Abstract
Sphingosine-1-phosphate (S1P) is a bioactive lysophospholipid that regulates numerous key cardiovascular functions. High-density lipoproteins (HDLs) are the major plasma lipoprotein carriers of S1P. Fibrinolysis is a physiological process that allows fibrin clot dissolution, and decreased fibrinolytic capacity may result from increased circulating levels of plasminogen activator inhibitor-1 (PAI-1). We examined the effect of S1P associated with HDL subfractions on PAI-1 secretion from 3T3 adipocytes. S1P concentration in HDL3 averaged twice that in HDL2. Incubation of adipocytes with increasing concentrations of S1P in HDL3, but not HDL2, or with S1P complexed to albumin stimulated PAI-I secretion in a concentration-dependent manner. Quantitative RT-PCR revealed that S1P1–3 are expressed in 3T3 adipocytes, with S1P2 expressed in the greatest amount. Treatment of adipocytes with the S1P1 and S1P3 antagonist VPC23019 did not block PAI-1 secretion. Inhibiting S1P2 with JTE-013 or reducing the expression of the gene coding for S1P2 using silencing RNA (siRNA) technology blocked PAI-1 secretion, suggesting that the S1P2 receptor mediates PAI-1 secretion from adipocytes exposed to HDL3 or S1P. Treatment with the phospholipase C (PLC) inhibitor U73122, the protein kinase C (PKC) inhibitor RO-318425, or the Rho-associated protein kinase (ROCK) inhibitor Y27632 all significantly inhibited HDL3- and S1P-mediated PAI-1 release, suggesting that HDL3- and/or S1P-stimulated PAI-1 secretion from 3T3 cells is mediated by activation of multiple, downstream signaling pathways of S1P2.
Keywords: plasminogen activator inhibitor-1, fibrinolysis, lipoprotein, high-density lipoprotein
Fibrinolysis is a physiological, antithrombotic process that allows dissolution of fibrin clots when the fibrin mesh is no longer needed for hemostasis. Decreased fibrinolytic capacity may result from a decrease in circulating levels of the activator of fibrinolysis, tissue plasminogen activator, or it may result from an increase in one of the main inhibitors of the reaction, plasminogen activator inhibitor-1 (PAI-1). A decreased fibrinolytic capacity as a result of increased circulating levels of PAI-1 is considered a cardiovascular risk factor (1, 2). There is clinical evidence that increased PAI-1 levels are associated with atherothrombosis (3), and increased levels have been identified as a predictor of myocardial infarction in epidemiological studies (1, 4). In addition to liver and vascular endothelium, PAI-1 is secreted by adipocytes cultured in vitro (5), and PAI-1 secreted by adipose tissue in vivo appears to be an important source of PAI-1 circulating in blood (6).
The expression of PAI-1 is regulated by various factors. Studies in vascular smooth muscle and endothelial cell cultures have shown that stimulation with some cytokines, such as transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), angiotensin II, or oxidized high-density lipoprotein (HDL), increased PAI-1 synthesis via activation of mitogen-activated protein kinase (MAPK), protein kinase C (PKC), and Rho kinase (7–10). Results from in vitro studies have shown that insulin and isolated lipoproteins, especially triglyceride-rich lipoproteins, stimulate PAI-1 production by cultured endothelial cells and hepatocytes (11, 12). Of particular relevance to the present study, sphingosine-1-phosphate (S1P) has been shown to increase the mRNA and protein expression of PAI-1 in glioblastoma cells (13).
S1P is a lysophospholipid that is emerging as key signaling molecule involved in the regulation of a variety of cellular functions, including cell growth and differentiation, proliferation, apoptotic cell death, and inflammation (14–19). S1P circulates at high nanomolar concentrations, with more than 50% of lipoprotein-associated S1P found in high-density lipoprotein (HDL) (20, 21) and with the greatest amounts associated primarily with small, dense HDL3 particles (20–22). Because of the high S1P concentrations found in HDL, this lipoprotein fraction has been considered a constant source of S1P which can mediate cellular signaling pathways that influence a multitude of cardiovascular functions (23). Regardless of the biological activity that is affected, the effects of extracellular S1P are clearly mediated through the activation of five specific G-protein-coupled receptors termed S1P1–5 (formerly termed endothelial differentiation gene [Edg]1, -3, -5, -6, and -8) (24). In this article, we report the biological effects of S1P and HDL-associated S1P on PAI-1 secretion in mouse 3T3 adipocytes, the specific S1P receptors that mediate the response, and the signaling pathways involved.
MATERIALS AND METHODS
Chemicals
Pertussis toxin (PTX), the protein kinase C (PKC) inhibitor Ro-31-8425, the phospholipase C (PLC) inhibitor U73122, and the Rho-associated protein kinase (ROCK) inhibitor Y27632 were obtained from Calbiochem-EMD Biosciences Inc. (San Diego, CA). The S1P2 antagonist JTE-013 and the S1P1,/S1P3/S1P4 inhibitor VPC23019 were purchased from Cayman Chemical (Ann Arbor, MI) and Avanti® Polar Lipids, Inc. (Alabaster, AL). Cell viability was evaluated using a commercially available luminescence-based assay (CellTiter-Glo® Luminecent Cell Viability Assay, Promega Corp., Madison, WI) and exceeded 89% in cultures in which chemical inhibitors were employed. S1P (Sigma-Aldrich, St. Louis, MO) was dissolved in methanol, a measured volume of the solution was transferred into a glass test tube, and the solvent was evaporated under a stream of nitrogen. The lipid residue was then dissolved in a solution containing fatty acid free BSA (0.4% w/v) in saline as recommended by the supplier, and this S1P-BSA solution was added to cultures at the indicated concentrations of S1P. S1P concentration in the solution was determined using the procedure described below for sphingolipid quantitation.
Isolation of HDL2 and HDL3
Blood (200 ml) was collected after an overnight fast from each of three or four donors who were free from clinically apparent disease in the presence of a lipoprotein preservative cocktail [EDTA (0.1% w/v), chloramphenicol (20 μg/ml), gentamycin sulfate (50 μg/ml), epsilon amino-caproic acid (0.13% w/v), and dithiobisnitrobenzoic acid (0.04% w/v)] to inhibit LCAT activity (final concentrations). Blood was then centrifuged (2,800 g, 20 min, 4°C) to obtain plasma. HDL2 (1.063<d<1.125 g/ml) and HDL3 (1.125<d<1.21 g/ml) were isolated by sequential ultracentrifugation of plasma with density adjusted with solid KBr in a Ti70 rotor (Beckman-Coulter Instruments, Palo Alto, CA) spun at 70,000 rpm for 18 h at 4°C. The floating HDL subfractions were harvested after tube slicing, and each isolated HDL subfraction was washed and concentrated by ultracentrifugation at its isolation density in a in a SW41 rotor (41,000 rpm, 36 h, 4°C). HDL subfractions were dialyzed against saline/EDTA (150 mM NaCl, 300 µM EDTA, pH 7.4), sterilized by filtering through a 0.22 µm membrane, and stored at 4°C until used. Each participant provided written informed consent, and the Institutional Review Board of the Medical University of South Carolina approved the experimental protocol.
Enrichment of HDL with S1P in vitro
HDL2 was incubated in vitro with S1P complexed to albumin for 24 h at 37°C under sterile conditions. After the incubation, the HDL2 subfraction was separated from albumin before incubating with adipocytes by centrifuging the mixture at d = 1.21 g/ml as described above. HDL2 not incubated with S1P but incubated with albumin for 24 h at 37°C under sterile conditions was included as a control. The in vitro modified HDL2 preparations and HDL3 were incubated with adipocytes at 800 µg HDL protein per ml media for 24 h, and the PAI-1 secreted into the media was determined using ELISA.
Cells and cell culture
Murine 3T3-L1 preadipocytes were purchased from the American Type Culture Collection (Manassas, VA) and maintained at low passage number in growth media consisting of DMEM that contained 25 mM glucose supplemented with 10% (v/v) fetal bovine serum (Gibco, Grand Island, NY) and penicillin-streptomycin (Gibco) in a humidified atmosphere containing 5% CO2. Two days after reaching confluence, cells were differentiated into adipocytes by incubation for 3 days in growth media modified by the addition of isobutylmethylxanthine (0.5 mM), dexamethasone (1 µM), and insulin (10 µg/ml), and then for another 2 days in growth media supplemented with insulin (10 µg/ml). Cultures were refed every 2 days for 10–14 days with fresh growth media to complete adipocyte maturation. At the start of an experiment, 90–95% of the cells exhibited the accumulation of Oil Red O positive droplets and significant adiponectin secretion (data not shown), consistent with adipocyte morphology and metabolism, respectively.
To conduct an experiment, the media was aspirated from each well, and the cultures were washed twice with DMEM. Fresh growth media was added to each well, and the cells were incubated with the indicated constituent for the specified period of time. At the end of an experiment, conditioned media was transferred from the culture dish into a plastic microfuge tube, which was centrifuged briefly to sediment cell debris. The supernatant was transferred to another microfuge tube and frozen at −70°C until analyzed.
Quantitative analyses
PAI-1.
The concentration of mouse PAI-1 protein secreted into the media by cultured adipocytes was determined using a commercially available ELISA assay (Innovative Research, Novi, MI). This assay was sensitive to PAI-1 in solution at 0.032 ng/ml, and a curvilinear solution was fit to the pattern of absorbance detected in wells with known amounts of PAI-1 using software supplied by the manufacturer of the microtiter plate reader (Molecular Devices, Sunnyvale, CA).
Sphingolipid extraction and analysis.
Analysis of endogenous sphingoid bases, sphingoid base-1-phosphates, and ceramide species was conducted in the Lipidomics Core Facility at Medical University of South Carolina using a Thermo Finnigan TSQ 7000 or SCIEX Q Trap triple-stage quadrupole mass spectrometers operating in a multiple reaction monitoring (MRM) positive ionization mode, as previously described (25). Briefly, 400 µg HDL protein was spiked with the following internal standards: 17C base D-erythro-sphingosine (17Sph); 17C D-erythro-sphingosine-1-phosphate (17S1P); D-erythro-N-palmitoyl-13C-D-erythro-sphingosine (13C/C16-Cer); N-heptadecanoyl-D-erythro-sphingosine (18C/C17-Cer); D-erythro-C6-SM (18C/C6-SM); D-erythro-C17-SM 18C/ C17-SM, D-erythro-C8-glucosylceramide (C8-GluCer); and D-erythro- C8-lactosylceramide (C8-LacCer).
The HDL lipids were extracted into a one-phase solvent system with ethyl acetate/iso-propanol/water (60/30/10% v/v). The internal standards were added prior to extraction and were added to all samples and calibration standards to correct for loss of any target analytes during sample preparation. The solvents were evaporated under nitrogen stream, and the residue was reconstituted in 150 µl of acidified (0.2% formic acid) methanol and injected onto the HP1100/TSQ 7000 LC/MS system and gradient-eluted from a BDS Hypersil C8, 150 × 3.2 mm, 3 µm particle size column using 1 mM methanolic ammonium formate/2 mM aqueous ammonium formate as the mobile phase system. Signal areas in elution peaks corresponding to target analytes and internal standards were processed using the Xcalibur™ software system (Thermo Fisher Scientific, Waltham, MA). Quantitative analyses were based on calibration curves generated by injecting known amounts of the target analytes and an equal amount of the internal standards. Final concentrations of analytes in samples were determined using the appropriate corrections for sample loss based on internal standard recovery calculations.
RT-PCR determination of adipocyte gene expression.
Total adipocyte RNA was extracted using a commercially available kit (RNeasy™ Lipid Tissue Mini Kit, Qiagen, Valencia, CA) according to the manufacturer's instructions. First-strand cDNA was synthesized from 0.75 µg total RNA using random hexamer primers according to the kit manufacturer's instructions (iScript™ cDNA Synthesis Kit, Bio-Rad, Hercules, CA). The complete reaction was cycled for 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C (MJ Mini, Bio-Rad). The reverse transcription reaction mixture was then diluted 1:40 with nuclease-free water and quantitative real-time PCR was performed using an iQ5 Real-Time PCR Detection System (Bio-Rad). Reactions were carried out in triplicate in a total volume of 20 µl using iQ™ SYBR Green Supermix (Bio-Rad). Primer sets were designed (Beacon Designer, PREMIER Biosoft, Int., Palo Alto, CA) to span intron-exon borders to distinguish amplified cDNA from genomic DNA. The hot start enzyme was activated (95°C for 2 min), and cDNA was then amplified for 40 cycles consisting of denaturation at 95°C for 10 s and annealing/extension at 50°C for 45 s. A melt curve assay was then performed (55°C for 1 min, and then the temperature was increased by 0.5°C every 10 s) to detect the formation of primer-derived trimers and dimers. The average starting quantity of fluorescence units was used for analysis. Quantification was calculated using the starting quantity of the cDNA of interest relative to that of GAPDH cDNA in the same sample. Primer pairs (Integrated DNA Technologies, Inc., Coralville, IA) used to quantitate the expression of each gene by RT-PCR are shown in Table 1.
TABLE 1.
PCR primer sets
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| S1P1 | GCA AGA ACA TCT CCA AGG | GAA GAC ACT CAG GAC AAT G |
| S1P2 | TTA GCA TCC TTC TCT TAG ACT C | GTC ACT CCC TTC CCT CTC |
| S1P3 | TGG ACT GTT GAA GTA ACC | AGA GTG TCA TTT CCC AAG |
| S1P4 | TAC TGC CTG CTG AAC ATC | AGC TGG AAG GTA CGA GTC |
| S1P5 | TCC TTC ACT ACA ACT ACA C | AAG TTC TCC AGC ACA ATG |
| GAPDH | ATC TTG GGC TAC ACT GAG | GCC GTA TTC ATT GTC ATA C |
Abbreviations: GAPDH, glyceraldehyde phosphate dehydrogenase S1P1–5, sphingosine-1-phosphate receptor1–5.
Transfection of adipocytes with siRNA.
Transformed adipocytes were transfected using the DharmaFECT™ 3 silencing RNA (siRNA) Transfection Reagent (Thermo Fisher Scientific, Lafayette, CO) following a modification of the protocol recommended by the manufacturer. Briefly, 2 µM siRNA were prepared with Opti-MEM™ media (Invitrogen, Carlsbad, CA). The silencing RNA (siRNA) and the DharmaFECT™ 3 solutions were combined and then incubated for an extended period (90 minutes) at room temperature before being added to the wells containing the cells incubated in 800 µL antibiotic-free growth media. Following incubation for 24 h, the media containing the siRNA was removed, the cells were washed, and fresh growth media was added to each well. Transfection efficiency was determined using BLOCK-IT™ fluorescent oligo (Invitrogen) and averaged more than 85% in all experiments conducted. Commercial siRNA oligonucleotides (SureSilencing™ siRNA, SABiosciences, Frederick, MD) were used for all experiments. The cells were used for experiments 72 h after transfection.
Statistical analysis.
Each experiment shown is representative of three to five experiments, each incubated in triplicate with similar results. Statistical significance of observed differences was analyzed using Student's t-test or ANOVA (SigmaStat, v3.0, SPSS, Chicago, IL).
RESULTS
HDL3, but not HDL2, stimulates PAI-I secretion from 3T3 adipocytes
There was a concentration-dependent increase in PAI-1 secretion into the medium when adipocytes were incubated with the HDL3 subfraction but not with HDL2 (Fig. 1). The effect of lipoproteins added to the culture media on PAI-1 secretion from adipocytes was specific for the HDL3 subfraction because the addition of the apolipoprotein B-containing fraction of lipoproteins (VLDL + LDL) only marginally stimulated PAI-1 secretion (<1.3-fold at 400 µg protein/ml) (data not shown).
Fig. 1.
Effect of increasing concentrations of HDL2 and HDL3 protein in cell culture media on the secretion of PAI-1 from 3T3-L1 adipocytes. HDL3, but not HDL2, stimulates PAI-1 secretion from 3T3 adipocytes. PAI-1 concentration in the media was determined using ELISA with media from each well assayed in duplicate. Cells were incubated with HDL subfractions at the indicated concentrations for 24 h. PAI-1 concentration in control incubations without lipoprotein added to the medium averaged 109 ± 51 ng/mg cell protein. Data shown are the mean ± SD for eight experiments using HDL subfractions prepared from different donor pools and with HDL subfractions at each concentration incubated in duplicate or triplicate with different 3T3 adipocyte preparations used for each experiment. *P < 0.01 for HDL2 versus HDL3 at the indicated HDL concentration. Abbreviation: PAI-1, plasminogen activator inhibitor-1.
S1P stimulates PAI-1 secretion in adipocytes
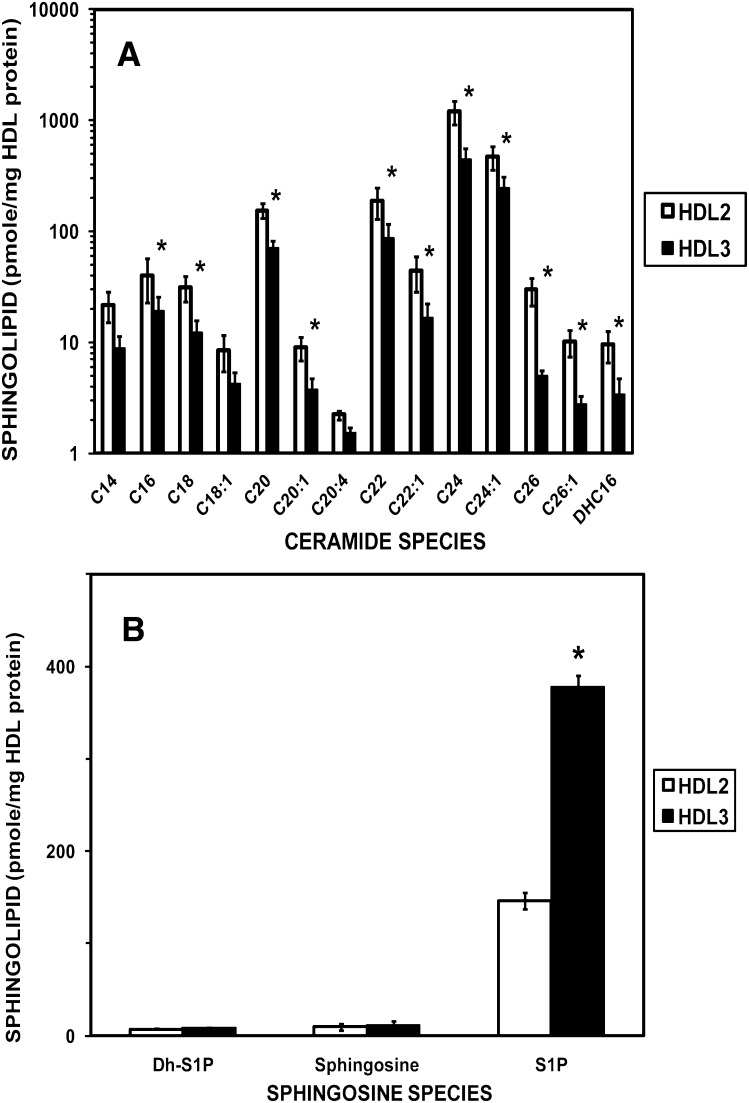
We determined the levels of sphingoid bases, sphingoid base-1-phosphates, and ceramide species in HDL subfractions using ESI/MS/MS (Fig. 2). The levels of the ceramide species were uniformly greater in HDL2 compared with HDL3 with the increase being statistically significant for all except the C14, C18:1, and CC20:4 ceramide species (Fig. 2A). In direct contrast to the ceramide content of HDL subfractions, the levels of sphingoid bases, sphingosine, dihydrosphingosine, and dihydrosphingosine-1-phosphate were uniformly greater in HDL3 compared with HDL2 (Fig. 2B). Most notably, the level of sphingosine-1-phosphate in HDL3 was significantly greater (2.6-fold) (P < 0.005) compared with that in HDL2. S1P levels averaged 23.4 ± 5.0 mmol/mole HDL2 and 41.5 ± 13.1 mmol/mole HDL3 in HDL preparations isolated from eight pools.
Fig. 2.
Amounts of ceramide species (A) and sphingolipid bases and their 1-phosphates (B) in HDL2 and HDL3 lipoprotein subfractions. HDL subfractions were isolated from plasma pools (N = 6), each obtained by combining blood from three normolipidemic donors. The assignment for ceramide subspecies is based on the amide-linked fatty acid found in the molecule. Values shown are mean ± SEM. Note the use of a logarithmic scale on the ordinate in panel A. *P < 0.05 for paired t-test of data for HDL2 versus HDL3. Abbreviations: DH-Sph, dihydrosphingosine; DH-S1P, dihydrosphingosine-1-phosphate; DHC16, dihydroceramide linked to C16 fatty acid; S1P, sphingosine-1-phosphate; Sph, sphingosine.
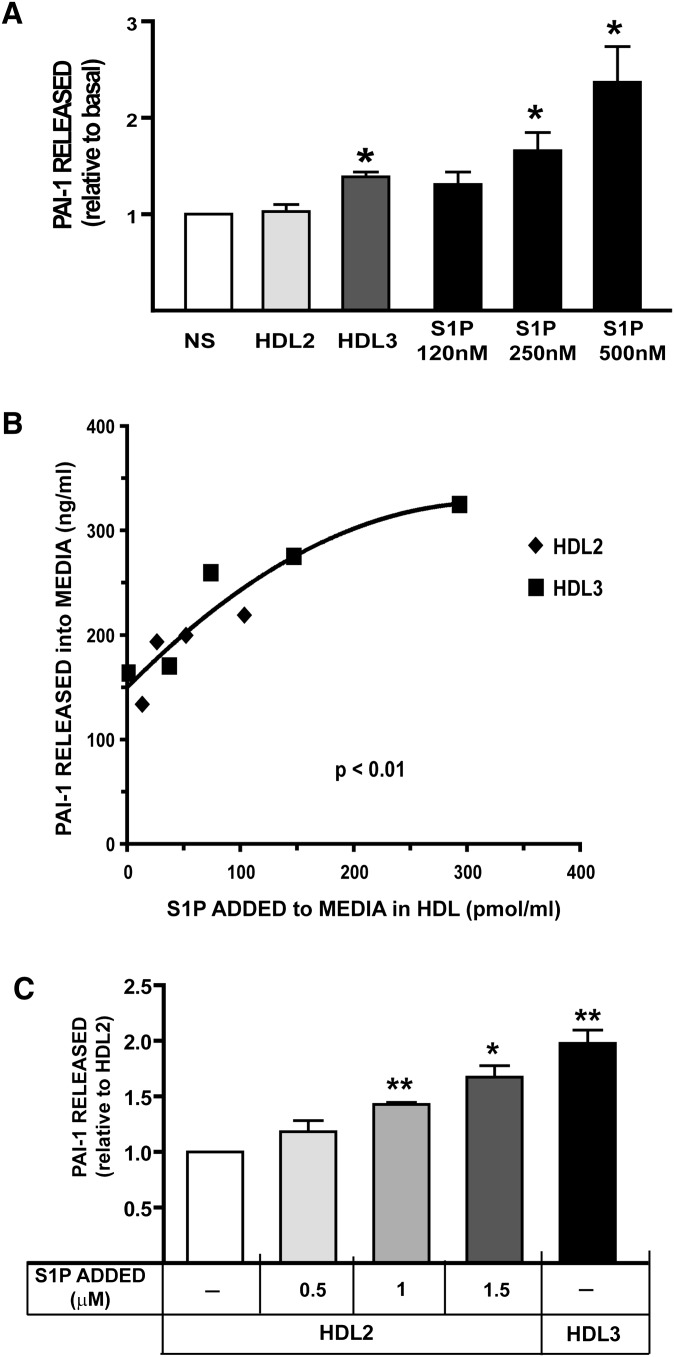
Because the S1P level in HDL3 was significantly greater than that in HDL2 and because HDL3 but not HDL2 stimulated PAI-1 secretion from adipocytes, we investigated the effect of S1P on PAI-1 secretion from adipocytes. S1P in plasma is found primarily either complexed to albumin or transported in the lipoprotein fraction bound to HDL, and especially to HDL3 (Fig. 2). We determined the effect of S1P complexed to albumin on PAI-1 secretion from adipocytes by incubating cells with increasing concentrations of S1P-albumin. As shown in Fig. 3A, there was a concentration-dependent increase in PAI-1 secretion into the medium when adipocytes were incubated with increasing concentrations of S1P-albumin at S1P concentrations comparable to those observed in isolated HDL3. Because increasing concentrations of HDL3 with its increased S1P content stimulated more PAI-1 secretion from adipocytes than did HDL2 (Fig. 1), we further investigated the association between the S1P concentration in HDL with PAI-1 secretion from adipocytes. As shown in Fig. 3B, there was a statistically significant, positive association between the concentration of PAI-1 released into the medium and the concentration of S1P in HDL incubated with adipocytes in the experiments reported in Fig. 1. To confirm and extend the data presented in Fig. 3B, which suggest that increasing S1P content in HDL stimulated PAI-1 secretion from adipocytes, we enriched HDL2 in vitro with increasing amounts of S1P. As shown in Fig. 3C, increasing S1P content in the HDL2 subfraction increased the concentration of PAI-1 secreted into the medium. Thus, when the S1P content of the lipoprotein is artificially increased by the addition of S1P in vitro, HDL2 stimulates PAI-1 release from adipocytes at an amount comparable to that observed when cells are incubated with native HDL3. In summary, increasing S1P concentration in the media stimulates PAI-1 secretion from adipocytes in a concentration-dependent manner. This occurs whether the S1P is present free in the media or if it is associated with HDL. HDL3, which contains significantly higher amounts of S1P compared with HDL2, stimulated more PAI-1 secretion from adipocytes than HDL2.
Fig. 3.
S1P stimulates PAI-1 secretion in adipocytes. A: Effect of increasing concentration of S1P added to the culture media and of HDL2 compared with HDL3 on PAI-1 release from adipocytes. HDL2 and HDL3 were isolated from pooled blood as described in “Methods” and incubated with adipocytes at 800 µg HDL protein/ml culture media for 24 h. S1P complexed to albumin was prepared as described in “Methods” and incubated at the indicated concentrations in companion cultures for 24 h. The concentrations of S1P were chosen to approximate the concentrations of S1P present in HDL2 compared with HDL3. Data shown are the mean ± SE for five experiments using HDL subfractions prepared from three different donor pools and with cells incubated in duplicate with each HDL subfraction and at each S1P concentration. PAI-1 concentration in the media was determined using ELISA with media from each well assayed in duplicate. PAI-1 concentration in cultures without any addition averaged 97 ± 20 ng/ml. Data are presented as mean ± SE. The data were normalized to basal PAI-level obtained from untreated cells (NS). *P < 0.05 and **P < 0.005 compared with untreated cells analyzed using ANOVA. B: PAI-1 released into the media as a function of the concentration of S1P in HDL subfractions. The S1P content of the HDL2 and HDL3 subfractions was determined as described in “Methods.” PAI-1 released into the media is plotted as a function of the experimentally determined concentration of S1P in HDL when the HDL subfraction was incubated with cells at the same protein concentrations reported in Fig. 1. The nonlinear, least-squares-regression line of best fit is plotted for reference. C: HDL2 supplemented in vitro with S1P stimulates PAI-1 release from adipocytes. HDL2 (8 ml at 7.5 mg protein/ml) was incubated in vitro with a 5 µl, 10 µl, or 15 µl volume of S1P (100 µM) complexed to albumin for 24 h at 37°C. The HDL2 subfraction was separated from albumin before incubating with adipocytes by centrifuging the mixture at d = 1.21 g/ml. The floating HDL2 preparation was harvested after tube slicing. The in vitro-modified HDL2 preparations and native HDL3 were incubated with adipocytes at 800 µg HDL protein per ml media for 24 h, and the PAI-1 secreted into the media was determined using ELISA. The PAI-1 concentration in the media from cells incubated with HDL2 supplemented with S1P in vitro or native HDL3 was normalized to PAI-levels obtained from adipocytes incubated with native HDL2. PAI-1 concentration in cultures incubated with native HDL2 not supplemented with S1P averaged 125 ± 20 ng/ml. Values shown are mean ± SD (n = 4). *P < 0.05 and **P < 0.005 for the level compared with PAI-1 level in the media when adipocytes were incubated with native HDL2. Abbreviations: PAI-1, plasminogen activator inhibitor-1; S1P, sphingosine-1-phosphate.
S1P-stimulated PAI-1 secretion from adipocytes is mediated by the G-protein-coupled receptor S1P2
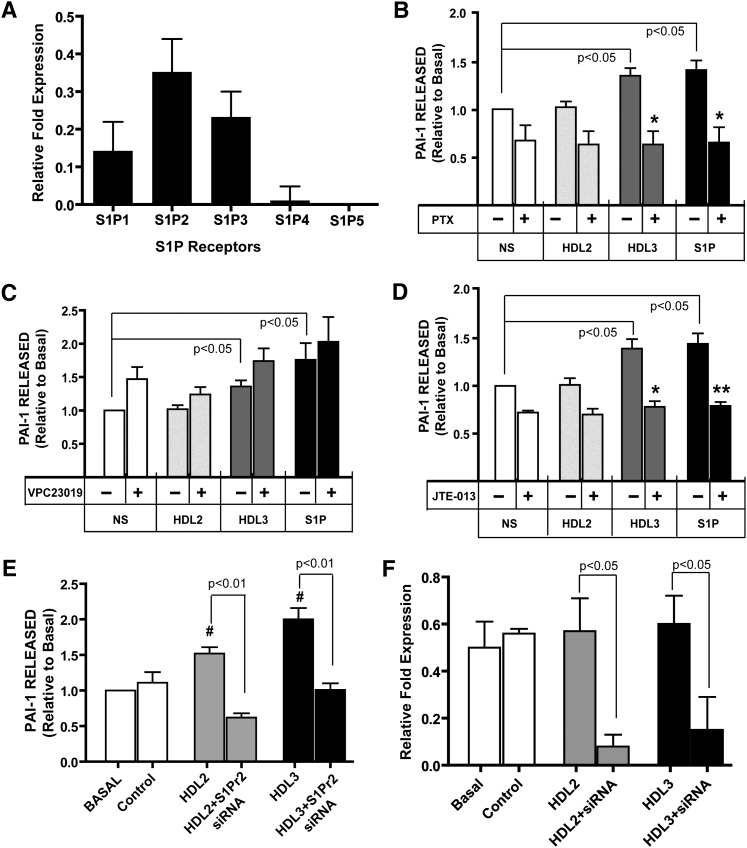
The effects of S1P on cell metabolism are mediated by S1P binding to the cell surface receptors S1P1-5, which have been reported to be variably expressed and tissue-specific (14, 26). We evaluated expression of the genes coding for S1P1-5 in 3T3-L1 adipocytes using quantitative RT-PCR. We determined that the S1P1, S1P2, and S1P3 genes were expressed in 3T3 adipocytes with the gene coding for S1P2 expressed in greatest abundance (Fig. 4A).
Fig. 4.
Relative abundance of S1P receptor subtype mRNA in 3T3-L1 adipocytes and the effect of inhibition of Gi/o or S1P2 on HDL3- and S1P-mediated release of PAI-I from adipocytes. A: Expression levels of S1P receptors. The fold expression of the specific S1P receptor transcript is expressed relative to that for the GAPDH gene (ΔΔCt). Data expressed are the mean ± SD of three experiments. The expression of S1P4 and S1P5 was highly variable and was at the limit of detection for the methods employed. B–D: To investigate the role of individual S1P receptors in S1P/HDL3-stimulated PAI-1 secretion from adipocytes, cultures were incubated overnight with 0.7 ng/ml PTX (B), 5 μM VPC23019 (C), or 10 μM JTE-013 (D) for 1 h before incubation with 800 μg/ml HDL2, HDL3, or S1P (0.25 μM) for 24 h. The conditioned medium was harvested, and the concentration of PAI-1 in the media was measured using ELISA. Data are presented as mean ± SE. (n = 6). The data were normalized to basal PAI-level obtained in media from untreated cells (NS). PAI-1 concentration in cultures without any addition averaged 252 ± 42 ng/ml. *P < 0.05 and **P < 0.01 obtained from ANOVA evaluation of PAI-1 level in culture medium from cells incubated with or without HDL or S1P in the presence compared with the absence of the chemical inhibitor. E: Results of experiments employing chemical inhibitors were confirmed and expanded by evaluating PAI-1 secretion from adipocytes after reducing the expression of the gene coding for S1P2 using siRNA technology. PAI-1 secretion was not altered in cells incubated with siRNA with a scrambled sequence (Control). F: Relative expression of the gene coding for S1P2 in adipocytes incubated with or without siRNA targeted to S1P2. Experiments reported in panels E and F were conducted using HDL2 and HDL3 obtained from one donor. #P < 0.05 ANOVA versus Basal for three studies with cultures incubated in triplicate. Abbreviations: PAI-1, plasminogen activator inhibitor-1; S1P, sphingosine-1-phosphate; S1P2, sphingosine-1-phosphate receptor2.
S1P receptors are G-protein-coupled receptors, and the diversity of S1P action depends upon the combination of the tissue- and cell type-specific expression patterns of the S1P receptor family and the subtype-specific, distinct repertoire of heterotrimeric G-proteins to which they are coupled. S1P1, S1P2, and S1P3 are coupled to the Gi/o-protein (18, 19, 27). To confirm that HDL3 and S1P-stimulated PAI-1 secretion are mediated by G-protein-coupled receptors for S1P, adipocytes were preincubated with the Gi/o inhibitor PTX before incubation with HDL2, HDL3, or S1P, and then the concentration of PAI-1 released in the cell culture medium was determined (Fig. 4B). PTX inhibited HDL3- and S1P-stimulated PAI-1 release from adipocytes. These data support the role of Gi/o protein coupling to S1P receptors in PAI-1 release from adipocytes.
To identify which of the three S1P receptors expressed in adipocytes mediate the secretion of PAI-1, two pharmacologic antagonists—VPC23019 (Fig. 4C) and JTE-013 (Fig. 4D)—were used to inhibit S1P1/S1P3 and S1P2, respectively. The inhibition of S1P1 and S1P3 by VPC23019 did not block the PAI-1 release into medium; in fact, PAI-1 release was moderately increased (Fig. 4C). In contrast, JTE-013 inhibition of S1P2 blocked PAI-1 secretion from adipocytes (Fig. 4D). These observations suggest that PAI-1 secretion from adipocytes upon exposure to HDL3 or S1P is mediated by the S1P2 receptor but not S1P1 or S1P3. To confirm and extend these studies, we conducted additional experiments. We reduced the expression of the gene coding for S1P2 using siRNA technology and determined the effect on the secretion of PAI-1 from adipocytes incubated with HDL subfractions (Fig. 4E). There was a statistically significant reduction in PAI-1 secretion from adipocytes (Fig. 4E) with reduced expression of the gene coding for S1P2 (Fig. 4F), further supporting the role of the S1P2 receptor in S1P/HDL3-stimulated secretion of PAI-1 from adipocytes. The rates of secretion of PAI-1 (Fig. 4E) and the relative expression of S1P2 (Fig. 4F) in adipocytes transfected with a scrambled sequence siRNA (control) were similar to those exhibited by cells incubated in growth media without any additions (basal), suggesting there were minimal off-target effects of the transfection procedures employed. The levels of expression of genes coding for S1P1 and S1P3 were not altered in cells transfected with siRNA directed against the S1P2 gene compared with the expression levels in basal or control incubations (data not shown), further suggesting minimal off-target effects of the transfection.
HDL3 and S1P stimulate PAI-1 secretion via multiple signaling pathways
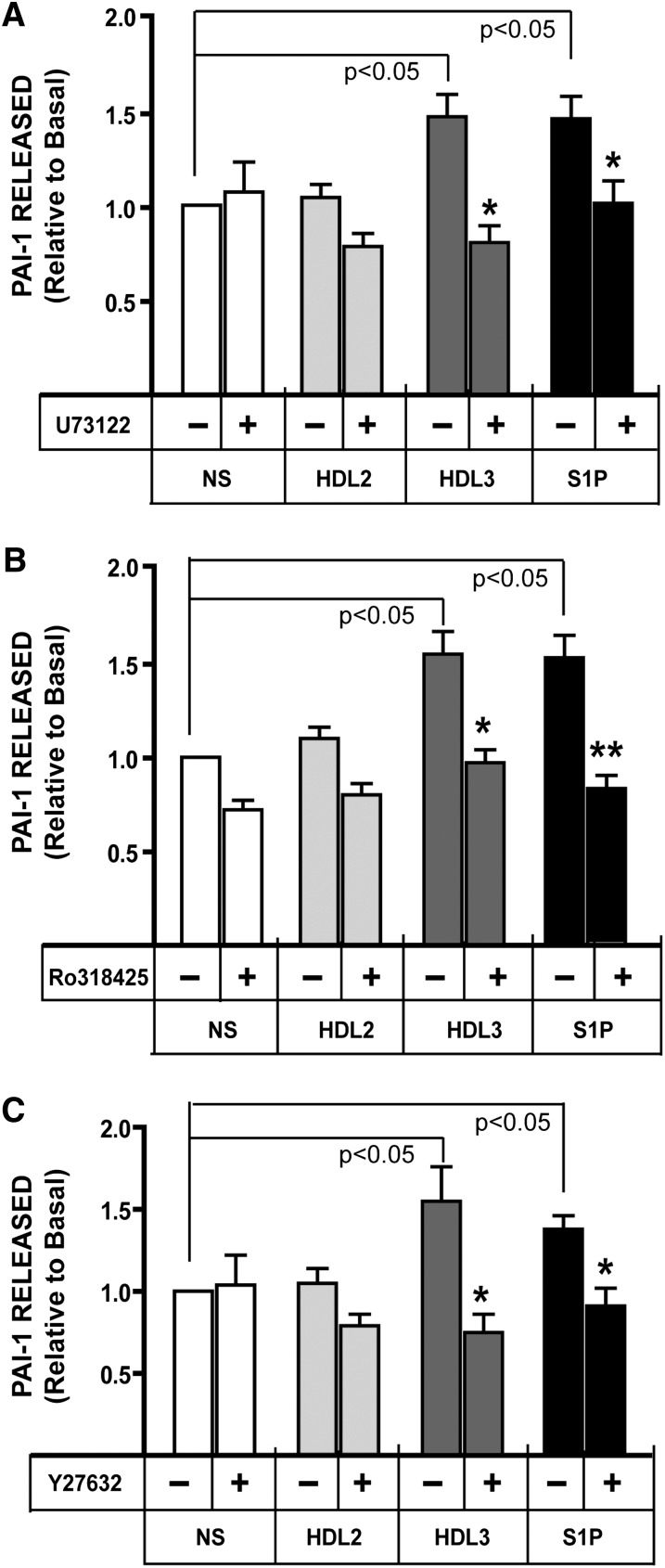
To investigate the downstream signaling pathways involved in HDL3- and S1P-induced PAI-1 release from adipocytes, we employed a panel of selective inhibitors targeting PLC (Fig. 5A), PKC (Fig. 5B), and Rho kinase (Fig. 5C). As shown in Fig. 5A, treatment of the cells with the PLCβ inhibitor U73122 significantly inhibited HDL- and S1P-stimulated PAI-1 release. One consequence of PLC activation is increased activity of classical PKC isoforms. As shown in Fig. 5B, pretreatment with the PKC inhibitor Ro-318425 mirrored the inhibitory effects of U73122 on HDL- and S1P-stimulated PAI-1 release. ROCK is a Rho effector downstream of G12/13 that might also be expected to be activated by S1P2 receptors. As shown in Fig. 5C, inhibition of ROCK using Y27632 was sufficient to inhibit PAI-1 release as was observed during chemical inhibition of the PLC-PKC pathways. These data suggest that multiple downstream signaling pathways of S1P2 are required for PAI-1 release from adipocytes.
Fig. 5.
Treatment of adipocytes with the PLC inhibitor U73122 (A), the PKC inhibitor RO-31-8425 (B), or the Rho inhibitor Y-27623 (C) blocks the secretion of PAI-I stimulated by incubation with HDL3 or S1P. 3T3 cells were pretreated with 10 μM of U73122 (n = 5), 1 μM RO-31-8425 (n = 4), or 5 μM Y-27623 (n = 7) for 1 h before incubation with HDL2 (800 μg/ml), HDL3 (800 μg/ml), or S1P (0.25 μM) for 24 h. The conditioned medium was removed from each well, and the concentration of PAI-1 was measured using ELISA. Data are presented as mean ± SE. The data were normalized to basal PAI-level obtained from untreated cells (NS). PAI-1 concentration in cultures without any addition averaged 303 ± 51 ng/ml. *P < 0.05 and **P < 0.01 obtained from ANOVA of PAI-1 level in culture medium from cells incubated with/without the indicated addition in the presence compared with the absence of the chemical inhibitor. Abbreviations: PAI-1, plasminogen activator inhibitor-1; PKC, protein kinase C; PLC, phospholipase C; S1P, sphingosine-1-phosphate.
DISCUSSION
The HDL fraction customarily is considered to be atheroprotective, predominantly because of it pivotal role in the transport of cholesterol from peripheral tissues to the liver, with the larger diameter HDL2 subfraction viewed as the more atheroprotective subfraction compared with the smaller-sized HDL3 subfraction (28, 29). A rapidly expanding literature further suggests that HDL-associated S1P is responsible, at least in part, for many of the beneficial effects of HDL on vasorelaxation, cell survival, cell adhesiveness, angiogenesis, and the synthesis of two powerful antiatherogenic and antithrombotic molecules, nitric oxide and prostacyclin (30). There is emerging literature, however, to suggest that S1P may also mediate proatherogenic metabolism (20) in part because of its integral involvement in inflammation (31) and may even function as a biomarker of obstructive coronary artery disease (32). We now demonstrate that S1P, especially the HDL3 subfraction which contains higher amounts of S1P than the HDL2 subfraction, significantly increases PAI-1 secretion from adipocytes and, thus, may negatively modulate fibrinolysis in vivo.
Because of its pivotal role in regulating fibrinolysis and the impact of decreased fibrinolysis on cardiovascular complications, increased circulating levels of PAI-1 are considered a cardiovascular risk factor (33). The prevalence of obesity in adults has increased rapidly in Western countries during the last decade, and it has long been known that fibrinolytic activity is decreased among obese subjects (34), a phenomenon that has been attributed to increased levels of PAI-1 (35). Although both endothelial cells and hepatocytes contribute to circulating PAI-1 levels, adipose tissue is thought to make a major contribution to circulating levels, and it may contribute proportionately more with the development of obesity due to the increase in adipose tissue mass associated with this metabolic disorder (5, 33, 35–38). Therefore, knowledge of the factors regulating PAI-1 production by adipose tissue is critical. We have demonstrated that S1P increases the secretion of PAI-1 from adipocytes (Fig. 3). In addition, S1P present in HDL, the main transport lipoprotein for S1P, stimulates PAI-1 release from adipocytes in a concentration-dependent manner (Fig. 3B). HDL3 stimulated significantly more PAI-1 release from adipocytes (Figs. 1, 3), presumably because the S1P concentration in the HDL3 subfraction averaged twice that observed in HDL2 isolated from the same subjects. While the concentrations of other constituents in the HDL2 and HDL3 lipoprotein subfractions certainly differ, the increased PAI-1 secretion from adipocytes incubated with HDL2 supplemented in vitro with S1P (Fig. 3) strongly supports the role of S1P in HDL- dependent release of PAI-1 from adipocytes. Importantly, while total HDL concentration customarily decreases with the hypertriglyceridemia that is associated with obesity, it is primarily the larger-sized HDL2 subfraction that is reduced. Thus, the remaining S1P, which is transported predominantly in the smaller HDL3 subfraction, could still affect PAI-1 release from adipocytes and provide one mechanism for the increased plasma PAI-1 concentrations observed with the hypertriglyceridemia of obesity.
Comparatively little is known regarding S1P transport in lipoproteins (21, 39), with no information available regarding the metabolic factors that influence the distribution of S1P in HDL subfractions. Although our study investigated relatively few subjects, the S1P content of HDL2 was consistently lower than that of HDL3 in all subjects. However, the distribution of S1P between the HDL2 and HDL3 subfractions was variable, and the S1P concentration in HDL3 ranged from 1.4 to 2.6 times that in HDL2 (data not shown). The factors that contribute to this broad range in S1P distribution are unknown, and in view of the negative impact of S1P on fibrinolysis, additional study is warranted. In addition, subjects donating HDL for the present studies were nondiabetic and free of clinically evident disease. The impact of altered lipoprotein metabolism that occurs in patients with the metabolic syndrome, hypertriglyceridemia, or diabetes on S1P transport in lipoprotein is unknown and more study is required.
The differential expression of S1P receptor subtypes and the subtype-specific repertoire of heterotrimeric G-proteins and effectors to which they are coupled determine the biological effects of S1P. We found that S1P1, S1P2, and S1P3 are expressed on 3T3 adipocytes, with S1P2 being most abundant. S1P has been shown to inhibit cell migration in embryonic fibroblast via S1P2-mediated signaling. This inhibition of migration via S1P2 was mediated by G12/13-dependent Rac inactivation and Rho-dependent PTEN (phosphatase and Tensin homolog inhibitory) pathway. The stimulation of glioblastoma cells with S1P increased PAI-1 expression via S1P2 receptor-mediated by activation of ERK1/2 and Rho pathways (13). We have confirmed and extended these observations and now report that HDL3 and S1P stimulate the release of PAI-1 from 3T3 adipocytes via the S1P2 receptor (Fig. 4). Interestingly, PAI-1 secretion from adipocytes was markedly increased when HDL2, HDL3, or S1P were added to adipocytes in the presence of VPC23019, a S1P1/S1P3 receptor antagonist (Fig. 4C). The reduced binding of S1P to S1P1 and S1P3 presumably resulted in less S1P binding to the cell via these receptor pathways, which we demonstrated were not involved in the PAI-1 response in adipocytes (Fig. 4); this may have increased the availability of S1P for binding to the S1P2 receptor, which was shown to mediate this metabolism in adipocytes. We also demonstrate for the first time that HDL3- and S1P-mediated PAI-1 secretion from adipocytes is sensitive to inhibition of either the PLC-PKC or the Rho-ROCK pathway, suggesting that PAI-1 secretion requires the coordinate actions of several S1P2 receptor signaling pathways (Fig. 5).
The various biological functions of HDL may be mediated, at least in part, by the interaction of HDL with one of its receptors (40–42). Recent evidence also supports the role of S1P transported in HDL in modifying multiple cellular metabolic pathways via its binding to one or more of the receptors in the S1P family of receptors (42–48). However, the mechanism whereby HDL-associated S1P can signal through S1P receptors is not well understood. Nofer et al. (47, 49) have shown that HDL particles dock with the scavenger receptor class BI (SR-BI), and lysosphingolipids in HDL activate endothelial gene expression through the S1P3 receptor. In addition, Kimura et al. (42) have shown that the ability of HDL to inhibit TNFα-induced adhesion molecule expression was attenuated following downregulation of either SR-BI or S1P receptor expression, and when the expression of both receptors was suppressed, HDL- mediated inhibition was abolished. These data suggest that the SR-BI and S1P receptor systems may cooperate in HDL-induced signaling and alterations in cellular metabolism. The present data do not establish a role for SR-BI or other HDL receptors in the HDL3/S1P-mediated increase in PAI-1 secretion from adipocytes; the possibility of cooperativity between HDL receptors and S1P receptors in regulating PAI-1 release from adipocytes remains to be determined.
While we have demonstrated that S1P that originates from exogenous sources, such as that bound to HDL or albumin, can interact with its receptors on the cell surface and effect cell metabolism, S1P also may be produced endogenously via sphingosine kinase (SK). It is not known if HDL (or HDL subfraction-specific) binding to adipocytes alters SK activity and, thus, endogenous S1P production. If HDL binding to the cell alters SK activity, this endogenously produced S1P also could potentially alter PAI-1 secretion. Studies indicate that S1P in the nucleus specifically binds to the histone deacetylases 1 (HDAC1) and HDAC2 and inhibits their enzymatic activity. Most importantly, SK2 has been shown to be associated with HDAC1 and HDAC2 in repressor complexes and is selectively enriched in the promotors of several genes. Thus, HDACs are direct intracellular targets of S1P and link endogenous, nuclear S1P to epigenetic regulation of gene expression (50). Alternatively, endogenous S1P may be exported out of cells by members of the ABC transporter family where it can act via “inside-out” signaling and bind to its receptors in an autocrine or paracrine fashion to stimulate G-protein-regulated signaling pathways (51). Thus, S1P from either endogenous or exogenous sources potentially may influence adipocyte metabolism. Again, additional investigation is required.
Lastly, there was an unanticipated, but interesting, statistically significant increase in PAI-1 released into the medium from cells incubated with HDL2 compared with basal incubations in the series of experiments in which S1P2 gene expression was reduced using siRNA technology (Fig. 4E) (although PAI-1 secretion from cells incubated with HDL3 continued to remain elevated relative to HDL2 as was observed in previous studies). To investigate the potential cause of this unexpected increase in PAI-1 secretion from adipocytes incubated with this particular HDL2 preparation, we determined the levels of S1P in the HDL2 and HDL3 preparations used in these studies. The S1P level in HDL2 isolated from this donor averaged 1.4-fold higher (202 pmol S1P/mg HDL2 protein) compared with S1P levels determined in the other eight HDL2 pools used in these studies. Thus, the S1P concentration in the media when adipocytes were incubated with this HDL2 preparation exceeded the S1P level in the cultures in all experiments in which the other eight HDL2 pools were employed and may have contributed to the observed increase in PAI-1 secretion. The results of this limited study further support our observations reported in Fig. 3B and suggest that when adipocytes are incubated with HDL at the same concentration, HDL subfractions containing higher levels of S1P can stimulate the release of more PAI-1 from adipocytes than HDL subfractions with lower S1P content. Although the S1P level in HDL3 was uniformly higher than that in HDL2 in every HDL preparation used in these studies and, furthermore, it uniformly stimulated more PAI-1 secretion from adipocytes than HDL2 from the same individual, the factor or factors which contributed to the observed differences in S1P level in HDL subfractions between individuals remain to be determined and are under investigation.
Footnotes
Abbreviations:
- PAI-1
- plasminogen activator inhibitor-1
- PKC
- protein kinase C
- PLC
- phospholipase C
- ROCK
- Rho-associated protein kinase
- siRNA
- silencing RNA
- S1P
- sphingosine-1-phosphate
- S1Pn
- sphingosine-1-phosphate receptor1–5
This work was supported by National Institutes of Health Grants P01 HL-55782 (M.L.V), DK-081352 (M.L.V.), and HL-079274 (S.M.H.). This work was also supported by the Merit Review Program of the Department of Veterans Affairs (R.L.K. and M.L.V.), the South Carolina COBRE in Lipidomics and Pathobiology (P20 RR-17677 from the National Center for Research Resources (NCRR) (S.M.H.), and the Lipidomics Core Facility at the Medical University of South Carolina. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Kohler H. P., Grant P. J. 2000. Plasminogen activator inhibitor type 1 and coronary artery disease. N. Engl. J. Med. 342: 1792–1801. [DOI] [PubMed] [Google Scholar]
- 2.Tsantes A. E., Nikolopoulos G. K., Bagos P. G., Rapti E., Mantzios G., Kapsimali V., Travlou A. 2007. Association between the plasminogen activator inhibitor-1 4G/5G polymorphism and venous thrombosis. A meta-analysis. Thromb. Haemost. 97: 907–913. [PubMed] [Google Scholar]
- 3.Lundgren C. H., Brown S. L., Nordt T. K., Sobel B. E., Fujii S. 1996. Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation. 93: 106–110. [DOI] [PubMed] [Google Scholar]
- 4.Hamsten A., de Faire U., Walldius G., Szamosi G. D., Landou C., Blomback M., Wiman B. 1987. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet. 2: 3–9. [DOI] [PubMed] [Google Scholar]
- 5.Le Lay S., Lefère I., Trautwein C., Dugail I., Krief S. 2002. Insulin and sterol-regulatory element-binding protein-1c (SREBP-1C) regulation of gene expression in 3T3–L1 adiopcytes. Identification of CCAAT/enhancer-binding protein beta as an SREBP-1C target. J. Biol. Chem. 277: 35625–35634. [DOI] [PubMed] [Google Scholar]
- 6.Morange P. E., Alessi M. C., Verdier M., Casanova D., Magalon G., Juhan-Vague I. 1999. PAI-1 produced ex vivo by human adipose tissue is relevant to PAI-1 blood level. Arterioscler. Thromb. Vasc. Biol. 19: 1361–1365. [DOI] [PubMed] [Google Scholar]
- 7.Samarakoon R., Higgins S. P., Higgins C. E., Higgins P. J. 2008. TGF-beta1-induced plasminogen activator inhibitor-1 expression in vascular smooth muscle cells requires pp60(c-src)/EGFR(Y845) and Rho/ROCK signaling. J. Mol. Cell. Cardiol. 44: 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakakuki T., Ito M., Iwasaki H., Kureishi Y., Okamoto R., Moriki N., Kongo M., Kato S., Yamada N., Isaka N., et al. 2005. Rho/Rho-kinase pathway contributes to C-reactive protein-induced plasminogen activator inhibitor-1 expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 25: 2088–2093. [DOI] [PubMed] [Google Scholar]
- 9.Takeda K., Ichiki T., Tokunou T., Iino N., Fujii S., Kitabatake A., Shimokawa H., Takeshita A. 2001. Critical role of Rho-kinase and MEK/ERK pathways for angiotensin II-induced plasminogen activator inhibitor type-1 gene expression. Arterioscler. Thromb. Vasc. Biol. 21: 868–873. [DOI] [PubMed] [Google Scholar]
- 10.Alessi M. C., Juhan-Vague I., Kooistra T., Declerck P. J., Collen D. 1988. Insulin stimulates the synthesis of plasminogen activator inhibitor 1 by the human hepatocellular cell line Hep G2. Thromb. Haemost. 60: 491–494. [PubMed] [Google Scholar]
- 11.Kooistra T., Bosma P. J., Tons H. A., van den Berg A. P., Meyer P., Princen H. M. 1989. Plasminogen activator inhibitor 1: biosynthesis and mRNA level are increased by insulin in cultured human hepatocytes. Thromb. Haemost. 62: 723–728. [PubMed] [Google Scholar]
- 12.Stiko-Rahm A., Wiman B., Hamsten A., Nilsson J. 1990. Secretion of plasminogen activator inhibitor-1 from cultured human umbilical vein endothelial cells is induced by very low density lipoprotein. Arteriosclerosis. 10: 1067–1073. [DOI] [PubMed] [Google Scholar]
- 13.Bryan L., Paugh B. S., Kapitonov D., Wilczynska K. M., Alvarez S. M., Singh S. K., Milstien S., Spiegel S., Kordula T. 2008. Sphingosine-1-phosphate and interleukin-1 independently regulate plasminogen activator inhibitor-1 and urokinase-type plasminogen activator receptor expression in glioblastoma cells: implications for invasiveness. Mol. Cancer Res. 6: 1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiegel S., Milstien S. 2003. Sphingosine-1-phosphate: an enigmatic signaling lipid. Nat. Rev. Mol. Cell Biol. 4: 397–407. [DOI] [PubMed] [Google Scholar]
- 15.Hla T. 2004. Physiological and pathological actions of sphingosine 1-phosphate. Semin. Cell Dev. Biol. 15: 513–520. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez S. E., Milstien S., Spiegel S. 2007. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol. Metab. 18: 300–307. [DOI] [PubMed] [Google Scholar]
- 17.Watterson K., Sankala H., Milstien S., Spiegel S. 2003. Pleiotropic actions of sphingosine-1-phosphate. Prog. Lipid Res. 42: 344–357. [DOI] [PubMed] [Google Scholar]
- 18.Takuwa Y., Okamoto Y., Yoshioka K., Takuwa N. 2008. Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system. Biochim. Biophys. Acta. 1781: 483–488. [DOI] [PubMed] [Google Scholar]
- 19.Rosen H., Gonzalez-Cabrera P. J., Sanna M. G., Brown S. 2009. Sphingosine 1-phosphate receptor signaling. Annu. Rev. Biochem. 78: 743–768. [DOI] [PubMed] [Google Scholar]
- 20.Okajima F. 2002. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim. Biophys. Acta. 1582: 132–137. [DOI] [PubMed] [Google Scholar]
- 21.Murata N., Sato K., Kon J., Tomura H., Yanagita M., Kuwabara A., Ui M., Okajima F. 2000. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 352: 809–815. [PMC free article] [PubMed] [Google Scholar]
- 22.Kontush A., Therond P., Zerrad A., Couturier M., Negre-Salvayre A., de Souza J. A., Chantepie S., Chapman M. J. 2007. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler. Thromb. Vasc. Biol. 27: 1843–1849. [DOI] [PubMed] [Google Scholar]
- 23.Argraves K. M., Argraves W. S. 2007. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J. Lipid Res. 48: 2325–2333. [DOI] [PubMed] [Google Scholar]
- 24.Hla T. 2001. Sphingosine 1-phosphate receptors. Prostaglandins Other Lipid Mediat. 64: 135–142. [DOI] [PubMed] [Google Scholar]
- 25.Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. 2006. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 39: 82–91. [DOI] [PubMed] [Google Scholar]
- 26.Takuwa Y., Takuwa N., Sugimoto N. 2002. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J. Biochem. 131: 767–771. [DOI] [PubMed] [Google Scholar]
- 27.Windh R. T., Lee M. J., Hla T., An S., Barr A. J., Manning D. R. 1999. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. J. Biol. Chem. 274: 27351–27358. [DOI] [PubMed] [Google Scholar]
- 28.Assmann G., Gotto A. M., Jr 2004. HDL cholesterol and protective factors in atherosclerosis. Circulation. 109(Suppl. 1): III8–III14. [DOI] [PubMed] [Google Scholar]
- 29.Rader D. J. 2003. Regulation of reverse cholesterol transport and clinical implications. Am. J. Cardiol. 92: 42J–49J. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez C., Gonzalez-Diez M., Badimon L., Martinez-Gonzalez J. 2009. Sphingosine-1-phosphate: a bioactive lipid that confers high-density lipoprotein with vasculoprotection mediated by nitric oxide and prostacyclin. Thromb. Haemost. 101: 665–673. [PubMed] [Google Scholar]
- 31.Nixon G. F. 2009. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br. J. Pharmacol. 158: 982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deutschman D. H., Carstens J. S., Klepper R. L., Smith W. S., Page M. T., Young T. R., Gleason L. A., Nakajima N., Sabbadini R. A. 2003. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am. Heart J. 146: 62–68. [DOI] [PubMed] [Google Scholar]
- 33.Faber D. R., de Groot P. G., Visseren F. L. 2009. Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes. Rev. 10: 554–563. [DOI] [PubMed] [Google Scholar]
- 34.Shaw D. A., Machaughton D. 1963. Relationship between blood fibrinoytic activity and body fatness. Lancet. 1: 352–354. [DOI] [PubMed] [Google Scholar]
- 35.Skurk T., Hauner H. 2004. Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. Int. J. Obes. Relat. Metab. Disord. 28: 1357–1364. [DOI] [PubMed] [Google Scholar]
- 36.Loskutoff D. J., Samad F. 1998. The adipocyte and hemostatic balance in obesity: studies of PAI-1. Arterioscler. Thromb. Vasc. Biol. 18: 1–6. [DOI] [PubMed] [Google Scholar]
- 37.Alessi M. C., Morange P., Juhan-Vague I. 2000. Fat cell function and fibrinolysis. Horm. Metab. Res. 32: 504–508. [DOI] [PubMed] [Google Scholar]
- 38.Janand-Delenne B., Chagnaud C., Raccah D., Alessi M. C., Juhan-Vague I., Vague P. 1998. Visceral fat as a main determinant of plasminogen activator inhibitor 1 level in women. Int. J. Obes. Relat. Metab. Disord. 22: 312–317. [DOI] [PubMed] [Google Scholar]
- 39.Kontush A., Chantepie S., Chapman M. J. 2003. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 23: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 40.Li X. A., Titlow W. B., Jackson B. A., Giltiay N., Nikolova-Karakashian M., Uittenbogaard A., Smart E. J. 2002. High density lipoprotein binding to scavenger receptor, class B, type I activates endothelial nitric-oxide synthase in a ceramide-dependent manner. J. Biol. Chem. 277: 11058–11063. [DOI] [PubMed] [Google Scholar]
- 41.Yuhanna I. S., Zhu Y., Cox B. E., Hahner L. D., Osborne-Lawrence S., Lu P., Marcel Y. L., Anderson R. G., Mendelsohn M. E., Hobbs H. H., et al. 2001. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 7: 853–857. [DOI] [PubMed] [Google Scholar]
- 42.Kimura T., Tomura H., Mogi C., Kuwabara A., Damirin A., Ishizuka T., Sekiguchi A., Ishiwara M., Im D. S., Sato K., et al. 2006. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J. Biol. Chem. 281: 37457–37467. [DOI] [PubMed] [Google Scholar]
- 43.Mihovilovic M., Robinette J. B., DeKroon R. M., Sullivan P. M., Strittmatter W. J. 2007. High-fat/high-cholesterol diet promotes a S1P receptor-mediated antiapoptotic activity for VLDL. J. Lipid Res. 48: 806–815. [DOI] [PubMed] [Google Scholar]
- 44.Frias M. A., James R. W., Gerber-Wicht C., Lang U. 2009. Native and reconstituted HDL activate Stat3 in ventricular cardiomyocytes via ERK1/2: role of sphingosine-1-phosphate. Cardiovasc. Res. 82: 313–323. [DOI] [PubMed] [Google Scholar]
- 45.Kimura T., Sato K., Kuwabara A., Tomura H., Ishiwara M., Kobayashi I., Ui M., Okajima F. 2001. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J. Biol. Chem. 276: 31780–31785. [DOI] [PubMed] [Google Scholar]
- 46.Kimura T., Sato K., Malchinkhuu E., Tomura H., Tamama K., Kuwabara A., Murakami M., Okajima F. 2003. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler. Thromb. Vasc. Biol. 23: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 47.Nofer J. R., van der Giet M., Tolle M., Wolinska I., von Wnuck Lipinski K., Baba H. A., Tietge U. J., Godecke A., Ishii I., Kleuser B., et al. 2004. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Invest. 113: 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nofer J. R., Bot M., Brodde M., Taylor P. J., Salm P., Brinkmann V., van Berkel T., Assmann G., Biessen E. A. 2007. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 115: 501–508. [DOI] [PubMed] [Google Scholar]
- 49.Nofer J. R., Levkau B., Wolinska I., Junker R., Fobker M., von Eckardstein A., Seedorf U., Assmann G. 2001. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J. Biol. Chem. 276: 34480–34485. [DOI] [PubMed] [Google Scholar]
- 50.Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., et al. 2009. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 325: 1254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim R. H., Takabe K., Milstien S., Spiegel S. 2009. Export and functions of sphingosine-1-phosphate. Biochim. Biophys. Acta. 1791: 692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]