Abstract
An imaging biomarker that would provide for an early quantitative metric of clinical treatment response in cancer patients would provide for a paradigm shift in cancer care. Currently, non-image based clinical outcome metrics include morphology, clinical and laboratory parameters however, these are obtained relatively late following treatment. Diffusion-weighted MRI (DW-MRI) holds promise for use as a cancer treatment response biomarker as it is sensitive to macromolecular and microstructural changes which can occur at the cellular level earlier than anatomical changes during therapy. Studies have shown that successful treatment of a many tumor types can be detected using DW-MRI as an early increase in the apparent diffusion coefficient (ADC) values. Additionally, low pre-treatment ADC values of various tumors are often predictive of better outcome. These capabilities, once validated, could provide for an important opportunity to individualize therapy thereby minimizing unnecessary systemic toxicity associated with ineffective therapies with the additional advantage of improving overall patient health care and associated costs. In this report, we provide a brief technical overview of DW-MRI acquisition protocols, quantitative image analysis approaches and review studies which have implemented DW-MRI for the purpose of early prediction of cancer treatment response.
Keywords: diffusion-weighted MRI, oncology, treatment monitoring, biomarker
Introduction
Patients suffering from a malignant tumor or metastases undergo extensive therapies including various side effects. Up to date treatment response is evaluated by morphological, clinical and laboratory outcome. Radiological tumor response is based on the extent of tumor size reduction as measured by anatomical imaging modalities such as CT or MRI. However, therapy is typically delivered in fractionated doses thus requiring sufficient time for enough treatment to be delivered to kill cells within the tumor as well as additional time needed for immunological removal of the macromolecular debri. Thus therapeutic-induced changes in tumor volumes often occurs relatively late in the time course of treatment.
Current therapeutic strategies in oncology include surgery, radiation therapy, and chemotherapy which include molecularly targeted agents against oncogenic signaling pathways as well as vascular targets. However, timely evaluation of treatment response in patients undergoing radiation therapy or chemotherapy depending on the underlying pathology or treatment strategy is often very difficult. Evaluation of the response to chemotherapy based on changes in the size of solid tumors is typically evaluable after six to eight weeks.
Development of antiangiogenic agents, vascular and molecularly-targeted agents may not lead to significant reduction in tumor size. Antiangiogenic agents inhibit neovascularisation of tumors, vascular targeting agents selectively destroy pathological vessels without interfering with normal vessels. Molecularly targeted agents for example, are directed at oncogenic signaling molecules which can for example lead to a variety of biological effects including inhibition of growth or apoptotic-induced cell death. In 2000 RECIST (response evaluation criteria in solid tumors) defined the response to treatment in terms of alteration of tumor size only (1). However as newer agents might provide therapeutic benefit by other mechanisms than size reduction new evaluation criteria are of utmost importance. Recently these RECIST criteria were reconsidered and are considering imaging biomarkers such as PET (2). In these articles it has already been considered that the up to date anatomic unidimensional assessment of tumor burden has to be changed to volumetric anatomical assessment as well as functional assessment including PET and MRI.
Clinical assessment of new treatments in oncology is evaluated by numerous response criteria including tissue and plasma biomarker readouts, improvement in quality of life, and survival among others. As the process to quantify response of agents undergoing clinical trials may be lengthy there is a significant need for timelier outcome measures to be identified and validated. The ability to quantify the effectiveness of experimental therapies would significantly impact the overall drug development process and offers the potential of using DW-MRI to provide for early evaluation and go, no go decisions in phase 1 clinical trials thus saving money and time. Thus early surrogate indicators which correlate with long term outcome metrics are urgently needed ((3)). Clinical trial measures that could provide early readouts of drug-target interactions and subsequent efficacy would facilitate quantification of outcomes in clinical trials and ultimately provide assistance in the regulatory approval process.
The current functional methods for response assessment as suggested by RECIST 1.1 includes PET, PET-CT, magnetic resonance spectroscopy as well as dynamic contrast enhanced MRI. PET and PET-CT are quite expensive methods and the differentiation between residual tumor and inflammation is often quite difficult using traces such as [F18]fluorodeoxyglucose. Furthermore, radiation exposure is a drawback mainly in younger patients undergoing several follow-up studies for response assessment. Magnetic resonance spectroscopy is a very promising method however, its limitations include low spatial resolution, restricted availability and lack of expertise of many radiologists. Dynamic contrast enhanced MRI provides information on changes in vascularization of the tumor during treatment however, image analysis is relatively complicated and therefore not suited for daily clinical routine. Furthermore, the risk of nephrogenic systemic fibrosis – although being very small - has to be taken into account in patients with renal insufficiency.
Diffusion-weighted MRI (DW-MRI) has not been considered in RECIST 1.1 however this noninvasive MR technique has been discussed as cancer biomarker in a consensus meeting at the ISMRM 2008 and a publication on consensus and recommendations for DW-MRI as a cancer biomarker has been published recently highlighting the potential of this promising technique in cancer patients (4).
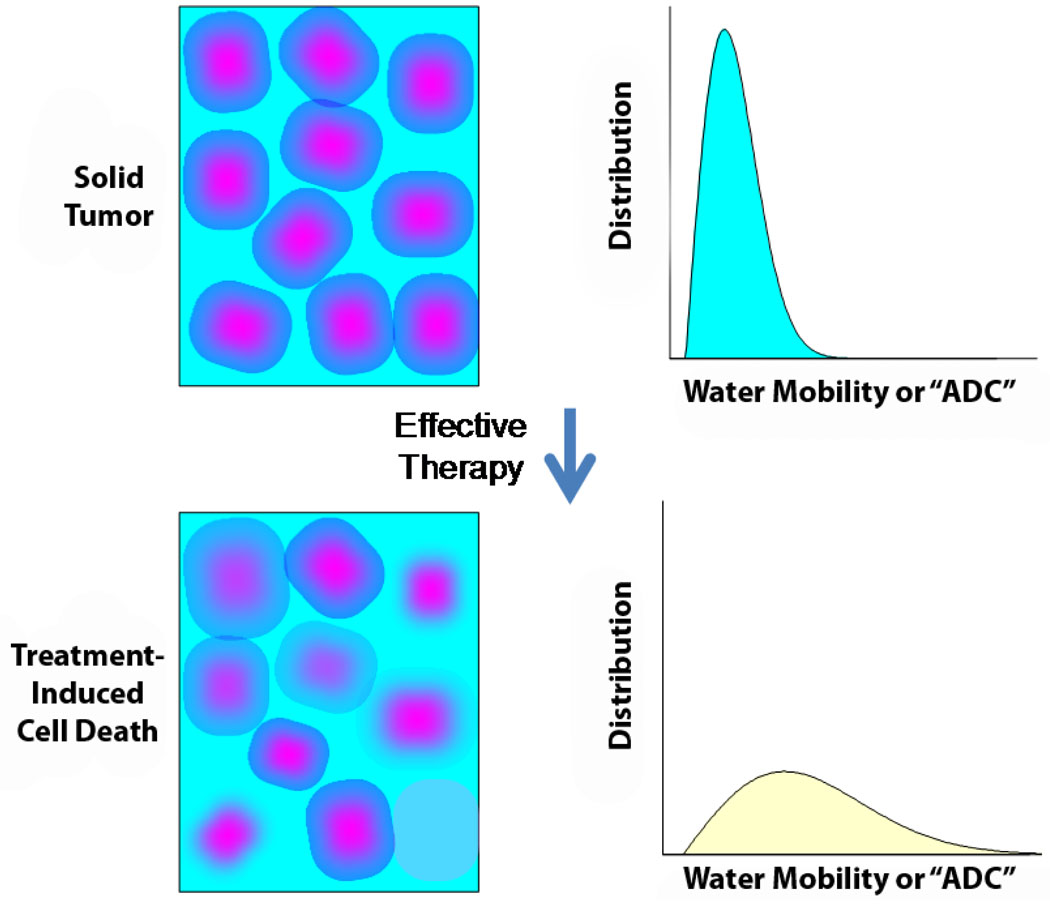
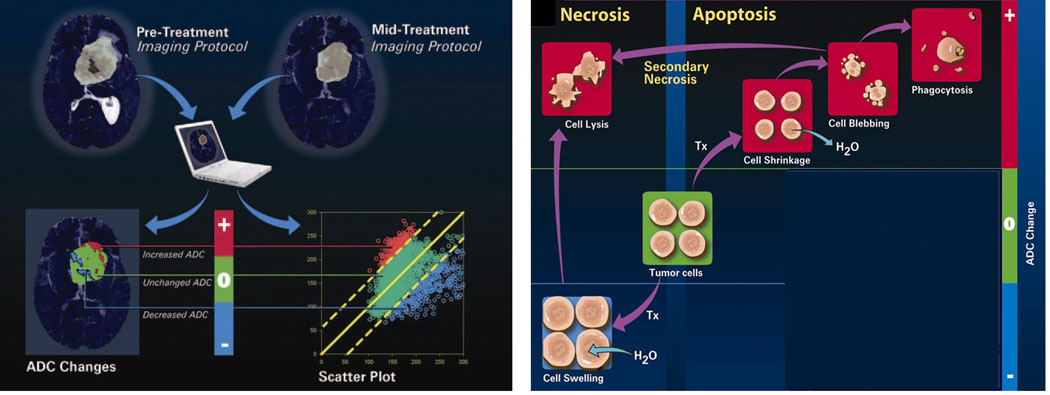
DW-MRI for the evaluation of early treatment response is very promising. As diffusion within tumors is impeded by the presence of cellular membranes and macromolecular structures, treatment with for example radiation and/or chemotherapy can result in the loss of cell membrane integrity which can be detected as an increase in mean diffusion value for the tumor as shown in Figure 1. Thus DW-MRI can provide microstructural information on the cellular level. Animal and clinical studies to date have revealed that successful treatments of a wide variety of tumor types can be detected as an increase in tumor ADC values due to the loss of cellular density.
Figure 1.
Schematic representation of the relationship between change in cellular density following an effective therapy and the corresponding distribution of water diffusion values within the tumor. Note that the mean diffusion value of a tumor increases early following the loss of cellular density.
Technical Requirements
Several technical requirements to perform follow-up of cancer patients using DW-MRI have to be considered. Standardized techniques including patient preparation for follow-up scans are important e.g. when evaluating the pelvis the filling of the urinary bladder has to be taken into consideration. Parallel imaging techniques offer several advantages for DWI, particularly at high magnetic fields where magnetic field inhomogeneity leads to large spatial distortion when using single-shot EPI. Parallel imaging techniques utilize previously acquired receiver coil sensitivity patterns to reduce the quantity of phase-encode lines required to reconstruct an image (5–7). This allows a shorter single-shot echo train interval thereby reducing inhomogeneity-induced phase accumulation manifest as geometric distortion. There is a SNR penalty associated with collecting fewer phase-encode lines but can be offset by the SNR gain achieved by the reduced minimum echo time afforded by parallel imaging. Further on the negative side, parallel imaging may introduce artefacts characterized by non-uniform noise and wrap-like artefacts that occur at spurious locations within the field-of-view. However usually the benefits of parallel imaging methods outweigh the drawbacks when applied to single-shot EPI-based DWI of the body, therefore incorporation and trial of parallel imaging in these protocols is encouraged, especially on 3T systems.
There are several options to reduce respiratory motion artefact when performing DWI of the chest and abdomen. When performing DW-MRI with free breathing multiple averaging is necessary to increase SNR and reduce respiratory effects, although this can lead to partial volume averaging and blur of internal structures (8). Breath-hold images are another option, however, in cancer patients free breathing is often easier to perform in severely ill individuals. Even if the patient is able to hold their breath, signal averaging within the breathold interval is severely limited leading to low SNR. At the expense of increased scan time, respiratory triggering or cardiac gating may be used when performing DW-MRI in the chest or upper abdomen. Although in a recent analysis of motion control techniques it was noted respiratory-triggering yielded significantly higher ADCs and lower ADC reproducibility than breath hold or non-breath hold scans (9,10). Anti-peristaltic agents can be administered mainly when examining the lower abdomen and the pelvis to avoid or minimize bowel motion artefacts.
DW-MRI is usually performed in the three orthogonal diffusion directions to minimize the influence of anisotropy although the degree of diffusion anisotropy in non-neural tissues and tumor is relatively low. By acquisition of DWI in three orthogonal directions, a rotationally-invariant diffusion coefficient can be calculated – that is the diffusion measurement is independent of the relative orientation of the tissue architecture and gradient axes. Optimization of fat suppression should also be considered. Presence of fat signal in single-shot EPI DWI is particularly problematic since the apparent location of fat structures are displaced many pixels along the phase-encode direction thus may superimpose water-based signals. This not only obscures the tissues of interest, but also greatly distorts ADC measurement since lipid protons have a very low diffusion coefficient. Standard fat suppression techniques are applicable to DWI and include: frequency-selection saturation of fat signal; use of adiabatic pulses to reduce sensitivity to B1 inhomogeneity in frequency-selection saturation/inversion of fat; and non-frequency selective Short TI Inversion Recovery (STIR) methods which are less affected by poor shim quality (11–13).
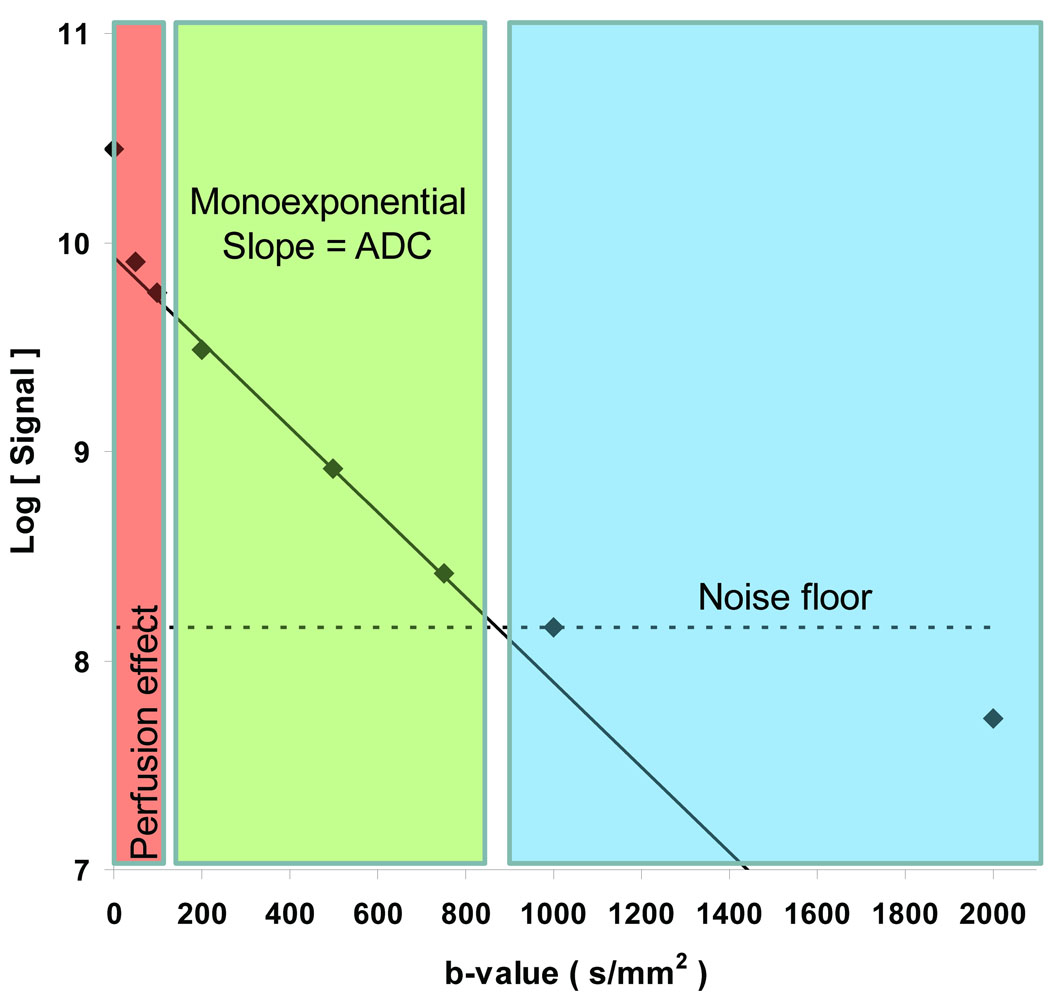
Diffusion weighted images at a minimum of two sensitivity levels defined by the sequence b-value must be acquired to quantify ADC. Proper selection of b-values depend on the given body DWI application and objective. The majority of clinical neuro imaging DWI studies have been performed at b = 0 and 1000s/mm2 and has grown acceptance as the default b-value set for brain DWI. However microcirculation through randomly-oriented capillaries in the presence of diffusion-sensitization gradients will also appear as a hyper diffusion-like attenuation particularly in the low b-value regime (i.e. b=0 to 100 s/mm2). Certainly perfusion must be considered fundamentally distinct from thermally-driven diffusion, but perfusion effects can be observed in DWI particularly in vascular-rich tissues/lesions thus should be considered in design of DWI protocols. Therefore if one seeks to disentangle perfusion effects from molecular diffusion, additional b-values are required in the low b-value range (0–100s/mm2), which are sensitive to perfusion effects, for contrast against nominal high b-value decay which is dominated by diffusion. The y-intercept extrapolated from fitting signal decay vs b-value using intermediate to high b-values compared to the b=0 s/mm2 signal can be used as an estimate of the perfusion signal component. Therefore collection of the b=0 DWI (ie T2-weighted image) is valuable to elucidate perfusion and diffusion influences. Figure 2 illustrates this perfusion effect in human kidney. Diffusion estimates are derived from the exponential decay of DWI signal vs b-value. If a simple two-point estimate of signal decay is used (as often is the case), then
| [1] |
where b1 and b2 represent the low and high b-values. By estimating ADC via the slope of log(signal) vs b-value using b-values > 100s/mm2, most of the perfusion signal is attenuated leading to an ADC estimate relatively free of perfusion contamination. At high b-values, however, the signal may falsely exhibit a multi-exponential shape due to the signal falling beyond the noise floor (see Figure 2 for b=1000 to 2000 s/mm2). The maximum b-value should be set such that signals recorded at that b-value are adequately above the noise floor. This maximum b-value depends on the SNR achievable for the target organ/tissue and the water diffusion coefficient of these tissues – the lower the diffusion value, the higher the b-value achieved before the signal approaches the noise floor. Tissues do exhibit true multi-exponential diffusion decays over a wide b-value range (b=500 to > 3000s/mm2), although very high SNR is required to document these features (14).
Figure 2.
Measured signal decay in kidney cortex of as a function of b-value. Perfusion effect is dominant in the low b-value range (0–100s/mm2) and would inflate ADC if included in slope calculations. By extinguishing most of the vascular signal at b values greater than 100s/mm2, a true estimate of ADC is derived. Using b-values 100 to 750s/mm2, the estimated kidney ADC = 2.03×10−3mm2/s. The dashed line represents the noise floor. DWI measurements where signal falls within the noise floor lead to erroneous ADC estimates and a false multi-exponential appearance to the log(signal) versus b-value curve. Tissues do exhibit true multi-exponential diffusion decays over a wide b-value range (b=500 to > 3000s/mm2), although very high SNR is required to document these features.
Image Analysis
To evaluate diffusion-weighted MRI there are two general categories for image analysis: qualitative and quantitative. Moreover, the “image” may represent several quantities derived from diffusion-weighted images. For example, ADC maps may be calculated over a specified b-value range, the image may represent an anisotropy index derived from a series of multiple directions of diffusion-weighted images or the image may represent an index that quantifies the multiexponential features of the diffusion decay over a wide range of b-values.
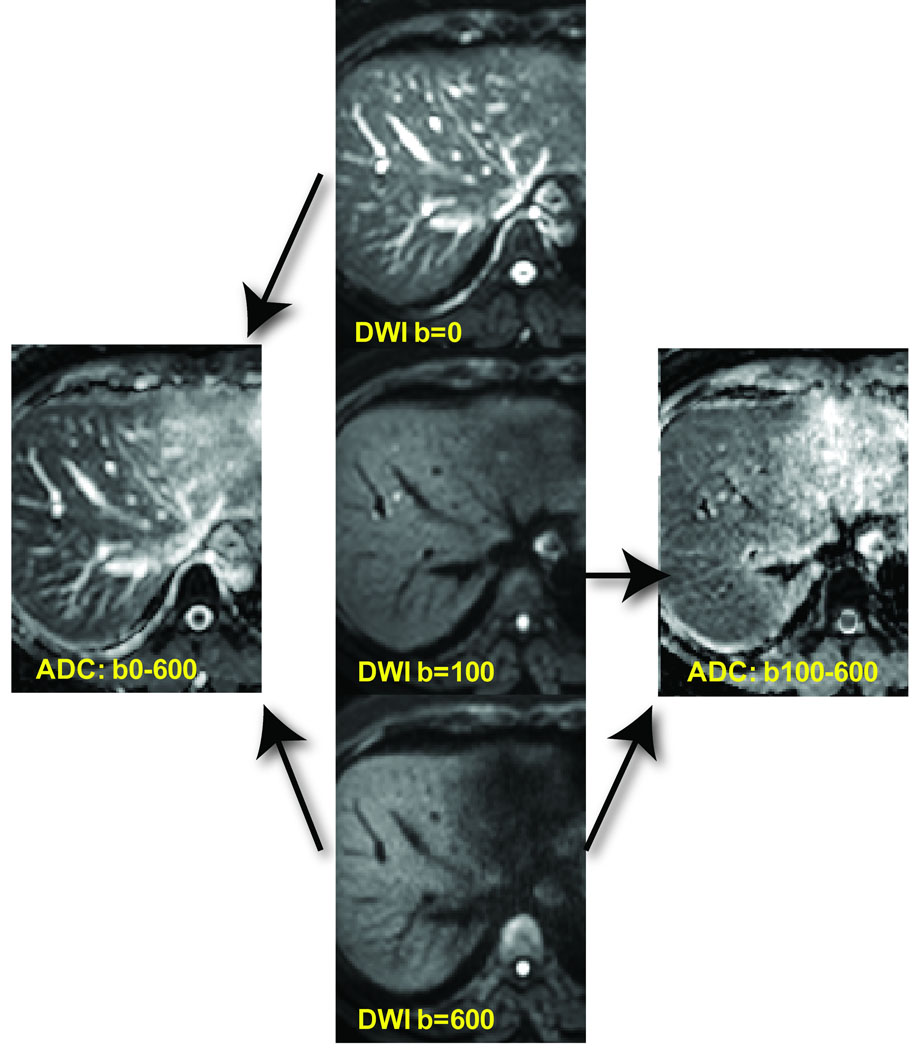
Shown in Figure 3 are a series of liver images acquired with varying b-values of 0, 100 and 600 s/mm2. Note the heavily T2-weighted contrast at b-value of 0 s/mm2 which includes bright vascular signal. The vascular signal is greatly attenuated at moderate diffusion weighting of 100 and 600 s/mm2. Calculated ADC maps derived from low using the low b-value data (0 and 600 s/mm2) yield ADC maps highly influenced by flowing blood. Use of moderate to high b-values (b= 100 to 600 s/mm2) provide a more accurate measure of water diffusion free of vascular contamination (Figure 3).
Figure 3.
Effects of b-values on blood flow contribution to images and ADC maps. DW-MRI of the liver with b-values of 0, 100 and 600 s/mm2 (center column) revealing suppression of vascular signal with increasing b-value. ADC maps generated using b-value images of 0 and 600 s/mm2 as well as 100 and 600 s/mm2. ADC maps generated using b-values of 100 and 600 s/mm2 have attenuated contribution/contamination from perfusion effects (right ADC map) compared with the ADC map generated using b-values of 0 and 600 s/mm2.
In general, water diffusion in tissue may be anisotropic; that is directionally dependent on the natural underlying directionality of cellular elements that impede water mobility. Neural tissue in particular is highly anisotropic where the diffusion coefficient may vary several-fold dependent on the relative orientation of the diffusion encoding gradient and the tissue structure. Most non-neural tissue exhibit nearly isotropic water diffusion; although in general one should not assume tissue is isotropic when performing diffusion measurements. Diffusion encoding should be performed along at least 3 orthogonal gradient directions to properly account for possible anisotropy.
Qualitative Assessment
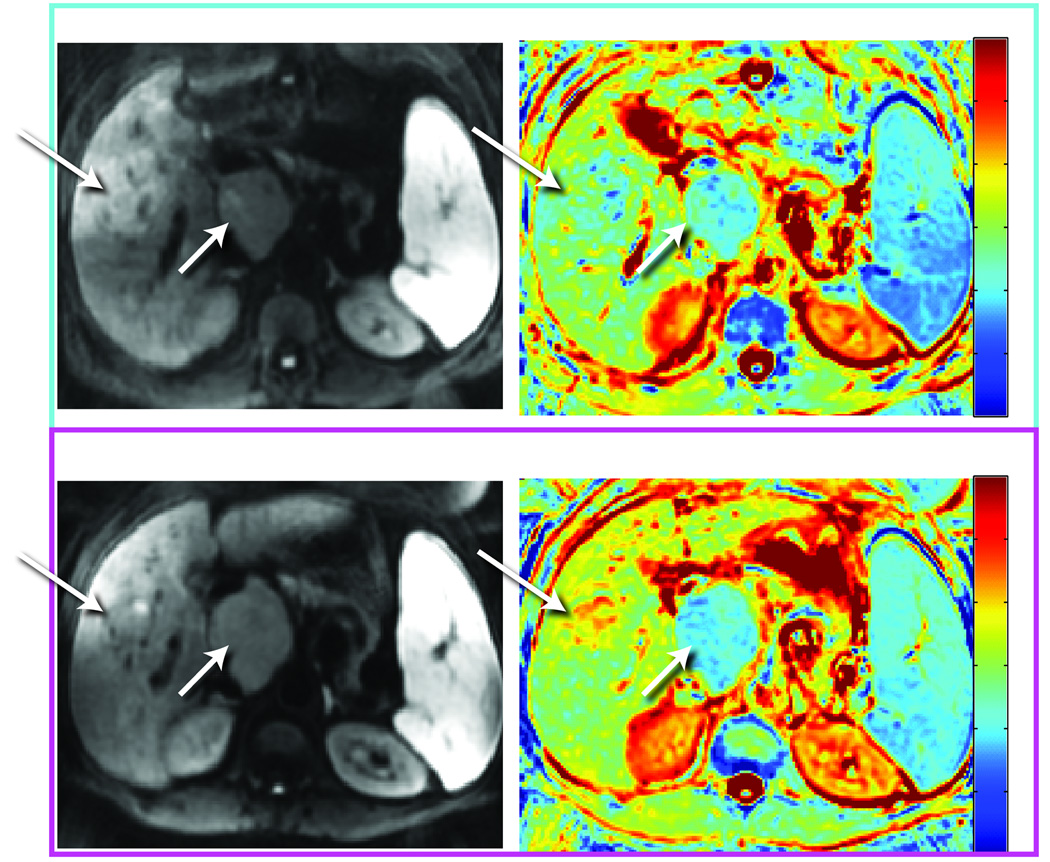
The signal intensity change during treatment can be assessed by visual analysis of the underlying pathology as shown in Figure 3. In this example of a patient with two distinct hepatocellular carcinomas treated with transarterial chemoembolization (TACE), the solid tumors before treatment a high b-value image shows high signal intensity while the corresponding ADC maps exhibit low signal intensity. During successful treatment the loss of cellularity in the upper tumor resulted in the high b-value image to shift towards lower signal intensity or high signal intensity on the corresponding ADC map. While these are early changes, formation of fibrosis in the extracellular matrix can also occur later on during therapy and in some types of tumors if this occurs, it would result in low signal intensity on the high b-value image and on the corresponding ADC map. Post-therapeutic changes such as edema during radiation therapy demonstrate high signal intensity on the high b-value image as well as on the corresponding ADC map. An additional issue arising when using b-images as the singular image to base disease or treatment response assessment is the “T2 shine-through” effect. This effect can result in a tissue region appearing to exhibit higher signal intensity on b-images in the range of 500–1000sec/m2 due to complicating factors such as the long intrinsic T2-relaxation time of the tissue. Avoidance of miss-interpretation of images due to artifactual T2-shine through effects can be accomplished by comparison with the corresponding ADC map.
Quantitative Assessment
In general, advancement of a biomarker must be demonstrated in multicenter trials with standardized acquisition techniques and with appropriate quality control (QC) standards to provide quantitative agreement across systems and assess overall repeatability. These quality issues require investigators to agree upon an appropriate QC phantom. A specific phantom for this purpose has not been decided upon by the broader community although phantoms for DW-MR have been proposed (15,16). Recent consensus has been reached as to a variety of issues by a consortium of investigators brought together by the National Institutes of Health to form overall recommendations (4).
Quantification of diffusion properties as a potential early surrogate response metric of clinical efficacy is commonly accomplished. There are two primary approaches to perform quantitative analysis of DW-MRI derived maps:
Change in mean ADC value over the entire tumor or select spatial zones of the tumor.
A voxel-by-voxel change in ADC maps or other diffusion parametric maps.
In oncologic imaging applications these two areas are particularly noteworthy to assess treatment response. The two distinct approaches involved in quantitative analyses of tumor ADC changes mentioned above are highlighted in the discussion below.
Change in ADC Following Therapy
This is a simple method that utilizes the whole tumor average ADC value where an increase in ADC following therapy due to a decrease in cellularity suggests a positive therapeutic response (Figure 1) (17,18). This approach has been widely used in animal tumor studies where tumor treatments are very effective and tumor cell lines tend to be more responsive to interventions and in a shorter time span than is observed clinically (17,19–30). Moreover, extracting ADC values from ADC maps by drawing ROI’s around the margin of the tumor is relatively simple to perform. The resultant distribution of ADC values are typically plotted in a histogram format and the mean or median value is used to evaluate changes following treatment over time. However, histogram-based detection of treatment-induced diffusion changes can be attenuated if spatially varying diffusion values occur wherein both an increase and a decrease occur within the tumor. In this situation, competing factors would reduce the mean shift in ADC using a histogram-based approach. Furthermore, if large cystic or necrotic regions are present within the tumor mass, these would also have the potential to reduce detectable changes in tumor ADC values following treatment as they would “weight” the number of unchanged values according to their proportion of the total voxels thus reducing the overall net change in ADC value. Therefore, the histogram-based approach for detection of treatment response can be attenuated by pre- and post-treatment spatial heterogeneity within the tumor.
Parametric Response Map Analysis
In an effort to address the effects of spatial heterogeneity within the tumor mass, a voxel-by-voxel approach was proposed (31). This voxel-based approach spatially registered the pre-treatment ADC map to a post-treatment initiation ADC map to provide for quantification of diffusion changes on the voxel level. Using this approach, changes in individual voxels can be tested for significance and superimposed on anatomic maps to reveal the spatial information within the context of the 3-dimensional view of the tumor. ADC changes are encoded as color maps and overlaid on the anatomical MRI to obtain spatial information. This approach provides for the possibility of also guiding the therapist in adapting therapy early as well as assisting in the ability to spatially-guide a directed therapy approach. The voxel-based analytical method was termed the functional diffusion map (fDM) and more recently the parametric response map (PRM) as it allows for quantification of the relative area or percent of tumor volume in which significant change had occurred as the response metric (either a decrease or increase in ADC values) (Figure 4). Shown in Figure 4, changes in tumor cellular structures can occur due to an apoptotic cell death process which would result in the decrease in tumor cellularity thus causing an increase in tumor ADC values in those regions. Swelling of tumor cells would produce a drop in ADC values transiently which upon conversion to necrosis, would produce an elevated ADC value. Image registration of pre-treatment ADC maps with a follow up ADC map acquired following treatment initiation allows for presentation of the PRMADC data both in terms of a three color overlay where red indicates increased ADC values, blue decreased ADC values and green unchanged ADC values as shown in Figure 4. This data can also be shown as a scatter plot and quantification of changes presented as a percent of tumor voxels (i.e. volume) with increased ADC values (Figure 4). This technique is readily applied in tumor sites where tumor growth is modest and tumors can be spatially registered with well established algorithms such as the affine transform. Extension of PRM to monitoring of tumors located at other sites where tumor growth and/or surrounding tissues are more elastic can be implemented using nonlinear warping algorithms for image registration (32).
Figure 4.
A patient with a hepatocellular carcinoma in the liver undergoing treatment with transcatheter arterial chemoembolization (TACE) (top row) pre and (bottom row) post-therapy. Each set of images consists of the DW-MRI (left images) along with their corresponding color ADC overlay maps (right column).
Applications of PRMADC tumor treatment response monitoring have been reported in both animal and clinical studies. Correlation of increased PRM values following treatment in a brain tumor glioma model revealed that measurement of early changes at 4 days post-treatment of the PRMADC metric correlated with overall survival in a dose-response chemotherapy study (33). This animal study validated the PRM imaging biomarker as an early response predictor for animal studies. Several clinical studies have also been reported in grade III/IV glioma patients (31,34,35) and in head and neck tumor patients (32). Overall, these clinical studies have reported correlation of early PRMADC changes with late, traditional clinical outcome measures revealing the potential utility of this voxel-based approach as an imaging oncologic response biomarker. Finally, extension of the voxel-by-voxel analysis approach has been reported using MRI perfusion maps from brain tumor patients undergoing therapy wherein changes as early as one week into therapy could be used to predict patient outcome (36). This study reveals that the voxel-by-voxel approach may provide improved sensitivity for treatment response assessment for a broad range of imaging modalities which includes DW-MRI.
Multiexponential Diffusion/Perfusion Properties of Treated Tumors
The signal loss in DW-MRI with increasing b-value does not necessarily follow a monoexponential decay (37). The perfusion component of tissue appears as a “hyper diffusion-like” decay with b-value over the low b-value regimen (0–100 sec/mm2). At higher b-values (>100sec/mm2) the perfusion signal is largely extinguished thus ADC measurements are more heavily influenced by the water diffusion properties within the cellular matrix. The apparent diffusion coefficient provides us information on diffusion and perfusion provided that these two entities can be separated.
In an experimental study in rats DW-MRI was performed for monitoring the effect of a vascular targeting agent on rabdomyosarcomas (38). As vascular targeting agents selectively destroy pathological vessels the aim of the study was to perform DW-MRI to detect changes in diffusion and perfusion noninvasively and without contrast medium administration. For that reason three different ADC values were calculated out of a large series of b-values: to approximate true diffusion an ADC was calculated only out of high b-values (500, 750, 1000sec/mm2 = ADChigh). An ADC including b-values from 0 – 100sec/mm2 (perfusion and diffusion) was considered as ADClow whereas the difference between ADClow and ADChigh was hypothesized to approximate perfusion. In addition, when ADC is used, it is assumed to mean from values generated using b-values between 100–1,000 sec/m2.
In this study performed on rhabdomyosarcomas in rats imaging was performed before administration of this vascular targeting agent, one hour, six hours, two days and nine days after start of therapy, where morphological images, including images before and after contrast medium administration as well as diffusion-weighted images were performed during treatment and histopathological correlation at each time point were presented (38).
One hour after vascular targeting injection leading to disruption of the pathological vessels, no perfusion of the solid tumor could be detected. However, the corresponding ADC map still showed vital tumor cells reflected as a low signal intensity of the solid tumor confirmed by histology. Similar findings were observed six hours after injection of the vascular targeting agents. The reason for the lack of enhancement as seen on conventional post-contrast T1-weighted fat saturated images was determined to be vasoconstriction and vasocongestion which was identified on histological tumor sections. Only two days after therapy the morphological images showing necrosis corresponded to tumor death on the ADC map which was also confirmed histologically. Nine days after treatment the tumor started regrowing from the periphery as seen on morphological images, the same results were also seen on ADC maps and corresponding histological specimens.
When measuring the ADC values early after treatment (one and six hours) a decrease in ADChigh was seen and attributed to cell swelling and consequent impeded diffusion in the extravascular space. The decrease in perfusion was due to vascular shutdown. During true necrosis at two days the ADChigh increased, whereas ADChigh during relapse decreased again due to increase in cellularity of the tumor. In another study the difference between ADClow and ADChigh as suggested corresponding to perfusion was correlated with the volume transfer constant k calculated from dynamic contrast enhanced images during follow-up of treatment. A correlation between these two parameters calculated from these two different techniques was demonstrated suggesting that dynamic contrast enhanced MRI provided information on perfusion changes during treatment whereas DW-MRI was able to provide information on perfusion changes and also allowed for differentiation between viable and necrotic regions within the tumor mass (39).
As it has already been suggested by Le Bihan in Radiology 1988 separation of diffusion and perfusion in intravoxel incoherent motion MR imaging should be performed and this was reconsidered in a recent paper published in Radiology from the same author (40). As many treatment strategies lead to changes on different levels of the tumor the differentiation between diffusion and perfusion calculated from the diffusion-weighted images might help to provide information on additional and more subtle changes that might occur during treatment.
DW-MRI as Biomarker
DW-MRI as biomarker has several advantages because it provides microstructural changes related to treatment effects over time that usually precede change in size. No ionizing radiation and no injection of isotope or any other contrast medium is necessary. Furthermore, the acquisition time to perform diffusion-weighted MRI lasts only a few minutes, the method is easily repeatable providing quantitative information and information on spatial distribution including heterogeneity of the tumor and its response. In addition this technique is magnetic field independent and therefore multicenter and longitudinal studies can be performed easily.
Early detection of treatment response might change therapeutic strategies in case of non-responders in order to avoid toxicity and other negative side effects; it might allow individualizing treatment and hopefully increase long term survival. In addition money for ineffective treatment can be saved. DW-MRI in oncology has several key applications including diagnosis, tumor staging, and early evaluation of treatment response and detection of relapse.
Diffusion-weighted MRI for monitoring therapy has already been applied in a wide variety of cancer types and organ sites, including the liver, breast, bone, soft tissue tumors, cervical tumors, head and neck tumors, as well as rectal cancer (41–46).
Clinical Applications of DW-MRI for Monitoring Treatment Response in Different Organ Sites Liver Metastases
Hepatic metastases are the most common neoplasms in the liver. Only selected lesions can be surgically resected therefore the vast majority of tumors are treated by chemotherapy. Size reduction as ultimate response to successful treatment often occurs relatively late hence early detection of response based on DW-MRI findings would be helpful in order to avoid unnecessary treatment in case of therapy failure. Several articles already showed that systemic chemotherapy of liver metastases had a good correlation of response with increased ADC values following treatment (28,47,48).
In a study that has been performed in 2004 in patients with liver metastases from breast cancer a correlation between ADC changes and tumor reduction was found at four and eleven days after start of therapy. The authors concluded that DW- MRI could be used to predict the response of liver metastases to effective chemotherapy (28). Similar results were reported in a study of twenty patients with potential operable hepatic lesions larger than 1 cm in diameter metastatic from colorectal carcinoma. In this study quantitative ADC maps were calculated separately with b-values from 0 to 500 and with b-values from 150 to 500sec/mm2. The non-responding lesions had a significantly higher pretreatment mean ADC (ADC 0 – 500 and mean ADC 150 – 500) than did responding lesions. After chemotherapy, responding lesions had a significant increase in ADC, whereas no significant change was observed in non-responding metastatic lesions after chemotherapy (47). The authors conclude that high pretreatment mean ADC values of liver metastases from colorectal cancer were predictive of poor response to chemotherapy. Responding lesions showed a significant increase in mean ADC in contrast to non-responding lesions indicating the potential of this method for the development of individualized therapy. These results were confirmed by a recent study performing diffusion-weighted MRI as potential imaging biomarker for prediction and early detection of response to chemotherapy in a total of 87 liver metastases (from colorectal and gastric carcinomas). The pretreatment mean ADC in responding lesions were significantly lower than those of non-responding lesions (p = 0,003). An early increase in ADC (on day three and seven) was observed in responding lesions in contrast to non-responding lesions (p = 0,002), preceding the effects of change in size that was only possible at a later time point. The authors concluded that ADC seems to be a promising tool for helping to predict and monitor the early response to chemotherapy of hepatic metastases from colorectal and gastric carcinomas (48).
In 12 patients with 48 metastatic liver lesions from colorectal cancer DW-MRI was applied for the evaluation of therapeutic response to hepatic arterial infusion chemotherapy (HAIC) with 5-fluorouracil (49). Imaging was performed before and 9 days after HAIC. Positive correlations were observed for relative change between %min ADC and reduction ratio (r=0.709) and between %mean ADC and reduction ratio (r=0.536). Both parameters (%min ADC and %mean ADC) were significantly greater in the responder group than in the nonresponder group as evaluated on follow-up CT after 3 months. These results show the usefulness of DW-MRI for early detection of response of liver metastases undergoing treatment with HAIC.
Malignant Liver Lesions
In recent years interventional techniques such as transcatheter arterial chemoembolization (TACE) and hepatic radiofrequency (RF) ablation gained importance in the curative or palliative treatment of hepatocellular carcinoma (HCC) or of liver metastases. The early assessment of successful treatment or relapse is important in patient management however the differentiation between residual or recurrent tumor from nontumoral tissue changes following thermotherapy or chemoembolization is a challenging issue when applying conventional techniques including CT or MRI.
Navigator respiratory-triggered DW-MRI in the follow-up of hepatic RF ablation in 54 patients with 77 liver lesions (17 primary tumors, 60 metastases due to different underlying tumors in 37 patients) showed promising results in the detection of local tumor progression compared to nontumoral post-treatment tissue changes (50). Hyperintensities in the periphery of the ablation zone of the tumor on DW-MR images on 58 of 148 examinations corresponded to local tumor progression confirmed by follow-up in only 17 of these lesions whereas the remaining signal alterations disappeared during follow-up. However, when performing quanitative analysis of these suspicious hyperintense areas on the corresponding ADC map the ADC values were significantly lower in case of local tumor progression than in cases without tumor progression (102.1 ± 22.4 vs. 130.8 ± 47.6 × 10−5 mm2/sec; P=0.00124) suggesting the potential of DW-MRI in this particular setting.
In 24 patients with unresectable HCC undergoing TACE contrast-enhanced MRI and DW-MRI were performed before, 24 hours and 1, 2, 3 and 4 weeks after therapy (51). Mean tumor size was unchanged up to 4 weeks after TACE whereas reduction in tumor enhancement in the arterial and portal venous phase occurred immediately after therapy with a consistent reduction in the first three weeks. The increase in tumor ADC value was significant 1–2 weeks after therapy (P=0.004), borderline significant after 3 weeks and insignificant after 24 hours and 4 weeks after therapy. In another study in 23 patients with 26 HCCs treated with TACE DW-MRI improved the sensitivity of DCE-MRI (85% to 92%) in the detection of peri-lesional tumor recurrence, however its specificity decreased from 65% to 50% after adding DW-MRI (52). In this study b-values of 50, 400 and 800 sec/mm2 were applied. As biexponential fitting provides more detailed information on perfusion and diffusion changes separately this technique might be helpful in this particular setting were perfusion changes are expected to appear however this can only be done on the expense of longer imaging times.
Overall the utility of DW-MRI for monitoring treatment response in HCC and metastases is very promising and may provide the possibility of individualizing treatments for this population of patients.
Pancreatic Cancer
As pancreatic cancer is often diagnosed at an advanced stage a curative surgical approach is often to late and chemotherapy or chemoradiotherapy are the only treatment options. The stratification of responders and nonresponders at an early stage after initiation of treatment might allow to change or stop therapy avoiding the high risk of complications and side effects and ultimately save unnecessary expenses for useless treatments.
In a recent study a consecutive group of 63 patients with advanced pancreatic cancer who were treated with chemotherapy underwent DW-MRI before therapy and follow-up was performed by CT (53). The patients were classified into two groups according to the findings during follow-up: a) those with progressive disease and b) those with stable disease at 3 and 6 months after initial treatment. DW-MRI was applied using 3 b-values (0, 400 and 1000sec/mm2) and a middle (including a b-value of 400 sec/mm2) and high (including a b-value of 1000 sec/mm2) ADC were calculated in the solid parts of the tumor. The results of this study showed that the rate of tumor progression was significantly higher in those with a lower high b-value ADC than in those with a higher b-value ADC. The authors concluded that a lower high b-value ADC in patients with advanced pancreatic carcinoma may be a predictive of early progression in chemotherapy-treated patients. Interestingly, these findings are contradictory to liver metastases where a low pretreatment ADC value was correlated with better outcome after treatment (47,48), however, similar to findings reported in rectal cancer (43,54). In pancreatic cancers a low ADC corresponding to early progression might be attributed to a high cellularity with consequent higher aggressiveness of the tumor but due to the worse response might probably be related to desmoplastic reaction in the tumor before treatment.
Breast Cancer
Neoadjuvant chemotherapy in patients with breast cancer is a relatively controversial issue however this treatment is helpful to reduce size of the tumor before surgery with a consequent improved rate for breast conserving surgery. DW-MRI is ideal for monitoring treatment response, because the results can be correlated with histology. In responding primary breast cancers increased ADC values have been reported. Changes in ADC after the first cycle of chemotherapy significantly correlated with volume and diameter (42,55). The pretreatment ADC values in a study including a control group, the contralateral normal breast as well as benign lesions and malignant lesions before therapy, the lowest ADC values in the malignant breast lesions were reported compared to all the other groups. A change in ADC after the first cycle was statistically significant compared with volume and diameter at the end of treatment indicating the potential in assessing early response. It has been concluded, that the results of this study show that the ADC is more useful for predicting early tumor response to new adjuvant chemotherapy than morphological variables suggesting its potential in effective treatment management (42).
In a breast cancer human xenograft model treated with chemotherapy, the mean change in tumor ADC values was found to be significantly increased by 44% at day 4 post-therapy (56). The increase in ADC values were correlated with both activation of apoptosis and presentation of cell death within the tumor mass in this study. More recently in a study performed in breast tumor xenografts applied DW-MRI (9.4T, b-values of 5 and 1000sec/mm2) to apoptosis-inducing anti-DR5 antibody. In this experimental study performed on nude mice bearing luciferase-positive breast tumors the effect to increasing doses of treatment were evaluated at different time points. The mean ADC increase was linearly proportional to the mean apoptotic cell density whereas the tumor volume changes were not different at day three after treatment in contrast to the ADC increase. The analysis of the peripheral shell of 1mm from the outer surface eliminated the ADC increase resulting from central tumor necrosis, improving the specificity of ADC quantification. In this study DW-MRI as early imaging biomarker for effective apoptosis-induction therapy has been shown in a preclinical breast cancer model (57). Therapeutic efficacy of the apoptosis-inducing strategy was detected as early as three days after increased dosing by using ADC quantification at the time when tumor volume changes were not apparent. An improved method of using the ADC data was demonstrated by using analysis of the peripheral shell of the tumor instead of analysis of the entire tumor region. ADC increases in tumor were dose dependant and were persistent with histological markers of apoptosis. The mean ADC increase in tumors was linearly proportional to the mean apoptotic cell density and was inversely proportional to the mean proliferating cell density. Optimizing the time point of imaging after start of treatment is crucial to maximize the accuracy of measuring therapeutic response. (57). Based on these data there is potential in the future to optimize patient therapy on the basis of DW-MRI to monitor effective response at an early interval after initiation of therapy and to prevent unnecessary treatment and establishing individualized therapeutic strategies. This study was performed on a 9.5 T MR unit using b-values of 5 and 1000.
Another recently published animal study performed in mice with BT474 breast tumor xenografts analyzed different MRI methods for evaluating the effects of different treatment regimens with tyrosine kinase inhibitor gefitinib (58). Tumor volume, ADC, transendothelial permeability (Kps), and fractional plasma volume (fPV) were measured in three groups receiving a) control animals for 10 days, or gefitinib as b) a single daily dose for 10 days or c) a 2-day pulsed dose. A significant tumor growth delay (pulsed: 439 ± 93; daily: 404 ± 53; controls: 891 ± 174 mm3, P<0.050) and lower cell density (P<0.050) were observed 9 days after treatment with gefitinib. Tumor ADC increased in treated groups but decreased in controls (P>0.050). Tumor Kps decreased with pulse treatment at day 4, but increased afterwards, increased from baseline to day 9 in the daily dose group and decreased in the control group 9 days after therapy. Tumor fPV increased in both treated groups, with a decrease afterwards with pulsed treatment (P>0.050). The authors of this animal study concluded that quantitative MRI can provide sensitive information as to tyrosine kinase inhibitor induced tumor changes suggesting the potential of distinguished treatment regimens and ultimately helping in the determination of optimal treatment scheduling for enhancing chemotherapy delivery.
Bone and Soft Tissue Tumors
The response to therapy in bone tumors has been performed by qualitative analysis looking at the signal intensity changes of bone marrow after therapy as well as by quantitative analysis measuring the ADC values. In 24 patients with spine metastases diffusion-weighted MRI and spin-echo MRI was performed before and after radiotherapy. Treatment response has been evaluated by qualitative analysis looking at the signal intensity changes of bone marrow after therapy (59). Metastases before radiotherapy showed a hyperintense signal intensity on the images at a b-value of 165 sec/mm2 applied in one direction. A signal decrease one month after therapy was defined as response to therapy. In 23 out of 24 patients hypointense signal intensity after successful treatment could be detected whereas 1 of 24 patients showed persistent hyperintense bone marrow corresponding to no clinical improvement. The authors concluded that diffusion-weighted MR imaging allows detecting decreased signal intensity in metastatic disease to the vertebral body marrow corresponding to successful treatment.
In 18 osteogenic and Ewing sarcomas monitoring of therapeutic response of primary bone tumors has been performed by using DW-MRI. ADC measurements were performed before and 10 to 14 days after finishing chemotherapy. Definitive surgery was performed within 3 days after the post-chemotherapy MR study. Necrosis was evaluated on histology. Significantly greater ADC change was observed in the group with tumor necrosis of more than 90% compared to the group with less than 90% necrosis on histology after treatment. However no statistical significant difference in tumor volume between the two groups could be detected. Therefore the ADC value of diffusion-weighted MRI could be used as promising tool for monitoring the therapeutic response to primary bone sarcomas (41). In a preclinical model of metastatic prostate cancer the functional diffusion maps (fDM) as an imaging biomarker for assessing early treatment response has been evaluated in order to quantify especially distinct therapeutic induced changes in the Brownian motion. In contrast to control animals a significant increase in ADC could already be detected early after treatment. The results of this study have shown the possibility of functional diffusion maps as a biomarker for the detection of bone cancer treatment efficacy with the aim to switch to an alternative therapy in a timelier manner in case of ineffective therapy (60).
These results were confirmed in a patient with metastatic prostate cancer to the bone where DW-MRI was performed at the start of therapy and two and eight weeks post treatment initiation to quantify changes in tumor diffusion values. In all three metastatic lesions that were analyzed an early increase in diffusion values at two weeks which increased further at 8 weeks post treatment initiation could be observed correlating with the decrease in the patients prostate specific antigen (PSA) levels suggestive of patient response. Although this has been shown in only one patient the fDM imaging biomarker may provide a quantifiable therapeutic end point to assess response to patients with metastatic bone cancer (61).
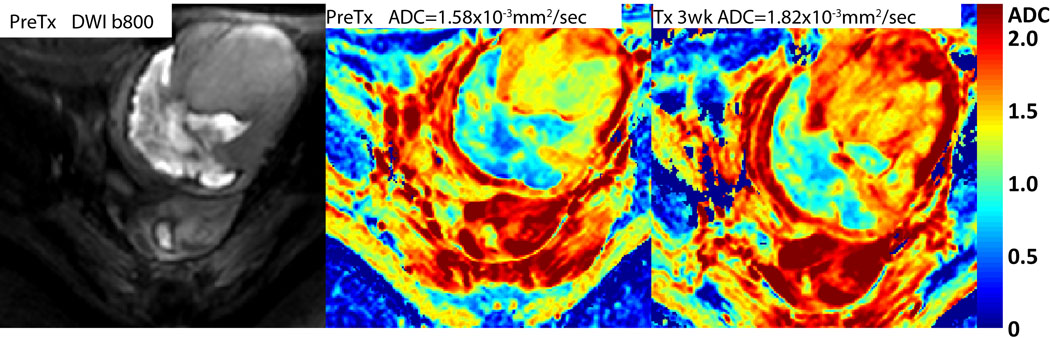
DW-MRI also resulted as useful noninvasive method to monitor anticancer treatment effects in 23 consecutive patients with soft-tissue sarcomas (44). DW-MRI was performed before and after initiation of chemotherapy. A high degree of correlation was found comparing changes in tumor volumes and ADC values (r=−0.925, P<0.0001), regardless of the effectiveness of anticancer treatment expressed as changes in tumor volume. As microstructural changes evaluated by DW-MRI are expected to precede change in tumor size and volume, DW-MRI performed at an early stage of fractionated therapy might provide unique prognostic information of effectiveness (44). An example of DW-MRI results obtained following chemotherapy of a patient with a soft tissue sarcoma is shown in Figure 3. In this example, a color ADC map is shown prior to therapy with a whole tumor average ADC value of 1.58×10−3 mm2/s. At 3 weeks into chemotherapy, the mean ADC value for the sarcoma increased by 15.2% to a value of 1.82×10−3 mm2/s which is clearly evident from the color overlay maps (see arrows in Figure 3) indicating a positive response to therapy which was clinically observed for this patient.
Head and Neck Cancer
In head and neck cancer early evaluation of treatment response may provide prognostic information about treatment efficacy and subsequent tailoring of treatment based on individual response might follow (62). Diffusion-weighted MRI as an imaging biomarker of treatment response of squamous cell head and neck cancer has been evaluated in a mouse model undergoing treatment with chemotherapy, ionizing radiation and combined therapy compared to control animals. The animals were examined during and after treatment for changes in tumor volumes, diffusion values and survival (63). Radiation therapy had only minimal effect on volumetric growth rate diffusion and survival whereas a combination of chemotherapy and radiotherapy showed an increase in tumor diffusion values which correlated with improved survival. The authors of this animal study concluded that diffusion MRI as an imaging biomarker has the potential for early evaluation of the response to chemoradiation treatment in squamous cell carcinoma of the head and neck (63). There is one study performed in 40 patients with newly diagnosed head and neck cancer which performed diffusion-weighted MRI before, during and after chemoradiation therapy of head and neck metastases to lymph nodes. In this study 33 patients with head and neck squamous cell carcinoma and metastatic cervical lymph nodes were included and the analysis of ADC values in metastatic lymph nodes were measured before, one week after start of chemoradiation therapy and post treatment (46). The pretreatment ADC values of complete responders (1.04 ± 0.19 × 10−3 mm2/sec) were significantly lower than those from partial responders (1.35 ± 0.30 × 10−3 mm2/sec). In complete responders significant increase in ADC was observed within one week of treatment which continued until the end of treatment. Furthermore, a significantly larger increase in ADC values was found in complete responders as compared to partial responders by the first week of chemoradiation. The results of this study were quite interesting however the ADC values were measured in the metastatic lymph nodes and not on the primary site which would be more challenging and also very helpful for daily decision making in clinical routine. However, the authors of this study concluded that the results suggest that ADC can be used as a marker for prediction and early detection of response to concurrent chemoradiation therapy in head and neck squamous cell carcinoma (46).
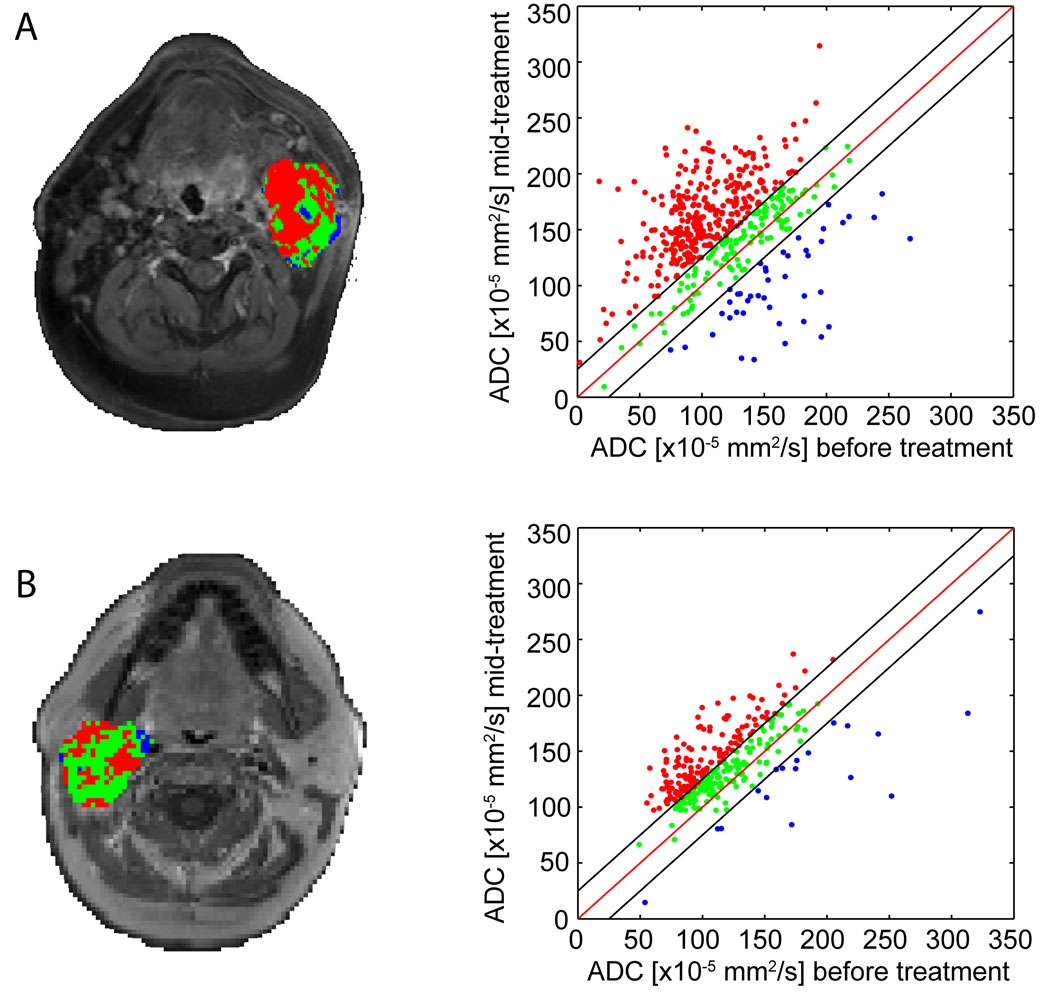
In a recent study, PRMADC was evaluated as an imaging response biomarker in head and neck cancer patients at 3 weeks post-initiation of nonsurgical organ preservation therapy (NSOPT) (32). A total of 15 patients were studied in which primary site as well as metastatic lymph nodes were evaluated. Shown in Figure 4 are PRMADC examples of two different patients which were subsequently found to have different clinical responses to NSOPT. The patient with the most robust therapeutic response exhibited a much larger shift in the overall number of increased ADC voxels at 3 weeks following treatment initiation (Figure 4A) versus the comparative patient (Figure 4B). In this study the degree of PRMADC shift exhibited by individual patients at 3 weeks was found to correlate with tumor control at 6 months. This study reveals the feasibility of using voxel-based assessment of tumor diffusion changes in patients where nonlinear warping algorithms need to be used for image registration of interval image examinations.
Cervical Cancer
In patients suffering from cervical cancer DW-MRI has already been performed for treatment monitoring in patients undergoing chemotherapy. In a study of 20 patients suffering from cervical cancer MRI was performed before treatment, two weeks after initiation of radio- and chemotherapy and at the end of therapy and results were correlated with the change in tumor size in MRI and conventional clinical response (45). In this prospective study performed on a 1.5 T MR unit correlation between change in ADC after 14 days of therapy and tumor size evaluated by conventional MRI was shown. Therefore, DW-MRI has the potential to provide the surrogate marker of treatment response in advanced cervical cancers. The use of ADC offers an early and reproducible indication of tumor response which may ultimately allow the development of individual treatment regimens. Similar results have been described in a study performed in 47 patients with cervical cancer undergoing chemoradiation therapy compared to 26 normal controls. Response to treatment was evaluated if no tumor was visible three to six months following completion of therapy. In patients with squamous cell carcinomas the 90th percentile of ADC values was lower in responders than non-responders (P<0.05). The median ADC in cervix carcinoma was significantly lower compared to normal cervix whereas the ADC may have predictive value in squamous cell tumors, but further long-term study will determine the ultimate clinical utility of this technique (64).
Rectal cancer
In rectal cancer DW-MRI was performed for prediction of response to chemoradiation. Pretreatment ADC values in rectal cancer patients were found to be negatively correlated with response: the presence of higher pretreatment ADC values reflected necrotic tumors that were resistant to therapy (43). The ADC values following treatment were consistently lower than prior to treatment which was attributed to possible increase in fibrosis and scar tissue formation in response to treatment. Similar results were observed in another study performed on rectal cancer(64).
Conclusion
Changes in tumor volume is currently the clinical response metric used for assessing response of a wide variety of tumor types which must be done late in the time course of treatment. DW-MRI has been shown to be able to detect microstructural changes which precede changes in tumor size. The effects of treatment can be variable: Necrosis as potential effect of treatment can be detected as a decrease in perfusion reflecting a decrease in the perfusion fraction (when performing biexponential fitting) as well as an increase in diffusion corresponding to an increase in the true diffusion coefficient. Fibrosis as other potential treatment response can be reflected in a decrease in perfusion (corresponding to decrease in the perfusion fraction Fp) as well as a decrease in diffusion corresponding to an ADC decrease. These findings can also be evaluated by visual analysis of the corresponding high b-value images as well as the ADC map.
For monitoring disease in most tumors and treatment strategies an increased ADC value during treatment corresponds to response whereas predicting the outcome of response is in most cases correlated with a low pre-treatment ADC value showing favorable outcome in most tumors and with most treatment options. However, in rectum carcinoma a decrease in ADC during treatment corresponded to response due to fibrosis and scar tissue formation. Whereas predicting of outcome also depends on the underlying therapy, high pretreatment ADC values have been shown to better correlate with the outcome in tumors treated by vascular targeting agents (65).
DW-MRI has significant potential to be applied as an imaging biomarker of treatment response at an early time interval following treatment initiation. DW-MRI protocols and analyses have to be standardized and need to be tailored to individual tumor types and anatomic sites and therapies. The time point of maximal response has to be evaluated for different tumors and treatment strategies. DW-MRI as ultimate surrogate tumor marker has to be confirmed by comparison to progression free survival and overall survival. Therefore multicenter studies to confirm these promising results are warranted.
Figure 5.
(Left panel) Diagrammatic representation of two possible pathways of cell death associated with therapy. Induction of apoptosis which would lead to an increase in tumor ADC values due to cell shrinkage and loss of cellular density. Processes involved with the induction of necrosis which might exhibit a transient initial drop in ADC values due to cell swelling following by an increase in ADC values during cell lysis during the later stages of this death pathway. (Right panel) The voxel-by-voxel analytical approach (functional diffusion map (fDM) or parametric response map (PRMADC)) used to quantify diffusion changes following therapy within a tumor using ADC maps pre- and post-treatment initiation. (Used with permission. Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A 2005;102:5524–5529. Copyright (2005) National Academy of Sciences, U.S.A.).
Figure 6.
Patient with a soft tissue sarcoma. (Left) Pre-treatment high b-value image shows revealing large dense (fibrosis) region on the lower left along with a high cellular region. ADC color overlay maps of the sarcoma (Middle) before and (Right) 3 weeks following chemotherapy. Note that the tumor mass (arrow) exhibited a large increase in tumor ADC values following treatment indicating positive response. (Image kindly provided by T.L. Chenevert, University of Michigan).
Figure 7.
Representative slices of PRMADC for patients whose conditions were diagnosed as (A) complete response (CR) and (B) partial response (PR); color-coded VOIs are shown as overlays on contrast-enhanced T1-weighted MR images before therapy and corresponding scatter plots for quantification and distribution of ADC before and 3 weeks after treatment initiation for the entire tumor volume. Unity and threshold designating significant change in ADC within the scatter plot are presented by red and black lines, respectively. Voxels with significant increased, decreased, or unchanged ADC values were assigned as red, blue, and green, respectively. Used with permission from Neoplasia Press. Galbán CJ, Mukherji SK, Chenevert TL, et al. Parametric Response Map Analysis of DW-MRI Scans of Head and Neck Cancer Patients Provides for Early Detection of Therapeutic Efficacy. Translational Oncology 2009;2:184–190.).
Acknowledgments
Grant Support: This manuscript was supported by the Swiss National Science Foundation for Scientific Research grant No 320000-113512, Carigest SA Foundation, Geneva, Switzerland and the National Institute of Health grants P50CA093990 and P01CA085878.
References
- 1.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 2.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verweij J, Therasse P, Eisenhauer E. Cancer clinical trial outcomes: any progress in tumour-size assessment? Eur J Cancer. 2009;45:225–227. doi: 10.1016/j.ejca.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Padhani AR, Liu G, Mu-Koh D, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 6.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- 7.Sodickson DK, Griswold MA, Jakob PM. SMASH imaging. Magn Reson Imaging Clin N Am. 1999;7:237–254. vii–viii. [PubMed] [Google Scholar]
- 8.Ivancevic MK, Kwee TC, Takahara T, et al. Diffusion-weighted MR imaging of the liver at 3.0 Tesla using TRacking Only Navigator echo (TRON): a feasibility study. J Magn Reson Imaging. 2009;30:1027–1033. doi: 10.1002/jmri.21939. [DOI] [PubMed] [Google Scholar]
- 9.Kwee TC, Takahara T, Koh DM, Nievelstein RA, Luijten PR. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J Magn Reson Imaging. 2008;28:1141–1148. doi: 10.1002/jmri.21569. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama T, Yoshida S, Fujii Y, et al. Use of diffusion-weighted MRI in monitoring response of lymph node metastatic bladder cancer treated with chemotherary. Nippon Hinyokika Gakkai Zasshi. 2008;99:737–741. doi: 10.5980/jpnjurol1989.99.737. [DOI] [PubMed] [Google Scholar]
- 11.Hardy PA, Recht MP, Piraino DW. Fat suppressed MRI of articular cartilage with a spatial-spectral excitation pulse. J Magn Reson Imaging. 1998;8:1279–1287. doi: 10.1002/jmri.1880080615. [DOI] [PubMed] [Google Scholar]
- 12.Bydder GM, Hajnal JV, Young IR. MRI: use of the inversion recovery pulse sequence. Clin Radiol. 1998;53:159–176. doi: 10.1016/s0009-9260(98)80096-2. [DOI] [PubMed] [Google Scholar]
- 13.Kuroda K, Oshio K, Mulkern RV, Jolesz FA. Optimization of chemical shift selective suppression of fat. Magn Reson Med. 1998;40:505–510. doi: 10.1002/mrm.1910400402. [DOI] [PubMed] [Google Scholar]
- 14.Clark CA, Le Bihan D. Water diffusion compartmentation and anisotropy at high b values in the human brain. Magn Reson Med. 2000;44:852–859. doi: 10.1002/1522-2594(200012)44:6<852::aid-mrm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Tofts PS, Lloyd D, Clark CA, et al. Test liquids for quantitative MRI measurements of self-diffusion coefficient in vivo. Magn Reson Med. 2000;43:368–374. doi: 10.1002/(sici)1522-2594(200003)43:3<368::aid-mrm8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Delakis I, Moore EM, Leach MO, De Wilde JP. Developing a quality control protocol for diffusion imaging on a clinical MRI system. Phys Med Biol. 2004;49:1409–1422. doi: 10.1088/0031-9155/49/8/003. [DOI] [PubMed] [Google Scholar]
- 17.Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3:1457–1466. [PubMed] [Google Scholar]
- 18.Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 19.Ross BD, Chenevert TL, Kim B, Ben-Yoseph O. Magnetic Resonance Imaging and Spectroscopy: Application to Experimental Neuro-Oncology. Q Magn Reson Biol Med. 1994:89–106. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao M, Pipe JG, Bonnett J, Evelhoch JL. Early detection of treatment response by diffusion-weighted 1H-NMR spectroscopy in a murine tumour in vivo. Br J Cancer. 1996;73:61–64. doi: 10.1038/bjc.1996.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poptani H, Puumalainen AM, Grohn OH, et al. Monitoring thymidine kinase and ganciclovir-induced changes in rat malignant glioma in vivo by nuclear magnetic resonance imaging. Cancer Gene Ther. 1998;5:101–109. [PubMed] [Google Scholar]
- 22.Moffat BA, Hall DE, Stojanovska J, et al. Diffusion imaging for evaluation of tumor therapies in preclinical animal models. MAGMA. 2004;17:249–259. doi: 10.1007/s10334-004-0079-z. [DOI] [PubMed] [Google Scholar]
- 23.Galons JP, Altbach MI, Paine-Murrieta GD, Taylor CW, Gillies RJ. Early increases in breast tumor xenograft water mobility in response to paclitaxel therapy detected by non-invasive diffusion magnetic resonance imaging. Neoplasia. 1999;1:113–117. doi: 10.1038/sj.neo.7900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall DE, Moffat BA, Stojanovska J, et al. Therapeutic efficacy of DTI-015 using diffusion magnetic resonance imaging as an early surrogate marker. Clin Cancer Res. 2004;10:7852–7859. doi: 10.1158/1078-0432.CCR-04-1218. [DOI] [PubMed] [Google Scholar]
- 25.Chinnaiyan AM, Prasad U, Shankar S, et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci U S A. 2000;97:1754–1759. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamstra DA, Lee KC, Tychewicz JM, et al. The use of 19F spectroscopy and diffusion-weighted MRI to evaluate differences in gene-dependent enzyme prodrug therapies. Mol Ther. 2004;10:916–928. doi: 10.1016/j.ymthe.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Stegman LD, Rehemtulla A, Hamstra DA, et al. Diffusion MRI detects early events in the response of a glioma model to the yeast cytosine deaminase gene therapy strategy. Gene Ther. 2000;7:1005–1010. doi: 10.1038/sj.gt.3301199. [DOI] [PubMed] [Google Scholar]
- 28.Theilmann RJ, Borders R, Trouard TP, et al. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia. 2004;6:831–837. doi: 10.1593/neo.03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schepkin VD, Lee KC, Kuszpit K, et al. Proton and sodium MRI assessment of emerging tumor chemotherapeutic resistance. NMR Biomed. 2006;19:1035–1042. doi: 10.1002/nbm.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larocque MP, Syme A, Yahya A, Wachowicz K, Allalunis-Turner J, Fallone BG. Temporal and dose dependence of T2 and ADC at 9.4 T in a mouse model following single fraction radiation therapy. Med Phys. 2009;36:2948–2954. doi: 10.1118/1.3147258. [DOI] [PubMed] [Google Scholar]
- 31.Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A. 2005;102:5524–5529. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galbán CJ, Mukherji SK, Chenevert TL, et al. Parametric Response Map Analysis of DW-MRI Scans of Head and Neck Cancer Patients Provides for Early Detection of Therapeutic Efficacy. Translational Oncology. 2009;2:184–190. doi: 10.1593/tlo.09175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moffat BA, Chenevert TL, Meyer CR, et al. The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia. 2006;8:259–267. doi: 10.1593/neo.05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamstra DA, Chenevert TL, Moffat BA, et al. Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci U S A. 2005;102:16759–16764. doi: 10.1073/pnas.0508347102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamstra DA, Galban CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol. 2008;26:3387–3394. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galban CJ, Chenevert TL, Meyer CR, et al. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat Med. 2009;15:572–576. doi: 10.1038/nm.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 38.Thoeny HC, De Keyzer F, Chen F, et al. Diffusion-weighted MR imaging in monitoring the effect of a vascular targeting agent on rhabdomyosarcoma in rats. Radiology. 2005;234:756–764. doi: 10.1148/radiol.2343031721. [DOI] [PubMed] [Google Scholar]
- 39.Thoeny HC, De Keyzer F, Vandecaveye V, et al. Effect of vascular targeting agent in rat tumor model: dynamic contrast-enhanced versus diffusion-weighted MR imaging. Radiology. 2005;237:492–499. doi: 10.1148/radiol.2372041638. [DOI] [PubMed] [Google Scholar]
- 40.Le Bihan D. Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology. 2008;249:748–752. doi: 10.1148/radiol.2493081301. [DOI] [PubMed] [Google Scholar]
- 41.Hayashida Y, Yakushiji T, Awai K, et al. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: Initial results. Eur Radiol. 2006;16:2637–2643. doi: 10.1007/s00330-006-0342-y. [DOI] [PubMed] [Google Scholar]
- 42.Sharma U, Danishad KK, Seenu V, Jagannathan NR. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009;22:104–113. doi: 10.1002/nbm.1245. [DOI] [PubMed] [Google Scholar]
- 43.Dzik-Jurasz A, Domenig C, George M, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307–308. doi: 10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- 44.Dudeck O, Zeile M, Pink D, et al. Diffusion-weighted magnetic resonance imaging allows monitoring of anticancer treatment effects in patients with soft-tissue sarcomas. J Magn Reson Imaging. 2008;27:1109–1113. doi: 10.1002/jmri.21358. [DOI] [PubMed] [Google Scholar]
- 45.Harry VN, Semple SI, Gilbert FJ, Parkin DE. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111:213–220. doi: 10.1016/j.ygyno.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 46.Kim S, Loevner L, Quon H, et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15:986–994. doi: 10.1158/1078-0432.CCR-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh DM, Scurr E, Collins D, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007;188:1001–1008. doi: 10.2214/AJR.06.0601. [DOI] [PubMed] [Google Scholar]
- 48.Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248:894–900. doi: 10.1148/radiol.2483071407. [DOI] [PubMed] [Google Scholar]
- 49.Marugami N, Tanaka T, Kitano S, et al. Early detection of therapeutic response to hepatic arterial infusion chemotherapy of liver metastases from colorectal cancer using diffusion-weighted MR imaging. Cardiovasc Intervent Radiol. 2009;32:638–646. doi: 10.1007/s00270-009-9532-8. [DOI] [PubMed] [Google Scholar]
- 50.Schraml C, Schwenzer NF, Clasen S, et al. Navigator respiratory-triggered diffusion-weighted imaging in the follow-up after hepatic radiofrequency ablation-initial results. J Magn Reson Imaging. 2009;29:1308–1316. doi: 10.1002/jmri.21770. [DOI] [PubMed] [Google Scholar]
- 51.Kamel IR, Liapi E, Reyes DK, Zahurak M, Bluemke DA, Geschwind JF. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009;250:466–473. doi: 10.1148/radiol.2502072222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu JS, Kim JH, Chung JJ, Kim KW. Added value of diffusion-weighted imaging in the MRI assessment of perilesional tumor recurrence after chemoembolization of hepatocellular carcinomas. J Magn Reson Imaging. 2009;30:153–160. doi: 10.1002/jmri.21818. [DOI] [PubMed] [Google Scholar]
- 53.Niwa T, Ueno M, Ohkawa S, et al. Advanced pancreatic cancer: the use of the apparent diffusion coefficient to predict response to chemotherapy. Br J Radiol. 2009;82:28–34. doi: 10.1259/bjr/43911400. [DOI] [PubMed] [Google Scholar]
- 54.Kremser C, Judmaier W, Hein P, Griebel J, Lukas P, de Vries A. Preliminary results on the influence of chemoradiation on apparent diffusion coefficients of primary rectal carcinoma measured by magnetic resonance imaging. Strahlenther Onkol. 2003;179:641–649. doi: 10.1007/s00066-003-1045-9. [DOI] [PubMed] [Google Scholar]
- 55.Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006;24:843–847. doi: 10.1016/j.mri.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Lee KC, Moffat BA, Schott AF, et al. Prospective early response imaging biomarker for neoadjuvant breast cancer chemotherapy. Clin Cancer Res. 2007;13:443–450. doi: 10.1158/1078-0432.CCR-06-1888. [DOI] [PubMed] [Google Scholar]
- 57.Kim H, Morgan DE, Zeng H, et al. Breast tumor xenografts: diffusion-weighted MR imaging to assess early therapy with novel apoptosis-inducing anti-DR5 antibody. Radiology. 2008;248:844–851. doi: 10.1148/radiol.2483071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aliu SO, Wilmes LJ, Moasser MM, et al. MRI methods for evaluating the effects of tyrosine kinase inhibitor administration used to enhance chemotherapy efficiency in a breast tumor xenograft model. J Magn Reson Imaging. 2009;29:1071–1079. doi: 10.1002/jmri.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Byun WM, Shin SO, Chang Y, Lee SJ, Finsterbusch J, Frahm J. Diffusion-weighted MR imaging of metastatic disease of the spine: assessment of response to therapy. AJNR Am J Neuroradiol. 2002;23:906–912. [PMC free article] [PubMed] [Google Scholar]
- 60.Lee KC, Sud S, Meyer CR, et al. An imaging biomarker of early treatment response in prostate cancer that has metastasized to the bone. Cancer Res. 2007;67:3524–3528. doi: 10.1158/0008-5472.CAN-06-4236. [DOI] [PubMed] [Google Scholar]
- 61.Lee KC, Bradley DA, Hussain M, et al. A feasibility study evaluating the functional diffusion map as a predictive imaging biomarker for detection of treatment response in a patient with metastatic prostate cancer to the bone. Neoplasia. 2007;9:1003–1011. doi: 10.1593/neo.07954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forastiere AA, Ang K, Brizel D, et al. Head and neck cancers. J Natl Compr Canc Netw. 2005;3:316–391. doi: 10.6004/jnccn.2005.0019. [DOI] [PubMed] [Google Scholar]
- 63.Hamstra DA, Lee KC, Moffat BA, Chenevert TL, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: an imaging treatment response biomarker to chemoradiotherapy in a mouse model of squamous cell cancer of the head and neck. Transl Oncol. 2008;1:187–194. doi: 10.1593/tlo.08166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McVeigh PZ, Syed AM, Milosevic M, Fyles A, Haider MA. Diffusion-weighted MRI in cervical cancer. Eur Radiol. 2008;18:1058–1064. doi: 10.1007/s00330-007-0843-3. [DOI] [PubMed] [Google Scholar]
- 65.Thoeny HC, De Keyzer F, Chen F, et al. Diffusion-weighted magnetic resonance imaging allows noninvasive in vivo monitoring of the effects of combretastatin a-4 phosphate after repeated administration. Neoplasia. 2005;7:779–787. doi: 10.1593/neo.04748. [DOI] [PMC free article] [PubMed] [Google Scholar]