Abstract
Subunit antigens are attractive candidates for vaccine development as they are safe, cost-effective, and rapidly produced. Nevertheless, subunit antigens often need to be adjuvanted and/or formulated to produce products with acceptable potency and efficacy. Here we describe a simple method for improving the potency and efficacy of a recombinant subunit antigen by its immobilization on nickel-chelating nanolipoprotein particles (NiNLPs). NiNLPs are membrane mimetic nanoparticles that provide a delivery and presentation platform amenable to binding any recombinant subunit immunogens featuring a poly-histidine tag. A His-tagged, soluble truncated form of the West Nile virus (WNV) envelope protein (trE-His) was immobilized on NiNLPs. Single inoculations of the NiNLP-trE-His produced superior anti-WNV immune responses and provided significantly improved protection against a live WNV challenge compared to mice inoculated with trE-His alone. These results have broad implications in vaccine development and optimization, as NiNLP technology is well suited to many types of vaccines, providing a universal platform for enhancing the potency and efficacy of recombinant subunit immunogens.
Rational design of vaccines, made possible by an enhanced understanding of the mechanisms that trigger an effective immune response, includes the optimization of immunogen generation, addition of adjuvants, and improvement of delivery. Numerous vaccine formulations have been very successful, but no single technology covers the key requirements of the ideal vaccine, namely impeccable safety, low cost, rapid preparation, and high potency (1). The three former characteristics are found with subunit antigens (i.e. purified protein components from pathogen candidates) (2), though these immunogens suffer from poor potency and thus often are formulated with adjuvants to improved their immunogenicity (3, 4). However, adjuvants themselves can have unwanted side-effects, and few are approved for use in humans (4). The development of an adjuvant-free technology to improve potency through antigen presentation or delivery would accelerate the development of new subunit vaccines.
Multivalency and shape are likely important determinants of vaccine potency and efficacy since valency may increase affinity and/or aggregate receptors, leading to activation (5), and the immune system has likely evolved to react more strongly to particles of the size of invading microorganisms. Based on these concepts multiple investigators have developed multimeric, particulate antigens as vaccine candidates against hepatitis B virus and the human papilloma virus, respectively produced in yeast and mammalian cells to form 10–20 nm diameter particles (6, 7). While these approaches have been highly effective and widely utilized, their multimeric nature is dictated by the properties of the virus-encoded antigens, and is not readily manipulated to produce vaccines for other agents. Other approaches utilizing particulate platforms (8, 9) to achieve multivalent presentation of antigens have shown promise, including liposomes (10), nano-scale microemulsions (11), and nanoparticles (12).
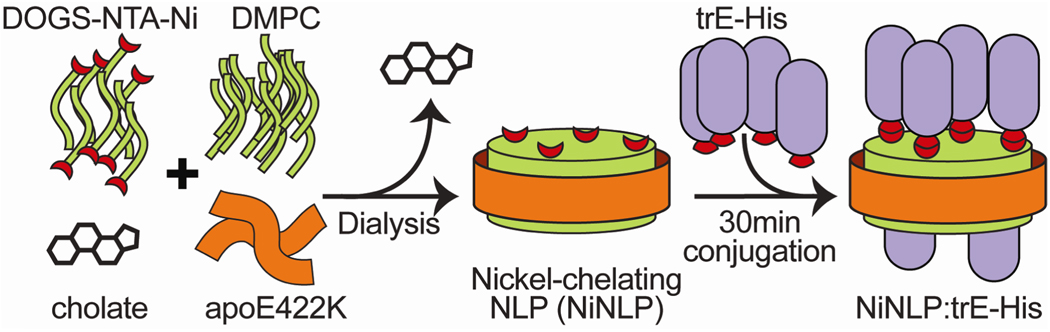
Here we describe a simple method to improve the potency and efficacy of His-tagged recombinant subunit vaccines using nickel-functionalized nanolipoprotein particles (NiNLPs) as a versatile antigen presentation and delivery platform to improve immune response to subunit antigens without requiring adjuvants (Figure 1). To harness multivalent and particulate characteristics for a universal vaccine platform, NiNLPs represent a promising technology. NLPs, also referred to as reconstituted high or low density lipoproteins (rHDLs and rLDLs, respectively), are self-assembled, nanometer-sized discoidal particles comprised of lipoproteins and phospholipids (13–15). The lipoproteins are amphipathic, α-helical bundles. Upon lipid binding, the hydrophobic faces of the helices bind the exposed hydrophobic core of the phospholipid bilayer, effectively protecting the lipid patches, producing discrete, soluble particles. The versatility of these biological nanoparticles is underscored by the ability to form NLPs with different apolipoproteins (13, 16, 17) and lipids (13, 18, 19), including functionalized lipids (20–22). Traditionally, NLPs have been used as model membrane mimetics for stabilizing and studying membrane proteins (23–27), although diagnostic (21, 22, 28) and drug delivery (29–34) applications have also been explored.
Figure 1.
Schematic of NiNLP assembly and His-tagged antigen (trE-His) conjugation.
We recently reported the facile functionalization of NLPs by incorporating lipids featuring nickel-chelating headgroups during the assembly process (20). The resulting particles present a lipid bilayer surface decorated with chelated nickel ions capable of binding recombinant proteins incorporating a poly-His peptide (Figure 1). NiNLPs were prepared by incubating a human apolipoprotein (apoE422K) with a cholate-solubilized mixture of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and the nickel-chelating lipid 1,2-dioleoyl-sn-glycero-3-{[N(5-amino-1-carboxypentyl) iminodiacetic acid]succinyl} (nickel salt) (DOGS-NTA-Ni). A 9:1 molar ratio of DMPC to DOGS-NTA-Ni was used to ensure that 10% of the NiNLP bilayer lipids feature chelated nickel (20). After dialysis to remove excess cholate, the assembled NiNLPs were purified by size exclusion chromatography (SEC) (Figure 2a). SEC fractions were subsequently analyzed by nondenaturing gradient gel electrophoresis (NDGGE) to identify fractions containing homogeneous populations of NiNLPs, which were then pooled. This approach yields NiNLPs of high purity and homogeneity, as assessed by native gel electrophoresis (Figure 2a, inset). Atomic force microscopy (AFM) confirmed the discoidal nature of the NiNLPs, and indicated an average diameter of 23.2 ± 2.3 nm (see Supporting Information). Based on previous modeling simulations of apoE422K-based NLPs, the molecular weight of 23.5 nm NLPs is estimated at 860 kDa (35), whereas the approximate molecular weight extrapolated from native gel mobility was ~500 kDa (Figure 2a, inset). This discrepancy is due in part to the effects of lipid composition on NLP native gel migration (unpublished observations) as well as the comparison of discoidal NLPs to globular molecular weight markers (35).
Figure 2.
NiNLP characterization and trE-His immobilization. (a) NiNLPs were purified by SEC (shaded region) and analyzed for purity by native gel electrophoresis (inset). (b) Conjugation of WNV trE-His antigen to NiNLPs was verified by centrifugal filtration using a 100 kDa molecular weight cut-off membrane. Total sample (T) and retentate after filtration (R) were resolved by SDS-PAGE and developed using SyproRuby.
To determine the ability of NiNLP technology to enhance the antigenicity of recombinant protein immunogens, the envelope protein (E) of West Nile virus (WNV) was identified as a candidate immunogen. Flaviviruses are responsible for serious diseases, including encephalitis (caused by WNV and Japanese encephalitis virus) as well as hemorrhagic fevers (caused by yellow fever virus and dengue viruses) (36). Two types of vaccines are currently available against flaviviruses: live-attenuated vaccines (LAVs) and formalin inactivated vaccines (INVs). While effective, LAVs are not recommended for all individuals and has been associated with adverse side effects (37). INVs are considered to be a safer type of vaccine, although multiple doses are required to induce high and long-lasting immunity (38). Therefore, safer and more potent vaccines need to be developed.
Vaccine-induced immunity to flavivirus infection has been linked to the E protein (36). In particular, vaccines based on subviral particles, characterized as 25 nm-diameter cell-derived lipid bilayers coated with E, have been effective against live viral challenges (39–44). These studies have confirmed the potential of subviral particles as vaccine candidates, but they are currently limited to vaccine formulations against viral targets. Recombinantly-expressed E protein has been shown to elicit immunity, however only in the presence of various adjuvants (45–48). A broadly adaptable platform for the presentation of antigens from a wide range of pathogens (e.g. viruses, bacteria, and parasites) in the absence of adjuvants would therefore present an important complementary approach to vaccine delivery. To adapt the E protein as a model antigen for use with the NiNLP platforms, a truncated variant of E, in which the native C-terminal membrane-binding domains were substituted with a poly-histidine tag (trE-His), was used in this study (49).
We have previously shown that His-tagged proteins are readily immobilized on NiNLPs (20). To verify that WNV trE-His was successfully bound to the NiNLPs after 45 minute incubation at room temperature, samples were isolated post-conjugation by centrifugal separation using a 100 kDa molecular weight cut-off filter. By this approach, all NiNLP constituents, including apoE422K and any immobilized proteins, are retained by the membrane whereas unconjugated proteins are removed. Analyses of total (T) and retentate (R) samples by SDS polyacrylamide electrophoresis are shown in Figure 2b, documenting that 1) the NiNLP was retained by the filter, 2) trE-His alone freely passed through the filter, 3) trE-His incubated with NiNLP was retained by the filter, and 4) the presence of EDTA in the mixture disrupted the association of the NiNLP with trE-His, permitting the trE-His to pass through the filter. AFM was used to independently verify immobilization of trE-His on the NiNLPs. An increase in NiNLP height was measured only in the presence of trE-His. No height increase was observed between trE-His and NiNLPs in the presence of EDTA (see Supporting Information).
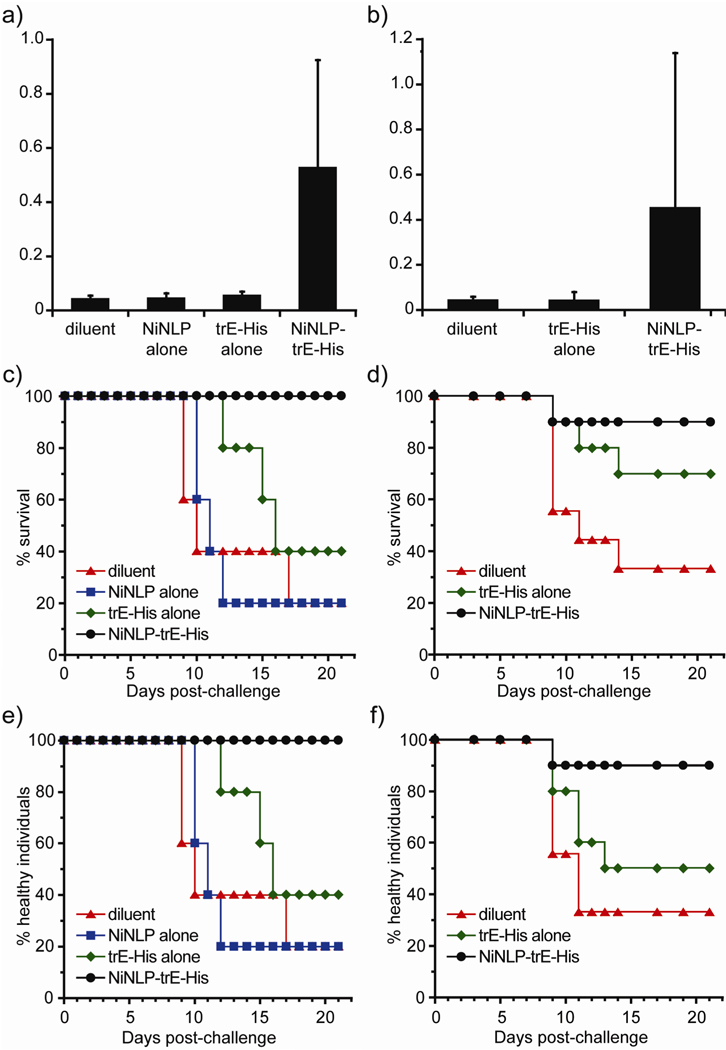
To determine whether NiNLP conjugation enhanced the immunogenicity of the trE-His to elicit WNV-specific immune responses, a pilot study was performed in 6-week-old mice. The groups of 5 mice were vaccinated once (i.e. no boost) with formulations containing 2.5 µg total trE-His protein, either as free protein (trE-His alone) or conjugated to the NiNLPs (NiNLP-trE-His). In addition, groups of animals were vaccinated with diluent or NiNLP alone. Figure 3a shows the results of the serological trE-His responses detected in the animals in these groups 3 weeks post-vaccination. At this dose, only the sera from mice vaccinated with NiNLP-trE-His produced readily detectable IgG responses to trE (Figure 3a). The large standard deviations in the IgG responses are indicative of variable responses by individual mice, rather than experimental error of the ELISAs (see Supporting Information). No response against apoE422K was detected by ELISA assays (data not shown), suggesting that apoE422K is either not immunogenic in mice or that too little protein is used in NLP formation so an immune response is not observed. To test the ability of NiNLP conjugation to increase the efficacy of trE-His, the animals in this pilot study were challenged with 1,000 ffu of live WNV NY99 (corresponding to 10 LD50 in 8-week-old mice) two weeks after the collection of sera (5 weeks post vaccination, 11 weeks old), and observed daily for three weeks. The Kaplan-Meier survival curve shown in Figure 3c shows that all mice inoculated with the NiNLP-trE-His preparation survived to 21 days post challenge when the experiment was terminated (at which time none of these animals displayed any morbidity – including weight loss, Figure 3e). All of the animals in the other groups succumbed to infection with the exception of two animals that received trE-His, and one animal in each of the NiNLP or diluent groups.
Figure 3.
NiNLP conjugation increases the potency of WNV trE vaccines. (a), (c) and (e) first experiment, n=5, (b), (d) and (f) second experiment, n=10. (a) and (b) WNV trE-His ELISA reactivity of sera from mice collected 21 or 28 days post vaccination (first and second experiment, respectively) with various formulations, containing either 2.5 µg of trE-His or no trE-His antigen. Bars represent average OD values obtained from 1:100 dilutions of sera assayed for reactivity to trE-His using standard methods (37). Error bars represent s.d. determined from OD values for all animals in each group. (c) and (d) Kaplan-Meier survival curves demonstrating that NiNLP conjugation improved trE-His efficacy. Animals were challenged with 1,000 ffu of WNV with various formulations containing 2.5 µg of trE-His and observed daily for 21 days. Animals that needed to be euthanized due to disease signs predictive of death within 24 hours were scored as dead the following day (37). (e) and (f) Morbidity curves reflecting the health of the challenged mice. Individuals were scored as unhealthy if indications of morbidity were observed (weight loss of more than 5 %, paralysis, ruffled fur, or inactivity).
To further assess the efficacy of NiNLP-trE-His, a larger trial using 5-week-old mice in groups of 10 animals was conducted. As with the pilot study, 2.5 µg total trE-His protein, either as free protein or as NiNLP-trE-His, were administered in a single dose. Serological responses were determined 4 weeks after vaccination, and the results mirrored those of the pilot experiment; only those animals receiving the NiNLP-trE-His constructs produced detectable IgG responses to trE (Figure 3b). In contrast to the pilot study, animals were challenged 4 weeks after vaccination. However, the exact same challenge dose used in the pilot study (1,000 ffu of live WNV) was administered. The animals were monitored for 21 days for signs of morbidity and mortality. Importantly, 90% of animals (9/10) receiving the NiNLP-trE-His inoculum survived (Figure 3d) with no indications of morbidity (weight loss of more than 5%, paralysis, ruffled fur, or inactivity) (Figure 3f). Among the animals receiving the unconjugated trE-His subunit in the second experiment, 70% (7/10) survived, however two animals displayed signs of morbidity (Figure 3f). Of the animals receiving only diluent controls (n=9), only three animals survived.
Taking the health of surviving individuals into account, the two experiments demonstrated a similar outcome. In the first experiment, 100% of NLP-trE-His treated mice survived and were healthy (5/5), whereas only 40% of those receiving trE-His alone (2/5) remained healthy. In the second experiment, 90% of mice receiving the NLP construct (9/10) were healthy following challenge, whereas only 50% of the trE-His vaccinated mice (5/10) remained healthy. Application of Fisher’s exact test to the combined 21-day survival data from the two experiments showed a significant difference between the NiNLP-trE-His groups and diluent and a significant difference between the NiNLP-trE-His and trE-His alone (in both cases, p < 0.01).
In order for NLPs to gain general use in the preparation of vaccines, a number of items require further investigation, including stability, biodistribution, and elimination. Preliminary stability assessments have demonstrated the ability to lyophilize and rehydrate NiNLPs with no loss in the ability to conjugate His-tagged proteins (data not shown). In vivo applications of rHDLs suggest significant stability over the length of the experiments (21). The in vivo fate of NiNLPs will be an important factor in determining the potential of this delivery vehicle. NLPs are most likely eliminated through the same pathway as native HDLs, i.e. processing through the liver. However, toxicology and biodistribution studies are currently underway to address this issue, as are additional stability and storage analyses. Since this vaccine preparation does not require infectious agents, inactivation procedures as well as controlled containment system for infectious agents are avoided, reducing potential costs significantly. Furthermore, we estimate that the process of NLP formation is scalable and the eventual cost of NiNLP-based vaccine platforms will reflect benefits of manufacturing.
We have demonstrated that the immunogenicity of the trE-His antigen is markedly increased when conjugated to NiNLPs. This approach is representative of a more general strategy wherein any protein with a His-tag can be used to generate a NLP-based construct in a matter of a few hours and can be administered to afford protective immunity. Furthermore, NiNLPs offer the potential to co-deliver immune stimulators similarly derivatized with a His-tag, providing for enhanced, specific, rapid immune stimulation at the site of delivery and uptake of the NiNLPantigen construct.
Supplementary Material
Acknowledgement
The authors thank Dr. Karl Weisgraber for providing apoE422k. This work was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory (LLNL-JRNL-420436) under Contract DE-AC52-07NA27344 with support from Lawrence Livermore National Laboratory (LDRD 06-SI-003 awarded to P.D.H and LDRD 09-LW-077 awarded to C.D.B.). P.W.M acknowledges support from NIAID through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research (NIH grant number U54 AI057156) and R21 AI077077.
Footnotes
Supporting Information Available: Experimental details, Figure S1 showing NiNLP purification, Figure S2 showing AFM characterization of NiNLP, Figure S3 showing AFM analyses of trE-His binding, Figure S4 showing individual serological responses. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Bae K, Choi J, Jang Y, Ahn S, Hur B. Innovative vaccine production technologies: The evolution and value of vaccine production technologies. Arch. Pharm. Res. 2009;32:465–480. doi: 10.1007/s12272-009-1400-1. [DOI] [PubMed] [Google Scholar]
- 2.Toth I, Simerska P, Fujita Y. Recent Advances in Design and Synthesis of Self-Adjuvanting Lipopeptide Vaccines. Int. J. Pept. Res. Ther. 2008;14333:333–340. [Google Scholar]
- 3.Kwissa M, Kasturi SP, Pulendran B. The science of adjuvants. Expert Rev. Vaccines. 2007;6:673–684. doi: 10.1586/14760584.6.5.673. [DOI] [PubMed] [Google Scholar]
- 4.Vogel FR, Hem SL. Immunologic Adjuvants. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Saunders; 2008. pp. 59–72. [Google Scholar]
- 5.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 6.Mast EE, Ward JW. Immunologic Adjuvants. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Saunders; 2008. pp. 177–204. [Google Scholar]
- 7.Lenz P, Day PM, Pang YYS, Frye SA, Jensen PN, Lowy DR, Schiller JT. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 8.Bramwell VW, Perrie Y. Particulate delivery systems for vaccines. Crit. Rev. Ther. Drug Carr. Syst. 2005;22:151–214. doi: 10.1615/critrevtherdrugcarriersyst.v22.i2.20. [DOI] [PubMed] [Google Scholar]
- 9.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008;60:915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrie Y, Mohammed AR, Kirby DJ, McNeil SE, Bramwell VW. Vaccine adjuvant systems: Enhancing the efficacy of sub-unit protein antigens. Int. J. Pharm. 2008;364:272–280. doi: 10.1016/j.ijpharm.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Patel JD, O'Carra R, Jones J, Woodward JG, Mumper RJ. Preparation and characterization of nickel nanoparticles for binding to his-tag proteins and antigens. Pharm. Res. 2007;24:343–352. doi: 10.1007/s11095-006-9154-7. [DOI] [PubMed] [Google Scholar]
- 12.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neill CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 13.Chromy BA, Arroyo E, Blanchette CD, Bench G, Benner H, Cappuccio JA, Coleman MA, Henderson PT, Hinz AK, Kuhn EA, Pesavento JB, Segelke BW, Sulchek TA, Tarasow T, Walsworth VL, Hoeprich PD. Different apolipoproteins impact nanolipoprotein particle formation. J. Am. Chem. Soc. 2007;129:14348–14354. doi: 10.1021/ja074753y. [DOI] [PubMed] [Google Scholar]
- 14.Jonas A, Kezdy KE, Wald JH. Defined apolipoprotein A-I conformations in reconstituted high-density lipoprotein disks. J. Biol. Chem. 1989;264:4818–4824. [PubMed] [Google Scholar]
- 15.Matz CE, Jonas A. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J. Biol. Chem. 1982;257:4535–4540. [PubMed] [Google Scholar]
- 16.Fang YL, Gursky O, Atkinson D. Lipid-binding studies of human apolipoprotein A-I and its terminally truncated mutants. Biochemistry. 2003;42:13260–13268. doi: 10.1021/bi0354031. [DOI] [PubMed] [Google Scholar]
- 17.Weers PMM, Ryan RO. Apolipophorin III: Role model apolipoprotein. Insect Biochem. Mol. Biol. 2006;36:231–240. doi: 10.1016/j.ibmb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Bhat S, Sorci-Thomas MG, Tuladhar R, Samuel MP, Thomas MJ. Conformational adaptation of apolipoprotein A-I to discretely sized phospholipid complexes. Biochemistry. 2007;46:7811–7821. doi: 10.1021/bi700384t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denisov IG, McLean MA, Shaw AW, Grinkova YV, Sligar SG. Thermotropic phase transition in soluble nanoscale lipid bilayers. J. Phys. Chem. B. 2005;109:15580–15588. doi: 10.1021/jp051385g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer NO, Blanchette CD, Chromy BA, Kuhn EA, Segelke BW, Corzett M, Bench G, Mason PW, Hoeprich PD. Immobilization of His-tagged proteins on nickel-chelating nanolipoprotein particles. Bioconjugate Chem. 2009;20:460–465. doi: 10.1021/bc8003155. [DOI] [PubMed] [Google Scholar]
- 21.Frias JC, Ma YQ, Williams KJ, Fayad ZA, Fisher EA. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett. 2006;6:2220–2224. doi: 10.1021/nl061498r. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Gray BD, Corbin I, Lebherz C, Choi H, Lund-Katz S, Wilson JM, Glickson JD, Zhou R. MR and fluorescent imaging of low-density lipoprotein receptors. Acad. Radiol. 2004;11:1251–1259. doi: 10.1016/j.acra.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Baker SE, Hopkins RC, Blanchette CD, Walsworth VL, Sumbad R, Fischer NO, Kuhn EA, Coleman M, Chromy BA, Letant SE, Hoeprich PD, Adams MWW, Henderson PT. Hydrogen production by a hyperthermophilic membrane-bound hydrogenase in water-soluble nanolipoprotein particles. J. Am. Chem. Soc. 2009;131:7508–7509. doi: 10.1021/ja809251f. [DOI] [PubMed] [Google Scholar]
- 24.Cappuccio JA, Blanchette CD, Sulchek TA, Arroyo ES, Kralj JM, Hinz AK, Kuhn EA, Chromy BA, Segelke BW, Rothschild KJ, Fletcher JE, Katzen F, Peterson TC, Kudlicki WA, Bench G, Hoeprich PD, Coleman MA. Cell-free co-expression of functional membrane proteins and apolipoprotein, forming soluble nanolipoprotein particles. Mol. Cell. Proteomics. 2008;7:2246–2253. doi: 10.1074/mcp.M800191-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzen F, Fletcher JE, Yang JP, Kang D, Peterson TC, Cappuccio JA, Blanchette CD, Sulchek T, Chromy BA, Hoeprich PD, Coleman MA, Kudlicki W. Insertion of membrane proteins into discoidal membranes using a cell-free protein expression approach. J. Proteome Res. 2008;7:3535–3542. doi: 10.1021/pr800265f. [DOI] [PubMed] [Google Scholar]
- 26.Lyukmanova EN, Shenkarev ZO, Paramonov AS, Sobol AG, Ovchinnikova TV, Chupin VV, Kirpichnikov MP, Blommers MJJ, Arseniev AS. Lipid-protein nanoscale bilayers: A versatile medium for NMR investigations of membrane proteins and membrane-active peptides. J. Am. Chem. Soc. 2008;130:2140-+. doi: 10.1021/ja0777988. [DOI] [PubMed] [Google Scholar]
- 27.Nath A, Atkins WM, Sligar SG. Applications of phospholipids bilayer nanodiscs in the study of membranes and membrane proteins. Biochemistry. 2007;46:2059–2069. doi: 10.1021/bi602371n. [DOI] [PubMed] [Google Scholar]
- 28.Cormode DP, Chandrasekar R, Delshad A, Briley-Saebo KC, Calcagno C, Barazza A, Mulder WJM, Fisher EA, Fayad ZA. Comparison of Synthetic High Density Lipoprotein (HDL) Contrast Agents for MR Imaging of Atherosclerosis. Bioconjugate Chem. 2009;20:937–943. doi: 10.1021/bc800520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng MQ, Cai QS, Shi XL, Huang H, Zhou P, Guo X. Recombinant high-density lipoprotein complex as a targeting system of nosiheptide to liver cells. J. Drug Target. 2008;16:502–508. doi: 10.1080/10611860802200938 . [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TS, Weers PMM, Raussens V, Wang Z, Ren G, Sulchek T, Hoeprich PD, Ryan RO. Amphotericin B induces interdigitation of apolipoprotein stabilized nanodisk bilayers. Biochim. Biophys. Acta-Biomembr. 2008;1778:303–312. doi: 10.1016/j.bbamem.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan RO. Nanodisks: hydrophobic drug delivery vehicles. Expert Opin. Drug Deliv. 2008;5:343–351. doi: 10.1517/17425247.5.3.343. [DOI] [PubMed] [Google Scholar]
- 32.Tufteland M, Ren G, Ryan R. Nanodisks derived from amphotericin B lipid complex. J. Pharm. Sci. 2008;97:4425–4432. doi: 10.1002/jps.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConathy WJ, Nair MP, Paranjape S, Mooberry L, Lacko AG. Evaluation of synthetic/reconstituted high-density lipoproteins as delivery vehicles for paclitaxel. Anti-Cancer Drugs. 2008;19:183–188. doi: 10.1097/CAD.0b013e3282f1da86. [DOI] [PubMed] [Google Scholar]
- 34.Nikanjarn M, Gibbs AR, Hunt A, Budinger TF, Forte TM. Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme. J. Control. Release. 2007;124:163–171. doi: 10.1016/j.jconrel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Blanchette CD, Law R, Benner WH, Pesavento JB, Cappuccio JA, Walsworth V, Kuhn EA, Corzett M, Chromy BA, Segelke BW, Coleman MA, Bench G, Hoeprich PD, Sulchek TA. Quantifying size distributions of nanolipoprotein particles with single-particle analysis and molecular dynamic simulations. J. Lipid Res. 2008;49:1420–1430. doi: 10.1194/jlr.M700586-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Gubler D, Kuno G, Markoff L. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 37.Widman DG, Ishikawa T, Fayzulin R, Bourne N, Mason PW. Construction and characterization of a second-generation pseudoinfectious West Nile virus vaccine propagated using a new cultivation system. Vaccine. 2008;26:2762–2771. doi: 10.1016/j.vaccine.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Tsai TF. Inactivated Japanese encephalitis virus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) M. M. W. R. Recomm. Rep. 1993;42:1–15. [PubMed] [Google Scholar]
- 39.Aberle JH, Aberle SW, Allison SL, Stiasny K, Ecker M, Mandl CW, Berger R, Heinz FX. A DNA immunization model study with constructs expressing the tick-borne encephalitis virus envelope protein E in different physical forms. J. Immunol. 1999;163:6756–6761. [PubMed] [Google Scholar]
- 40.Fonseca BAL, Pincus S, Shope RE, Paoletti E, Mason PW. Recombinant vaccinia viruses co-expressing dengue-1 glycoproteins prM and E induce neutralizing antibodies in mice. Vaccine. 1994;12:279–285. doi: 10.1016/0264-410x(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 41.Konishi E, Pincus S, Paoletti E, Shope RE, Burrage T, Mason PW. Mice immunized with a subviral particle containing the Japanese encephalitis virus prM/M and E proteins are protected from lethal JEV infection. Virology. 1992;188:714–720. doi: 10.1016/0042-6822(92)90526-u. [DOI] [PubMed] [Google Scholar]
- 42.Mason PW, Pincus S, Fournier MJ, Mason TL, Shope RE, Paoletti E. Japanese encephalitis virus-vaccinia recombinants produce particulate forms of the structural membrane proteins and induce high levels of protection against lethal JEV infection. Virology. 1991;180:294–305. doi: 10.1016/0042-6822(91)90034-9. [DOI] [PubMed] [Google Scholar]
- 43.Pincus S, Mason PW, Konishi E, Fonseca BAL, Shope RE, Rice CM, Paoletti E. Recombinant vaccinia virus producing the prM and E proteins of yellow fever virus protects mice from lethal yellow fever encephalitis. Virology. 1992;187:290–297. doi: 10.1016/0042-6822(92)90317-i. [DOI] [PubMed] [Google Scholar]
- 44.Qiao M, Ashok M, Bernard KA, Palacios G, Zhou ZH, Lipkin WI, Liang TJ. Induction of sterilizing immunity against west nile virus (WNV), by immunization with WNV-like particles produced in insect cells. J. Infect. Dis. 2004;190:2104–2108. doi: 10.1086/425933. [DOI] [PubMed] [Google Scholar]
- 45.Chu JHJ, Chiang CCS, Ng ML. Immunization of flavivirus West Nile recombinant envelope domain III protein induced specific immune response and protection against West Nile virus infection. J. Immunol. 2007;178:2699–2705. doi: 10.4049/jimmunol.178.5.2699. [DOI] [PubMed] [Google Scholar]
- 46.Lieberman MM, Nerurkar VR, Luo HY, Cropp B, Carrion R, de la Garza M, Coller BA, Clements D, Ogata S, Wong T, Martyak T, Weeks-Levy C. Immunogenicity and Protective Efficacy of a Recombinant Subunit West Nile Virus Vaccine in Rhesus Monkeys. Clin. Vaccine Immunol. 2009;16:1332–1337. doi: 10.1128/CVI.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martina BE, Koraka P, van den Doel P, van Amerongen G, Rimmelzwaan GF, Osterhaus A. Immunization with West Nile virus envelope domain III protects mice against lethal infection with homologous and heterologous virus. Vaccine. 2008;26:153–157. doi: 10.1016/j.vaccine.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonald WF, Huleatt JW, Foellmer H, Hewitt D, Tang J, Desai P, Price A, Takahashi VN, Huang Y, Nakaar V, Alexopoulou L, Fikrig E, Powell TJ. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J. Infect. Dis. 2007;195:1607–1617. doi: 10.1086/517613. [DOI] [PubMed] [Google Scholar]
- 49.Mason PW, Shustov AV, Frolov I. Production and characterization of vaccines based on flaviviruses defective in replication. Virology. 2006;351:432–443. doi: 10.1016/j.virol.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.