Abstract
Objective
We examined the efficacy and tolerability of ethyl-eicosapentaenoate (EPA-E) monotherapy for major depressive disorder (MDD) in a double-blind, randomized controlled pilot study.
Methods
57 adults with DSM-IV MDD were randomized from 1/2003-6/2006 to receive 1 gram/day of EPA or placebo (PBO) for 8 weeks. Response criteria were based on the Hamilton-D-17 scale. Subjects' plasma lipid profiles were examined by gas chromatography.
Results
35 subjects (63% female; mean age 45+/-13 yrs) were eligible for the intent to treat (ITT) analysis. In the ITT sample, mean HAM-D-17 scores decreased from 21.6+/-2.7 to 13.9+/-8.9 for the EPA group (n=16) and from 20.5+/-3.6 to 17.5+/-7.5 for the PBO group (n=19) (p=0.123); the effect size for EPA was 0.55. ITT response rates were 38% (6/16) for EPA, and 21% (4/19) for PBO (p=0.45). Among the 24 study completers, mean HAM-D-17 scores decreased from 21.3+/-3.0 to 11.1+/-8.1 for the EPA group and from 20.5+/-3.8 to 16.3+/-6.9 for the PBO group (p=0.087); the effect size for EPA was 0.73. Completer response rates were 45% (5/11) for EPA, and 23% (3/13) for PBO (p=0.39). Among EPA subjects, baseline n-6/n-3 ratio was associated with decrease in HAM-D-17 score (r= -0.686, p=0.030) and with treatment response (p=0.032); change in n-6/n-3 ratio was associated with change in HAM-D-17 score (r=0.784, p=0.032). Side effects, reported in 2 EPA subjects and 5 PBO subjects, were exclusively gastrointestinal, mild, and not associated with discontinuation.
Conclusions
EPA demonstrated an advantage over placebo that did not reach statistical significance, possibly due to the small sample and low completer rates, which were the major study limitations.
Keywords: eicosapentaenoic acid, docosahexaenoic acid, DHA, EPA, omega-3, n-3, depression
Introduction
There is increasing evidence that omega-3 (n-3) polyunsaturated fatty acids (PUFAs) may be an effective treatment for major depressive disorder (MDD). Countries with high fish intake have been associated with lower rates of depression, and the n-3 fatty acids, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are proposed to be among the protective factors1-6. Omega-3-deficient states such as alcoholism, pregnancy, and the post-partum period have been linked with depression7-11, and depressed individuals have demonstrated marked depletions in omega-3 FAs in red blood cell phospholipids and lower docosahexanoic acid (DHA) levels in adipose, compared to controls11. As a whole, these data strongly suggest a psychotropic role for the omega-3 fatty acids.
Several double blind treatment studies12-27 with eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and mixtures of the two have generally supported their antidepressant efficacy at doses of 0.6-6.0 g/day, primarily as an adjunct to standard antidepressants28. The current consensus is that pure EPA and EPA-DHA combinations at doses of about 1 g/day may be effective antidepressants29-30, but the evidence for pure DHA has been mixed14,27.
There are relatively fewer studies that have examined omega-3 monotherapy for treatment or prevention of depression. A 6-week randomized controlled trial (RCT) with 36 subjects showed lack of efficacy of 2 g/day DHA monotherapy for depression14, with response rates of 27.8% for DHA and 23.5% for placebo. Rees and colleagues23 found no significant benefit with omega-3 over placebo in 26 women with perinatal depression. Marangell and colleagues31 found no preventive effect of post-partum depression with open 2960 mg/day EPA-DHA mix in a small sample of pregnant women. Freeman and colleagues25 examined an EPA-DHA mix of 1.9 g/day as a monopharmacologic adjunct to psychotherapy in 59 women with perinatal depression; while both treatment groups improved significantly, the difference between the omega-3 and placebo arms was not significant.
On the other hand, Su and colleagues22 found significant benefit of 3.4 g/day omega-3 compared to placebo in a sample of 36 women with postpartum depression. Freeman and colleagues17 carried out a dose-finding trial of omega-3 in 16 women with postpartum depression, using doses of 0.5 g/day, 1.4 g/day, or 2.8 g/day. Hamilton-D scores and the Edinburgh Post Natal Depression Scale both decreased by approximately 50% for all groups, with no apparent dose-response effect. Our group recently demonstrated greater efficacy of 1g/day of DHA compared to 2 g/day and 4 g/day in a dose-finding study of DHA in 35 subjects with MDD27. Nemets and colleagues19 found significant benefit with omega-3 compared to placebo in 28 children with major depressive disorder. The monotherapy studies reviewed here were generally limited by small sample sizes, and sometimes by a lack of a placebo arm.
In view of the encouraging evidence for efficacy of n-3s as adjunctive therapy, and more mixed results with n-3 monotherapy, there is a need to better investigate the latter. We therefore examined the efficacy and safety of EPA in subjects with MDD in a pilot, double-blind, placebo-controlled randomized clinical trial. In line with past findings, we hypothesized that EPA monotherapy would be more effective than placebo. We also assessed the effect size of EPA, so as to inform future larger-scale investigations on this compound.
Previous studies have examined the impact of n-3 supplementation on plasma lipid profiles in various populations32, but there is a relative dearth of such investigations in depressed samples. Given the strong link between omega-3 deficiencies and depressed states, an understanding of the impact of EPA supplementation on plasma lipid profiles in depressed patients could yield insights into the antidepressant mechanism of action of the n-3 fatty acids. We therefore examined the relationship between subjects' severity of depression and their baseline plasma levels of EPA, DHA, and the n-6/n-3 ratio, as well as the impact of EPA therapy on these parameters. We hypothesized that subjects with lower baseline levels of omega-3 and/or higher n-6/n-3 ratios would have greater severity of depression and would be more likely to have a favorable response to EPA treatment compared to subjects with higher plasma omega-3 levels, and that response would correlate with improvement in plasma omega-3 profiles. Finally, we examined the impact of consumption of omega-3 rich foods on depression severity and response to EPA. We hypothesized that subjects with diets low in omega-3 would have higher baseline severity of depression and would also have a more robust response to EPA supplementation compared to subjects with adequate dietary n-3 consumption.
Method
The study was approved by the Massachusetts General Hospital's (MGH) Institutional Review Board (IRB). Subjects with major depressive disorder (MDD) were recruited by advertisements and referrals to our MGH Depression Clinical and Research Program, beginning in January 2003 and ending in June 2006.
Subjects were required to meet criteria for MDD, as per the Structured Clinical Interview for DSM-IV (SCID-patient edition33). The following conditions were also required: ability to provide written IRB-approved informed consent; ages between 18-80 years; a baseline 17-item Hamilton-D scale34 (HAM-D-17) score of 18 or greater; and a baseline Clinical Global Impression-Severity scale35 (CGI-S) score of 3 or greater.
Exclusion criteria included: pregnancy or no use of a medically accepted means of contraception in women of child bearing potential; breastfeeding; a current, serious suicidal or homicidal risk; serious or unstable medical illness including cardiovascular, hepatic, renal, respiratory, endocrine, neurologic, or hematologic disease; history of unstable seizure disorder; use of anticoagulants such as heparin or warfarin; DSM-IV diagnoses including organic mental disorders, substance use disorders, including alcohol (active within the last six months), schizophrenia, delusional disorder, psychotic disorders not elsewhere classified; bipolar disorder; history of multiple adverse drug reactions or allergy to the study drugs; psychotic features; current use of antidepressants, lithium, or anticonvulsants for mood stabilization; clinical or laboratory evidence of hypothyroidism; current use of other psychotropic drugs; having failed to respond during the course of their current major depressive episode to at least one adequate antidepressant trial, defined as six weeks or more of treatment with citalopram 40mg/day (or its antidepressant equivalent); having taken at least 1 g/day of an omega-3 product, or any current use of supplements enriched with n-3 fatty acids, e.g. flax seed oil; history of electroconvulsive therapy (ECT) within the 6 months preceding study entry. Subjects were allowed concurrent psychotherapy if they were already receiving it prior to study entry, but were not allowed to initiate psychotherapy during the study.
Fifty-seven subjects (65% female; mean age 42 ±14 yrs) were randomized after being deemed eligible by a study clinician at the screening visit. Randomization and treatment assignment were performed by the MGH Research Pharmacy using the website www.randomization.com; all study clinicians and subjects remained blinded to the assignment for the duration of the study. Subjects received either 1 gram/day of EPA or placebo for 8 weeks. Each EPA capsule (donated by Amarin Neuroscience Ltd. [Laxdale], Oxford, UK) contained approximately 500 mg ethyl-ester of eicosapentaenoic acid (LAX-101) of greater than 95% purity (equivalent to 485mg pure ethyl-eicosapentaenoate) with 0.2% dl-alpha-tocopherol as an antioxidant. Placebo (containing paraffin oil and 0.2% dl-alpha-tocopherol) was provided for oral administration within identical 500mg soft gelatin capsules. All participants received a total of 2 capsules per day, to be taken twice daily or both at once, depending on patient preference. Packaging, storage and handling conditions were identical for LAX-101 and placebo. Pill counts were performed at each visit to ensure treatment adherence. Following screening and baseline visits, subjects were seen every 2 weeks for 8 weeks. Study completers who responded to treatment continued on their double-blind medication for an additional 8 weeks of maintenance, if desired. Non-responders were offered 8 weeks of rescue therapy with open label escitalopram.
Participants were given food diaries (Mallinkrodt General Clinical and Research Center Food Record) to fill out each day in order to estimate dietary intake of n-3 fatty acids and control for any influence of diet on the response to the study medication. Intakes were stratified into three categories: low = 0-1 serving/week of n-3-rich food; intermediate = 2-3 servings/week; high = 4 or more servings/week. Subjects were encouraged not to modify their regular diet during the study.
Plasma Fatty Acid Isolation, Identification and Quantification
Plasma levels of EPA, DHA, total n-3, total n-6, and the n-6/n-3 ratio were measured at the baseline visit, at 8 weeks (or final study visit), and at 16 weeks (or final maintenance visit). Plasma fatty acids were isolated and methylated as detailed in Mischoulon et al27 and Moser and Moser36. The fatty acid methyl ester (FAME) mixture was analyzed by gas chromatography, using a SHIMADZU GC-2014, with an FID detector and a Restek Stabilwax column, 30m l, 0.25mm i.d., 0.25um df. Standards from NU-CHEK Prep, INC. (P.O. Box 295, Elysian, MN 56028, U.S.A.) included: 1.Methyl-heptadecanoic acid internal standard (i.s.). 2. Reference standard #GLC642 for n-3 FAMEs. 3. Reference standard #GLC643 for n-6 FAMEs. The GLC-642 and GLC-643 FAME Mixes were combined in equal proportions (v/v) with a known amount of the FAME i.s. added.
The oven temperature was kept at 150°C for 2min, ramped at 10°C/min to 200°C and held for 4min, ramped again at 5°C/min to 240°C and held for 3min. Finally it was ramped to 250°C at 10°C /min and held there for 7min. The injection volume was 1ul, the carrier gas was Helium and the column flow was 1ml/min. The detector temperature was at 280°C. The total runtime was 30min. The Fame Standard Mix of n-3 and n-6 fatty acids containing the methyl-hepta-decanoic acid as i.s. was run in triplicate, on three different days, using several different dilutions. Only one dilution, which was closest in peak areas to the plasmas, was selected for use in calculations. The plasma FAME samples were run singly. Peak identification was based upon the retention time (RT) of each unknown peak and comparing it to the RTs of the standard peaks. Quantitation of each n-3 and n-6 fatty acid in the plasma was obtained using the individual peak areas of the fatty acids in the plasma and the standard Mix to obtain the masses. The internal standard peak area was used to apply correction factors to nullify the day-to-day variations in machine performance. Results were expressed as ug fatty acid / ml plasma.
Mean levels of plasma EPA, DHA, total n-3, total n-6, and n-6/n-3 ratio were calculated for all analyzable samples and significance of lipid changes within each treatment group was assessed by paired samples t-test and Wilcoxon Signed Ranks test. Comparison of lipid changes between treatment groups was assessed by the Mann Whitney U Test.
Outcome Measures
The primary outcome measure was the change in HAM-D-17 score from the baseline visit to study completion (8 weeks), with clinical response defined as a 50% or greater decrease in HAM-D-17 score. Remission was defined as a final HAM-D-17 score of 7 or less. Completer and intent-to-treat (ITT) analyses of patients with at least one post-baseline evaluation visit were carried out. The last-observation-carried-forward (LOCF) approach was used to define endpoint severity for patients who discontinued prematurely. Chi square and Fisher's exact test were used for comparing response and remission rates, and for comparing the difference in dropout rates, between the two treatment groups. Patients were routinely asked about side effects at each clinical visit, using a standard adverse events questionnaire developed at MGH.
In view of the small sample size, non-parametric procedures were used for some statistical comparisons. The Mann-Whitney U test was used to compare the degree of clinical improvement between treatment arms. One-way between-groups ANOVA and the Mann-Whitney U test were used to compare the degrees of improvement between subjects consuming different levels of dietary n-3. These techniques were also used to assess significance of changes in plasma lipid parameters (DHA, EPA, total n-3, total n-6, and n-6/n-3).
Linear regression was used to assess the relationship between baseline plasma lipid parameters (independent variables) and baseline severity of depression as well as improvement in HAM-D-17 score (dependent variables). Logistic regression was used to assess the relationship between baseline plasma lipid parameters (independent variables) and treatment response (dependent variable). Associations between degree of change in plasma fatty acid levels, improvement in HAM-D-17 score, and treatment response were similarly examined.
The Mann Whitney U test was used to assess the relationship between dietary n-3 and baseline severity of depression as well as improvement in HAM-D-17 score. Relationships between dietary n-3 intake, baseline plasma lipid parameters, and treatment response were similarly assessed.
For all analyses, two-sided significance was set at p<0.05. Statistical analyses were performed using SPSS version 16.
Results
Fifty-seven patients (65% female; mean age 42 ±14) were randomized to EPA (n=28) or Placebo (n=29). Treatments were assigned by the MGH Research Pharmacy upon successful completion of the screen visit. Six subjects were lost to follow up after the screen visit; seven subjects were disqualified at baseline because of significant clinical improvement or worsening; and three chose not to enter the study after completing the baseline visit (only one of them specified a reason, a lengthy commute). This resulted in 41 subjects (63% female; mean age 43 ± 13 yrs) entering double blind treatment (17 on EPA, and 24 on placebo). Thirty-five subjects (63% female; mean age 45 ± 13 yrs, 16 on EPA, 19 on Placebo) remained for at least one post-baseline visit and were evaluable in the intent to treat (ITT) analysis. Three evaluable subjects (1 on EPA, 2 on placebo) withdrew because of non-response; one EPA subject withdrew because of commuting difficulties; and one placebo subject chose to discontinue early because he was feeling better. The remaining early discontinuers were lost to follow-up and no reasons for their discontinuation were available. There was no significant difference in dropout rates between the two treatment groups (p>0.05). Twenty-four subjects (11 on EPA, 13 on placebo) completed the full 8 weeks of treatment.
Degree of Improvement in HAM-D-17 Scores, and Response and Remission Rates
Among the 24 study completers (63% female; 11 on EPA, 13 on Placebo), mean HAM-D-17 scores decreased from 21.3 ± 3.0 to 11.1 ± 8.1 for the EPA group (p=0.004) and from 20.5 ± 3.8 to 16.3 ± 6.9 for the placebo group (p=0.06), with a trend to significance in the difference between the two groups (U=42.00, z=-1.71, p=0.087) (Figure 1); the effect size for EPA was 0.73. Completer response rates, based on 50% or greater decrease in HAM-D-17 score, were 45% (5/11) for the EPA group, and 23% (3/13) for the placebo group (Figure 2) (Fisher's p=0.39, OR=2.78, 95% CI, 0.48-16.03). Remission rates, based on a final HAM-D-17 score of 7 or less, were 36% (4/11) for the EPA group and 15% (2/13) for the placebo group (Figure 2) (Fisher's p=0.357, OR=3.14, 95% CI, 0.45-21.74).
Figure 1. Change in HAM-D-17 score over time for each treatment group (completers).
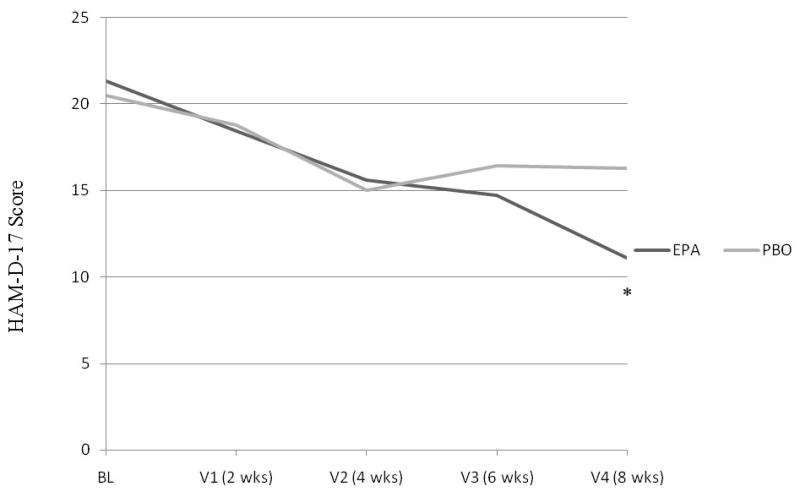
*EPA group had a significant decrease in HAM-D-17 score after 8 weeks of treatment (p=0.004)
Figure 2. Response and Remission Rates for Each Treatment Group (completers).
* Fisher's p=0.39 for EPA group vs Placebo (PBO)
+ Fisher's p=0.36 for EPA group vs Placebo (PBO)
In the ITT sample (63% female; 16 on EPA, 19 on Placebo), mean HAM-D-17 scores decreased from 21.6 ± 2.7 to 13.9 ± 8.9 for the EPA group (p=0.005) and from 20.5 ± 3.6 to 17.5 ± 7.5 for the placebo group (p=0.12), with a non-significant difference between the two groups (U=105.50, z=-1.54, p=0.123); the effect size for EPA was 0.55. ITT response rates were 38% (6/16) for the EPA group, and 21% (4/19) for the Placebo group (Fisher's p=0.45, OR=2.25, 95% CI, 0.50-10.10). Remission rates were 25% (4/16) for the EPA group and 16% (3/19) for the Placebo group (Fisher's p=0.677, OR=1.78, 95% CI, 0.33-9.43).
Only four EPA responders and three placebo responders entered the 8-week maintenance phase. This small sample did not allow for significant analyses or comparisons, but all these subjects maintained their response during this phase, with no relapses or significant depressive worsening.
Effect of EPA Administration on Plasma Fatty Acid Profiles
A total of 37 analyzable samples were available for baseline visit, 14 for the week 8 visit, and 9 for the week 16 visit (including the 7 double-blind responders, and two non-responders on escitalopram rescue). Lipid samples could not be obtained for certain patients due to unavailability for blood draws, and some samples were not analyzable due to damage during storage.
In EPA subjects, mean plasma EPA level increased significantly over the 8 weeks of treatment, from 7.62 ± 8.21 ug/ml to 22.13 ± 7.04 ug/ml (Z=-2.028, p=0.043), and the n-6/n-3 ratio decreased significantly, from 13.78 ± 3.92 to 9.05 ± 1.57 (Z= -2.197, p=0.028). Plasma DHA showed no significant change, from 40.93 ± 15.13 ug/ml to 44.83 ± 10.62 (p>0.05), nor did total n-3 and n-6 (p>0.05). No significant changes in any plasma lipid parameters were observed for placebo subjects (p>0.05). The change in plasma EPA level in EPA subjects was significantly higher than the one from 4.25 ± 3.17 ug/ml to 4.39 ± 5.29 ug/ml observed in the placebo group (U=5.00, Z=-2.03, p=0.042), but differences in changes in the other lipid parameters between the two treatment groups did not reach significance (p>0.05). Among subjects who entered the maintenance/rescue treatment phase, only one EPA responder and two placebo responders had analyzable plasma lipid samples, so no significant comparisons in lipid parameters could be made for the EPA group; for the placebo subjects, changes in lipid parameters were not significant (p>0.05).
Relationship Between Plasma Lipid Levels, Severity of Depression, and Response to Treatment
Linear regression was carried out to determine the association between baseline plasma EPA, DHA, total n-3, total n-6, and n-6/n-3 ratio (independent variables) and baseline HAM-D-17 score (dependent variable). For all subjects with available baseline lipid data and HAM-D-17 scores (n=37), we found no significant associations between any of the baseline lipid parameters and severity of depression (p>0.05).
In study completers, we similarly examined the relationship between baseline plasma lipid parameters (independent variables) and the change in HAM-D-17 score (dependent variable) with treatment. For EPA group completers who had baseline lipid data available (n=8), we found a significant Pearson correlation between the baseline n-6/n-3 ratio and the change in the HAM-D-17 score with treatment (r= -0.686, p=0.030). No significant associations were observed between any of the other lipid parameters and the change in the HAM-D-17 score (p>0.05). Among placebo group completers who had baseline lipid data available (n=8), we found a significant Pearson correlation between baseline DHA and the change in the HAM-D-17 score (r=0.677, p=0.033), and between baseline total n-3 and the change in the HAM-D-17 score (r=0.694, p=0.028).
We examined the relationship between changes in plasma lipid parameters (independent variables) and change in HAM-D-17 score (dependent variable) with treatment. For EPA subjects, we found a significant Pearson correlation between change in n-6/n-3 ratio and change in HAM-D-17 score (r=0.784, p=0.032). No significant associations were observed between the changes in the other lipid parameters and the change in the HAM-D-17 score (p>0.05). In placebo subjects, no significant associations were observed between any changes in lipid parameters and changes in HAM-D-17 score (p>0.05).
Logistic regression was carried out to examine the relationship between mean baseline plasma lipid parameters (independent variables) and treatment response (dependent variable). For completers in the EPA and placebo groups, we found no significant association between treatment response and any of the baseline lipid parameters (p>0.05). In the ITT sample, we found a significant association between baseline n-6/n-3 ratio and treatment response (p=0.032) in EPA subjects, but no significant associations among placebo subjects (p>0.05).
We similarly examined the relationship between treatment-related changes in mean plasma lipid parameters (independent variables) and response (dependent variable). We found no significant associations between treatment response and changes in any lipid parameters in either treatment group, both for completers and the ITT sample (p>0.05).
Effect of Dietary Omega-3 Intake on Depression Severity, Plasma Lipid Parameters, and Response to Treatment
25 subjects (11 from the EPA group, and 14 from the placebo group) consistently filled out their food diaries. Thirteen subjects (6 from EPA, 7 from PBO) met criteria for low dietary n-3 (0-1 serving/week), nine (4 from EPA, 5 from PBO) for medium n-3 (2-3 servings/week), and three (1 from EPA, 2 from PBO) for high n-3 (4 or more servings/week). To simplify the analysis, in some cases we pooled the medium and high dietary n-3 groups (n =12) (“adequate”) to compare against the low dietary n-3 group (n =13) (“inadequate”).
Subjects consuming low dietary n-3 (n=13) had a mean baseline HAM-D-17 score of 21.8 ± 3.0; subjects consuming intermediate n-3 levels (n=9) had a mean baseline HAM-D-17 score of 20.8 ± 2.8; and subjects consuming high n-3 levels (n=3) had a mean baseline HAM-D-17 score of 19.33 ± 6.7. One-way between-groups ANOVA showed no significant difference between baseline HAM-D-17 scores across the three dietary groups (p>0.05). After pooling medium and high dietary n-3, the mean baseline HAM-D-17 score for the group receiving adequate dietary n-3 (n=12) was 20.4 ± 3.8. The Mann-Whitney U test showed no significant difference in severity of depression at baseline between low and adequate dietary n-3 groups (z = -0.91; U = 61.5; p = 0.37).
Plasma lipid data were available for 18 of the subjects who filled out food diaries. In subjects with adequate dietary n-3 consumption, baseline plasma EPA (9.56 ± 9.11 ug/ml), and DHA (44.58 ± 19.46 ug/ml) were higher than in subjects with low consumption (EPA = 3.10 ± 2.28 ug/ml, and DHA = 35.19 ± 8.31 ug/ml). The n-6/n-3 ratio was slightly lower in subjects with adequate dietary n-3 consumption (12.57 ± 2.85) than in those with inadequate n-3 consumption (15.04 ± 2.58). The Mann-Whitney U test showed a significant difference between low and adequate n-3 consumers for plasma EPA levels only (z = -2.845; U = 8.00; p = 0.004), and a trend to significance for n-6/n-3 (z=-1.77, U=20.00, p= 0.076).
As an exploratory investigation, depression severity and response to treatment were examined for subjects at each level of dietary n-3 consumption. Among EPA subjects in the ITT group, the ones with low dietary n-3 consumption had the most robust response rate of 33% (n=2/6), with progressively lower response rates in the groups with medium (25%; n=1/4) and high n-3 consumption (0%; n=0/1), and the pooled (adequate n-3) group having a response rate of 20% (n=1/5). The same trend was observed for completers, with the low dietary n-3 group responding at a rate of 50% (n=2/4), the medium n-3 group at 33% (n=1/3), and the high n-3 group at 0% (n=0/1). The pooled group of subjects with adequate dietary n-3 had a response rate of 25% (n=1/4). Among placebo subjects, only the medium n-3 group had a moderately robust response rate, with 40% (n=2/5) in the ITT group and 33% (n=1/3) in completers, compared to the other two dietary groups, among which completers and ITT subjects all had response rates of 20% or less. None of the differences in response and remission rates between dietary n-3 groups were significant by Fisher's Exact test (p>0.05). No comparisons of HAM-D-17 changes, using one-way between-groups ANOVA (for the 3 dietary groups) and the Mann-Whitney U Test (for adequate vs low n-3 groups) reached significance (p>0.05 for all comparisons).
Impact of Smoking Status on Outcomes
Because smoking is known to lower omega-3 levels, we examined tobacco use in our subjects to rule out any potential confounding effects. Only four of the 35 evaluable subjects reported smoking 10 or more cigarettes per day on a regular basis, which was unlikely to have a significant impact on our findings overall.
Tolerability and Side Effects
Seven subjects (2 on EPA, and 5 on placebo) reported mild side effects, all gastrointestinal. EPA subjects reported gas and an unspecified GI upset. Placebo subjects reported GI upset and increased bowel movements or diarrhea. Three of these subjects discontinued in the acute phase, and two in the maintenance/rescue phase. One cited non-response as the reason for his termination, and the rest were lost to follow-up. No subjects attributed their discontinuation to side effects.
Discussion
While several studies have investigated the efficacy of omega-3 as adjunctive therapy for partial responders to conventional antidepressants, this is one of the relatively fewer RCTs investigating the efficacy of EPA monotherapy for major depressive disorder. Peet and Horrobin12 reported antidepressant efficacy of adjunctive use of the same EPA preparation used in our study, and we found a strong trend to significance for 1 g/day of EPA compared to placebo. The observed decrease in HAM-D-17 score was significant for the EPA group, but not for the placebo group, both among study completers and the ITT sample. Response and remission rates gave approximately a 2:1 advantage to EPA over placebo, both for completers and the ITT sample. The differences in HAM-D change and response/remission rates between groups did not reach significance, perhaps because of the small sample size and small number of completers. However, Peet and Horrobin12 also had a relatively small sample, and their more robust efficacy findings might be explained by their use of EPA as an adjunct rather than as monotherapy.
Although our response rates were lower than those generally reported for synthetic antidepressants, the effect size for EPA was within the moderate range for completers and for the ITT sample. The observed placebo response rates (21% in the ITT sample and 23% in completers), were consistent with those documented in the depression literature37-39, suggesting that the efficacy observed with the active treatment was a true drug effect. We unfortunately did not question the patients to determine whether the blinding of EPA and placebo were effective. However, given the composition of the study capsules, and the few complaints of adverse effects, we have no reason to think that subjects were able to readily discriminate between placebo and active treatment, which makes it unlikely that this impacted on our findings. The recent evidence that the efficacy gap between antidepressants and placebo is less than previously thought, based on meta-analyses40 and examination of publication biases41 lends further support for investigating omega-3s as viable antidepressants.
Administration of EPA resulted in a significant increase of mean plasma EPA, and a significant decrease in the n-6/n-3 ratio after 8 weeks, but had limited effect on plasma DHA, total n-3, and total n-6. Previous investigations have shown that EPA and DHA are interconvertible, with the biochemical pathway favoring conversion from EPA to DHA and allowing limited retroconversion of DHA to EPA32. EPA supplementation of 4 g/day has been shown to increase plasma EPA concentration, but has had little impact on DHA concentration, which has been explained by poor enzymatic conversion of EPA to DHA in humans32. Our findings are therefore consistent with past investigations; our more modest 1 g/day dose of EPA was likely too small to have a significant impact on plasma DHA levels.
We found a significant association between baseline n-6/n-3 ratio and the decrease in HAM-D-17 score with EPA treatment, as well as with treatment response, suggesting that a higher baseline n-6/n-3 ratio may be associated with greater response to EPA, a reflection of the impact of omega-3 supplementation on individuals with relative deficiencies of omega-3. We also found a significant association between magnitude of treatment response and the decrease in n-6/n-3 ratio in EPA subjects. These results are consistent with our hypothesis and with our pilot investigation of DHA administration27, in which we found a trend to association between a lower baseline n-6/n-3 ratio and less depressive severity. There may be an “optimal” n-6/n-3 ratio in humans that maintains a balance between pro- and anti-inflammatory forces, represented by omega-6 and omega-3 fatty acids, respectively42-44, and its proper equilibration may prevent or reverse a depressed state.
Consumption of adequate levels of dietary n-3 was associated with higher baseline plasma EPA and DHA and a lower n-6/n-3 ratio. A non-significant trend of modestly increasing severity of depression with decreased dietary n-3 intake was observed for the sample as a whole, suggesting that dietary n-3 may have at least a modest impact on depression severity. A similar trend was observed in our previous study with DHA supplementation27. Levels of dietary n-3 consumption had no significant impact on treatment response, though it was noted, particularly among EPA subjects, that subjects with higher omega-3 consumption appeared to respond less well than those with lower consumption, which again may reflect a greater impact of EPA supplementation in a “deficient” population. However, the analyzable sample is too small to draw any definitive conclusions, and these findings must therefore be considered exploratory and very preliminary. Replication in larger samples seems warranted, however.
EPA appeared to be well tolerated, though this observation must be made with caution, given the relatively high rate of loss to follow-up. Only two subjects receiving EPA reported side effects, which were mild and exclusively in the gastrointestinal category. Among subjects who gave a reason for early termination, none attributed it to treatment-related side effects, though adverse effects may have contributed to the loss of other subjects for which reasons for termination were not obtained. The apparently infrequent and benign nature of side effects is consistent with the n-3 literature45.
The study was originally powered to recruit 80 subjects. Fewer patients were ultimately enrolled, due to recruitment challenges that prevented attainment of the full complement within the financial and temporal constraints of the grant. Our experience appears to be representative of a growing problem in clinical research: increasing competition for study subjects between and within research groups46; subjects becoming more selective and preferring studies that offer monetary compensation47; and growing public and political skepticism about placebo use in clinical trials48.
The low completer rate was particularly surprising, given the few complaints of adverse effects, a good response rate for the active treatment, and a placebo response rate comparable to that seen in most antidepressant studies. The more common reasons for early discontinuation of antidepressants include lack of tolerability, feeling better or believing that the medication is not necessary, and perceived lack of response49-51. In our sample, lack of tolerability did not seem to be an issue. Likewise, only one patient attributed his discontinuation to non-response, and the similar response rates between the ITT group and completers would suggest that inadequate response was not likely a cause for early discontinuation. In our studies of natural treatments, we have seen many patients enter with a large degree of enthusiasm over the prospect of getting well with a complementary medicine. This is consistent with prior reports about the public's faith in complementary and alternative medicine52, and may perhaps contribute to an early placebo response and discontinuation due to the belief that one is cured, or conversely, to a rapid disappointment and discontinuation in cases where early improvement is not observed. Nonetheless, because most patients who discontinued early were lost to follow-up, it is difficult to ascertain the exact causes that led to their discontinuation.
When this pilot study was designed in 2000-2001, there was limited guidance about accuracy of the target effect of omega-3s, and the study was intended to provide an effect size estimate. For the calculation of sample size relative to power, we selected a targeted treatment effect of a difference in the proportions of responders of 0.30, with at least 60% of patients receiving EPA and up to 30% of patients receiving placebo expected to meet criteria for response. A power of 80% to achieve a statistically significant result was estimated, though there was not enough efficacy data to say with confidence whether the difference in treatment effectiveness would be 30%. Nonetheless, computation of effect size should assist in the planning of more definitive larger scale studies.
In summary, EPA appears to be a well-tolerated, potentially effective monotherapy for MDD at doses of 1 g/day. Our efficacy findings are consistent with prior investigations, which collectively suggest that doses of 1 gram per day of n-3, usually pure EPA or an EPA-DHA combination, alleviate depressive symptoms28-30, though these recommendations are based in large part on augmentation studies, which are often characterized by greater treatment resistance and potential interactions between n-3 and standard antidepressants that may affect response rates and observed “effective” n-3 doses. Though EPA subjects who entered the maintenance phase were too few to draw generalizable conclusions, it was encouraging that they remained depression-free, which suggests that EPA may have longer-term benefit.
Our results must be interpreted with caution, in view of the small sample and modest number of completers, which limited statistical power. Further study in larger samples with adequate placebo controls is warranted. At this time, combination treatment with EPA and DHA remains the optimal recommendation for use29, and reflects the natural dietary availability of these fatty acids. However, we must emphasize that at this point in time, there is still not enough evidence to unequivocally recommend the omega-3s as a first line monotherapy or even as adjunctive agents. The study of DHA and EPA independently may eventually clarify their respective roles and mechanisms in the prevention of depression, and such investigations are currently in progress.
Acknowledgments
Ethyl-eicosapentaenoic acid and placebo were kindly provided by Amarin (Laxdale). Lipid analyses were kindly performed by Jeff Breu, PhD, and Ambalini Selvaraj, PhD, of the Massachusetts Institute of Technology's Clinical Research Center. Drs Breu and Selvaraj were supported in part by a General Clinical Research Center grant from the National Institutes of Health (MO1-RR01066) awarded to the Massachusetts Institute of Technology General Clinical Research Center.
Support: This work was supported by grant K23 AT001129 from the National Center for Complementary and Alternative Medicine (NCCAM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine, National Institutes of Health.
Footnotes
Previous Presentation: A preliminary analysis of this work was presented in poster form at the Annual Meeting of the American Psychiatric Association, Washington, DC, May 7, 2008 and at the NCDEU, Phoenix, AZ, May 29, 2008.
Financial Disclosures: Dr. Mischoulon has received research support for other clinical trials, in the form of donated medications from Amarin (Laxdale), NordicNaturals, Lichtwer Pharma GmbH, Bristol-Myers Squibb Company, Cederroth, and SwissMedica. He has received consulting and writing honoraria from Pamlab. He has received speaking honoraria from Bristol-Meyers Squibb Company, Pfizer, and Pamlab, Virbac, and NordicNaturals. He has received speaker's honoraria from the MGH-Psychiatry Academy. Commercial entities currently supporting the MGH Psychiatry Academy are listed on the Academy's website www.mghcme.org, and include Astra Zeneca, Eli Lilly, and Janssen Pharmaceuticals. He has received royalty income from Back Bay Scientific for PMS Escape (patent application pending).
Dr Papakostas has served as a consultant for the Aphios Corporation, Bristol-Myers Squibb Company, GlaxoSmithKline, Eli Lilly & Company, Evotec Ltd, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, PAMLAB LLC, Pfizer Inc., Pierre Fabre Medicament, and Wyeth, Inc., has received honoraria from Bristol-Myers Squibb Company, Eli Lilly & Company, Evotec Ltd, GlaxoSmithKline, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, Lundbeck, PAMLAB LLC, Pfizer, Pierre Fabre Medicament, Titan Pharmaceuticals, and Wyeth Inc., and has received research support from Bristol-Myers Squibb Company, PAMLAB LLC, Pfizer Inc, and Precision Health Biosystems.
Dr Dording has received speaker's honoraria from Wyeth.
Dr. Nierenberg has received grant/research support from Bristol-Myers Squibb, Cederroth, Cyberonics, Forest Pharmaceuticals, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutica, Lichtwer Pharma, NARSAD, NIMH, Pfizer, Stanley Foundation, and Wyeth-Ayerst. He has served as advisor/Consultant to Abbott Laboratories, Brain Cells, Inc., Bristol-Myers Squibb, Cederroth, Eli Lilly, Genaissance, GlaxoSmithKline, Innapharma, Janssen Pharmaceutica, Eli Lilly, Novartis, Pfizer, Sepracor, Shire, and Somerset. He has received speaking honoraria from Bristol-Myers Squibb, Cyberonics, Forest Pharmaceuticals, GlaxoSmithKline, Eli Lilly, and Wyeth-Ayerst.
Dr Alpert has received research support from Abbott Laboratories, Alkermes, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Aspect Medical Systems, Astra-Zeneca, Bristol-Myers Squibb Company, Cephalon, Cyberonics, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, J & J Pharmaceuticals, Novartis, Organon Inc., PamLab, LLC, Pfizer Inc, Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., and Wyeth-Ayerst Laboratories. He has received speakers' honoraria from: Eli Lilly & Company, Janssen, Organon. He has advisory/consultative relationships with: Eli Lilly & Company, Pamlab LLC, and Pharmavite LLC.
Dr Fava has received research support from Abbott Laboratories, Alkermes, Aspect Medical Systems, Astra-Zeneca, Bristol-Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, J & J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Novartis, Organon Inc., PamLab, LLC, Pfizer Inc, Pharmavite, Roche, Sanofi/Synthelabo, Solvay Pharmaceuticals, Inc., Wyeth-Ayerst Laboratories. He has served as advisor/consultant for Aspect Medical Systems, Astra-Zeneca, Bayer AG, Biovail Pharmaceuticals, Inc., BrainCells, Inc. Bristol-Myers Squibb Company, Cephalon, Compellis, Cypress Pharmaceuticals, Dov Pharmaceuticals, Eli Lilly & Company, EPIX Pharmaceuticals, Fabre-Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals Inc., GlaxoSmithkline, Grunenthal GmBH, Jansse Pharmaceutica, Jazz Pharmaceuticals, J & J Pharmaceuticals, Knoll Pharmaceutical Company, Lundbeck, MedAvante, Inc., Neuronetics, Novartis, Nutrition 21, Organon Inc., PamLab, LLC, Pfizer Inc, PharmaStar, Pharmavite, Roche, Sanofi/Synthelabo, Sepracor, Solvay Pharmaceuticals, Inc., Somaxon, Somerset Pharmaceuticals, Wyeth-Ayerst Laboratories. He has received speaking honoraria from Astra-Zeneca, Boehringer-Ingelheim, Bristol-Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithkline, Novartis, Organon Inc., Pfizer Inc., PharmaStar, Wyeth-Ayerst Laboratories. He holds equity in Compellis, and MedAvante.
All other authors declare that they have no conflicts of interest.
References
- 1.Cross- National Collaborative Group. The changing rate of major depression: cross national comparisons. JAMA. 1992;268:3098–3105. doi: 10.1001/jama.1992.03490210080039. [DOI] [PubMed] [Google Scholar]
- 2.Hibbeln JR, Salem N. Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr. 1995;62:1–9. doi: 10.1093/ajcn/62.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ration in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31:157–161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 4.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. Letter. [DOI] [PubMed] [Google Scholar]
- 5.Hibbeln JR. Long-chain polyunsaturated fatty acids in depression and related conditions. In: Peet M, Glen I, Horrobin DF, editors. Phospholipid Spectrum Disorder in Psychiatry. Marius Press; Carnforth, England: pp. 195–210. [Google Scholar]
- 6.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 7.Salem N, Ward G. The effects of ethanol on polyunsaturated fatty acid composition. In: Alling C, Diamond I, Leslie SW, et al., editors. Alcohol, Cell Membranes and Signal Transduction in Brain. New York: Plenum Press; 1993. pp. 33–46. [Google Scholar]
- 8.Weissman MW. The treatment of depressive symptoms secondary to alcoholism, opiate addiction and schizophrenia: evidence for the efficacy of tricyclics. In: Clayton PJ, Barret JE, editors. Treatment of Depression: Old Controversies and New Approaches. New York: Raven Press; 1983. pp. 207–216. [Google Scholar]
- 9.Houwelingen AC, Honstra G. Scientific conference on omega-3 fatty acids in nutrition, vascular biology and medicine. Houston, TX: American Heart Association; 1994. Docosahexanoic acid, 22:6(n3), cervonic acid (CA), and hypertension in pregnancy: consequences for mother and child; pp. 17–19. abstr 56. [Google Scholar]
- 10.Cohen LS, Altshuler LL. Pharmacologic management of psychiatric illness during pregnancy and the postpartum period. Psychiatric Clinics of North America: Annual of Drug Therapy. 1997;4:21–60. [Google Scholar]
- 11.Mischoulon D, Fava M. Docosahexanoic acid and omega-3 fatty acids in depression. Psychiatric Clinics of North America. 2000;23:785–794. doi: 10.1016/s0193-953x(05)70197-0. [DOI] [PubMed] [Google Scholar]
- 12.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 13.Nemets B, Stahl ZM, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159:477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- 14.Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160:996–998. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- 15.Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. EurNeuropsychopharmacol. 2003;13:267–271. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 16.Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomized double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;72:211–218. doi: 10.1016/j.plefa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Freeman MP, Hibbeln JR, Wisner KL, Brumbach BH, Watchman M, Gelenberg AJ. Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr Scand. 2006;113:31–35. doi: 10.1111/j.1600-0447.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 18.Frangou S, Lewis M, Mccrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 19.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 20.Grenyer BF, Crowe T, Meyer B, Owen AJ, Grigonis-Deane EM, Caputi P, Howe PR. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1393–1396. doi: 10.1016/j.pnpbp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Hallahan B, Hibbeln JR, Davis JM, Garland MR. Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double-blind randomised controlled trial. Br J Psychiatry. 2007;190:118–122. doi: 10.1192/bjp.bp.106.022707. [DOI] [PubMed] [Google Scholar]
- 22.Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC, Pariante CM. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69:633–634. doi: 10.4088/jcp.v69n0418. [DOI] [PubMed] [Google Scholar]
- 23.Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42:199–205. doi: 10.1080/00048670701827267. [DOI] [PubMed] [Google Scholar]
- 24.Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, Jalali M, Peet M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42:192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- 25.Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: A randomized placebo-controlled study. J Affect Disord. 2008;110:142–148. doi: 10.1016/j.jad.2007.12.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, Heatherley SV, Christian LM, McNaughton SA, Ness AR. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99:421–431. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 27.Mischoulon D, Best-Popescu C, Laposata M, Merens W, Murakami JL, Wu S, Papakostas GI, Dording CM, Sonawalla SB, Nierenberg AA, Alpert JE, Fava M. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharm. 2008;18:639–645. doi: 10.1016/j.euroneuro.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 29.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Marangell LB, Richardson AJ, Lake J, Stoll AL. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatr. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 30.Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatr. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- 31.Marangell LB, Martinez JM, Zboyan HA, Chong H, Puryear LJ. Omega-3 fatty acids for the prevention of postpartum depression: negative data from a preliminary, open-label pilot study. Depress Anxiety. 2004;19:20–23. doi: 10.1002/da.10148. [DOI] [PubMed] [Google Scholar]
- 32.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(6 Suppl):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders - Patient Edition (With Psychotic Screen) (SCID-I/P (w/ psychotic screen)) (Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; 722 West 168th Street, New York, NY 10032: 1995. [Google Scholar]
- 34.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 35.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology, revised. National Institute of Mental Health; Rockville, MD: 1976. DHEW Pub. No. (ADM) 76-338. [Google Scholar]
- 36.Moser HW, Moser AB. Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual. Wiley-Liss Inc.; New York: 1991. Measurement of saturated very long chain fatty acids in plasma; pp. 177–191. [Google Scholar]
- 37.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 38.Yang H, Cusin C, Fava M. Is there a placebo problem in antidepressant trials? Curr Top Med Chem. 2005;5:1077–1086. doi: 10.2174/156802605774297092. [DOI] [PubMed] [Google Scholar]
- 39.Rihmer Z. Drug-placebo difference: in antidepressant drug trials could be 50% greater than previously believed. Neuropsychopharmacol Hung. 2007;9:35–37. [PubMed] [Google Scholar]
- 40.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 42.Stoll AL. Omega-3 fatty acids in mood disorders: a review of neurobiological and clinical actions. In: Mischoulon D, Rosenbaum J, editors. Natural Medications for Psychiatric Disorders: Considering the Alternatives. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 39–67. [Google Scholar]
- 43.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 45.Freeman MP, Sinha P. Tolerability of omega-3 fatty acid supplements in perinatal women. Prostaglandins Leukot Essent Fatty Acids. 2007;77:203–208. doi: 10.1016/j.plefa.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Kittredge C. A Shrinking Target: Clinical trials are taking longer, and costing more, as competition for patients heats up. The Scientist. 2005;19:46. [Google Scholar]
- 47.Elliott C. Guinea-pigging: healthy human subjects for drug-safety trials are in demand. But is it a living? The New Yorker. 2008 January 7;:36–41. [PubMed] [Google Scholar]
- 48.Kotzalidis G, Pacchiarotti I, Manfredi G, Savoja V, Torrent C, Mazzarini L, Tatarelli C, Amann B, Di Marzo S, Sánchez-Moreno J, Sani G, Girardi P, Colom F, Vieta E. Ethical questions in human clinical psychopharmacology: should the focus be on placebo administration? J Psychopharmacol. 2008;22:590–597. doi: 10.1177/0269881108089576. [DOI] [PubMed] [Google Scholar]
- 49.Lin EH, Von Korff M, Katon W, Bush T, Simon GE, Walker E, Robinson P. The role of the primary care physician in patients' adherence to antidepressant therapy. Med Care. 1995;33:67–74. doi: 10.1097/00005650-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Hunot VM, Horne R, Leese MN, Churchill RC. A cohort study of adherence to antidepressants in primary care: the influence of antidepressant concerns and treatment preferences. Prim Care Companion J Clin Psychiatry. 2007;9:91–99. doi: 10.4088/pcc.v09n0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demyttenaere K, Enzlin P, Dewé W, Boulanger B, De Bie J, De Troyer W, Mesters P. Compliance with antidepressants in a primary care setting, 1: Beyond lack of efficacy and adverse events. J Clin Psychiatry. 2001;62 22:30–33. [PubMed] [Google Scholar]
- 52.Mischoulon D. Nutraceuticals in psychiatry, Part 1: social, technical, economic, and political perspectives. Contemporary Psychiatry. 2004;2(11):1–6. [Google Scholar]



