Abstract
Cholesteryl ester transfer protein (CETP) has been identified as a novel target for increasing HDL cholesterol levels. In this report, we describe the biochemical characterization of anacetrapib, a potent inhibitor of CETP. To better understand the mechanism by which anacetrapib inhibits CETP activity, its biochemical properties were compared with CETP inhibitors from distinct structural classes, including torcetrapib and dalcetrapib. Anacetrapib and torcetrapib inhibited CETP-mediated cholesteryl ester and triglyceride transfer with similar potencies, whereas dalcetrapib was a significantly less potent inhibitor. Inhibition of CETP by both anacetrapib and torcetrapib was not time dependent, whereas the potency of dalcetrapib significantly increased with extended preincubation. Anacetrapib, torcetrapib, and dalcetrapib compete with one another for binding CETP; however anacetrapib binds reversibly and dalcetrapib covalently to CETP. In addition, dalcetrapib was found to covalently label both human and mouse plasma proteins. Each CETP inhibitor induced tight binding of CETP to HDL, indicating that these inhibitors promote the formation of a complex between CETP and HDL, resulting in inhibition of CETP activity.
Keywords: triglyceride, anacetrapib, torcetrapib, dalcetrapib, atherosclerosis
Numerous epidemiological studies over the past decades have demonstrated that HDL cholesterol (HDL-C) is inversely correlated with the incidence of coronary heart disease (CHD) (1–4). An increase of 1 mg/dl of HDL-C is associated with a 2–3% decrease in the risk of death from CHD, independent of LDL cholesterol (LDL-C) levels (1, 5). In addition to epidemiological evidence supporting an atheroprotective role for HDL-C, preclinical and clinical studies involving either overexpression of apolipoprotein (apo) A-I (6–9), the predominant protein component of HDL-C, or infusion of reconstituted HDL-C particles (10–18) both indicate that therapeutically raising HDL-C in humans will significantly reduce CHD risk. Although increasing HDL-C is a promising strategy for reducing CHD risk, there are currently few treatment options for increasing HDL-C levels, including statins, fibrates, and niacin. Both statins and fibrates provide only modest increases in HDL-C (5–20%) and niacin is poorly tolerated, thereby limiting its therapeutic potential in raising HDL-C (19–23). Therefore, there is great interest in developing novel therapies that will raise circulating HDL-C levels in humans for the treatment of cardiovascular disease.
Cholesteryl ester transfer protein (CETP), a hydrophobic plasma glycoprotein, is a promising target for raising circulating HDL-C concentrations in humans. CETP is secreted primarily from the liver and plays a critical role in HDL metabolism by facilitating the exchange of cholesteryl esters (CE) from HDL for triglycerides (TG) in apoB-containing lipoproteins, such as LDL and VLDL (24, 25). This activity both directly lowers the cholesterol levels of HDL-C and enhances HDL catabolism by providing HDL with the TG substrate of hepatic lipase (26, 27). Thus, CETP helps regulate circulating levels of HDL-C, LDL-C, and apo-AI. Findings in preclinical animal models have generally supported a role for CETP in lowering HDL-C levels and, importantly, in promoting atherosclerosis. Mice naturally lack CETP, carrying most of their cholesterol in HDL-C, and are relatively resistant to atherosclerosis. Transgenic mice carrying either the human or cynomolgus monkey CETP gene have dramatically lower HDL-C levels and are more susceptible to diet-induced atherosclerosis (28, 29). In the context of hypertriglyceridemia, however, transgenic expression of CETP was shown to reduce atherosclerosis despite lower HDL-C levels (30, 31). In contrast to mice, rabbits have CETP activity levels comparable to humans and are prone to diet-induced atherosclerosis (32). Inhibition of CETP in rabbits via the administration of anti-CETP monoclonal antibodies, anti-sense oligonucleotides, small molecules, or vaccine-induced antibodies results in increased HDL-C levels and a decrease in atherosclerosis (33–36).
Interest in CETP as a therapeutic target for raising HDL-C began with the discovery of genetic CETP deficiency in a small Japanese population with hyperalphalipoproteinemia or very high HDL-C levels (37–39). However, findings regarding the relationship between CETP deficiency and CHD risk have been conflicting, indicating either an increase (40) or decrease (41) in CHD risk. Several reports describing single nucleotide polymorphisms (SNPs) within CETP and their effect on CETP activity and expression levels, in addition to HDL-C concentrations, have been described, illustrating a key role of CETP in regulating circulating HDL-C levels in humans (42–45). Whereas the relationship between SNPs within CETP and both CETP and HDL-C levels has been established, findings regarding the influence of such SNPs on CHD risk remain inconsistent (45–49). Therefore, to firmly establish a causal link between CETP and CHD risk, either additional genetic analyses with larger populations of individuals or pharmacological inhibition of CETP will be required.
To date, clinical studies with 3 CETP inhibitors have been reported, including anacetrapib, torcetrapib, and dalcetrapib. Torcetrapib is a potent inhibitor of CETP transfer activity, presumably through the formation of a complex between CETP and HDL (50). Although treatment with torcetrapib in humans significantly elevates HDL-C and lowers LDL-C levels (51, 52), its clinical development recently ended due to an increase in both mortality and morbidity rates in the active treatment group compared with the placebo group within the ILLUMINATE trial (53). Following termination of the ILLUMINATE trial, it was found that treatment with torcetrapib results in increased systolic blood pressure, increased levels of serum aldosterone, sodium, and bicarbonate, and decreased serum potassium levels (53). Preclinical studies have clearly demonstrated that the effect of torcetrapib on blood pressure and aldosterone release is the result of molecule-specific, off-target effects; these effects have not been observed with anacetrapib or dalcetrapib (54, 55). Dalcetrapib is an inhibitor of CETP transfer activity and is currently in phase III clinical studies (35). Inhibition of CETP by dalcetrapib requires formation of a covalent disulfide bond with Cys13 of CETP (35, 56). Treatment of humans with dalcetrapib results in increases in HDL-C (19–37%) and a modest decrease (∼6%) in LDL-C levels (57). Anacetrapib is an inhibitor of CETP transfer activity and is currently in phase III clinical studies (58). Treatment with anacetrapib results in a significant increase in HDL-C (∼130%) while also significantly decreasing LDL-C (∼40%) (59–61). Importantly, no differences in either blood pressure or aldosterone levels have been observed with anacetrapib and dalcetrapib (54, 55, 57, 59, 61).
Whereas the CETP inhibitors anacetrapib, torcetrapib ,and dalcetrapib have been studied in the clinic for their effects on lipids, a detailed comparison of their biochemical properties has not been described. In this study, we sought to provide a detailed comparison of these CETP inhibitors in regards to both their mechanism of inhibiting CETP and binding to other plasma proteins.
MATERIALS AND METHODS
Protein expression and purification
cDNA encoding human CETP-glycosylation-site mutation N341Q (62) was cloned into pS2-neo and stably transfected into S2 cells (63). Initially, the cells were cultured in Schneider's Drosophila medium (Gibco) supplemented with 10% fetal calf serum, 2 mM glutamate, and 1.5 mg/ml G418 and then were gradually adapted to serum-free, protein-free medium (Drosophila SFM from Invitrogen) containing 2 mM glutamate and 1.5 mg/ml G418. Cells were delivered to Kemp Biotechnologies (Frederick, MD) for large scale production. The stably transfected S2 cells were subsequently adapted to Invitrogen Sf-900 serum-free medium (SFM). Expression of recombinant CETP was induced by growing the cells in stirred tank bioreactors in SFM supplemented with 1 mM CuSO4 for 4 days. The medium containing secreted CETP was harvested and concentrated 20-fold with 10 kDa MWCO ultra-filtration. Media was stored at −70°C.
Purification of CETP was performed in two steps using an AKTA Explorer fast-protein liquid chromatography (FPLC). Briefly, after clarification of the media by centrifugation (1,000 g 10 min), 50 ml was adjusted to 250 mM NaCl. The sample was filtered (0.45 μm, Millipore) and injected onto an equilibrated (250 mM NaCl, 1 mM EDTA) 5 ml Hi Trap Butyl FF column (GE Healthcare). After loading, the column was rinsed with 50 ml of washing solution (250 mM NaCl, 1 mM EDTA) followed by 15 ml of washing buffer (50 mM Tris, pH 7.5, 1 mM EDTA). Protein was eluted with 35 ml of water. Protein-containing fractions were pooled and concentrated 10-fold (30 kDa MWCO concentrators, Sartorius). The pooled fractions were immediately adjusted to 50 mM Tris, pH 7.5, 150 mM NaCl, and 500 μl of filtered sample (0.65 mm, Amicon Ultra Free MC) was injected onto a 24 ml Superdex 75 HR 10/300 GL (GE Healthcare) equilibrated with 50 mM Tris, pH 7.5, 150 mM NaCl, and 1 mM EDTA. Fractions containing CETP were pooled and concentrated 10-fold and stored at 4°C.
The identity of the purified CETP was confirmed by MS. Protein concentration was determined by optical density at 280nm (O.D.280) and was confirmed by amino acid analysis (BioSynthesis, Inc., Lewisville, TX). Purity of protein preparations was assessed using an Experion Automated Electrophoresis System (BioRad) and was typically ≥95%.
In vitro fluorogenic assays of CETP-mediated CE and TG transfer
For determination of in vitro CETP transfer activity, a continuous fluorogenic assay described previously (64) was used. In brief, this assay measures the CE or TG transfer half-reaction using a synthetic donor particle similar in size and density to HDL-C, which bears a core of fluorescent CE or TG. The high concentration of Bodipy®-CE or Bodipy®-TG and the addition of DabcylN(C18) (in the case of the CE donor particles only) cause quenching of the fluorescent signal in the donor particles. Native VLDLs and LDLs are used as an acceptor. As a molecule of fluorescent substrate is removed from the donor and transferred to an acceptor, it escapes quench and becomes fully fluorescent. Compounds were preincubated with CETP and HDL donor particles for either 1 or 24 h before addition of VLDL/LDL acceptor particles.
In vitro radioactive assays of CETP-mediated CE and TG transfer
Reagents were obtained from commercial sources as indicated: [3H] cholesteryl oleate (GE Healthcare), [3H] triolein (Perkin-Elmer), butylated hydroxyl toluene (Aldrich), DOPC (Sigma), sodium bromide (Fisher Scientific), PEG 8000 (Fisher Scientific), and human HDL (Intracel Corp.).
The ability of inhibitors to alter CETP activity in 95% human serum was evaluated by measuring the transfer of [3H] cholesteryl oleate or [3H] triolein from exogenous LDL to HDL by CETP in the human serum. The lipoproteins in human serum were labeled with [3H] lipid and the labeled LDL was isolated by density gradient ultracentrifugation as described (65). The assays were performed by incubating human serum with or without inhibitors at 37°C for 1 h. The [3H] labeled exogenous LDL was then added to the reaction for 60 min at 37°C. The transfer reaction was terminated by precipitation of LDL with 20% w/v PEG 8000. The samples were centrifuged and an aliquot of the HDL-containing supernatant was counted by liquid scintillation. Counts present in the supernatant for controls (incubated at 4°C) were subtracted from those incubated at 37°C to correct for nonspecific transfer.
The ability of inhibitors to block CETP-mediated CE and TG transfer was also measured by radioactive CETP transfer assay with 2% human serum. The procedure was similar to the 95% human serum transfer assay described above, except that purified human HDL (128 µg/ml) and [3H] cholesteryl oleate or [3H] triolein-labeled LDL were used as acceptor and donor particles, respectively. The [3H] cholesteryl oleate or [3H] triolein from exogenous LDL to HDL was transferred by purified recombinant CETP (30 nM) in standard CETP buffer (50 mM Tris, pH 7.4, 100 nM NaCl, and 1 mM EDTA) with 2% human serum. The transfer reaction was terminated by precipitation of LDL with 1 vol of ice-cold human serum and 2 vols of 20% w/v PEG 8000. The samples were centrifuged and an aliquot of the HDL-containing supernatant was counted by liquid scintillation. Counts present in the supernatant for controls (without CETP addition) were subtracted from those reactivated with CETP to correct for nonspecific transfer.
CETP inhibitor binding assay
The saturable and specific binding of [3H]labeled inhibitors to purified CETP and their ability to compete with other inhibitors was determined as follows. Assay conditions similar to the in vitro fluorogenic CE transfer assay (64) were chosen to allow direct comparison of an inhibitor's binding and biochemical activity. Binding assays contained 1× CETP buffer (50 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA) with 0.005% Tween20, and 20 nM purified recombinant CETP in a 100 μl final volume. Sheep serum, lacking endogenous CETP, at a final concentration of 2% was added to act as a carrier. Final concentrations of [3H]labeled inhibitor were 15 nM and competing cold inhibitors were added in the range of 1–2,000 nM. Cold inhibitors were added as DMSO solutions such that the final concentration of DMSO did not exceed 1.0%. Dalcetrapib was preincubated with the recombinant CETP for 1 h at 25°C before addition of the [3H]labeled inhibitor. Binding reactions were incubated for 2 h at 25°C, whereupon bound ligand was separated from free by processing 80 μl on a Pierce ZebaTM 96-well Desalt Spin Plate (Pierce) according to the manufacturer's instructions. The 50 μl of the column eluate containing CETP and bound inhibitor was quantitated in 5 ml Perkin Elmer Ultima Gold MV using a Perkin Elmer TriCarb 2900TR liquid scintillation analyzer. Specific binding was calculated as the difference between the amount of inhibitor bound in the presence of CETP (eluate) minus the amount of inhibitor bound in the absence of CETP (eluate). Similar levels of nonspecific binding were observed in reactions lacking CETP or in which an excess of unlabeled torcetrapib (1 uM) or an unrelated protein (prolylcarboxypeptidase) was added, indicating that the binding observed with CETP was specific (data not shown).
Liquid chromatography-mass spectrometry of covalent modification of CETP
N341Q CETP (18 μM) was incubated with either 120 μM dalcetrapib disulfide inhibitor or anacetrapib in 50 mM Tris, pH 7.5, with 150 mM NaCl for 36 h at room temperature. Intact mass spectra were collected by ultra performance (UP) LC-MS using a polyhydroxyethyl A column (100 × 2.1 mm; 5 μ, 1,000 Å) from PolyLC Inc. (Columbia, MD) running isocratically at 0.05 ml/min on a Waters Acquity with a Synapt mass spectrometer. The UPLC solvent was 0.1% formic acid, 1% m-nitrobenzyl alcohol in 20% acetonitrile. For reactions containing DTT, 10 mM DTT was added to samples after the 36 h incubation with dalcetrapib and incubated for an additional 1 h at room temperature. For reactions containing iodoacetamide, a final concentration of 10 mM iodoacetamide was incubated with CETP for 6 h at room temperature.
In vitro covalent reaction of CETP inhibitors with human and mouse plasma proteins
The covalent labeling of bulk plasma proteins by CETP inhibitors was assessed using a method similar to those previously reported (66). [14C-phenyl]dalcetrapib and [14C]anacetrapib (at 3, 10, and 50 μM final concentration) were incubated at 37°C with pooled fresh human or mouse plasma for 0, 0.5, 1, 2, 16, and 24 h. At the end of each incubation time, aliquots of plasma in triplicate were quenched with 2 ml of acetonitrile. Samples were vortex mixed, refrigerated to precipitate the protein, and centrifuged at 1800 g for 15 min. The supernatant was discarded, and the protein pellet was saved for subsequent washings. Then 1 ml of distilled and deionized water was added to each pellet and the samples were sequentially sonicated and vortex mixed for 10 min each. Absolute ethanol (4 ml) was added to the samples and the tubes were vortex mixed again for 10 min. The samples were then refrigerated again to reprecipitate the protein, centrifuged, and the supernatant was discarded. The above washing sequence was repeated until the radioactivity in the washings was near background levels. After the final washing, the protein pellet was dissolved in 0.7 ml of 0.1 N NaOH by gentle vortex mixing. The radioactivity content in a 0.2 ml aliquot of the pellet solution was determined by direct liquid scintillation counting following neutralization with 0.2 ml of 0.1 N HCl. Protein concentrations in the pellet solution were measured using the Pierce protein assay kit. The final amount of 14C radioactivity irreversibly bound to proteins was expressed as pmol-equivalent bound/mg of protein.
For SDS-PAGE analysis of protein labeling, the binding method was also modified from those previously reported (66). [14C-phenyl]dalcetrapib and [14C]anacetrapib (at 50 μM final concentration) were incubated at 37°C with pooled fresh human or mouse plasma for 2 and 24 h. Identical experiments were performed with fresh plasma from humans and either wild-type C57Bl/6 mice or from C57Bl/6 mice carrying the cynomolgus monkey CETP gene (28). At each time point, aliquots of human or mouse plasma in triplicate were diluted to a final volume of 1,275 μl with ultrapure water and precipitated with 225 μl of tricholoracetic acid. Samples were mixed and stored on ice for 30 min to precipitate the protein and centrifuged at 1,700 g for 5 min. The supernatant was discarded and the protein pellet was saved for subsequent washings. Then 1 ml of ice-cold acetone was added to each pellet, and the samples were sequentially sonicated and vortexed, centrifuged for 5 min and the supernatant was discarded. The above washing sequence was repeated until the radioactivity in the washings was near background levels. After the final washing, one of the triplicates was saved for covalent binding determination and the other two were prepared for SDS-PAGE analysis. For covalent binding determination, the protein pellets were dissolved in 0.3 ml of 0.1 N NaOH by gentle vortex mixing. The radioactivity content in a 0.15 ml aliquot of the pellet solution was determined by direct liquid scintillation counting following neutralization with 0.15 ml of 0.1 N HCl. Protein concentrations in the pellet solution were measured using the Pierce protein assay kit (Pierce). The final amount of 14C radioactivity irreversibly bound to proteins was expressed as pmol-equivalent bound/mg protein. For SDS-PAGE analysis, the remaining acetone over the protein pellets was air dried and proteins were solubilized in 7 M urea, 2 M thiourea, and 4% CHAPS for 30 min at room temperature with occasional vortexing. Samples were ultracentrifuged at 50,000 rpm for 30 min at 4°C (Beckman TL-100). Cleared lysates were transferred to fresh tubes, and proteins were quantified with Bradford reagent (Bio-Rad). Samples of equal protein amounts were loaded onto 10–20 or 8% Tris-glycine SDS-PAGE gels (Invitrogen) using a nonreducing 4× Laemmli SDS-Sample buffer (Boston BioProducts). For radioactivity detection, gels were stained with GelCode (Thermo Scientific), destained in water, incubated in Amplify (GE Healthcare) for 30 min, and dried using the DryEase mini-gel drying system (Invitrogen). Dried gels were exposed for 12 days to a storage phosphor screen, and signal was detected using the Typhoon 9400 (GE Healthcare). For Western blots, proteins were transferred from gels onto a nitrocellulose filter. CETP was detected by first blocking the membrane with TBS containing 0.1% Tween (TBST) and 1% BSA. The blot was then incubated with the mouse TP2 anti-CETP antibody (Ottawa Heart Institute Research Corp., Canada) in TBST with 1% BSA overnight, washed with TBST, incubated with an anti-mouse-AP secondary antibody (Promega) for 1 h, and lastly washed with TBST. Detection was performed with the 1-step NBT/BCIP reagent for 15 min.
CETP-HDL interaction gel mobility shift assay
Reagents were obtained from commercial sources: CETP TP2 (Ottawa Heart Institute Research Corp.,), ECL anti-mouse IgG-HRP (GE Healthcare), and ECL plus detection system (GE Healthcare).
To measure binding of CETP to HDL induced by inhibitors, the electrophoretic mobility of CETP in the presence of HDL was examined by native gel electrophoresis followed by Western blotting. The assays were performed by incubating mixtures of purified N341Q CETP (30 nM) and density-purified HDL (128 ng/μl) with or without inhibitors at 37°C for 1 h. The 4–12% Tris-glycine gels were run at 125 V for 2 h and then electro-blotted to nitrocellulose membrane by iBlot (Invitrogen) dry transfer. The blots were blocked in 5% nonfat milk in 0.1% Tween 20 in PBS (PBST), washed with PBST, probed with the primary CETP TP2 antibody in PBST for 1 h, washed, incubated with HRP-coupled anti-mouse IgG secondary antibody, and then developed with ECL plus detection system.
FPLC fractionation of human plasma
CETP distribution in human plasma was examined using gel filtration chromatography. Human plasma from a donor with high HDL was incubated with or without CETP inhibitor for 1 h at 37°C. A 500 μl aliquot was injected inline to dual Superose 6 10/300 GL columns (GE Healthcare) connected in tandem. The plasma was fractionated by FPLC at 4°C using an AKTA Purifier (GE Healthcare). To obtain good recovery of CETP from plasma fractionated by FPLC, a low ionic strength buffer was used (65 mM sucrose, 225 mM mannitol, 10 mM Tris, pH 7.4, and 1 mM EDTA) as the eluent at a flow rate of 0.2 ml/min. Then 0.5 ml fractions were collected after the injection loop was emptied with 2.5 ml of buffer.
Total cholesterol was assayed using the Wako Total Cholesterol E kit per the manufacturer's protocol in fractions collected between 10 and 50 ml.
CETP was assayed in the same fractions using a CETP Delfia ELISA. Microflour 2 plates (ThermoLabsystems) were coated with 50 μl 4 μg/ml anti-TP20 (Ottawa Heart Institute) overnight at 4°C. The FPLC fractions and human serum CETP standards (Wako CETP kit) were treated with SDS (final concentration 0.25%) for 1 h at 37°C. The detection antibody was 50 μl of 4 μg/ml biotinylated anti-TP2 (Ottawa Heart Institute) followed by the addition of DELFIA® Eu-Labeled Streptavidin diluted in Delfia Assay Buffer. Delfia Enhance solution was added and read in the Envision 2104 Multilabel Reader (Perkin Elmer).
RESULTS
Anacetrapib is a potent inhibitor of CETP neutral lipid transfer activity
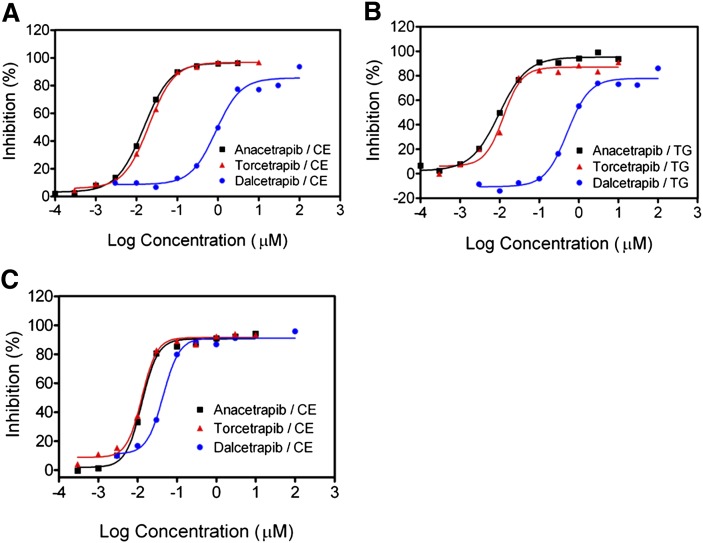
Anacetrapib has been shown to inhibit CETP activity, thereby significantly raising HDL-C levels in humans (59, 61, 67). However, a description of anacetrapib's biochemical characterization and mechanism of CETP inhibition has not been reported. To better understand both the similarities and differences between anacetrapib and two additional CETP inhibitors, torcetrapib and dalcetrapib, we characterized each inhibitor in a series of biochemical assays. Although each CETP inhibitor increases circulating HDL-C levels in humans, anacetrapib is structurally distinct from both torcetrapib and dalcetrapib. We first compared the relative potencies of each inhibitor in reducing CETP neutral lipid transfer activity using a fluorogenic CETP assay (Table 1) (64). As shown in Fig. 1A, anacetrapib is a potent inhibitor of the CETP-dependent transfer of CE (IC50 = 17 ± 4.8 nM). Torcetrapib inhibited CE transfer with a similar potency (IC50 = 13 ± 2.7 nM), whereas dalcetrapib displayed a 70- to 80-fold weaker potency in inhibiting CE transfer (IC50 = 1,178 ± 443 nM). The potencies of these compounds in inhibiting CETP-dependent TG transfer were similar to those observed with CE transfer, indicating that each compound blocked both CE and TG transfer activities of CETP (Fig. 1B, Table 1). We next determined if the ability of these compounds to inhibit CETP-mediated transfer of CE was time dependent by preincubating each compound with CETP and HDL donor particles for 24 h prior to the addition of VLDL/LDL acceptor particles. The potencies of both anacetrapib (IC50 = 13 ± 1.4 nM) and torcetrapib (IC50 = 14 ± 0.7 nM) in inhibiting CE transfer were not significantly different than when preincubated for only 1 h (Fig. 1C). In contrast, dalcetrapib was 26-fold more potent (IC50 = 45 ± 2.1 nM) in inhibiting CETP-mediated transfer of CE when preincubated for 24 h (Fig. 1C).
Table 1.
Testing of CETP inhibitors in the fluorogenic CETP transfer assaya
| Assay Formatb | ||||
|---|---|---|---|---|
| CE |
TG |
Preincubation Time, h | ||
| Compound | IC50 (c | IC50 (nM) | % Serum | |
| Anacetrapib | 17 ± 4.8 | 15 ± 5.3 | 2 | 1 |
| Torcetrapib | 13 ± 2.7 | 15 ± 2.8 | 2 | 1 |
| Dalcetrapib | 1178 ± 443 | 850 ± 8.0 | 2 | 1 |
| Anacetrapib | 13 ± 1.4 | n.d.d | 2 | 24 |
| Torcetrapib | 14 ± 0.7 | n.d. | 2 | 24 |
| Dalcetrapib | 45 ± 2.1 | n.d. | 2 | 24 |
The potencies of CETP inhibitors anacetrapib, torcetrapib, and dalcetrapib were determined in the fluorogenic transfer assays as described in “Methods.”
Inhibitors were tested in reactions measuring transfer of either CE or TG.
Concentration of CETP inhibitor at which 50% of either CE or TG transfer was inhibited; values are means ± SD.
n.d., not determined.
Fig. 1.
Inhibition of CETP-dependent neutral lipid transfer using a fluorogenic transfer assay. Increasing amounts of the indicated compounds were assayed in the fluorogenic assay using purified recombinant N341Q CETP as described in “Methods.” A: Dose response of inhibitors reducing CETP-mediated CE transfer. Inhibitors were preincubated with CETP and HDL donor particles for 1 h before addition of acceptor particles. B: Dose response of inhibitors reducing CETP-mediated TG transfer. Inhibitors were preincubated with CETP and HDL donor particles for 1 h before addition of acceptor particles. C: The effect of 24 h preincubation on the potency of inhibitors in reducing CETP-mediated CE transfer. Inhibitors were preincubated with CETP and HDL donor particles for 24 h before addition of acceptor particles. Typical results representative of at least three independent experiments are shown and are fit to a sigmoidal dose-response curve by nonlinear regression.
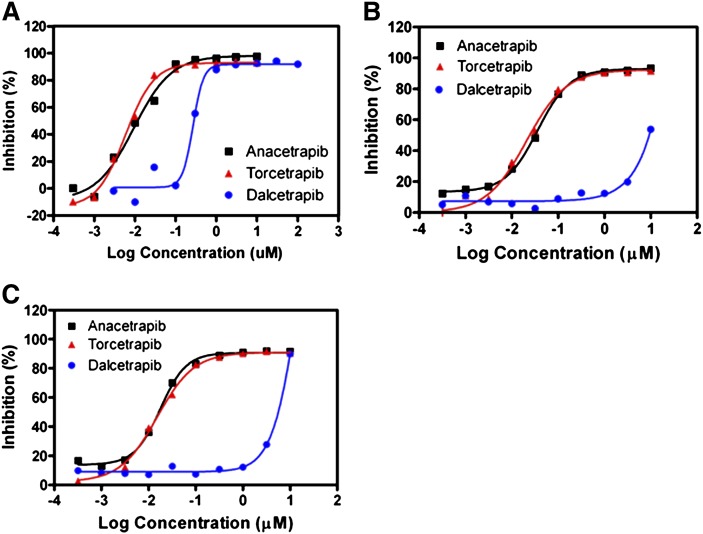
The potency of each inhibitor in reducing CETP neutral lipid transfer activity was next determined using a radioactive neutral lipid transfer assay in either 2 or 95% human serum (Fig. 2, Table 2). When tested in 2% human serum, we found that the relative potency of each inhibitor was similar in both the radioactive and fluorogenic transfer assays (Table 1, 2). Whereas anacetrapib and torcetrapib were potent inhibitors of both CE (IC50 = 10 ± 4.1 and 8 ± 3.1 nM, respectively) and TG (IC50 = 11 ± 5.9 and 7 ± 1.0 nM, respectively) transfer, dalcetrapib was approximately 27-fold less potent in inhibiting CETP-mediated neutral lipid transfer (CE IC50 = 270 ± 38 nM; TG IC50 = 268 ± 48 nM) (Fig. 2A, Table 2). Both anacetrapib and torcetrapib continued to potently block CE and TG transfer in 95% human serum, whereas the potency of dalcetrapib was diminished >37-fold (Fig. 2B, Table 2). Further preincubation of each inhibitor for 24 h did not significantly change their potencies in blocking either CE or TG transfer by CETP in 95% human serum (Fig. 2C, Table 2). Together, these results indicate that both anacetrapib and torcetrapib are potent inhibitors of CETP neutral lipid transfer activity. In addition, dalcetrapib was shown to be a less potent inhibitor of CETP activity and displayed a time-dependence of inhibition and a significant loss of potency when tested in 95% human serum.
Fig. 2.
Inhibition of CETP-dependent CE transfer using a radioactive neutral lipid transfer assay. Increasing amounts of the indicated compounds were assayed in the in vitro radioactive assay using either 2% human serum with additional (30 nM) purified N341Q CETP protein added, or in 95% human serum as described in “Methods.” Inhibitors were preincubated for either 1 or 24 h before addition of LDL containing labeled tracer neutral lipids. A: Dose response of inhibitors reducing CETP-mediated CE transfer in 2% human serum. Inhibitors were preincubated with CETP in 2% human serum for 1 h before addition of 3H-labeled LDL. B: Dose response of inhibitors reducing CETP-mediated CE transfer in 95% human serum. Inhibitors were preincubated in 95% human serum for 1 h before addition of 3H-labeled LDL. C: The effect of 24 h preincubation on the potency of inhibitors in reducing CETP-mediated CE transfer in 95% human serum. Inhibitors were preincubated in 95% human serum for 24 h before addition of 3H-labeled LDL. Typical results representative of at least three independent experiments are shown and are fit to a sigmoidal dose-response curve by nonlinear regression.
Table 2.
Testing of CETP inhibitors in the radioactive CETP transfer assaya
| Assay Formatb |
||||
|---|---|---|---|---|
| CE |
TG |
Preincubation Time, h | ||
| Compound | IC50 (c | IC50 (nM) | % Serum | |
| Anacetrapib | 10 ± 4.1 | 11 ± 5.9 | 2 | 1 |
| Torcetrapib | 8 ± 3.8 | 7 ± 1.0 | 2 | 1 |
| Dalcetrapib | 270 ± 38 | 268 ± 48 | 2 | 1 |
| Anacetrapib | 45 ± 18 | 59 ± 25 | 95 | 1 |
| Torcetrapib | 21 ± 0.4 | 29 ± 1 | 95 | 1 |
| Dalcetrapib | >10,000 | >10,000 | 95 | 1 |
| Anacetrapib | 18 ± 0.6 | 21 ± 0.6 | 95 | 24 |
| Torcetrapib | 15 ± 1 | 16 ± 0.1 | 95 | 24 |
| Dalcetrapib | >10,000 | 4,583 ± 672 | 95 | 24 |
The potencies of CETP inhibitors anacetrapib, torcetrapib, and dalcetrapib were determined in the radioactive transfer assay as described in “Methods.”
Inhibitors were tested in reactions measuring transfer of either CE or TG.
Concentration of CETP inhibitor at which 50% of either CE or TG transfer was inhibited; values are means ± SD.
Anacetrapib and dalcetrapib compete with torcetrapib for binding CETP
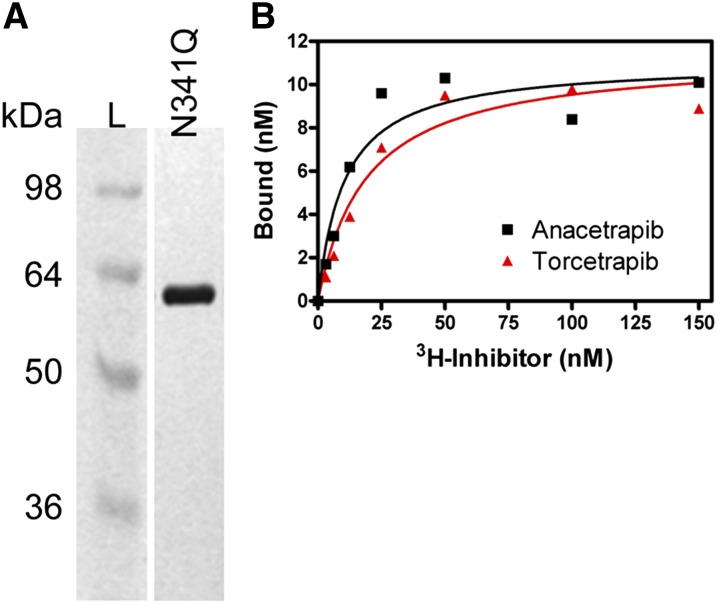
Binding of torcetrapib to CETP has previously been demonstrated using either a [3H]torcetrapib-CETP binding assay (50) or by surface plasmon resonance (Biacore) (56). To measure the specific binding of anacetrapib to CETP, we used a similar assay with purified CETP protein and either [3H]anacetrapib or [3H]torcetrapib. Recombinant human CETP containing a N341Q substitution was expressed in Drosophila S2 cells and purified to homogeneity (Fig. 3A). The N341Q substitution was engineered to reduce the complexity of CETP glycosylation (62). The purified N341Q protein was found to be identical to wild-type CETP in its specific activity of CE and TG transfer in addition to its relative sensitivity to various CETP inhibitors [(62) and data not shown]. The apparent binding affinity purified N341Q CETP protein and [3H]anacetrapib was 19 nM (Fig. 3B). This binding affinity is in good agreement with the potency of unlabeled anacetrapib for inhibiting CETP neutral lipid transfer (anacetrapib CE IC50 = 17 ± 4.8 nM; TG IC50 = 15 ± 5.3 nM) (Table 1). In addition, we found that [3H]torcetrapib had a similar apparent binding affinity for CETP (19 nM), in agreement with its potency in inhibiting CETP activity (Fig. 3B, Table 1).
Fig. 3.
Binding of [3H]anacetrapib to purified CETP. A: Silver-stained gel of purified N341Q CETP protein. Media from a stable S2 cell line overexpressing N341Q mutant CETP protein was used for purification of secreted protein as described in “Methods.” The results shown are from 3 μg of recombinant N341Q CETP protein loaded and are run next to a standard protein molecular weight ladder (L). B: Dose response of [3H]anacetrapib and [3H]torcetrapib binding to CETP. Increasing amounts of [3H]anacetrapib or [3H]torcetrapib were combined with purified N341Q CETP protein (20 nM) and samples were processed as described in “Methods.” Specific binding activity was calculated as the difference between the amount of inhibitor bound in the presence of CETP minus the amount of inhibitor bound in the absence of CETP.
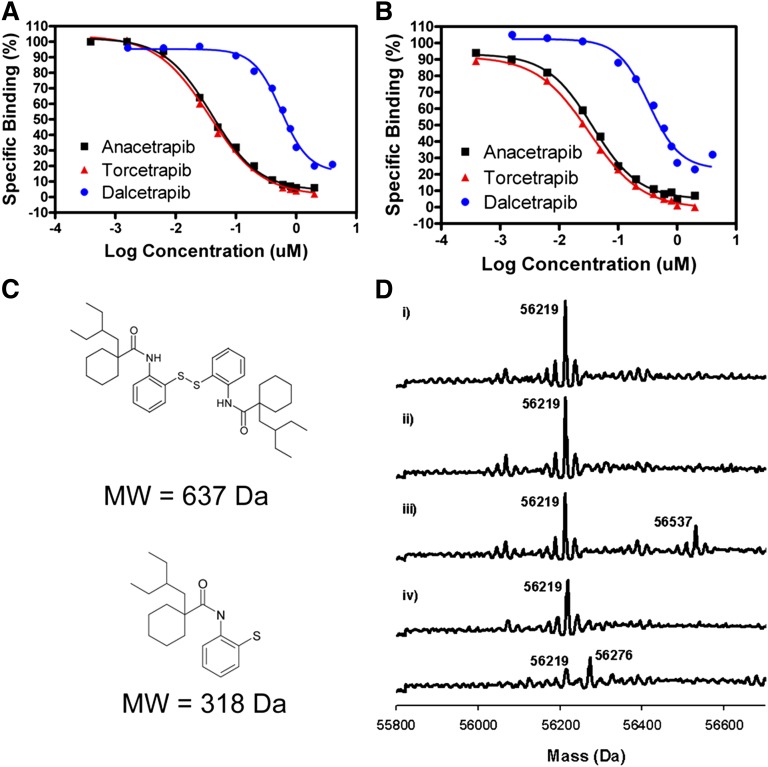
Using the direct binding assay, we next determined if each CETP inhibitor competes with one another for binding CETP (Fig. 4). For these experiments, a constant concentration of [3H]anacetrapib or [3H]torcetrapib (15 nM) was combined with increasing concentrations of unlabeled anacetrapib, torcetrapib, or dalcetrapib and the amount of [3H]inhibitor remaining bound to CETP was determined. As shown in Fig. 4A, each compound competes with [3H]torcetrapib for binding to CETP, with EC50s similar to their IC50s in neutral lipid transfer assays. In addition, each compound also competes with [3H]anacetrapib for binding to CETP (Fig. 4B). These results demonstrate that anacetrapib, torcetrapib, and dalcetrapib each compete with one another for binding CETP.
Fig. 4.
Competition of inhibitors for binding CETP and reversibility of binding. A: Competition between [3H]torcetrapib and unlabeled inhibitors for binding CETP. Increasing concentrations of indicated unlabeled compounds were incubated with 15 nM [3H]torcetrapib and CETP and CETP-bound [3H]torcetrapib was measured as detailed in “Methods.” B: Competition between [3H]anacetrapib and unlabeled inhibitors for binding CETP. Increasing concentrations of indicated unlabeled compounds were incubated with 15 nM [3H]anacetrapib and CETP and CETP-bound [3H]anacetrapib was measured as detailed in “Methods.” C: Chemical structures of dalcetrapib. Dalcetrapib is represented both as a disulfide-linked dimer of 637 Da molecular weight and as a half-molecule of 318 Da molecular weight. D: MS of CETP. i) CETP control; ii) CETP incubated with anacetrapib; iii) CETP with dalcetrapib disulfide inhibitor showed a modification of 318 Da corresponding to a half-molecule of the inhibitor. This modification was reversed by DTT (iv). Incubation with iodoacetamide (v) illustrated the susceptibility of a free cysteine in CETP for modification; the appearance of a peak with a difference of 57 Da corresponding to iodoacetamide modification was detected.
It has previously been reported that inhibition of CETP activity by dalcetrapib requires disulfide bond formation between dalcetrapib and Cys13 of CETP (35, 56). In contrast, torcetrapib has been shown to be a reversible inhibitor of CETP activity (50). To measure the reversibility of anacetrapib's interaction with CETP, we used LC-MS to detect covalent labeling of CETP by dalcetrapib. When the disulfide form of dalcetrapib (Fig. 4C) was incubated with CETP, a gain in mass of 318 Da was observed, consistent with the addition of a single half-molecule (Fig. 4D). Under identical conditions, we were unable to detect covalent labeling of CETP by anacetrapib, consistent with it being a reversible inhibitor of CETP (Fig. 4D). The addition of a reducing agent (DTT) in the labeling reaction resulted in a loss of CETP labeling by dalcetrapib, confirming that the labeling was due to disulfide bond formation. CETP was also covalently labeled by the cysteine-modifying reagent iodoacetamide, confirming the susceptibility of a free cysteine in CETP for modification. These results confirm that dalcetrapib covalently labels CETP via disulfide bond formation and also demonstrates that anacetrapib does not covalently label CETP.
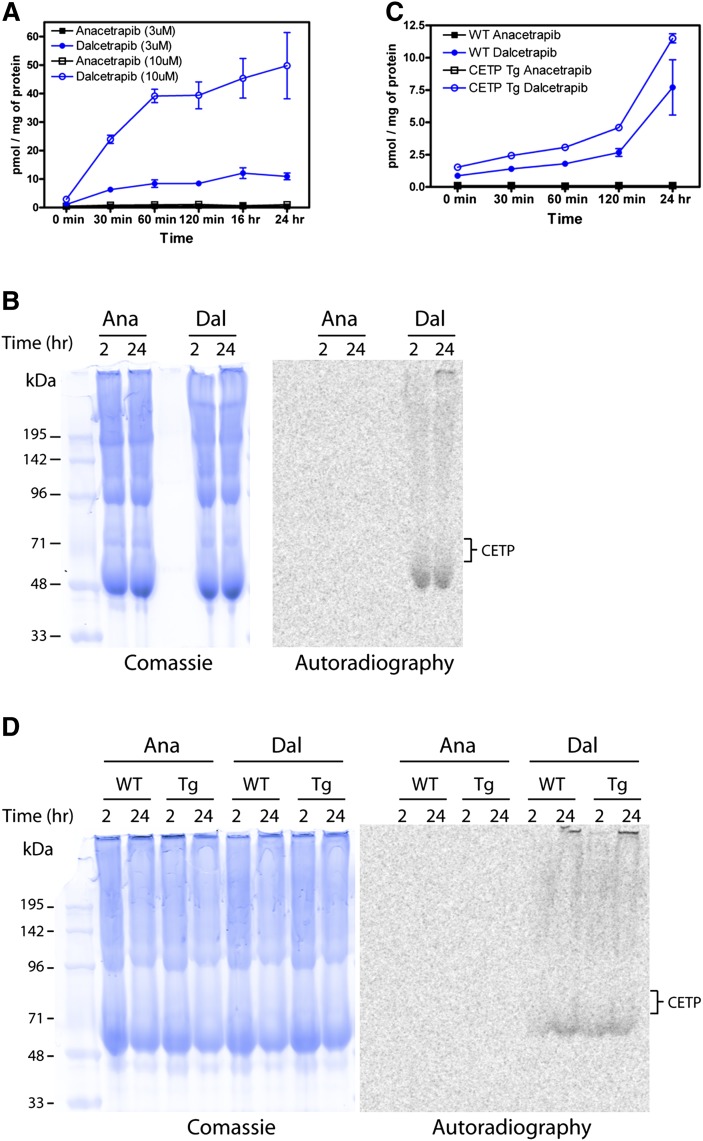
Dalcetrapib covalently labels bulk proteins from human and mouse plasma
Given that dalcetrapib forms a disulfide bond with CETP and that dalcetrapib shows a significant loss of inhibitory potency in the presence of 95% serum, we questioned whether dalcetrapib was capable of covalently labeling other plasma proteins. Therefore, we examined the ability of both anacetrapib and dalcetrapib to form covalent interactions with human serum proteins (Fig. 5A). [14C]labeled versions of both anacetrapib and dalcetrapib were first incubated with 100% human serum for up to 24 h. During the incubation, aliquots were collected, total protein was extracted, and [14C]labeled inhibitor covalently bound to protein was measured. As shown in Fig. 5A, there were increasing amounts of [14C]dalcetrapib present in the protein fraction over the time of incubation. In contrast, there was no evidence of covalent protein labeling by [14C]anacetrapib when tested under identical conditions. Labeling of human plasma proteins by [14C]dalcetrapib was also shown to be dependent on disulfide bond formation, because the addition of a reducing agent (DTT) to labeling reactions resulted in a loss of [14C]labeled protein (data not shown). In addition to measuring total [14C]inhibitor in protein fractions, samples were separated by SDS-PAGE to visualize labeled proteins and determine if CETP was the only plasma protein labeled (Fig. 5B). Covalent labeling of plasma proteins was evident in samples in which [14C]dalcetrapib was incubated for either 2 or 24 h, whereas there was no evidence of protein labeling in samples treated with [14C]anacetrapib. Importantly, based on the expected molecular weight of human CETP (65–71 kDa), proteins labeled by dalcetrapib run at distinct molecular weights (Fig. 5B, band at ∼50 kDa), indicating that dalcetrapib is capable of covalently labeling additional proteins in human plasma.
Fig. 5.
Covalent binding of dalcetrapib to human and mouse plasma proteins in vitro. Human and mouse plasma was incubated with either [14C]anacetrapib or [14C]dalcetrapib and the degree of protein labeling was determined after various times of incubation as described in “Methods.” A: Labeling of human plasma proteins. B: Analysis of human plasma proteins labeled by [14C]dalcetrapib. Following incubation of 50 μM [14C]anacetrapib or [14C]dalcetrapib, proteins were separated via nonreducing SDS-PAGE. Analysis of total protein loaded (Coomassie), [14C]labeled proteins, or CETP (anti-CETP immunoblot) was carried out as described in “Methods.” C: Labeling of mouse plasma proteins. Plasma from either wild-type mice lacking CETP (WT) or CETPTg mice was incubated with 3 μM [14C]anacetrapib or [14C]dalcetrapib, and the degree of protein labeling was determined after various times of incubation as described in “Methods.” D: Following incubation of 50 μM [14C]anacetrapib or [14C]dalcetrapib, proteins were separated via nonreducing SDS-PAGE. Analysis of total protein loaded (Coomassie), [14C]labeled proteins, or CETP (anti-CETP immunoblot) was carried out as described in “Methods.”
We next determined if [14C]dalcetrapib is capable of labeling mouse plasma proteins in either the presence or absence of CETP (Fig. 5C). For these studies, plasma was isolated from either wild-type C57BL/6 mice lacking CETP or from transgenic mice carrying the cynomolgus monkey CETP gene (CETPTg) (68). As shown in Fig. 5C, [14C]dalcetrapib covalently labeled mouse plasma proteins from either wild-type or CETPTg mice to a similar degree. Similar to results with human plasma, there was no evidence of protein labeling in samples treated with [14C]anacetrapib. When proteins were separated by SDS-PAGE and labeled proteins visualized, we again observed plasma protein labeling by [14C]dalcetrapib from both wild-type and CETPTg mouse samples but no evidence of protein labeling by [14C]anacetrapib (Fig. 5D). Western-blot analysis of protein samples from wild-type and CETPTg mice confirmed that cynomolgus CETP migrates at an apparent molecular weight of 68–71 kDa (69), which was not detected in plasma from wild-type mice. Importantly, the pattern of protein labeling by dalcetrapib was similar in both wild-type and CETPTg samples, indicating that additional proteins to CETP are covalently labeled by dalcetrapib (Fig. 5D). These data demonstrate that dalcetrapib, but not anacetrapib, is capable of forming covalent bonds with both human and mouse plasma proteins.
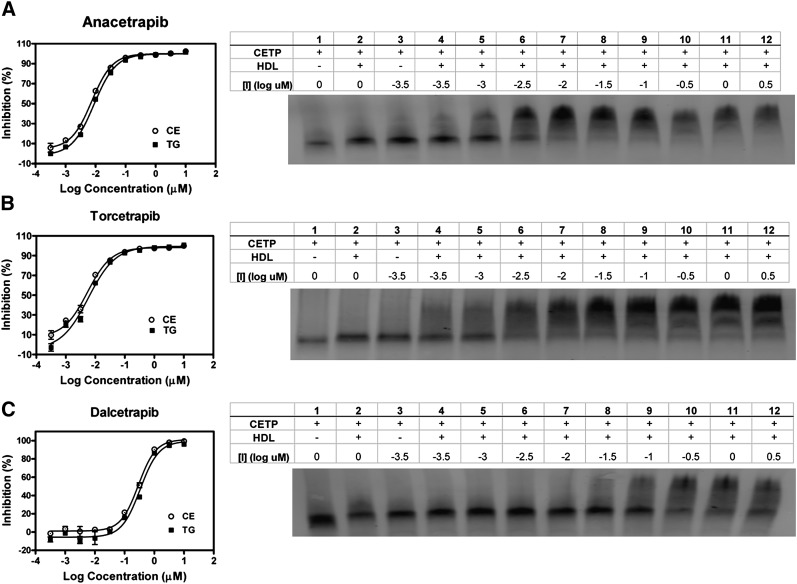
CETP inhibitors induce the formation of a complex between CETP and HDL
Torcetrapib has been shown to inhibit the neutral lipid transfer activity of CETP by inducing a stable complex between CETP and HDL (50, 56). One method used to measure the formation of this complex is native gel electrophoresis, which can detect differences in the effective size of CETP upon binding both inhibitor and HDL. To compare the relative potencies of CETP inhibitors in blocking neutral lipid transfer and formation of a CETP-HDL complex, binding assay conditions were chosen to be similar to those used in the fluorogenic CETP activity assay described in Fig. 1. As shown in Fig. 6A, addition of anacetrapib results in a significant shift in the apparent molecular weight of CETP due to formation of a stable complex between CETP and HDL. The potency with which anacetrapib promotes the shift in CETP's apparent molecular weight correlates with its potency in inhibiting CETP-mediated neutral lipid transfer under similar conditions (Fig. 6A, Table 1). In parallel, we also found that addition of either torcetrapib or dalcetrapib also results in a shift of CETP's apparent molecular weight (Fig. 6B, C). Similar to its relatively weaker potency in blocking CETP neutral lipid transfer, dalcetrapib was significantly less potent than both anacetrapib and torcetrapib in promoting CETP-HDL complex formation.
Fig. 6.
Effect of inhibitors on formation of a stable CETP-HDL complex. Binding assay conditions were designed to mimic the neutral lipid fluorescence transfer assay in order to compare inhibition of transfer activity with formation of a CETP-HDL complex. Each panel includes representative dose responses for inhibition of neutral lipid transfer by each compound in the fluorogenic neutral lipid transfer assay and native-PAGE/CETP Western-blot analysis as described in “Methods.” A: Dose response of anacetrapib. B: Dose response of torcetrapib. C: Dose response of dalcetrapib. CETP-HDL binding assay reactions consisted of CETP alone or with HDL and increasing concentrations of each inhibitor.
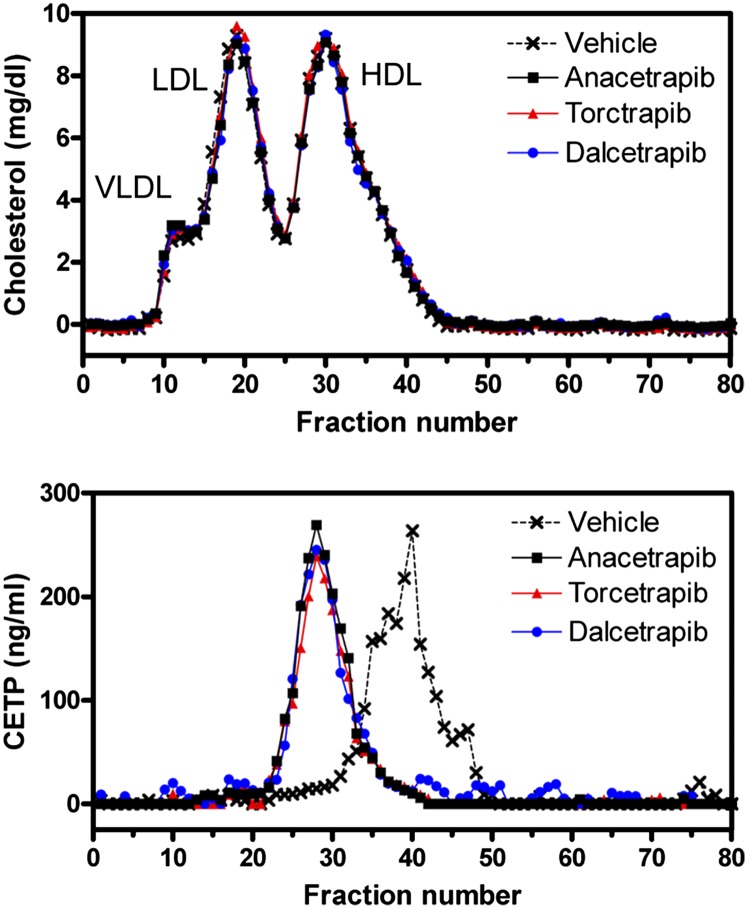
In addition to native gel electrophoresis, we also measured the apparent size of CETP in human plasma following treatment with anacetrapib, torcetrapib, or dalcetrapib (Fig. 7). In the absence of inhibitor, CETP eluted in a size range smaller than HDL. In contrast, when inhibitor-treated human plasma was fractionated by FPLC, we found the apparent size of CETP to be greater than HDL, consistent with each inhibitor promoting the association of CETP and HDL (Fig. 7) (50). This association was consistent whether plasma was treated with anacetrapib, torcetrapib, or dalcetrapib. These data indicate that each inhibitor blocks CETP-mediated neutral lipid transfer by inducing the formation of a stable complex between CETP and HDL.
Fig. 7.
Effect of inhibitors on CETP-HDL complex formation in human plasma. FPLC fractionation of human plasma after treatment with either vehicle or CETP inhibitor was carried out as described in “Methods.” Samples were treated with 1 μM anacetrapib or torcetrapib or 10 μM dalcetrapib. Shown are representative FPLC profiles from three independent experiments.
DISCUSSION
Because anacetrapib is currently in late-stage clinical development, a detailed description of its mechanism of action has not been reported. In the present study, we have conducted a detailed biochemical analysis of anacetrapib in addition to two structurally distinct CETP inhibitors, torcetrapib and dalcetrapib. Anacetrapib was a potent inhibitor of CETP-mediated neutral lipid transfer when tested in both fluorogenic and radioactive transfer assays. Similarly, torcetrapib was also a potent inhibitor of CETP activity, whereas dalcetrapib was a less potent inhibitor of lipid transfer. The potency of dalcetrapib in inhibiting CETP activity was also found to be time dependent and sensitive to the presence of human serum. Although anacetrapib, torcetrapib, and dalcetrapib competed with one another for binding CETP, anacetrapib's binding was reversible and dalcetrapib's binding was covalent. In addition, dalcetrapib was capable of covalently labeling both human and mouse plasma proteins. Lastly, each inhibitor was found to promote the formation of a stable CETP-HDL complex at concentrations relevant to their inhibition of neutral lipid transfer. Together, these results demonstrate that anacetrapib is a potent, reversible inhibitor of CETP that is well suited to determine the pharmacological potential of inhibiting CETP for treatment of CHD.
To determine the potency of each inhibitor in blocking CETP activity, we tested them in both a fluorogenic and radioactive neutral lipid transfer assay (64, 65). In agreement with previous reports (35, 50), we found torcetrapib was a potent inhibitor of both CE and TG transfer, whereas dalcetrapib was a significantly weaker inhibitor (Table 1, 2). Similar to torcetrapib, anacetrapib was also a potent inhibitor of CE and TG transfer, with IC50 values in the range of 10–17 nM when tested in 2% human serum. Inhibition of CETP by torcetrapib has been shown to be reversible and not time dependent (50). In agreement with these findings, the inhibitory potency of anacetrapib and torcetrapib was not significantly affected by 24 h preincubation of compound and CETP (Fig. 1C, 2C). In contrast, the potency of dalcetrapib in blocking CETP-dependent transfer of CE increased 26-fold when preincubated for 24 h (Fig. 1C). Thus, dalcetrapib shares the characteristic of time-dependent inhibition with a number of hydrophobic cysteine-modifying reagents (70, 71). When tested in 95% human serum, both anacetrapib and torcetrapib were somewhat less potent (4- to 5-fold) in blocking neutral lipid transfer, while the potency of dalcetrapib was reduced by >37-fold (Fig. 2B, Table 2). This apparent loss of activity in 95% serum indicates that dalcetrapib may be more likely than anacetrapib and torcetrapib to bind plasma proteins or lipoproteins.
Using a modified CETP binding assay (50), we found that anacetrapib, torcetrapib, and dalcetrapib each compete with one another for binding CETP. The potency of each inhibitor (EC50) in replacing either [3H]torcetrapib or [3H]anacetrapib bound to CETP corresponded well with their potencies in blocking CETP activity (Fig. 4A, B). Although these results demonstrate competition in binding CETP, they do not address the manner of such competition (direct vs. indirect) or the location of inhibitor binding CETP. Based on the crystal structure of CETP, the cysteine forming a disulfide bond with dalcetrapib (Cys13) is near one end of the lipid-binding tunnel (72). The question of whether or not each CETP inhibitor binds an overlapping site on CETP awaits X-ray structural determination of a CETP-inhibitor complex. Previous reports using site-directed mutagenesis have suggested that dalcetrapib, in addition to other cysteine-modifying reagents, requires disulfide bond formation with Cys13 of CETP to inhibit activity (35, 71). In addition, direct evidence for dalcetrapib modifying Cys13 of CETP was recently obtained using peptide mapping and MS (56). Using MS, we confirmed that CETP is modified by a single half-molecule of the dalcetrapib disulfide inhibitor and that this modification is dependent on disulfide bond formation (Fig. 4D). In addition, we also confirmed that anacetrapib does not covalently modify CETP.
While this report was under preparation, Maugeais et al. (73) reported that dalcetrapib did not compete with either anacetrapib or torcetrapib for binding CETP. It is important to note key differences between the two CETP-inhibitor binding studies. Binding studies in the present report were carried out in solution under conditions similar to the CETP activity assay. As previously described, the apparent binding affinities of both [3H]anacetrapib and [3H]torcetrapib for CETP under these conditions were similar to their potencies in blocking CETP activity (Fig. 1A, 3B). In addition, the potencies of torcetrapib, anacetrapib, and dalcetrapib (EC50) in replacing either [3H]torcetrapib or [3H]anacetrapib bound to CETP corresponded well with their potencies in blocking CETP activity (Fig. 1A, 4A, B). In studies described by Maugeasis et al., (73) inhibitor binding was measured using CETP that was first immobilized to Sepharose or by amine coupling to a Biacore sensor chip. Both the nonspecific immobilization of CETP on a solid surface and the immobilization conditions used (e.g., low pH) could alter the interaction between CETP and inhibitors. For example, specific binding pockets for each inhibitor could be altered, or nonspecific binding sites could be introduced due to partial denaturing or unfolding of immobilized CETP. In addition, our results are in agreement with previously reported Biacore studies demonstrating that preincubation of CETP with dalcetrapib prevented the interaction of CETP and an immobilized analog of torcetrapib (56).
Incubation of dalcetrapib in 95% human serum significantly decreased its potency in inhibiting CETP neutral lipid transfer (Fig. 2B). In addition, dalcetrapib was confirmed to covalently label CETP (Fig. 4D), presumably via a disulfide bond with Cys13. Together, these findings suggest that dalcetrapib may be capable of covalently labeling additional proteins with accessible cysteines. To determine whether dalcetrapib and anacetrapib are capable of covalently labeling plasma proteins, we incubated [14C]labeled inhibitors with human plasma and determined the levels of covalent protein labeling (Fig. 5). Dalcetrapib was found to covalently label human plasma proteins in both a time- and concentration-dependent manner, whereas there was no evidence of protein labeling by anacetrapib (Fig. 5A). Similar results were also observed with plasma from both wild-type and CETPTg mice (Fig. 5C). While qualitative, imaging of [14C]labeled proteins by nonreducing SDS-PAGE suggests that CETP is not the only protein covalently labeled by dalcetrapib (Fig. 5B, D). While it is expected that CETP will be labeled in human plasma or in plasma from CETPTg mice, evidence in support of dalcetrapib labeling additional proteins includes: 1) the apparent molecular weights of predominant [14C]labeled proteins do not overlap with the expected size of CETP (Fig. 5B, D); 2) equivalent levels of protein labeling were observed in plasma samples from both wild-type mice lacking CETP and CETPTg mice (Fig. 5C) the pattern of labeled proteins was identical from both wild-type and CETPTg mouse plasma samples (Fig. 5D). In addition, MD-2, a part of the Toll-like 4 signaling cascade, was recently shown to be modified by dalcetrapib in addition to other hydrophobic compounds with a cysteine-reactive group (74). Altogether, these results indicate that dalcetrapib is capable of covalently labeling both CETP and additional plasma proteins.
Given the differences in the nature of CETP binding by these inhibitors, with dalcetrapib being an irreversible inhibitor and the other two being reversible, it seems possible they might exhibit different mechanisms of inhibition. Clark et al. (50) reported that torcetrapib inhibits CETP neutral lipid transfer via the promotion of a stable complex between CETP and HDL. In agreement with this finding, the addition of increasing amounts of torcetrapib resulted in CETP-HDL complex formation, as determined using both native PAGE and FPLC (Fig. 6B, 7). We also found that both anacetrapib and dalcetrapib promote the formation of a higher molecular weight CETP-HDL complex (Fig. 6A, C, 7). No inhibitor, at any concentration, changed the mobility of CETP in the absence of HDL (data not shown). Consistent with the interpretation that the formation of a CETP-HDL complex is part of the inhibitory mechanism of these inhibitors, the IC50 of each inhibitor in the fluorogenic neutral lipid transfer assay strongly correlates with the concentration of inhibitor required to cause one-half of the CETP to complex with HDL (Fig. 6). This mode of inhibition was also observed with a structurally distinct N,N-disubstituted trifluoro-3-amino-2-propanol CETP inhibitor (Searle 1) (50). In the clinic, treatment of human subjects with anacetrapib, torcetrapib, or dalcetrapib has also been shown to increase the concentration of CETP in circulation despite inhibiting CETP activity (51, 67, 75). This increase in CETP concentration has been postulated to be due to an increased association of CETP with HDL, thereby resulting in CETP taking on a significantly longer half-life (76–78). These results demonstrate that despite their biochemical differences (e.g., differences in potency, reversible vs. irreversible binding), each CETP inhibitor tested prevents CETP from being released from its lipoprotein substrate.
In summary, these studies describe the biochemical characterization of the CETP inhibitors anacetrapib, torcetrapib, and dalcetrapib and compare their effects on CETP inhibition and protein binding. We find that anacetrapib and torcetrapib potently block both CE and TG transfer activities of CETP. Dalcetrapib was a comparably weaker inhibitor of CETP in vitro and displayed time-dependent inhibition of CETP neutral lipid transfer activity. Although each inhibitor competed for binding purified CETP, both anacetrapib and torcetrapib bound reversibly and dalcetrapib bound CETP covalently, in agreement with its time-dependent inhibition. Using a covalent plasma labeling assay, we also found that dalcetrapib covalently labels both human and mouse plasma proteins other than CETP. Each of the inhibitors tested promoted formation of a stable complex between CETP and HDL, indicating that the formation of a complex between CETP and HDL is a key mechanism whereby these inhibitors block CETP transfer activity. Together, these findings detail both shared characteristics of tested CETP inhibitors, such as blocking both CE and TG transfer and promoting CETP-HDL complex formation, and also demonstrate differences between them, such as noncovalent versus covalent binding to CETP and covalent labeling of bulk plasma proteins. Further clinical studies are needed to determine whether the observed changes in lipids in response to CETP inhibition will translate into an improved clinical benefit in patients with dyslipidemia.
Acknowledgments
The authors acknowledge Florida Kallashi, Roy Helmy, and Xinchun Tong for their assistance in experiments.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- CHD
- coronary heart disease
- FPLC
- fast-protein liquid chromatography
- HDL-C
- HDL cholesterol
- LDL-C
- LDL cholesterol
- PBST
- 0.1% Tween 20 in PBS
- SNP
- single nucleotide polymorphism
- TBST
- TBS containing 0.1% Tween
- TG
- triglyceride
- UP
- ultra performance
REFERENCES
- 1.Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R., Bangdiwala S., Tyroler H. A. 1989. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 79: 8–15. [DOI] [PubMed] [Google Scholar]
- 2.Assmann G., Schulte H., Eckardstein A., Huang Y. 1996. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 124: S11–S20. [DOI] [PubMed] [Google Scholar]
- 3.Sharrett A. R., Ballantyne C. M., Coady S. A., Heiss G., Sorlie P. D., Catellier D., Patsch W. 2001. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 104: 1108–1113. [DOI] [PubMed] [Google Scholar]
- 4.Luc G., Bard J., Ferrieres J., Evans A., Amouyel P., Arveiler D., Fruchart J., Ducimetiere P., on behalf of the PRIME Study Group. 2002. Value of HDL cholesterol, apolipoprotein A-I, lipoprotein A-I, and lipoprotein A-I/A-II in prediction of coronary heart disease: The PRIME Study. Arterioscler. Thromb. Vasc. Biol. 22: 1155–1161. [DOI] [PubMed] [Google Scholar]
- 5.Boden W. E. 2000. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High–Density Lipoprotein Intervention Trial. Am. J. Cardiol. 86: 19L–22L. [DOI] [PubMed] [Google Scholar]
- 6.Rubin E. M., Krauss R. M., Spangler E. A., Verstuyft J. G., Clift S. M. 1991. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 353: 265–267. [DOI] [PubMed] [Google Scholar]
- 7.Plump A. S., Scott C. J., Breslow J. L. 1994. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc. Natl. Acad. Sci. USA. 91: 9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pászty C., Maeda N., Verstuyft J., Rubin E. 1994. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J. Clin. Invest. 94: 899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu A. C., Lawn R. M., Verstuyft J. G., Rubin E. M. 1994. Human apolipoprotein A-I prevents atherosclerosis associated with apolipoprotein[a] in transgenic mice. J. Lipid Res. 35: 2263–2267. [PubMed] [Google Scholar]
- 10.Badimon J. J., Badimon L., Fuster V. 1990. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Invest. 85: 1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazaki A., Sakuma S., Morikawa W., Takiue T., Miake F., Terano T., Sakai M., Hakamata H., Sakamoto Y., Naito M., et al. 1995. Intravenous injection of rabbit apolipoprotein A-I inhibits the progression of atherosclerosis in cholesterol-fed rabbits. Arterioscler. Thromb. Vasc. Biol. 15: 1882–1888. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson M., Carlson L. A., Miettinen T. A., Angelin B. 1999. Stimulation of fecal steroid excretion after infusion of recombinant proapolipoprotein A-I: potential reverse cholesterol transport in humans. Circulation. 100: 594–598. [DOI] [PubMed] [Google Scholar]
- 13.Parolini C., Marchesi M., Lorenzon P., Castano M., Balconi E., Miragoli L., Chaabane L., Morisetti A., Lorusso V., Martin B., et al. 2008. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A-I Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: in vivo assessment by intravascular ultrasound and magnetic resonance imaging. J. Am. Coll. Cardiol. 51: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 14.Ibanez B., Vilahur G., Cimmino G., Speidl W., Pinero A., Choi B., Zafar M., Santos-Gallego C., Krause B., Badimon L., et al. 2008. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein A-I Milano (ETC-216) administration: magnetic resonance imaging study in an experimental model of atherosclerosis. J. Am. Coll. Cardiol. 51: 1104–1109. [DOI] [PubMed] [Google Scholar]
- 15.Shaw J. A., Bobik A., Murphy A., Kanellakis P., Blombery P., Mukhamedova N., Woollard K., Lyon S., Sviridov D., Dart A. M. 2008. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ. Res. 103: 1084–1091. [DOI] [PubMed] [Google Scholar]
- 16.Nanjee M. N., Cooke C. J., Garvin R., Semeria F., Lewis G., Olszewski W. L., Miller N. E. 2001. Intravenous apoA-I/lecithin discs increase pre-{beta}-HDL concentration in tissue fluid and stimulate reverse cholesterol transport in humans. J. Lipid Res. 42: 1586–1593. [PubMed] [Google Scholar]
- 17.Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., Grines C. L., et al. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 290: 2292–2300. [DOI] [PubMed] [Google Scholar]
- 18.Tardif J-C., Gregoire J., L'Allier P. L., Ibrahim R., Lesperance J., Heinonen T. M., Kouz S., Berry C., Basser R., Lavoie M-A., et al. 2007. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 297: 1675–1682. [DOI] [PubMed] [Google Scholar]
- 19.Birjmohun R. S., Hutten B. A., Kastelein J. J., Stroes E. S. 2005. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 45: 185–197. [DOI] [PubMed] [Google Scholar]
- 20.Jones P. H., Davidson M. H., Stein E. A., Bays H. E., McKenney J. M., Miller E., Cain V. A., Blasetto J. W., STELLAR Study Group. 2003. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am. J. Cardiol. 92: 152–160. [DOI] [PubMed] [Google Scholar]
- 21.Vrecer M., Turk S., Drinovec J., Mrhar A. 2003. Use of statins in primary and secondary prevention of coronary heart disease and ischemic stroke. Meta-analysis of randomized trials. Int. J. Clin. Pharmacol. Ther. 41: 567–577. [DOI] [PubMed] [Google Scholar]
- 22.Robins S. J., Collins D., Wittes J. T., Papademetriou V., Deedwania P. C., Schaefer E. J., McNamara J. R., Kashyap M. L., Hershman J. M., Wexler L. F., et al. 2001. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 285: 1585–1591. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg A., Alagona P. J., Capuzzi D., Guyton J., Morgan J., Rodgers J., Sachson R., Samuel P. 2000. Multiple-dose efficacy and safety of an extended-release form of niacin in the management of hyperlipidemia. Am. J. Cardiol. 85: 1100–1105. [DOI] [PubMed] [Google Scholar]
- 24.Pattnaik N. M., Montes A., Hughes L. B., Zilversmit D. B. 1978. Cholesteryl ester exchange protein in human plasma isolation and characterization. Biochim. Biophys. Acta. 530: 428–438. [DOI] [PubMed] [Google Scholar]
- 25.Drayna D., Jarnagin A. S., McLean J., Henzel W., Kohr W., Fielding C., Lawn R. 1987. Cloning and sequencing of human cholesteryl ester transfer protein cDNA. Nature. 327: 632–634. [DOI] [PubMed] [Google Scholar]
- 26.Clay M. A., Hopkins G. J., Ehnholm C. P., Barter P. J. 1989. The rabbit as an animal model of hepatic lipase deficiency. Biochim. Biophys. Acta. 1002: 173–181. [DOI] [PubMed] [Google Scholar]
- 27.Collet X., Tall A. R., Serajuddin H., Guendouzi K., Royer L., Oliveira H., Barbaras R., Jiang X., Francone O. L. 1999. Remodeling of HDL by CETP in vivo and by CETP and hepatic lipase in vitro results in enhanced uptake of HDL CE by cells expressing scavenger receptor B-I. J. Lipid Res. 40: 1185–1193. [PubMed] [Google Scholar]
- 28.Marotti K. R., Castle C. K., Boyle T. P., Lin A. H., Murray R. W., Melchior G. W. 1993. Severe atherosclerosis in transgenic mice expressing simian cholesteryl ester transfer protein. Nature. 364: 73–75. [DOI] [PubMed] [Google Scholar]
- 29.Plump A. S., Masucci-Magoulas L., Bruce C., Bisgaier C. L., Breslow J. L., Tall A. R. 1999. Increased atherosclerosis in ApoE and LDL receptor gene knock-out mice as a result of human cholesteryl ester transfer protein transgene expression. Arterioscler. Thromb. Vasc. Biol. 19: 1105–1110. [DOI] [PubMed] [Google Scholar]
- 30.Hayek T., Masucci-Magoulas L., Jiang X., Walsh A., Rubin E., Breslow J. L., Tall A. R. 1995. Decreased early atherosclerotic lesions in hypertriglyceridemic mice expressing cholesteryl ester transfer protein transgene. J. Clin. Invest. 96: 2071–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kako Y., Masse M., Huang L., Tall A. R., Goldberg I. J. 2002. Lipoprotein lipase deficiency and CETP in streptozotocin-treated apoB-expressing mice. J. Lipid Res. 43: 872–877. [PubMed] [Google Scholar]
- 32.Ha Y. C., Barter P. J. 1982. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp. Biochem. Physiol. B. 71: 265–269. [DOI] [PubMed] [Google Scholar]
- 33.Sugano M., Makino N., Sawada S., Otsuka S., Watanabe M., Okamoto H., Kamada M., Mizushima A. 1998. Effect of antisense oligonucleotides against cholesteryl ester transfer protein on the development of atherosclerosis in cholesterol-fed rabbits. J. Biol. Chem. 273: 5033–5036. [DOI] [PubMed] [Google Scholar]
- 34.Rittershaus C. W., Miller D. P., Thomas L. J., Picard M. D., Honan C. M., Emmett C. D., Pettey C. L., Adari H., Hammond R. A., Beattie D. T., et al. 2000. Vaccine-induced antibodies inhibit CETP activity in vivo and reduce aortic lesions in a rabbit model of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 20: 2106–2112. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto H., Yonemori F., Wakitani K., Minowa T., Maeda K., Shinkai H. 2000. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 406: 203–207. [DOI] [PubMed] [Google Scholar]
- 36.Morehouse L. A., Sugarman E. D., Bourassa P., Sand T. M., Zimetti F., Gao F., Rothblat G. H., Milici A. J. 2007. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. J. Lipid Res. 48: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 37.Koizumi J., Mabuchi H., Yoshimura A., Michishita I., Takeda M., Itoh H., Sakai Y., Sakai T., Ueda K., Takeda R. 1985. Deficiency of serum cholesteryl-ester transfer activity in patients with familial hyperalphalipoproteinaemia. Atherosclerosis. 58: 175–186. [DOI] [PubMed] [Google Scholar]
- 38.Inazu A., Brown M., Hesler C., Agellon L., Koizumi J., Takata K., Maruhama Y., Mabuchi H., Tall A. R. 1990. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N. Engl. J. Med. 323: 1234–1238. [DOI] [PubMed] [Google Scholar]
- 39.Brown M. L., Inazu A., Hesler C. B., Agellon L. B., Mann C., Whitlock M. E., Marcel Y. L., Milne R. W., Koizumi J., Mabuchi H., et al. 1989. Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteins. Nature. 342: 448–451. [DOI] [PubMed] [Google Scholar]
- 40.Zhong S., Sharp D., Grove J., Bruce C., Yano K., Curb J., Tall A. R. 1996. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J. Clin. Invest. 97: 2917–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curb J. D., Abbott R. D., Rodriguez B. L., Masaki K., Chen R., Sharp D. S., Tall A. R. 2004. A prospective study of HDL-C and cholesteryl ester transfer protein gene mutations and the risk of coronary heart disease in the elderly. J. Lipid Res. 45: 948–953. [DOI] [PubMed] [Google Scholar]
- 42.Nagano M., Yamashita S., Hirano K., Takano M., Maruyama T., Ishihara M., Sagehashi Y., Kujiraoka T., Tanaka K., Hattori H., et al. 2004. Molecular mechanisms of cholesteryl ester transfer protein deficiency in Japanese. J. Atheroscler. Thromb. 11: 110–121. [DOI] [PubMed] [Google Scholar]
- 43.Boekholdt S. M., Thompson J. F. 2003. Natural genetic variation as a tool in understanding the role of CETP in lipid levels and disease. J. Lipid Res. 44: 1080–1093. [DOI] [PubMed] [Google Scholar]
- 44.Boekholdt S. M., Kuivenhoven J. A., Hovingh G. K., Jukema J. W., Kastelein J. J., van Tol A. 2004. CETP gene variation: relation to lipid parameters and cardiovascular risk. Curr. Opin. Lipidol. 15: 393–398. [DOI] [PubMed] [Google Scholar]
- 45.Ordovas J. M., Cupples L. A., Corella D., Otvos J. D., Osgood D., Martinez A., Lahoz C., Coltell O., Wilson P. W. F., Schaefer E. J. 2000. Association of cholesteryl ester transfer protein-TaqIB polymorphism with variations in lipoprotein subclasses and coronary heart disease risk: The Framingham Study. Arterioscler. Thromb. Vasc. Biol. 20: 1323–1329. [DOI] [PubMed] [Google Scholar]
- 46.Thompson A., Di Angelantonio E., Sarwar N., Erqou S., Saleheen D., Dullaart R. P., Keavney B., Ye Z., Danesh J. 2008. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 299: 2777–2788. [DOI] [PubMed] [Google Scholar]
- 47.Brousseau M. E., O'Connor J. J., Ordovas J. M., Collins D., Otvos J. D., Massov T., McNamara J. R., Rubins H. B., Robins S. J., Schaefer E. J. 2002. Cholesteryl ester transfer protein TaqI B2B2 genotype is associated with higher HDL cholesterol levels and lower risk of coronary heart disease end points in men with HDL deficiency: Veterans Affairs HDL Cholesterol Intervention Trial. Arterioscler. Thromb. Vasc. Biol. 22: 1148–1154. [DOI] [PubMed] [Google Scholar]
- 48.Liu S., Schmitz C., Stampfer M., Sacks F., Hennekens C., Lindpaintner K., Ridker P. 2002. A prospective study of TaqIB polymorphism in the gene coding for cholesteryl ester transfer protein and risk of myocardial infarction in middle-aged men. Atherosclerosis. 161: 469–474. [DOI] [PubMed] [Google Scholar]
- 49.Boekholdt S. M., Sacks F. M., Jukema J. W., Shepherd J., Freeman D. J., McMahon A. D., Cambien F., Nicaud V., de Grooth G. J., Talmud P. J., et al. 2005. Cholesteryl ester transfer protein TaqIB variant, high-density lipoprotein cholesterol levels, cardiovascular risk, and efficacy of pravastatin treatment: Individual patient meta-analysis of 13 677 subjects. Circulation. 111: 278–287. [DOI] [PubMed] [Google Scholar]
- 50.Clark R. W., Ruggeri R. B., Cunningham D., Bamberger M. J. 2006. Description of the torcetrapib series of cholesteryl ester transfer protein inhibitors, including mechanism of action. J. Lipid Res. 47: 537–552. [DOI] [PubMed] [Google Scholar]
- 51.Clark R. W., Sutfin T. A., Ruggeri R. B., Willauer A. T., Sugarman E. D., Magnus-Aryitey G., Cosgrove P. G., Sand T. M., Wester R. T., Williams J. A., et al. 2004. Raising high-density lipoprotein in humans through inhibition of cholesteryl ester transfer protein: an initial multidose study of torcetrapib. Arterioscler. Thromb. Vasc. Biol. 24: 490–497. [DOI] [PubMed] [Google Scholar]
- 52.Brousseau M. E., Schaefer E. J., Wolfe M. L., Bloedon L. T., Digenio A. G., Clark R. W., Mancuso J. P., Rader D. J. 2004. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N. Engl. J. Med. 350: 1505–1515. [DOI] [PubMed] [Google Scholar]
- 53.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J-C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 54.Forrest M. J., Bloomfield D., Briscoe R. J., Brown P. N., Cumiskey A-M., Ehrhart J., Hershey J. C., Keller W. J., Ma X., McPherson H. E., et al. 2008. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br. J. Pharmacol. 154: 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu X., Dietz J. D., Xia C., Knight D. R., Loging W. T., Smith A. H., Yuan H., Perry D. A., Keiser J. 2009. Torcetrapib induces aldosterone and cortisol production by an intracellular calcium-mediated mechanism independently of cholesteryl ester transfer protein inhibition. Endocrinology. 150: 2211–2219. [DOI] [PubMed] [Google Scholar]
- 56.Cunningham D., Lin W., Hoth L. R., Danley D. E., Ruggeri R. B., Geoghegan K. F., Chrunyk B. A., Boyd J. G. 2008. Biophysical and biochemical approach to locating an inhibitor binding site on cholesteryl ester transfer protein. Bioconjug. Chem. 19: 1604–1613. [DOI] [PubMed] [Google Scholar]
- 57.Stein E. A., Stroes E. S., Steiner G., Buckley B. M., Capponi A. M., Burgess T., Niesor E. J., Kallend D., Kastelein J. J. 2009. Safety and tolerability of dalcetrapib. Am. J. Cardiol. 104: 82–91. [DOI] [PubMed] [Google Scholar]
- 58.Cannon C. P., Dansky H. M., Davidson M., Gotto A. M., Brinton E. A., Gould A. L., Stepanavage M., Liu S. X., Shah S., Rubino J., et al. 2009. Design of the DEFINE trial: determining the EFficacy and tolerability of CETP INhibition with anacEtrapib. Am. Heart J. 158: 513–519. [DOI] [PubMed] [Google Scholar]
- 59.Krishna R., Anderson M. S., Bergman A. J., Jin B., Fallon M., Cote J., Rosko K., Chavez-Eng C., Lutz R., Bloomfield D. M., et al. 2007. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet. 370: 1907–1914. [DOI] [PubMed] [Google Scholar]
- 60.Krishna R., Garg A., Jin B., Keshavarz S. S., Bieberdorf F. A., Chodakewitz J., Wagner J. A. 2009. Assessment of a pharmacokinetic and pharmacodynamic interaction between simvastatin and anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Br. J. Clin. Pharmacol. 67: 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bloomfield D., Carlson G.L., Sapre A., Tribble D., McKenney J.M., Littlejohn T.W., Sisk C.M., Mitchel Y., Pasternak R.C. 2009. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am. Heart J. 157: 352–360. [DOI] [PubMed] [Google Scholar]
- 62.Stevenson S. C., Wang S., Deng L., Tall A. R. 1993. Human plasma cholesteryl ester transfer protein consists of a mixture of two forms reflecting variable glycosylation at asparagine 341. Biochemistry. 32: 5121–5126. [DOI] [PubMed] [Google Scholar]
- 63.LoGrasso P. V., Frantz B., Rolando A. M., O'Keefe S. J., Hermes J. D., O'Neill E. A. 1997. Kinetic mechanism for p38 MAP kinase. Biochemistry. 36: 10422–10427. [DOI] [PubMed] [Google Scholar]
- 64.Eveland S. S., Milot D. P., Guo Q., Chen Y., Hyland S. A., Peterson L. B., Jezequel-Sur S., O'Donnell G. T., Zuck P. D., Ferrer M., et al. 2007. High-precision fluorogenic cholesteryl ester transfer protein assay compatible with animal serum and 3456-well assay technology. Anal. Biochem. 368: 239–249. [DOI] [PubMed] [Google Scholar]
- 65.Morton R. E., Zilversmitg D. B. 1981. A plasma inhibitor of triglyceride and cholesteryl ester transfer activities. J. Biol. Chem. 256: 11992–11995. [PubMed] [Google Scholar]
- 66.Evans D. C., Watt A. P., Nicoll-Griffith D. A., Baillie T. A. 2004. Drug-protein adducts: an industry perspective on minimizing the potential for drug bioactivation in drug discovery and development. Chem. Res. Toxicol. 17: 3–16. [DOI] [PubMed] [Google Scholar]
- 67.Krishna R., Bergman A., Jin B., Fallon M., Cote J., Van Hoydonck P., Laethem T., Gendrano I., Van Dyck K., Hilliard D., et al. 2008. Multiple-dose pharmacodynamics and pharmacokinetics of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Clin. Pharmacol. Ther. 84: 679–683. [DOI] [PubMed] [Google Scholar]
- 68.Marotti K., Castle C., Murray R., Rehberg E., Polites H., Melchior G. 1992. The role of cholesteryl ester transfer protein in primate apolipoprotein A-I metabolism. Insights from studies with transgenic mice. Arterioscler. Thromb. 12: 736–744. [DOI] [PubMed] [Google Scholar]
- 69.Sarcich J. L., Fischer H. D., Babcock M. S., Leone J. W., Tomasselli A. G. 1995. Expression and purification of recombinant cynomolgus monkey cholesteryl ester transfer protein from Chinese hamster ovary cells. J. Protein Chem. 14: 73–80. [DOI] [PubMed] [Google Scholar]
- 70.Connolly D. T., Heuvelman D., Glenn K. 1996. Inactivation of cholesteryl ester transfer protein by cysteine modification. Biochem. Biophys. Res. Commun. 223: 42–47. [DOI] [PubMed] [Google Scholar]
- 71.Hope H. R., Heuvelman D., Duffin K., Smith C., Zablocki J., Schilling R., Hegde S., Lee L., Witherbee B., Baganoff M., et al. 2000. Inhibition of cholesteryl ester transfer protein by substituted dithiobisnicotinic acid dimethyl ester: involvement of a critical cysteine. J. Lipid Res. 41: 1604–1614. [PubMed] [Google Scholar]
- 72.Qiu X., Mistry A., Ammirati M., Chrunyk B., Clark R., Cong Y., Culp J., Danley D., Freeman T., Geoghegan K., et al. 2007. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat. Struct. Mol. Biol. 14: 106–113. [DOI] [PubMed] [Google Scholar]
- 73.Maugeais C., Magg C., Dernick G., Matile H., von der Mark E., Pflieger P., Huber W., Thoma R., Kakutani M., Okamoto H., et al. 2009. Abstract 1092: Dalcetrapib binds to and changes the conformation of CETP in a unique manner (differing to that observed with torcetrapib). Circulation. 120: S445. [Google Scholar]
- 74.Mancek-Keber M., Gradisar H., Pestana M. I., de Tejada G. M., Jerala R. 2009. Free thiol group of MD-2 as the target for inhibition of the lipopolysaccharide-induced cell activation. J. Biol. Chem. 284: 19493–19500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Grooth G. J., Kuivenhoven J. A., Stalenhoef A. F. H., de Graaf J., Zwinderman A. H., Posma J. L., van Tol A., Kastelein J. J. P. 2002. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans: a randomized phase II dose-response study. Circulation. 105: 2159–2165. [DOI] [PubMed] [Google Scholar]
- 76.McPherson R., Lau P., Kussie P., Barrett H., Tall A. R. 1997. Plasma kinetics of cholesteryl ester transfer protein in the rabbit. Effects of dietary cholesterol. Arterioscler. Thromb. Vasc. Biol. 17: 203–210. [DOI] [PubMed] [Google Scholar]
- 77.Herbert P. N., Bernier D. N., Cullinane E. M., Edelstein L., Kantor M. A., Thompson P. D. 1984. High-density lipoprotein metabolism in runners and sedentary men. JAMA. 252: 1034–1037. [PubMed] [Google Scholar]
- 78.Daerr W. H., Pethke W., Windler E. T., Greten H. 1990. Biotinyl-high-density lipoproteins as a probe for the determination of high-density lipoprotein turnover in humans. Biochim. Biophys. Acta. 1043: 311–317. [DOI] [PubMed] [Google Scholar]