Abstract
Background
Although increased apoptosis is a central feature of AIDS, little is known about its kinetics or relationship to the early host response in acute HIV/SIV infection.
Methods
Ex vivo apoptosis in freshly isolated peripheral blood and lymph node lymphocytes was monitored longitudinally in six SIVmac239-infected rhesus macaques by flow-cytometric detection of active caspase-3, cleaved poly (ADP-ribose) polymerase, and fragmented DNA.
Results
Increased apoptosis of multiple lymphocyte subsets was observed in the first two weeks following SIV infection. Apoptosis of CD4+ T lymphocytes was of low magnitude but peaked earlier than other T lymphocyte subsets. A ten-to 36-fold increase in CD8+ T lymphocyte apoptosis coincided temporally with onset of the SIV-specific cellular immune response and enrichment of caspase-3-positive cells within recently proliferating, activated CD8+ T lymphocytes.
Conclusions
The virus-specific T lymphocyte response to primary infection and generalized non-specific immune activation contribute to the pathogenesis of apoptosis in acute SIV infection.
Keywords: AIDS pathogenesis, apoptosis detection, active caspase-3, TUNEL, cleaved PARP, SIV-specific cellular immune response
Introduction
Several studies in HIV-infected humans and SIV-infected macaques with chronic infection have reported an increased susceptibility of peripheral blood lymphocytes to undergo apoptosis when cultured in medium or activated in vitro (7, 17, 18, 20, 22, 24, 27, 28, 33, 40). This phenomenon is restricted or absent in the setting of nonpathogenic HIV or SIV infection (17, 18, 23, 44). Increased apoptosis of both infected and uninfected CD4+ and CD8+ T lymphocytes has been observed and implicated as a mechanism for loss of CD4+ T lymphocytes, loss or dysfunction of HIV-specific CD8+ T lymphocytes, and disease progression in AIDS (15, 21, 22, 41, 43, 45, 46).
Relatively little is known of apoptosis during acute pathogenic lentiviral infection. A few published studies on apoptosis in acute HIV infection conducted around the time of seroconversion have shown a similar susceptibility to increased T lymphocyte apoptosis on stimulation or culture (14, 34, 52). The SIV macaque model provides a useful animal model to study early events from the time of acquisition of SIV infection. While increased apoptosis in the peripheral blood and lymph node has been demonstrated in several studies in experimentally SIV-infected non-human primates (16, 19, 36, 44, 46), data on the kinetics of apoptosis and its relationship to the host immune response in primary SIV infection are limited. In published studies, the kinetics of apoptosis in early SIV infection were only examined after peripheral blood lymphocytes had been cultured in vitro for 24-72 hours (19, 25, 44). Thus, these studies do not provide information on the in vivo frequency of apoptotic lymphocytes at any given time-point post SIV infection.
In this study we investigated the early kinetics of apoptosis in ex vivo and cultured lymphocytes in the peripheral blood and lymph node of SIVmac239-infected rhesus macaques. Lymphocytes undergoing apoptosis were identified at the single-cell level by flow-cytometric detection of intracellular active caspase-3, cleaved poly (ADP-ribose) polymerase (PARP), and terminal deoxynucleotidyl transferase-mediated biotin-dUTP nick end labeling (TUNEL). Additionally, the interferon-γ ELISPOT assay and Ki67 antigen were used to examine the relationship of the SIV-specific cellular immune response and immune activation to lymphocyte apoptosis.
We demonstrate a generalized increase in ex vivo apoptosis of multiple lymphocyte subsets in the first four weeks after SIVmac239 infection. Contrary to published studies on apoptosis in HIV and SIV infection, we show that increased apoptosis in acute SIV infection was best detected ex vivo in freshly isolated lymphocytes instead of after overnight culture. This allowed precise quantitation and relative comparison of the frequency of apoptotic cells within phenotypically defined lymphocyte subsets. At its peak, CD4+ T lymphocyte apoptosis was significantly lower in magnitude compared to apoptosis of CD8+ and other T lymphocyte subsets. Increase in CD8+ T lymphocyte apoptosis coincided with onset of the SIV-specific cellular immune response, an increase in proliferating, Ki67 antigen-positive CD8+ T lymphocytes, and an enrichment of apoptotic CD8+ T lymphocytes within the recently proliferating CD8+ T lymphocytes. These data indicate a direct contribution of virus-specific T lymphocytes to increased CD8+ T lymphocyte apoptosis in primary SIV infection, suggesting that both virus-specific and non-specific generalized immune activation contribute to the pathogenesis of increased lymphocyte apoptosis in AIDS.
Materials and Methods
Animals and SIV infection
Rhesus macaques were housed at the New England Primate Research Center (NEPRC), Southborough, Massachusetts. Six specific pathogen-free rhesus macaques that had previously been vaccinated with a control replication defective HSV-1 d106 vector (48) were inoculated intra-rectally with 3000 TCID50 of the pathogenic molecular clone SIVmac239 (provided by Ron Desrosiers, New England Primate Research Center). All animals were maintained in accordance with institutional and federal guidelines for animal care (4).
Sample collection and processing
Blood was collected in heparin vacutainer tubes (Becton Dickinson Vacutainer systems, Franklin Lakes, N.J.) and peripheral blood mononuclear cells (PBMC) isolated using density gradient centrifugation (Lymphocyte Separation Medium; MP Biomedicals Inc., Solon, OH). Lymph node biopsy tissue collected in R-10 medium, consisting of RPMI 1640 medium (Cellgro, Herndon, VA) supplemented with 10% FCS, 2 mM L-glutamine (Cellgro), 10 mM HEPES buffer (Cellgro), 50 I.U/ml penicillin (Cellgro) and 50 μg/ml streptomycin (Cellgro), was subjected to mechanical dissection and homogenization using sterile techniques. Lymphocytes released from the lymph node tissue were separated from cell debris by straining through a 70 μm cell strainer (BD Biosciences, San Jose, CA) and used for flow cytometry and ELISPOT assays.
Antibodies
Flourochrome conjugated antibodies of anti-human specificity were obtained from BD Biosciences unless stated otherwise. Monoclonal antibodies used for surface staining included anti-CD3 (clone SP34-2) allophycocyanin (APC) or APC-Cy7, anti-CD4 (clone L200) APC, phycoerythrin (PE), or peridinin chlorophyll protein (PerCP), anti-CD8 (clone SK1) PerCP, and anti-CD8 (clone RPA-T8) Alexa700. Monoclonal antibodies used for intracellular staining included anti-Ki67 (clone B56) fluorescein isothiocyanate (FITC), anti-active caspase-3 (Clone C92-605) FITC or PE, and anti-cleaved PARP (clone F21-852) PE.
Detection of apoptosis
Four-color and polychromatic flow cytometry was used for lymphocyte immunophenotyping and detection of apoptosis at the single-cell level. Intracellular expression of active caspase-3 was used for longitudinal analysis of lymphocyte apoptosis at all time-points in all rhesus macaques. Additionally, intracellular expression of cleaved PARP and the TUNEL assay were used in two rhesus macaques to confirm the presence of apoptosis. Apoptosis was measured immediately following lymphocyte isolation (ex vivo apoptosis), as well as after overnight incubation in R-10 medium in 48 well plates at 37°C in a 5%CO2 incubator (overnight apoptosis).
Intracellular staining was performed using standard procedures. Briefly, 1×106 lymphocytes were washed with phosphate-buffered saline supplemented with 2% fetal calf serum (wash buffer), stained for 30 min at 4°C with fluorochrome-conjugated monoclonal antibodies specific for cell surface molecules, washed, and fixed by incubation with FIX & PERM Medium A (Caltag Labs, Burlingame, CA) for 15 min at room temperature followed by permeabilization with FIX & PERM Medium B (Caltag Labs) along with intracellular antibodies for active caspase-3, cleaved PARP, or Ki67 antigen for another 30 min at 4°C. After a final wash, cells were fixed in fresh 2% paraformaldehyde.
TUNEL staining by flow cytometry was performed according to the manufacturer’s recommendations (Roche, Mannheim, Germany). Briefly, 2 × 106 surface-stained lymphocytes were fixed for 1 hour at RT in 2% paraformaldehyde, washed two times with wash buffer and centrifuged at 300 g for 10 min. Cells were permeabilized by incubation with 0.1% Triton X-100 in 0.1% sodium citrate on ice for 5 min. Cells were washed twice with wash buffer and incubated at 37°C in a humidified atmosphere in the dark for 1 hour along with 50 μl TUNEL reaction mix (enzyme solution and label solution 1:1). After a final wash, cells were resuspended in wash buffer and run on a FACS Calibur. Cells incubated with 50 μl of label solution served as a negative control.
Isotype and fluorescent minus one (FMO) controls were included to establish gates for positive active caspase-3 or cleaved PARP signals. In all instances, cells induced to undergo apoptosis in the presence of 5 μM Camptothecin or 10 μM Dexamethasone (Sigma-Aldrich, Steinheim, Germany) for 18 hours were used as positive controls. Samples were run on a FACS Calibur or LSR II (BD Biosciences) and at least 200,000 events were acquired. Data was analyzed using FlowJo software 8.4.6 (Tree Star, Inc., San Carlos, Calif.).
Measurement of plasma SIV RNA
Blood collected in tubes containing acid citrate dextrose was spun at 2000 rpm for 10 min within one hour of collection and stored at −80° C for quantification of SIV RNA. SIV RNA was quantified using a real time RT PCR assay, as previously described (13). As used in the present study, the assay has a threshold sensitivity of 30 SIV RNA copies/ml of plasma.
Peptides
Fifteen-amino-acid (aa) peptides overlapping by 11 aa and spanning all nine SIV proteins corresponding to the sequence of SIVmac239 were synthesized at the Massachusetts General Hospital peptide core facility (Charlestown, Mass.) by 9-fluorenylmethoxy carbonyl chemistry using an automated peptide synthesizer (MBS 396; Advanced Chemtech, Inc., Louisville, Ky.); they were also obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH. Individual peptides were suspended at 100 mg/ml in 100% dimethyl sulfoxide and subsequently pooled together for each SIV protein. A total of 10 peptide pools representing Gag, Pol (2 pools), Env, Rev, Tat, Nef, Vpr, Vpx, and Vif were used to stimulate PBMC and measure the total SIV-specific IFN-γ ELISPOT response as previously described (47). Peptides were used at a final concentration of 1 to 2 μg/ml, with the dimethyl sulfoxide concentration being maintained at <0.5% in all functional assays.
ELISPOT assay
IFN-γ ELISPOT assays were performed as previously described (47). The capture- and biotinylated-detector-matched MAb pair for IFN-γ were clones GZ-4 and 7-B6-1 (Mabtech, Nack Strand, Sweden) respectively. Briefly, sterile 96-well polyvinylidene difluoride MultiScreen-IP plates (Millipore, Bedford, Mass.) coated with anti-cytokine MAb were plated with unfractionated PBMC at 200,000 to 300,000 cells/well for SIV-specific stimulation. Stimulation of 100,000 cells with staphylococcal enterotoxin B (SEB) (100 ng/ml; Sigma, St. Louis, Mo.) were used as a positive control, while stimulation with medium alone was used as a negative control. After overnight stimulation at 37°C in a 5% CO2 incubator, cells were removed by extensive washing and incubated for 2 h at room temperature with biotinylated detector monoclonal antibodies. Spots were developed by successive incubation with streptavidin-alkaline phosphatase followed by the substrate nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate buffer (Moss, Inc., Pasadena, Maryland). Spots were counted on a KS ELISPOT Automated Reader System (Carl Zeiss, Inc., Thornwood, N.Y.) using KS ELISPOT 4.2 software (performed by ZellNet Consulting, Inc., Fort Lee, N.J.). Frequencies of responding cells obtained after subtracting background spots in negative control wells were expressed as spot-forming cells (SFC) per million PBMC. ELISPOT responses to individual SIV proteins that were more than two fold above those of negative control wells and greater than two standard deviations (SD) above the mean in SIV-seronegative rhesus macaques were considered positive.
Statistical analysis
The two-tailed paired t-test was used for comparison of changes pre-SIV infection to time-points post SIV infection. Correlation analysis was performed by the Pearson test. The Kruskal-Wallis analysis of variance and Dunn’s post test was used for comparison of more than two unpaired groups. All statistical analysis was performed using the GraphPad Prism software version 4.0c (GraphPad Software, Inc.).
Results
Flow cytometric detection of lymphocyte apoptosis in acute SIV infection
Although several studies have demonstrated an increased susceptibility of lymphocytes to undergo apoptosis in the chronic phase of pathogenic lentiviral infection (7, 18, 24, 33, 40), relatively little data is available on apoptosis in the setting of acute infection. In this study we used experimental SIV infection of rhesus macaques to study the kinetics of apoptosis during acute pathogenic SIV infection in a host species that progresses to AIDS.
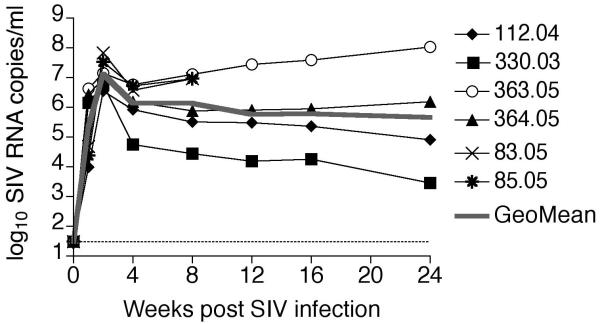
Six Indian rhesus macaques (112.04, 330.03, 363.05, 364.05, 83.05 and 85.05) were inoculated intra-rectally with 3000 TCID50 of pathogenic SIVmac239 and followed longitudinally from the time of inoculation until six months after SIV infection. SIVmac239 replicated to high levels in the rhesus macaques; peak plasma viremia ranged between 3.6 × 106 and 6.7 × 107 SIV RNA copies/ml plasma (mean 2.2 × 107 copies/ml) while set-point (week12) plasma SIV RNA levels ranged between 1.6 × 104 and 2.8 × 107 copies per ml/plasma (mean 7.3 × 106 copies/ml; Figure 1).
Figure 1. Kinetics of SIVmac239 viremia in six experimentally infected rhesus macaques.
Plasma SIV RNA levels in six SIVmac239-infected rhesus macaques determined by real time PCR are shown. Rhesus macaques 83.05 and 85.05 were only followed until eight weeks post SIV infection. The grey line represents the geometric mean of the viral loads. The limit of detection of the assay denoted by the dotted line was 30 SIV RNA copies/ml plasma.
Since published studies on lymphocyte apoptosis in HIV / SIV infection have observed increased apoptosis only after a period of in vitro culture (7, 14, 24, 34, 40, 52), we first examined whether similar conditions for detecting apoptosis were required in the setting of acute SIV infection. In this study flow-cytometric detection of CD3+ lymphocytes with intracellular expression of active caspase-3 was used for identification of apoptotic T lymphocytes. Caspase-3 is an effector caspase, that is synthesized as an inactive pro-enzyme (32kDa) and activated by self-proteolysis or cleavage into a heterodimer of 17- and 12-kDa subunits during the early stages of apoptosis (39). The cleavage of caspase-3 has been shown to be a reliable marker for the presence of apoptotic cell death (1, 8, 42, 50).
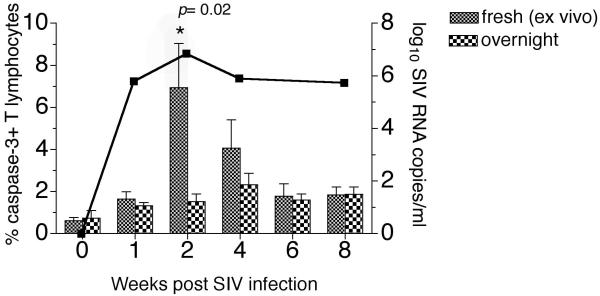
In four rhesus macaques, apoptosis measured in freshly isolated lymphocytes (fresh, ex vivo apoptosis) was compared to apoptosis following overnight incubation of lymphocytes in R-10 culture medium (overnight apoptosis) for the first eight weeks following SIV inoculation (Figure 2). In contrast to chronic infection where increased lymphocyte apoptosis is only revealed after a period of in vitro culture or stimulation, in acute SIV infection increased lymphocyte apoptosis was readily detected in freshly isolated lymphocytes and often masked after overnight culture (Figure 2 and data not shown). At two weeks, coincident with the peak of plasma SIV viremia, the frequency of apoptotic lymphocytes after overnight culture were significantly lower compared to freshly examined lymphocytes (Figure 2). These differences were not apparent after six to eight weeks post SIV infection (Figure 2). The discrepancy in apoptosis detected in fresh versus cultured lymphocytes at the peak of viral replication raises the possibility of significant activation-induced apoptosis during acute SIV infection. Thus, the presence of a large pool of circulating activated T lymphocytes prone to undergo rapid apoptosis and death would explain the underestimation of apoptosis after overnight culture. These findings point to the necessity of measuring apoptosis directly ex vivo in freshly isolated lymphocytes for evaluation during the acute phase of SIV infection.
Figure 2. Comparison of apoptosis in freshly isolated versus cultured lymphocytes in relation to plasma viremia in acute SIV infection.
Longitudinal analysis of the frequency of caspase-3-positive T lymphocytes detected in freshly isolated lymphocytes (fresh, ex vivo) and following 18 hours in vitro culture without any additional stimuli (overnight). Columns and error bars represent the mean and standard error of mean (SEM) values obtained in four animals. Asterisk denotes significant differences between the two methods of apoptosis detection calculated by the paired t-test. The solid overlay line represents the geometric mean plasma SIV RNA viral load in the four animals.
In order to confirm the reliability of using intracellular active caspase-3 as a marker of lymphocyte apoptosis in SIV-infected macaques, two additional markers of apoptosis were investigated by flow cytometry. Intracellular staining for active caspase-3 was combined with detection of cleaved PARP, as well as detection of DNA fragmentation by the TUNEL assay. All three markers of apoptosis were investigated longitudinally for the first eight weeks following SIVmac239 infection in two rhesus macaques, 83.05 and 85.05.
Following SIV infection, TUNEL-positive T lymphocytes increased from baseline values of 0.2 and 0.4% pre-SIV infection to 7.41 and 3.96% at week two post SIV infection in macaques 83.05 and 85.05 respectively (Figure 3A-B). Coincident with the increase in TUNEL-positive T lymphocytes, a comparable increase in caspase-3-positive T lymphocytes was observed (Figure 3A-B). The presence of apoptosis was further confirmed by intracellular detection of cleaved PARP, a by-product of the proteolytic action of active caspase-3 on its substrate PARP (Figure 3A). Cleaved PARP was detected in 4% (83.05) and 2.91% (85.05) of all T lymphocytes at week two post SIV infection as compared to 0.46% (83.05) and 0.38% (85.05) pre-SIV infection (Figure 3B). As expected, cleaved PARP co-localized with active caspase-3 (Figure 3A). While almost all cleaved PARP-positive lymphocytes were positive for active caspase-3, a subset of lymphocytes expressing active caspase-3 did not yet express cleaved PARP (Figure 3A-B). Similar findings were detected in lymph node lymphocytes (data not shown). Finally, the frequency of active caspase-3-positive T lymphocytes showed a strong positive correlation with both TUNEL-positive and cleaved PARP-positive T lymphocytes indicating that it was a reliable marker for detection of apoptosis (Figure 3C).
Figure 3. Comparison of active caspase-3, cleaved PARP, and TUNEL for detection of T lymphocyte apoptosis in SIV-infected rhesus macaques.
(A) Representative flow plots of CD3+ T lymphocytes stained for TUNEL-positive cells (on the left), active caspase-3-positive cells (in the middle) and cleaved PARP-positive cells, including PARP and caspase-3 colocalization (on the right). Data are shown for pre-SIV and two weeks post SIV infection time-points in one rhesus macaque. (B) Kinetics of active caspase-3-, TUNEL- and cleaved PARP-positive T lymphocytes following SIVmac239 infection. Data on two rhesus macaques, 83.05 (on the left) and 85.05 (on the right) are shown. (C) Correlation between active caspase-3-positive and TUNEL-positive T lymphocytes (on the left) and between active caspase-3-positive and cleaved PARP-positive T lymphocytes (on the right). Data on peripheral blood and lymph node of two SIVmac239-infected rhesus macaques shown. Correlation analysis was performed with the Pearson test. “p.i.” – post-infection
Global increase in lymphocyte apoptosis following SIVmac239 infection in rhesus macaques
Having established optimal conditions for flow-cytometric detection of apoptosis in acute SIV infection, we used intracellular active caspase-3 expression in freshly isolated lymphocytes to examine the kinetics of apoptosis in multiple lymphocyte subsets in the peripheral blood and lymph node of SIVmac239-infected rhesus macaques (Figure 4).
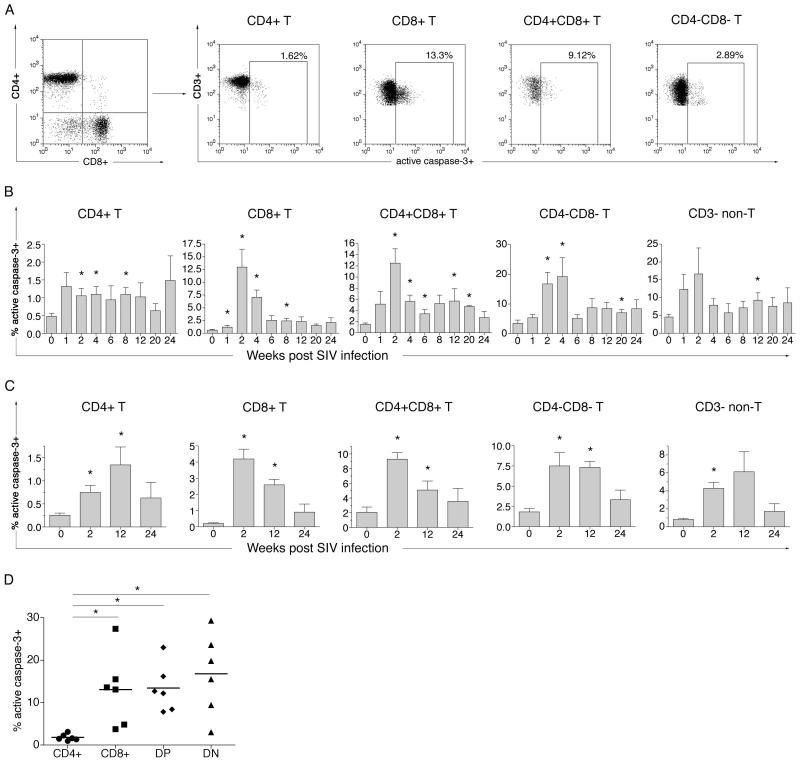
Figure 4. Increase in apoptosis of T and non-T lymphocyte subsets following SIVmac239 infection.
(A) Representative flow cytometric analysis of active caspase-3 in freshly isolated T lymphocyte subsets at week two post SIV infection. Lymphocytes gated on CD3+ T lymphocyte were analyzed for intracellular expression of active caspase-3 on CD4+, CD8+, CD4+CD8+ (DP) and CD4−CD8− (DN) T lymphocytes. (B) Longitudinal analysis of apoptosis in peripheral blood lymphocytes. Percentage caspase-3-positive cells prior to and at different time-points following SIV infection are shown for T lymphocyte subsets and CD3− non-T lymphocytes. (C) Longitudinal analysis of apoptosis of T lymphocyte subsets and CD3− non-T lymphocytes in the peripheral lymph node. Time-points pre SIV, and at weeks two, 12 and 24 post SIV infection are shown. Columns and error bars represent the mean and SEM values of six (weeks 0 to12) or four (weeks 16 to 24) rhesus macaques. Asterisks denote significant differences (P value <0.05) between pre-SIV and post-SIV infection time-points determined by the two-tailed paired t-test. (D) Comparison of maximal frequency of apoptotic cells in different T lymphocyte subsets in the first four weeks post SIV infection. Asterisks denotes significant differences (P value <0.05) between T lymphocytes subsets determined by the one way AVOVA Kruskal-Wallis and Dunn’s post test.
Coincident with the detection of plasma viremia, increased apoptosis of multiple lymphocyte subsets was observed in the peripheral blood and lymph node of all six infected rhesus macaques (Figure 4A-C). The overall frequency of apoptotic (active caspase-3-positive) CD3+ T lymphocytes in the peripheral blood increased five-to 22-fold at two weeks post SIV infection (data not shown). This increase involved all four T lymphocytes subsets (Figure 4A-C). Additionally, a two- to nine-fold increase in apoptosis of non-T (CD3−) lymphocytes was also observed in all macaques at one or two weeks following SIV infection (Figure 4B), suggesting a common mechanism driving the global increase in apoptosis in acute SIV infection.
The magnitude and kinetics of apoptosis of CD4+ T lymphocytes differed from that of other T lymphocyte subsets in several respects (Figure 4B-D). A two-to six-fold increase in CD4+ T lymphocyte apoptosis was observed as early as one week after SIVmac239 infection in four of six rhesus macaques. At its peak (one week post SIV infection in three macaques), 0.96 to 3.13% (mean 1.8%) of peripheral CD4+ T lymphocytes were undergoing apoptosis (Figure 4B). Levels of CD4+ T lymphocyte apoptosis were slow to decline and at eight weeks post SIV infection continued to be elevated in more than half of the SIV-infected macaques (Figure 4B). In contrast to CD4+ T lymphocytes, the increase in CD8+, CD4+CD8+ (DP) and CD4−CD8− (DN) T lymphocyte apoptosis peaked only two weeks or later after SIV infection (Figure 4B) and was of significantly greater magnitude (Figure 4D). While pre-SIV infection frequencies of active caspase-3-positive cells were similar for CD4+ and CD8+ T lymphocytes (mean 0.48% and 0.62% respectively), 3.7 to 27.4% (mean 12.9%) of CD8+ T lymphocytes were undergoing apoptosis at two weeks post SIV infection (Figure 4D). There was no correlation between the magnitude of CD4+ or CD8+ T lymphocyte apoptosis and the level of peak SIV viremia (data not shown). A similar pattern of increased lymphocyte apoptosis was observed in the peripheral lymph node of all animals (Figure 4C). The frequency of apoptotic lymphocytes in the lymph node were lower compared to peripheral blood, likely reflecting the lower frequency of memory T lymphocytes in the lymph node.
Relationship between caspase-3-positive CD8+ T lymphocytes and the SIV-specific cellular immune response in acute SIV infection
The acute phase of HIV and SIV infection is characterized by an initial burst of viral replication followed by partial control of viremia that is temporally associated with increased lymphocyte proliferation and the onset of a virus-specific cellular immune response (29, 30, 32).
To investigate the relationship between the SIV-specific cellular immune response and the increase in CD8+ T lymphocyte apoptosis in acute SIV infection, the IFN-γ ELISPOT response to the entire SIV proteome was measured using overlapping SIVmac239 sequence-based peptides as previously described (47). Concordant with previous studies in SIV-infected rhesus macaques, SIV-specific ELISPOT responses were detectable in the peripheral blood and lymph node as early as two weeks following SIV infection (Figure 5A-B; top panels). The total magnitude of the SIV-specific IFN-γ ELISPOT response at two weeks post infection ranged between 877 to 8589 SFC/106 lymphocytes (mean 3702) in the peripheral blood and between 203 to 1960 SFC/106 lymphocytes (mean 608) in the peripheral lymph node (Figure 5A-B; top panels). At two weeks post infection, 53.0 to 95.8% (mean 68.8%) of the total anti-SIV IFN-γ ELISPOT response in the peripheral blood was directed towards the SIV structural proteins Gag, Pol and Env.
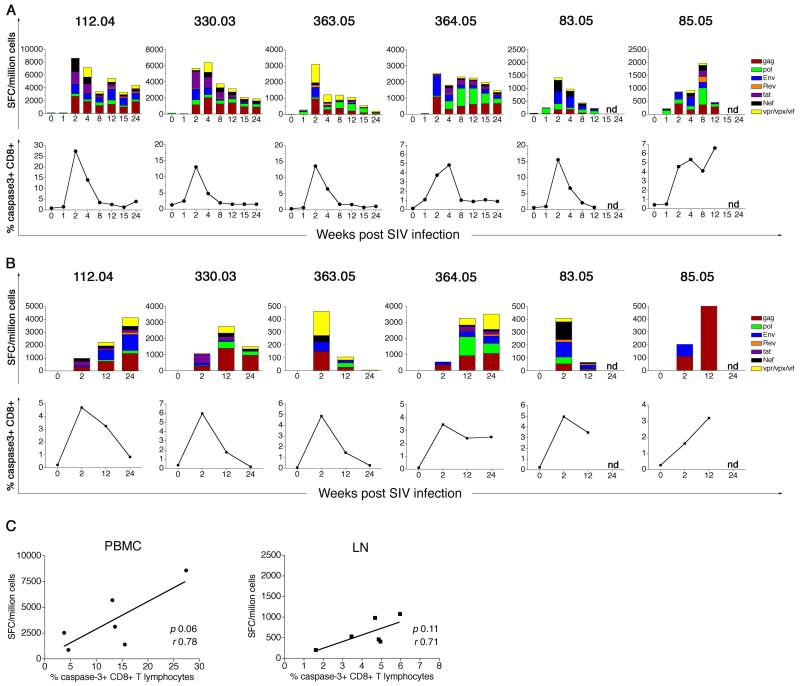
Figure 5. SIV-specific IFN-γ ELISPOT responses and CD8+ T lymphocyte apoptosis in SIVmac239-infected rhesus macaques.
(A) Kinetics of peripheral blood SIV-specific IFN-γ ELISPOT responses (top panel) and CD8+ T lymphocyte apoptosis (bottom panel) for the first 24 weeks following SIV infection. (B) Kinetics of peripheral lymph node SIV-specific IFN-γ ELISPOT responses (top panel) and CD8+ T lymphocyte apoptosis (bottom panel) for the first 24 weeks following SIV infection. Data on individual SIVmac239-infected rhesus macaques shown. (C) Relationship between the magnitude of the SIV-specific IFN-γ ELISPOT response and frequency of active caspase-3-positive CD8+ T lymphocytes at two weeks post SIV infection in PBMC (left panel) and lymph node (right panel). Correlation analysis was performed with the Pearson test. SFC: Spot forming cells
The onset of the SIV-specific cellular immune response in both peripheral blood and lymph node coincided temporally with the increase in frequency of active caspase-3-positive CD8+ T lymphocytes (Figure 5A-B; bottom panels), suggesting that the expanded pool of apoptotic CD8+ T lymphocytes early in SIV infection likely contained SIV-specific CD8+ T lymphocytes. Although a trend towards a positive correlation between the magnitude of active caspase-3-positive CD8+ T lymphocytes and the SIV-specific IFN-γ ELISPOT response at two weeks post SIV infection was observed, it did not reach statistical significance (Figure 5C).
Relationship between CD8+ T lymphocyte apoptosis and immune activation
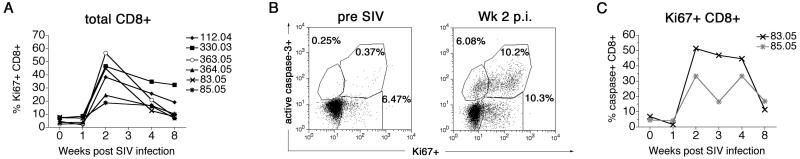
We next investigated the relationship between increased CD8+ T lymphocyte apoptosis and immune activation in acute SIV infection. Immune activation has been implicated as a major factor in the pathogenesis of apoptosis in pathogenic lentiviral infection (23, 38). Coincident with the onset of the SIV-specific cellular immune response and a ten- to 36-fold increase in CD8+ T lymphocyte apoptosis, a three- to 23-fold increase in activated CD8+ T lymphocytes expressing the proliferation marker Ki67 antigen was observed at two weeks post SIV infection (Figure 6A).
Figure 6. Relationship between intracellular Ki67 antigen and active caspase-3 expression in CD8+ T lymphocytes following SIVmac239 infection.
(A) Kinetics of activated Ki67-positive CD8+ T lymphocytes in six SIVmac239-infected rhesus macaques. (B) Representative flow analysis demonstrating co-localization of Ki67 antigen and active caspase-3 on CD8+ T lymphocytes prior to and two weeks post SIV infection. (C) Frequency of active caspase-3-positive cells within Ki67-positive CD8+ T lymphocytes in the first eight weeks following SIVmac239 infection. Data on rhesus macaques 83.05 and 85.05 shown. “p.i.” – post-infection
In two SIVmac239-infected rhesus macaques, 83.05 and 85.05, the relationship between CD8+ T lymphocyte apoptosis and activation was further investigated by double-staining CD8+ T lymphocytes for intracellular Ki67 antigen and active caspase-3 in the first eight weeks post SIV infection (Figure 6B-C). Prior to SIV infection, <7% of Ki67-positive CD8+ T lymphocytes showed evidence of apoptosis (Figure 6B-C). Two weeks post SIV infection, at the time of peak increase in total Ki67-positive CD8+ T lymphocytes (Figure 6A), there was a marked and preferential increase in the frequency of active caspase-3-positive cells within the Ki67 antigen-positive CD8+ T lymphocyte population, and Ki67hi CD8+ T lymphocytes expressing high levels of active caspase-3 were observed (Figure 6B). At this time-point, 33 (85.05) to 51% (83.05) of Ki67-positive CD8+ T lymphocytes were undergoing apoptosis (Figure 6C), while the corresponding frequency of apoptotic cells within total CD8+ T lymphocytes in these macaques was 4.6 and 15.5% respectively (Figure 5A; bottom panel). These data suggest that the rapidly expanding pool of proliferating, activated CD8+ T lymphocytes characteristic of acute pathogenic SIV infection in rhesus macaques, are enriched for cells undergoing activation-induced apoptosis. Thus, the increase in CD8+ T lymphocyte apoptosis soon after SIV infection is in part related to the presence of activated SIV-specific CD8+ T lymphocytes.
Discussion
By measuring apoptosis ex vivo at the single-cell level in lymphocytes of SIVmac239-infected macaques for six months from the time of SIV inoculation, this study provides the first longitudinal analysis of quantitative estimates of apoptosis in multiple T lymphocyte subsets during primary pathogenic SIV infection. A generalized increase in apoptosis of T and non-T lymphocytes was observed in the peripheral blood, and to a lesser extent in peripheral lymph node lymphocytes during the first twelve weeks post SIV inoculation, suggesting a common initiating mechanism, likely immune activation. These findings are consistent with previous studies on lymphocyte apoptosis in primary HIV and SIV infection (19, 25, 34). However, the results of this analysis have important differences from published studies. To date, studies on apoptosis in peripheral blood lymphocytes in primary HIV or SIV infection have been confined to examination of cultured lymphocytes (14, 19, 25, 34, 44, 52). Analysis of apoptosis after culture or stimulation is subject to in vitro artefact and is unlikely to provide meaningful information on in vivo levels of apoptosis. In this study we first compared measurement of apoptosis directly ex vivo in freshly isolated uncultured lymphocytes with overnight culture and found that increased apoptosis was detected in ex vivo lymphocytes during acute SIV infection and importantly, was a more sensitive and reproducible measure of apoptosis compared to overnight cultured lymphocytes. Thus, the kinetics of peripheral blood lymphocyte apoptosis reported in this study are likely to be a more precise representation of the in vivo frequency of T lymphocyte subsets undergoing apoptosis following SIV infection.
Despite the generalized increase in lymphocyte apoptosis soon after SIV inoculation, important differences in apoptosis between T lymphocyte subsets were observed. Notably, peripheral blood CD4+ T lymphocyte apoptosis peaked earlier and decayed at a slower rate compared to CD8+ T lymphocyte apoptosis. Furthermore, the peak magnitude of CD4+ T lymphocyte apoptosis was significantly lower compared to that of CD8+ and other (double-positive and double-negative) T lymphocyte subsets. A two- to five-fold increase in CD4+ T lymphocyte apoptosis contrasted with a ten- to 36-fold increase in CD8+ T lymphocyte apoptosis. The magnitude of apoptosis in the lymph node lymphocytes at two weeks post SIV infection also showed a similar trend. Such a difference in the early magnitude of apoptosis between CD4+ and CD8+ T lymphocytes was reported in one cross-sectional study on primary HIV infection (52), but has not previously been reported in primary SIV infection. In two studies on primary SIVmac251 infection in Chinese rhesus macaques, Annexing V labeling of freshly isolated lymph node lymphocytes (46), or of in vitro cultured peripheral blood lymphocytes (19), did not show differences in apoptosis in the two lymphocyte subsets early in infection. Peripheral blood lymphocytes showed increases in apoptosis of CD4+ and CD8+ T lymphocytes as early as one week post SIV inoculation that appeared to be of comparabe magnitude (19). In the lymph nodes, a significant increase in CD4+ but not CD8+ T lymphocyte apoptosis was observed at two weeks post infection (46).
Differences between CD4+ and CD8+ T lymphocytes observed in our study suggest additional, possibly differential mechanisms of apoptosis for the two T lymphocyte subsets. It has been shown that CD4+ T lymphocytes in HIV-infected humans are particularly susceptible to activation-induced apoptosis (24) and Fas-mediated apoptosis (27, 28). Different from CD8+ T lymphocytes, CD4+ T lymphocyte apoptosis can also be attributed to direct virus infection. However, in contrast to primary SIVmac251 infection in Chinese rhesus macaques (36), we did not observe a correlation between the magnitude of peak viremia and CD4+ T lymphocyte apoptosis in SIVmac239-infected Indian rhesus macaques. Infected and uninfected CD4+ T lymphocytes are also more susceptible to apoptosis mediated by cross-linking of gp120 with the CD4 molecule and co-receptors (3). In this context, it would appear paradoxical that CD8+ T lymphocyte apoptosis exceeded CD4+ T lymphocyte apoptosis during acute SIV infection. There may be several explanations for this phenomenon. Double-positive and double-negative T lymphocytes showed a high frequency of apoptotic cells early in SIV infection. It is conceivable that these respective subsets included activated CD4+ T lymphocytes with up-regulation of the CD8αα chain, and infected CD4+ T lymphocytes with down-regulation of surface CD4 molecules. Other possibilities for differential CD4+ and CD8+ T lymphocyte apoptosis in acute SIV infection include differences in the proportion of naïve and memory cells within each subset. A higher frequency of naïve cells within CD4+ T lymphocytes may account for lower frequencies of apoptotic cells. Finally, a quantitatively greater representation of CD8+ T lymphocytes in the primary SIV-specific T lymphocyte response could account for a greater magnitude of CD8+ T lymphocyte apoptosis at two weeks post SIV infection.
The kinetic studies on lymphocyte apoptosis and the SIV-specific cellular immune response measured by the IFN-γ ELISPOT assay suggest a significant contribution of the SIV-specific CD8+ T lymphocyte response to apoptosis in acute SIV infection. We observed that increased CD8+ T lymphocyte apoptosis in the peripheral blood and lymph node coincided temporally with detection of SIV-specific IFN-γ ELISPOT responses and an increase in Ki67 antigen-positive CD8+ T lymphocytes. Furthermore, there was an enrichment of apoptotic CD8+ T lymphocytes within recently activated, proliferating CD8+ T lymphocytes as evidenced by co-localization of active caspase-3 and Ki67-positive CD8+ T lymphocytes following SIVmac239 infection. This scenario is similar to acute viral infections such as HIV and EBV which are characterized by an initial rapid, massive expansion of activated CD8+ T lymphocytes followed by a contraction phase in which activated cells are eliminated by apoptosis in order to maintain T cell homeostasis (5, 10, 52). Although the precise contribution of antigen-specific cells to the pool of activated T cells remains controversial, studies in the lymphocytic choriomeningitis virus mouse model, and in infectious mononucleosis have shown that a substantial majority (>50%) of activated cells can be antigen-specific (9, 11, 37). We observed a trend for a positive correlation between the SIV-specific IFN-γ ELISPOT response and the peak of CD8+ T lymphocytes apoptosis, suggesting that the increase in CD8+ T lymphocyte apoptosis in the acute phase is at least in part due to an expansion of SIV-specific CD8+ T lymphocytes. Thus, the increase in CD8+ T lymphocyte apoptosis during the acute phase of SIV infection observed in our study could be attributed to (i) a physiological process, in which activated SIV specific CD8+ T lymphocytes are being cleared to maintain normal T lymphocyte homeostasis, (ii) abnormal apoptosis of antigen-specific CD8+ T lymphocytes that prevents the virus from being cleared, or (iii) apoptosis of activated bystander CD8+ T lymphocytes. In the absence of studies on active caspase-3 expression on tetramer-positive CD8+ T lymphocytes we cannot determine the precise contribution of activated non-virus-specific T lymphocytes to the increased pool of apoptotic cells. Overall, our findings implicates the virus-specific host immune response to SIV in the pathogenesis of increased CD8+ T lymphocyte apoptosis during primary SIV infection.
In the present study flow-cytometric detection of active caspase-3 was used as the primary marker for identifying lymphocyte apoptosis. Caspase-3 is an effector caspase, that is activated by both the intrinsic and extrinsic apoptotic pathways. Activation of caspase-3 triggers cleavage of cellular substrates, which eventually leads to chromatin condensation, DNA fragmentation, cell shrinkage and the formation of apoptotic bodies (39). Although cleavage of caspase-3 constitutes a reliable marker for the presence of apoptotic cell death (1, 8, 42), and has been used to investigate apoptosis in HIV infection (12, 51, 52), there are certain caveats related to its usage. Active caspase-3 can accumulate in cells during T lymphocyte activation, proliferation and differentiation in the absence of apoptosis (2, 31, 35, 49) and thus could potentially overestimate apoptosis. Conversely, apoptosis could be underestimated in the presence of caspase-independent apoptosis (6, 26). In the present study, these two scenarios are unlikely since apoptosis detection by the TUNEL assay and detection of cleaved PARP, two reliable assays for detection of apoptosis, were highly concordant with detection of active caspase-3 in T lymphocytes.
In conclusion, our study has comprehensively defined the kinetics of T lymphocyte apoptosis in relation to the host immune response and immune activation in the setting of pathogenic SIV infection. Comparison with nonpathogenic infection should provide a better understanding of the role of apoptosis in AIDS pathogenesis.
Acknowledgements
This work was supported in part by Public Health Services grants RR00168, AI49809, and AI46006. We gratefully acknowledge Michael Piatak Jr, AIDS Vaccine Program, SAIC Frederick Inc., for plasma SIV RNA measurements; Angela Carville and the department of Primate Resources NEPRC for assistance with animal studies, and Michelle Connolle and Jackie Gillis for flow cytometry support.
References
- 1.Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26:390–7. doi: 10.1016/s0968-0004(01)01844-8. [DOI] [PubMed] [Google Scholar]
- 2.Alam A, Cohen LY, Aouad S, Sekaly RP. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J Exp Med. 1999;190:1879–90. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alimonti JB, Ball TB, Fowke KR. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol. 2003;84:1649–61. doi: 10.1099/vir.0.19110-0. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous . Guide for care and use of laboratory animals. Institute of Laboratory Animal Resources, National Council; Washington, D.C.: 1996. pp. 86–123. [Google Scholar]
- 5.Appay V, Papagno L, Spina CA, Hansasuta P, King A, Jones L, Ogg GS, Little S, McMichael AJ, Richman DD, Rowland-Jones SL. Dynamics of T cell responses in HIV infection. J Immunol. 2002;168:3660–6. doi: 10.4049/jimmunol.168.7.3660. [DOI] [PubMed] [Google Scholar]
- 6.Arnoult D, Petit F, Lelievre JD, Lecossier D, Hance A, Monceaux V, Hurtrel B, Ho Tsong Fang R, Ameisen JC, Estaquier J. Caspase-dependent and -independent T-cell death pathways in pathogenic simian immunodeficiency virus infection: relationship to disease progression. Cell Death Differ. 2003;10:1240–52. doi: 10.1038/sj.cdd.4401289. [DOI] [PubMed] [Google Scholar]
- 7.Baumler CB, Bohler T, Herr I, Benner A, Krammer PH, Debatin KM. Activation of the CD95 (APO-1/Fas) system in T cells from human immunodeficiency virus type-1-infected children. Blood. 1996;88:1741–6. [PubMed] [Google Scholar]
- 8.Budd RC. Activation-induced cell death. Curr Opin Immunol. 2001;13:356–62. doi: 10.1016/s0952-7915(00)00227-2. [DOI] [PubMed] [Google Scholar]
- 9.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–75. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callan MF, Fazou C, Yang H, Rostron T, Poon K, Hatton C, McMichael AJ. CD8(+) T-cell selection, function, and death in the primary immune response in vivo. J Clin Invest. 2000;106:1251–61. doi: 10.1172/JCI10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callan MF, Steven N, Krausa P, Wilson JD, Moss PA, Gillespie GM, Bell JI, Rickinson AB, McMichael AJ. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–11. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 12.Cicala C, Arthos J, Rubbert A, Selig S, Wildt K, Cohen OJ, Fauci AS. HIV-1 envelope induces activation of caspase-3 and cleavage of focal adhesion kinase in primary human CD4(+) T cells. Proc Natl Acad Sci U S A. 2000;97:1178–83. doi: 10.1073/pnas.97.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cline AN, Bess JW, Piatak M, Jr., Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–12. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 14.Cossarizza A, Mussini C, Mongiardo N, Borghi V, Sabbatini A, De Rienzo B, Franceschi C. Mitochondria alterations and dramatic tendency to undergo apoptosis in peripheral blood lymphocytes during acute HIV syndrome. Aids. 1997;11:19–26. doi: 10.1097/00002030-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Cotton MF, Ikle D, Rapaport E, Marschner S, Tseng P, Kurrle R, Finkel TH, Terri H. Apoptosis of CD4+ and CD8+ T Cells Isolated Immediately ex Vivo Correlates with Disease Severity in Human Immunodeficiency Virus Type 1 Infection. Pediatric Research. 1997;42:656–664. doi: 10.1203/00006450-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Cumont MC, Diop O, Vaslin B, Elbim C, Viollet L, Monceaux V, Lay S, Silvestri G, Le Grand R, Muller-Trutwin M, Hurtrel B, Estaquier J. Early divergence in lymphoid tissue apoptosis between pathogenic and non-pathogenic SIV infections of non-human primates. J Virol. 2007 doi: 10.1128/JVI.00450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dittmer U, Petry H, Stahl-Hennig C, Nisslein T, Spring M, Luke W, Bodemer W, Kaup FJ, Hunsmann G. T cell apoptosis in human immunodeficiency virus type 2- and simian immunodeficiency virus-infected macaques. J Gen Virol. 1996;77(Pt 10):2433–6. doi: 10.1099/0022-1317-77-10-2433. [DOI] [PubMed] [Google Scholar]
- 18.Estaquier J, Idziorek T, de Bels F, Barre-Sinoussi F, Hurtrel B, Aubertin AM, Venet A, Mehtali M, Muchmore E, Michel P, Mouton Y, Girard M, Ameisen JC. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci U S A. 1994;91:9431–5. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estaquier J, Monceaux V, Cumont MC, Aubertin AM, Hurtrel B, Ameisen JC. Early changes in peripheral blood T cells during primary infection of rhesus macaques with a pathogenic SIV. J Med Primatol. 2000;29:127–35. doi: 10.1034/j.1600-0684.2000.290305.x. [DOI] [PubMed] [Google Scholar]
- 20.Estaquier J, Tanaka M, Suda T, Nagata S, Golstein P, Ameisen JC. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood. 1996;87:4959–66. [PubMed] [Google Scholar]
- 21.Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–34. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 22.Gougeon ML, Lecoeur H, Dulioust A, Enouf MG, Crouvoiser M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–20. [PubMed] [Google Scholar]
- 23.Gougeon ML, Lecoeur H, Heeney J, Boudet F. Comparative analysis of apoptosis in HIV-infected humans and chimpanzees: relation with lymphocyte activation. Immunol Lett. 1996;51:75–81. doi: 10.1016/0165-2478(96)02558-8. [DOI] [PubMed] [Google Scholar]
- 24.Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen JC. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–40. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iida T, Ichimura H, Ui M, Shimada T, Akahata W, Igarashi T, Kuwata T, Ido E, Yonehara S, Imanishi J, Hayami M. Sequential analysis of apoptosis induction in peripheral blood mononuclear cells and lymph nodes in the early phase of pathogenic and nonpathogenic SIVmac infection. AIDS Res Hum Retroviruses. 1999;15:721–9. doi: 10.1089/088922299310818. [DOI] [PubMed] [Google Scholar]
- 26.Jaattela M, Tschopp J. Caspase-independent cell death in T lymphocytes. Nat Immunol. 2003;4:416–23. doi: 10.1038/ni0503-416. [DOI] [PubMed] [Google Scholar]
- 27.Katsikis PD, Garcia-Ojeda ME, Wunderlich ES, Smith CA, Yagita H, Okumura K, Kayagaki N, Alderson M, Herzenberg LA, Herzenberg LA. Activation-induced peripheral blood T cell apoptosis is Fas independent in HIV-infected individuals. Int Immunol. 1996;8:1311–7. doi: 10.1093/intimm/8.8.1311. [DOI] [PubMed] [Google Scholar]
- 28.Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA, Herzenberg LA. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–36. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaur A, Grant RM, Means RE, McClure H, Feinberg M, Johnson RP. Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques. J Virol. 1998;72:9597–611. doi: 10.1128/jvi.72.12.9597-9611.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur A, Hale CL, Ramanujan S, Jain RK, Johnson RP. Differential dynamics of CD4(+) and CD8(+) T-lymphocyte proliferation and activation in acute simian immunodeficiency virus infection. J Virol. 2000;74:8413–24. doi: 10.1128/jvi.74.18.8413-8424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190:1891–6. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyaard L, Otto SA, Jonker RR, Mijnster MJ, Keet RP, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–9. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 34.Meyaard L, Otto SA, Keet IP, Roos MT, Miedema F. Programmed death of T cells in human immunodeficiency virus infection. No correlation with progression to disease. J Clin Invest. 1994;93:982–8. doi: 10.1172/JCI117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miossec C, Dutilleul V, Fassy F, Diu-Hercend A. Evidence for CPP32 activation in the absence of apoptosis during T lymphocyte stimulation. J Biol Chem. 1997;272:13459–62. doi: 10.1074/jbc.272.21.13459. [DOI] [PubMed] [Google Scholar]
- 36.Monceaux V, Estaquier J, Fevrier M, Cumont MC, Riviere Y, Aubertin AM, Ameisen JC, Hurtrel B. Extensive apoptosis in lymphoid organs during primary SIV infection predicts rapid progression towards AIDS. Aids. 2003;17:1585–96. doi: 10.1097/00002030-200307250-00002. [DOI] [PubMed] [Google Scholar]
- 37.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–87. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 38.Muro-Cacho CA, Pantaleo G, Fauci AS. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995;154:5555–66. [PubMed] [Google Scholar]
- 39.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 40.Oyaizu N, McCloskey TW, Coronesi M, Chirmule N, Kalyanaraman VS, Pahwa S. Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type-1 infected patients and in CD4 cross-linked PBMCs from normal individuals. Blood. 1993;82:3392–400. [PubMed] [Google Scholar]
- 41.Petrovas C, Mueller YM, Katsikis PD. Apoptosis of HIV-specific CD8+ T cells: an HIV evasion strategy. Cell Death Differ. 2005;12(Suppl 1):859–70. doi: 10.1038/sj.cdd.4401595. [DOI] [PubMed] [Google Scholar]
- 42.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 43.Prati E, Gorla R, Malacarne F, Airo P, Brugnoni D, Gargiulo F, Tebaldi A, Castelli F, Carosi G, Cattaneo R. Study of spontaneous apoptosis in HIV+ patients: correlation with clinical progression and T cell loss. AIDS Res Hum Retroviruses. 1997;13:1501–8. doi: 10.1089/aid.1997.13.1501. [DOI] [PubMed] [Google Scholar]
- 44.Reinberger S, Spring M, Nisslein T, Stahl-Hennig C, Hunsmann G, Dittmer U. Kinetics of lymphocyte apoptosis in macaques infected with different simian immunodeficiency viruses or simian/human immunodeficiency hybrid viruses. Clin Immunol. 1999;90:141–6. doi: 10.1006/clim.1998.4630. [DOI] [PubMed] [Google Scholar]
- 45.Tateyama M, Oyaizu N, McCloskey TW, Than S, Pahwa S. CD4 T lymphocytes are primed to express Fas ligand by CD4 cross-linking and to contribute to CD8 T-cell apoptosis via Fas/FasL death signaling pathway. Blood. 2000;96:195–202. [PubMed] [Google Scholar]
- 46.Viollet L, Monceaux V, Petit F, Ho Tsong Fang R, Cumont MC, Hurtrel B, Estaquier J. Death of CD4+ T cells from lymph nodes during primary SIVmac251 infection predicts the rate of AIDS progression. J Immunol. 2006;177:6685–94. doi: 10.4049/jimmunol.177.10.6685. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Metcalf B, Ribeiro RM, McClure H, Kaur A. Th-1-type cytotoxic CD8+ T-lymphocyte responses to simian immunodeficiency virus (SIV) are a consistent feature of natural SIV infection in sooty mangabeys. J Virol. 2006;80:2771–83. doi: 10.1128/JVI.80.6.2771-2783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe D, Brockman MA, Ndung’u T, Mathews L, Lucas WT, Murphy CG, Felber BK, Pavlakis GN, Deluca NA, Knipe DM. Properties of a herpes simplex virus multiple immediate-early gene-deleted recombinant as a vaccine vector. Virology. 2007;357:186–98. doi: 10.1016/j.virol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Wilhelm S, Wagner H, Hacker G. Activation of caspase-3-like enzymes in non-apoptotic T cells. Eur J Immunol. 1998;28:891–900. doi: 10.1002/(SICI)1521-4141(199803)28:03<891::AID-IMMU891>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 50.Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–52. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 51.Yue FY, Kovacs CM, Dimayuga RC, Gu XX, Parks P, Kaul R, Ostrowski MA. Preferential apoptosis of HIV-1-specific CD4+ T cells. J Immunol. 2005;174:2196–204. doi: 10.4049/jimmunol.174.4.2196. [DOI] [PubMed] [Google Scholar]
- 52.Zaunders JJ, Moutouh-de Parseval L, Kitada S, Reed JC, Rought S, Genini D, Leoni L, Kelleher A, Cooper DA, Smith DE, Grey P, Estaquier J, Little S, Richman DD, Corbeil J. Polyclonal proliferation and apoptosis of CCR5+ T lymphocytes during primary human immunodeficiency virus type 1 infection: regulation by interleukin (IL)-2, IL-15, and Bcl-2. J Infect Dis. 2003;187:1735–47. doi: 10.1086/375030. [DOI] [PubMed] [Google Scholar]