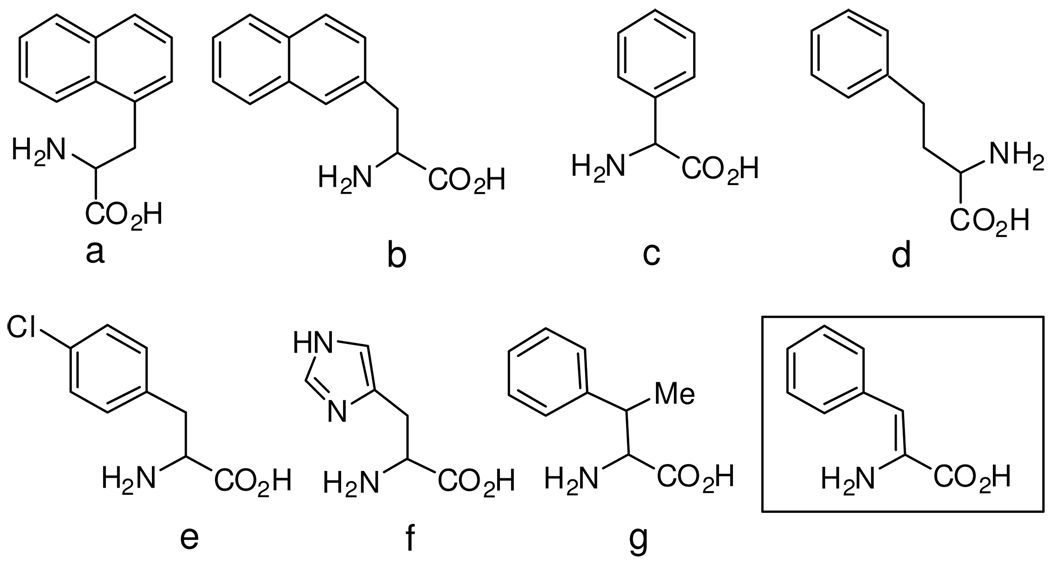
In accordance with the above reported observations, a literature examination reveals that several papers, have described structural modifications performed on the EM native sequences, which specifically focused on replacement of the Phe3 and Phe4 residues. In Fig. 1 most of the so far incorporated Phe mimic residues are illustrated.
Figure 1.
Structures a–g illustrate examples of aromatic amino acids so far used to replace native residues at positions 3 and/or 4 of endomorphins. The structure in the box refers to ΔZPhe, the achiral α,β-didehydro-amino acid adopted in the present study. (a): 3-(1′-naphthyl)-alanine (1-Nal);8 (b): 3-(2′-naphthyl)-alanine (2′-Nal);8 (c): phenylglycine (Phg);9,7b (d): homophenylalanine (Hfe);9 (e): p-Cl phenylalanine;10 (f): histidine;7a (g): β-methyl-phenylalanine (β-MePhe).11
Of particular interest is the enhancement of activity and selectivity of EM analogues obtained through incorporation of β-methylated Phe residues (Fig. 1) performed by Tomboly and coworkers.11 The interesting rationale followed by these authors is based on the reduction of the conformational mobility of the Phe aromatic side chains by biasing, according to the original proposals of Hruby et al.,12,13 the population of the χ1 (torsion angle) rotamers. By following a related and still unexplored approach we decided to synthesize and investigate the properties of a series of EM analogues incorporating α,β-unsaturated phenylalanine residues at the position 3 and 4. In analogues with this structure the aromatic ring, and each of its two adjacent backbone amide groups, are bound to a sp2 hybridized Cβ or Cα atom, respectively. As a consequence, the achiral ΔPhe residue exerts conformational constraint on the backbone and restricts, at the same time, the β-aromatic substituent to the either (Z) or (E) orientation. These steric and stereochemical features, together with the chemical properties connected with the electronic distribution within the involved peptide bonds, render α,β-dehydroamino acids an appealing tool for the development of variants of naturally occurring bioactive peptides. The consequences of this structural alteration appear quite relevant in the case of the tetrapeptidic EMs molecule. Here in fact three closely located aromatic side chains are present and their location involves both the “message” Tyr-Pro-Phe- N-terminal moiety and the Phe-NH2 “address” C-terminal fragment.14
Chemistry
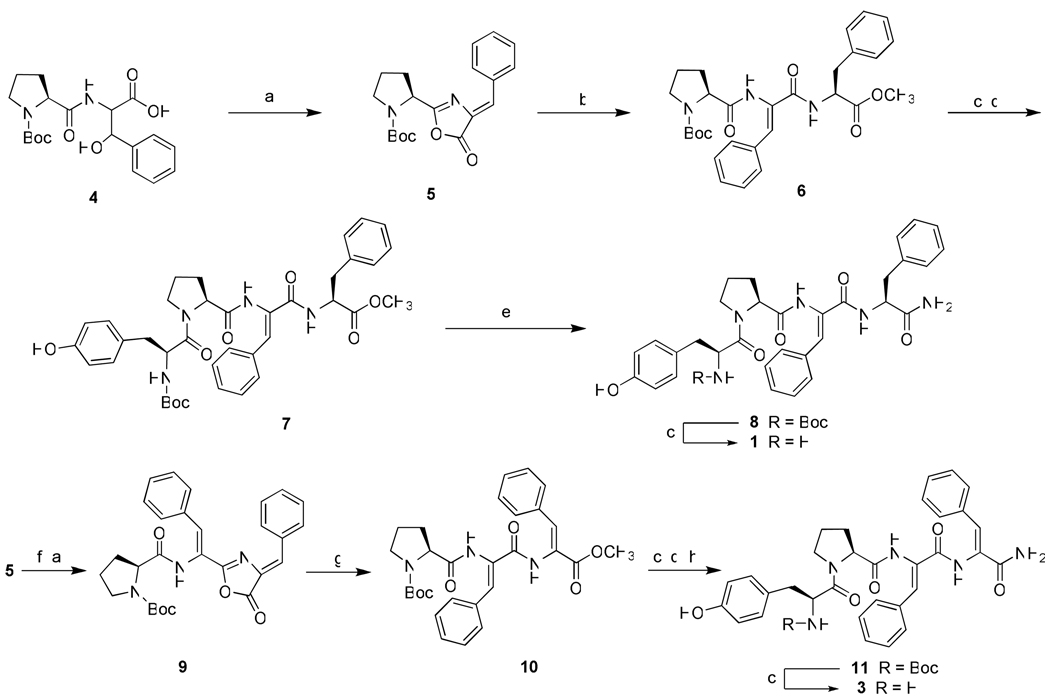
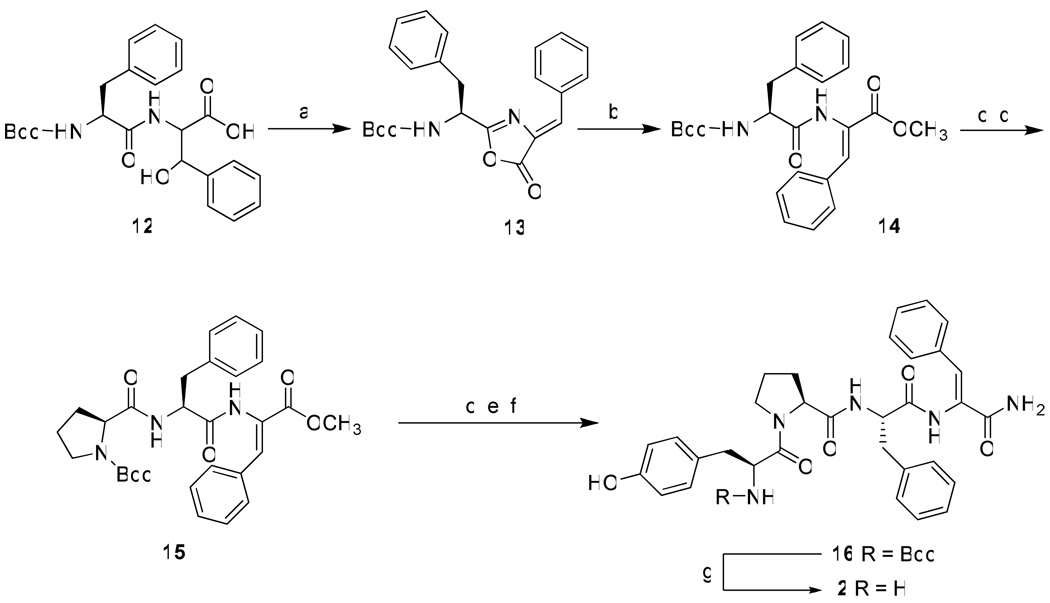
Based on the above considerations the syntheses and biological activities of three EM-2 analogues Tyr-Pro-ΔZPhe-Phe-NH2 {[ΔZPhe3]EM-2} (1), Tyr-Pro-Phe-ΔZPhe-NH2 {[ΔZPhe4]EM-2} (2) and Tyr-Pro-ΔZPhe-ΔZPhe-NH2 {[ΔZPhe3,4]EM-2}(3) are here reported, together with the X-ray crystal structure of Boc-Tyr-Pro-ΔZPhe-Phe-NH2 (8), the N-protected analogue of (1). Incorporation of the ΔZPhe residue has been accomplished by performing an acetic anhydride mediated azlactonization-dehydration reaction on dipeptide or tripeptide units containing C-terminal β-hydroxy-d,l-phenylalanine.15 Scheme 1 outlines the synthesis of the [ΔZPhe3] and [ΔZPhe3,4] EM-2 analogs. The common intermediate 5 is used to obtain both the final tetrapeptides 1 and 3. In the case of the synthesis of the tetrapeptide 3 the incorporation of the two consecutive ΔZPhe residues was accomplished by ring opening of the C-terminal unsaturated azlactone 5 with the sodium salt of β-hydroxy-d,l-phenylalanine followed by azlactonization and subsequent treatment with MeOH/DMAP. The analogue 2, containing the unsaturated Phe residue at position 4, has been obtained by following an analogous synthetic strategy which is illustrated in Scheme 2.
Scheme 1.
Synthesis of the EM-2 analogues Tyr-Pro-ΔZPhe-Phe-NH2 {[ΔZPhe3]EM-2} (1), Tyr-Pro-ΔZPhe-ΔZPhe-NH2 {[ΔZPhe3,4]EM-2}(3)a and Boc-Tyr-Pro-ΔZPhe-Phe-NH2 (8)
aReagents and conditions: (a) (CH3CO)2O/CH3COONa, room temp, 24 h, (95%); (b) HCl·Phe-OMe, DIEA, DMAP, DCM, room temp, 12 h, (86%); (c) TFA/DCM (1:1), room temp, 1 h, (quantitative); (d) Boc-Tyr-OH, EDC, HOBt, NMM, DCM, room temp, 12 h, (40%); (e) NH3/MeOH, room temp, 48 h, (95%); (f) dl-3-β(OH)Phe-OH, 1N NaOH, acetone, room temp,12 h, (90%); (g) DMAP, MeOH, room temp, 12 h, (60%); (h) NH3/MeOH, room temp, 48 h, (56%).
Scheme 2.
Synthesis of the EM-2 analogue Tyr-Pro Phe-ΔZPhe-NH2 {[ΔZPhe4]EM-2} (2)a
a Reagents and conditions: (a) (CH3CO)2O/CH3COONa, room temp, 24 h, (75%); (b) DMAP, MeOH, room temp, 12 h, (95%); (c) TFA/DCM (1:1), room temp, 1 h, (quantitative); (d) Boc-Pro-OH, EDC, HOBt, NMM, DCM, room temp, 12 h, (44%); (e) Boc-Tyr-OH, EDC, HOBt, NMM, DCM, room temp, 12 h, (40%); (f) NH3/MeOH, room temp, 48 h, (55%).
Results and Discussion
Table 1 summarizes binding affinities and functional bioactivities for µ and δ opioid receptors of the here studied EM-2 analogues. Data reported indicate that 1 and 3 bind weakly to µ receptors (Kiµ = 202 and 128, respectively) and are, although with substantial µ selectivity, weakly active in the GPI assay. Conversely, the analogue 2 shows potent µ binding affinity and high µ versus δ selectivity (Kiδ /Kiµ = 1200 ca.) with high potency in the GPI assay, comparable to the parent EM-2.16 Thus, binding and bioassays data indicate that, the incorporation of the ΔZPhe at position 3 or at positions 3 and 4 simultaneously, are both detrimental structural modifications. The corresponding alteration, performed at position 4 only, leads to the ligand 2 which maintains high affinity and biological activity, together with the µ selectivity typical of the native ligand.
Table 1.
Binding affinity and in vitro activity for compounds 1–3 and EM-2.
| Compound | Receptor affinity a,b (nM) | Selectivity | Functional Bioactivity | ||
|---|---|---|---|---|---|
| K iδ | K iµ | δ/µ | MVDb (IC50) | GPIb (IC50) | |
| EM-2 c | -- | 9.6 ± 0.98 | -- | 510 ± 35 | 15 ± 2 |
| 1 [ΔZPhe3]EM-2 | n.c.d | 200 ± 16 | -- | 1,900 ± 430 | 170 ± 13 |
| 2 [ΔZPhe4]EM-2 | > 10,000 | 8.4 ± 1.2 | > 1,200 | 390 ± 87 | 25 ± 2.8 |
| 3 [ΔZPhe3,4]EM-2 | 7,300 ± 890 | 130 ± 36 | 57 | 1,100 ± 120 | 330 ± 59 |
Displacement of [3H]DAMGO (µ-selective) and [3H]DPDPE (δ-selective) using membranes preparations from transfected cells expressing rat µ-opioid receptor or human δ-opioid receptor, respectively.
± S.E.M.
Data from reference 16. Binding affinity based on competition against [3H]naloxone in rat brain membranes. Data were not presented on the affinity of EM2 at the δ-opioid receptor in that preparation, as EM was shown to be highly selective for µ-opioid receptors (see also reference 1).
n.c. = no competition, i.e., compound (up to a concentration of 10−4 M) did not displace the specific binding of radioligand.
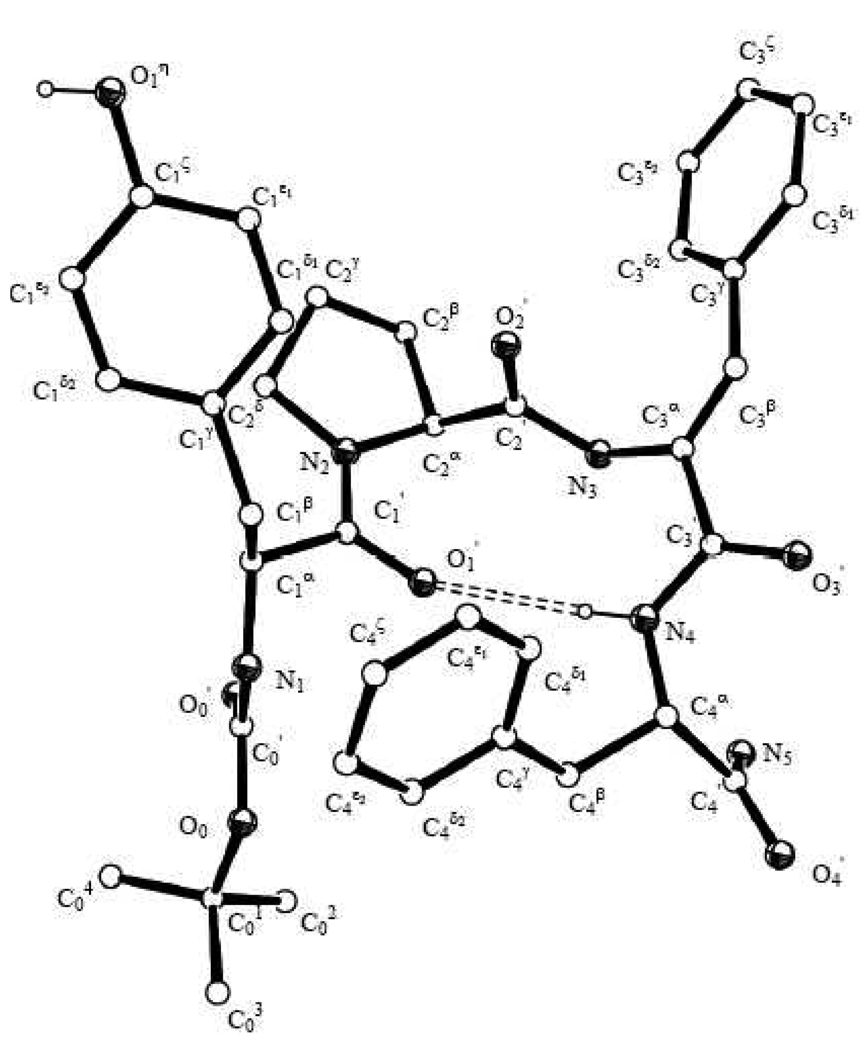
In order to gain further information on the preferred conformation of the new ligands we examined the 2D 1H NMR structures of the new ligands 1–3, the conformation adopted in solution, and in the crystal structure of 8, the Nα-Boc derivative of 1 (see Scheme 1). Single crystals of 8 were successfully obtained by slow evaporation from a solution in MeOH. The X-ray structure of the molecule (Fig. 2 and Table 2) presents a trans Tyr-Pro amide bond, and adopts a H-bond stabilized β-turn structure with the Pro and ΔZPhe residues at the i+1 and i+2 corner positions, respectively, of the turn. As for the endomorphin µ-receptor agonists, only two other crystal structures have been reported, i.e. [D-Tic2]EM-217 and [Chx2]EM-218 in addition to the C-terminal free acid Tyr-Pro-Phe-Phe-OH,19 the latter completely devoid of µ-opioid receptor agonist activity. The X-ray crystal structure of 8 (Figure 2) gives then the first available information on the solid state conformation adopted by an N-protected EM analogue.
Figure 2.
X-ray crystal structure of Boc-Tyr-Pro-ΔZPhe-Phe-NH2 (8), with numbering of the atoms. The intramolecular H-bond is shown as a dashed double line.
Table 2.
Main backbone and side chains torsion angles (°) of the X-ray crystal structure of 8a
| Backbone | ||
| O0-C0'-N1C1α | ω0 | 177.5(4) |
| C0'-N1-C1α-C1' | φ1 | −62.7(6) |
| N1-C1α-C1'-N2 | ψ1 | 150.3(4) |
| C1α-C1'-N2-C2α | ω1 | 175.5(5) |
| C1-N2-C2α-C2' | φ2 | −59.4(6) |
| N2-C2α-C2'-N3 | ψ2 | 127.9(4) |
| C2α-C2'-N3-C3α | ω2 | −175.5(4) |
| C2'-N3-C3α-C3' | φ3 | 113.8(5) |
| N3-C3α-C3'-N4 | ψ3 | −14.2(7) |
| C3α-C3'-N4-C4α | ω3 | −179.3(5) |
| C3'-N4-C4α-C4' | φ4 | −78.1(6) |
| N4-C4α-C4'-N5 | ψ4 | −19.3(6) |
| Side chains | ||
| N1-C1α-C1β-C1γ(Tyr) | χ11,1 | −156.2(4) |
| N2-C2α-C2β-C2γ (Pro) | χ21,1 | 18.2(6) |
| N3-C3α-C3β-C3 (ΔZPhe) | χ31,1 | −8.0(11) |
| N4-C4α-C4β-C4γ (Phe) | χ41,1 | −72.6(5) |
Numbers in parenthesis are e.s.d. values.
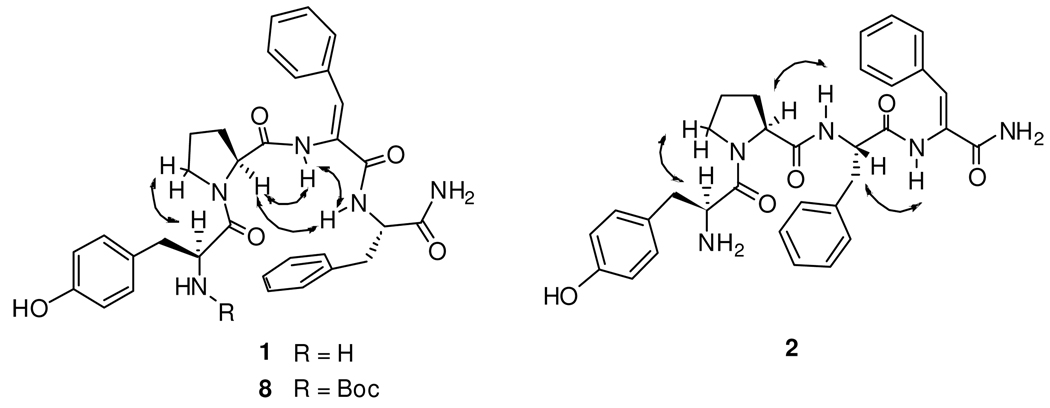
As shown in Table 3, 1 and its Nα-Boc protected derivative 8 show strong sequential NOEs ProCαH… ΔZPhe3NH. This effect is observed, for both 1 and 8, by two interresidue NOEs which are not found in the case of 2 and 3: the first between the NH groups of ΔZPhe3 and Phe4 and the second between the ProCαH and Phe4NH (i.e. between the” i+2 and i+3” and “i+1 and i+3” residues, respectively, of a β-turn). These data strongly suggest that the Nα-Boc derivative 8 maintains in DMSO-d6 solution the folded conformation found in the crystal, and that the same conformational preference is also shown by its N-terminal free analog 1. It is worth noting that the findings on 1 and 8 are those expected on the basis of literature on peptides containing α,β-dehydro amino acid where the strong tendency of the ΔZPhe residue to occupy the i+2 position of β-turns is well documented.20 No NOEs indicative of folded structures could be found in the spectra of the two peptides 2 and 3. Furthermore, the presence of the sequential NOEs ProCαH… PheNH and PheNH…ΔZPheNH in the spectrum of 2 are consistent with an extended structure.
Table 3.
Observed NOE cross peaks and intensities of analogs 1–3 and 8a in DMSO-d6
| 1 | 2 | 3 | 8 | |
|---|---|---|---|---|
| ΔZPhe3 NH… Phe4 NH | m | - | - | m |
| Pro CαH …Phe4 NH | mb | - | - | m |
| Pro CαH…ΔZPhe3 NH | sb | - | S | s |
| Pro CαH… Phe3 NH | - | s | - | - |
| Phe3 CαH…αZPhe4 NH | - | m | - | - |
| Tyr CαH… Pro CδH2 | m | m | m | m |
NOE intensities are classified as weak (1.6–5.0 Å), medium (1.6–3.6 Å) and strong (1.6–2.9 Å).
The Pro CαH is partially overlapped with Phe CαH.
Conclusions
As revealed by literature data, the tetrapeptide EMs are characterized by considerable conformational flexibility involving both the backbone and the aromatic side chains. Although the optimal three-dimensional arrangement of the EM pharmacophoric elements favouring selectivity and potency as µ agonist is not yet resolved, the relevant role of a proper spatial orientation of the aromatic rings, and in particular of the benzylic side chains at position 3 and 4, is well established. Here we have examined the properties of new EM analogues in which Phe mimics, with the aromatic ring locked in the (Z) spatial orientation, have been incorporated. A remarkable difference is observed in the receptor affinity and functional bioactivity between 2, possessing the aromatic at position 3 free to adopt a proper spatial orientation, and the analogue 1 in which the Phe at position 3 is forced to the rigid (Z) orientation imposed by the olefinic geometry of the residue. These results underline the importance of the correct orientation of the aromatic rings, and suggest, at the same time, that the preformed modification of Phe3, in the Tyr-Pro-Phe- “message” moiety, leads to the ligand 1 in which both the folded conformation of the backbone and the third aromatic ring spatial orientation are unfavourable features for proper interaction with the receptor. The analogue 2, which maintains unchanged the native EM “message” sequence and an extended backbone conformation exhibits a high activity, comparable to that of EM-2. In this analogue the aromatic side chain at position 3 can adopt a proper orientation in the receptor pocket. This interpretation appears to be in agreement with the previously described EM analogues containing β-MePhe stereoisomers.11 Here, the highest activity is shown by the ligand Tyr-Pro-Phe-(2S,3S)-β-MePhe-NH2 which maintains the native Phe3 residue and possesses at position 4 an aromatic side chain properly oriented by the stereochemistry of the β-MePhe residue.
Studies are now in progress to further investigate conformational and pharmacological consequences of EM analogues obtained by following this general approach.
Experimental Section
Chemistry. General Methods
Preparative layer chromatography (PLC) were performed on Merck 60 F254 silica gel plates. The purity of all tested compounds was determined by combustion analysis and they are ≥ 95% pure. HRMS data are also obtained.
Synthesis. General Procedures for Preparation of Peptide Amides
The peptide methyl ester is allowed to stand in a pressure bottle at room temperature for 48 h in anhydrous methanol previously saturated with ammonia gas at 0 °C. The solution is then concentrated to dryness in vacuo at a temperature not exceeding 40° C. Compound 8 was purified by crystallization from MeOH and the amides 11 and 16 were purified by PLC.
General Procedure for Deprotection of Boc-Derivatives 1, 2 and 3
The Boc group was removed by treatment with TFA in DCM (1:1) for 1 h at room temperature. Removal of solvent and precipitation of the residue with ether gave the TFA salt.
NMR Experiments
All 1D and 2D 1H NMR experiments were performed at 400 MHz on a Bruker Avance 400 NMR spectrometer with a constant temperature at 298 K. The ROESY spectra were obtained using standard pulse programs, with a mixing times of 300 ms. The 2D NMR matrixes were created and analyzed using the TOPSPIN 3.0a computer program (Bruker Biospin - 2009). Each two-dimensional spectrum was acquired in a 1024 × 1024 data matrix complex points in F1 and F2. Zero filling in F1 and sine windows in both dimensions were applied before Fourier transformation. Chemical shifts (δ) are quoted in parts per million (ppm) downfield from tetramethylsilane, and values of coupling constants are given in Hz.
TFA·H2N-Tyr-Pro-ΔZPhe-Phe-NH2 (1)
1H NMR (400 MHz, DMSO-d6): δ 1.85–2.13 (4H, m, Pro C3H2 and Pro C4H2), 2.7–3.2 (4H, m, Phe4 CβH2 and Tyr CβH2), 3.41 and 3.61 (2H, m, Pro C5H2), 4.21 (1H, m, Tyr CαH), 4.5 (2H, m, Phe4 CαH and Pro CαH), 6.6–7.6 (17H, m, aromatics, ΔZPhe3 CβH and CONH2), 7.77 (1H, d, J = 8, Phe4 NH), 8.09 (3H, br, Tyr NH3+), 9.36 (1H, s, Tyr OH), 9.82 (1H, s, ΔZPhe3 NH); HRMS for [M+H]+: m/z calcd 570.2716; found 570.2710.
TFA·H2N-Tyr-Pro-Phe-ΔZPhe-NH2 (2)
1H NMR (400 MHz, DMSO-d6): δ 1.69–2.08 (4H, m, Pro C3H2 and Pro C4H2), 2.7–3.2 (4H, m, Phe3 CβH2 and Tyr CβH2), 3.4–3.68 (2H, m, Pro C5H2), 4.17 (1H, m, Tyr CαH), 4.41 (1H, m, Pro CαH), 4.63 (1H, m, Phe3 CαH), 6.6–7.55 (17H, m, aromatics, ΔZPhe4 CβH and CONH2), 8.09 (3H, br, Tyr NH3+), 8.3 (1H, d, J = 7.8, Phe3 NH), 9.38 (1H, s, Tyr OH), 9.7 (1H, s, ΔZPhe4 NH); HRMS for [M+H]+: m/z calcd 570.2716; found 570.2721.
TFA·H2N-Tyr-Pro-ΔZPhe-ΔZPhe-NH2 (3)
1H NMR (400 MHz, DMSO-d6): δ 1.71–2.2 (4H, m, Pro C3H2 and Pro C4H2), 2.68–3.07 (2H, m, Tyr CβH2), 3.4–3.68 (2H, m, Pro C5H2), 4.17 (1H, m, Tyr CαH), 4.53 (1H, m, Pro CαH), 6.6–7.8 (18H, m, aromatics, ΔZPhe3 CβH, ΔZPhe4 CβH and CONH2), 8.09 (3H, br, Tyr NH3+), 9.35 (1H, s, Tyr OH), 9.42 (1H, s, ΔZPhe4 NH), 10.13 (1H, s, ΔZPhe3 NH); HRMS for [M+H]+: m/z calcd 568.2560; found 568.2555.
Boc-Tyr-Pro-ΔZPhe-Phe-NH2 (8)
White solid: mp = 228–232 °C; 95% yield; 1H NMR (400 MHz, DMSO-d6): δ 1.3 [9H, s, C(CH3)3]1.85–2.31 (4H, m, Pro C3H2 and Pro C4H2), 2.58–3.2 (4H, m, Phe3 CβH2 and Tyr CβH2), 3.58–3.7 (2H, m, Pro C5H2), 4.17 (1H, m, Tyr CαH), 4.39 (1H, m, Pro CαH), 4.5 (1H, m, Phe3 CαH), 6.6–7.55 (18H, m, aromatics, Tyr NH, ΔZPhe4 CβH and CONH2), 7.90 (1H, d, J = 8.4, Phe3 NH), 9.18 (1H, s, Tyr OH), 9.7 (1H, s, ΔZPhe4 NH); HRMS for [M+H]+: m/z calcd 670.3241; found 670.3248.
Boc-Tyr-Pro-ΔZPhe-ΔZPhe-NH2 (11)
Eluent mixture: chloroform/methanol 95:5; 56% yield; 1H NMR (400 MHz, DMSO-d6): δ 1.3 [(9H, s, C(CH3)3], 1.71–2.26 (4H, m, Pro C3H2 and Pro C4H2), 2.62–2.75 (2H, m, Tyr CβH2) 3.48–3.72 (2H, m, Pro C5H2), 4.25 (1H, m, Tyr CαH), 4.46 (1H, m, Pro CαH), 6.6–7.8 (19H, m, aromatics, Tyr NH, ΔZPhe3 CβH, ΔZPhe4 CβH and CONH2), 9.22 (1H, s, Tyr OH), 9.52 (1H, s, ΔZPhe4 NH), 10.12 (1H, s, ΔZPhe3 NH); HRMS for [M+H]+: m/z calcd 668.3084; found 668.3091.
Boc-Tyr-Pro-Phe-ΔZPhe-NH2 (16)
Eluent mixture: chloroform/methanol 95:5; 55% yield; 1H NMR (400 MHz, DMSO-d6): δ 1.3 [(9H, s, C(CH3)3], 1.7–2 (4H, m, Pro C3H2 and Pro C4H2), 2.5–3.1 (4H, m, Phe4 CβH2 and Tyr CβH2), 3.48 and 3.58 (2H, m, Pro C5H2), 4.21 (1H, m, Tyr CαH), 4.32 (1H, m, Pro CαH), 4.53 (1H, m, Phe4 CαH) 6.6–7.51 (18H, m, aromatics, Tyr NH, ΔZPhe3 CβH and CONH2), 8.31 (1H, d, J = 7.2, Phe4 NH), 9.66 (1H, s, ΔZPhe3 NH), 9.45 (1H, s, Tyr OH); HRMS for [M+H]+: m/z calcd 670.3241; found 670.3238.
Binding and Functional Assays
All binding assays used crude membrane preparations from transfected HEK293 cells expressing the human δ-opioid receptor or HN9.10 cells expressing the rat µ-opioid receptor. Binding affinities of the compounds were determined by competitive binding analysis against the δ-selective agonist [3H]DPDPE, and the µ-selective agonist [3H]DAMGO in the respective membrane preparations. Data from three independent experiments were fitted by non-linear regression analysis using GraphPad Prism. Ki values were calculated from IC50 values by the Cheng and Prusoff equation.21 The in vitro tissue bioassays (MVD and GPI/LMMP) were performed as described previously.22 IC50 values represent means of no less than four experiments. IC50 values, relative potency estimates, and their associated standard errors were determined by fitting the data to the Hill equation by a computerized non-linear least-squares method.
Supplementary Material
Figure 3.
Relevant interproton correlations as deduced by ROESY experiments of compounds 1, 2 and 8.
Acknowledgment
This research was supported in part by grants of the U. S. Public Health Service, National Institutes of Health, DA006284 and DA13449 (VJH).
Abbreviations: DCM, dichloromethane; DMAP, 4-(dimethylamino)pyridine; DMF, N,N-dimethylformamide; DMSO, dimethylsulfoxide; EDC, 1-ethyl-(3-dimethylaminopropyl)carbodiimide; EM-1, Tyr-Pro-Trp-Phe-NH2 (endomorphine-1); EM-2, Tyr-Pro-Phe-Phe-NH2 (endomorphine-2); 3H-DAMGO, [3H]-[d-Ala2, N-Me-Phe4, Gly-ol5]enkephalin; 3H-DPDPE, [3H]-c[d-Pen2, d-Pen5]enkephalin; GPI/LMMP, guinea pig ileum/longitudinal muscle myenteric plexus (µ opioid receptors); hMOR, human µ opioid receptor; HOBt, 1-hydroxybenzo-triazole; MVD, mouse vas deferens (δ opioid receptors); NMM, N- methyl morpholine; rDOR, rat δ opioid receptor; TEA, triethylamine; TFA, trifluoroacetic acid.
Supporting Information Available
Elemental analysis of final products, details on experimental procedures for the synthesis of intermediates, X-ray crystallographic data. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the µ-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 2.Hackler L, Zadina JE, Ge LJ. Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex. Peptides. 1997;18:1635–1639. doi: 10.1016/s0196-9781(97)00259-3. [DOI] [PubMed] [Google Scholar]
- 3.For recent reviews see: Janecka A, Staniszewska R, Fichna J. Endomorphin analogs. Curr. Med. Chem. 2007;14:3201–3208. doi: 10.2174/092986707782793880. Fichna J, Janecka A, Costentin J, do Rego J-C. The endomorphin system and its evolving neurophysiological role. Pharmacol. Rev. 2007;59:88–123. doi: 10.1124/pr.59.1.3.
- 4.(a) Leitgeb B, Tóth G. Aromatic-aromatic and proline-aromatic interactions in endomorphin-1 and endomorphin-2. Eur. J. Med. Chem. 2005;40:674–686. doi: 10.1016/j.ejmech.2004.10.015. [DOI] [PubMed] [Google Scholar]; (b) Leitgeb B. Structural investigation of endomorphins by experimental and theoretical methods: hunting for the bioactive conformation. Chemistry & Biodiv. 2007;4:2703–2724. doi: 10.1002/cbdv.200790221. [DOI] [PubMed] [Google Scholar]; (c) Yu Y, Shao X, Cui Y, Liu H-m, Wang C, Fan Y, Liu J, Dong S, Cui Y-x, Wang R. Structure activity study on the spatial arrangement of the third aromatic ring of endomorphins 1 and 2 using an atypical constrained C terminus. Chem Med. Chem. 2007;2:309–317. doi: 10.1002/cmdc.200600274. [DOI] [PubMed] [Google Scholar]
- 5.(a) Staniszewska R, Fichna J, Gach K, Toth G, Poels J, Vanden Broeck J, Janecka A. Synthesis and biological activity of endomorphin-2 analogs incorporating piperidine-2, 3- or 4-carboxylic acids instead of proline in position 2. Chem. Biol. Drug Des. 2008;72:91–94. doi: 10.1111/j.1747-0285.2008.00678.x. [DOI] [PubMed] [Google Scholar]; (b) Perlikowska R, Gach K, Fichna J, Toth G, Walkowiak B, do Rego J-C, Janecka A. Biological activity of endomorphin and [Dmt1]endomorphin analogs with six-membered proline surrogates in position 2. Bioorg. Med. Chem. 2009;17:3789–3794. doi: 10.1016/j.bmc.2009.04.046. [DOI] [PubMed] [Google Scholar]; (c) Torino D, Mollica A, Pinnen F, Lucente G, Feliciani F, Peg D, Lai J, Ma S-w, Porreca F, Hruby VJ. Synthesis of new endomorphin analogues modified at the Pro2 residue. Bioorg. Med. Chem. Lett. 2009;19:4115–4118. doi: 10.1016/j.bmcl.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Cardillo G, Gentilucci L, Melchiorre P, Spampinato S. Synthesis and binding activity of endomorphin-1 analogues containing β-amino acids. Bioorg. Med. Chem. Lett. 2000;10:2755–2758. doi: 10.1016/s0960-894x(00)00562-x. [DOI] [PubMed] [Google Scholar]; (b) Cardillo G, Gentilucci L, Qasem AR, Sgarzi F, Spampinato S. Endomorphin-1 analogues containing β-proline are µ-opioid receptor agonists and display enhanced enzymatic hydrolysis resistance. J. Med. Chem. 2002;45:2571–2578. doi: 10.1021/jm011059z. [DOI] [PubMed] [Google Scholar]; (c) Keresztes A, Szűcs M, Borics A, Kövér KE, Forró E, Fülöp F, Tömböly Cs, Péter A, Páhi A, Fábián G, Murányi M, Tóth G. New endomorphin analogues containing alicyclic β-amino acids: influence on bioactive conformation and pharmacological profile. J. Med. Chem. 2008;51:4270–4279. doi: 10.1021/jm800223t. [DOI] [PubMed] [Google Scholar]
- 7.(a) Honda T, Shirasu N, Isozaki K, Kawano M, Shigehiro D, Chuman Y, Fujita T, Nose T, Shimohigashi Y. Differential receptor binding characteristics of consecutive phenylalanines in µ-opioid specific peptide ligand endomorphin-2. Bioorg. Med. Chem. 2007;15:3883–3888. doi: 10.1016/j.bmc.2007.03.009. [DOI] [PubMed] [Google Scholar]; (b) Shao X, Gao Y, Zhu C, Liu X, Yao J, Cui Y, Wang R. Conformational analysis of endomorphin-2 analogs with phenylalanine mimics by NMR and molecular modeling. Bioorg. Med. Chem. 2007;16:3539–3547. doi: 10.1016/j.bmc.2007.02.050. [DOI] [PubMed] [Google Scholar]; (c) Del Borgo MP, Blanchfield JT, Toth I. Internalisation of the µ-opioid receptor by endomorphin-1 and leu-enkephalin is dependant on aromatic amino acid residues. Bioorg. Med. Chem. Lett. 2008;16:4341–4346. doi: 10.1016/j.bmc.2008.02.074. [DOI] [PubMed] [Google Scholar]
- 8.Fichna J, do-Rego J-C, Chung NN, Lemieux C, Schiller PW, Poels J, Vanden Broeck J, Costentin J, Janecka A. Synthesis and characterization of potent and selective µ-opioid receptor antagonists, [Dmt1, D-2-Nal4] EM-1 (Antanal-1) and [Dmt1, D-2-Nal4] EM-2 (Antanal-2) J. Med. Chem. 2007;50:512–520. doi: 10.1021/jm060998u. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Liu X, Liu W, Qi Y, Liu X, Zhou Y, Wang R. Opioid receptor binding and antinociceptive activity of the analogues of endomorphin-2 and morphiceptin with phenylalanine mimics in the position 3 or 4. Bioorg. Med. Chem. Lett. 2006;16:3688–3692. doi: 10.1016/j.bmcl.2006.04.063. [DOI] [PubMed] [Google Scholar]
- 10.Fichna J, do-Rego J-C, Kosson P, Costentin J, Janecka A. Characterization of antinociceptive activity of novel endomorphin-2 and morphiceptin analogs modified in the third position. Biochem. Pharmacol. 2005;69:179–185. doi: 10.1016/j.bcp.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Tömböly Cs, Kövér KE, Péter A, Tourwé D, Biyashev D, Benyhe S, Borsodi A, Al-Khrasani M, Rónai AZ, Tóth G. Structure activity study on the Phe side chain arrangement of endomorphins using conformationally constrained analogues. J. Med. Chem. 2004;47:735–743. doi: 10.1021/jm0310028. [DOI] [PubMed] [Google Scholar]
- 12.Hruby VJ, Al-Obeidi F, Kazmierski W. Emerging approaches in the molecular design of receptor-selective peptide ligands: conformational, topographical and dynamic considerations. Biochem. J. 1990;268:249–262. doi: 10.1042/bj2680249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hruby VJ, Li G, Haskell-Luevano C, Shenderovich M. Design of peptides, proteins and peptidomimetics in chi space. Biopolymers. 1997;43:219–266. doi: 10.1002/(SICI)1097-0282(1997)43:3<219::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 14.Schwyzer R. ACTH: a short introductory review. Ann. N. Y. Acad. Sci. 1977;297:3–26. doi: 10.1111/j.1749-6632.1977.tb41843.x. [DOI] [PubMed] [Google Scholar]
- 15.Goel VK, Guha M, Baxla AP, Dey S, Singh TP. Design of peptides with α,β-dehydro-residues: synthesis, crystal structure and molecular conformation of a peptide N-tertiary-butyloxycarbonyl-L-Leu-ΔPhe-L-Ile-OCH3. J. Molecular Structure. 2003;658:135–141. [Google Scholar]
- 16.Biondi B, Giannini E, Negri L, Melchiorri P, Lattanzi R, Rosso F, Ciocca L, Rocchi R. Opioid peptides: synthesis and biological activity of new endomorphin analogs. Int. J. Pept. Res. Ther. 2006;12:145–151. [Google Scholar]
- 17.Flippen-Anderson JL, Deschamps JR, George C, Reddy PA, Lewin AH, Brine GA, Sheldrick G, Nikiforovich G. X-ray structure of Tyr-D-Tic-Phe-Phe-NH2(D-TIPP-NH2), a highly potent µ-receptor selective opioid agonist: comparison with proposed model structures. J. Pept. Res. 1997;49:384–393. doi: 10.1111/j.1399-3011.1997.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 18.Doi M, Asano A, Komura E, Ueda Y. The structure of endomorphin analogue incorporating 1-aminicyclohexane-1-carboxylic acid for proline is similar to the β-turn of Leu-enkephalin. Biochem. Biophys. Res. Cmmun. 2002;297:138–142. doi: 10.1016/s0006-291x(02)02087-9. [DOI] [PubMed] [Google Scholar]
- 19.Y, Minoura K, Tomoo K, Sasaki Y, Lazarus LH, Okada Y, Ishida T. Structural function of C-terminal amidation of endomorphin. FEBS J. 2005;272:5079–5097. doi: 10.1111/j.1742-4658.2005.04919.x. In. [DOI] [PubMed] [Google Scholar]
- 20.(a) Piazzesi AM, Bardi R, Crisma M, Bonora GM, Toniolo C, Chauhan VS, Kaur P, Uma K, Balaram P. Conformational restrictions of peptides via backbone modification: solution and crystal-state analysis of Boc-L-Pro-ΔZPhe-Gly-NH2. Gazz. Chim. Ital. 1991;121:1–7. [Google Scholar]; (b) Ramagopal UA, Ramakumar S, Joshi RM, Chauhan VS. Crystal structure of Boc-Ala-(ΔPhe)4-Phe-NHMe a left-handed helical peptide. J. Peptide Res. 1998;52:208–215. doi: 10.1111/j.1399-3011.1998.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan BB, Hochgeschwender U, Hruby VJ, Malan TP, Jr., Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neurophatic pain. J. Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer TH, Davis P, Hruby VJ, Burks TF, Porreca F. In vitro potency, affinity and agonist efficacy of highly selective delta opioid receptor ligands. J. Pharmacol. Exp. Ther. 1993;266:577–584. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.