Abstract
The rare disease cerebrotendinous xanthomatosis (CTX) is due to a lack of sterol 27-hydroxylase (CYP27A1) and is characterized by cholestanol-containing xanthomas in brain and tendons. Mice with the same defect do not develop xanthomas. The driving force in the development of the xanthomas is likely to be conversion of a bile acid precursor into cholestanol. The mechanism behind the xanthomas in the brain has not been clarified. We demonstrate here that female cyp27a1−/− mice have an increase of cholestanol of about 2.5- fold in plasma, 6-fold in tendons, and 12-fold in brain. Treatment of cyp27a1−/− mice with 0.05% cholic acid normalized the cholestanol levels in tendons and plasma and reduced the content in the brain. The above changes occurred in parallel with changes in plasma levels of 7α-hydroxy-4-cholesten-3-one, a precursor both to bile acids and cholestanol. Injection of a cyp27a1−/− mouse with 2H7-labeled 7α-hydroxy-4-cholesten-3-one resulted in a significant incorporation of 2H7-cholestanol in the brain. The results are consistent with a concentration-dependent flux of 7α-hydroxy-4-cholesten-3-one across the blood-brain barrier in cyp27a1−/− mice and subsequent formation of cholestanol. It is suggested that the same mechanism is responsible for accumulation of cholestanol in the brain of patients with CTX.
Keywords: CYP27A1, 7α-hydroxy-4-cholesten-3-one, blood-brain barrier, tendon xanthomas
Sterol 27-hydroxylase (CYP27A1) is a mitochondrial cytochrome P-450 present in most cells and tissues (1). In the liver, the enzyme catalyzes the initial step in the degradation of the steroid side-chain in bile acid biosynthesis. Part of the latter synthesis may start extrahepatically with a 27-hydroxylation of cholesterol followed by a flux of 27-hydroxycholesterol or cholestenoic acid to the liver. This mechanism may be regarded as an alternative to reversed cholesterol transport, and CYP27A1 can thus be regarded to be an antiatherogenic enzyme.
CYP27A1 deficiency [cerebrotendinous xanthomatosis (CTX)] is a rare familial lipid storage disease characterized by accumulation of cholesterol and cholestanol in most tissues, in particular in tendon and brain xanthomas [for a review, see (2)]. The most serious symptoms: dementia, cerebellar ataxia, and spinal cord paresis, are thought to be caused by the brain xanthomas. It is noteworthy that the xanthomas develop in spite of normal circulating levels of cholesterol. As a consequence of the reduced capacity to form normal bile acids and the reduced negative feedback inhibition of the cholesterol 7α-hydroxylase, CTX patients may excrete gram amounts of 7α-hydroxylated bile alcohols in feces.
In contrast to humans, disruption of the CYP27A1 gene in mice does not lead to formation of xanthomas in tendons or in brain (3–6). In similarity with CTX patients, Cyp27a1−/− mice have a reduced formation of bile acid, but there is a low excretion only of 7α-hydroxylated bile alcohols. As a consequence of the lack of bile acids and reduced cholesterol absorption, CYP7A1 is modestly upregulated and cholesterol synthesis is increased, at least in the liver.
It is well documented that treatment of CTX patients with bile acids, in particular chenodeoxycholic acid, reverses the cholestanol accumulation and the symptoms (2, 7). Even the size of the xanthomas in the brain may be reduced as a result of such treatment (8). In cyp27a1−/− mice, treatment with cholic acid normalizes CYP7A1 activity and cholesterol synthesis (9).
To understand the pathogenetic mechanism behind formation of xanthomas in patients with CTX, it is of interest to compare the effect of CYP27A1 deficiency on some key enzyme systems and some key metabolic products in mice and humans. It has been reported that the bile acid deficiency leads to a 22-fold upregulation of cholesterol 7α-hydroxylase activity in CTX patients but only 2-7-fold in Cyp27a1−/− mice (4). In accordance with this, the circulating levels of 7α-hydroxycholesterol are increased about 4-fold in Cyp27a1−/− mice (6) but often more than 50-fold in CTX-patients (unpublished observation).
Another interesting difference between CTX patients and Cyp27a1−/− mice is the degree of accumulation of cholestanol in the circulation. In patients with CTX, the levels of plasma cholestanol are generally increased about 7-fold or more, whereas the levels in Cyp27a1−/− mice have been reported to be increased by a factor of about 2-fold (5, 7).
There is a clear link between the increased cholesterol 7α -hydroxylase activities in patients with CTX and the accumulation of cholestanol. By injecting 7α-tritium-labeled cholesterol and measuring incorporation of the label in cholestanol, we could demonstrate that the major part of the cholestanol formed in CTX involves 7α-hydroxylated intermediates (10). We have also shown that the bile acid precursor 7α-hydroxy-4-cholesten-3-one is metabolized into cholestanol under in vitro conditions, with cholesta-4,6-dien-3-one and 4-cholesten-3-one as intermediates (10). The latter intermediates are markedly accumulated in the circulation of patients with CTX (7).
The origin of the cholestanol present in the brain of patients is not obvious. The simplest mechanism, direct blood-to-brain passage of cholestanol formed extracerebrally, is dependent upon the ability of cholestanol to cross the blood-brain barrier. Buchmann and Claussen (11) reported that rabbits fed a diet enriched with cholestanol for 8 weeks had brain cholestanol about twice that of animals fed a control diet. Additionally, Buyn et al. (12) showed that feeding mice with 1% cholestanol for 8 months led to a significant enrichment of this sterol in the cerebellum. Because of the possibility of a contamination from blood vessels, it is difficult to draw firm conclusions from these studies. If a passage of cholestanol does occur from the circulation into the brain, the efficiency of such transfer must be very low. There is a close structural similarity between cholesterol and cholestanol, and it is well established that there is no significant transfer of cholesterol over the blood-brain barrier [for a review, see ref (13)].
We have suggested an alternative mechanism in which there is a passage of a circulating precursor such as 7α-hydroxycholesterol or 7α-hydroxy-4-cholesten-3-one into the brain, with subsequent conversion into cholestanol. In accordance with this hypothesis, we recently demonstrated a very efficient transfer of 7α-hydroxy-4-cholesten-3-one across cultured porcine brain endothelial cells (a model for the blood-brain barrier) that was about 100 times more efficient than the transfer of cholestanol (14). Furthermore, there was an efficient conversion of 7α-hydroxy-4-cholesten-3-one into cholestanol in cultured neuronal and glial cells as well as in monocyte-derived macrophages of human origin.
In the present work, we measured the content of cholestanol in the circulation, liver, brain, and tendons of cyp27a1−/− mice. In accordance with previous reports, no xanthomas were formed, but there was a marked and hitherto unreported accumulation of cholestanol in brain and tendons of the cyp27a1−/− mice. The accumulation was less marked in liver and plasma. In addition, we measured the concentration of the cholestanol precursor, 7α-hydroxy-4-cholesten-3-one, in plasma under different conditions. The latter precursor was also injected in cyp27a1−/− mice. The results are consistent with the hypothesis that 7α-hydroxy-4-cholesten-3-one is an important precursor of cholestanol in the brain of the cyp27a1−/− mice.
MATERIALS AND METHODS
Materials
7α-Hydroxy-4-cholesten-3-one was obtained from Steraloids. 2H7-labeled 7α-hydroxycholesterol, with a purity of >99%, was obtained from Avanti. 2H7-labeled 7α-hydroxy-4-cholesten-3-one was synthesized from 2H7-labeled 7α-hydroxycholesterol by enzymatic oxidation as described (15). The 2H7-labeled 7α-hydroxy-4-cholesten-3-one obtained was purified by preparative thin-layer chromatography using toluene/ethyl acetate 1/1 (v/v) as moving phase.
CYP27A1 knockout mice
Generation of these mice in Israel has been described previously (6). The breeding of these cyp27a1−/− mice in our Swedish animal facility became complicated by low mothering nurture instincts and aggressive behavior toward pups. Because of this, we generated the cyp27a1−/− mice from heterozygotes on a C57BL/6J background. This breeding was uncomplicated and also generated the cyp27a1+/+ mice that were used as controls. The mice had free access to normal chow and water. In one set of experiments, mice were fed with normal chow containing 5% cholestyramine or 0.05% cholic acid. In the case of treatment with cholestyramine, the mice were 8–9 weeks old at the start of the experiment. In the case of treatment with cholic acid, the mice were 3 weeks old at the start of the experiment. The treatment continued for 8–9 weeks, following which they were euthanized by carbon dioxide inhalation. Cerebrum, cerebellum, liver, and tendons were collected immediately after collection of blood by cardiac puncture. There were 4–5 mice in each group, with the exception of the group of cyp27a1−/− females treated with cholestyramine that consisted of three animals and the group with male cyp27a1−/− mice on chow diet, which consisted of eight animals.
In one experiment, a cyp27a1−/− mouse was subjected to daily injections of unlabeled 7α-hydroxy-4-cholesten-3-one, 200 μg dissolved in 500 μl saline containing BSA 1% (w/v) and 10% ethanol (v/v) for 3 months. In another experiment, a cyp27a1−/− mouse was injected daily with 2H7-labeled 7α-hydroxy-4-cholesten-3-one, 100 μg dissolved in 500 μl saline containing BSA 1% (w/v) and 10% ethanol (v/v) for 9 days. In accordance with previous work (6), there were no signs of xanthomas in the tendons, liver, or brain of the cyp27a1−/− mice.
All experimental protocols were approved by the local ethics committee for animal experiments.
Lipid extractions
Lipids were extracted from the brain as described elsewhere with some modifications (16, 17). Approximately 50–100 mg of brain tissue was added to 1 ml of homogenization buffer (5 mM EDTA, 50 μg/ml butylated hydroxytoluene in phosphate-buffered saline, pH 7.4) in a clean glass tube, and the tissue was disrupted using a polytron homogenizer. Five milliliters of chloroform:methanol (2:1, v:v) were added to the homogenate, and the vials were mixed by vortexing and shaken at room temperature overnight. Mouse plasma (25 μl) was added to 1 ml of NaCl (0.9% solution) and 4 ml of chloroform:methanol (2:1, v:v) and incubated on a shaking platform for 1 h at room temperature. Mouse livers were extracted in 3 ml of chloroform:methanol (2:1, v:v) for 24 h at room temperature. Liver pieces were removed and 1 ml of NaCl (0.9% solution) was added. Samples were centrifuged at 10,000 g for 10 min. The organic phase was transferred to a new vial. The aqueous phase was reextracted one more time. Mouse tendons were extracted in 3 ml of chloroform:methanol (2:1, v:v) for 5 days at +4°C. The organic phases from different tissues were dried under a stream of nitrogen gas, and chloroform:methanol (2:1, v:v) was added to the dried samples to achieve suitable concentrations.
Sterol analysis
Sterols in the lipid extract were assayed by combined gas chromatography-MS of the trimethylsilyl ether derivative similar to previously described methods from our laboratory (14). The assay of cholestanol was different from the previously used method, however, by using 2H4-lathosterol (18) as internal standard and the ion at m/z 460 and m/z 462 in the selected ion monitoring of cholestanol and 2H4 lathosterol, respectively. The latter compound was used as internal standard also in the assay of lathosterol. Cholesterol was assayed by isotope dilution-MS as described previously using 2H7-cholesterol as internal standard (14). Cholesterol precursors were analyzed by isotope dilution MS as previously described (19). Content of deuterium in cholestanol isolated from the mouse injected with 2H7-7α-hydroxy-4-cholesten-3-one was calculated by use of combined gas chromatography-MS and selected monitoring of the molecular ions at m/z 460 and m/z 467. 7α-Hydroxy-4-cholesten-3-one was analyzed by LC-MS-MS as described previously (15).
Isolation and measurement of mRNA levels
RNA was isolated from mouse brains with TRIzol Reagent according to the manufacturer's instructions (Invitrogen). The mRNA levels of the different genes relative to the housekeeping gene, hprt, were measured as described previously (20).
Analysis of data
Data are reported as the mean ± SEM in the specified number of individual animals. Differences between mean values were tested for statistical significance (* P ≤ 0.05, ** P ≤ 0.01, ***P ≤ 0.001) by the two-tailed Student's t-test assuming equal variance.
RESULTS
Cholestanol levels in plasma, liver, tendons, and brain of wild-type and cyp27a1-deficient mice
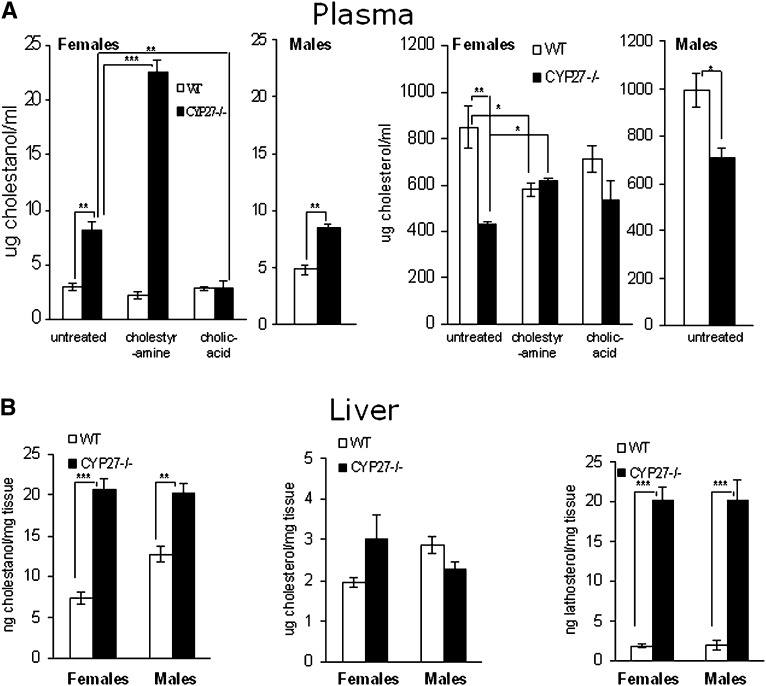
In accordance with previous work (5), the levels of cholestanol in the circulation of both male and female cyp27a1−/− mice were increased about two-fold in relation to wild type (Fig. 1). Also in accordance with previous investigations (3), the levels of cholesterol were significantly reduced by about 50% in female mice. The levels of cholestanol were increased 2- to 3-fold in the liver of the female cyp27a1−/− mice and about 2-fold in the liver of the males (Fig. 1). The cholesterol content in the liver was significantly increased in the female cyp27a1−/− mice and decreased in the liver of the male cyp27a1−/− mice (Fig. 1). Cholesterol synthesis was markedly increased in the liver of both male and female cyp27a1−/− mice as shown by the lathosterol levels (Fig. 1).
Fig. 1.
Levels of sterols (A) in plasma of female wild-type (WT) and CYP27KO mice on different treatments (WT and KO; ctrl n3 and n3, cholestyramine n3 and n3, cholic acid n5 and n5) and male WT (n5) and CYP27KO (n3) mice on control diet (B) in liver of female WT (n4) and CYP27KO (n5) and male WT (n5) and CYP27KO (n8) on control diet.
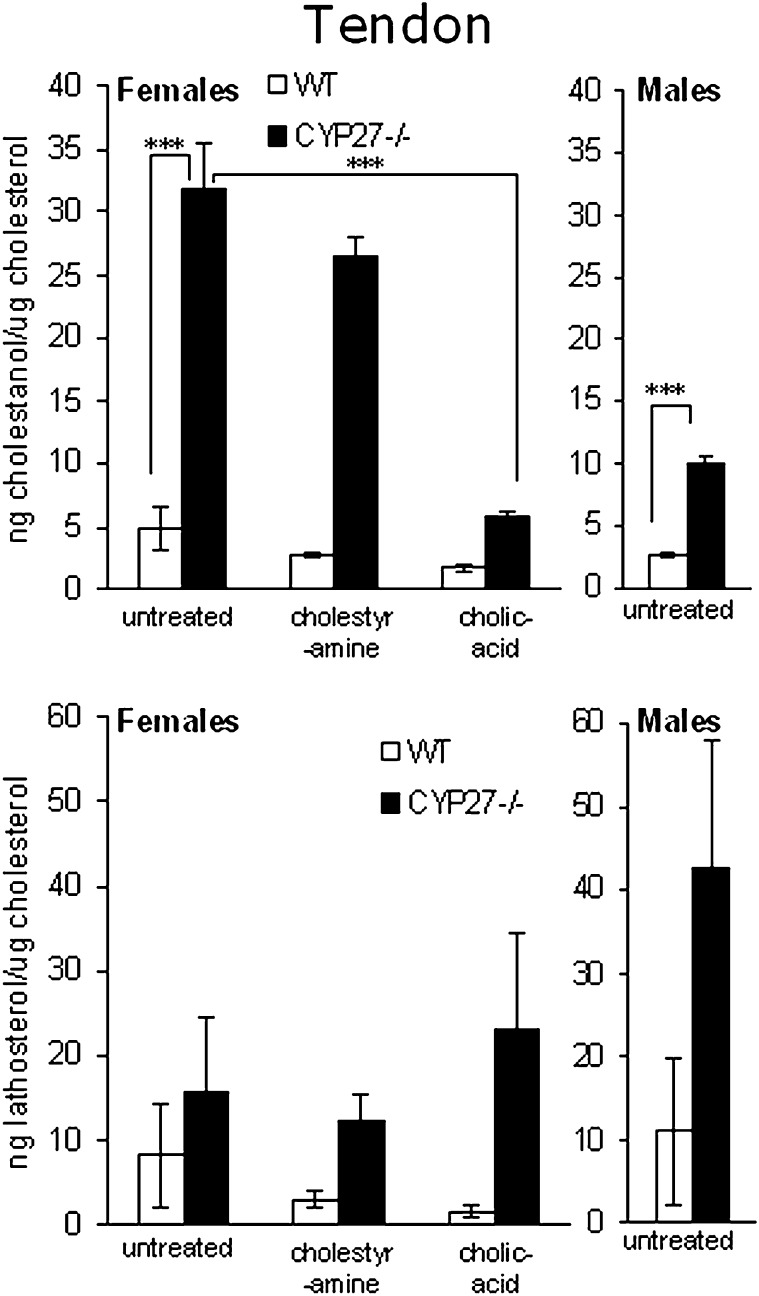
In view of the fact that tendons are preferential sites for formation of cholestanol-containing xanthomas in patients with CTX, we also measured the cholestanol content in the Achilles tendons of the cyp27a1−/− mice and wild-type mice. In this case, we got more reproducible results when expressing the levels of cholestanol in relation to cholesterol than to weight of tissue. The levels of cholesterol in the tendons were not significantly affected by the knockout of cyp27a1, but the individual variations were great (results not shown). As shown in Fig. 2, the content of cholestanol in relation to cholesterol was about 6-fold higher in female cyp27a1−/− mice than in wild-type mice. The corresponding figure for male mice was about 4-fold. The lathosterol levels were increased under all conditions, suggesting increased cholesterol synthesis (Fig. 2). In the case of female mice on a control diet, this level did not reach statistical significance due to the great inter-individual variations (P > 0.05).
Fig. 2.
Levels of sterols in tendons of female wild-type (WT) and CYP27KO mice on different treatments (WT and KO; ctrl n4 and n5, cholestyramine n4 and n3, cholic acid n5 and n5) and male WT (n5) and CYP27KO (n8) mice on control diet.
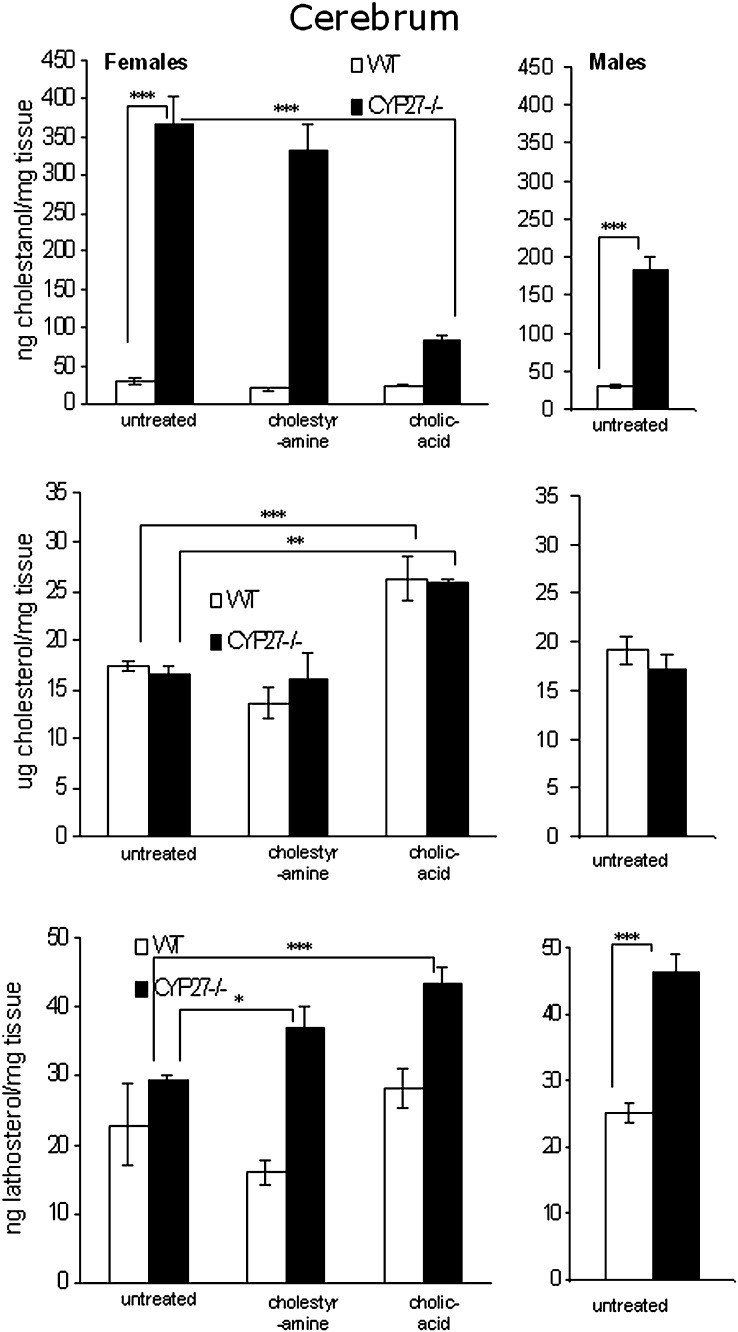
The levels of cholestanol in the brain of the cyp27a1-deficient female mice were increased about 12-fold compared with their wild-type littermates (Fig. 3). In cyp27a1-deficient males, the corresponding increase was about 6-fold. The lack of cyp27a1 did not affect the cholesterol levels in the brain (Fig. 3). The levels of lathosterol were, however, increased about 2-fold, indicating an increased rate of cholesterol synthesis (Fig. 3).
Fig. 3.
Levels of sterols in cerebrum of female wild-type (WT) and CYP27KO mice on different treatments (WT and KO; ctrl n4 and n5, cholestyramine n4 and n3, cholic acid n5 and n5) and male WT (n5) and CYP27KO (n8) mice on control diet.
In view of the fact that the cerebellum is a preferential site for accumulation of cholestanol-containing xanthomas in the brain of CTX patients, we also measured the cholestanol content in the cerebellum of cyp27a1-deficient female mice. These levels were similar to those in the whole brain, and there was no effect on the cholesterol content (results not shown).
It is evident from above that the accumulation of cholestanol is higher in plasma and tissues of female than in male cyp27a1-deficient mice. Because of this, a more detailed investigation was performed with female mice only.
Levels of 7 α-hydroxy-4-cholesten-3-one in the circulation of cyp27a1 −/− mice and wild-type mice
As shown in Table 1, the levels of 7α-hydroxy-4-cholesten-3-one were about 35 times higher in the circulation of cyp27a1−/− mice than in the circulation of the corresponding control mice. Treatment with cholestyramine would be expected to increase the activity of the cholesterol 7α-hydroxylase due to a reduced negative feedback by bile acids. This would be expected to increase the levels of 7α-hydroxy-4-cholesten-3-one. In accordance with this, the levels of 7α-hydroxy-4-cholesten-3-one increased about 3-fold in the wild-type mice and about twice in the cyp27a1−/− mice treated with cholestyramine. Treatment with cholic acid, the major suppressor of cholesterol 7α-hydroxylase activity in mice (21), would be expected to reduce cholesterol 7α-hydroxylase activity as well as the levels of 7α-hydroxy-4-cholesten-one. Under the conditions employed, with a dose of cholic acid corresponding to the normal production of cholic acid (21), there was a very small reduction only of the levels of 7α-hydroxy-4-cholesten-3-one in the wild-type mice. In cyp27a1−/− mice, however, with a very low endogenous production of cholic acid (6), the treatment caused a marked reduction in the levels of 7α-hydroxy-4-cholesten-3-one by about 75% (Table 1).
TABLE 1.
Plasma 7α-hydroxy-4-cholesten-3-one levels in wild-type and cyp27−/− female mice on different treatments
| Mice | Diet | 7α-Hydroxy-4-cholesten-3-one |
|---|---|---|
| μg/ml | ||
| WT | Control | 0.053 |
| WT | Cholestyramine | 0.19 |
| WT | Cholic acid | 0.036 ± 0.014 |
| CYP27−/− | Control | 1.9 ± 0.15 |
| CYP27−/− | Cholestyramine | 3.8 ± 0.38 |
| CYP27−/− | Cholic acid | 0.49 ± 0.093 |
Mice were treated as indicated in the table. Data from wild-type control and cholestyramine-treated mice were received from pooled plasma from four animals per group. Individual animals analyzed per group: wild-type and CYP27−/− cholic acid fed, n = 5, CYP27−/− control and cholestyramine fed, n = 3.
Effect of cholestyramine and cholic acid on cholestanol levels in tissue and plasma of female wild-type and cyp27a1 −/− mice
As shown in Figs. 1 and 3, neither treatment with cholestyramine nor treatment with cholic acid had any significant effect on cholestanol levels in plasma or brain of the wild-type mice. In tendons, treatment with cholic acid reduced the levels of cholestanol in the wild-type mice (Fig. 2). In the cyp27a1−/− mice, treatment with cholestyramine increased the levels of cholestanol in plasma but not in tendons or brain (Figs. 1–3). Treatment with cholic acid markedly reduced the levels of cholestanol in plasma as well as in tendons and brain of the cyp27a1−/− mice (Figs. 1, 3, and 4).
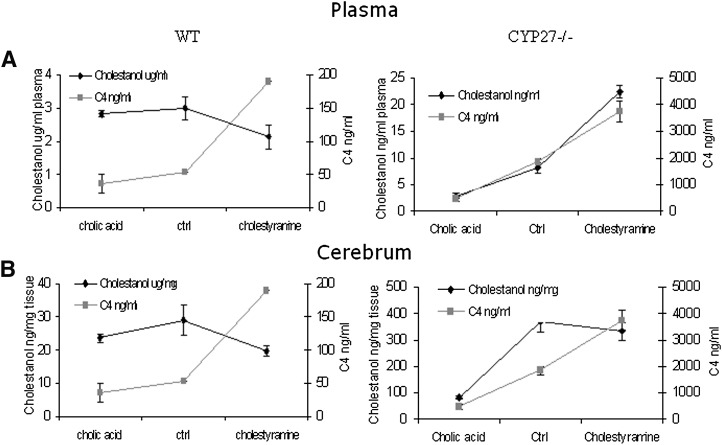
Fig. 4.
Levels of cholestanol and 7α-hydroxy-4-cholesten-3-one (C4) in female wild-type (WT) and CYP27KO mice (A) in plasma. B: Cerebrum cholestanol levels and plasma 7α-hydroxy-4-cholesten-3-one levels shown in the same diagrams. Number of mice, used to measure 7α-hydroxy-4-cholesten-3-one levels in plasma, per group were: WT and KO; ctrl n4 (pool) and n3, cholestyramine n4 (pool) and n3, cholic acid n5 and n5). Number of mice, used to measure cholestanol levels in plasma and cerebrum, per group were: WT and KO; ctrl n4 and n5, cholestyramine n4 and n3, cholic acid n5 and n5.
As shown in Fig. 4, there was no relation between levels of cholestanol and 7α-hydroxy-4-cholesten-3-one in plasma or brain of wild-type mice under the different conditions. In plasma of the cyp27a1−/− mice, however, the changes in levels of cholestanol closely followed the changes in levels of cholestanol under the different conditions. In brain of the cyp27a1−/− mice, the changes in levels of cholestanol induced by treatment with cholic acid were similar to those of 7α-hydroxy-4-cholesten-3-one in plasma. The increased levels of 7α-hydroxy-4-cholesten-3-one in plasma induced by treatment of the cyp27a1−/− mice with cholestyramin were, however, not followed by a similar increase in cholestanol.
Direct evidence for a transfer of 7 α-hydroxy-4-cholesten- 3-one from the circulation into the brain and its conversion into cholestanol
The above results are consistent with a transfer of 7α-hydroxy-4-cholesten-3-one from the circulation into the brain under conditions when the level of this oxysterol is sufficiently high (at least higher than about 200 ng/ml plasma). To demonstrate such a transfer, we treated a female cyp27a1−/− mouse with daily injections with 200 μg of 7α-hydroxy-4-cholesten-3-one during 3 months. As a result of this treatment, the levels of cholestanol in the brain of this mouse increased to 1.47 μg/mg, almost 4-fold higher than the corresponding level in untreated cyp27a1−/− mice. In spite of this high level, corresponding to about 10% of the sterol fraction, no xanthomas could be found in the brain. Further direct evidence for a formation of cholestanol from 7α-hydroxy-4-cholesten-3-one in the circulation was obtained by treating a cyp27a1−/− mouse with daily injections of 100 μg of 2H7-labeled 7α-hydroxy-4-cholesten-3-one for 10 days. After this treatment, the content of 2H7 in plasma cholestanol was 2.6% and in brain cholestanol was 0.19%.
Effect of the cyp27a1 deficiency on cholesterol homeostasis in the brain
There was no significant difference between cyp27a1−/− mice and wild-type mice with respect to content of cholesterol in the brain (Fig. 3). The levels of lathosterol were, however, increased in the brain of the cyp27a1−/− mice, suggesting an increased cholesterol synthesis. Such increase was observed under all conditions, including treatment with cholic acid. It was shown that not only lathosterol but also some other precursors to cholesterol (lanosterol, 24,25-dihydroxycholesterol, and 7-dehydrocholesterol) were increased in the cyp27a1−/− mice (results not shown). The apparent increase in cholesterol synthesis was not, however, associated with increased mRNA levels of 3-hydroxy-3-methyl-glutaryl-CoA reductase or synthase (results not shown). The mRNA levels of a gene involved in the metabolism of cholesterol in the brain, cyp46a1, was also not affected by cyp27a1 deficiency (results not shown).
DISCUSSION
Mice with a disruption of the CYP27A1 are not ideal model systems for CTX because of the fact that no xanthomas are formed in tendons and brain. We show here, however, that both the brain and tendons of cyp27−/− mice contain a relatively high accumulation of cholestanol, which is the most specific feature of the xanthomas in CTX.
Female mice were found to have higher accumulation of cholestanol than male mice, and a detailed investigation was therefore made on female mice only. The reason for the gender difference is unknown. Although there is no obvious direct link to atherosclerosis, it is noteworthy that female mice are more sensitive than male mice to develop atherosclerosis under different experimental conditions (22, 23). Evidently, this is due to a reduced capacity for reverse cholesterol transfer, and female mice tend to have lower levels of HDL. In view of our finding that cholestanol can be eliminated from cultured cells by the same lipoprotein-mediated mechanism as cholesterol, this could be the explanation for the gender difference.
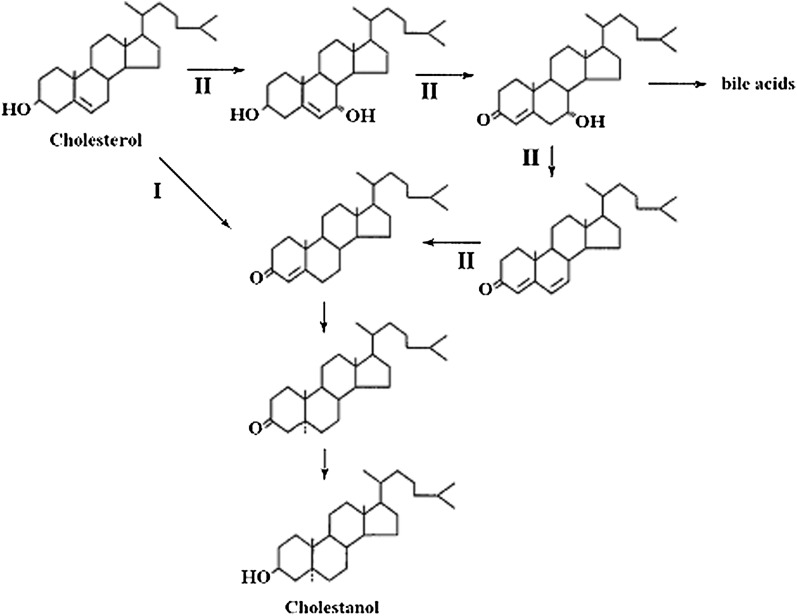
The two pathways involved in the formation of cholestanol from cholesterol are shown in Fig. 5. Under normal conditions, cholestanol is formed from cholesterol by a pathway involving the action of a 3β-hydroxy-Δ-5-dehydrogenase as a first step (24) (pathway I in the figure). This reaction leads to formation of 4-cholesten-3-one, which is further converted into cholestanol in two reductive steps (2). In the alternative pathway (pathway II in the figure), 4-cholesten-3-one is not formed directly from cholesterol but from 7α-hydroxy-4-cholesten-3-one by dehydration followed by saturation of the Δ 6 double bond (2, 10). The enzymes involved in the latter two reactions have been characterized with respect to reaction mechanisms (25) and are analogous to reactions involved in the bacterial 7α-dehydroxylation of cholic acid into deoxycholic acid (26).
Fig. 5.
Sequence of reactions in connection with formation of cholestanol by the “classical” pathway (I) and the “alternative” pathway (II) involving intermediates in bile acid synthesis.
It is evident that cholestanol is mainly formed by the classical pathway in the wild-type mouse and not by the alternative pathway. The small changes in plasma cholestanol levels induced by cholestyramine and cholic acid were similar to those of cholesterol. The slight reducing effect of treatment with cholestyramine is likely to be due to increased activity of the cholesterol 7α-hydroxylase with increased consumption of substrate. It is well established that cholestanol is a substrate for this enzyme (27). When treating the cyp27a1−/− mice with cholestyramine, however, opposite effects were obtained on plasma levels of cholestanol. Under these conditions, the plasma levels of 7α-hydroxy-4-cholesten-3-one increased about 3-fold with a parallel increase in the levels of cholestanol. This is consistent with 7α-hydroxy-4-cholesten-3-one as a precursor of cholestanol under conditions when its level in plasma is considerably higher than about 200 ng/ml (corresponding to the levels obtained in a wild-type mouse treated with cholestyramin).
Increased brain levels of cholestanol were found in the cyp27a1-deficient mice when the plasma levels of 7α-hydroxy-4-cholesten-3-one were between 500 and 4,500 ng/ml. Reducing the plasma levels of 7α-hydroxy-4-cholesten-3-one in the cyp27a1-deficient mice by treatment with cholic acid led to expected reduction in levels of cholestanol in the brain.
Surprisingly, an increase of the levels of 7α-hydroxy-4-cholesten-3-one, by a factor of two, by treating the cyp27a1−/− mice with cholestyramine did not lead to the expected increase of cholestanol concentration in the brain. The reason for this may have been the relatively short duration of the treatment. Another possibility could be presence of a metabolism of cholestanol in the brain that may be activated when the accumulation reaches a certain level.
The major mechanism by which cholesterol is removed from the brain involves a 24S-hydroxylation catalyzed by the brain-specific cholesterol 24S-hydroxylase. This enzyme is also active toward cholestanol (28), but the role of this enzyme in connection with the accumulation of cholestanol is uncertain. A treatment of a cyp27a1-deficient mouse with daily injections of very large amounts of 7α-hydroxy-4-cholesten-3-one during 3 months led, however, to a marked increased in brain levels of cholestanol. If a mechanism is present in the brain of the cyp27−/− mice that counteracts the accumulation, it is evident that this mechanism can be overcome by a sufficiently high flux of the cholestanol precursor into the brain. Further evidence for a direct flux of 7α-hydroxy-4-cholesten-3-one into the brain with subsequent formation of cholestanol was obtained by the demonstration of incorporation of deuterium in brain cholestanol in a mouse injected with 2H7-labeled 7α-hydroxy-4-cholesten-3-one.
The mechanism for accumulation of cholestanol in the brain of cyp27a1−/− mice demonstrated here is likely to also be valid for patients with CTX. The suppressive effect of cholic acid on the accumulation of cholestanol in the brain of the cyp27a1−/− mice is analogous to the suppressive effect of chenodeoxycholic acid on brain xanthomas reported for patients with CTX (8). It should be noted that cholic acid is the major suppressor of cholesterol 7α-hydroxylase in mice (21), whereas chenodeoxychoic acid is the major suppressor in humans (1). Unexpectedly, the treatment with cholic acid appeared to increase, rather than decrease, lathosterol levels in the cyp27−/− mice. The reason for this is unknown, and further experiments have been initiated to elucidate the mechanism behind this effect.
The accumulation of cholestanol in relation to cholesterol was considerably higher in tendons and brain of the cyp27a1−/− mice than in circulation or liver. Tendons and brain are also the preferential sites for formation of xanthomas in patients with CTX. The CYP27A1-mediated conversion of cholesterol into 27-hydroxycholesterol and cholestenoic acid can be regarded as an antiatherogenic mechanism by which cholesterol can be eliminated from macrophages (29) and possibly also from glial cells. Not only cholesterol but also cholestanol can be eliminated by this mechanism (30). The classical HDL-mediated mechanism for reversed cholesterol transfer may be less effective in tendons and brain than in other tissues and organs, increasing the importance of CYP27A1 for removal of excess cholesterol and cholestanol. We have shown that human monocyte-derived macrophages eliminate cholestanol less efficiently than cholesterol, both by the classical HDL- mediated mechanism and by CYP27A1 (30). A reduced capacity for removal of sterols is thus likely to result in a preferential accumulation of cholestanol.
The reason for development of cholesterol-containing xanthomas in the brain and tendons of patients with CTX but not in cyp27a1-deficient mice is still not known. The accumulation of cholestanol in brain and tendons was thus not accompanied by a parallel accumulation of cholesterol in our mouse model. The lathosterol levels were slightly increased, suggesting increased cholesterol synthesis. Whether this increase is a consequence of the accumulation of cholestanol or the lack of 27-hydroxycholesterol is not possible to evaluate from the present study. In CTX, the upregulation of the cholesterol 7α-hydroxylase due to the low production of chenodeoxycholic acid is considerably higher than the corresponding upregulation in the mouse model. As a consequence, both production and accumulation of cholestanol are considerably higher in CTX than in the mouse model. The production of cholestanol in patients with CTX has been estimated to be about 40–50 mg/24 h (31, 32), which is about 10% of the normal rate of formation of bile acids in humans. The accumulation of cholestanol in the brain of CTX patients has been reported to be 20–40% of the sterol fraction (31). In our untreated cyp27a1-deficient mice, cholestanol corresponded only to about 3% of the sterol fraction. In the cyp27a1-deficient mouse treated with 7α-hydroxy-4-cholesten-3-one, the corresponding enrichment was about 10%.
In contrast to the situation in human deficiency of the CYP27A1, cyp27−/− mice have a marked upregulation of the nuclear xenobiotic receptor pregnane X receptor and its target genes (33). 7α-Hydroxy-4-cholesten-3-one is one of the activators of this receptor. One of the metabolic consequences of this activation is an increased capacity to convert bile acid intermediates into more polar 25-hydroxylated alcohols. The excretion of such bile alcohols is, however, very modest in cyp27−/− mice compared to the situation in CTX (4, 6). The reason for this is probably that 7α-hydroxylation of cholesterol is rate limiting not only for the synthesis of cholestanol by the alternative pathway but also for the production of 25-hydroxylated bile alcohols. The cholesterol 7α-hydroxylase is not upregulated in cyp27−/− mice to the same extent as in patients with CTX. In any case, it is difficult to link differences in the degree of activation of the pregnane X receptor to the differences in the accumulation of cholestanol and cholesterol at the present state of knowledge.
To summarize, we have an explanation for the accumulation of cholestanol in the brain of CYP27A1-deficient mice and humans and for the higher accumulation in the human situation. We still do not know, however, why the accumulation of cholestanol in the human situation is associated with accumulation of cholesterol in the form of xanthomas.
Footnotes
A. Båvner and M. Shafaati contributed equally to this work.
This study was funded by the Swedish Research Council, Swedish Brain Power (to I.B.), and The Sarah and Moshe Mayer Foundation for Research (to E.L.).
REFERENCES
- 1.Russell D. W. 2003. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72: 137–174. [DOI] [PubMed] [Google Scholar]
- 2.Björkhem I., Muri-Boberg K., Leitersdorf E. 2001. Inborn errors in bile acid biosynthesis an storage of sterols other than cholesterol. Metabolic and Molecular Bases of Inherited Diseases. Scriver C. R., Beaudet A. L., Sly W. S., Valle D., McGraw-Hill, 2961–2988. [Google Scholar]
- 3.Dubrac S., Lear S. R., Ananthanarayanan M., Balasubramaniyan N., Bollineni J., Shefer S., Hyogo H., Cohen D. E., Blanche P. J., Krauss R. M., et al. 2005. Role of CYP27A in cholesterol and bile acid metabolism. J. Lipid Res. 46: 76–85. [DOI] [PubMed] [Google Scholar]
- 4.Honda A., Salen G., Matsuzaki Y., Batta A. K., Xu G., Leitersdorf E., Tint G. S., Erickson S. K., Tanaka N., Shefer S. 2001. Side chain hydroxylations in bile acid biosynthesis catalyzed by CYP3A are markedly up-regulated in Cyp27−/− mice but not in cerebrotendinous xanthomatosis. J. Biol. Chem. 276: 34579–34585. [DOI] [PubMed] [Google Scholar]
- 5.Honda A., Salen G., Matsuzaki Y., Batta A. K., Xu G., Leitersdorf E., Tint G. S., Erickson S. K., Tanaka N., Shefer S. 2001. Differences in hepatic levels of intermediates in bile acid biosynthesis between Cyp27(−/−) mice and CTX. J. Lipid Res. 42: 291–300. [PubMed] [Google Scholar]
- 6.Rosen H., Reshef A., Maeda N., Lippoldt A., Shpizen S., Triger L., Eggertsen G., Bjorkhem I., Leitersdorf E. 1998. Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin D metabolites in mice with disrupted sterol 27-hydroxylase gene. J. Biol. Chem. 273: 14805–14812. [DOI] [PubMed] [Google Scholar]
- 7.Bjorkhem I., Skrede S., Buchmann M. S., East C., Grundy S. 1987. Accumulation of 7 alpha-hydroxy-4-cholesten-3-one and cholesta-4,6-dien-3-one in patients with cerebrotendinous xanthomatosis: effect of treatment with chenodeoxycholic acid. Hepatology. 7: 266–271. [DOI] [PubMed] [Google Scholar]
- 8.Berginer V. M., Salen G., Shefer S. 1989. Cerebrotendinous xanthomatosis. Neurol. Clin. 7: 55–74. [PubMed] [Google Scholar]
- 9.Repa J. J., Lund E. G., Horton J. D., Leitersdorf E., Russell D. W., Dietschy J. M., Turley S. D. 2000. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia. Reversal by cholic acid feeding. J. Biol. Chem. 275: 39685–39692. [DOI] [PubMed] [Google Scholar]
- 10.Skrede S., Bjorkhem I., Buchmann M. S., Hopen G., Fausa O. 1985. A novel pathway for biosynthesis of cholestanol with 7 alpha-hydroxylated C27-steroids as intermediates, and its importance for the accumulation of cholestanol in cerebrotendinous xanthomatosis. J. Clin. Invest. 75: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchmann M. S., Clausen O. P. 1986. Effects of cholestanol feeding and cholestyramine treatment on the tissue sterols in the rabbit. Lipids. 21: 738–743. [DOI] [PubMed] [Google Scholar]
- 12.Byun D. S., Kasama T., Shimizu T., Yorifuji H., Seyama Y. 1988. Effect of cholestanol feeding on sterol concentrations in the serum, liver, and cerebellum of mice. J Biochem. 103: 375–379. [DOI] [PubMed] [Google Scholar]
- 13.Bjorkhem I., Meaney S. 2004. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 24: 806–815. [DOI] [PubMed] [Google Scholar]
- 14.Panzenboeck U., Andersson U., Hansson M., Sattler W., Meaney S., Bjorkhem I. 2007. On the mechanism of cerebral accumulation of cholestanol in patients with cerebrotendinous xanthomatosis. J. Lipid Res. 48: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 15.Lovgren-Sandblom A., Heverin M., Larsson H., Lundstrom E., Wahren J., Diczfalusy U., Bjorkhem I. 2007. Novel LC-MS/MS method for assay of 7alpha-hydroxy-4-cholesten-3-one in human plasma. Evidence for a significant extrahepatic metabolism. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 856: 15–19. [DOI] [PubMed] [Google Scholar]
- 16.Folch J., Ascoli I., Lees M., Meath J. A., Le B. N. 1951. Preparation of lipide extracts from brain tissue. J. Biol. Chem. 191: 833–841. [PubMed] [Google Scholar]
- 17.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 18.Lund E., Sisfontes L., Reihner E., Bjorkhem I. 1989. Determination of serum levels of unesterified lathosterol by isotope dilution-mass spectrometry. Scand. J. Clin. Lab. Invest. 49: 165–171. [DOI] [PubMed] [Google Scholar]
- 19.Acimovic J., Lovgren-Sandblom A., Monostory K., Rozman D., Golicnik M., Lutjohann D., Bjorkhem I. 2009. Combined gas chromatographic/mass spectrometric analysis of cholesterol precursors and plant sterols in cultured cells. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2081–2086. [DOI] [PubMed] [Google Scholar]
- 20.Heverin M., Meaney S., Brafman A., Shafir M., Olin M., Shafaati M., von Bahr S., Larsson L., Lovgren-Sandblom A., Diczfalusy U., et al. 2007. Studies on the cholesterol-free mouse: strong activation of LXR-regulated hepatic genes when replacing cholesterol with desmosterol. Arterioscler. Thromb. Vasc. Biol. 27: 2191–2197. [DOI] [PubMed] [Google Scholar]
- 21.Li-Hawkins J., Gafvels M., Olin M., Lund E. G., Andersson U., Schuster G., Bjorkhem I., Russell D. W., Eggertsen G. 2002. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J. Clin. Invest. 110: 1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caligiuri G., Nicoletti A., Zhou X., Tornberg I., Hansson G. K. 1999. Effects of sex and age on atherosclerosis and autoimmunity in apoE-deficient mice. Atherosclerosis. 145: 301–308. [DOI] [PubMed] [Google Scholar]
- 23.Paigen B., Morrow A., Holmes P. A., Mitchell D., Williams R. A. 1987. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 68: 231–240. [DOI] [PubMed] [Google Scholar]
- 24.Bjorkhem I., Karlmar K. E. 1974. Biosynthesis of cholestanol: conversion of cholesterol into 4-cholesten-3-one by rat liver microsomes. Biochim. Biophys. Acta. 337: 129–131. [DOI] [PubMed] [Google Scholar]
- 25.Bjorkhem I., Buchmann M., Bystrom S. 1992. Mechanism and stereochemistry in the sequential enzymatic saturation of the two double bonds in cholesta-4,6-dien-3-one. J. Biol. Chem. 267: 19872–19875. [PubMed] [Google Scholar]
- 26.Hylemon P. B., Harder J. 1998. Biotransformation of monoterpenes, bile acids, and other isoprenoids in anaerobic ecosystems. FEMS Microbiol. Rev. 22: 475–488. [DOI] [PubMed] [Google Scholar]
- 27.Shefer S., Hauser S., Mosbach E. H. 1968. 7-alpha-hydroxylation of cholestanol by rat liver microsomes. J. Lipid Res. 9: 328–333. [PubMed] [Google Scholar]
- 28.Mast N., Norcross R., Andersson U., Shou M., Nakayama K., Bjorkhem I., Pikuleva I. A. 2003. Broad substrate specificity of human cytochrome P450 46A1 which initiates cholesterol degradation in the brain. Biochemistry. 42: 14284–14292. [DOI] [PubMed] [Google Scholar]
- 29.Babiker A., Andersson O., Lund E., Xiu R. J., Deeb S., Reshef A., Leitersdorf E., Diczfalusy U., Bjorkhem I. 1997. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high density lipoprotein-mediated reverse cholesterol transport. J. Biol. Chem. 272: 26253–26261. [DOI] [PubMed] [Google Scholar]
- 30.von Bahr S., Movin T., Papadogiannakis N., Pikuleva I., Ronnow P., Diczfalusy U., Bjorkhem I. 2002. Mechanism of accumulation of cholesterol and cholestanol in tendons and the role of sterol 27-hydroxylase (CYP27A1). Arterioscler. Thromb. Vasc. Biol. 22: 1129–1135. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharyya A. K., Lin D. S., Connor W. E. 2007. Cholestanol metabolism in patients with cerebrotendinous xanthomatosis: absorption, turnover, and tissue deposition. J. Lipid Res. 48: 185–192. [DOI] [PubMed] [Google Scholar]
- 32.Salen G., Grundy S. M. 1973. The metabolism of cholestanol, cholesterol, and bile acids in cerebrotendinous xanthomatosis. J. Clin. Invest. 52: 2822–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodwin B., Gauthier K. C., Umetani M., Watson M. A., Lochansky M. I., Collins J. L., Leitersdorf E., Mangelsdorf D. J., Kliewer S. A., Repa J. J. 2003. Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc. Natl. Acad. Sci. USA. 100: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]