Abstract
We previously reported that the endogenous ATP-binding cassette transporter (ABC)A7 strongly associates with phagocytic function rather than biogenesis of high-density lipoprotein (HDL), being regulated by sterol-regulatory element binding protein (SREBP)2. Phagocytic activity was found enhanced by apolipoprotein (apo)A-I and apoA-II more than twice the maximum in J774 and mouse peritoneal macrophages. Therefore we investigated the molecular basis of this reaction in association with the function of ABCA7. Similar to ABCA1, ABCA7 was degraded, likely by calpain, and apoA-I and apoA-II stabilize ABCA7 against degradation. Cell surface biotinylation experiments demonstrated that endogenous ABCA7 predominantly resides on the cell surface and that the apolipoproteins increase the surface ABCA7. The increase of phagocytosis by apolipoproteins was retained in the J774 cells treated with ABCA1 siRNA and in the peritoneal macrophages from ABCA1-knockout mice, but it was abolished in the J774 cells treated with ABCA7 siRNA and in the peritoneal macrophages from ABCA7-knockout mice. Phagocytosis was decreased in the cells in the peritoneal cavity of the ABCA7-knockout mouse compared with the wild-type control. We thus concluded that extracellular helical apolipoproteins augment ABCA7-associated phagocytosis by stabilizing ABCA7. The results demonstrated direct enhancement of the host defense system by HDL components.
Keywords: ABCA7, apoA-I, HDL, phagocytosis, ABCA1, cholesterol
ATP-binding cassette transporter (ABC)A7 (1) is a membrane protein highly homologous to ABCA1 that mediates biogenesis of high-density lipoprotein (HDL) with cellular lipid and helical apolipoproteins (2–7). ABCA7 mediates formation of HDL when exogenously transfected and expressed (8–10). However, endogenous ABCA7 was shown to have no significant impact on generation of HDL (11–13) and was found associated with phagocytosis (13, 14). Interestingly, expression of ABCA7 is regulated by sterol-regulatory element binding protein (SREBP)2 (13), in an opposite direction to the regulation of the ABCA1 gene by the liver X receptor (LXR) with respect to the induction by change in cellular sterol level. Given these divergent findings, function of ABCA7 may be involved in more fundamental cellular processes other than just elimination of cell cholesterol.
Phagocytosis is one of the fundamental functions of animal cells. It is an important responsive reaction to infection, injury, and apoptosis. Rapid and efficient clearance of foreign bodies/exogenous materials or apoptotic cells by phagocytic reactions of scavenger cells prevents the release of proinflammatory components. Some ABC transporters were reported to relate to the phagocytic function of cells (15–18). It is important to investigate the involvement of ABCA7 in phagocytic function in detail, especially as the ABCA7 gene is apparently regulated by a sterol- related mechanism, to shed light on the relationship between sterol homeostasis and the host defense system.
MATERIALS AND METHODS
Reagents and antibodies
Apolipoporteins (apo)A-I and apoA-II were isolated from human plasma HDL (19, 20). Cycloheximide was purchased from Wako. Calpeptin was purchased from Calbiochem. Antibodies against HSP110 (610510) and BIP/GRP78 (610978) were purchased from BD Transduction Laboratories. Anti-ATPase (Na/K) α1 antibody (NB300-146) and anti β-actin antibody (A5441) were from Novus and Sigma, respectively. Monoclonal antibodies against mouse ABCA1 (MABI98-4) and ABCA7 (MABI97-17) were generated in rats against peptides corresponding to the C terminus of each protein, NF AKD QSD DDH LKD LSL HKN (ABCA1) (21) and PG RQH PKR VSR FLE DPS SVE TMI (ABCA7) (13), respectively, at the MAB Institute (Yokohama, Japan) as described previously.
Animals and cells
ABCA7-knockout mouse was generated and backcrossed to C57BL/6 background more than 10 times at Lipid Metabolism Unit and Partners Center for Genetics and Genomics, Massachusetts General Hospital (11), and bred on the same background at the Animal Experiment Facility of Nagoya City University Graduate School of Medical Sciences. ABCA1-knockout mice were bred from the heterozygous mice (DBA/1-Abca1tm1jdm/J) (22) purchased from Jackson's Animal Laboratories (Stony Brook, NY) and backcrossed at least 10 times to make the background of C57BL/6 at the Nagoya City University facility (21). Each mouse-derived fibroblast was obtained from the embryos between 12.5 and 14.5 days, and each cell line was established after the thirtieth subculture. The cells were cultured in DF medium (1:1 mixture of DMEM and Ham's F12 medium) with 10% fetal calf serum (10% FCS/DF). Mouse resident peritoneal macrophages were obtained from over 11-week-old mice after overnight starvation. The cells were seeded in a 96-well tray and cultured in 10% FCS/RPMI1640 medium. J774 cells and BALB/3T3 cells were obtained from the Riken Cell Bank and cultured in 10% FCS/RPMI1640 medium and 10% FCS/DF medium, respectively. All cells were maintained at 37°C in humidified atmosphere of 5% CO2. The animal experiment protocols were approved by the Institutional Animal Welfare Committee.
Cell surface biotinylation assay
Surface ABCA7 was probed by its biotinylation (23, 24). J774 cells, BALB/3T3 cells, and mouse fibroblasts were subcultured at a density of each 3 × 106, 1 × 106, and 2 × 106 cells/60 mm dish for one day. After overnight incubation in the presence of apoA-I, 10 μg/ml, cells were washed twice with ice-cold Dulbecco's PBS containing 1 mM CaCl2 and 1 mM MgCl2 (PBS-CM), and surface protein was biotinylated with sulfosuccinimidobiotin (sulfo- NHS-SS-biotin; Pierce) for 30 min at 4°C. The biotinylation reaction was quenched for 10 min at 4°C by removal of the sulfo-NHS-SS-biotin solution. Cells were washed with ice-cold PBS-CM, lysed with RIPA buffer, and homogenized. Then 150 μg of protein was added to 40 μl of streptavidin-Sepharose beads (NeutrAvidin Agarose Resin; Thermo Scientific) and incubated for 1 h on a platform mixer at 4°C. After pull-down of streptavidin, the supernatant was incubated with another 40 μl of streptavidin-Sepharose beads for 1 h on a platform mixer at 4°C. The pellet (biotinylated) and supernatant (not biotinylated) were prepared for Western blotting.
RNA interference
SiRNAs (Stealth Select RNAi) for ABCA1 and ABCA7 were purchased from Invitrogen. They were transfected by nucleofection (Nucleofector Kit V for J774, MEF Nucleofector Kit 2 for mouse fibroblast; AMAXA Biosystems). Two different siRNAs were tested for each and yielded similar results. The data presented represent a typical set.
Cellular lipid and lipid release assay
J744 cells were subcultured in a 6-well tray in 10% FCS/RPMI1640 medium for two days. The cells were washed with PBS and incubated in 1 ml/well of the RPMI1640 medium containing 0.02% BSA for 24 h in the presence of 10 μg/ml apoA-I. Lipid content of cells was determined by colorimetric enzymatic assay (25). Mouse fibroblasts were treated with ABCA7 siRNA and incubated in 0.02% BSA/DF medium overnight. The cells were then incubated with 10 μg/ml apoA-I in the medium for 24 h, and lipid in the medium was measured (25). Cellular protein was dissolved in RIPA buffer and then determined with the BCA Protein Assay Kit (Pierce).
Quantitative analysis of mRNA
Total RNA was isolated by ISOGEN (Wako) and reverse-transcribed by SuperScript III (Invitrogen) with oligo dT primers. Quantitative expression analysis by real-time reverse transcription PCR was performed in StepOnePlus Real-Time PCR system (Applied Biosystems) using SYBR Green technology. The following PCR primers were used for amplification of mouse RNAs: ABCA7, 5′-GCC AGT ATG GAA TCC CTG AA-3′ (forward) and 5′-ATG GAG ACA CCA GGA ACC AG-3′ (reverse); β-actin, 5′- CTG TAT TCC CCT CCA TCG TG-3′ (forward) and 5′-AGG TGT GGT GCC AGA TCT TC-3′ (reverse).
Quantitative phagocytosis assay
We used fluorescent polystyrene microspheres, 1 μm diameter, with carboxylate groups on the surface (15702, Polysciences) that could be activated for the covalent coupling of proteins to quantify in vitro phagocytosis. Alternatively, Staphylococcus aureus conjugate of Alexa Flour 488 (S23371, Invitrogen) was used as a fluorescent phagocytosis probe. For polystyrene beads uptake, J774 cells or mouse peritoneal macrophages subcultured in a 96-well tray in 10% FCS/RPMI1640 medium were washed twice with PBS, and then incubated with 50 μl of 0.02% BSA/RPMI1640 medium, with or without 10 μg/ml apoA-I or apoA-II overnight at 37°C, and the same medium, 50 μl, was added. Another 50 μl of each medium containing 4.55 × 106 of the microspheres preincubated in the medium containing 10 μg/ml apoA-I, apoA-II, or BSA at 37°C for 1 h was added 30 min later and incubated for 1 h. Alternatively, microspheres precoated with apoA-I or apoA-II as above were washed before adding to the cells pretreated without apolipoproteins. Cells were washed carefully four times with PBS to remove remaining extracellular beads, and then fixed by 4% paraformaldehyde. Photographs were taken at 10 regular positions of each well at 40-fold magnification in a fluorescence microscope BZ-800 (Keyence) with a Plan Fluor ELWD 20× lens (Nicon). Numbers of cells and engulfed beads in the cells were counted in the fields, and the average number of beads engulfed per cell was calculated. For Staphylococcus aureus uptake, J744 cells were cultured in a 24-well tray as above and incubated with 6 × 106 bacteria/well for 1 h. Cells were washed thoroughly and carefully with PBS twice, and then treated with 200 μl of 1 mg/ml lysozyme for 25 min at 37°C. After washing cells, fluorescence intensity was measured in a plate reader FL600 (BioTek Inc.). Validity of the method was confirmed by inhibition of the phagocytic reaction by adding 10 μM of cytochalasin D (Merck), a known phagocytosis inhibitor, for 30 min at 37°C before the phagocytic assay (by 33% and 60% significant inhibition for J774 cells and mouse peritoneal macrophages, respectively).
In vivo phagocytosis assay
Carbon ink (Platinum Carbon Ink Black) was diluted 10-fold with PBS and injected into the mouse peritoneal cavity by 10 μl/g body weight. After overnight starvation of mice, peritoneal macrophages were recovered. Over 400 cells of each sample were counted, and the phagocytosis index was calculated as the relative number of cells that engulfed carbon ink particles per total cells.
Other methods
Western blotting was performed as described previously (21) by using loading controls of Na/K ATPase as a membrane protein and β-actin as a housekeeping cellular protein. Chemiluminescence signal of the bands were integrated by an LAS-3000mini Image Reader (Fuji Film) and analyzed by Multi Gauge v. 3.0 software (Fuji Film). Density of the ABCA7 band was largely proportional to dose. Samples for polyacrylamide gel electrophoresis were treated with RIPA buffer containing protease inhibitors cocktail (Sigma P2714) unless described otherwise. Data were statistically analyzed by Wilcoxon signed rank test, t-test, or one-way ANOVA with Tukey's test. P < 0.05 was accepted as statistically significant.
RESULTS
We developed a quantitative phagocytosis assay system using carboxylate polystyrene microspheres (see “Materials and Methods”) and examined the effects of apolipoproteins on phagocytotic uptake of the spheres. In the presence of apoA-I or apoA-II, uptake of the microspheres by J774 cells and peritoneal macrophages prepared from wild-type mice was increased (Fig. 1A, B). ApoA-I facilitated phagocytosis in a dose-dependent manner in both of these cell types (Fig. 1A, B). Inhibition of the reaction by cytochalasin D confirmed that this is a phagocytic reaction. The pretreatment of the microspheres by coating with apoA-I, apoA-II, or BSA (as a control) for 1 h resulted in irreversible binding of apolipoproteins (data not shown) but did not enhance the phagocytosis of J774 cells (Fig. 1C) or mouse peritoneal macrophages (data not shown), indicating this effect was not mediated by the apolipoproteins bound to the beads.
Fig. 1.
Enhancement of phagocytosis by apoA-I and apoA-II. J774 cells (A) were subcultured in a 96-well tray at a density of 4 × 103cells/well for one day. Mouse peritoneal macrophages (B) were subcultured in a 96-well tray at a density of 1 × 104cells/well for six days. After overnight incubation in the presence or absence of 10 μg/ml of apoA-I or apoA-II, quantitative phagocytosis assay was performed as described. The results were expressed as phagocytic activity relative to the blank background control (without apolipoprotein, 0.02% BSA). Dose-dependent data of apoA-I (μg/ml) are also displayed with a negative control of 10 μM CytD in the presence of apoA-I (10 μg/ml) to inhibit phagocytosis (A and B). The microspheres were precoated with apoA-I or apoA-II prior to incubation with J774 cells as described (C). The results are expressed as the activity relative to each control, activity of the cells for beads precoated and preincubated in 0.02% BSA only as the blank control. The data represent the means ± SD for eight samples. Statistical analysis was performed by Tukey's test to give significance levels of ***P < 0.001; **P < 0.01; *P < 0.05 to the control or between the data indicated. The microscopic photo data represent typical results of three independent experiments performed. ApoA, apolipoprotein A; CytD, cytochalasin D.
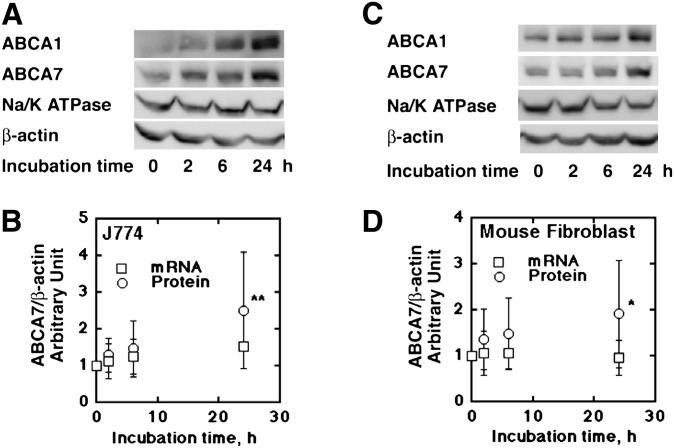
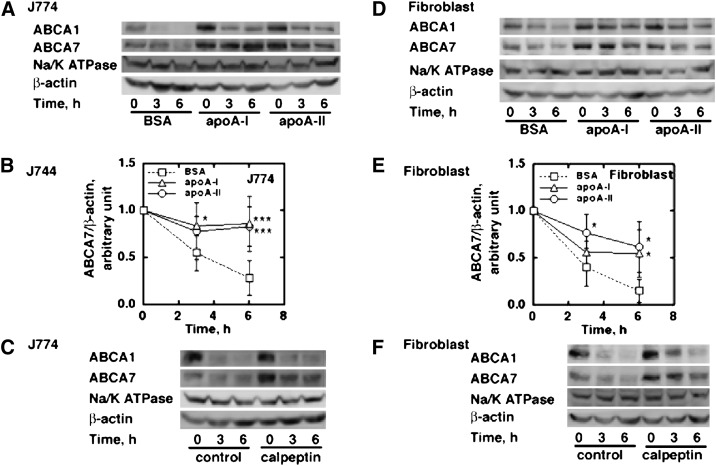
ABCA7 increased upon incubation with apoA-I in a time-dependent manner in J774 cells and mouse fibroblasts (Fig. 2A, C). However, this increase was not accompanied by significant change in its mRNA (Fig. 2B, D), indicating that the effects were not on the transcriptional level. Decay of ABCA7 protein examined in the presence of the protein synthesis inhibitor cycloheximide was retarded by apoA-I and apoA-II, similar to the findings with ABCA1 (Fig. 3A, B, D, E). Calpeptin increased ABCA7 protein as well as ABCA1 protein by retarding the decaying rate (Fig. 3C, F) (with significance of P < 0.05 for three experiments at each time point), indicating that calpain is likely to mediate degradation of ABCA7 similar to ABCA1 (24, 26). In our previous work, immunofluorescent probing indicated that endogenous ABCA7 may be located intracellularly (13). To seek greater clarity, ABCA7 in the cell surface was estimated by a biotinylation technique (24) in J774 cells, mouse fibroblasts, and BALB/3T3 cells as described in “Materials and Methods.” A large portion of endogenous ABCA7 was expressed in the surface cells (Fig. 4A). Incubation of cells with apoA-I increased cell surface ABCA7 (Fig. 4B).
Fig. 2.
Increase of ABCA7 by incubation of the cells with apoA-I. J774 cells (A and B) and mouse fibroblasts (C and D) in a 6-well tray (2 × 106 and 1 × 106, respectively) were pretreated overnight in the serum-free medium (RPMI1640 and DF, respectively) containing 0.02% BSA, and added with apoA-I, 10 μg/ml. Proteins and mRNAs of ABCA1 and ABCA7 were analyzed at the time points of incubation with apolipoproteins as indicated. The mRNA was extracted and quantitative expression analysis was performed in a StepOnePlus Real-Time PCR system using SYBR Green technology. The protein was extracted with RIPA buffer for Western blotting (A and C). Chemiluminescence of ABCA7 band was quantified as described. Results were expressed as fold change relative to the mRNA level and the protein level at 0 h (B and D). Data represent the mean ± SD for 12 samples. Statistical analysis was performed by Wilcoxon signed rank test, to give significance levels of **P < 0.01; *P < 0.05 to the zero time data. ABC, ATP-binding cassette transporter; ApoA, apolipoprotein A.
Fig. 3.
Degradation of ABCA7 in J774 cells (A–C) and mouse fibroblasts (D–F). A and D: The cells were pretreated overnight with apoA-I or apoA-II in a 6-well tray (2 × 106 and 1 × 106, respectively). BSA indicates blank background control of 0.02% BSA. Protein synthesis was inhibited by cycloheximide, 50 μg/ml, and proteins were analyzed by Western blotting for ABCA7 and ABCA1 at 0, 3, and 6 h of incubation. B and E: ABCA7 bands in the experiments above were quantified as described. Data represent the mean ± SD for six samples. Statistical analysis by t-test against the BSA control at each incubation time gave significance levels of ***P < 0.001; **P < 0.01; *P < 0.05. C and F: After overnight starvation of J774 cells and mouse fibroblasts in the BSA control condition, the cells were preincubated with and without calpeptin, 50 μM, for 1 h, and then incubated for 0, 3, and 6 h under inhibition of protein synthesis by cycloheximide. Protein was analyzed by Western blotting for ABCA7 and ABCA1. ABC, ATP-binding cassette transporter; ApoA, apolipoprotein A.
Fig. 4.
Localization of endogenous ABCA1 and ABCA7. Surface protein was biotinylated with sulfo-NHS-SS-biotin for 30 min at 4°C for the cells in 60 mm dish. The biotinylated (surface) and nonbiotinylated (intracellular) proteins were prepared for Western blotting after the treatment of cells with biotin as described. The letter “W” indicates the analysis of the whole cell lysate without biotynilation. A: J774 cells and mouse fibroblasts were analyzed after the treatment with various concentrations of biotin for ABCA7 (the data represent the mean ± SD). B: J774 cells and BALB/3T3 cells were preincubated overnight in the absence and presence of apoA-I, 10 μg/ml, prior to biotinylation (with 1 mM biotin). HSP110 and BIP/GRP78 are controls for intracellular protein. Lower bands should be considered as ABC proteins according to their molecular weights. ABC, ATP-binding cassette transporter.
In these experiments, there was no significant change in cellular lipid in J774 cells (supplementary Fig. I), so the effect of apolipoproteins was not directly related to sterol-mediated transcriptional regulation of ABCA7. ApoA-I had no influence on glycosylation of ABCA1 or ABCA7 (supplementary Fig. II).
ABCA7 was downregulated by specific siRNAs of ABCA7 in mouse fibroblasts as described in “Materials and Methods” (supplementary Fig. III-A), and apoA-I-mediated cell lipid release was measured. No change was observed by knockdown of ABCA7 in release of either cholesterol or phospholipid (supplementary Fig. III-B). Thus, endogenous ABCA7 did not play a significant role in apolipoprotein-mediated lipid release or generation of HDL, which was consistent with our previous findings in BALB/3T3 cells (13).
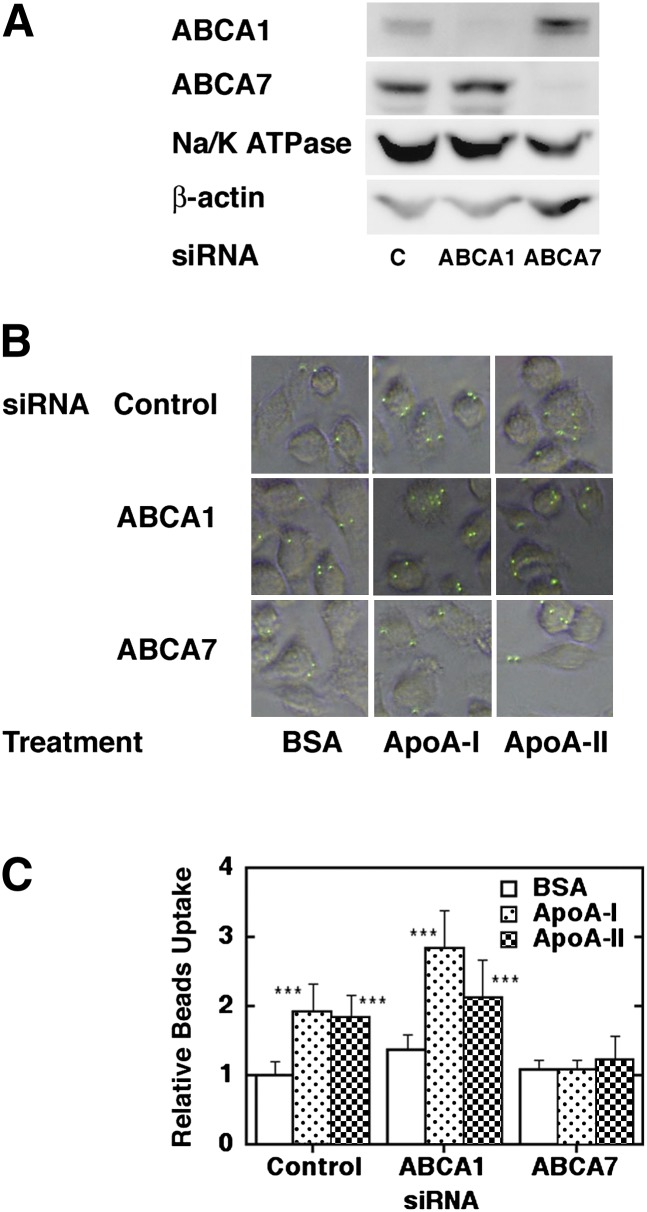
Expression of ABCA1 or ABCA7 was downregulated by siRNA in J774 (Fig. 5A), and quantitative phagocytosis assay was performed. Phagocytosis enhancement by apoA-I and apoA-II was ablated in the cells by knockdown of ABCA7 but remained after knockdown of ABCA1 (Fig. 5B, C). Uptake of Staphylococcus aureus was also examined in J775 cells (Fig. 6). It was also enhanced by apoA-I, but knockdown of ABCA7 abolished this effect, reproducing the results with polystyrene beads.
Fig. 5.
Knockdown of ABCA7 and ABCA1 and phagocytosis. siRNA specific for ABCA7 and ABCA1 was transfected into J774 cells at a density of 6.2 × 106 cells/cuvette. The cells were seeded at 3 × 106/well in a 6-well tray for SDS-PAGE analysis (A), and the remaining cells were subcultured in a 96-well tray at a density of 1 × 105 cells/well. After overnight incubation in the presence or absence of 10 μg/ml apoA-I and apoA-II, quantitative phagocytosis assay was performed (B). The results were shown as phagocytic activity relative to that of control siRNA without apoA-I (0.02% BSA) (C). The data represent the mean ± SD for eight samples. Statistical analysis was performed by Tukey's test to give significance level of ***P < 0.001 to the BSA data of each group. The microscopic photo data represent a typical set of the two independent experiments preformed. ABC, ATP-binding cassette transporter.
Fig. 6.
Phagocytosis of Staphylococcus aureus by J774 cells. The cells were seeded in a 24-well plate and incubated with or without apoA-I (10 μg/ml) overnight. CytD (10 μM) was added to the apoA-I samples for 30 min as a negative control. ABCA7 expression was knocked down as described in Fig. 5. Cell density was 5 × 104/well and 1 × 106/well for CytD and siRNA experiments, respectively. The medium containing Staphylococcus aureus was added to each well and incubated for 1 h. After careful and thorough washing, the cells were treated with lysozyme (2 mg/ml × 200 μl, for 25 min at 37°C) to remove remaining extracellular Staphylococcus aureus, and fluorescence intensity was measured by a plate reader. A: Increase by apoA-I and inhibition by CytD of the phagocytic uptake. B: Canceling the effect of apoA-I on the phagocytosis by knockdown of ABCA7 expression by a specific siRNA. The microscopic photos represent a typical set of the two independent experiments. The data in the graph represent the results of their quantification as the mean ± SD for four samples. Statistical analysis was performed by Tukey's test to give significance levels of **P < 0.01 and ***P < 0.001 to the blank background control (C) of 0.02% BSA. ABC, ATP-binding cassette transporter; ApoA, apolipoprotein A; CytD, cytochalasin D.
Enhancement of phagocytosis by apoA-I and apoA-II was retained in the peritoneal macrophages prepared from ABCA1-knockout mice, whereas it was lost in those obtained from ABCA7-knockout mice (Fig. 7A, B). The effect of apoA-I on phagocytosis was demonstrated in the peritoneal macrophages of ABCA1-knockout mice in a dose-dependent manner but not in the peritoneal macrophages of ABCA7-knockout mice (Fig. 7B). In aggregate, these results indicate that apolipoproteins stimulate macrophage phagocytosis and that this stimulation depends on ABCA7, but not ABCA1, expression. Phagocytic activity of macrophages in mice peritoneal cavity in vivo was examined by measuring the uptake of carbon microparticles after injecting them into the peritoneal cavity. Uptake of the particles by the peritoneal cells was decreased in ABCA7-knockout mice compared with that in the wild-type mice (Fig. 7C). Uptake of the ink particles was also decreased in ABCA1-knockout mice. Expression of ABCA7 was decreased in ABCA1-knockout mice peritoneal cells in vivo (Fig. 7C), although it was not decreased in the peritoneal macrophages transferred to the culture (Fig. 7A, B), agreeing with the apparent decrease in phagocytosis in vivo. The decrease of phagocytosis in ABCA1-knockout mice could also be consistent with a rational assumption that HDL and, consequently, apoA-I levels in the peritoneal fluid should extremely be low in these mutant mice (21), while they should be unchanged in ABCA7-knockout mice (11) from their plasma HDL levels.
Fig. 7.
Phagocytosis by the peritoneal macrophages prepared from the ABCA1-knockout mouse and the ABCA7-knockout mouse. A: Peritoneal macrophages were collected from the ABCA7-knockout mouse and the ABCA1-knockout mouse. The cells were subcultured in a 96-well tray at a density of 3 × 104 and 4 × 104 cells/well, respectively, for four days. After overnight incubation in the presence or absence of 10 μg/ml apoA-I or apoA-II, quantitative phagocytosis assay was performed. B: The results were shown as phagocytic activity relative to that without apolipoproteins (0.02% BSA as a background) (left). Dose-dependent data on apoA-I (μg/ml) are also displayed as phagocytic activity relative to the blank background control (BSA) with a negative control of 10 μM CytD in the presence of apoA-I (10 μg/ml) to inhibit phagocytosis (right). The data represent the means ± SD for eight samples. The data represent a typical set of three independent experiments performed. C: Phagocytic activity was measured directly in the peritoneal cavity of the mice in vivo. Diluted carbon ink was injected into the mouse peritoneal cavity as described. After overnight starvation of mice, peritoneal macrophages were recovered and over 400 cells were counted for calculation of phagocytosis index as a relative number of cells that engulfed carbon ink particles. Data represents mean ± SD of n = 9, 6, and 23 for wild-type, ABCA1-knockout, and ABCA7-knockout mice, respectively. Statistical analysis was performed by Tukey's test to give significance levels of ***P < 0.001; **P < 0.01 compared to the control or between the groups indicated. Western blotting data show expression of ABCA1 and ABCA7 in mouse peritoneal cells from wild-type, ABCA1-knockout, and ABCA7-knockout mice, analyzed immediately after collection. J774 cells were analyzed as a control. ABC, ATP-binding cassette transporter; ApoA, apolipoprotein A; CytD, cytochalasin D; KO, knockout.
DISCUSSION
The findings in this study are summarized as follows: (1) We confirmed that endogenous ABCA7 is strongly associated with cellular phagocytic function; (2) Endogenous ABCA7 is predominantly located on the cell surface, and extracellular helical apolipoproteins, such as apoA-I and apoA-II, stabilize it against the degradation presumably mediated by calpain; and (3) Helical apolipoproteins increase surface ABCA7 and enhance phagocytic function. The results thus indicated direct involvement of HDL apolipoproteins in regulation of the host defense system through modulation of the ABCA7 function.
Association of ABCA7 expression with phagocytic function of cells rather than cholesterol release is consistent with previous observations (13, 14). The current study, however, demonstrated that ABCA7 is expressed predominantly on the cell surface, which is inconsistent with our previous conjecture based on immunofluorescence staining (13) and the inconclusive findings in other reports (12, 14). Nevertheless, the present findings by surface biotinylation and stabilization of ABCA7 by extracellular apolipoproteins against calpain strongly support its surface localization and not our previous data. This discrepancy may be explained if ABCA7 rapidly turns over in the surface. An exon 5 splicing variant of ABCA7 was found to remain intracellularly (27), but the primers and antibody employed in the current study for detection of ABCA7 mRNA and protein do not differentiate the variant. The current findings with ABCA7 is very similar to ABCA1, stabilized against its calpain-mediated degradation by helical apolipoproteins when the HDL biogenesis reaction is ongoing (26, 28, 29), perhaps forming a complex prior to the endocytotic internalization for its intracellular proteolysis to recycle ABCA1 to the cell surface (24). HDL apolipoproteins were shown to stabilize ABCA7 in an apparently similar manner and to enhance phagocytosis, although the detailed mechanism has not been demonstrated. This hypothesis should be confirmed by more direct evidence, such as a pulse-labeling experiment of ABCA7. Phagocytosis is one of the most primitive but important host defense reactions, and the results indicate its link with HDL metabolism and, therefore, indirect link with cholesterol homeostasis.
ABCA1, a key protein for cellular cholesterol release, generates HDL particles from cellular phospholipids and cholesterol (30). Its gene is relevantly upregulated by LXR (31), and its activating ligand is oxysterol (32), a signal for the increase of cellular cholesterol (33). However, as ABCA1 is also negatively regulated by sterol/SREBP2 in hepatocytes (34), perhaps its opposite regulation is needed in the liver to maintain cholesterol homeostasis for the whole body. ABCA7, a protein highly homologous to ABCA1 (1), mediates generation of HDL, but it is cholesterol-poor with helical apolipoproteins only when transfected and overexpressed (8–10). However, endogenous ABCA7 does not support HDL generation (11–13), and it was associated with phagocytic function (13, 14). The ABCA7 gene is regulated by SREBP2 (13), so ABCA7 may be a key molecule to link cellular cholesterol homeostasis and the host defense system.
Association of ABCA7 with phagocytosis was demonstrated at least in fibroblast (13) and macrophage cells or cell lines for polystyrene beads, Staphylococcus aureus, and carbon microparticles. Our preliminary experiments yielded similar results with zymosan and Escherichia coli, indicating that ABCA7 seems to be a fundamental factor involved in broad spectrum of the phagocytic pathways, although it is not evident yet. Nevertheless, the present findings are additional evidence that ABCA7 links cholesterol metabolism and the host defense system through its interaction with the HDL components.
Helical apolipoproteins of HDL, such as apoA-I, stabilized ABCA7 similar to ABCA1, and they increased the function of this membrane protein. The current findings provided a strong indication with a concrete molecular background that HDL plays a role in regulating cellular phagocytic function through the action of ABCA7. Helical apolipoproteins are carried by HDL, and they may dissociate from the lipoprotein particles in equilibrium (35). The presence of HDL in interstitial fluid always provides a certain amount of free apolipoproteins, especially apoA-I, and the interaction of free apoA-I with ABCA1 was shown to be responsible for HDL biogenesis (36). It is quite relevant to assume that HDL also provides ABCA7 with free apoA-I to stabilize it and enhance phagocytosis. When the cells are exposed to HDL, helical apolipoprotein dissociated from HDL interacts with ABCA1, directly or indirectly, to stabilize it and generate HDL, and to reduce cell cholesterol. Consequently, ABCA7 is upregulated via the SREBP2 system. HDL also stabilizes ABCA7, perhaps similarly to ABCA1, resulting in the increase of ABCA7 as shown in this study. It is not completely clear yet whether helical apolipoproteins bind directly to ABCA1 or ABCA7 to achieve these effects (9, 24), and it is possible that the ABC transporters may be stabilized by alteration of membrane microenvironment by modification of its lipid composition. This is a new insight to relationship among cholesterol metabolism, helical apolipoproteins, and the host defense system. The present observations may be related to other, previous reports indicating association of macrophage functions with its cholesterol homeostasis (37–39).
Interesting findings were described for human ABCA7: it contains the extracellular domain homologous to SS-N, an epitope of Sjögren's syndrome (40), and ABCA7-positive cells were detected in plasma cells from the salivary glands of patients with Sjögren's syndrome (41). The incidence of single nucleotide polynucleotide allele of HA1 was different between Sjögren syndrome patients and control patients (42). These findings may not link directly to our observations, but they might suggest a relationship between ABCA7 and the immune system.
Supplementary Material
Acknowledgments
The authors thank Rika Hayashi and Haruka Hayashi for macrophage preparation and mouse genotyping; Shizuka Nishimoto for establishing fibroblast cell lines; and Mariko Hato and Kuniko Okumura-Noji for preparation of human apolipoproteins.
Footnotes
Abbreviations:
- ABC
- ATP-binding cassette transporter
- ApoA
- apolipoprotein A
- CytD
- cytochalasin D
- LXR
- liver X receptor
- sulfo-NHS-SS-biotin
- sulfosuccinimidobiotin
- SREBP
- sterol regulatory element binding protein
This study was supported by grants-in-aid and by the program for developing the supporting system for upgrading the education and research from the Ministry of Education, Science, Technology, Culture and Sports and Ministry of Health, Welfare and Labor of Japan; by the program for promotion of fundamental studies in health sciences of the National Institute of Biomedical Innovation of Japan; and by grant-in-aid for research at Nagoya City University of Japan. Generation of the ABCA7-knockout mice was funded by National Institutes of Health Grant HL-074136 (M.L.F.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Kaminski W. E., Orso E., Diederich W., Klucken J., Drobnik W., Schmitz G. 2000. Identification of a novel human sterol- sensitive ATP-binding cassette transporter (ABCA7). Biochem. Biophys. Res. Commun. 273: 532–538. [DOI] [PubMed] [Google Scholar]
- 2.Hara H., Yokoyama S. 1991. Interaction of free apolipoproteins with macrophages. Formation of high density lipoprotein-like lipoproteins and reduction of cellular cholesterol. J. Biol. Chem. 266: 3080–3086. [PubMed] [Google Scholar]
- 3.Francis G. A., Knopp R. H., Oram J. F. 1995. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier Disease. J. Clin. Invest. 96: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remaley A. T., Schumacher U. K., Stonik J. A., Farsi B. D., Nazih H., Brewer H. B., Jr 1997. Decreased reverse cholesterol transport from Tangier disease fibroblasts. Acceptor specificity and effect of brefeldin on lipid efflux. Arterioscler. Thromb. Vasc. Biol. 17: 1813–1821. [DOI] [PubMed] [Google Scholar]
- 5.Bodzioch M., Orso E., Klucken J., Langmann T., Bottcher A., Diederich W., Drobnik W., Barlage S., Buchler C., Porsch-Ozcurumez M., et al. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22: 347–351. [DOI] [PubMed] [Google Scholar]
- 6.Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O., et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22: 336–345. [DOI] [PubMed] [Google Scholar]
- 7.Rust S., Rosier M., Funke H., Real J., Amoura Z., Piette J. C., Deleuze J. F., Brewer H. B., Duverger N., Denefle P., et al. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22: 352–355. [DOI] [PubMed] [Google Scholar]
- 8.Abe-Dohmae S., Ikeda Y., Matsuo M., Hayashi M., Okuhira K., Ueda K., Yokoyama S. 2004. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J. Biol. Chem. 279: 604–611. [DOI] [PubMed] [Google Scholar]
- 9.Wang N., Lan D., Gerbod-Giannone M., Linsel-Nitschke P., Jehle A. W., Chen W., Martinez L. O., Tall A. R. 2003. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J. Biol. Chem. 278: 42906–42912. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi M., Abe-Dohmae S., Okazaki M., Ueda K., Yokoyama S. 2005. Heterogeneity of high density lipoprotein generated by ABCA1 and ABCA7. J. Lipid Res. 46: 1703–1711. [DOI] [PubMed] [Google Scholar]
- 11.Kim W. S., Fitzgerald M. L., Kang K., Okuhira K., Bell S. A., Manning J. J., Koehn S. L., Lu N., Moore K. J., Freeman M. W. 2005. Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J. Biol. Chem. 280: 3989–3995. [DOI] [PubMed] [Google Scholar]
- 12.Linsel-Nitschke P., Jehle A. W., Shan J., Cao G., Bacic D., Lan D., Wang N., Tall A. R. 2005. Potential role of ABCA7 in cellular lipid efflux to apoA-I. J. Lipid Res. 46: 86–92. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto N., Abe-Dohmae S., Sato R., Yokoyama S. 2006. ABCA7 expression is regulated by cellular cholesterol through the SREBP2 pathway and associated with phagocytosis. J. Lipid Res. 47: 1915–1927. [DOI] [PubMed] [Google Scholar]
- 14.Jehle A. W., Gardai S. J., Li S., Linsel-Nitschke P., Morimoto K., Janssen W. J., Vandivier R. W., Wang N., Greenberg S., Dale B. M., et al. 2006. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J. Cell Biol. 174: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson P., Schlegel R. A. 2002. Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim. Biophys. Acta. 1585: 53–63. [DOI] [PubMed] [Google Scholar]
- 16.Hamon Y., Chambenoit O., Chimini G. 2002. ABCA1 and the engulfment of apoptotic cells. Biochim. Biophys. Acta. 1585: 64–71. [DOI] [PubMed] [Google Scholar]
- 17.Reddien P. W., Horvitz H. R. 2004. The engulfment process of programmed cell death in caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 20: 193–221. [DOI] [PubMed] [Google Scholar]
- 18.Jouret F., Devuyst O. 2009. CFTR and defective endocytosis: new insights in the renal phenotype of cystic fibrosis. Pflugers Arch. 457: 1227–1236. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama S., Tajima S., Yamamoto A. 1982. The process of dissolving apolipoprotein A-I in an aqueous buffer. J. Biochem. 91: 1267–1272. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama S., Tajima S., Yamamoto A. 1984. Association of apolipoprotein A-II with egg phosphatidylcholine unilamellar vesicles. J. Biochem. 96: 871–880. [DOI] [PubMed] [Google Scholar]
- 21.Hu W., Abe-Dohmae S., Tsujita M., Iwamoto N., Ogikubo O., Otsuka T., Kumon Y., Yokoyama S. 2008. Biogenesis of HDL by SAA is dependent on ABCA1 in the liver in vivo. J. Lipid Res. 49: 386–393. [DOI] [PubMed] [Google Scholar]
- 22.McNeish J., Aiello R. J., Guyot D., Turi T., Gabel C., Aldinger C., Hoppe K. L., Roach M. L., Royer L. J., de Wet J., et al. 2000. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl. Acad. Sci. USA. 97: 4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Boxberg Y., Wutz R., Schwarz U. 1990. Use of the biotin-avidin system for labelling, isolation and characterization of neural cell-surface proteins. Eur. J. Biochem. 190: 249–256. [DOI] [PubMed] [Google Scholar]
- 24.Lu R., Arakawa R., Ito-Osumi C., Iwamoto N., Yokoyama S. 2008. ApoA-I facilitates ABCA1 recycle/accumulation to cell surface by inhibiting its intracellular degradation and increases HDL generation. Arterioscler. Thromb. Vasc. Biol. 28: 1820–1824. [DOI] [PubMed] [Google Scholar]
- 25.Abe-Dohmae S., Suzuki S., Wada Y., Aburatani H., Vance D. E., Yokoyama S. 2000. Characterization of apolipoprotein-mediated HDL generation induced by cAMP in a murine macrophage cell line. Biochemistry. 39: 11092–11099. [DOI] [PubMed] [Google Scholar]
- 26.Arakawa R., Yokoyama S. 2002. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J. Biol. Chem. 277: 22426–22429. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda Y., Abe-Dohmae S., Munehira Y., Aoki R., Kawamoto S., Furuya A., Shitara K., Amachi T., Kioka N., Matsuo M., et al. 2003. Posttranscriptional regulation of human ABCA7 and its function for the apoA-I-dependent lipid release. Biochem. Biophys. Res. Commun. 311: 313–318. [DOI] [PubMed] [Google Scholar]
- 28.Arakawa R., Hayashi M., Remaley A. T., Brewer B. H., Yamauchi Y., Yokoyama S. 2004. Phosphorylation and stabilization of ATP binding cassette transporter A1 by synthetic amphiphilic helical peptides. J. Biol. Chem. 279: 6217–6220. [DOI] [PubMed] [Google Scholar]
- 29.Wang N., Chen W., Linsel-Nitschke P., Martinez L. O., Agerholm-Larsen B., Silver D. L., Tall A. R. 2003. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J. Clin. Invest. 111: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokoyama S. 2006. Assembly of high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 26: 20–27. [DOI] [PubMed] [Google Scholar]
- 31.Venkateswaran A., Laffitte B. A., Joseph S. B., Mak P. A., Wilpitz D. C., Edwards P. A., Tontonoz P. 2000. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc. Natl. Acad. Sci. USA. 97: 12097–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costet P., Luo Y., Wang N., Tall A. R. 2000. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 275: 28240–28245. [DOI] [PubMed] [Google Scholar]
- 33.Bjorkhem I., Diczfalusy U. 2002. Oxysterols: friends, foes, or just fellow passengers? Arterioscler. Thromb. Vasc. Biol. 22: 734–742. [DOI] [PubMed] [Google Scholar]
- 34.Tamehiro N., Shigemoto-Mogami Y., Kakeya T., Okuhira K., Suzuki K., Sato R., Nagao T., Nishimaki-Mogami T. 2007. Sterol regulatory element-binding protein-2- and liver X receptor-driven dual promoter regulation of hepatic ABC transporter A1 gene expression: mechanism underlying the unique response to cellular cholesterol status. J. Biol. Chem. 282: 21090–21099. [DOI] [PubMed] [Google Scholar]
- 35.Fukushima D., Yokoyama S., Kezdy F. J., Kaiser E. T. 1981. Binding of amphiphilic peptides to phospholipid/cholesterol unilamellar vesicles: a model for protein–cholesterol interaction. Proc. Natl. Acad. Sci. USA. 78: 2732–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okuhira K., Tsujita M., Yamauchi Y., Abe-Dohmae S., Kato K., Handa T., Yokoyama S. 2004. Potential involvement of dissociated apoA-I in the ABCA1-dependent cellular lipid release by HDL. J. Lipid Res. 45: 645–652. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Schwabe R. F., DeVries-Seimon T., Yao P. M., Gerbod-Giannone M. C., Tall A. R., Davis R. J., Flavell R., Brenner D. A., Tabas I. 2005. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J. Biol. Chem. 280: 21763–21772. [DOI] [PubMed] [Google Scholar]
- 38.Li Y., Gerbod-Giannone M. C., Seitz H., Cui D., Thorp E., Tall A. R., Matsushima G. K., Tabas I. 2006. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the Mer receptor. J. Biol. Chem. 281: 6707–6717. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y., Ishibashi M., Seimon T., Lee M., Sharma S. M., Fitzgerald K. A., Samokhin A. O., Wang Y., Sayers S., Aikawa M., et al. 2009. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ. Res. 104: 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka A. R., Ikeda Y., Abe-Dohmae S., Arakawa R., Sadanami K., Kidera A., Nakagawa S., Nagase T., Aoki R., Kioka N., et al. 2001. Human ABCA1 contains a large amino-terminal extracellular domain homologous to an epitope of Sjogren's Syndrome. Biochem. Biophys. Res. Commun. 283: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 41.Toda Y., Aoki R., Ikeda Y., Azuma Y., Kioka N., Matsuo M., Sakamoto M., Mori S., Fukumoto M., Ueda K. 2005. Detection of ABCA7-positive cells in salivary glands from patients with Sjogren's syndrome. Pathol. Int. 55: 639–643. [DOI] [PubMed] [Google Scholar]
- 42.Harangi M., Kaminski W. E., Fleck M., Orso E., Zeher M., Kiss E., Szekanecz Z., Zilahi E., Marienhagen J., Aslanidis C., et al. 2005. Homozygosity for the 168His variant of the minor histocompatibility antigen HA-1 is associated with reduced risk of primary Sjogren's syndrome. Eur. J. Immunol. 35: 305–317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.