Abstract
Researchers and clinicians with expertise in autosomal recessive polycystic kidney disease and congenital hepatic fibrosis (ARPKD/CHF) and related fields met on May 5-6, 2005, on the National Institutes of Health (NIH) campus for a 1.5-day symposium sponsored by the NIH Office of Rare Diseases, the National Human Genome Research Institute (NHGRI), and in part by the ARPKD/CHF Alliance. The meeting addressed the present status and the future of ARPKD/CHF research.
BACKGROUND
Autosomal recessive polycystic kidney disease/congenital hepatic fibrosis (ARPKD/CHF) is a developmental disorder of the kidneys and liver caused primarily, if not exclusively, by mutations in the PKHD1 gene.1-5 Fibrocystin/polyductin, the protein encoded by PKHD1, is expressed on the primary cilia of renal and bile duct epithelial cells and is believed to function to maintain the 3-dimensional tubular architecture.6 Kidney cysts in ARPKD are nonobstructive dilations of the collecting ducts. CHF, which typically occurs in ARPKD, results from malformation of the developing ductal plate.7 Approximately 30% of patients with ARPKD present perinatally with enlarged kidneys.1,2 Although severely affected neonates may die in infancy from pulmonary insufficiency or sepsis, survival rates have dramatically increased because of improved neonatal care and antihypertensive therapy.2 However, both kidney and liver disease are progressive in most patients, with variable rates of deterioration. More than half of patients with ARPKD/CHF require kidney transplantation before reaching 20 years of age.1,8 Portal hypertension, the major clinical problem associated with CHF, causes hypersplenism and esophageal varices. A subset of patients also exhibit cystic dilation of the bile ducts (Caroli's syndrome) with risk of recurrent cholangitis.9 Early-onset severe hypertension, renal insufficiency, bleeding esophageal varices, and recurrent cholangitis are the major sources of morbidity; only symptomatic therapy is available at present.1,2,8,9 Despite what is known, the natural history of ARPKD has not been elucidated in a detailed, longitudinal fashion.
WHY A WORKSHOP ON ARPKD/CHF?
The face of ARPKD/CHF has changed because of the wide availability of prenatal ultrasonography, which enables fetal diagnosis, permitting anticipatory treatment and, potentially, improved outcome. Recent advances in the molecular genetics and cell biologic study of polycystic kidney disease (PKD), on the basis of the identification of PKD genes, offer the potential to understand the functions of PKD proteins and to propose directed therapies. A workshop creates an opportunity for experts in the field to take advantage of synergistic contributions and participate in focused discussions bridging basic science and patient care. Such a meeting may lead to comprehensive, prospective, longitudinal studies that complement existing retrospective studies of ARPKD/CHF and enhances ongoing intramural NIH protocol, which addresses these issues and attempts to identify outcome parameters for therapeutic interventions.
HIGHLIGHTS/KNOWLEDGE GAPS
Genetics and Cell Biology of ARPKD/CHF
PKHD1 is a large and complex gene, with a complicated transcription profile that likely generates multiple protein isoforms.3,4 Mutations are distributed throughout the gene, and polymorphisms are common.5,10 The current mutation detection rate is 80% to 85%.5,10 There is marked allelic heterogeneity, and most affected patients appear to be compound heterozygotes. Denaturing high-performance liquid chromatography (DHPLC) has been successfully used for mutation screening.10 In general, sequence analysis, combined with haplotype analysis in multiply affected families provides the most comprehensive results.11 The roles of the various splice forms in determining disease severity have yet to be determined.
Prenatal diagnosis of ARPKD /CHF, on the basis of haplotype analysis and on mutation analysis, has been performed.12 Preimplantation genetic diagnosis is becoming available, and there is some consideration that diagnostic centers coordinate their services.
Like other human and murine PKD proteins (ie, polycystin-1, polycystin-2, polaris, inversin), fibrocystin is localized to the primary cilia (mostly at the basal body) and apical membranes of renal tubular and biliary epithelial cells.6,13 The role of fibrocystin remains uncertain, but some evidence indicates that it functions as a part of the polycystin complex. The roles of polycystin-1 and polycystin-2 appear to involve calcium flux-dependent signaling.14 The interaction of fibrocystin with calcium ion (Ca2+)–modulating cyclophilin ligand suggests it may also be involved in regulation of Ca2+ homeostasis.15 Three-dimensional modeling of fibrocystin might give clues regarding its function and association with other proteins.
Certain gross characteristics of cyst formation in ARPKD differ from those of autosomal dominant polycystic kidney disease (ADPKD). ARPKD cysts originate from the proximal tubule during the first trimester of intrauterine life, but by birth they are localized exclusively to the collecting ducts, maintaining their continuity with the nephron.16,17 In contrast, ADPKD cysts may originate from any segment of the nephron, although cysts from the distal nephron and collecting duct often predominate.16,17 ADPKD cysts rapidly close off from urinary flow and continue to expand by transepithelial secretion. Nevertheless, several intracellular characteristics are shared by ARPKD and ADPKD cystic epithelial cells. In both disorders, normal, well-differentiated, polarized epithelia with low rates of cell division and apoptosis become cystic epithelia comprising partially dedifferentiated cells with polarization defects and high rates of division and apoptosis.16,17
Cyst development and progression require epithelial cell proliferation, transepithelial fluid secretion and extracellular matrix remodeling.16,17 Increased proliferation of PKD cyst epithelia is mediated, at least in part, by cyclic adenosine monophosphate (cAMP), lipid factors, and the epidermal growth factor family of ligands and receptors.16,17 Fibrocystin and several other human and rodent PKD proteins appear to play a role in mechanosensation18 of the extracellular environment and are considered to interact with actin, tubulin, and plasma membrane proteins. There is also emerging evidence indicating that the primary cilium and basal body may help to regulate the switch from canonical to non-canonical signaling in the developing kidney.17,19 Collectively, the available data suggest that at least some of the PKD proteins may function to help regulate planar orientation.
Many of the downstream intracellular signaling and trafficking pathways involved in ADPKD and ARPKD also appear to be shared. PKD complex proteins transduce signals by means of intracellular phosphorylation cascades, regulating nuclear gene transcription. There is evidence that these signal-transduction cascades include those of the canonical Wnt pathway (β-catenin), c-Src and focal adhesion kinase signaling, and the MAP Kinase, JAK-STAT pathway.16,17,20 Integration of complex signaling events are essential for the control of cellular proliferation, apoptosis, epithelial differentiation and for the development of polarity, intercellular adhesion, cell migration, and tubule formation.16,17,20 Within the cells, certain proteins are properly localized, but others are apically mislocalized, suggesting that specific sorting pathways or cargo vesicles are altered in PKD.16,17
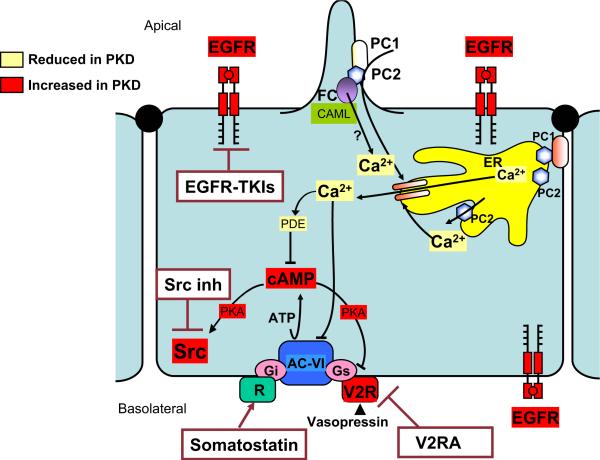
Overexpression and apical mislocalization of the epidermal growth factor receptor (EGFR) have been observed in both dominant and recessive forms of human and murine PKD21 (Figure). ARPKD cystic epithelia have exhibited EGFR-mediated fluid secretion and proliferation21 and biliary epithelia in ARPKD/CHF are also hyperresponsive to EGF.21 The relationship of the polycystin complex to EGFR function has not been satisfactorily elucidated.
Figure.
Hypothetical representation of regulation of calcium ion [Ca2+]i homeostasis by PKD-related proteins and summary of potential novel therapeutic interventions targeting major downstream effects of mutations disrupting this pathway. In response to mechanical stimulation of primary cilium, polycystin-1/2 complex mediates Ca2+ entry into cell. This triggers Ca2+ release from endoplasmic reticulum (ER). Fibrocystin (FC) may regulate [Ca2+]i homeostasis by its interaction with polycystin-2 (PC2) and Ca2+-modulating cyclophilin ligand (CAML). Major downstream effects from disruptions of this pathway include elevated cAMP, up-regulated and apically mislocalized epidermal growth factor receptor (EGFR) and up-regulated vasopressin V2 receptor (V2R). Cellular content of cAMP is determined by balance between activities of synthesizing adenylyl cyclases (AC) and catabolizing cAMP phosphodiesterases (PDE). Adenylyl cyclase VI (AC-VI), predominant AC in collecting duct epithelial cells, is stimulated by αs-subunit of heteromeric G proteins (Gs) and is inhibited by Gi and by Ca2+. Activation of V2R by vasopressin increases cAMP production. Somatostatin and vasopressin V2 receptor antagonists inhibit cAMP production. PDE1 and PDE4 are predominant PDEs in collecting duct principal cells. PDE1 is activated by Ca2+/calmodulin. PKA, Protein kinase A; Src, membrane-associated tyrosine-specific kinase; V2RA, vasopressin 2 receptor antagonist; Src inh, Src inhibitors; EGFRTKI, epidermal growth factor receptor tyrosine kinase inhibitor. Modified from VE Torres and PC Harris: Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2006;2:40-55.
Increased intracellular cAMP is a common feature of both ARPKD and ADPKD renal cells22 (Figure). Renal cAMP concentrations are increased in the pck rat (a model in which the rat orthologue of PKHD1 is disrupted), as well as the pcy muse (modeling human nephronophthisis) and the Pkd2WS25 mouse (modeling human ADPKD). Cystic renal epithelia are selectively sensitive to proliferative stimulation by increased intracellular cAMP.
Some studies have revealed that PKD cells have abnormal cell-matrix interactions,16,17 including an increased proliferative response to collagens, alterations in the expression and localization of matrix metalloproteinases, abnormal focal adhesion complex assembly, composition and phosphorylation, and increased interstitial fibroblast proliferation. Abnormalities in matrix production, receptors, adhesion proteins and complex formation may lead to increased adhesion and decreased migration. The role of extracellular matrix proteins in cyst formation requires further investigation.
Variability of the Clinical Phenotype and Modifying Genes
There can be marked variability of the severity of the clinical phenotype of ARPKD/CHF, even within the same family.23 Quantitative trait locus (QTL) mapping using the cpk mouse model suggests the presence of a modifying gene complex on mouse chromosome 4.24 Human homologues of modifying gene(s) are likely to be identified in the future. These might encode other proteins that function on the primary cilia or basal body or proteins involved in intracellular pathways downstream of fibrocystin.
Clinical Features and Management
Management of chronic renal insufficiency in ARPKD is symptomatic and similar to the management of renal insufficiency resulting from other causes. However, the presence of hypertension and the urinary concentration defect in ARPKD may require special attention. Early-onset severe hypertension, the pathophysiological condition of which remains poorly understood, occurs in approximately two thirds of children with ARPKD.1,2,8 Activation of the local reninangiotensin system25 and enhanced sodium retention by the distal collecting ducts, perhaps mediated by heightened epithelial sodium channel (EnaC) expression26,27 may be involved based on information to date. Further research into the pathogenesis of hypertension in ARPKD is necessary to delineate the complex mechanisms involved. Unlike in ADPKD, no data currently exist on the role of hypertension in disease progression and its control in ARPKD. Hypertension in patients with ARPKD is treated empirically, typically with a combination of agents. Angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and lipid-lowering agents are believed to slow progression of chronic renal insufficiency in all forms of PKD, although controlled studies are not available.
The diagnosis of those patients with ARPKD/CHF with Caroli's syndrome requires a high index of suspicion. Neither consensus statements nor guidelines exist for management strategies such as the use of prophylactic antibiotics and ursodeoxycholic acid to prevent recurrent cholangitis, sepsis, and cholelithiasis in those patients with liver involvement.28 Indications for portosystemic shunting and approaches to combined liver and kidney transplantation are complex and should be individualized.28 Recurrent cholangitis or refractory complications of portal hypertension are strong indications to consider liver transplantation. Children with ARPKD/CHF may have advanced failure of more than one organ. Immediate post-liver transplantation care can be difficult in children on dialysis, and kidney transplantation is challenging in the presence of severe portal hypertension.28 Long-term considerations related to liver transplantation, such as the potential risk of extrahepatic cholangiocarcinoma with duct-to-duct anastomoses in immunosuppressed patients, also require attention.
Some patients with ARPKD have splenic dysfunction, placing them at risk for infections with encapsulated bacteria. Patients with portal hypertension may benefit from vaccination against pneumococcus, meningococcus, and Haemophilus influenzae.
Areas related to CHF that require future research include defining the basic biology of ductal plate malformation as it pertains to bile duct epithelium and portal vein development, the prospective natural history of portal hypertension and biliary disease, the pathogenesis of extrahepatic biliary dilation29 and increased risk of ascending cholangitis, and genotype/phenotype correlations for liver disease. Cholangiocytes appear to provide a robust system in which to study ciliary function and dysfunction.
DEVELOPING SYMPTOMATIC TREATMENT AND THERAPIES DIRECTED AT THE BASIC DEFECT IN ARPKD/CHF
Therapies effective in animal models of PKD that may be effective in human PKD include angiotensin blockade (ACE inhibitors and ARBs), lipid-lowering medications (HMG CoA reductase inhibitors), vasopressin-2 receptor antagonists (V2RA; renal only), EGFR inhibitors (renal and liver), and dietary modifications (low protein, high soy protein, flaxseed oil, fish oil).30,31
Studies indicate that deficiency of fibrocystin causes enhanced downstream effects of EGFR tyrosine kinase, making EGFR-specific tyrosine kinase inhibitors (EGFR-TKIs) candidates for therapy (Figure). EGFR-TKIs are small molecules that competitively bind to the intracellular tyrosine kinase domain of EGFR, preventing receptor autophosphorylation.32 EGFR-TKIs, which can be administered orally, have been shown to retard the progression of kidney and liver disease in murine models of ARPKD, reducing renal cyst size and preserving renal function.21 This class of drugs appears relatively safe in adult patients with cancer; dose-dependent diarrhea and skin rash are the most common side effects.32 However, the potential side effects of EGFR-TKIs in the pediatric age group are not known. Specifically, we do not know the long-term effects on growth and maturation of cells with a high mitotic index, such as gastrointestinal tract epithelia. Skin biopsy might potentially be used to monitor tissue levels of EGFR-TKIs.
V2RAs decrease intracellular cAMP within renal collecting duct cells via the stimulatory G protein, Gs31 (Figure). Preclinical trials with a particular V2RA, OPC-31260, on the pck rat, the pcy mouse, and the Pkd2WS25 mouse resulted in decreased renal cAMP levels, renal cyst volume, kidney weight, plasma BUN, mitotic and apoptotic indexes, and renal fibrosis volume.31 Preclinical trials with OPC-41061 (human V2RA) produced similar effects in the pck rat.31 V2RA therapy might prove beneficial for ameliorating or preventing the renal cysts in ARPKD but not for the bile duct disease, since there are no V2 receptors in the liver. Tolerance to the effects of these agents with chronic use is a possibility. Another concern is that V2RA may result in decreased renal medullary blood flow or dehydration, especially in infants. On the other hand, potential benefits of V2RAs may be that they might contribute to the control of systemic hypertension via down-regulation of ENaC and slow the progression of chronic renal insufficiency. Another therapeutic approach might be to decrease cAMP using agonists to inhibitory G protein (Gi) such as somatostatin31 (Figure). The potential advantages and disadvantages of somatostatin treatment have not been studied.
Inhibition of c-Src, which is a component of the intra-cellular pathways common to EGFR and cAMP signaling, has the potential to be a most effective treatment33 (Figure). Some preclinical studies of c-Src inhibition in the pck rat have demonstrated amelioration of both renal and hepatic abnormalities.33 Further studies are required to explore the safety and efficacy of this approach.
Currently, we do not know how the EGFR receptor, water channels, and ion transporters are mistargeted to their ectopic locations. Identification of intermediary trafficking pathways involved in this mislocalization may provide additional new targets for treatment.
It appears premature to initiate a clinical therapeutic trial for ARPKD. Outcome parameters must be defined but might include measurements of renal functional reserve, tubular function (including maximal concentrating ability), ambulatory blood pressure, urinary biomarkers of fibrosis, high-resolution renal ultrasonography, and magnetic resonance imaging. For congenital hepatic fibrosis and portal hypertension, one might follow the platelet count, leukocyte count, spleen size, elastography of the liver, endoscopy in selected patients, upright oxygen saturation as a measure of hepatopulmonary syndrome, and bile duct imaging with magnetic resonance cholangiography.
THE NIH ARPKD/CHF PROTOCOL
The National Human Genome Research Institute (NHGRI) at the NIH has an ongoing intramural research protocol on ARPKD/CHF. The objective of this protocol is to produce comprehensive longitudinal data on the kidney and liver disease in ARPKD/CHF that will in turn provide the basis for more focused studies and for novel therapeutic interventions in the future. The protocol enrolls children and adults with a clinical diagnosis of ARPKD/CHF, which requires the presence of characteristic kidney involvement based upon clinical or biopsy findings plus either liver involvement based upon clinical or biopsy findings, or a normal parental renal ultrasound and family history compatible with autosomal recessive inheritance. This inpatient study requires subjects to be admitted to the NIH Clinical Center for 4 to 5 days, with follow-up visits every 1 to 2 years. Evaluations include 24-hour urine collections for determination of renal tubular and glomerular function, measurement of plasma renin, aldosterone, erythropoietin, cystatin C, norepinephrine, PTH, Vitamin D, IGF1 and IGF-BP3 levels. Imaging studies include high-resolution ultrasound, Doppler, MRI of the kidneys and the liver, MR cholangiography and echocardiogram. A 24-hour ambulatory blood pressure monitoring study is performed on patients with hypertension. To date, 35 patients have been evaluated under this protocol. Currently, the protocol is accepting new patients (see www.clinicaltrials.gov, where this protocol is registered as trial NCT00068224).
The NIH ARPKD/CHF protocol offers a unique opportunity to obtain detailed cross-sectional and longitudinal data through inpatient admissions and specialized, prospectively determined studies assessing the rates of change of renal and hepatic pathology. Some outcomes of the protocol to date include documentation of normal proximal tubular function (using the Fanconi Syndrome Index and fractional excretions of sodium and phosphate), impaired distal tubule function (increased fractional excretion of magnesium), normal peripheral sympathetic activity, and a specific pattern of bile duct dilation in the form of lacy cystic dilations of the very peripheral intrahepatic bile ducts in the face of normal sized segmental and common bile ducts.
Some modifications of the protocol are being pursued. For example, both renal concentrating and diluting capacity can be measured using challenge tests involving the use of DDAVP after overnight dehydration. It would be useful to measure glomerular filtration rate more precisely. When possible, patients may be placed on antihypertensive medications that do not block the renin-angiotensin system (replacement of ACE inhibitors with vasodilators, for example) for more accurate evaluation of the renin-angiotensin system. Measurement of plasma vasopressin levels may be helpful. Proteomics will be performed on urinary exosomes, small low-density vesicles enriched in membrane and cytosolic proteins from renal epithelia.34 For example, following urinary levels of aqua-porin 2, abundant in exosomes, might improve understanding about the mechanism of protein misrouting in general and apical mislocalization of EGFR in particular.
Transient liver elastography is a new, noninvasive method of evaluating liver fibrosis by measuring liver stiffness; it appears to correlate significantly with clinical, biologic, and morphologic parameters of liver disease in adults.35 Applying this noninvasive method to patients with ARPKD/CHF would likely generate pediatric elastography data that might potentially be used to monitor the degree of liver fibrosis and serve as a useful outcome parameter. To follow mild hepatic encephalopathy, a modified tracking test adapted for children may be used. Measurement of upright oxygen saturation and examination of the tricuspid valve for regurgitation on echo-cardiogram may be useful to evaluate hepatopulmonary syndrome/portopulmonary hypertension.
FUTURE DIRECTIONS
The most critical need in the field of ARPKD/CHF is to define the basic defect including the precise function of the fibrocystin protein more completely. In addition the acquisition of detailed longitudinal clinical data via the continued entry of new patients into the NIH ARPKD/CHF Protocol was endorsed as an important goal by the participants.
Acknowledgments
Supported in part by the Intramural Research Program of the NIH, specifically, that of the National Human Genome Research Institute, and by the Office of Rare Diseases, Office of the Director, NIH, and ARPKD/CHF Alliance.
Glossary
- ACE
Angiotensin converting enzyme
- ADPKD
Autosomal dominant polycystic kidney disease
- ARB
Angiotensin II receptor blocker
- ARPKD/CHF
Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis
- cAMP
Cyclic adenosine monophosphate
- CHF
Congenital hepatic fibrosis
- DHPLC
Denaturing high-performance liquid chromatography
- EGF
Epidermal growth factor
- NHGRI
National Human Genome Research Institute
- NIH
National Institutes of Health
- PKD
Polycystic kidney disease
- QTL
Quantitative trait locus
REFERENCES
- 1.Zerres K, Rudnik-Schoneborn S, Deget F, Holtkamp U, Brodehl J, Geisert J, et al. Clinical course of 115 children with autosomal recessive polycystic kidney disease. Acta Pediatr. 1996;85:437–45. doi: 10.1111/j.1651-2227.1996.tb14056.x. [DOI] [PubMed] [Google Scholar]
- 2.Capisonda R, Phan V, Traubuci J, Daneman A, Balfe JW, Guay-Woodford LM. Autosomal recessive polycystic kidney disease: outcomes from a single-center experience. Pediatr Nephrol. 2003;18:119–26. doi: 10.1007/s00467-002-1021-0. [DOI] [PubMed] [Google Scholar]
- 3.Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, et al. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immuneglobulin-like plexin transcription factor domain and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–17. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large receptor-like protein. Nat Genet. 2002;30:259–69. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann C, Senderek J, Windelen E, Kupper F, Middeldorf I, Schneider F, et al. Clinical consequences of PKHD1 mutations in 164 patients with autosomal-recessive polycystic kidney disease (ARPKD). Kidney Int. 2005;67:829–48. doi: 10.1111/j.1523-1755.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 6.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, et al. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12:2703–10. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- 7.Desmet VJ. Pathogenesis of ductal plate abnormalities. Mayo Clinic Proc. 1998;73:80–9. doi: 10.4065/73.1.80. [DOI] [PubMed] [Google Scholar]
- 8.Roy S, Dillon MJ, Trompeter RS, Barrat TM. Autosomal recessive polycystic kidney disease: long-term outcome of neonatal survivors. Pediatr Nephrol. 1997;11:302–6. doi: 10.1007/s004670050281. [DOI] [PubMed] [Google Scholar]
- 9.Summerfield JA, Nagafuchi Y, Sherlock S, Canafalch J, Scheuer PJ. Hepatobiliary fibropolycystic disease: a clinical and histological review of 51 patients. J Hepatol. 1986;2:141–56. doi: 10.1016/s0168-8278(86)80073-3. [DOI] [PubMed] [Google Scholar]
- 10.Sharp AM, Messiaen LM, Page G, Antignac C, Gubler MC, Onuchic LF, et al. Comprehensive genomic analysis of PKHD1 mutations in ARPKD cohorts. J Med Genet. 2005;42:336–49. doi: 10.1136/jmg.2004.024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consugar MB, Anderson SA, Rosetti S, Pankratz VS, Ward CJ, Torra R, et al. Haplotype analysis improves molecular diagnostics of autosomal recessive polycystic kidney disease. Am J Kidney Dis. 2005;45:77–87. doi: 10.1053/j.ajkd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Zerres K, Senderek J, Rudnik-Schoneborn S, Eggermann T, Kunze J, Mononen T, et al. New options for prenatal diagnosis in autosomal recessive polycystic kidney disease by mutation analysis of the PKHD1 gene. Clin Genet. 2004;66:53–7. doi: 10.1111/j.0009-9163.2004.00259.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Luo Y, Wilson PD, Witman GB, Zhou J. The autosomal recessive polycystic kidney disease protein is localized to primary cilia, with concentration in the basal body area. J Am Soc Nephrol. 2004;15:592–602. doi: 10.1097/01.asn.0000113793.12558.1d. [DOI] [PubMed] [Google Scholar]
- 14.Anyatonwu GI, Ehrlich BE. Calcium signaling and polycystin-2. Biochem Biophys Res Commun. 2004;322:1364–73. doi: 10.1016/j.bbrc.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 15.Nagano J, Kitamura K, Hujer KM, Ward CJ, Bram RJ, Hopfer U, et al. Fibrocystin interacts with CAML, a protein involved in Ca2+ signaling. Biochem Biophys Res Commun. 2005;338:880–9. doi: 10.1016/j.bbrc.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–64. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 17.Torres VE, Harris PC. Mechanism of disease: autosomal dominant and recessive polycystic kidney diseases. Nature Clinical Practice Nephrology. 2006;2:40–55. doi: 10.1038/ncpneph0070. [DOI] [PubMed] [Google Scholar]
- 18.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li Xiaogang, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 19.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;3:537–43. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delmas P. Polycystins: polymodal receptor/ion-channel cellular sensors. Pflugers Arch. 2005;451:264–76. doi: 10.1007/s00424-005-1431-5. [DOI] [PubMed] [Google Scholar]
- 21.MacRae Dell K, Nemo R, Sweeney WE, Jr, Avner ED. EGF-related growth factors in the pathogenesis of murine ARPKD. Kidney Int. 2004;65:2018–29. doi: 10.1111/j.1523-1755.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 22.Torres V. Cyclic AMP, at the hub of the cystic cycle. Kidney Int. 2004;64:1283–5. doi: 10.1111/j.1523-1755.2004.00945.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan BS, Kaplan P, Chadarevian J-P, Jequier S, O'Regan S, Russo P. Variable expression of autosomal recessive polycystic kidney disease and congenital hepatic fibrosis within a family. Am J Med Genet. 1988;29:639–47. doi: 10.1002/ajmg.1320290323. [DOI] [PubMed] [Google Scholar]
- 24.Mrug M, Li R, Cui X, Schoeb TR, Churchill GA, Guay-Woodford LM. Kinesin family member 12 is a candidate polycystic kidney disease modifier in the cpk mouse. J Am Soc Nephrol. 2005;16:905–16. doi: 10.1681/ASN.2004121083. [DOI] [PubMed] [Google Scholar]
- 25.Loghman-Adham M, Carlos ES, Tadashi I, Sotelo-Avila Expression of components of the renin-angiotensin system in autosomal recessive polycystic kidney disease. J Histochem Cytochem. 2005;53:979–88. doi: 10.1369/jhc.4A6494.2005. [DOI] [PubMed] [Google Scholar]
- 26.Rohatgi R, Zavilowitz B, Vergara M, Woda C, Kim P, Satlin LM. Cyst fluid composition in human autosomal recessive polycystic kidney disease. Pediatr Nephrol. 2005;20:552–3. doi: 10.1007/s00467-004-1728-1. [DOI] [PubMed] [Google Scholar]
- 27.Olteanu D, Yoder BK, Liu W, Croyle MJ, Welty EA, Rosborough K, et al. Heightened ENaC-mediated sodium absorption in a murine polycystic kidney disease model epithelium lacking apical monocilia. Am J Physiol Cell Physiol. 2005 doi: 10.1152/ajpcell.00339.2005. doi:10.1152/ajpcell.00339.2005. [DOI] [PubMed] [Google Scholar]
- 28.Shneider B, Magid M. Liver disease in autosomal recessive polycystic kidney disease. Pediatr Transplantation. 2005;9:634–9. doi: 10.1111/j.1399-3046.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 29.Goilav B, Norton K, Satlin L, Guay-Woodford L, Chen F, Magid M, et al. Predominant extrahepatic biliary disease in autosomal recessive polycystic kidney disease (ARPKD): a new association. Pediatr Transplantation. doi: 10.1111/j.1399-3046.2005.00456.x. in press. [DOI] [PubMed] [Google Scholar]
- 30.Davis ID, MacRae Dell K, Sweeney WE, Avner ED. Can progression of autosomal dominant or autosomal recessive polycystic kidney disease be prevented? Semin Nephrol. 2001;21:430–40. doi: 10.1053/snep.2001.24937. [DOI] [PubMed] [Google Scholar]
- 31.Gattone VH. Emerging therapies for polycystic kidney disease. Curr Opin Pharmacol. 2005;5:535–42. doi: 10.1016/j.coph.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Ranson M. Epidermal growth factor receptor tyrosine kinase inhibitors. Br J Cancer. 2004;90:2250–5. doi: 10.1038/sj.bjc.6601873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweeney WE, Frost P, Avner ED. Inhibition of c-Src ameliorates cyst progression in the PCK rat.. Platform Presentation. American Society of Pediatric Nephrology. Annual Meeting.; Washington, DC. May 14-17 2005. [Google Scholar]
- 34.Hoorn EJ, Pisitkun T, Zietse R, Gross P, Frokiaer J, Wang NS, et al. Prospects for urinary proteomics: exosomes as a source of urinary biomarkers. Nephrology. 2005;10:283–90. doi: 10.1111/j.1440-1797.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- 35.Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (Fibroscan): a prospective study. Gut. 2006;55:403–8. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]