Abstract
Fatty acid profiles of biological specimens from epidemiological/clinical studies can serve as biomarkers to assess potential relationships between diet and chronic disease risk. However, data are limited regarding fatty acid stability in archived specimens following long-term storage, a variable that could affect result validity. Our objective was to determine the effect of prolonged storage at −80°C on the fatty acid profiles of serum cholesteryl ester (CE), triglyceride (TG), and phospholipid (PL) fractions. This was accomplished by determining the fatty acid profile of frozen, archived, previously unthawed serum samples from 22 subjects who participated in a controlled feeding trial. Initial analysis was performed after trial completion and the repeat analysis after 8–10 years of storage using GC. No significant differences were observed among the majority of fatty acids regardless of lipid fraction. Reliability coefficients were high for the fatty acid classes (saturated fatty acid : 0.70, MUFA : 0.90, PUFA : 0.80). When differences were identified, they were limited to low abundance fatty acids (≤1.5 mol%). These differences were quantitatively small and likely attributable to technical improvements in GC methodology rather than sample degradation. Thus, our data demonstrate that storage at −80°C up to 10 years does not significantly influence serum CE, TG, or PL fatty acid profiles.
Keywords: serum fatty acids, storage time, auto-oxidation
There is considerable clinical and public health interest on the relationship between dietary fatty acids and cardiovascular disease risk. Consequently, the saturated (SFA), monounsaturated (MUFA), and polyunsaturated (PUFA) fatty acid profiles of various biological specimens are increasingly being used as biomarkers of dietary fat quality as well as chronic disease risk (1–4). This measurement overcomes some of the inherent limitations of subjective self-reported data by providing a more objective assessment (5–7). It has been previously established that the fatty acid profile of adipose tissue triglyceride (TG) is a good indicator of long-term dietary fat quality in weight-stable individuals due to its slow turnover time (2, 8–10). A limitation of these data is that the measure is useful only for essential fatty acids and not for those that can be synthesized de novo. Additionally, the invasive nature of sample collection precludes routine use in most studies.
Analysis of the fatty acid profile of serum/plasma fractions, including cholesteryl ester (CE), TG, and phospholipid (PL), is considered minimally invasive, necessitating only a single blood draw and has been shown to be responsive to dietary fat modifications (2, 11–13). Specifically, the fatty acid profile of the PL fraction is considered to be an indicator of medium-term intake (weeks), whereas the CE and TG fatty acid profiles reflect shorter-term intake (days) (4). Although it is recommended that analysis be performed on fresh or recently collected samples, most studies collect and store samples for subsequent analysis. Storage time can vary from days to months and even years. Additionally, once the analytical work has commenced, it may take several years to complete for very large cohorts. Although significant changes have been documented in the fatty acid profile of samples stored at 4°C and −20°C (14–16), data are limited and inconsistent regarding the stability of fatty acids stored at very low temperatures (−80°C) over prolonged periods of time (17–19). This is important, because the reliability of the analysis affects the validity of the results and subsequently the association with outcome measures. Our aim was to document the reliability of CE, TG, and PL fatty acid profile determinations in previously unthawed serum aliquots stored at −80°C, without an added antioxidant, over an 8- to 10-year period.
METHODS
Subjects
Archived serum samples were from subjects who participated in a previously reported randomized, controlled trial of dietary fat type (20, 21). These papers describe the intervention variables and study design in detail. The protocol for the trial was approved by the Human Investigation Review Committee of Tufts University/Tufts Medical Center. All subjects gave written informed consent. The data reported here are for archived serum samples from the soybean oil (control) phase of the trial.
Blood collection
At the end of each 5 week diet phase, fasting blood samples were collected and centrifuged at 1,800 g for 20 min. The serum was removed, separated into multiple aliquots, and stored at −80°C. All samples were processed and frozen at −80°C within 1 h after collection. The initial fatty acid analysis was performed on one aliquot of serum approximately 8–12 months after the samples were stored. The repeat analysis was performed on another aliquot of the same serum sample approximately 8–10 years later over a 2 month period. Although 36 subjects participated in the diet intervention, an unthawed aliquot was available for only 22 subjects at the time of the repeat analyses. The mean age of these subjects was 64 years and BMI was 28 kg/m2 (Table 1). Per the recruitment inclusion criteria, all subjects were moderately hypercholesterolemic, free from chronic illness, not taking medications known to affect lipid metabolism, and did not report consuming ≥2 alcoholic drinks per day or smoking. All women were postmenopausal.
TABLE 1.
Baseline characteristics and serum lipid profile of subjects with an archived serum sample for fatty acid analysis
| Variables | N = 22 (11 F/ 11 M) |
|---|---|
| Age (years) | 64 ± 5 |
| Weight (kg) | 79.6 ± 14.1 |
| Body mass index (kg/m ) | 27.6 ± 2.7 |
| Total cholesterol (mg/dl) | 231.7 ± 35.7 |
| LDL-cholesterol (mg/dl) | 159.2 ± 30.6 |
| HDL-cholesterol (mg/dl) | 43.0 ± 8.1 |
| TG (mg/dl) | 149.5 ± 75.2 |
Fatty acid analysis
For the initial and repeat analyses, the same protocols were used for serum lipid extraction (22, 23), separation of lipid fractions (CE, TG, and PL) (24) as well as fatty acid methylation (25). The resulting fatty acid methyl esters in each fraction were quantified using GC. Details regarding the initial GC analysis have been described previously (21). Modifications to the GC analysis at the time of the repeat analysis included the use of a newer machine (Autosystem XL gas chromatograph, Perkin Elmer, Boston, MA) and GC capillary column (30m × 0.25 mm inner diameter × 0.25 µm film thickness; HP INNOWAX, Agilent Technologies, DE), as well as refinements to the GC methodological parameters to improve fatty acid resolution as described below. Briefly, the injector and flame ionization detector temperatures were 250° and 260°C, respectively. Helium was used as the carrier gas (2 ml/min) and the split ratio was 2:1. The oven temperature was programmed at 80°C, held for 2 min, and then increased to 160°C at a rate of 10°C/min. After 5 min, the temperature was increased to 222°C at a rate of 2°C/min and held for 5 min. The final temperature was 252°C held for 5 min.
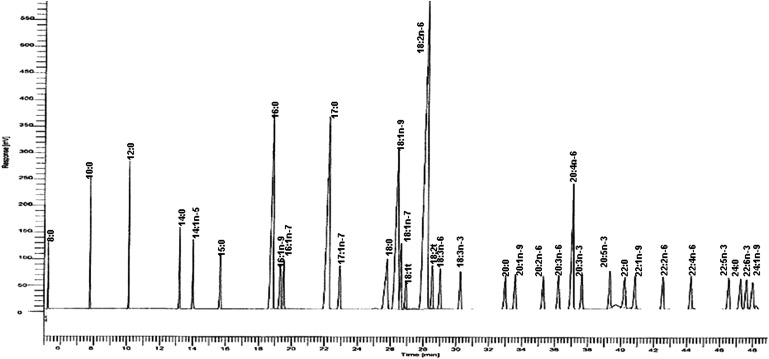
Peaks of interest were identified by comparison with authentic fatty acid standards (National Institutes of Health Fatty Acid Standards A, B, C, and GLC 461, Nu-Check-Prep, Elysian, MN) (Fig. 1). Response factors were calculated, and the fatty acid composition expressed as molar percentage (mol %) proportions of total fatty acids relative to the internal standard. One external quality control sample (pooled serum) was extracted and analyzed for every six experimental samples. Instrument precision was determined by analyzing 10 serial injections from each of three extracted control samples. The mean (± SD) percent recovery of the internal standard was 91% for cholesteryl heptadecanoate, 92% for triheptadecanoin glyceride, and 95% for 1,2 diheptadecanoyl-glycero-3-phosphocholine. For the repeat analysis, a subset of 10 samples was analyzed in triplicate, and the coefficient of variations ranged from 0.5 to 4.3% for fatty acids present at levels > 5%, 1.8–7.1% for fatty acids present at levels between 1 and 5 mol%, and 2.8–11.1% for fatty acids present at levels <1 mol%.
Fig. 1.
Chromatogram of the external standard mixture of fatty acid methyl esters.
Statistical analyses
All values are expressed as mean ± SD. Differences for each fatty acid, within the three lipid fractions, related to storage time, were analyzed using the nonparametric signed-rank test (SAS version 9.1, SAS Institute Inc., Cary, NC). The significance criterion was set P < 0.05. The reliability coefficient or intraclass correlation for the repeated measures was calculated using restricted maximum-likelihood techniques in SAS PROC VARCOMP (SAS version 9.1). Of note, within-subject variability was reduced by using serum aliquots derived from a single blood sample drawn after subjects were provided with a controlled diet for a 5 week period for both analyses. Thus, the sources contributing to the variability of the repeated measure can be considered to be primarily due to the method component and storage time. Method repeatability was assessed using the Bland and Altman method (26), and the coefficient of repeatability was derived by multiplying the SD of the differences by two. The SD of the differences was calculated as the square root of the sum of the squared differences divided by n. The coefficient of repeatability is a precision measure that represents the value below which the absolute difference between two repeated test results may be expected to lie with a probability of 95% (27). Bland-Altman plots were generated for selected fatty acids by plotting the difference between the initial and repeat analysis against the mean of all initial and repeat analysis for each subject. Upper and lower limits of agreement were calculated as previously described (26).
RESULTS
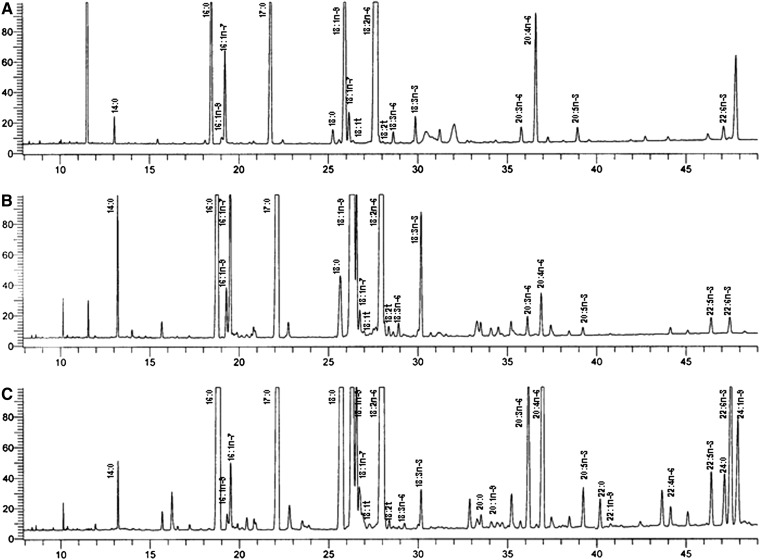
In the initial analysis, a total of 15 fatty acids were identified in the CE fraction, 16 in the TG fraction, and 23 in the PL fraction. Chain lengths ranged from 14:0 to 24:1n-9. All fatty acids detected were used to determine the sum of the fatty acids in each lipid faction. Fatty acids were also grouped as SFA, MUFA, PUFA, n-6, n-3, and total trans. Due to refinement of the gas chromatographic method parameters, a greater number of fatty acids (chain lengths ranging from 8:0 to 26:1n-9) were resolved and identified in the repeat analysis. Consequently, fatty acids not measured as part of the initial analysis were excluded from the mol % calculation in the repeat analysis. Also, fatty acids that were not resolved individually (e.g., 16:1n-7 and 16:1n-9) in the initial analysis were aggregated in the repeat analysis to facilitate comparison. Figure 2 shows the chromatogram of the serum CE, TG, and PL fatty acid profile of one subject at the time of the repeat analysis.
Fig. 2.
Representative gas chromatogram of the serum CE (A), TG (B), and PL (C) fatty acid profile of one subject at the time of the repeat analysis. The x axis denotes the run time in minutes and the y axis the response time in millivolts (mV). The scale has been magnified to highlight the fatty acid peaks of interest.
The reliability coefficients for the individual fatty acids in the CE, TG, and PL fractions are depicted in Table 2. Overall, high reliability coefficients were obtained for the majority of the fatty acids in all three fractions. In the CE fraction, with the exception of 18:1n-7, which had an intermediate reliability coefficient of 0.55, all the other fatty acids had high values (≥0.70). In the TG fraction, three fatty acids had an intermediate value, between 0.50 and 0.70, while the remaining 13 fatty acids had high values (≥0.70). In the PL fraction, 5 had low (≤0.50), 7 had intermediate (0.50–0.70), and 10 had high values (≥0.70). Among the fatty acid classes, the reliability coefficients were ≥0.70 for SFAs, ≥0.90 for MUFAs, and ≥0.80 for PUFAs. The reliability coefficient for total trans was high in the TG (0.89) and PL (0.84) fractions and intermediate in the CE (0.64) fraction.
TABLE 2.
Reliability coefficient of serum CE, TG, and PL fatty acid profiles measured at two time points
| Fatty Acid | CE | TG | PL |
|---|---|---|---|
| SFA | 0.91 | 0.99 | 0.72 |
| 14:0 | 0.88 | 0.96 | 0.69 |
| 16:0 | 0.90 | 0.98 | 0.58 |
| 18:0 | 0.71 | 0.92 | 0.92 |
| 20:0 | — | — | 0.40 |
| 22:0 | — | — | 0.18 |
| 24:0 | — | — | 0.57 |
| MUFA | 0.94 | 0.98 | 0.91 |
| 16:1n-9+7 | 0.72 | 0.88 | 0.56 |
| 18:1n-9 | 0.90 | 0.98 | 0.91 |
| 18:1n-7 | 0.55 | 0.58 | 0.49 |
| 20:1n-9 | — | — | 0.50 |
| 22:1n-9 | — | — | 0.50 |
| 24:1n-9 | — | — | 0.60 |
| PUFA | 0.96 | 0.99 | 0.66 |
| n-6 | 0.96 | 0.99 | 0.80 |
| 18:2n-6 | 0.98 | 0.99 | 0.95 |
| 18:3n-6 | 0.96 | 0.94 | 0.40 |
| 20:3n-6 | 0.82 | 0.88 | 0.74 |
| 20:4n-6 | 0.96 | 0.80 | 0.97 |
| 22:4n-6 | — | — | 0.33 |
| n-3 | 0.93 | 0.80 | 0.94 |
| 18:3n-3 | 0.91 | 0.80 | 0.89 |
| 20:5n-3 | 0.98 | 0.89 | 0.98 |
| 22:5n-3 | — | 0.50 | 0.59 |
| 22:6n-3 | 0.77 | 0.89 | 0.93 |
| Trans | 0.64 | 0.89 | 0.84 |
| 18:1t | 0.75 | 0.92 | 0.82 |
| 18:2t | 0.35 | 0.62 | 0.89 |
No significant differences (P > 0.05) were observed in the magnitude of the absolute change for the majority of the fatty acids in the CE (Table 3) and TG (Table 4) fractions. The exceptions were two fatty acids present in relatively low abundance: 22:6n-3 in the CE fraction (mean difference 0.09 mol%, P = 0.03) and 22:5n-3 in the TG fraction (mean difference 0.12 mol%, P = 0.01). Among the PL fatty acids (Table 5), no significant differences were detected for 18 of 23 fatty acids measured. Among the five fatty acids for which significant differences were detected, three had lower values (16:0, 22:0, and 22:1n-9) and two had higher values (18:1n-7 and 22:4n-6) in the repeat relative to the initial analysis.
TABLE 3.
Mean (SD) serum CE fatty acid profile measured at two time points with corresponding P-values and repeatability coefficient
| Fatty Acid | Initial Analyses | Repeat Analyses | Mean Difference | P | Repeatability Coefficient |
|---|---|---|---|---|---|
| SFA | 13.5 ± 1.3 | 13.8 ± 1.3 | 0.26 | 0.25 | 1.03 |
| 14:0 | 0.75 ± 0.21 | 0.73 ± 0.17 | −0.02 | 0.46 | 0.18 |
| 16:0 | 11.9 ± 1.0 | 12.2 ± 1.2 | 0.26 | 0.26 | 1.00 |
| 18:0 | 0.81 ± 0.24 | 0.84 ± 0.33 | 0.03 | 0.49 | 0.42 |
| MUFA | 16.1 ± 2.5 | 16.3 ± 2.3 | 0.14 | 0.44 | 1.64 |
| 16:1n-9+n-7 | 2.0 ± 0.7 | 2.1 ± 0.6 | 0.07 | 0.38 | 0.96 |
| 18:1n-9 | 11.9 ± 2.8 | 12.1 ± 2.3 | 0.22 | 0.49 | 2.24 |
| 18:1n-7 | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.06 | 0.33 | 0.42 |
| PUFA | 70.4 ± 3.4 | 69.9 ± 3.1 | −0.45 | 0.28 | 1.80 |
| n-6 | 68.2 ± 3.6 | 67.6 ± 3.4 | −0.60 | 0.22 | 1.92 |
| 18:2n-6 | 57.5 ± 4.5 | 57.0 ± 4.5 | −0.53 | 0.33 | 1.79 |
| 18:3n-6 | 0.87 ± 0.37 | 0.91 ± 0.40 | 0.03 | 0.39 | 0.19 |
| 20:3n-6 | 0.62 ± 0.19 | 0.63 ± 0.15 | 0.02 | 0.23 | 0.21 |
| 20:4n-6 | 7.3 ± 2.0 | 7.4 ± 2.1 | 0.06 | 0.41 | 1.14 |
| n-3 | 2.2 ± 0.8 | 2.6 ± 0.8 | 0.15 | 0.18 | 0.55 |
| 18:3n-3 | 0.76 ± 0.33 | 0.78 ± 0.29 | 0.01 | 0.45 | 0.25 |
| 20:5n-3 | 0.91 ± 0.53 | 0.95 ± 0.55 | 0.04 | 0.27 | 0.19 |
| 22:6n-3 | 0.52 ± 0.20 | 0.61 ± 0.23 | 0.09 | 0.03 | 0.30 |
| Trans | 3.1 ± 2.1 | 2.7 ± 1.5 | −0.39 | 0.32 | 3.06 |
| 18:1t | 1.2 ± 1.4 | 1.0 ± 1.0 | −0.22 | 0.35 | 1.72 |
| 18:2t | 1.9 ± 0.8 | 1.7 ± 0.5 | −0.18 | 0.44 | 1.45 |
TABLE 4.
Mean (SD) serum TG fatty acid profile measured at two time points with corresponding P-values and repeatability coefficient
| Fatty Acid | Initial Analyses | Repeat Analyses | Mean Difference | P | Repeatability Coefficient |
|---|---|---|---|---|---|
| SFA | 29.6 ± 4.9 | 30.1 ± 5.1 | 0.50 | 0.50 | 1.66 |
| 14:0 | 2.3 ± 0.7 | 2.2 ± 0.8 | −0.06 | 0.33 | 0.39 |
| 16:0 | 24.4 ± 4.1 | 24.7 ± 4.2 | 0.31 | 0.34 | 1.44 |
| 18:0 | 2.9 ± 0.8 | 2.8 ± 0.7 | −0.13 | 0.31 | 0.61 |
| MUFA | 35.4 ± 4.8 | 35.5 ± 4.5 | 0.05 | 0.42 | 1.78 |
| 16:1n-9+n-7 | 3.3 ± 1.0 | 3.5 ± 1.0 | 0.28 | 0.22 | 0.98 |
| 18:1n-9 | 27.0 ± 4.5 | 26.8 ± 4.3 | −0.17 | 0.38 | 1.48 |
| 18:1n-7 | 1.9 ± 0.4 | 1.7 ± 0.4 | −0.18 | 0.07 | 0.76 |
| PUFA | 35.0 ± 5.9 | 34.5 ± 5.9 | −0.51 | 0.47 | 1.54 |
| n-6 | 31.3 ± 5.6 | 30.5 ± 5.6 | −0.80 | 0.41 | 1.44 |
| 18:2n-6 | 27.0 ± 5.2 | 26.4 ± 5.2 | −0.27 | 0.45 | 1.08 |
| 18:3n-6 | 0.7 ± 0.3 | 0.7 ± 0.4 | 0.02 | 0.33 | 0.22 |
| 20:3n-6 | 0.4 ± 0.1 | 0.4 ± 0.2 | 0.01 | 0.45 | 0.13 |
| 20:4n-6 | 1.5 ± 0.5 | 1.7 ± 0.6 | 0.11 | 0.36 | 0.71 |
| n-3 | 3.7 ± 1.2 | 4.0 ± 1.0 | 0.30 | 0.26 | 1.27 |
| 18:3n-3 | 2.3 ± 0.9 | 2.3 ± 0.7 | −0.02 | 0.31 | 0.92 |
| 20:5n-3 | 0.4 ± 0.2 | 0.4 ± 0.3 | 0.05 | 0.31 | 0.24 |
| 22:5n-3 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.12 | 0.01 | 0.32 |
| 22:6n-3 | 0.7 ± 0.6 | 0.9 ± 0.6 | 0.18 | 0.07 | 0.55 |
| Trans | 4.8 ± 2.3 | 4.7 ± 2.0 | −0.12 | 0.45 | 1.60 |
| 18:1t | 3.3 ± 1.9 | 3.1 ± 1.7 | −0.16 | 0.40 | 0.96 |
| 18:2t | 1.5 ± 0.4 | 1.7 ± 0.6 | −0.05 | 0.48 | 0.92 |
TABLE 5.
Mean (SD) serum PL fatty acid profile measured at two time points with corresponding P-values and repeatability coefficient
| Fatty Acid | Initial Analyses | Repeat Analyses | Mean Difference | P | Repeatability Coefficient |
|---|---|---|---|---|---|
| SFA | 47.2 ± 1.6 | 46.5 ± 1.4 | −0.72 | 0.07 | 2.16 |
| 14:0 | 0.54 ± 0.12 | 0.57 ± 0.14 | 0.03 | 0.19 | 0.19 |
| 16:0 | 30.5 ± 1.1 | 29.8 ± 1.4 | −0.75 | 0.03 | 2.31 |
| 18:0 | 14.0 ± 1.1 | 14.2 ± 1.1 | 0.27 | 0.20 | 0.83 |
| 20:0 | 0.38 ± 0.04 | 0.35 ± 0.09 | −0.02 | 0.14 | 0.16 |
| 22:0 | 1.1 ± 0.1 | 0.9 ± 0.3 | −0.19 | 0.003 | 0.53 |
| 24:0 | 0.74 ± 0.13 | 0.69 ± 0.20 | −0.05 | 0.19 | 0.31 |
| MUFA | 11.3 ± 1.4 | 11.5 ± 1.6 | 0.18 | 0.40 | 1.24 |
| 16:1n-9+n-7 | 0.58 ± 0.14 | 0.56 ± 0.21 | −0.03 | 0.28 | 0.34 |
| 18:1n-9 | 6.2 ± 1.2 | 6.3 ± 1.2 | 0.17 | 0.31 | 0.99 |
| 18:1n-7 | 1.2 ± 0.2 | 1.4 ± 0.3 | 0.19 | 0.02 | 0.51 |
| 20:1n-9 | 0.24 ± 0.06 | 0.22 ± 0.07 | −0.02 | 0.25 | 0.10 |
| 22:1n-9 | 0.04 ± 0.01 | 0.03 ± 0.01 | −0.01 | 0.01 | 0.03 |
| 24:1n-9 | 1.4 ± 0.2 | 1.4 ± 0.2 | −0.01 | 0.44 | 0.35 |
| PUFA | 42.2 ± 1.5 | 42.1 ± 1.2 | −0.10 | 0.41 | 2.12 |
| n-6 | 35.7 ± 2.0 | 35.3 ± 1.5 | −0.40 | 0.24 | 2.23 |
| 18:2n-6 | 22.8 ± 2.6 | 22.8 ± 2.5 | −0.01 | 0.47 | 1.57 |
| 18:3n-6 | 0.15 ± 0.06 | 0.18 ± 0.08 | 0.02 | 0.15 | 0.16 |
| 20:3n-6 | 3.5 ± 1.0 | 3.0 ± 0.9 | −0.50 | 0.07 | 1.36 |
| 20:4n-6 | 9.3 ± 1.8 | 9.4 ± 1.9 | 0.09 | 0.47 | 0.95 |
| 22:4n-6 | 0.21 ± 0.05 | 0.25 ± 0.05 | 0.04 | 0.01 | 0.12 |
| n-3 | 5.6 ± 1.4 | 5.9 ± 1.5 | 0.33 | 0.13 | 1.00 |
| 18:3n-3 | 0.30 ± 0.09 | 0.31 ± 0.11 | 0.01 | 0.48 | 0.11 |
| 20:5n-3 | 0.98 ± 0.64 | 1.03 ± 0.67 | 0.06 | 0.31 | 0.23 |
| 22:5n-3 | 0.74 ± 0.19 | 0.85 ± 0.19 | 0.11 | 0.06 | 0.33 |
| 22:6n-3 | 3.6 ± 0.9 | 3.7 ± 1.0 | 0.16 | 0.20 | 0.71 |
| Trans | 2.4 ± 1.1 | 2.2 ± 1.0 | −0.19 | 0.28 | 1.16 |
| 18:1t | 1.7 ± 0.8 | 1.6 ± 1.5 | −0.11 | 0.31 | 0.95 |
| 18:2t | 0.69 ± 0.36 | 0.61 ± 0.30 | −0.08 | 0.09 | 0.30 |
Given that modifications to improve fatty acid resolution were made to the GC method at the time of repeat analysis, we also assessed method repeatability by calculating the repeatability coefficient. The results for all fatty acids measured in each fraction are summarized in Tables 3–5 and depicted graphically for selected fatty acids in supplementary. All measurements were within the coefficient of repeatability, indicating that 95% of differences were <2 SD of the mean measurement of the combined initial and repeat analyses.
DISCUSSION
The fatty acid profiles of serum lipid fractions are biomarkers of dietary fat quality and have been used to estimate diet-chronic disease risk (1–4). However, in many studies, the analysis is performed several years after sample collection. The published data on the stability of these fatty acids during prolonged storage is limited. To address this issue, we examined the reliability of the fatty acid profile of serum CE, TG, and PL in samples stored at −80°C over an 8–10 year period without an added antioxidant. Of particular interest was the potential differential degradation rate among SFA, MUFA, and PUFA. Results indicate that the majority of the fatty acids in all three serum fractions did not change significantly and there was no trend toward greater loss of PUFA in any of the fractions. For those fatty acids for which differences were identified, the variability was modest and primarily restricted to fatty acids present at <1.5 mol%. This is likely a reflection of low endogenous abundance and/or improved GC sensitivity at the time of the repeat analysis rather than sample deterioration.
To our knowledge, this is the first study to report the effect of storage longer than 8 years at a very low temperature (−80°C) on the fatty acid profile of all three lipid fractions. Two prior studies have examined fatty acid stability in various lipid fractions, but storage time was limited to 1–3 years and conditions were different. In the first study, the fatty acid composition of plasma CE, TG, and PL was analyzed before and after 3 years of storage at −20°C (16). Considerable loss (14–46%) was reported in all three fractions, particularly for PUFA with three or more double bonds. Additionally, the proportions of SFA and MUFA showed an increasing trend. The changes tended to be greatest in the TG fraction and smallest in the PL fraction. In the second study, the fatty acid profile of the three plasma lipid fractions was documented after 1 year of storage at −60°C (15). Numerous small differences were reported between the first and second determinations in all three fractions. These were attributed to minor methodological modifications rather than auto-oxidation. Thus, the available data suggest that storage at a very low temperature is crucial to ensure accurate assessment of fatty acid profiles after long-term storage.
We identified two studies (18, 19) that reported reliability coefficients for either the CE and/or PL fractions of samples stored at −80°C. The New York University Women's Health Study determined the reliability of nonfasting serum PL fatty acids, collected at three yearly visits and stored at −80°C for 7–12 years (19). For the 20 individual PL fatty acids measured, the reliability coefficients were <0.50 for 4 (15:0, 17:0, 18:0, and 18:3n-6), between 0.50 and 0.70 for 9 (16:0, 18:1t, 18:2n-6, 20:2n-6, 20:3n-6, 22:4n-6, 20:5n-3, 22:5n-3, and 22:6n-3), and >0.70 for 7 (20:0, 22:0, 24:0, 16:1n-7, 18:1n-9, 18:3n-3, and 20:4n-6). Overall, 80% of the fatty acids had reliability coefficients > 0.50. However, the reliability coefficients for total SFA (0.31), MUFA (0.66), and PUFA (0.43) tended to be lower than for the individual fatty acids. The Atherosclerosis Risk in Communities study assessed the reliability of the CE and PL fatty acid determinations using fasting samples collected 2 years apart (18). The reliability coefficient was >0.65 for the major fatty acids (16:0, 18:0, 18:2n-6, and 20:4n-6) in both lipid fractions, although the reliability tended to be higher for fatty acids in the CE than in the PL fraction. There was a trend for the reliability coefficients to be lower for fatty acids present at levels <1.0 mol%. Data was not available for the fatty acid classes. Our reliability data for the individual fatty acids was higher than reported for the prior studies. The exceptions tended to be limited to low abundance fatty acids (20:0, 22:0, 24:0, 16:1, 22:4n-6, and 22:5n-3). The reason for the high reliability is most likely attributable to the fact that we analyzed aliquots from the same sample from each subject at both time points and that all subjects were on a controlled feeding regime.
The data on the stability of fatty acids in the TG fraction is limited. This is probably due to the fact that the fatty acid composition of TG represents dietary intake from the preceding days and therefore is not considered suitable by most investigators as a measure of long-term dietary fat quality, unless the subjects are on a stable dietary regime (2). In the one study (17) that determined the fatty acid composition of the plasma TG fraction after storage for nearly 4 years at −80°C, very little change was observed overall in the fatty acid pattern. Specifically, a decrease in 14:1 of 0.11 mol % and an increase in 22:5n-3 of 0.04 mol% occurred between the original and repeat analyses. Interestingly, in the present study, an increase was also observed in 22:5n-3 of 0.12 mol% at the repeat analysis. However, it is more likely that the change is a result of increased method sensitivity rather than any known biological mechanism. Data on 14:1 could not be compared, because this fatty acid was not measured at the time of the initial analysis.
A limitation of the present study is that the stability of the fatty acids from the time of collection until the initial analysis (8–12 months) was not determined. However, based on prior data (15) that reported no difference in plasma fatty acid composition measured immediately and after 12 months of storage at −60°C, it is reasonable to assume that little deterioration occurred in our samples during the time period prior to the initial analysis. Second, a different GC machine and capillary column were used for the repeat analysis. Refinements had also been made to the GC methodological parameters to improve detection of low abundance fatty acids, as well as resolution of cis and trans isomers. Hence, there was concern that it would be difficult to ferret out the potential contribution of sample deterioration from methodological changes, were any to occur. To address this issue, we measured method repeatability and found it to be high for the majority of the fatty acids. Finally, our data was derived from subjects who participated in a controlled feeding trial. Thus, results might not be generalizable to free-living populations where the higher within-subject biological and dietary variability may result in lower reliability of the fatty acid profile. This is of particular concern for fatty acids present at levels < 1% of the total, where these components constitute the major parts of the variability.
In conclusion, our findings suggest that serum fatty acid profiles generated from samples collected up to 10 years prior to the analysis and stored at −80°C yield reliable data that may be useful in establishing dietary fat-disease relationships.
Supplementary Material
Acknowledgments
We are indebted to the following for the availability of the archived serum samples used in this study: the Metabolic Research Unit staff, including the study volunteer recruiters; the nurses for their expert care of the study subjects; the dieticians and kitchen staff for their meticulous planning and preparation of the study foods for each dietary phase; and to the study subjects without whom this investigation would not be possible.
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- mol %
- molar percentage
- PL
- phospholipid
- SFA
- saturated fatty acid
- TG
- triglyceride
This work was supported by grant HL-54727 from the National Institutes of Health, and by the US Department of Agriculture, under agreement no. 58-1950-4-401. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of authors, and do not necessarily reflect the views of the US Department of Agriculture, the National Institutes of Health, or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Baylin A., Campos H. 2006. The use of fatty acid biomarkers to reflect dietary intake. Curr. Opin. Lipidol. 17: 22–27. [DOI] [PubMed] [Google Scholar]
- 2.Hodson L., Skeaff C. M., Fielding B. A. 2008. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 47: 348–380. [DOI] [PubMed] [Google Scholar]
- 3.Wolk A., Furuheim M., Vessby B. 2001. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J. Nutr. 131: 828–833. [DOI] [PubMed] [Google Scholar]
- 4.Arab L. 2003. Biomarkers of fat and fatty acid intake. J. Nutr. 133: 925S–932S. [DOI] [PubMed] [Google Scholar]
- 5.Fuhrman B. J., Barba M., Krogh V., Micheli A., Pala V., Lauria R., Chajes V., Riboli E., Sieri S., Berrino F., et al. 2006. Erythrocyte membrane phospholipid composition as a biomarker of dietary fat. Ann. Nutr. Metab. 50: 95–102. [DOI] [PubMed] [Google Scholar]
- 6.Arab L., Akbar J. 2002. Biomarkers and the measurement of fatty acids. Public Health Nutr. 5: 865–871. [DOI] [PubMed] [Google Scholar]
- 7.Prentice R. L., Sugar E., Wang C. Y., Neuhouser M., Patterson R. 2002. Research strategies and the use of nutrient biomarkers in studies of diet and chronic disease. Public Health Nutr. 5: 977–984. [DOI] [PubMed] [Google Scholar]
- 8.Poppitt S. D., Kilmartin P., Butler P., Keogh G. F. 2005. Assessment of erythrocyte phospholipid fatty acid composition as a biomarker for dietary MUFA, PUFA or saturated fatty acid intake in a controlled cross-over intervention trial. Lipids Health Dis. 4: 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leaf D. A., Connor W. E., Barstad L., Sexton G. 1995. Incorporation of dietary n-3 fatty acids into the fatty acids of human adipose tissue and plasma lipid classes. Am. J. Clin. Nutr. 62: 68–73. [DOI] [PubMed] [Google Scholar]
- 10.Garland M., Sacks F. M., Colditz G. A., Rimm E. B., Sampson L. A., Willett W. C., Hunter D. J. 1998. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am. J. Clin. Nutr. 67: 25–30. [DOI] [PubMed] [Google Scholar]
- 11.Mensink R. P., Hornstra G. 1995. The proportion of trans monounsaturated fatty acids in serum triacylglycerols or platelet phospholipids as an objective indicator of their short-term intake in healthy men. Br. J. Nutr. 73: 605–612. [DOI] [PubMed] [Google Scholar]
- 12.Zock P. L., Mensink R. P., Harryvan J., de Vries J. H., Katan M. B. 1997. Fatty acids in serum cholesteryl esters as quantitative biomarkers of dietary intake in humans. Am. J. Epidemiol. 145: 1114–1122. [DOI] [PubMed] [Google Scholar]
- 13.Skeaff C. M., Hodson L., McKenzie J. E. 2006. Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J. Nutr. 136: 565–569. [DOI] [PubMed] [Google Scholar]
- 14.Brown V. L., Shay J. C., Morse-Fisher N. L. 1992. Effect of heat inactivation and freezing on fatty acid composition of plasma and red blood cells. Prostaglandins Leukot. Essent. Fatty Acids. 47: 203–207. [DOI] [PubMed] [Google Scholar]
- 15.Moilanen T., Nikkari T. 1981. The effect of storage on the fatty acid composition of human serum. Clin. Chim. Acta. 114: 111–116. [DOI] [PubMed] [Google Scholar]
- 16.Salo M. K., Grey F., Nikkari T. 1986. Stability of plasma fatty acids at -20oC and its relationship to antioxidants. Int. J. Vitam. Nutr. Res. 56: 231–239. [PubMed] [Google Scholar]
- 17.Hodson L., Skeaff C. M., Wallace A. J., Arribas G. L. B. 2002. Stability of plasma and erythrocyte fatty acid composition during cold storage. Clin. Chim. Acta. 321: 63–67. [DOI] [PubMed] [Google Scholar]
- 18.Ma J., Folsom A. R., Eckfeldt J. H., Lewis L., Chambless L. E., and the Atherosclerosis Risk in Communties (ARIC) Study Investigators. 1995. Short- and long-term repeatibility of fatty acid composition of human plasma phospholipids and cholesterol esters. Am. J. Clin. Nutr. 62: 572–578. [DOI] [PubMed] [Google Scholar]
- 19.Zeleniuch-Jacquotte A., Chajes V., Van Kappel A. L., Riboli E., Toniolo P. 2000. Reliability of fatty acid composition i human serum phospholipids. Eur. J. Clin. Nutr. 54: 367–372. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein A. H., Ausman L. A., Jalbert S. M., Schaefer E. J. 1999. Comparison of different forms of hydrogenated fats on serum lipid levels in moderately hypercholesterolemic female and male subjects. N. Engl. J. Med. 340: 1933–1940. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein A. H., Erkkila A. T., Lamarche B., Schwab U. S., Jalbert S. M., Ausman L. M. 2003. Influence of hydrogenated fat and butter on CVD risk factors: remnant-like particles, glucose and insulin, blood pressure and C-reactive protein. Atherosclerosis. 171: 97–107. [DOI] [PubMed] [Google Scholar]
- 22.Folch J., Lees M., Sloane-Stanley G. H. 1957. A simple method for the isolation and purification of total lipid from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 23.Erkkila A. T., Matthan N. R., Herrington D. M., Lichtenstein A. H. 2006. Higher plasma docosahexaenoic acid is associated with reduced progression of coronary atherosclerosis in women with CAD. J. Lipid Res. 47: 2814–2819. [DOI] [PubMed] [Google Scholar]
- 24.Agren J. J., Julkunen A., Penttila I. 1992. Rapid separation of serum lipids for fatty acid analysis by a single aminopropyl column. J. Lipid Res. 33: 1871–1876. [PubMed] [Google Scholar]
- 25.Morrison W. R., Smith L. M. 1964. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 5: 600–608. [PubMed] [Google Scholar]
- 26.Bland J. M., Altman D. G. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1: 307–310. [PubMed] [Google Scholar]
- 27.Britsih Standards Institution. 1979. Precision of test methods I: guide for the determination and reproducibility for a standard test method. BS 5497, part 1. BSI, London. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.