Abstract
An online, two-dimensional (2D) liquid chromatography (LC) quadrupole time-of-flight mass spectrometry (QToF-MS) method was developed for lipid profiling of rat peritoneal surface layers, in which the lipid classes and species could be simultaneously separated in one injection with a significantly increased sensitivity. Different lipid classes were separated on a normal-phase column in the first dimension and lipid molecular species were separated on a reversed-phase column in the second dimension, so that the ion suppression effects were reduced while the detection sensitivity was improved. Identified were 721 endogenous lipid species from 12 lipid classes, in which 415 structures were confirmed using tandem mass spectra, and the other 306 lipid molecular species were identified by accurate masses. The linearity, limit of detection, and repeatability were all satisfactory. The method was applied to the investigation of the lipid changes in rat peritoneal surface layer after peritoneal dialysis, and 32 potential lipid biomarkers were identified, as their concentrations in the dosed group were 2.2–12.5 times of those in the control group. The results revealed that this 2D LC-MS system was a promising tool for lipid profiling of complex biological samples.
Keywords: lipidomic, free fatty acid, phospholipid, lyso-phospholipid, plasmalogen, peritoneal dialysis, peritoneum, biomarker, two-dimensional liquid chromatography, mass spectrometry
Lipids contain a wide variety of hydrophobic or amphoteric biological molecules, which are fundamental structural components of the cellular membranes that form a protective barrier around cells and modulate the membrane fluidity and trafficking. Lipids play a crucial role in numerous physiological processes in virtually all living organisms (1–3). It is known that lipids are involved in intracellular signal transduction and affect the function of integral membrane proteins by interacting with the protein molecules. On the peritoneal membrane, the lipid layer acts as a barrier to the transport of water-soluble solutes while permitting water flux (4). Peritoneal dialysis may induce changes of the composition and structure of the peritoneal surface layer and the peritoneal transport function (5). Qualitative and quantitative investigation of those lipids will promote a better understanding of the mechanism of increased peritoneal transport after flushed by the dialysate.
The traditional strategies for the analysis of lipids are thin-layer chromatography (TLC) (6–8) or high-performance liquid chromatography (HPLC) coupled with UV absorbance or evaporative light-scattering detection (9–12). To obtain lipid structural information, gas chromatography (GC) and gas-chromatography mass spectrometry (GC-MS) were utilized (13–16), prior to which time consuming hydrolysis and derivatization reactions of lipids should have been performed. This problem was solved with the advent of electrospray ionization (ESI), resulting in the indispensability of MS today in lipid research. For the analysis of lipids extracted from complex biological samples, there are two major strategies based on the ESI-MS approach. One is the direct infusion “shotgun” method, which has a shorter analytical time due to the lack of chromatography separation (17, 18); however, the ion suppression effects among lipids decrease the detection sensitivity, especially for low-abundance lipid species. The other is liquid-chromatography mass spectrometry (LC-MS), which is often employed to avoid ionization suppression and mass overlapping. In normal-phase liquid chromatography (NPLC), lipids are separated by adsorption mechanisms and eluted from the column by class, in the order of neutral lipids to polar lipids (19–21). In reversed-phase liquid chromatography (RPLC), compounds are separated based on hydrophobicity. In this case, the elution sequence of lipid molecules is determined by both the chain length and the degree of unsaturation in the fatty-acyl chains. To avoid coelution of molecular species from the same lipid class, RPLC is usually performed to separate individual molecular species of a particular lipid class (22–26). The combination of NPLC and RPLC was suggested by Pulfer and Murphy (27) to achieve complete separation of extracted lipids from a complex biological matrix. Lesnefsky (28), Houjou (29), and Peterson (30) separated individual lipid classes by NPLC, and the fraction of each class of interest was collected and rerun on an reversed-phase (RP) column for the further separation of lipid molecule species. Although more lipid species could be separated by these offline, two-dimensional (2D) LC methods, it is obvious that these methods were time consuming and labor intensive. To overcome these shortcomings and obtain reliable quantitative results, online combination of NPLC and RPLC should be used, but the immiscibility of two mobile phases has resulted in only a few published articles on this topic so far. In 2004, Dugo (31) first realized an online comprehensive NPLC and RPLC system for the separation of lemon essential oil. The flow rate of the first dimension NPLC was only 20 μl/min and a much higher flow rate, 4 ml/min, was applied in the second dimension RPLC. The effluent from normal-phase column collected in the interface loop was so greatly diluted by the reversed-phase solvents that immiscibility and band spreading were solved. However, small injection volume and too much dilution largely decreased the sensitivity of this 2D LC system. Recently, Tian et al. (32) designed a solvent-evaporating interface for online combination of NPLC and RPLC. The mobile phase from the NPLC was evaporated under vacuum in the interface loop, which not only solved the mobile phase immiscibility but also enriched the components. So far, this method has not been applied to the lipid analysis and its repeatability, which needs further improvement and systematical optimization, was not satisfactory for quantitative analysis of lipids.
In this study, the valve and loop of the evaporating interface of the above-mentioned device were redesigned and prepared to achieve better repeatability and recovery for determination of lipids. The temperature and vacuum conditions of the interface were also systematically optimized. In addition, the columns and chromatographic conditions were investigated. As a result, online separation of different lipid classes and different molecular species within one injection through this NP/RP 2D LC was achieved with satisfactory repeatability, sensitivity, and recovery. We applied this method to profile lipids of rat peritoneal surface layers, and eight times as much lipid species were detected compared with our previous 1D LC results (33). Furthermore, 32 potential biomarkers from two lipid subclasses were identified after comparing the lipids from peritoneal surface layers of control rats and peritoneal dialysis rats, which would be helpful to the investigation of the correlation between the peritoneum structure and its function. Therefore, the 2D LC-MS method established in this work could be a useful and promising method in lipidomic research.
MATERIALS AND METHODS
Key compounds and reagents
The following synthetic lipid standards were purchased from Avanti Polar Lipids (Alabaster, AL): 1,2-dimyristoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (sodium salt) [PG(14:0/14:0)]; 1,2- dimyristoyl-sn-glycero-3-phosphoethanolamine [PE(14:0/14:0)]; 1,2-dimyristelaidoyl-sn-glycero-3-phosphocholine [PC(14:1/14:1)]; N-(dodecanoyl)-heptadecasphing-4-enine-1-phosphoethanolamine, Sphingosyl PE(d17:1) [SPE(d17:1/12:0)]; 1-(10Z-heptadecenoyl)-sn-glycero-3-phospho-(1'-rac-glycerol) (sodium salt) [LPG(17:1)]; and 1-(10Z-heptadecenoyl)-sn-glycero-3-phosphoethanolamine [LPE(17:1)]. N-hexane, isopropanol, and chloroform of HPLC grade from Dikma Technology (US) and purified water from Hangzhou Wahaha Group Co., (Zhejiang, China) were used. Ammonium formate was from Sigma-Aldrich (US). The peritoneal dialysis solution containing 4.25% glucose was obtained from Baxter Healthcare (Beijing, China).
Preparation of lipids from Sprague-Dawley rat peritoneal surface
All animal protocols used were approved by the Animal Care and Use Committee of Peking University. Lipids in the rat peritoneal surface layer were prepared as previously described (33). Four male Sprague-Dawley (SD) rats (220–250 g body weight) were euthanized. Then 20 ml of Folch solution (chloroform/methanol, 2:1, v/v) was infused into the peritoneal cavity for 30 s and drained completely. This procedure was used to extract the peritoneal surface layer completely without damaging the mesothelial cells (34). The same amounts of six lipid standards [0.1 μg PG(14:0/14:0), 0.1 μg PE(14:0/14:0), 0.1 μg PC(14:1/14:1), 0.1 μg SPE(d17:1/12:0), 0.1 μg LPG(17:1), 0.1 μg LPE (17:1)] were added into 0.5 ml crude solution. The lipids in the crude solution were extracted by a modified Folch extraction method (35, 36). The lipid extracts were dried by evaporation under nitrogen. Then the dried lipid extracts were redissolved in 0.5 ml Folch solution for HPLC injection.
Preparation of lipidomics application
The 12 SD rats (220–250 g body weight) were divided into two equivalent groups, one as the control group and the other as the dosed group. Each rat of the dosed group received one intraperitoneal injection of 25 ml 4.25% glucose dialysis solution per day. On day 7, 4 h after the last intraperitoneal injection, the six rats of the dosed group were euthanized, and the dialysate was drained completely. Lipids in peritoneal surfaces of all 12 rats were extracted with the method described above.
LC system
1D LC system.
The experiments were performed on an Agilent 1100 series HPLC system equipped with quaternary pump, online degasser, autosampler, and thermostated column compartment (Agilent Technologies, Karlsruhe, Germany). Aliquots (20 μl) of lipid samples were injected onto a Rx-SIL silica column (2.1 × 150 mm, 5 μm, Agilent, US) with mobile phase flow rate of 0.1 ml/min. Mobile phase A1 and B1 were hexane/isopropanol/water (30/70/2, v/v/v) and methanol/water (100/2, v/v), respectively, both containing 5 mmol/l ammonium formate. The gradient elution began 100% A1 for 23 min, followed by a change to 100% solvent B1 at 23.01 min, which lasted until 39 min. The thermostatted column compartment was operated at 25°C.
2D LC system.
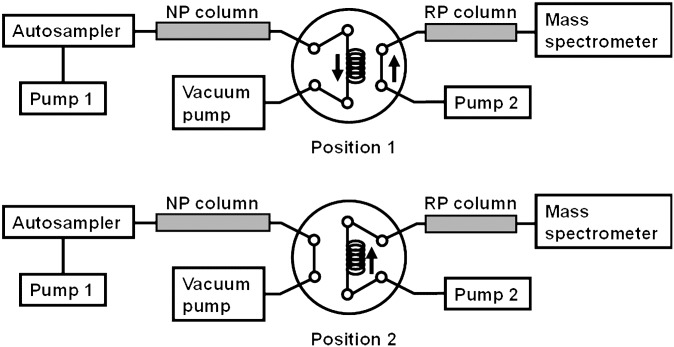
The 2D LC system for lipid profiling (Fig. 1) was built based on the configuration reported previously (32), on which some alterations and modifications were performed to make it more suitable for the lipid separation. The same conditions of above 1D LC, including the instrument, column, and mobile phase, were utilized to perform the separation in the first dimension of the 2D LC system. In the second dimension, an Agilent 1200-series binary pump connected to an online degasser (Agilent) delivered the mobile phase through an Eclipse Plus C8 column (2.1 × 10 mm, 3.5 μm, Agilent). Between the two dimensions, there was a six-port, two-position manual valve (IDEX Health and Science, US) with the interface loop in which eluate from the first dimension was trapped and then transferred to second dimension. In the second dimension, solvent A2 and B2 were methanol/water (50/50, v/v) and methanol, both containing 5 mmol/l ammonium formate, respectively. The temperature of the loop was maintained at 50°C by a homemade, electric-heated, thermostatic water bath. The E2M2 rough pump, obtained from Edwards Vacuum (Crawley, UK), was utilized for the solvent evaporation in the interface. The gradient programs of both the first and the second dimension and the valve switching program in the 2D LC system were formulated in Table 1. The thermostated column compartment was operated at 25°C for the Rx-SIL and 40°C for the Eclipse Plus C8.
Fig. 1.
Scheme of the 2D LC system. Valve in position 1 (top): Mobile phase eluted from the NP column was evaporated by the vacuum pump when it flowed through the loop, and the analytes were trapped in the loop. Valve in position 2 (bottom): Trapped analytes were flushed onto the RP column by mobile phase from pump 2 for further separation. At the same time, the vacuum pump and pump 1 were both stopped until the valve was switched to position 1. 2D, two dimensional; LC, liquid chromatography; NP, normal phase; RP, reversed phase.
TABLE 1.
Configuration of online LC-MS systems for lipid profiling
| 2D LC system |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1D LC system |
First dimensional LC |
Interface |
Second dimensional LC |
|||||||||||
| Fraction | Time(min) | Flow rate(ml/min) | A1(%) | B1(%) | Time(min) | Flow rate(ml/min) | A1(%) | B1(%) | Loop position (dimension) | Time(min) | Flow rate(ml/min) | A2(%) | B2(%) | Remarks |
| 1 | 0–9 | 0.1 | 100 | 0 | 0–9 | 0.1 | 100 | 0 | first | 0 | 0.3 | 50 | 50 | First dimension: Loop trapping first-fraction anlytes |
| 9–21 | 0 | 100 | 0 | second | 9 | 0.3 | 50 | 50 | Second dimension: first-fraction anlytes were injected and separated on the second column | |||||
| 20 | 0.3 | 40 | 60 | |||||||||||
| 21 | 0.3 | 20 | 80 | |||||||||||
| 29 | 0.3 | 0 | 100 | |||||||||||
| 31 | 0.3 | 0 | 100 | |||||||||||
| 2 | 9–23 | 0.1 | 100 | 0 | 21–35 | 0.1 | 100 | 0 | first | 31.01 | 0.3 | 100 | 0 | First dimension: Loop trapping second-fraction anlytes |
| 35–37 | 0 | 100 | 0 | second | 35 | 0.3 | 100 | 0 | Second dimension: second-fraction anlytes were injected and separated on the second column | |||||
| 37 | 0.3 | 30 | 70 | |||||||||||
| 50 | 0.3 | 5 | 95 | |||||||||||
| 3 | 23–30 | 0.1 | 100 | 0 | 37–44 | 0.1 | 100 | 0 | first | 50.01 | 0.3 | 100 | 0 | First dimension: Loop trapping third-fraction anlytes |
| 30–40 | 0.1 | 0 | 100 | 44–54 | 0.1 | 0 | 100 | first | ||||||
| 54–75 | 0 | 0 | 100 | second | 54 | 0.3 | 100 | 0 | Second dimension: third-fraction anlytes were injected and separated on the second column | |||||
| 56 | 0.3 | 20 | 80 | |||||||||||
| 74 | 0.3 | 10 | 90 | |||||||||||
| 76 | 0.3 | 0 | 100 | |||||||||||
| 80 | 0.3 | 0 | 100 | |||||||||||
| 4 | 40–49 | 0.1 | 0 | 100 | 75–84 | 0.1 | 0 | 100 | first | 80.01 | 0.3 | 100 | 0 | First dimension: Loop trapping forth-fraction anlytes |
| 84–91 | 0 | 0 | 100 | second | 84 | 0.3 | 100 | 0 | Second dimension: forth-fraction anlytes were injected and separated on the second column | |||||
| 86 | 0.3 | 25 | 75 | |||||||||||
| 90 | 0.3 | 20 | 80 | |||||||||||
| 91 | 0.3 | 10 | 90 | |||||||||||
| 95 | 0.3 | 0 | 100 | |||||||||||
| 100 | 0.3 | 0 | 100 | |||||||||||
| 5 | 49–62 | 0.1 | 0 | 100 | 91–104 | 0.1 | 0 | 100 | first | 100.01 | 0.3 | 100 | 0 | First dimension: Loop trapping fifth-fraction anlytes |
| 62–72 | 0.4 | 0 | 100 | 104–114 | 0.4 | 0 | 100 | second | 104 | 0.3 | 100 | 0 | Second dimension: fifth-fraction anlytes were injected and separated on the second column | |
| 72–102 | 0.4 | 100 | 0 | 114–144 | 0.4 | 100 | 0 | second | 106 | 0.3 | 45 | 55 | ||
| 102–108 | 0.1 | 100 | 0 | 114–150 | 0.1 | 100 | 0 | second | 114 | 0.3 | 40 | 60 | ||
| 114.01 | 0.3 | 20 | 80 | |||||||||||
| 124 | 0.3 | 20 | 80 | |||||||||||
| 139 | 0.3 | 15 | 85 | |||||||||||
| 150 | 0.3 | 0 | 100 | |||||||||||
Left: The gradient program and the time segments of five fractions in 1D LC system. Right: The first and second dimensional gradient programs and loop positions in the 2D LC system. 1(2)D, one (two)-dimensional; LC, liquid chromatography; MS, mass spectrometry.
aLoop trapping first-fraction analytes.
bFirst-fraction analytes were injected and separated on the second column.
cLoop trapping second-fraction analytes.
dSecond-fraction analytes were injected and separated on the second column.
eLoop trapping third-fraction analytes.
fThird-fraction analytes were injected and separated on the second column.
gLoop trapping forth-fraction analytes.
hForth-fraction analytes were injected and separated on the second column.
iLoop trapping fifth-fraction analytes.
jFifth-fraction analytes were injected and separated on the second column.
Mass spectrometry system
In the 1D LC-MS system, the LC was coupled online to an Agilent 6530 Accurate-Mass Quadrupole Time-of-Flight mass spectrometer (QToF MS) equipped with an Agilent Jet Stream ESI source (Agilent). In the 2D LC-MS system, this Agilent 6530 QToF MS was coupled online with the second dimensional LC system, and the eluate from the second column flowed directly into the Jet Stream ESI source. Same MS parameters were set in the two systems. The Jet Stream ESI source was operated in negative mode, and instrument parameters were set as follows: sheath gas temperature, 350°C; sheath gas flow, 8 L/min; nebulizer, 20 psi; dry gas temperature, 300°C; dry gas flow, 5 L/min; and capillary entrance voltage, 3500 V. Fragmentor and Skimmer1 were operated at 190 V and 65 V, respectively. The MS scan data were collected at a rate of 1.02 spectra/s in the range of m/z 100–2000. The m/z of all ions in the mass spectra were corrected by two reference ions [Trifluoroacetate anion and Hexakis(1H, 1H, 3H-tetrafluoropropoxy)phosphazine, m/z 112.985587 and 966.000725, Agilent P/N G1969-85001], which insured mass error less than 3 ppm in our experiment. In the targeted MS/MS mode, the MS and the MS/MS information were collected in the same m/z range at the same rate as the MS scan, and collision energy was 40 V. Iso. width of precursor ion was set as narrow (∼1.3 m/z).
All the MS and MS/MS data were collected with MassHunter Data Acquisition B.02.00 (Agilent), and MassHunter Qualitative Analysis B.02.00 (Agilent) was applied to identify lipid species. Peak areas of the validation standards and potential biomarkers were integrated from extracted ion chromatograms (EIC) by MassHunter Quantitative Analysis B.03.01 (Agilent), and the linearity of six validation standards were constructed by this software. All EICs were obtained with ± 5 ppm m/z expansion. Mass Profiler Professional 2.0 (Agilent) and Microsoft Office Excel 2007 (Microsoft, Redmond, WA) were used for statistical data analysis and data visualization.
RESULTS AND DISCUSSION
1D and 2D separation of lipids
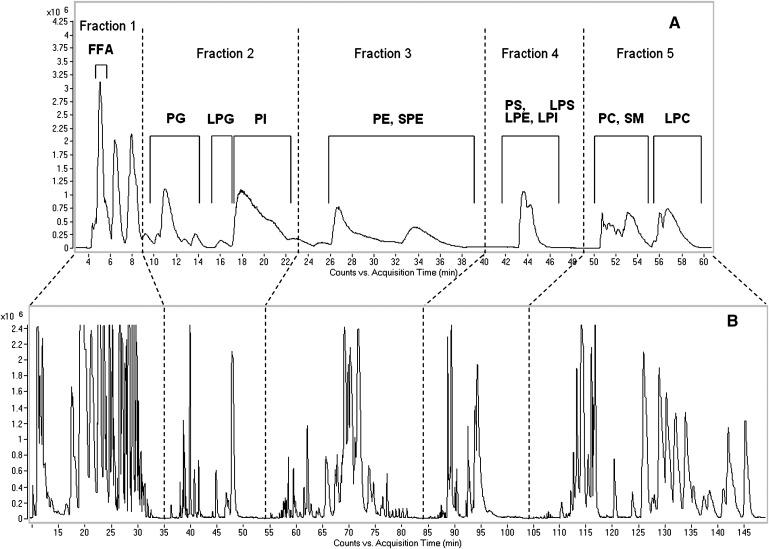
In the 1D LC system, the lipid extracts of SD rat peritoneal surface layers added with phosphatidylglyceral [PG(14:0/14:0)], phosphoethanolamine [PE(14:0/14:0)], phosphocholine [PC(14:1/14:1)], sphingosyl phosphoethanolamine [SPE(d17:1/12:0)], lyso-phosphatidylglyceral [LPG(17:1)], and lyso-phosphoethanolamine [LPE(17:1)] as validation standards were separated by the normal-phase column. Twelve endogenous lipid classes, including free fatty acid (FFA), glycerophosphoglycerol (PG), lysoglycerophosphoglycerol (LPG), glycerophosphoinositol (PI), glycerophosphoethanolamine (PE), lysoglycerophosphoethanolamine (LPE), lysoglycerophosphoinositol (LPI), lysoglycerophosphoserine (LPS), glycerophosphoserine (PS), glycerophosphocholine (PC), sphingomyelin (SM), and lysoglycerophosphocholine (LPC) were determined (Fig. 2A). In the 2D LC, the normal-phase eluate was divided into five fractions (Fig. 2A). Time segments of the five fractions are shown in Table 1 (left). With the coordination of gradient programs of the two pumps and the switching of the interface valve (Table 1, right), each fraction in the first dimension was individually collected in the interface loop and injected onto the second RP column for further separation. Consequently, eluates of the five fractions in the first dimension were separated, respectively, into five corresponding segments in the second dimensional LC (Fig. 2).
Fig. 2.
A: BPC of the lipids in rat peritoneal surface layer in 1D LC-MS system. Twelve endogenous lipid classes and one validation standard [SPE (d17:1/12:0)] were separated by NPLC. B: BPC of the lipids in rat peritoneal surface layer in 2D LC-MS system. In the first dimension, lipids were separated by NPLC, the same as in the 1D LC-MS system. The eluate from the normal-phase column was divided into five fractions and transferred onto the RPLC column, respectively, for further separation in the second dimension. 1(2)D, one (two)- dimensional; BPC, base peak chromatogram; LC, liquid chromatography; NPLC, normal-phase liquid chromatography; RPLC, reversed-phase liquid chromatography SPE, sphingosyl phosphoethanolamine.
The detailed operation was as follows. In the first fraction, the lipids eluted from the first column flowed into the valve and the mobile phase was continuously being evaporated (Fig. 1, position 1). At the end of the first fraction, the first dimensional LC pump stopped, and the interface loop was switched into the second dimensional LC system (Fig. 1, position 2). The mobile phase delivered by the second pump flushed the analytes of the first fraction out of the loop onto the second column. While the eluate of the first fraction was separated with gradient elution in the second dimension, the first pump was standby, and the separation in the first column was stopped until the interface loop was switched back to the first dimension LC system (Fig. 1, position 1) at the beginning of next fraction. The first pump restarted to deliver mobile phase for the separation in the first column, and simultaneously, the loop began to collect the eluate of the second fraction and the second column started a 4 min equilibration. With the same process, all analytes in the five fractions were separated individually in the second dimensional RPLC.
As shown in Fig. 2, more peaks could be obtained by the 2D LC-MS system than by the 1D LC-MS system. Two dimensional LC can provide a higher peak capacity by multiplying the separation capacity of each dimension, and the combination of NPLC and RPLC can further enhance the resolving power because it is the most orthogonal in nature (37). In addition to the higher peak capacity, a significant advantage of our 2D LC system is that the separation of lipid classes and species could be completed within one injection.
In lipidomic research, NPLC is used to separate lipid classes based on the difference of their polarity. The different lipid species of the same class eluted together from the column, however, would bring about ion suppression effects among these lipid species in the ion source of the mass spectrometer. RPLC is usually carried out to separate the lipid molecular species according to the lipophilicity, in which the lipid classes could not be separated. When lipids are analyzed only by RPLC, the lipid molecules of different classes detected together would result in decreased ionization efficiency because of the ionizing competition among lipid molecules. Meanwhile, identification of the lipids becomes more difficult due to coelution of the molecular species from different classes. Therefore, for the comprehensive analysis of complex lipid samples, it is better to utilize NPLC to perform the separation of lipid classes and RPLC to separate the downstream molecular species (27). In our 2D LC-MS system, all 12 endogenous lipid classes did not achieve baseline separation in the first dimension and the analytical speed of each class in the second dimension was not fast enough, so the separation in the first dimension were divided into five fractions for further separation on the RP column. Compared with the 1D LC-MS system, the 2D LC-MS method offered a much higher peak capacity, reduced ion suppression effects among lipid molecular species, and facilitated the identification of the lipid molecular species.
Qualitative analysis of lipids
All lipids in the rat peritoneal surface layer were identified with high-accuracy mass values measured by Agilent 6530 Accurate-Mass QToF MS. The abundant molecular species were confirmed by targeted MS/MS and retention time, and low-abundance lipid molecules were identified with retention time and m/z value. The measured accurate masses were applied for preliminary identification using Lipid MS Predict software (v1.5, LIPDMAPS) or compared with exact masses calculated by MassHunter Qualitative Analysis with a mass tolerance of less than 3 ppm on the basis of the predicted elemental composition.
All the lipids except for free fatty acids could generate MS/MS fragmentation in targeted MS/MS mode by Agilent 6530 QToF MS, so the structures of the most abundant lipid species could be confirmed by the tandem MS spectra with high accurate masses. The identification of one molecular species of PE is presented in detail as an example. According to the retention time of the PE standard, the PE class should be eluted from the column with retention time at 55–85 min, and our example's retention time was 69.95 min. Its measured m/z 790.5390 was searched in Lipid MS Predict as a deprotonated PE ion with 0.1 amu mass tolerance. Several candidates were listed in this software, but considering our 3 ppm mass tolerance, only one ion formula C45H77NO8P− (calculated mass: 790.5392) was acceptable; the error was −0.3 ppm. According to the search result, the lipid species was identified as PE(40:6). To confirm this species, targeted MS/MS of the ions (m/z = 790.54, Iso. width: ∼1.3 m/z) was performed by the QToF MS in negative mode, and the tandem mass spectrum is shown in Fig. 3. Based on the fragmentation of PE reported before (38), the m/z 327.2334 ion and the 283.2586 ion were 22:6-carboxylate anion and 18:0-carboxylate anion, respectively. The ions at m/z 480.3097 and 462.2987 corresponded to losing the 22:6-fatty acyl substituent as a ketene and as an acid, respectively. The neutral loss of the 18:0-fatty acyl chain as a ketene and as an acid yielded corresponding ions at m/z 524.2784 and 506.2681. The mass spectrum was featured by the m/z 196.0374 ion corresponding to the loss of one fatty acyl chain as an acid and the other as a ketene, along with the phosphoethanolamine anion at m/z 140.0115. The profile of the mass spectrum indicated that this compound was PE(18:0/22:6) also called PE(40:6). The fragmentations in negative mode of lipids, including plasmenyl and plasmanyl glycerophospholipids (pPL, subclasses of glycerophospholipids ) and other glycerophospholipids (PL), lysoplasmenyl and lysoplasmanyl glycerophospholipids (LpPL, subclasses of glycerophospholipids) and other lysoglycerophospholipids (LPL), and sphingomyelin (SM), have been well investigated (38–44). Based on the reported fragment pathways and feature fragment ions of each lipid class, structural information of the most abundant lipid species has been confirmed in our work.
Fig. 3.
Targeted MS/MS spectrum of the precursor ion (m/z = 790.54; Iso. width: ∼1.3 m/z) in the negative mode obtained by an Accurate-Mass QToF MS. MS, mass spectrometry; QToF, quadrupole time-of-flight.
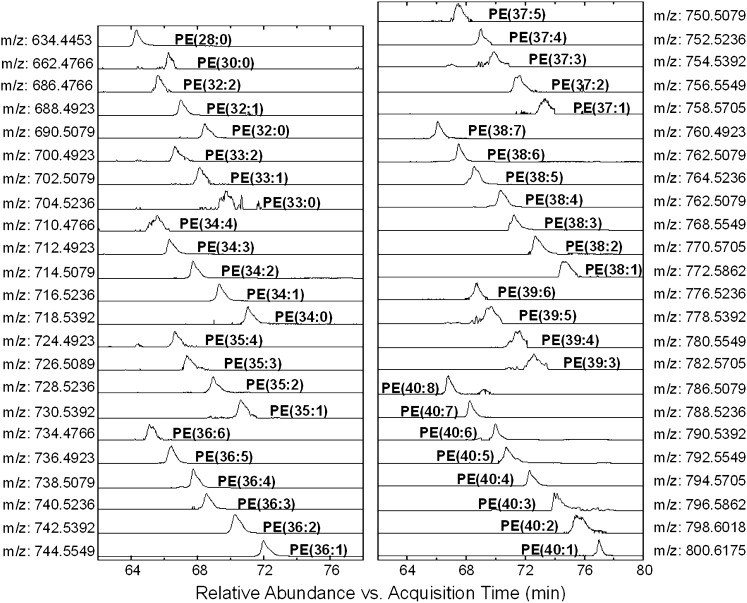
However, some lipid species with low abundance could not generate reliable MS/MS spectra and, consequently, could not be determined by the above approach. Kim's work (45) indicated that lipid species were separated according to chain length and degree of unsaturation on reversed- phase column. A mathematical relationship between the equivalent carbon number (ECN) of a lipid molecular species in fatty acid moieties and the relative retention time of the species in the isocratic RPLC system has been calculated by Brouwers (24). In our experiment, similar regulation was found in the second dimensional separation, which could aid the identification of those low abundance lipid species. Fig. 4 shows the EICs of PE molecular species extracted with calculated masses, from which we can observe that PE species having the same total carbon number in two fatty acid chains were eluted in the order of the decrease of double-bond number in aliphatic groups. Five of the PEs with carbon number 36, from PE(36:1) to PE(36:5), were abundant enough to generate MS/MS spectra and to be determined by those mass spectra (supplementary Table I); the low-abundance peak at m/z 734.4766 that was in a line with the five could thereby be identified as PE(36:6). Similarly, the peak (m/z 796.5862) was identified as PE(40:3) by comparing its retention time with the other seven peaks, which had been confirmed as PE species containing 40 carbon atoms.
Fig. 4.
EICs of the [M-H]− ions of PE molecular species which were classified based on acyl carbon chains. EIC, extract ion chromatogram; PE, glycerophosphoethanolamine.
In the end, a total of 721 endogenous lipid species from 12 lipid classes were determined by accurate mass and retention time, and 415 structures were further identified with tandem mass spectrometry data. Because of the separation of different lipid species and enrichment feature of the 2D LC system, along with the improvement of the sensitivities of low-abundance lipid species by the Agilent Jet Stream Technologies ESI source, more lipid species were detected and identified in rat peritoneal surface layer by this 2D LC QToF-MS system than by 1D LC ion trap MS (33).
Evaluation of the method
In preparation for the application of lipid profiling, the calibration curves (supplementary Fig. I) of this 2D LC-MS method were constructed for six nonendogenous lipid standards, including PG(14:0/14:0), PE(14:0/14:0), PC(14:1/14:1), SPE(d17:0/12:0), LPG(17:1), LPE(17:1), spiked to 0.5 ml crude solution at six concentrations (C1-C6: 0.01, 0.02, 0.1, 0.2, 1, 2 µg/ml). The average peak area of triplicate measurements at each concentration level was calculated from the extracted ion chromatograms as described in “Materials and Methods”, and the calibration equations of peak area (y) versus concentration (x) of six lipid standards are shown in Table 2. The linearity was satisfactory, with coefficients (R2) greater than 0.9917 for all the six validation standards. The LODs (at signal-to-noise ratio of 3) of six validation standards in this highly sensitive 2D LC-MS system could reach 2 ng/ml. As for repeatability, the same amounts (50 ng) of six lipid validation standards were spiked to 0.5 ml crude solution, and the relative standard deviation (RSD) of the peak areas and retention times of each lipid standard was calculated based on six injections of this prepared sample. The RSDs of peak area and retention time ranged 2.4–6.4% and 0.00– 0.03%, respectively. All the results indicated that our 2D LC-MS method could be applied for lipid profiling.
TABLE 2.
Calibration curves for six nonendogenous lipid standards
| Standards | Calibration Equationsa | Linear Range (µg/ml) | R2 | LOD (ng/ml) | RSD%b (area) | RSD%b (RT) |
|---|---|---|---|---|---|---|
| PG(14:0/14:0) | y = 4393x + 4859 | 0.01–2 | 0.9960 | 2 | 4.7 | 0.03 |
| PE(14:0/14:0) | y = 3496x + 1652 | 0.01–2 | 0.9983 | 2 | 5.5 | 0.03 |
| PC(14:1/14:1) | y = 5853x − 611 | 0.01–2 | 0.9973 | 2 | 2.4 | 0.01 |
| LPG(17:1) | y = 8502x − 46171 | 0.01–2 | 0.9937 | 2 | 6.4 | 0.01 |
| LPE(17:1) | y = 10692x − 36506 | 0.01–2 | 0.9917 | 2 | 5.0 | 0.00 |
| SPE(d17:1/12:0) | y = 10439x − 18975 | 0.01–2 | 0.9976 | 2 | 5.4 | 0.03 |
The calibration equations, linear regression coefficients, LOD, and RSD of peak areac and RT for each validation standard. EIC, extract ion chromatogram; LOD, limit of detection; LPE, lysoglycerophosphoethanolamine; LPG, lysoglycerophosphoglycerol; PC, glycerophosphocholine; PE, glycerophosphoethanolamine; PG, glycerophosphoglycerol; RSD, relative standard deviation; RT, retention time; SPE, sphingosyl phosphoethanolamine.
y = peak area and x = concentration of corresponding validation standard (µg/ml).
n = 6, 0.1 µg/ml.
Peak area represents the EIC.
Comparison of dosed group and control group
The surface lipid layer on the peritoneal membrane acts as a barrier to the transport of water-soluble solutes while permits water flux (4). Peritoneal dialysis, as a treatment to uremia and renal failure, affects the structure of the peritoneal surface layer and causes increasing transport (5). Therefore, study on changes of the lipids in the peritoneal surface layer will help us to understand the mechanism of increased peritoneal transport.
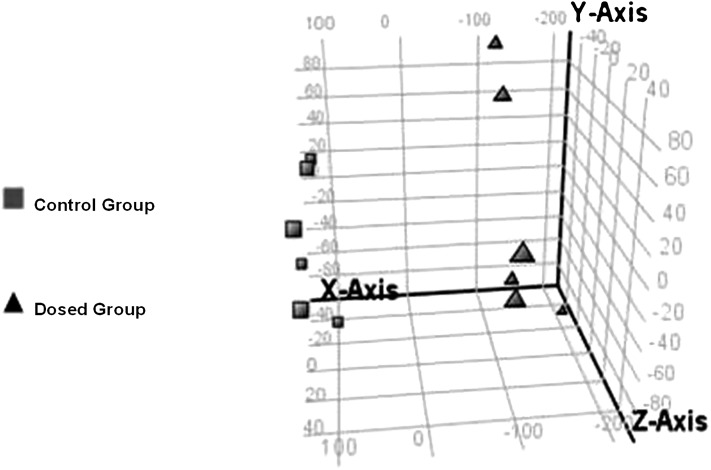
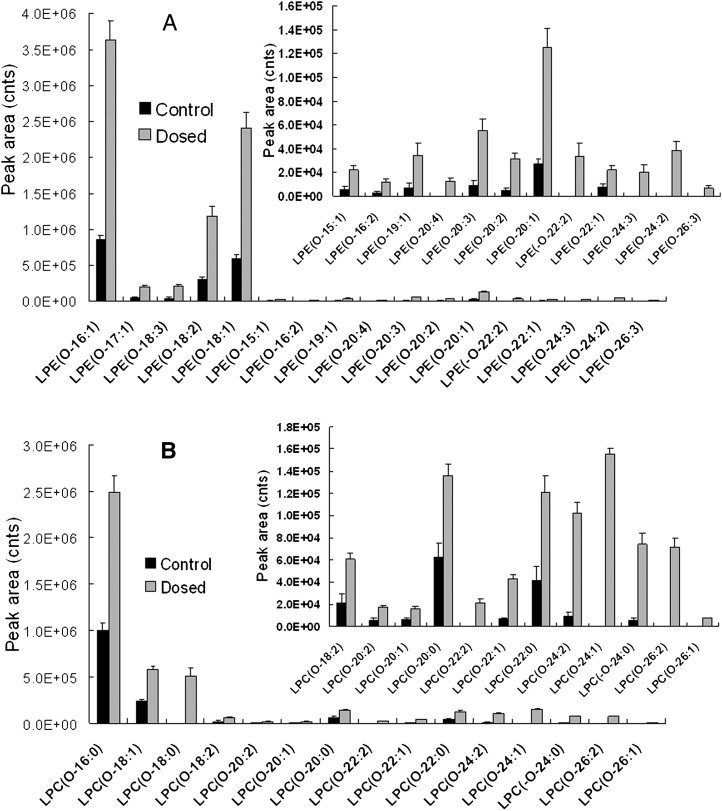
To investigate lipid changes after peritioneal dialysis, 12 SD rats were randomly separated into a dosed group (n = 6) and a control group (n = 6). All 12 samples were detected by the 2D LC-MS method. The dosed group and control group were alternately injected to reduce systemic error. During the sequence, one blank sample (20 μl Folch solution) was injected after every fourth sample, and no significant carryover of lipids was observed. MS data of all 12 samples were extracted by MassHunter Qualitative Analysis software and analyzed by Mass Profiler Professional software. The statistical results are presented in a 3D principal component analysis (PCA) score plot (Fig. 5). Fig. 5 shows that the 12 samples could be clearly separated into two groups: control and dosed. Through setting threshold parameters, the software presented a list of potential biomarkers whose absolute fold-change of peak area was larger than 2 and P value less than 0.05. By the above-mentioned approach, 32 potential biomarkers were further identified, all belonging to two subclasses: lyso-plasmenyl or lyso-plasmanyl PE and PC. The lipid species and mean peak areas of LpPE are given in Fig. 6A and those of LpPC in Fig. 6B. All mean peak areas of the potential biomarkers experienced increments in the dosed group. For LpPE, they were from 2.8- to 6.0-fold of those in control group; for LpPC, they were from 2.2- to 12.5-fold of those in the control group.
Fig. 5.
3D score plot of principal component analysis of MS data of the control group (▪) and dosed group (▴).
Fig. 6.
A: Mean peak areas of LpPE in control group (black bars) and dosed group (gray bars). B: Mean peak areas of LpPC in control group (black bars) and dosed group (gray bars). LpPC, lysoplasmenyl (lysoplasmanyl) PC; LpPE, lysoplasmenyl (lysoplasmanyl) PE.
Our experimental results suggest that lipid species of LpPE and LpPC increased significantly with the stimulation of dialysate. This conclusion partially agrees with the results of Wang et al. (5) that was achieved using TLC. According to the detection limitation of TLC, only abundant lipid classes were identified in their study. Using 2D LC QToF-MS method, we discovered not only molecular species of interest but also detailed qualitative and quantitative information about those potential biomarkers. For example, we found that only two subclasses of LPL, LpPE and LpPC, in the peritoneal surface layer were related to the degenerated peritoneal function. Previous surface-active phospholipid research in peritoneal dialysis (46) was focused on the correlation between PC and peritoneal function, such as changes of PC in peritoneal surface or dialysate and recovery of destroyed peritoneum by PC replenishment through intraperitoneal, intravenous, and oral administration. Combined with our research, there are two reasons for the focus: 1) PC was the major class both in rat peritoneal surface (33) and dialysate (47), which means even a small proportion of PC altered by some stimulation could be enough to be detected. It is reasonable that these changes are not crucial but detectable by traditional analytical approaches; and 2) previous detection methods, such as TLC and 1D LC-MS (5, 33, 47), cannot determine LpPL molecular species, let alone the discovery of their changes. In our study, significant PC differences have not been observed between the control and dosed groups, but the LpPL species was observed to be dramatically increased in rat peritoneal surface after dialysate stimulation, which will be very useful to better understand peritoneal degradation and improve the peritoneal dialyses treatment. The results of our study support the potential of this validated method for lipid profiling of different biological or clinical samples.
CONCLUSION
To profile lipids, we introduced a novel 2D LC-MS method equipped with a solvent evaporating interface. Compared with the previous 1D method, seven times more lipid molecular species from 12 endogenous lipid classes were determined with one injection after an efficient separation, which dramatically improves detection sensitivity. The results of method validation were satisfied for a 2D LC-MS system and acceptable for lipid profiling. Statistical analysis identified the differences between control and dosed groups. In total, 32 potential biomarkers with significant changes in the peak area were detected, which suggests a future for the 2D LC-MS method in lipidomic and clinical research.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Wei Chen and Dr. Xiaorong Ran (Agilent) for their useful interpretation of MPP software, and Dr. Xiaofang Fu for her kind suggestions about the manuscript.
Footnotes
Abbreviations:
- 1(2)D
- one (two)-dimensional
- ECN
- equivalent carbon number
- LC
- liquid chromatography
- LOD
- limit of detection
- LPC
- lysoglycerophosphocholine
- LPE
- lysoglycerophosphoethanolamine
- LPG
- lysoglycerophosphoglycerol
- LPI
- lysoglycerophosphoinositol
- LPL
- lysoglycerophospholipids
- LpPC
- lysoplasmenyl (lysoplasmanyl) PC
- LpPE
- lysoplasmenyl (lysoplasmanyl) PE
- LpPL
- lysoplasmenyl (lysoplasmanyl) glycerophospholipid
- LPS
- lysoglycerophosphoserine
- MS
- mass spectrometry
- NPLC
- normal-phase LC
- PC
- glycerophosphocholine
- PCA
- principal component analysis
- PE
- glycerophosphoethanolamine
- PG
- glycerophosphoglycerol
- PI
- glycerophosphoinositol
- PL
- glycerophospholipid
- pPL
- plasmenyl (plasmanyl) glycerophospholipid (subclass of glycerophospholipids)
- PS
- glycerophosphoserine
- QToF
- quadrupole time-of-flight
- RPLC
- reversed-phase LC
- RSD
- relative standard deviation
- SPE
- sphingosyl phosphoethanolamine
- SM
- sphingomyelin
This work was supported by National Natural Science Foundation of China Grants 20975005, 90717002, 20805001, and 20775090; and by the Fundamental Research Funds for the Central Universities.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and one table.
REFERENCES
- 1.van Meer G., Voelker D. R., Feigenson G. W. 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9: 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons K., Toomre D. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1: 31–39. [DOI] [PubMed] [Google Scholar]
- 3.Balla T. 2005. Inositol-lipid binding motifs: signal integrators through protein-lipid and protein-protein interactions. J. Cell Sci. 118: 2093–2104. [DOI] [PubMed] [Google Scholar]
- 4.Grahame G. R., Torchia M. G., Dankewich K. A., Ferguson I. A. 1985. Surface-active material in peritoneal effluent of CAPD patients. Perit. Dial. Int. 5: 109–111. [PubMed] [Google Scholar]
- 5.Wang T., Cheng H., Liu S., Wang Y., Wu J., Peng W., Zhong J., Lindholm B. 2001. Increased peritoneal membrane permeability is associated with abnormal peritoneal surface layer. Perit. Dial. Int. 21: S345–S348. [PubMed] [Google Scholar]
- 6.Mangold H. K. 1961. Thin-layer chromatography of lipids. J. Am. Oil Chem. Soc. 38: 708–727. [Google Scholar]
- 7.Ruiz J. I., Ochoa B. 1997. Quantification in the subnanomolar range of phospholipids and neutral lipids by monodimensional thin-layer chromatography and image analysis. J. Lipid Res. 38: 1482–1489. [PubMed] [Google Scholar]
- 8.Farwanah H., Neubert R., Zellmer S., Raith K. 2002. Improved procedure for the separation of major stratum corneum lipids by means of automated multiple development thin-layer chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 780: 443–450. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton J. G., Comai K. 1984. Separation of neutral lipids and free fatty-acids by high-performance liquid-chromatography using low wavelength ultraviolet detection. J. Lipid Res. 25: 1142–1148. [PubMed] [Google Scholar]
- 10.Murphy E. J., Rosenberger T. A., Horrocks L. A. 1996. Separation of neutral lipids by high-performance liquid chromatography: quantification by ultraviolet, light scattering and fluorescence detection. J. Chromatogr. B Biomed. Appl. 685: 9–14. [DOI] [PubMed] [Google Scholar]
- 11.McNabb T. J., Cremesti A. E., Brown P. R., Fischl A. S. 1999. The separation and direct detection of ceramides and sphingoid bases by normal-phase high-performance liquid chromatography and evaporative light-scattering detection. Anal. Biochem. 276: 242–250. [DOI] [PubMed] [Google Scholar]
- 12.Grizard G., Sion B., Bauchart D., Boucher D. 2000. Separation and quantification of cholesterol and major phospholipid classes in human semen by high-performance liquid chromatography and light-scattering detection. J. Chromatogr. B. 740: 101–107. [DOI] [PubMed] [Google Scholar]
- 13.Satouchi K., Saito K. 1979. Use of tert-butyldimethylchlorosilane-imidazole reagent for identification of molecular-species of phospholipids by gas-liquid-chromatography mass-spectrometry. Biomed. Mass Spectrom. 6: 396–402. [DOI] [PubMed] [Google Scholar]
- 14.Alexander L. R., Justice J. B., Madden J. 1985. Fatty-acid composition of human-erythrocyte membranes by capillary gas-chromatography mass-spectrometry. J. Chromatogr. B. 342: 1–12. [DOI] [PubMed] [Google Scholar]
- 15.Myher J. J., Kuksis A., Marai L., Sandra P. 1988. Identification of the more complex triacylglycerols in bovine-milk fat by gas-chromatography mass-spectrometry using polar capillary columns. J. Chromatogr. 452: 93–118. [DOI] [PubMed] [Google Scholar]
- 16.Bicalho B., David F., Rumplel K., Kindt E., Sandra P. 2008. Creating a fatty acid methyl ester database for lipid profiling in a single drop of human blood using high resolution capillary gas chromatography and mass spectrometry. J. Chromatogr. A. 1211: 120–128. [DOI] [PubMed] [Google Scholar]
- 17.Han X., Gross R. W. 2005. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom. Rev. 24: 367–412. [DOI] [PubMed] [Google Scholar]
- 18.Ejsing C. S., Sampaio J. L., Surendranath V., Duchoslav E., Ekroos K., Klemm R. W., Simons K., Shevchenko A. 2009. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA. 106: 2136–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christie W. W. 1985. Rapid separation and quantification of lipid classes by high-performance liquid-chromatography and mass (light-scattering) detection. J. Lipid Res. 26: 507–512. [PubMed] [Google Scholar]
- 20.Silversand C., Haux C. 1997. Improved high-performance liquid chromatographic method for the separation and quantification of lipid classes: application to fish lipids. J. Chromatogr. B. 703: 7–14. [DOI] [PubMed] [Google Scholar]
- 21.Homan R., Anderson M. K. 1998. Rapid separation and quantitation of combined neutral and polar lipid classes by high-performance liquid chromatography and evaporative light-scattering mass detection. J. Chromatogr. B. 708: 21–26. [DOI] [PubMed] [Google Scholar]
- 22.Aveldano M. I., Vanrollins M., Horrocks L. A. 1983. Separation and quantitation of free fatty-acids and fatty-acid methyl-esters by reverse phase high-pressure liquid-chromatography. J. Lipid Res. 24: 83–93. [PubMed] [Google Scholar]
- 23.Isaac G., Bylund D., Mansson J. E., Markides K. E., Bergquist J. 2003. Analysis of phosphatidylcholine and sphingomyelin molecular species from brain extracts using capillary liquid chromatography electrospray ionization mass spectrometry. J. Neurosci. Methods. 128: 111–119. [DOI] [PubMed] [Google Scholar]
- 24.Brouwers J. F., Vernooij E. A., Tielens A. G., van Golde L. M. 1999. Rapid separation and identification of phosphatidylethanolamine molecular species. J. Lipid Res. 40: 164–169. [PubMed] [Google Scholar]
- 25.Byrdwell W. C., Emken E. A., Neff W. E., Adlof R. O. 1996. Quantitative analysis of triglycerides using atmospheric pressure chemical ionization mass spectrometry. Lipids. 31: 919–935. [DOI] [PubMed] [Google Scholar]
- 26.Hu C., van Dommelen J., van der Heijden R., Spijksma G., Reijmers T. H., Wang M., Slee E., Lu X., Xu G., van der Greef J., et al. 2008. RPLC-ion-trap-FTMS method for lipid profiling of plasma: method validation and application to p53 mutant mouse model. J. Proteome Res. 7: 4982–4991. [DOI] [PubMed] [Google Scholar]
- 27.Pulfer M., Murphy R. C. 2003. Electrospray mass spectrometry of phospholipids. Mass Spectrom. Rev. 22: 332–364. [DOI] [PubMed] [Google Scholar]
- 28.Lesnefsky E. J., Stoll M. S. K., Minkler P. E., Hoppel C. L. 2000. Separation and quantitation of phospholipids and lysophospholipids by high-performance liquid chromatography. Anal. Biochem. 285: 246–254. [DOI] [PubMed] [Google Scholar]
- 29.Houjou T., Yamatani K., Imagawa M., Shimizu T., Taguchi R. 2005. A shotgun tandem mass spectrometric analysis of phospholipids with normal-phase and/or reverse-phase liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 19: 654–666. [DOI] [PubMed] [Google Scholar]
- 30.Peterson B. L., Cummings B. S. 2006. A review of chromatographic methods for the assessment of phospholipids in biological samples. Biomed. Chromatogr. 20: 227–243. [DOI] [PubMed] [Google Scholar]
- 31.Dugo P., Favoino O., Luppino R., Dugo G., Mondello L. 2004. Comprehensive two-dimensional normal-phase (adsorption)-reversed-phase liquid chromatography. Anal. Chem. 76: 2525–2530. [DOI] [PubMed] [Google Scholar]
- 32.Tian H., Xu J., Xu Y., Guan Y. 2006. Multidimensional liquid chromatography system with an innovative solvent evaporation interface. J. Chromatogr. A. 1137: 42–48. [DOI] [PubMed] [Google Scholar]
- 33.Gao F., Tian X., Wen D., Liao J., Wang T., Liu H. 2006. Analysis of phospholipid species in rat peritoneal surface layer by liquid chromatography/electrospray ionization ion-trap mass spectrometry. Biochim. Biophys. Acta. 1761: 667–676. [DOI] [PubMed] [Google Scholar]
- 34.Zhong J. H., Guo Q. Y., Ye R. G., Lindholm B., Wang T. 2000. Phospholipids in dialysate and the peritoneal surface layer. Adv. Perit. Dial. 16: 36–41. [PubMed] [Google Scholar]
- 35.Folch J., Lees M., Stanley G. H. S. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 36.Shaikh N. A. 1994. Assessment of various techniques for the quantitative extraction of lysophospholipids from myocardial tissues. Anal. Biochem. 216: 313–321. [DOI] [PubMed] [Google Scholar]
- 37.Dugo P., Cacciola F., Kumm T., Dugo G., Mondello L. 2008. Comprehensive multidimensional liquid chromatography: theory and applications. J. Chromatogr. A. 1184: 353–368. [DOI] [PubMed] [Google Scholar]
- 38.Hsu F. F., Turk J. 2000. Charge-remote and charge-driven fragmentation processes in diacyl glycerophosphoethanolamine upon low-energy collisional activation: a mechanistic proposal. J. Am. Soc. Mass Spectrom. 11: 892–899. [DOI] [PubMed] [Google Scholar]
- 39.Hsu F. F., Turk J. 2000. Characterization of phosphatidylinositol, phosphatidylinositol-4-phosphate, and phosphatidylinositol-4,5-bisphosphate by electrospray ionization tandem mass spectrometry: a mechanistic study. J. Am. Soc. Mass Spectrom. 11: 986–999. [DOI] [PubMed] [Google Scholar]
- 40.Hsu F. F., Turk J. 2001. Studies on phosphatidylglycerol with triple quadrupole tandem mass spectrometry with electrospray ionization: fragmentation processes and structural characterization. J. Am. Soc. Mass Spectrom. 12: 1036–1043. [Google Scholar]
- 41.Houjou T., Yamatani K., Nakanishi H., Imagawa M., Shimizu T., Taguchi R. 2004. Rapid and selective identification of molecular species in phosphatidylcholine and sphingomyelin by conditional neutral loss scanning and MS3. Rapid Commun. Mass Spectrom. 18: 3123–3130. [DOI] [PubMed] [Google Scholar]
- 42.Hsu F. F., Turk J. 2005. Studies on phosphatidylserine by tandem quadrupole and multiple stage quadrupole ion-trap mass spectrometry with electrospray ionization: Structural characterization and the fragmentation processes. J. Am. Soc. Mass Spectrom. 16: 1510–1522. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X., Reid G. E. 2006. Multistage tandem mass spectrometry of anionic phosphatidylcholine lipid adducts reveals novel dissociation pathways. Int. J. Mass Spectrom. 252: 242–255. [Google Scholar]
- 44.Hsu F. F., Turk J. 2007. Differentiation of 1-O-alk-1'-enyl-2-acyl and 1-O-alkyl-2-acyl glycerophospholipids by multiple-stage linear ion-trap mass spectrometry with electrospray ionization. J. Am. Soc. Mass Spectrom. 18: 2065–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim H. Y., Salem N. 1986. Phospholipid molecular-species analysis by thermospray liquid-chromatography mass-spectrometry. Anal. Chem. 58: 9–14. [DOI] [PubMed] [Google Scholar]
- 46.Hills B. A. 2000. Role of surfactant in peritoneal dialysis. Perit. Dial. Int. 20: 503–515. [PubMed] [Google Scholar]
- 47.Zhe X. W., Gao F., Nie H. G., Tian X. K., Chen W., Liu H. W., Lindholm B., Axelsson J., Wang T. 2008. Increased dialysate levels of phospholipids containing unsaturated fatty acid are associated with increased peritoneal transport rate. Am. J. Nephrol. 28: 1007–1013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.