Abstract
The T347S polymorphism in the human apolipoprotein (apo) A-IV gene is present at high frequencies among all the world's populations. Carriers of a 347S allele exhibit faster clearance of triglyceride-rich lipoproteins, greater adiposity, and increased risk for developing atherosclerosis, which suggests that this conservative amino acid substitution alters the structure of apo A-IV. Herein we have used spectroscopic and surface chemistry techniques to examine the structure, stability, and interfacial properties of the apo A-IV 347S isoprotein. Circular dichroism spectroscopy revealed that the 347S isoprotein has similar α-helical structure but lower thermodynamic stability than the 347T isoprotein. Fluorescence spectroscopy found that the 347S isoprotein exhibits an enhanced tyrosine emission and reduced tyrosine→tryptophan energy transfer, and second derivative UV absorption spectra noted increased tyrosine exposure, suggesting that the 347S isoprotein adopts a looser tertiary conformation. Surface chemistry studies found that although the 347S isoprotein bound rapidly to the lipid interface, it has a lower interfacial exclusion pressure and lower elastic modulus than the 347T isoprotein. Together, these observations establish that the T347S substitution alters the conformation of apo A-IV and lowers its interfacial activity—changes that could account for the effect of this polymorphism on postprandial lipid metabolism.
Keywords: genetic polymorphism, circular dichroism spectroscopy, fluorescence spectroscopy, fluorescence resonance energy transfer, UV absorption spectroscopy, surface chemistry, triglyceride, phospholipid, lipoprotein metabolism
Lipoprotein metabolism is regulated by the plasma apolipoproteins, a family of surface-active lipid binding proteins. The largest member of the family, apo B, evolved from ancient lipid transport proteins involved in oogenesis (1) to play a central role in the intracellular assembly of triglyceride-rich lipoproteins in the intestine and liver, whereas the smaller, exchangeable, members of this family evolved from a single primordial gene to control various processes in intravascular and peripheral lipid transport (2). Apo A-IV, a 46 kDa plasma glycoprotein of intestinal origin (3), is the largest of the exchangeable apolipoproteins (2). Although a broad spectrum of physiologic functions has been proposed for apo A-IV, a preponderance of evidence suggests that its primary biological role is in the regulation of dietary lipid absorption and metabolism (4). Apo A-IV is synthesized by intestinal enterocytes during lipid absorption, and it is incorporated into nascent chylomicrons (5). In the postprandial state, apo A-IV enters circulation on lymph chylomicrons (6) and is rapidly displaced from their surface by HDL-associated apolipoproteins (7); thereafter in the fasting state, apo A-IV is associated predominantly with lipid-poor HDL (8).
Like most apolipoproteins, apo A-IV is polymorphic (9). Carriers of the apo A-IV Q360H polymorphism, which is prevalent only in northwestern European Caucasian populations (9), exhibit delayed postprandial chylomicron clearance (10, 11), which we have previously proposed is due to the higher lipid affinity (12) and interfacial exclusion pressure (13) of the 360H isoprotein. However, the most common apo A-IV polymorphism is an ACT→TCT substitution at codon 367 in the APOA4 gene that encodes a threonine→serine substitution at residue 347, near the carboxyl terminus of the protein molecule (14). The T347S polymorphism is found at uniform frequencies of 0.12–0.22 among all the world's populations (9), suggesting that it is an ancient allele that appeared early in human evolution (15). Carriers of a 347S allele also display evidence of altered lipoprotein metabolism, including faster clearance of postprandial triglyceride-rich lipoproteins (11, 16), greater adiposity (17, 18), reduced plasma antioxidant activity (19, 20), and increased risk for atherosclerotic cardiovascular disease (21).
Structural considerations (13, 14) and the fact that most of the observed physiological effects of the 347S allele are the opposite of those conferred by the 360H allele (9–11, 16) lead us to predict that the 347S isoprotein should display an altered structure and lower lipid affinity. However, to-date its biophysical characteristics have not been examined. Herein we report the application of spectroscopic and surface chemistry techniques to investigate the structure and interfacial behavior of human apo A-IV 347S.
EXPERIMENTAL PROCEDURES
Lipids and apolipoproteins
Egg phosphatidylcholine (EPC) and triolein (Sigma, St. Louis, MO) were stored under nitrogen at −20°C. For monolayer studies, EPC was diluted to 0.1 mg/ml in HPLC-grade chloroform (Aldrich, Milwaukee, WI). Phospholipid concentration was confirmed by phosphorous analysis (22). Apo A-IV 347S and 347T isoproteins were isolated from donors homozygous for the respective 347S and 347T alleles (23). The protocol for obtaining blood from human subjects was approved by the Wake Forest University School of Medicine Institutional Review Board; informed consent was obtained from all human subjects. Apo A-IV 347T was purified from blood pooled from two 347T donors; apo A-IV 347S was purified from two individual 347S donors and yielded similar results. No donors carried the apo A-IV 360H allele. The protein concentration of apolipoprotein solutions was determined using bicinchoninic acid (24) and BSA as a standard. All isoprotein preparations were homogeneous by SDS-PAGE with Coomassie Blue staining (Fig. 1).
Fig. 1.
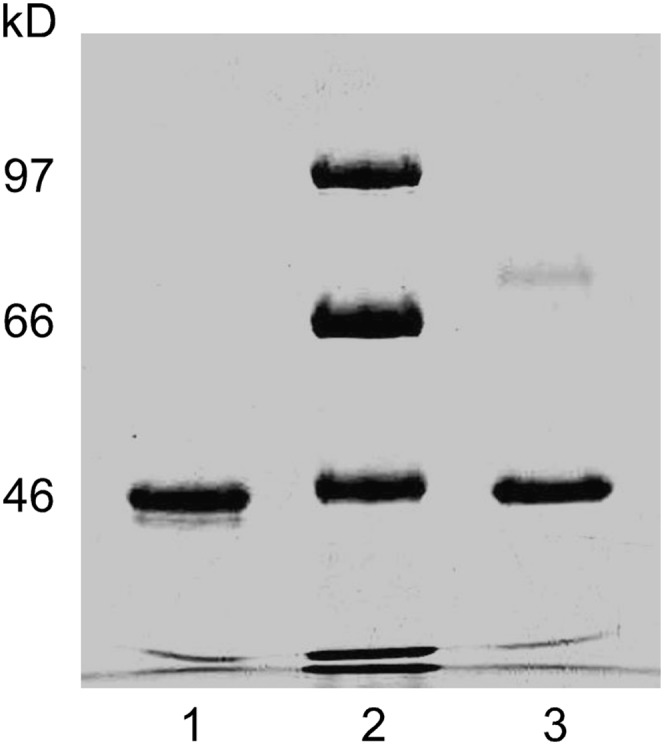
SDS-PAGE of purified apo A-IV 347S and apo A-IV 347T. Lane 1, apo A-IV 347T; lane 2, molecular weight standards; lane 3, apo A-IV 347S. Abbreviation: Apo, apolipoprotein.
Circular dichroism spectroscopy
Circular dichroism (CD) studies were performed on a Jasco J-720 Spectropolarimeter. Spectra of apo A-IV isoproteins at 3 μM in 10 mM Tris (pH 7.5) were recorded at 25°C from 190 to 260 nm using a 0.5 mm thermostated cell, 1 mm spectral band width, and 2 s time constant. Buffer blanks were digitally subtracted. Mean residue ellipticity at 222 nm ([Θ]222) was calculated as ([Θ]raw × MRW)/(10 × l × C), where l is path length in cm and C is the concentration in g/ml, using a mean residue weight (MRW) of 115.1; α-helicity was calculated as previously described (25). Thermal denaturation studies of apo A-IV isoproteins at 1 μM in 10 mM Tris (pH 7.5) were performed by monitoring ellipticity at 222 nm at 2 s intervals as the temperature of the cuvette was increased from 25°C to 75°C at 1°C/min. The cooperativity of unfolding was calculated from the sigmoidicity of the denaturation curves (26). The enthalpy of denaturation (ΔHD) and the thermal denaturation midpoint (Tm) were determined from the slope of Van't Hoff plots of ΔG versus 1/T (13). The entropy of folding (ΔS) was calculated at the denaturation midpoint temperature using the Gibbs-Helmholtz equation.
Fluorescence spectroscopy
Fluorescence studies were performed on an SLM 8000C spectrofluorometer as previously described (12, 25, 27). Emission spectra of apo A-IV isoproteins at 3 μM in 10 mM Tris (pH 7.5) were recorded at 25°C between 290 nm and 400 nm with excitation at 280 nm and 295 nm using a 1 cm cell, 1 s integration, and 4 nm slits for both monochromators. Spectra were excitation-corrected, and corrected for scatter and Raman emission by digital subtraction of buffer blanks. The tyrosine and tryptophan contributions to the emission spectra were deconvoluted by subtracting spectra obtained with excitation at 295 nm from spectra obtained with excitation at 280 after normalization at 370 nm, where tyrosine emission is negligible. Quenching studies were performed by addition of buffered 6M KI. Quenching constants (Kq) were calculated from the change in integrated emission intensity between 300 nm and 400 nm (12); fractional tryptophan exposure was calculated as a ratio to the Kq of an isomolar solution of N-acetyl tryptophanamide. The excitation wavelength dependence of tryptophan emission anisotropy at 270 nm and 305 nm (A305/A270) was measured using Glan-Thompson polarizers in the excitation and emission light paths (12). Red-edge excitation shift (REES) was measured by determining the dependence of the maximum fluorescence emission wavelength on the excitation wavelength from 275 nm to 310 nm (28).
UV absorption spectroscopy
UV absorption studies were performed on Cary 50 UV-Vis spectrophotometer. Spectra of apo A-IV isoproteins at 8.5 μM in 10 mM Tris (pH 7.5) were recorded in dual beam mode at 25°C from 270 to 300 nm using a 1 cm thermostated cell, a 0.15 nm spectral interval, and a 45 nm/min scan rate, and were baseline corrected by digital subtraction of buffer blanks. For each isoprotein, five individual spectra were averaged, smoothed with a polynomial regression using weights computed from a Gaussian density function, and differentiated using a filter length of 5.
Phospholipid monolayer studies
Adsorption of apo A-IV isoproteins at the phospholipid/water interface was examined at 25°C with a KSV 5000 Langmuir Film Balance (KSV Instruments, Helsinki, Finland) (13, 29). EPC monolayers were spread at the air/water interface over 5.65 mM Na2HPO4, 3.05 mM NaH2PO4, 80 mM NaCl (pH 7.0) and allowed to stabilize. Surface pressure at the interface was measured using a Wilhelmy plate with a precision of ±0.1 mN/m. Subphase saturating concentrations of each isoform were determined by injecting increasing amounts of protein in 2.0 M GdnHCl under EPC monolayers spread at 10 mN/m and recording the change in surface tension (ΔΠ). Interfacial exclusion pressure was determined by injecting saturating concentrations of protein under EPC monolayers spread at initial pressures between 5 and 30 mN/m and extrapolating plots of ΔΠ versus initial pressures to zero.
Dynamic interfacial activity
The dynamic interfacial behavior of apo A-IV isoproteins at the oil/water interface was studied at 25°C using a Tracker® tensiometer (TECLIS-ITConcept, Longessaigne, France). The instrument consists of a computer-controlled syringe that pumps fluids into an optical cuvette; a coherent light source that projects the drop image onto a video chip; and software that digitizes the image and calculates drop volume, surface area, and interfacial tension (30). Triolein drops (10 μl) were formed into 6 ml of 41.3 mM Tris (pH 7.5) containing apo A-IV isoproteins at 25 μg/ml. Protein adsorption to the triolein/water interface was recorded as the decrease in interfacial tension (γ) with time. Exponential adsorption rate constants were calculated by log transformation of the initial γ versus time curves. Interfacial elasticity (ϵ) was determined by sinusoidally oscillating the volume of 10 μl drops by ±5 μl at 6 cycles/min and analyzing the change in surface tension as a function of area (31).
RESULTS
Circular dichroism spectroscopy
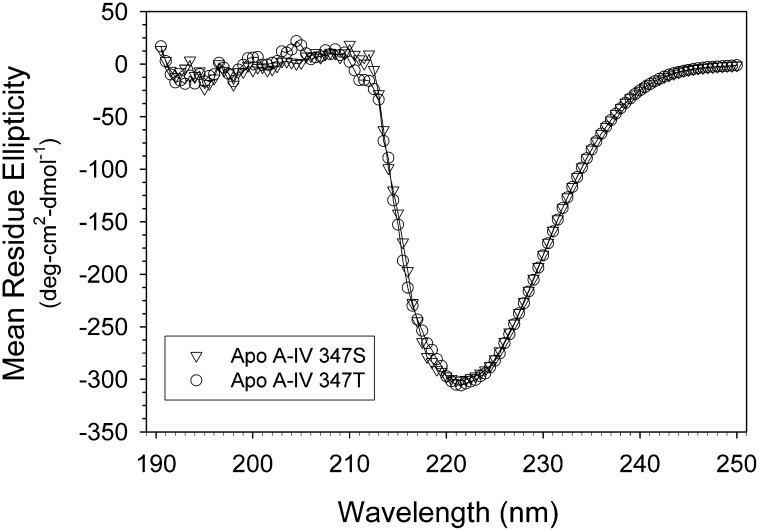
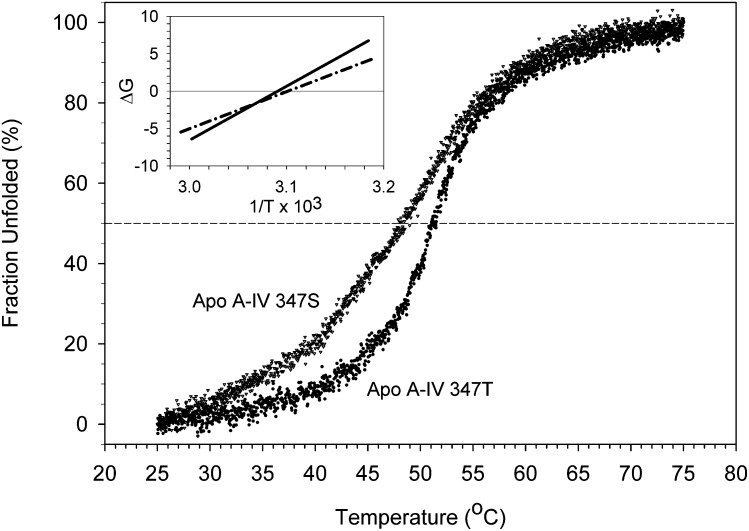
CD spectra of the apo A-IV isoproteins displayed mean residue ellipticity minima at 222 nm of 26,229 ± 849 deg·cm2·dmol−1 for apo A-IV 347S and 28,306 ± 2750 deg·cm2·dmol−1 for apo A-IV 347T (Fig. 2), corresponding to 76% and 77% α-helical structure, respectively. For comparison, the mean residue ellipticity of the apo A-IV 360H isoprotein was previously found to be 26,250 deg·cm2·dmol−1 (12). The 347S isoform unfolded between 35°C and 55°C, with a transition temperature midpoint at 48.8 ± 0.5°C. The 347T isoform was more resistant to thermal denaturation and unfolded between 40°C and 60°C (Fig. 3), with a transition midpoint at 50.2 ± 0.2°C. Cooling curves demonstrated little hysteresis (data not shown), indicating that the thermal denaturation was reversible. The sigmoidicity index of the 347S isoprotein thermal denaturation curve was 8.2, compared with 11.2 for the 347T isoprotein, suggesting that there is less intramolecular domain cooperativity stabilizing its secondary structure. Van't Hoff plots of the data (Fig. 3, inset) yielded thermodynamic stability parameters (Table 1): for apo A-IV 347S, the mean enthalpy of denaturation was 51.3 ± 2.8 kcal/mol, and the mean entropy of unfolding was 159.4 ± 9.0 cal/mol°K; for apo A-IV 347T, the mean enthalpy of denaturation was 70.7 ± 1.4 kcal/mol, and the mean entropy of unfolding was 218.7 ± 4.2 cal/mol°K. Together, these data suggest that the ordered structure of the apo A-IV 347S isoprotein is less stable than apo A-IV 347T.
Fig. 2.
CD spectra of apo A-IV 347S and apo A-IV 347T. The mean residue ellipticity of 3 μM solutions of apo A-IV 347S (▽) and apo A-IV 347T (○) was recorded at 25°C as a function of wavelength from 190 nm to 250 nm. Abbreviations: Apo, apolipoprotein; CD, circular dichroism.
Fig. 3.
Thermal denaturation of apo A-IV 347S and apo A-IV 347T. The fractional unfolding of 1 µM solutions of apo A-IV 347S and apo A-IV 347T was monitored at 222 nm at 2 sec intervals as the cuvette temperature was raised from 25 to 75°C. Inset: Van't Hoff plots of the data. Lines are linear curve fits of the data for apo A-IV 347S (dash-dot line) and apo A-IV 347T (solid line). The X-axis intercepts of the lines yield the reciprocals of the transition temperature midpoints. Abbreviation: Apo, apolipoprotein.
TABLE 1.
CD spectroscopy and derived thermodynamic parameters for apo A-IV 347S and apo A-IV 347T
| [Θ]222 |
Tm |
n |
ΔHD |
ΔS |
|
|---|---|---|---|---|---|
| deg-cm2-dmol−1 | °C | kcal/mo/ | cal/mol°K | ||
| apo A-IV 347S | 26,229 ± 849 | 48.8 ± 0.5 | 8.2 ± 0.9 | 51.3 ± 2.8a | 159.4 ± 9.0a |
| apo A-IV 347T | 28,306 ± 2750 | 50.2 ± 0.2 | 11.2 ± 0.2 | 70.7 ± 1.4 | 218.7 ± 4.2 |
[Θ]222, mean residue ellipticity at 222 nm; Tm, thermal denaturation midpoint temperature; n, denaturation curve sigmoidicity; ΔHD, enthalpy of denaturation; ΔS, entropy of folding. Values are mean ± SE for n = 2–3 determinations.
Abbreviations: Apo, apolipoprotein; CD, circular dichroism.
P = 0.03 for apo A-IV 347S versus apo A-IV 347T by Student's t-test.
Fluorescence spectroscopy
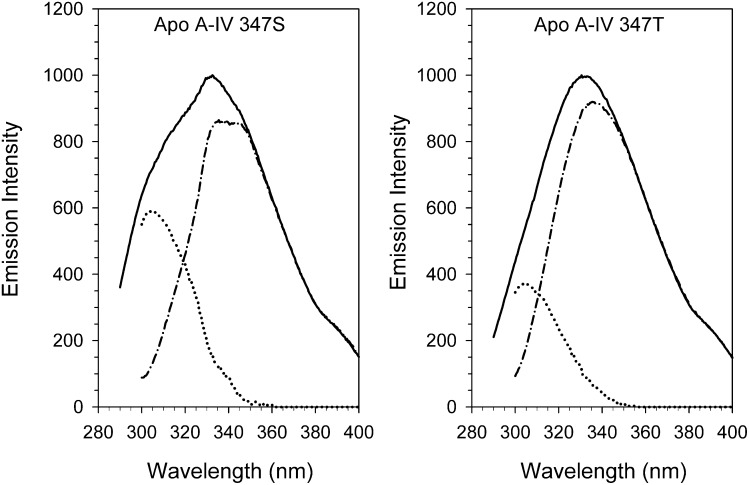
Fluorescence spectra of the apo A-IV 347S and 347T isoforms excited at 280 nm displayed maxima at 331.8 ± 0.3 and 332.1 ± 0.9 nm, respectively (Fig. 4); apo A-IV 347S also displayed a prominent shoulder at 303.5 nm, characteristic of enhanced tyrosine fluorescence emission. Spectral deconvolution showed that the single N-terminal tryptophan residue in both isoproteins exhibited blue-shifted emission maxima at 336 nm, typical of tryptophan in a hydrophobic environment. However, the tryptophan emission of the 347S isoprotein was slightly narrower than the 347T isoprotein (53 nm versus 58 nm at half height) and displayed a second maximum at 342 nm, suggesting the presence of a heterogeneous population of N-terminal conformers. The 347S isoprotein exhibited a more intense tyrosine emission peak and a correspondingly higher tyrosine/tryptophan peak intensity ratio (Table 2) than the 347T isoprotein. As tyrosine fluorescence in apo A-IV is suppressed by efficient energy transfer to the amino terminal tryptophan (27), this finding suggests that the average distance between the N-terminus and one or more tyrosine residues is greater in the 347S isoprotein. This is further suggested by the finding that the A305/A270 tryptophan emission anisotropy ratio, a qualitative measure of intramolecular tyrosine→tryptophan energy transfer efficiency and an indirect measure of the compactness of folding (27), was lower in apo A-IV 347S (Table 2). Interestingly, the tryptophan fluorescence of apo A-IV 347S was more resistant to iodide quenching than apo A-IV 347T, and it also displayed a larger red-edge excitation shift (Table 2), parameters that indicate greater hydrophobic shielding and restricted rotational freedom (28). For comparison, previous studies of the apo A-IV 360H isoprotein found an emission maxima at 330 ± 1 nm, a A305/A270 ratio of 2.32, and a normalized iodide quenching constant of 0.52 (12). Together, these findings further suggest that the global conformation of the apo A-IV 347S isoprotein is more loosely folded than the 347T isoprotein, although its N-terminal domain may paradoxically be locally enfolded more tightly.
Fig. 4.
Fluorescence spectra of apo A-IV 347S and apo A-IV 347T. The fluorescence emission of 3 µM solutions of apo A-IV 347S and apo A-IV 347T excited at 280 nm (solid line) and 295 nm (tryptophan emission, dash-dot line) was monitored from 290 nm to 400 nm. The tyrosine fluorescence emission (dotted line) was deconvoluted from the 280 nm and 295 nm spectra as described in the text. Abbreviation: Apo, apolipoprotein.
TABLE 2.
Fluorescence spectroscopy parameters for apo A-IV 347S and apo A-IV 347T
| λmax |
ImaxTyr/ImaxTrp |
A305/A270 |
REES |
Kq/KNATA |
|
|---|---|---|---|---|---|
| nm | nm | nm | |||
| apo A-IV 347S | 331.8 ± 0.3 | 0.68 ± 0.01a | 1.19 ± 0.04a | 16.5 | 0.16 |
| apo A-IV 347T | 332.1 ± 0.9 | 0.40 ± 0.00 | 2.58 ± 0.02 | 9.0 | 0.45 |
λmax, wavelength of maximum emission with excitation at 280 nm; ImaxTyr/ImaxTrp, tyrosine/tryptophan maximum emission intensity ratio; A305/A270, tryptophan emission anisotropy ratio with excitation at 305 and 270 nm. REES, red-edge excitation shift at 310 nm; Kq/KNATA, iodide quenching constant, normalized to an isomolar solution of N-acetyl tryptophanamide. Values are means ± SE for n = 2–4 determinations.
Abbreviation: Apo, apolipoprotein.
P = 0.001 for apo A-IV 347S versus apo A-IV 347T by Student's t-test.
UV absorption spectroscopy
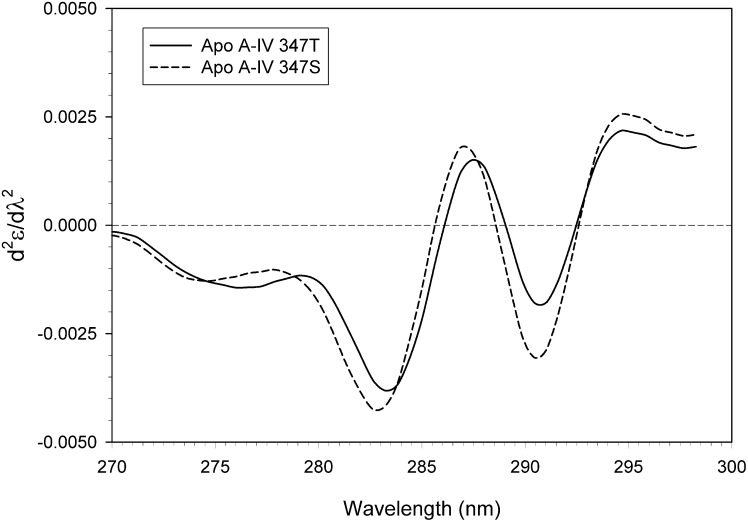
Second derivative UV absorption spectra of the apo A-IV 347S and 347T isoproteins (Fig. 5) displayed multiple positive and negative peaks characteristic of tyrosine and tryptophan absorption in globular proteins (32). The predominant tyrosine absorption peaks for apo A-IV 347T occurred at 279.2, 283.2, and 287.5 nm, whereas for apo A-IV 347S, they were blue-shifted to 277.8, 282.8, and 287.0 nm, respectively, indicative of increased tyrosine residue polarity and exposure (33). Conversely, although the major tryptophan absorption peaks were found at 290.7 nm and 294.7 nm for both isoproteins, the 290.7 nm peak for apo A-IV 347S displayed a deeper minimum, indicating that its single tryptophan was located in a less polar environment (34).
Fig. 5.
Second derivative UV absorption spectra of apo A-IV 347S and apo A-IV 347T. The absorption of 8 µM solutions of apo A-IV isoproteins was monitored from 270 nm to 300 nm. Five individual spectra were averaged, smoothed, and differentiated using a filter length of 5. Apo A-IV 347S (solid line); apo A-IV 347T (dashed line). Abbreviation: Apo, apolipoprotein.
Phospholipid monolayer studies
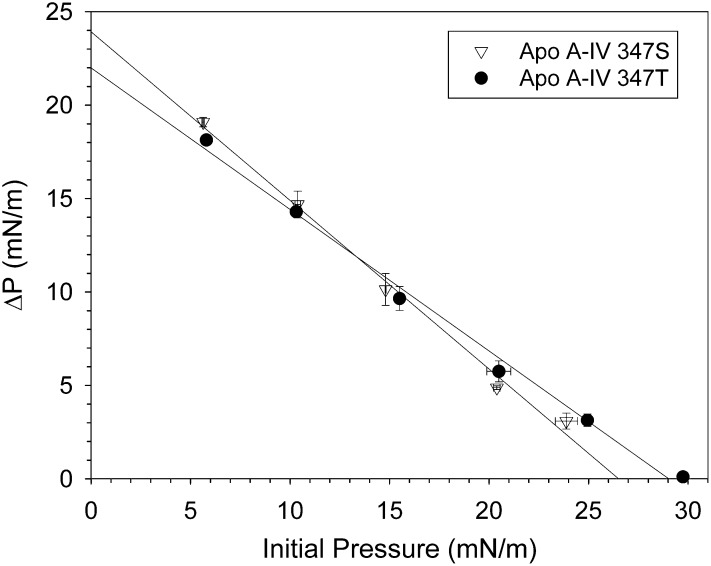
Adsorption of both apo A-IV isoproteins to EPC monolayers spread at 10 mN/m increased as a function of subphase concentration and reached plateau above 5.0 × 10−5 g/dl (data not shown); the exclusion pressure experiments were performed using this saturating subphase concentration. Binding of apo A-IV isoproteins to the EPC/water interface decreased linearly with increasing initial pressure, as evidenced by progressively smaller pressure changes. Extrapolation of the curves to zero yielded mean interfacial exclusion pressures of 26.5 ± 0.2 mN/m for apo A-IV 347S compared with 28.7 ± 0.2 mN/m for apo A-IV-T (Fig. 6). For comparison, the interfacial exclusion pressure of the apo A-IV 360H isoprotein was previously determined to be 30.8 mN/m (13).
Fig. 6.
Interfacial exclusion pressure of apo A-IV 347S and apo A-IV 347T. Apo A-IV 347S (▽) and apo A-IV 347T (•) were injected beneath EPC monolayers spread at increasing initial surface pressures, and the change in surface pressure was determined. Data points are the means ± SE of two separate experiments; the solid lines are linear regressions of the data. Extrapolation of the lines to the X axis yields exclusion pressures of 26.5 ± 0.2 mN/m for apo A-IV 347S and 28.7 ± 0.2 for apo A-IV 347T. Abbreviations: Apo, apolipoprotein; EPC, egg phosphatidylcholine.
Dynamic interfacial activity at the triolein/water interface
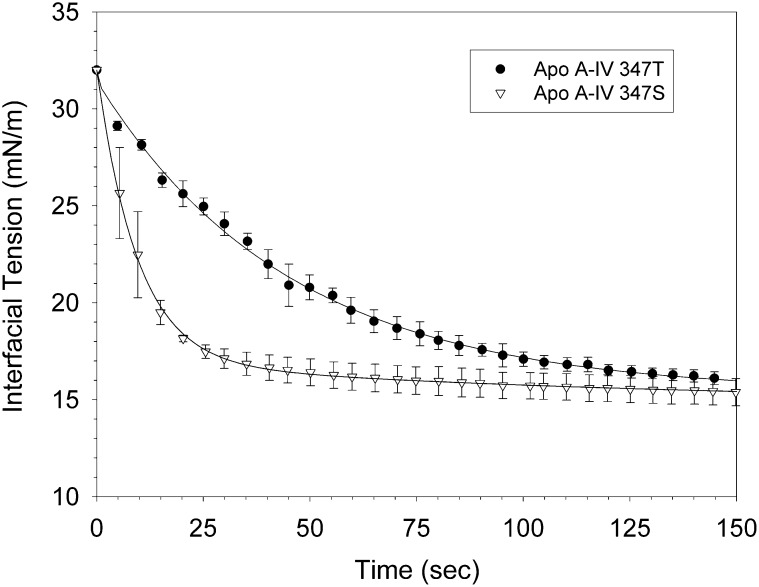
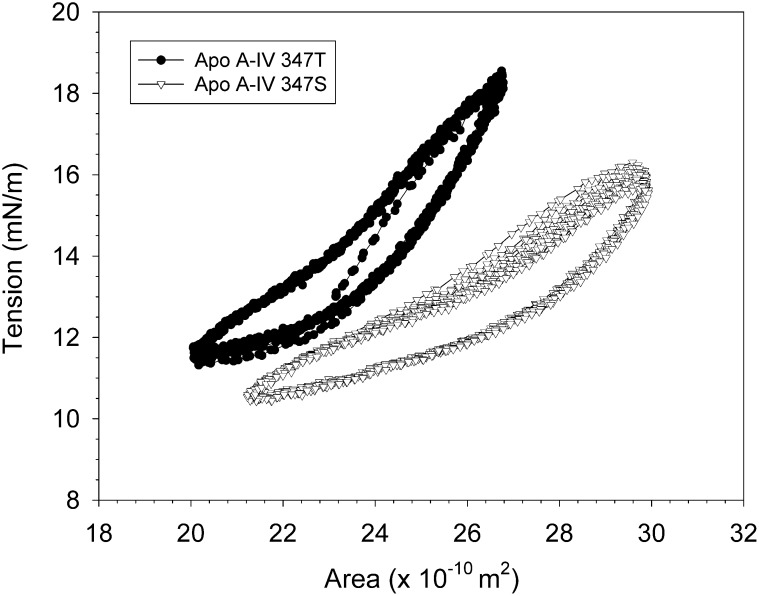
Apo A-IV 347S and apo A-IV 347T bound readily to the triolein/water interface, decreasing the initial surface tension by 13.8 ± 0.7 mN/m and 17.7 ± 0.4 mN/m, respectively (Fig. 7). Apo A-IV 347S bound much more rapidly, as indicated by a binding rate constant of 33.9 ± 1.4 × 10−3 sec−1, compared with 6.3 ± 1.4 × 10−3 sec−1 for apo A-IV 347T (Table 3). With sinusoidal oscillation of the drop volume, the surface area tension loops for A-IV 347S were shifted to the right relative to apo A-IV 347T (Fig. 8), which indicates a more expanded interfacial conformation. The calculated elasticity modulus for apo A-IV 347S, 14.9 ± 0.1 mN/m, was significantly lower than that for apo A-IV 347T, 32.4 ± 1.0 mN/m (Table 3). These findings suggest that at hydrophobic lipid interfaces the apo A-IV 347S isoprotein displays lower affinity and less molecular flexibility.
Fig. 7.
Binding of apo A-IV 347S and apo A-IV 347T) to the triolein/water interface. Triolein drops (10 μl) were injected into the optical cuvette of a ITC Tracker® tensiometer containing 25 μg/ml solutions of apo A-IV 347S (▽) and apo A-IV 347T (•), and the surface tension was continuously monitored. Data points are the means ± SE of two separate experiments. Abbreviation: Apo, apolipoprotein.
TABLE 3.
Interfacial properties of apo A-IV 347S and apo A-IV 347T
| EPC/Water Interface |
Triolein/Water Interface |
|||
|---|---|---|---|---|
| ΠEX | Δγ | Ka | ϵ | |
| mN/m | mN/m | (× 10−3 sec−1) | mN/m | |
| Apo A-IV 347S | 26.5 ± 0.2a | 13.8 ± 0.7b | 33.9 ± 1.4c | 14.9 ± 0.1c |
| Apo A-IV 347T | 28.7 ± 0.2 | 17.7 ± 0.4 | 6.3 ± 1.4 | 32.4 ± 1.0 |
ΠEX, EPC interfacial exclusion pressure; Δγ, change in interfacial tension at the triolein/water interface; Ka adsorption rate constant; ϵ, elastic modulus. Values are means ± SE for n = 2–3 determinations.
Abbreviation: Apo, apolipoprotein; EPC, egg phosphatidylcholine.
P = 0.02 for apo A-IV 347S versus apo A-IV 347T by Student's t-test.
P = 0.01 for apo A-IV 347S versus apo A-IV 347T by Student's t-test.
P < 0.001 for apo A-IV 347S versus apo A-IV 347T by Student's t-test.
Fig. 8.
Elastic behavior of apo A-IV 347S and apo A-IV 347T at the triolein/water interface. Triolein drops (10 μl) were injected into the optical cuvette of a ITC Tracker® tensiometer containing 25 μg/ml solutions of apo A-IV 347S (▽) and apo A-IV 347T (•). After the surface tension had stabilized, the drop volume was sinusoidally oscillated ± 5 μl at six cycles/min, and the change in surface tension was continuously monitored as a function of drop surface area. Abbreviation: Apo, apolipoprotein.
DISCUSSION
These data establish that the T347S polymorphism alters the stability, folding, and interfacial behavior of the apo A-IV molecule. Although CD spectroscopy found no effect of the threonine→serine substitution on the secondary structure of apo A-IV—in keeping with the conservative nature of this substitution and the fact that it resides in a region predicted to contain random coil structure (35)—thermal denaturation studies indicated that the 347S isoprotein is less thermodynamically stable than the 347T isoprotein. Moreover, the reduced cooperativity of thermal denaturation and the lower entropy of unfolding exhibited by apo A-IV 347S imply that its α-helical domains are folded less tightly in its native conformation. The basis for this perturbed stability is suggested by the fluorescence and UV spectroscopy studies.
The fluorescence properties of the single tryptophan at residue 12 in the N-terminus of apo A-IV serve as an informative intrinsic conformational probe (12, 25, 27). Based on the observation of efficient intramolecular tyrosine→tryptophan energy transfer in native apo A-IV, we proposed that the apo A-IV molecule adopts a conformation in which its amino terminal tryptophan is enfolded in the hydrophobic interior of a confluence of amphipathic α-helical domains, within energy transfer range of nonvicinal tyrosine residues (25, 27). Intramolecular cross-linking studies by Tubb et al. have confirmed these predictions and further suggest that apo A-IV adopts a classical “helical bundle” conformation in solution, stabilized by an interaction between the amino and carboxyl termini (36). A similar helical bundle motif has been described for apo A-I (26), apo E (26), and the insect lipid binding protein Lp-III (37). The increased tyrosine emission and decreased internal energy transfer seen in apo A-IV 347S suggests that this interaction is disrupted. Moreover, the blue-shifted tyrosine UV absorption bands indicate that this is accompanied by exposure of tyrosine residues to a more polar milieu. Together, these findings imply that the lower stability of the 347S isoprotein is a consequence of disrupted intramolecular interactions among the central α-helical domains in apo A-IV 347S, which results in a more loosely folded central helical bundle that is more exposed to the external aqueous environment.
How could such a conservative amino acid substitution alter the conformation and stability of the apo A-IV molecule? In mammals, residue 347 demarcates the end of a series of 13 α-helical domains and the beginning of a highly conserved C-terminal tail that contains a variable number of EQQQ repeats (35, 38). As this tail is absent in avian apo A-IV, we have proposed that it is a recent evolutionary adaptation that has functional significance (35). Although serine is slightly more hydrophilic than threonine (39), a single threonine→serine substitution clearly has negligible impact on total molecular hydrophobicity. However, because serine has a smaller molecular volume than threonine (89.0 Å3 versus 116.1 Å3) (40) and allows greater rotational flexibility around the peptide backbone (41), the presence of a serine residue at this critical structural transition point could confer greater rotational freedom on the carboxyl terminal “tail,” thereby disrupting its interaction with the N-terminus and destabilizing molecular folding. Indeed, recent studies by Pearson et al. (42) and Tubb et al. (43) have established that apo A-IV structure, stability, and function is exquisitely sensitive to deletions and single point mutations in either the N- or C-terminus.
In this regard, it was interesting that apo A-IV 347S displayed increased resistance to iodide quenching, which denotes decreased aqueous exposure of its amino terminal tryptophan. Similar increased resistance to iodide quenching is seen when the structure of apo A-IV is disrupted by deletion of α-helical domains (13). Apo A-IV 347S also displayed a large red-edge excitation shift, which indicates constrained tryptophan rotation and solvent dipole relaxation (28) and a deeper UV absorption peak near 290 nm, which is a signature of a nonpolar environment (34). Thus destabilized folding of apo A-IV 347S may enable the N-terminal to assume a locally more compact and/or shielded conformation. Nonetheless, the tryptophan emission data suggest that altered folding may also create distinct populations of conformers in which the N-terminal is exposed to different hydrophobic environments; reduced stability of one of these conformers could contribute to the lower global stability of the 347S isoprotein molecule.
Surface chemistry studies demonstrated that apo A-IV 347S has a lower interfacial exclusion pressure than apo A-IV 347T or apo A-IV 360H (the lowest exclusion pressure, in fact, of any of the exchangeable apolipoproteins (29)), and it also exhibits significantly lower interfacial elasticity. These properties may be the consequence of its decreased thermodynamic stability and looser folding. The interfacial activity of the exchangeable apolipoproteins is mediated by a cooperative interaction of tandem amphipathic α-helical domains (44). However, before apo A-IV can bind to lipid, its central helical bundle must first partially unfold so that the hydrophobic faces of its constituent amphipathic helices are exposed to the lipid/ water interface (29, 43, 45). The decreased stability of the apo A-IV 347S isoprotein lowers the energy barrier for this process, as evidenced by the higher rate constant for its binding to the triolein/water interface. In addition, as the amino terminal in apo A-IV appears to be critical for lipid binding (43), the looser folding of apo A-IV 347S may liberate its amino terminal to initiate interaction with lipid interfaces. However, once adsorbed to the oil/water interface, the 347S isoprotein exhibited lower interfacial elasticity and a more expanded surface conformation, as indicated by its right-shifted surface area tension loops. This inverse relationship between lipid binding rate and elasticity is also observed with apo A-IV C-terminal truncation mutants (R. B. Weinberg, unpublished observations), suggesting that disrupted helical domain interactions can compromise its interfacial elastic behavior.
Many of the functions of apolipoproteins are exerted at the lipid interface, and in this regard the association of apo A-IV with lipid is labile (29) and very sensitive to changes in its stability and conformation (13). Bearing in mind the caveats that the spectroscopic studies examined the structure of only lipid-free apo A-IV isoproteins and that lipid-free apo A-IV undergoes a conformational change when it binds to lipid (29, 36, 45), the surface chemistry studies nonetheless suggest that the interfacial activity of apo A-IV could modulate one of its important physiological functions. The displacement of apo A-IV from the surface of lymph chylomicrons upon their entry into plasma by HDL-associated apo C-II and apo E is an essential step in postprandial lipid metabolism, as it enables chylomicrons to be recognized as a substrate by lipoprotein lipase and targets chylomicron remnants for hepatic uptake (46). In carriers of an apo A-IV 360H allele, postprandial triglyceride clearance is delayed (10, 11); we have proposed that this is a consequence of the higher lipid affinity (12) and interfacial exclusion pressure (30.8 mN/m (13)) of the 360H isoprotein, which impedes transfer of apo C-II and apo E from HDL to the chylomicron surface. Thus, conversely, in carriers of an apo A-IV 347S allele, the lower surface activity and interfacial exclusion pressure of the 347S-isoform (26.5 mN/m) could instead facilitate the transfer of apo C-II and apo E, thereby accelerating postprandial chylomicron clearance (11, 16).
In summary, the commonly occurring and widely distributed T347S polymorphism alters the folding and thermodynamic stability of apo A-IV and lowers its affinity for hydrophobic lipid interfaces. These data, which complement previous biophysical (12, 13) and clinical (10, 11) studies of the apo A-IV Q360H polymorphism, suggest that differential lipid affinity and interfacial behavior of commonly occurring apo A-IV isoproteins may modulate the effect of this apolipoprotein on postprandial lipid metabolism.
Footnotes
Abbreviations:
- Apo
- apolipoprotein
- CD
- circular dichroism
- EPC
- egg phosphatidylcholine
This work was supported by grant HL-30897 from the National Heart, Lung and Blood Institute of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Mann C. J., Anderson T. A., Read J., Chester S. A., Harrison G. B., Kochl S., Ritchie P. J., Bradbury P., Hussain F. S., Amey J., et al. 1999. The structure of vitellogenin provides a molecular model for the assembly and secretion of atherogenic lipoproteins. J. Mol. Biol. 285: 391–408. [DOI] [PubMed] [Google Scholar]
- 2.Luo C. C., Li W. H., Moore M. N., Chan L. 1986. Structure and evolution of the apolipoprotein multigene family. J. Mol. Biol. 187: 325–340. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg R. B., Scanu A. M. 1983. The isolation and characterization of human apolipoprotein A-IV from lipoprotein depleted serum. J. Lipid Res. 24: 52–59. [PubMed] [Google Scholar]
- 4.Kalogeris T. J., Rodriquez M. D., Tso P. 1997. Control of synthesis and secretion of intestinal apolipoprotein A-IV. J. Nutr. 127: 537S–543S. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi H., Nutting D. F., Fujimoto K., Cardelli J. A., Black D., Tso P. 1990. Transport of lipid and apolipoproteins apo A-I and apo A-IV in intestinal lymph of the rat. J. Lipid Res. 31: 1613–1625. [PubMed] [Google Scholar]
- 6.Green P. H., Glickman R. M., Saudek C. D., Blum C. B., Tall A. R. 1979. Human intestinal lipoproteins: studies in chyluric subjects. J. Clin. Invest. 64: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberg R. B., Spector M. S. 1985. Human apolipoprotein A-IV: displacement from the surface of triglyceride-rich particles by HDL2-associated C-apoproteins. J. Lipid Res. 26: 26–37. [PubMed] [Google Scholar]
- 8.Bisgaier C. L., Sachdev O. P., Megna L., Glickman R. M. 1985. Distribution of apolipoprotein A-IV in human plasma. J. Lipid Res. 26: 11–25. [PubMed] [Google Scholar]
- 9.Weinberg R. B. 2002. Apolipoprotein A-IV and diet-gene interactions. Curr. Opin. Lipidol. 13: 125–134. [DOI] [PubMed] [Google Scholar]
- 10.Jansen S., Lopez-Miranda J., Ordovas J. M., Zambrana J. L., Marin C., Ostos M. A., Castro P., McPherson R., Lopez Segura F., Blanco A., et al. 1997. Effect of 360His mutation in apolipoprotein A-IV on plasma HDL-cholesterol response to dietary fat. J. Lipid Res. 38: 1995–2002. [PubMed] [Google Scholar]
- 11.Hockey K. J., Anderson R. A., Hantgan R. R., Weinberg R. B. 2001. Effect of the apolipoprotein A-IV Q360H polymorphism on post-prandial plasma triglyceride clearance. J. Lipid Res. 42: 211–217. [PubMed] [Google Scholar]
- 12.Weinberg R. B., Jordan M., Steinmetz A. 1990. Distinctive structure and function of human apolipoprotein variant, Apo A-IV-2. J. Biol. Chem. 265: 18372–18378. [PubMed] [Google Scholar]
- 13.Weinberg R. B., Anderson R. A., Cook V. R., Emmanuel F., Denefle P., Tall A. R., Steinmetz A. 2002. Interfacial exclusion pressure determines the ability of apolipoprotein A-IV truncation mutants to activate cholesterol ester transfer protein. J. Biol. Chem. 277: 21549–21553. [DOI] [PubMed] [Google Scholar]
- 14.Lohse P., Kindt M. R., Rader D. J., Brewer H. B. 1991. Three genetic variants of apolipoprotein A-IV. J. Biol. Chem. 266: 13513–13518. [PubMed] [Google Scholar]
- 15.Kamboh M. I., Haman R. F., Ferrell R. E. 1992. Two common polymorphisms in the apo A-IV coding gene: their evolution and linkage disequilibrium. Genet. Epidemiol. 9: 305–315. [DOI] [PubMed] [Google Scholar]
- 16.Ostos M. A., Lopez-Miranda J., Ordovas J. M., Marin C., Blanco A., Castro P., Lopez-Segura F., Jimenez-Pereperez J., Perez-Jimenez F. 1998. Dietary fat clearance is modulated by genetic variation in apolipoprotein A-IV gene locus. J. Lipid Res. 39: 2493–2500. [PubMed] [Google Scholar]
- 17.Wallace A. J., Humphries S. B., Fisher R. M., Mann J. I., Chisholm A., Sutherland W. H. F. 2000. Genetic factors associated with response of LDL subfractions to change in the nature of dietary fat. Atherosclerosis. 149: 387–394. [DOI] [PubMed] [Google Scholar]
- 18.Heilbronn L. K., Noakes M., Morris A. M., Kind K. L., Clifton P. M. 2000. 360His polymorphism of the apolipoprotein A-IV gene and plasma lipid response to energy restricted diets in overweight subjects. Atherosclerosis. 150: 187–192. [DOI] [PubMed] [Google Scholar]
- 19.Wong W. M., Stephens J. W., Acharya J., Hurel S. J., Humphries S. E., Talmud P. J. 2004. The APOA4 T347S variant is associated with reduced plasma TAOS in subjects with diabetes mellitus and cardiovascular disease. J. Lipid Res. 45: 1565–1571. [DOI] [PubMed] [Google Scholar]
- 20.Wong W. M., Gerry A. B., Putt W., Roberts J. L., Weinberg R. B., Humphries S. E., Leake D. S., Talmud P. J. 2007. Common variants of apolipoprotein A-IV differ in their ability to inhibit low density lipoprotein oxidation. Atherosclerosis. 192: 266–274. [DOI] [PubMed] [Google Scholar]
- 21.Wong W. M., Hawe E., Li L. K., Miller G. J., Nicaud V., Pennacchio L. A., Humphries S. E., Talmud P. J. 2003. Apolipoprotein AIV gene variant S347 is associated with increased risk of coronary heart disease and lower plasma apolipoprotein AIV levels. Circ. Res. 92: 969–975. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234: 466–468. [PubMed] [Google Scholar]
- 23.Weinberg R. B., Hopkins R. A., Jones J. B. 1996. Purification, isoform characterization, and quantitation of human apolipoprotein A-IV. Plasma Lipoproteins. Part C: Quantitation. Methods in Enzymology (series). Vol. 263 Bradley W. A., Gianturco S. H., Segrest J. P., Academic Press, New York: 282–296. [DOI] [PubMed] [Google Scholar]
- 24.Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. 1985. Protein assay using bicinchoninic acid. Anal. Biochem. 150: 76–85. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg R. B., Spector M. S. 1985. Structural properties and lipid binding of human apolipoprotein A-IV. J. Biol. Chem. 260: 4914–4921. [PubMed] [Google Scholar]
- 26.Saito H., Dhanasekaran P. D., Nguyen P., Holvoet S., Lund-Katz M. C., Phillips M. C. 2003. Domain structure and lipid interaction in human apolipoproteins A-I and E, a general model. J. Biol. Chem. 278: 23227–23232. [DOI] [PubMed] [Google Scholar]
- 27.Weinberg R. B. 1988. Exposure and electronic interaction of tyrosine and tryptophan residues in human apolipoprotein A-IV. Biochemistry. 27: 1515–1521. [DOI] [PubMed] [Google Scholar]
- 28.Chattopadhyay A. 2003. Exploring membrane organization and dynamics by the wavelength-selective fluorescence approach. Chem. Phys. Lipids. 122: 3–17. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg R. B., Ibdah J. A., Phillips M. C. 1992. Adsorption of apolipoprotein A-IV to phospholipid monolayers spread at the air/water interface. J. Biol. Chem. 267: 8977–8983. [PubMed] [Google Scholar]
- 30.Labourdenne S., Gaudry-Rolland N., Letellier S., Lin M., Cagna A., Esposito G., Verger R., Riviere C. 1994. The oil-drop tensiometer: potential applications for studying the kinetics of (phospho)lipase action. Chem. Phys. Lipids. 71: 163–173. [Google Scholar]
- 31.Benjamins J., Cagna A., Lucassen-Reynders E. H. 1996. Viscoelastic properties of triacylglycerol/water interfaces covered with proteins. Colloids Surf. 114: 245–254. [Google Scholar]
- 32.Balestrieri C., Colonna G., Giovane A., Irace G., Servillo L. 1978. Second-derivative spectroscopy of proteins. A method for the quantitative determination of aromatic amino acids in proteins. Eur. J. Biochem. 90: 433–440. [DOI] [PubMed] [Google Scholar]
- 33.Ragone R., Colonna G., Balestrieri C., Servillo L., Irace G. 1984. Determination of tyrosine exposure in proteins by second-derivative spectroscopy. Biochemistry. 23: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 34.Soulages J. L., Arrese E. L. 2000. Dynamics and hydration of the alpha-helices of apolipophorin III. J. Biol. Chem. 275: 17501–17509. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg R. B., Anderson R. A., Cook V. R., Emmanuel F., Denefle P., Hermann M., Steinmetz A. 2000. Structure and interfacial properties of chicken apolipoprotein A-IV. J. Lipid Res. 41: 1410–1418. [PubMed] [Google Scholar]
- 36.Tubb M. R., Silva R. A., Fang J., Tso P., Davidson W. S. 2008. A three-dimensional homology model of lipid-free apolipoprotein A-IV using cross-linking and mass spectrometry. J. Biol. Chem. 283: 17314–17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breiter D. R., Kanost M. R., Benning M. M., Wesenberg G., Law J. H., Wells M. A., Rayment I., Holden H. M. 1991. Molecular structure of an apolipoprotein determined at 2.5-A resolution. Biochemistry. 30: 603–608. [DOI] [PubMed] [Google Scholar]
- 38.Weinberg R. B. 1994. Identification of functional domains in the plasma apolipoproteins by analysis of inter-species amino acid sequence variability. J. Lipid Res. 35: 2212–2222. [PubMed] [Google Scholar]
- 39.Engelman D. M., Steitz T. A., Goldman A. 1986. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu. Rev. Biophys. Biophys. Chem. 15: 321–353. [DOI] [PubMed] [Google Scholar]
- 40.Zamyatnin A. A. 1972. Protein volume in solution. Prog. Biophys. Mol. Biol. 24: 107–123. [DOI] [PubMed] [Google Scholar]
- 41.Vihinen M., Torkkila E., Riikonen P. 1994. Accuracy of protein flexibility predictions. Proteins. 19: 141–149. [DOI] [PubMed] [Google Scholar]
- 42.Pearson K., Tubb M. R., Tanaka M., Zhang X. Q., Tso P., Weinberg R. B., Davidson W. S. 2005. Specific sequences in the N and C termini of apolipoprotein A-IV modulate its conformation and lipid association. J. Biol. Chem. 280: 38576–38582. [DOI] [PubMed] [Google Scholar]
- 43.Tubb M. R., Silva R. A., Pearson K. J., Tso P., Liu M., Davidson W.S. 2007. Modulation of apolipoprotein A-IV lipid binding by an interaction between the N and C termini. J. Biol. Chem. 282: 28385–28394. [DOI] [PubMed] [Google Scholar]
- 44.Segrest J. P., Jones M. K., De Loof H., Brouillette C. G., Venkatachalapathi Y. V., Anatharamaiah G. M. 1992. The amphipathic helix in exchangeable apolipoproteins: a review of secondary structure and function. J. Lipid Res. 33: 141–166. [PubMed] [Google Scholar]
- 45.Weinberg R. B., Jordan M. 1990. Effects of phospholipid on the structure of human apolipoprotein A-IV. J. Biol. Chem. 265: 8081–8086. [PubMed] [Google Scholar]
- 46.Hussain M. M., Kancha R. K., Zhou Z., Luchoomun J., Zu H., Bakillah A. 1996. Chylomicron assembly and catabolism: role of apolipoproteins and receptors. Biochim. Biophys. Acta. 1300: 151–170. [DOI] [PubMed] [Google Scholar]