Abstract
Objective:
Patients with acute to subacute neurologic decline undergo a battery of imaging and laboratory tests to determine a diagnosis and treatment plan. Often, after an extensive evaluation, a brain biopsy is recommended as yet another tool to assist in determining the diagnosis. The goal of this retrospective cohort analysis is to measure the sensitivity of open brain biopsy in this patient population, compare these results with the preoperative presumed diagnosis, and evaluate if the biopsy result significantly alters treatment.
Methods:
The authors reviewed the medical records of 135 consecutive patients who underwent open brain biopsies for acute to subacute progressive neurologic decline between January 1999 and September 2008 at a single institution. All patients with mass lesions, with HIV/AIDS, and who were younger than 20 years of age were excluded from the study. Fifty-one patients met these criteria and all preoperative tests, imaging, and treatment plans were examined and compared with postbiopsy interventions to determine the impact of the biopsy on patient outcome.
Results:
The sensitivity of open brain biopsy at our institution was 35%. The most common preoperative presumed diagnosis was vasculitis and the most common postoperative finding was Creutzfeldt-Jakob disease, followed by amyloid angiopathy. Postbiopsy hemorrhage was a complication in 4% of patients. Treatment plans changed as a direct result of the biopsy in 8% of patients, and in only 4% did the biopsy findings make a difference in disease course.
Conclusion:
In patients with progressive neurologic decline without a radiographic mass lesion or immunodeficiency, open brain biopsy often fails to provide a diagnosis and even more rarely does it significantly alter treatment.
GLOSSARY
- CJD
= Creutzfeldt-Jakob disease;
- PCNSL
= primary CNS lymphoma.
When a patient presents to a medical center with a history of progressive neurologic decline, an evaluation ensues that often includes blood and CSF studies, diagnostic imaging, as well as an EEG. After all the data have been analyzed and a diagnosis has not yet been reached, the decision may be made to proceed with an open brain biopsy. In the past, authors have examined the diagnostic yield of these biopsies.1–3 Patients with malignancies and immunodeficiencies were found to have benefited from this invasive procedure. Outside of this subset of patients, the sensitivity of brain biopsies has varied significantly from study to study. The circumstance for these patients is complicated further by the possibility of a nondiagnostic biopsy, the unveiling of an untreatable diagnosis, or the risk of insult to neighboring cortex or white matter. Often patients are treated with comfort care until they recover from the biopsy, or in the worst case, go on to die of the disease, having gained no benefit from the procedure.
The goal of this retrospective study was to not only determine the sensitivity of open brain biopsy in this population of patients with progressive neurologic decline, but also ascertain the overall role of the biopsy on patient care and treatment. Previous studies have demonstrated that in up to 60% of diagnostic biopsies, there was little to no impact on therapeutic treatment plans.1,4 In our series, we examined 51 consecutive open brain biopsies to help further evaluate the overall therapeutic benefit of this invasive procedure. All decisions to proceed with the biopsy were made through combined discussions between the neurology and neurosurgical services, in accordance with patient family wishes. This decision to proceed with biopsy often included many poorly understood biases and the data are examined in this context.
METHODS
Standard protocol approvals, registrations, and patient consents.
An application to the institutional review board of Emory University School of Medicine was approved for a retrospective review of all medical records, including operative reports and discharge summaries, for those patients who underwent a craniotomy for open brain biopsy at Emory University Hospital from January 1999 to September 2008 (IRB 00014122).
Patient identification.
A total of 135 cases were identified and patients with immunodeficiencies, mass lesions, or known malignancies thought to be associated with the presenting symptoms were eliminated from this review. To help eliminate potential inadequate sampling, all patients underwent a craniotomy and all stereotactic needle biopsies were excluded (6 patients). Intraoperatively, a 1-cm3 specimen of arachnoid, pia, cortex, and underlying white matter was sent to pathology for interpretation. The biopsy was preferentially removed from the right middle frontal lobe gyrus unless history, examination, or imaging asymmetry suggested another location would provide a higher diagnostic yield. After applying the above exclusion criteria and including only those patients with complete medical records available, a total of 51 patients were identified, with demographics shown in table 1. All included patients had documented evaluations available in their charts with complete laboratory results, operative reports, imaging, and pathology.
Table 1 Demographics
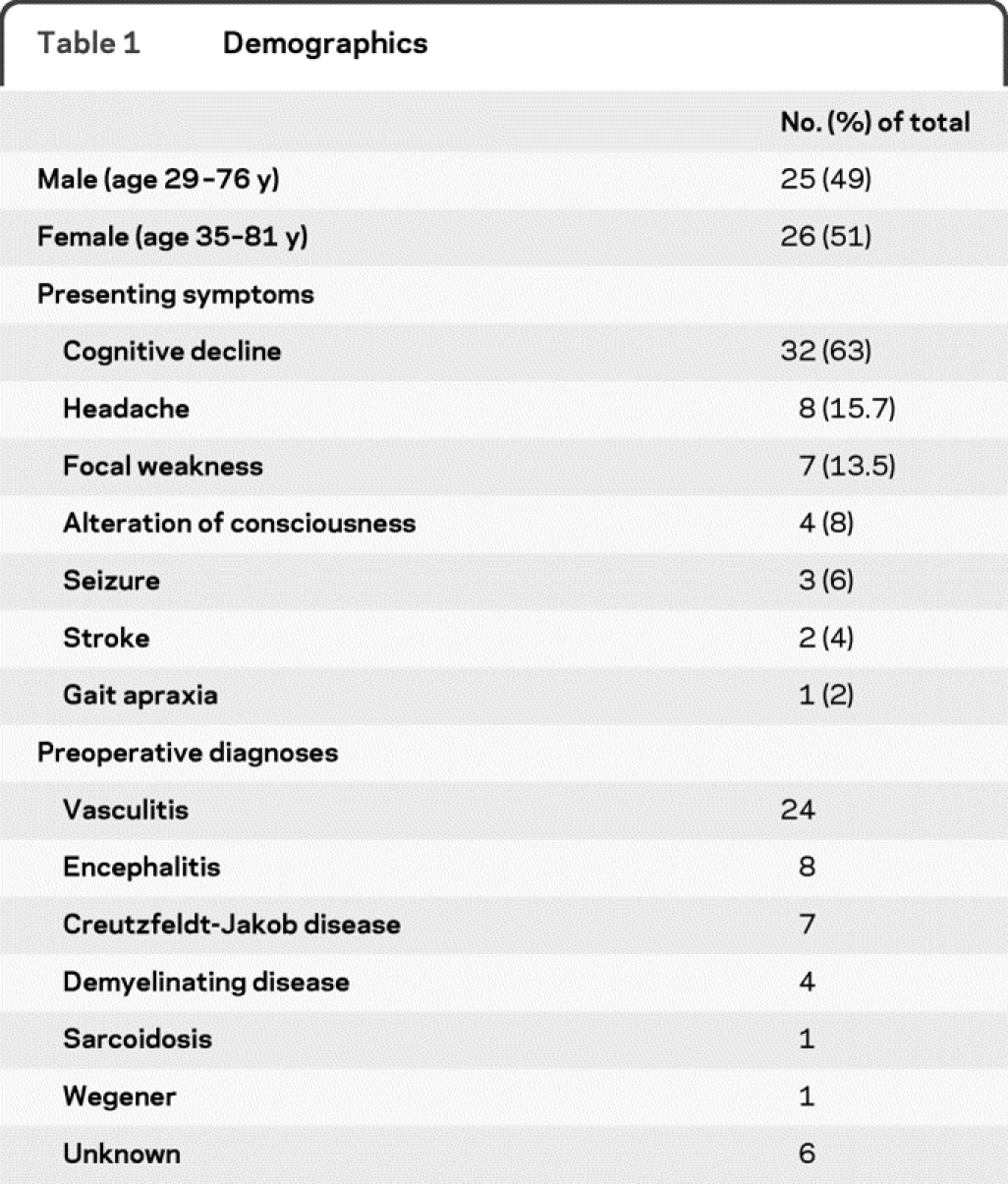
Presenting symptoms at time of hospitalization as listed in the medical records are documented in table 1. All radiographic imaging was interpreted by a neuroradiologist and pathology slides by a neuropathologist. Using discharge summaries and clinic notes at time of follow-up, changes made to the treatment regimen were recorded. On average, 15 months of follow-up were available for the included patients.
RESULTS
The most common presenting symptom in this population was rapid cognitive decline, defined as less than 6 months since onset, as seen in 63% of patients. Other symptoms including memory loss, headache, focal weakness, and ataxia occurred much less frequently (table 1).
Initial evaluation consisted of CSF analysis in 48 of 51 patients and EEG in 45 of 51 patients. In addition, 26 of 51 patients underwent cerebral diagnostic subtraction angiography, especially if vasculitis led the differential. All patients underwent comprehensive MRI prior to open biopsy. A presumed preoperative diagnosis of vasculitis was found in 24 of 51 patients (47%). Other less common preoperative diagnoses included encephalitis (8), Creutzfeldt-Jakob disease (CJD) (7), demyelinating diseases (4), and other (8) (table 1).
A right frontal craniotomy for sampling of the middle frontal gyrus was the most common site for biopsy (65%; 33 of 51 patients). Depending upon symptoms on presentation, other sites were preferentially sampled, including left frontal, right or left temporal, as well as right or left occipital lobes. After obtaining a postoperative noncontrast CT scan of the head on all patients, 2 of 51 (4%) patients were found to have a biopsy-related intraparenchymal hemorrhage. While 1 patient required the insertion of a ventriculostomy for intracranial pressure monitoring, the other was safely followed by examination and serial CT scans. Of note, neither of these individuals required further surgical intervention or reoperation.
The overall sensitivity, or likelihood of a pathologic abnormality, of a diagnostic brain biopsy was 35% (18 of 51 patients). The nondiagnostic biopsies were labeled either normal brain tissue (13 of 51 patients) or mild, nonspecific inflammatory changes (20 of 51 patients). Biopsy results are listed in table 2. The most common pathology in the diagnostic biopsies was CJD at 15.7% (8 of 51 patients), followed by amyloidosis at 6% (3 of 51 patients). Other results included demyelinating disease, primary CNS lymphoma (PCNSL), viral encephalitis, infarction, and sarcoidosis, as seen in figure 1.
Table 2 Final pathology
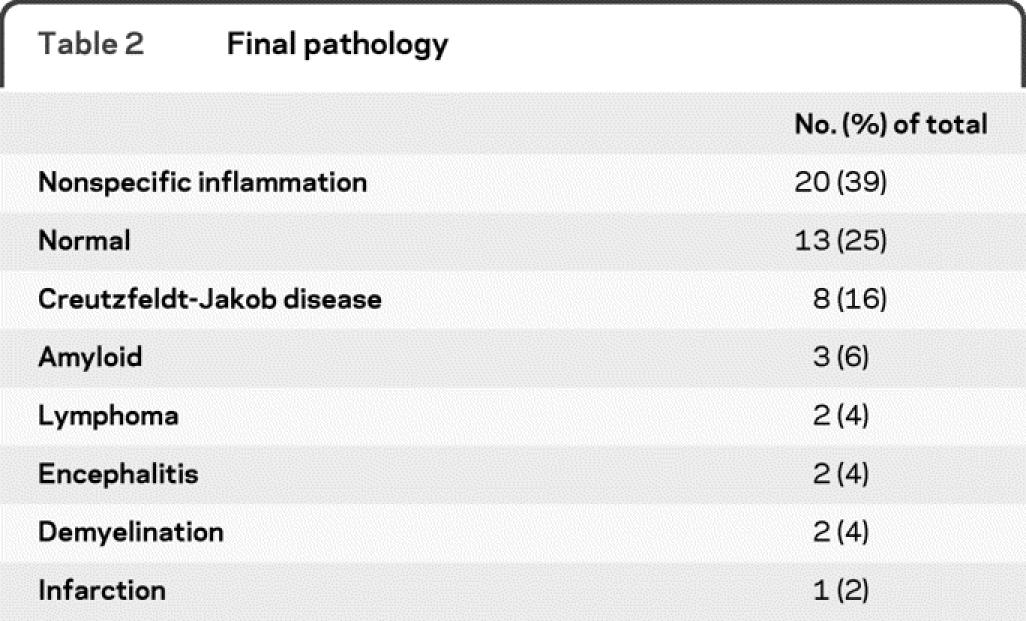
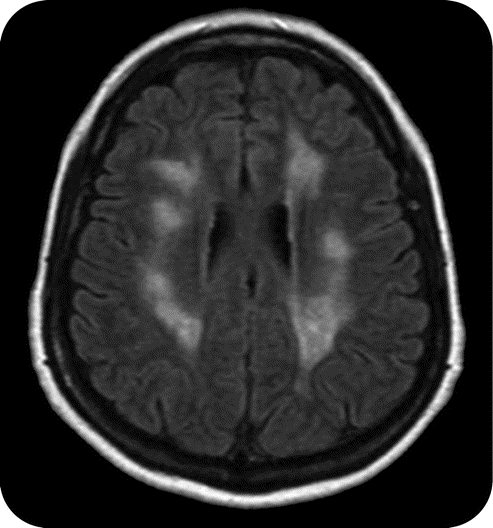
Figure 1 Encephalomyelitis
Axial fluid-attenuated inversion recovery image showing hyperintensities throughout the white matter in a patient diagnosed with acute disseminated encephalomyelitis by biopsy.
Eight percent (4 of 51 patients) of open biopsies performed resulted in a change of treatment or start of a new treatment. These included patients with diagnosed demyelinating disease, PCNSL,2 or sarcoidosis. The remaining diagnostic results, primarily CJD, amyloid, and chemical meningoencephalitis, were without additional meaningful treatment options. Overall, 25% (13 of 51) of the biopsied patients were started on a new treatment despite negative or inconclusive results, almost half of which (12%; 6 of 51) were started on an immunomodulatory therapy, including CellCept and Cytoxan for presumed vasculitis. Of the 4 patients started on a new treatment regimen as a result of their diagnostic biopsy, only 2 (4%, 2 of 51) gained a meaningful therapeutic benefit. This was only seen in the 2 patients who were newly diagnosed with lymphoma and subsequently initiated on chemotherapy.
DISCUSSION
The reported diagnostic yield of brain biopsies for rapidly deteriorating neurologic conditions varies significantly, ranging from 20% to 65%.1,2,4–6 Though the details of the methodology for these studies differed, the primary goals were similar. These studies all excluded individuals with suspected neoplastic processes, with the exception of PCNSL, as well as known immunodeficient patients. These heavily studied groups have cited diagnostic brain biopsy yields of 95% and 96%, respectively.7,8 Previous studies have also surmised that with the advancement of noninvasive imaging and serologic studies, biopsies could be avoided in many of these patients.4
Our study concentrated on 51 consecutive open brain biopsies with medical records available for thorough review, extensive presurgical evaluations, and postbiopsy follow-up at our institution.
The primary goal is to determine the number of positive biopsies in this population and identify the sensitivity of open biopsies in this patient population. We define sensitivity as the likelihood of any pathologic abnormality on biopsy. A secondary goal is to examine the overall value of the treatment changes directly associated with the biopsy findings. Additionally, we examined the prebiopsy evaluations themselves, and ascertained if there were certain groups where a biopsy would have a higher diagnostic yield. In contrast, we also aimed to identify patterns of presentation and other diagnostic testing that may improve treatment regimens and reaching a diagnosis, without the need for brain biopsy. This also includes avoiding unnecessary costs to the patient. For instance, our institution currently has a rigorous protocol for biopsies without a radiographic mass lesion, viewing all cases as possible CJD transmissible. This often unnecessary safety protocol adds an additional expense to the already high cost of a brain biopsy. The ability to increase diagnostic biopsy yield, minimize patient and surgeon risk, and minimize unnecessary procedures would be of great value.
The most common diagnostic pathology finding from our series was CJD. Initial evaluation of this patient subset included an MRI, CSF studies, and an EEG in all 8 patients. In 7 of 8 patients, an MRI demonstrated characteristic diffusion-weighted and T2 fluid-attenuated inversion recovery signal abnormalities centered in the caudate nucleus or putamen (figure 2). Previous studies have shown these characteristic radiographic changes in 92.3% of patients with CJD.9 In 4 of 8 patients, an EEG revealed classic periodic sharp-wave complexes, a finding positive in 50% of the CJD population in this same study.9 Finally, the 14-3-3 CSF immunoblot, testing for the presence of the same-named protein, has been shown to be positive in 77%–100% of patients with CJD.9–11 In our series, it was identified in 4 patients, ambiguous in 1, not tested in 2, and negative in only 1 patient. Of note, no inflammatory markers were found in the CSF specimens of all 8 patients.
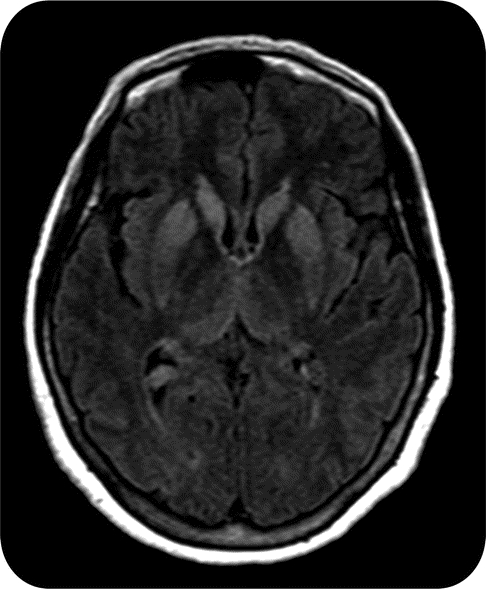
Figure 2 Creutzfeldt-Jakob disease
MRI axial image demonstrating classic fluid-attenuated inversion recovery hyperintensities in the caudate and putamen in a patient with Creutzfeldt-Jakob disease.
The culmination of the evaluations led to a presumed preoperative diagnosis of CJD in 7 of 8 (87.5%) patients, with the biopsy serving as a confirmatory test. A previous study12 reported that brain biopsies serve a role only in those cases where reliable clinical parameters fail to reveal the diagnosis. In our series, only 4 brain biopsies were performed after the addition of protein 14-3-3 immunoblot testing to the presurgical evaluation. In the 4 cases performed following positive immunoblot testing, the presumed preoperative diagnosis matched the postbiopsy findings. With the continued improvement in neuroimaging, serologic, and CSF studies such as 14-3-3 immunoblot assay, the number of patients with suspected CJD requiring diagnostic brain biopsy may be significantly reduced in the future.
Nearly half (24 of 51) of the studied patients initially presenting at our institution carried a prebiopsy diagnosis of primary CNS vasculitis. Eight of these patients (32%) underwent digital subtraction angiography with findings suggestive of, but not diagnostic of, vasculitis as defined by the interpreting neuroradiologist (figure 3). Those patients with angiograms diagnostic for cerebral vasculitis did not undergo biopsies and were therefore not included in this study. No patients for whom vasculitis was the primary diagnostic suspicion were found to have biopsy-proven cerebral vasculitis, with the majority of specimens showing only nonspecific inflammation. In an earlier study,4 only 1 of 7 patients believed to have primary CNS vasculitis had a diagnostic result on biopsy. Clearly, cerebral vasculitis continues to be a diagnostic dilemma as brain biopsies have a historically low yield, often cited as 35% to 53%.13,14 These numbers are probably optimistic and ophthalmologic fluorescein angiography, as well as other advanced radiologic and serologic testing, may serve as less-invasive, higher-yield diagnostic tools.15
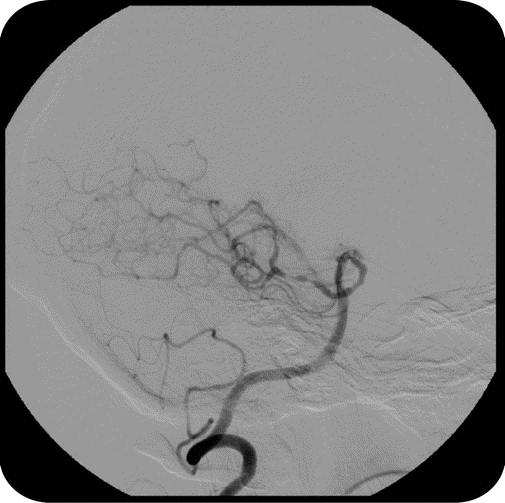
Figure 3 Presumed vasculitis: Nondiagnostic
Digital subtraction angiogram demonstrating multiple areas of alternating dilation and constriction in the bilateral posterior cerebral arteries read as suggestive of but not diagnostic of vasculitis. Open biopsy was nondiagnostic.
All efforts were made to biopsy in the region localized by MRI or angiographic abnormalities. However, most individuals had no clear focal abnormality and the biopsies were sampled from a site thought to be the least harmful to surrounding cerebral function. The previous studies demonstrating higher diagnostic results all had focal T2 and fluid-attenuated inversion recovery signal abnormalities from which specimens were removed.12,13 Interestingly, in our series, 9 of the 25 patients suspected to have vasculitis were started on steroids, as well as, in some cases, immunomodulatory medications despite nondiagnostic results. As treatment currently stands, medical regimens for CNS vasculitis have been extrapolated from systemic vasculitides with no controlled therapeutic trials.16 This holds with previous assertions that treatment should be approached on a patient-by-patient basis; however, the lack of a well-documented therapy for CNS vasculitis further clouds the real value of a biopsy in the diagnostic algorithm.
In our study, 8 patients were suspected to have viral or nonviral meningoencephalitis. Only 2 (25%) of the biopsies showed inflammation consistent with viral encephalitis, and CSF analysis of these patients revealed cell counts with 1 to 100 nucleated cells and lymphocyte predominance. Likewise, erythrocyte sedimentation rate results ranged from 11 to 59 (normal 1–20 mm/hour) in this patient group.
Past studies have shown equally low yield when meningoencephalitis is suspected. A study from 199417 compiled brain biopsy results in 49 patients and found a diagnostic yield of 24%. A second study from 199418 demonstrated a diagnostic yield of 39%; however, only 9% if the patient lacked enhancement on neuroimaging. Again, these low yields are likely due to improved serologic and CSF testing identifying herpes simplex virus and other viruses, further precluding the need for brain biopsy in these patients.
The most significant role of a brain biopsy in the diagnostic algorithm is the ability to alter treatment plans. More importantly, however, is the therapeutic benefit obtained by the change in the treatment regimen. In our study, 8% (4 of 51) of patients had an alteration in their treatment regimen as a direct result of an open brain biopsy. Previous studies have also demonstrated equally low numbers. For example, a 2005 publication studied 90 consecutive biopsies for progressive dementia and showed similar numbers, with 11% of biopsies leading to treatment alterations.2 These numbers differ greatly from a 2007 study,1 where 44% of biopsies in a series of 64 led to alterations in treatment. This substantial difference can be attributed to the large number of patients with PCNSL in the latter retrospective series. The focal nature of imaging abnormalities in PCNSL, along with its documented response to treatment, makes this study less comparable to our series of patients.
Apart from the alteration in treatment regimen, the true overall value of a brain biopsy must be weighed against the impact of the change in the outcome of the patient. In our study, only 4% of patients (2 of 51) had a direct therapeutic benefit as a result of the biopsy findings. In both instances, the diagnosis obtained from sampling was PCNSL, and the addition of chemotherapy improved their clinical status. Although difficult to compare among studies, only nominally higher numbers have been found in other series. For example, a 1997 study4 illustrated an 8% rate (4 of 50 patients) of meaningful therapeutic intervention following brain biopsy, while a 2008 study found a slightly increased 12% change.6 Once again, the question must be asked: Can the diagnosis found as a result of the brain biopsy be achieved through less-invasive means, such as CSF assays and serologic markers?
Although commonly underreported, the morbidity and mortality associated with this invasive cranial procedure should be discussed. While there were no deaths as a direct result of the biopsies in our series, 2 patients (4%) had postbiopsy hemorrhagic complications. Aside from these obvious radiographic morbidities, it is difficult to discern whether delayed postbiopsy physical examination manifestations are a consequence of the biopsy itself or simply progression of the disease process. Either way, it is important to weigh the 4% potential therapeutic benefit against the 4% risk of a postbiopsy hemorrhagic event.
As mentioned, 25% (13 of 51) of our patients were started on additional postbiopsy therapies despite normal results. The majority of these patients initiated immunomodulatory medications, with the presumptive diagnosis of vasculitis. This striking discovery further raises the question of the need for biopsy in this particular patient population. Often, these patients had equivocal angiographic results and were considered to improve clinically on steroids after biopsy. With proper serologic and CSF analysis, as well as patient and family counseling, these decisions can be made without the risk and expense of tissue samples in many cases.
There may be a limited role for open biopsy in a small subset of these patients. However, these patients must not have signs, symptoms, imaging, or serologic testing indicative of CJD or PCNSL. There are little data to support open brain biopsy as a confirmatory measure for the definitive diagnosis of these diseases. Nonetheless, if a coexisting medical condition precludes the use of certain therapeutic agents or the treating team, along with a fully informed family member, is hesitant to begin treatment without a biopsy, then it may be valuable to proceed.
Overall, the role of brain biopsy in patients with acute or subacute progressive neurologic decline is not clear in the literature. With modern advanced neuroimaging and CSF/serologic testing, the role of brain biopsy is steadily decreasing, as many clinicians are arriving at a diagnosis without the need for surgery. This retrospective study provides a current, critical examination with an emphasis on patient management. By concentrating on not only the diagnosis, but also the treatment options prior to surgery, we can avoid in many cases a potentially unwarranted surgical risk, added expense, and emotional hardship that accompanies an open brain biopsy.
DISCLOSURE
Dr. Schuette and Dr. Taub report no disclosures. Dr. Hadjipanayis has received funding for travel or speaker honoraria from Varian Inc.; serves on the editorial board of Cancer Nanotechnology; and receives research support from the NIH (NINDS K08NS053454 [PI]), the Georgia Cancer Coalition, the Dana Foundation, the Southeastern Brain Tumor Foundation, and the American Brain Tumor Association. Dr. Olson serves on the Data Safety Monitoring Board for Monteris, Inc., and has funded clinical research for which he is the Emory PI by Wyeth, Myriad Genetics, Inc., and Baxter International Inc.; and receives research support from the NIH (UO1CA137433-01 [Site PI], R01 CA116174 [Co-I], R01 CA121320-01 [Co-I], and R21 CA141836 [PI]).
Disclosure: Author disclosures are provided at the end of the article.
Address correspondence and reprint requests to Dr. Albert J. Schuette, 1365 Clifton Road NE, Suite 6200, Atlanta, GA 30322; ajschue@gmail.com
Received December 3, 2009. Accepted in final form April 9, 2010.
REFERENCES
- 1.Josephson SA, Papanastassiou AM, Berger MS, et al. The diagnostic utility of brain biopsy procedures in patients with rapidly deteriorating neurological conditions or dementia. J Neurosurg 2007;106:72–75. [DOI] [PubMed] [Google Scholar]
- 2.Warren JD, Schott JM, Fox NC, et al. Brain biopsy in dementia. Brain 2005;128:2016–2025. [DOI] [PubMed] [Google Scholar]
- 3.Burns JD, Cadigan RO, Russell JA. Evaluation of brain biopsy in the diagnosis of severe neurologic disease of unknown etiology. Clin Neurol Neurosurg 2009;111:235–239. [DOI] [PubMed] [Google Scholar]
- 4.Javedan SP, Tamargo RJ. Diagnostic yield of brain biopsy in neurodegenerative disorders. Neurosurgery 1997;41:823–828; discussion 8–30. [DOI] [PubMed]
- 5.Kaufman HH, Catalano LW Jr. Diagnostic brain biopsy: a series of 50 cases and a review. Neurosurgery 1979;4:129–136. [DOI] [PubMed] [Google Scholar]
- 6.Burns JD, Cadigan RO, Russell JA. Evaluation of brain biopsy in the diagnosis of severe neurologic disease of unknown etiology. Clin Neurol Neurosurg Epub 2008 Nov 18. [DOI] [PubMed]
- 7.Hall WA. The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer 1998;182:1749–1755. [DOI] [PubMed] [Google Scholar]
- 8.Levy RM, Russell E, Yungbluth M, et al. The efficacy of image-guided stereotactic brain biopsy in neurologically symptomatic acquired immunodeficiency syndrome patients. Neurosurgery 1992;30:186–189; discussion 9–90. [DOI] [PubMed]
- 9.Shiga Y, Miyazawa K, Sato S, et al. Diffusion-weighted MRI abnormalities as an early diagnostic marker for Creutzfeldt-Jakob disease. Neurology 2004;63:443–449. [DOI] [PubMed] [Google Scholar]
- 10.Castellani RJ, Colucci M, Xie Z, et al. Sensitivity of 14-3-3 protein test varies in subtypes of sporadic Creutzfeldt-Jakob disease. Neurology 2004;63:436–442. [DOI] [PubMed] [Google Scholar]
- 11.Collins SJ, Sanchez-Juan P, Masters CL, et al. Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt-Jakob disease. Brain 2006;129:2278–2287. [DOI] [PubMed] [Google Scholar]
- 12.Heinemann U, Krasnianski A, Meissner B, et al. Brain biopsy in patients with suspected Creutzfeldt-Jakob disease. J Neurosurg 2008;109:735–741. [DOI] [PubMed] [Google Scholar]
- 13.Benseler S, Schneider R. Central nervous system vasculitis in children. Curr Opin Rheumatol 2004;16:43–50. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese LH, Duna GF, Lie JT. Vasculitis in the central nervous system. Arthritis Rheum 1997;40:1189–1201. [DOI] [PubMed] [Google Scholar]
- 15.Scolding NJ, Jayne DR, Zajicek JP, et al. Cerebral vasculitis: recognition, diagnosis and management. QJM 1997;90:61–73. [DOI] [PubMed] [Google Scholar]
- 16.Hajj-Ali RA, Calabrese LH. Central nervous system vasculitis. Curr Opin Rheumatol 2009;21:10–18. [DOI] [PubMed] [Google Scholar]
- 17.Smith JE, Aksamit AJ Jr. Outcome of chronic idiopathic meningitis. Mayo Clin Proc 1994;69:548–556. [DOI] [PubMed] [Google Scholar]
- 18.Cheng TM, O'Neill BP, Scheithauer BW, et al. Chronic meningitis: the role of meningeal or cortical biopsy. Neurosurgery 1994;34:590–595; discussion 596. [DOI] [PubMed]




