Abstract
Objectives:
Depression and antidepressant use, especially selective serotonin reuptake inhibitors (SSRIs), are common in Parkinson disease (PD). The objective of this clinical trial was to assess the efficacy of atomoxetine, a selective norepinephrine reuptake inhibitor (SNRI), for the treatment of clinically significant depressive symptoms and common comorbid neuropsychiatric symptoms in PD.
Methods:
A total of 55 subjects with PD and an Inventory of Depressive Symptomatology–Clinician (IDS-C) score ≥22 were randomized to 8 weeks of atomoxetine or placebo treatment (target dosage = 80 mg/day). Depression response (>50% decrease in IDS-C score or Clinical Global Impression–Improvement [CGI-I] score of 1 or 2) was assessed using intention-to-treat modeling procedures. Secondary outcomes included global cognition, daytime sleepiness, anxiety, apathy, and motor function.
Results:
There were no between-groups differences in a priori–defined response rates. Using a more liberal response criterion of >40% decrease in IDS score from baseline, there was a trend (p = 0.08) favoring atomoxetine. Patients receiving atomoxetine experienced significantly greater improvement in global cognition (p = 0.003) and daytime sleepiness (p = 0.001), and atomoxetine was well-tolerated.
Conclusions:
Atomoxetine treatment was not efficacious for the treatment of clinically significant depressive symptoms in PD, but was associated with improvement in global cognitive performance and daytime sleepiness. Larger studies of SNRIs in PD for disorders of mood, cognition, and wakefulness are appropriate.
Classification of evidence:
This interventional study provides Class II evidence that atomoxetine (target dosage = 80 mg/day) is not efficacious in improving clinically significant depression in PD.
GLOSSARY
- ADHD
= attention-deficit/hyperactivity disorder;
- AS
= Apathy Scale;
- CGI-I
= Clinical Global Impression–Improvement;
- CI
= confidence interval;
- dPD
= depression in Parkinson disease;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- ESS
= Epworth Sleepiness Scale;
- GDS-15
= 15-item Geriatric Depression Scale;
- ICC
= intraclass correlation coefficient;
- IDS-C
= Inventory of Depressive Symptomatology–Clinician;
- LC
= locus ceruleus;
- MMSE
= Mini-Mental State Examination;
- NNT
= number needed to treat;
- OR
= odds ratio;
- PD
= Parkinson disease;
- SNRI
= selective norepinephrine reuptake inhibitor;
- SSRI
= selective serotonin reuptake inhibitor;
- STAI
= State Anxiety Inventory;
- TCA
= tricyclic antidepressant;
- UPDRS
= Unified Parkinson's Disease Rating Scale.
Depression occurs in up to 50% of patients with Parkinson disease (PD), and is associated with functional impairment,1,2 reduced quality of life,3 and caregiver distress.4 In addition, depression in PD (dPD) is frequently comorbid with anxiety, apathy, disorders of sleep and wakefulness, and cognitive impairment.
Up to 25% of patients with PD are taking an antidepressant at any given time, most commonly a selective serotonin reuptake inhibitor (SSRI).5 However, a recent meta-analysis found no evidence for SSRI efficacy in dPD, with a diminished effect compared with geriatric depression.6
Placebo-controlled studies with tricyclic antidepressants (TCAs) have been promising. In 2 studies, TCA-treated patients experienced significant improvement in depression compared with placebo,7,8 with 1 study also showing benefit for anxiety and disturbed sleep.7 However, TCAs can be difficult to utilize in PD due to aggravation of orthostatic hypotension, constipation, and cognitive impairment.
Given the significant noradrenergic deficits that occur in PD,9 the association between norepinephrine and depression in general, and a primary noradrenergic mechanism of many TCAs,10 we conducted a randomized, double-blind, placebo-controlled study of atomoxetine for clinically significant depressive symptoms in PD. Atomoxetine, a selective norepinephrine reuptake inhibitor (SNRI) approved for the treatment of attention-deficit/hyperactivity disorder (ADHD), has shown evidence of having antidepressant properties.11 A small open-label study of reboxetine, an SNRI used in Europe, for patients with dPD found significant decreases in depression scores with treatment.12 We hypothesized that atomoxetine would be efficacious for the treatment of depression and common comorbid nonmotor symptoms in PD.
METHODS
Participants.
The study population consisted of 55 patients with idiopathic PD recruited from The Parkinson's Disease and Movement Disorders Center at Pennsylvania Hospital (n = 41) and the Parkinson's Disease Research, Education and Clinical Center at the Philadelphia Veterans Affairs Medical Center (n = 14). The diagnosis of possible or probable PD13 was confirmed by the patient's movement disorder neurologist.
The primary measure of depression symptom severity was the Inventory for Depressive Symptomatology–Clinician Rated (IDS-C),14 a 30-item (scores 0–84, increasing scores indicating greater depression severity) comprehensive instrument that is increasingly used as a primary outcome measure in major depression treatment studies in the general population.15 In this study, patients were at least moderately depressed, as determined by a baseline IDS-C score ≥22.
Subjects were excluded for the following reasons: Mini-Mental State Examination (MMSE)16 score <15; consumption of >14 alcoholic beverages a week; a history of bipolar disorder, schizophrenia, or schizoaffective disorder; deep brain stimulation within the previous 6 months; current use of monoamine oxidase inhibitors; and current or planned pregnancy or nursing. Patients currently on an antidepressant were permitted to participate and continue the antidepressant if they had received an adequate antidepressant trial (minimum 6-week duration at a therapeutic dose).
Standard protocol approvals, registration, and patient consents.
The Institutional Review Board at each participating institution approved the study, and written informed consent was obtained from subjects prior to study participation. This clinical trial was posted on clinicaltrials.gov (NCT00304161).
Design.
This was an 8-week, randomized, double-blind, placebo-controlled study. The Investigational Drug Service at the University of Pennsylvania maintained the blind. The Investigational Drug Service was responsible for purchasing the study drug, preparing drug kits, and creating and implementing the randomization table. Numbered drug kits were provided by Investigational Drug Service and administered to study participants by trained research personnel. Everyone directly involved with the study participants as well as the participants themselves were blinded to study group assignment. Recruitment spanned a period of 4 years from July 2004 to August 2008, at which time the blind was broken and data analysis began.
All subjects were randomized to atomoxetine 40 mg/day or placebo. At the end of 2 weeks, the dosage of atomoxetine or placebo was increased to 80 mg/day for the duration of the study, although patients were allowed to continue study participation at 40 mg/day if a decrease in study medication was clinically indicated.
Eligibility was determined at screening. Patients provided informed consent and were assessed with the MMSE and the 15-item Geriatric Depression Scale (GDS-15).17 Patients with possible clinically significant depression, determined by GDS-15 score ≥5, were scheduled for a baseline visit after at least a 1-week delay. At baseline, eligible and willing patients with an IDS-C score ≥22 were entered into the clinical trial.
Assessments.
Study participants were assessed in person at baseline, week 2, week 4, and week 8 (study completion). Five trained research staff over the course of the study evaluated subjects with an extensive psychiatric battery including the IDS-C (intraclass correlation coefficient [ICC] ≥0.945 with Principal Investigator's (D.W.) assessment or training tape at 4 time points over the course of the study), a semi-structured interview to determine a DSM-IV-TR18 diagnosis of major depressive episode, the State Anxiety Inventory (STAI)19 to assess anxiety, the Apathy Scale (AS)20 as a measure of apathy, and the Epworth Sleepiness Scale (ESS)21 as a measure of daytime sleepiness. The primary and secondary outcome measures presented here were chosen on the basis of measuring severity of psychiatric symptoms or cognitive deficits that might respond to atomoxetine treatment.
The IDS-C was administered at all study visits, and the rest of the psychiatric measures and the MMSE were administered at baseline and study completion. D.W. administered the Clinical Global Impression–Improvement (CGI-I) scale at all postbaseline visits, the Unified Parkinson's Disease Rating Scale (UPDRS) at baseline, week 4, and study completion, and queried about possible side effects at all study visits using the Treatment Emergent Symptom Scale.22 Blood pressure (sitting and standing) and pulse were measured at each study visit.
Analyses.
The study was powered to detect a 30% differential in response rates between atomoxetine and placebo treatment, leading to a projected study population of 80 randomized subjects. Over the 4-year period of funding that supported the study, 55 subjects were recruited.
The criteria for the 2 primary measures of treatment response were a >50% decrease in IDS-C score from baseline and a final CGI-I score of 1 or 2. Remission was defined as a final IDS-C score ≤13. Secondary analyses involved examining treatment group differences in global cognition, daytime sleepiness, anxiety, apathy, and motor function.
Descriptive univariate analyses included calculating means and standard deviations for continuous outcomes and percentages for categorical outcomes. Tests of significance across treatment groups included t tests for equality of means for continuous measures and χ2 tests for categorical measures. Longitudinal, intent-to-treat mixed-effects modeling procedures were used to test and estimate treatment group differences in both continuous and binary outcome measures over time, regardless of dropout or missing data, thus making use of all available data and not limiting the analyses to complete cases. The models adjusted for clustering at the patient level and included main effect and interaction terms (i.e., time by treatment group) at each follow-up visit. To control for potential between-group baseline differences, the baseline score for each measure was included as a covariate in the appropriate model. Contrasts for continuous outcomes were modeled using SAS PROC MIXED, and for binary outcomes using SAS PROC GENMOD (SAS version 9.0, SAS Institute, Cary, NC). p < 0.05 was considered significant.
The study was conducted as part of a Career Development Award (K23) grant awarded to D.W. by the National Institute of Mental Health, and the funding source had no direct role in the study.
RESULTS
Clinical and demographic characteristics.
Study completion rates for the atomoxetine and placebo patients were 79% and 78%. Table 1 presents sociodemographic characteristics and baseline values for PD variables and primary and secondary outcome measures of interest (e.g., depression, apathy, daytime sleepiness, anxiety symptoms, and cognitive performance) for the full sample and stratified by treatment group. The majority of the sample was white (96.4%) and male (66.0%). Participants were on average 64.3 (SD = 10.5) years old, with an average PD duration of 6.9 (SD = 6.2) years. Approximately two-thirds (62%) of participants met criteria for major depression. Over half (53.1%; 15 in atomoxetine and 14 in placebo groups) were currently taking an antidepressant in the recommended therapeutic range for a minimum of 6 weeks. Comparisons across treatment arms indicate that there were no significant differences between patients receiving placebo and atomoxetine on any background or baseline variables, with the exception of a slightly higher mean MMSE score in the placebo group.
Table 1 Baseline study sample characteristics by randomized group assignment
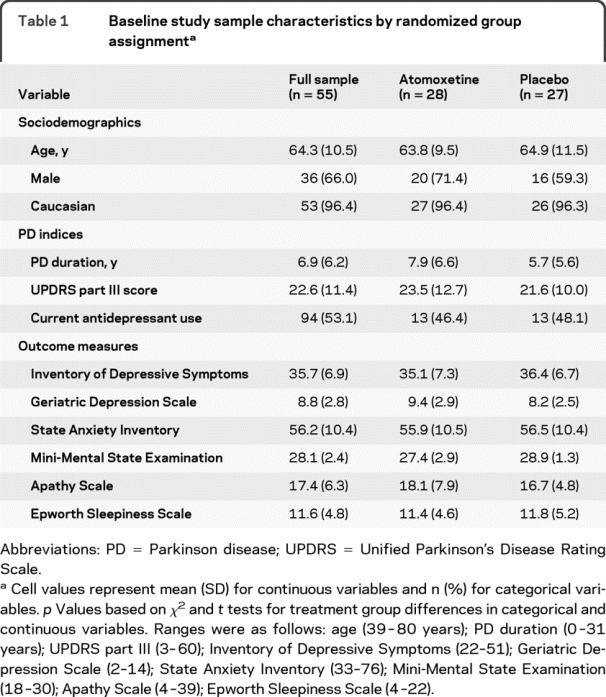
Of study completers, 97.7% (42/43) of subjects were taking 80 mg/day of atomoxetine or placebo equivalent at the final visit. For this group, mean (SD) medication adherence was 96.4% (8.6%) for the final 4 weeks of the study. Participant flow through the trial is shown in figure 1.
Figure 1 Participant flow through the trial
Depression outcomes.
Results from the longitudinal, mixed-effects models showed reductions in depressive symptoms (i.e., continuous IDS score) at each follow-up visit relative to baseline, but no treatment group differences for these outcomes (mean between-group difference for change in IDS score at week 8 adjusted for baseline score = 1.67 points [SE = 2.30], p = 0.47).
When modeling IDS response (i.e., ≥50% decrease in IDS score since baseline) as a binary outcome, results indicated that atomoxetine and placebo groups did not differ in response rates at 8 weeks (22.7% vs 9.5% for atomoxetine and placebo; odds ratio [OR] = 2.79; 95% confidence interval [CI] = 0.48–16.33; p = 0.25), which translates to a number needed to treat (NNT) of 7.6 (figure 2). However, using a more liberal response criterion of >40% decrease in IDS score since baseline to allow for nonresponse in IDS physical items that might have been present secondary to PD rather than depression, there was a trend for atomoxetine-treated patients to have a superior response (31.8% vs 9.5%; OR = 4.43; 95% CI = 0.80–24.54; p = 0.08).

Figure 2 Depression response at week 8 based on change in Inventory of Depressive Symptomatology (IDS) score by treatment group
On the a priori defined response criteria for the CGI-I (i.e., score of 1 or 2), there were no between-group differences in response rates at week 8 (45.5% and 33.3% for atomoxetine and placebo; OR = 1.67; 95% CI = 0.48–5.74; p = 0.42), a NNT of 7.6 (figure 3). Using a more liberal CGI-I response criterion (i.e., score of 1, 2, or 3), there still was no difference in response rates between the groups (63.6% and 42.9%; OR = 2.33; 95% CI = 0.69–7.95; p = 0.18).

Figure 3 Depression response at week 8 based on Clinical Global Impression–Improvement (CGI-I) score by treatment group
There were no between-group differences in remission rates (3/28 [10.7%] for atomoxetine vs 0/27 [0%] for placebo, Fisher exact p = 0.12), a NNT of 9.3. Using a more liberal remission definition of IDS-C score ≤19 (i.e., at most mild depressive symptoms), patients randomized to atomoxetine were more likely to experience remission (8/28 [28.6%] vs 2/27 [7.4%], Fisher exact p = 0.04). Concurrent antidepressant treatment did not have an impact on any of the depression outcome measures (data not shown).
Other outcomes.
Examination of cognitive status (MMSE score) and daytime sleepiness (ESS score) did find significant treatment group differences in change in outcome over time (table 2). Specifically, patients randomized to atomoxetine experienced an improvement in global cognition relative to patients on placebo over the course of 8 weeks (mean group difference = 1.31 [SE = 0.41], p = 0.003). In addition, patients receiving atomoxetine experienced greater reductions in daytime sleepiness from baseline to week 8 relative to placebo (mean group difference = −2.90 [SE = 0.83], p = 0.001). Finally, there was a suggestion for a greater reduction in anxiety with atomoxetine treatment (mean group difference = −4.69 [SE = 2.62], p = 0.08).
Table 2 Differences in secondary outcome measures by treatment group

Adverse events.
Constipation (25.9%) and insomnia (14.8%) were the 2 most common adverse events in atomoxetine-treated patients. There was a trend for constipation to be more common in atomoxetine-treated than in placebo-treated patients (p = 0.05), while nausea/vomiting was less common in atomoxetine-treated patients (p = 0.02). Regarding cardiovascular adverse events, only one study participant (randomized to placebo) experienced a >20 mm increase in either systolic or diastolic blood pressure compared with baseline, and no patients experienced a >20% increase in pulse compared with baseline. Four serious adverse events requiring hospitalization occurred during the study, 2 in atomoxetine-treated patients (suicide ideation several weeks after study termination in 1 patient, and exacerbation of congestive heart failure in another) and 2 in placebo-treated patients (chest pain and possible anxiety attacks in 1 patient, and urosepsis in another); none of the serious adverse events were thought to be related to study treatment. There were no between-group differences in UPDRS motor scores throughout the course of the study (p = 0.87).
DISCUSSION
Our primary finding was that atomoxetine did not demonstrate efficacy for the treatment of clinically significant dPD based on traditionally defined response criteria. Using a more liberal criterion for treatment response to allow for symptom overlap between depression and PD symptoms, there was a suggestion for improvement in depression with atomoxetine, which was paralleled by a trend in favor of decreased severity of anxiety in atomoxetine-treated patients. In addition, significant improvements in global cognition and daytime sleepiness were found with atomoxetine treatment.
Regarding study limitations, the sample size was relatively small, possibly precluding detection of a significant treatment effect on the 2 primary measures of treatment response. Second, the diagnostic criteria for depression which are typically used in clinical trials were not used as an inclusion criterion. However, as existing diagnostic criteria for major depression may not apply well to patients with neurodegenerative diseases,23,24 we chose to define our population by severity of depressive symptoms rather than by presence of a specific depression diagnosis. Third, antidepressant use at baseline was common in our population, suggesting a high percentage of patients who may have been refractory to antidepressant treatment. However, there was no difference in depression outcome measures on the basis of baseline antidepressant treatment status. Fourth, the IDS has not been previously validated as a depression rating scale in PD, but it is increasingly used in large depression treatment studies in the general population.15 Finally, the clinical significance of the improvements in daytime sleepiness and global cognition seen with atomoxetine treatment was not assessed, but the between-group end-of-study difference in ESS score crossed the recommended cutoff score for excessive daytime sleepiness,25 and the 1-point difference in MMSE scores may underestimate end-of-study differences in cognitive abilities, given the insensitivity of the MMSE to detect mild cognitive impairments in patients with PD.26
Depression and antidepressant use, particularly SSRIs, are common in PD.5 However, evidence for the efficacy of SSRIs for dPD is lacking.6 To date, stronger evidence, albeit from a limited number of small studies,7,8,27 exists for the efficacy of TCAs, which affect both the noradrenergic and serotonergic systems to varying degrees. While atomoxetine, which is selective for the noradrenergic system, has not undergone extensive study as an antidepressant, it has been shown to be beneficial for depressed patients in the general population who are nonresponsive to SSRIs.11 Studies with another SNRI, the European compound reboxetine, have demonstrated promising results for depression resistant to SSRI treatment, and a small open-label reboxetine study in patients with dPD found significant improvement in depression scores with treatment.12
The findings suggest that medications increasing noradrenergic tone may be beneficial for other common nonmotor symptoms in PD. There is prominent loss of norepinephrine neurons in the locus ceruleus (LC) in PD,9,28 and atomoxetine inhibition of norepinephrine reuptake likely enhances the postsynaptic effects of LC activation in each target area.29 The LC is recognized as a major wakefulness-promoting nucleus,29 so atomoxetine may have alerting effects in PD based on possible enhancement of LC activity. In addition, although atomoxetine is not classified as a stimulant, it appears to possess some stimulant-like effects,30 which may have contributed to decreased daytime sleepiness in our subjects. Excessive daytime sleepiness occurs in up to half of patients with PD, and atomoxetine may offer an alternative to modafinil and stimulants, for which there is mixed evidence for efficacy in PD.31,32
The LC is thought to play an important role in the regulation of cognitive performance, including attentional modulation,33 and LC neuronal loss is associated with dementia in PD.34 Given its primary attention-enhancing effects,35 it is not surprising that atomoxetine would be associated with improvements in global cognitive performance in our sample, and additional research is needed to replicate these findings and determine if atomoxetine treatment is associated with improvements in specific cognitive domains. In a preliminary open-label study, atomoxetine was shown to enhance executive functioning in patients with PD without dementia.36 Atomoxetine increases both norepinephrine and dopamine levels in the prefrontal cortex, as dopamine uptake in this region occurs via norepinephrine transporters.37 Therefore, if atomoxetine has cognitive enhancing effects in PD, a disease with cognitive deficits linked to impairments in cortico-striatal dopaminergic pathways,38 it may be due to enhanced noradrenergic or dopaminergic tone in frontal brain regions. It is possible that stimulants, also approved for ADHD, may have similar cognitive-enhancing effects in PD.
Compared with commonly used TCAs, which target not only the noradrenergic system but to varying degrees other neurotransmitter systems (e.g., serotonergic, cholinergic, and histaminergic), atomoxetine is a selective norepinephrine reuptake inhibitor. Thus, potentially troubling side effects that can occur with TCA use due to their anticholinergic or antihistaminergic effects would not be expected to occur commonly with atomoxetine use. In support of this, we found that atomoxetine was well-tolerated in this study. Larger studies of SNRIs in PD for disorders of mood, cognition, and wakefulness are appropriate given these preliminary findings, good tolerability, and limited options currently available for the treatment of these disorders.
AUTHOR CONTRIBUTIONS
D.W. participated in the conception and design of the study and drafted the manuscript. S.M. and T.R.T.H., both affiliated with the University of Pennsylvania, recommended statistical analysis and analyzed study data. A.D.S., J.E.D., H.I.H., A.C., S.S.H., and M.B.S. assisted with study design and subject recruitment. E.M. and S.N. recruited patients and administered study-related assessments. All authors participated in the writing of the manuscript. D.W. had access to all data in the study and held final responsibility for the decision to submit the paper.
ACKNOWLEDGMENT
The authors thank Dr. William McDonald (Emory University), who served as an external study monitor.
DISCLOSURE
Dr. Weintraub has served on a scientific advisory board for Boehringer Ingelheim; serves on the editorial board of Movement Disorders; has received speaker honoraria from Boehringer Ingelheim, ACADIA Pharmaceuticals, Novartis, Osmotica Pharmaceutical Corp., BrainCells Inc., Merck Serono, sanofi-aventis, and Pfizer Inc; and has received/receives research support from Avid Radiopharmaceuticals, Inc., Boehringer Ingelheim, NIH (NIMH K23 MH067894 [PI], NINDS P50 NS053488-01 [Co-Investigator], NIA RO1AG031348 [Site PI], and NINDS R01NS065087 [Co-Investigator]), and from the Michael J. Fox Foundation for Parkinson's Research. Dr. Mavandadi and Ms. Mamikonyan report no disclosures. Dr. Siderowf serves on a scientific advisory board for and has received speaker honoraria from Teva Pharmaceutical Industries Ltd.; serves as a consultant for Supernus Pharmaceuticals, Inc.; and receives research support from Avid Radiopharmaceuticals, Inc., the NIH (NINDS U10 NS044451-023 [Site PI], NINDS P50 NS053488-01 [Co-Core Leader and Project Leader], NINDS R43NS0636071 [Site PI], and NINDS R01NS065087 [Co-Investigator]), and from the Institute for Neurodegenerative Disorders. Dr. Duda serves on a grant review panel for the Michael J. Fox Foundation for Parkinson's Research; receives research support from the U.S. Department of Veterans Affairs (Merit Award [PI]), the Michael J. Fox Foundation, and the Samueli Institute; and holds stock in C.R. Bard, Inc., Celgene, Clarient, Inc., and Johnson & Johnson. Dr. Hurtig serves on a grant review panel for the Michael J. Fox Foundation for Parkinson's Research; serves on the editorial board of Parkinsonism and Related Disorders; receives publishing royalties from UpToDate, Inc.; has received speaker honoraria from Teva Pharmaceutical Industries Ltd.; receives research support from Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim, Bayer Schering Pharma, Kyowa Hakko Kirin Pharma, Inc., PRA International, Novartis, GlaxoSmithKline, Avid Radiopharmaceuticals, Inc., St Jude Medical, Amarin Corporation, Prestwick Pharmaceutical, Inc., HP Therapeutics Foundation, Inc., Cephalon, Inc., NIH (NINDS P50 NS053488-01 [Core Leader and PI] and NINDS U10 NS044451-023 [Site PI]); and holds stock in Teva Pharmaceutical Industries Ltd. Dr. Colcher has received speaker honoraria from Lundbeck Inc. and Teva Pharmaceutical Industries Ltd. Dr. Horn has received speaker honoraria from Teva Pharmaceutical Industries Ltd. Ms. Nazem reports no disclosures. Dr. Ten Have serves on a scientific advisory board for the NIH; served as a consultant for Abbott and Avid Radiopharmaceuticals, Inc.; and receives research support from the NIH (R01-MH078016 [PI], R01-HL073932 [Co-Investigator], R01-MH067498 [PI], U01-HL087072 [Co-Investigator], U49-CE001093 [Co-Investigator], R01-HD053270 [Co-Investigator], R01-MH079736 [Co-Investigator], R01-HL086830 [Co-Investigator], P30-AG031043 [Co-Investigator], R01-AA016187 [Co-Investigator], R01-MH083717 [Co-Investigator], R01-CA132656 [Co-Investigator], R01-AT004921 [Co-Investigator], R34-MH085906 [Co-Investigator], NHBLI R01-HL096651 [Co-Investigator], R01-HD061061 [Co-Investigator], R01-MH082799 [Co-Investigator], R01-DA027204 [Co-Investigator], R01-CE001615 [Co-Investigator], RC1-HL099612 [Co-Investigator], RC1-AA019092 [Co-Investigator], and R34-MH085880 [Co-Investigator]). Dr. Stern has served on scientific advisory boards for Boehringer Ingelheim, Teva Pharmaceutical Industries Ltd., Ipsen, Schering-Plough Corp., Novartis, and Adamas Pharmaceuticals; has received funding for travel or speaker honoraria from Novartis, Boehringer Ingelheim, and Teva Pharmaceutical Industries Ltd.; receives royalties from the publication of Surgical Management of Parkinson's Disease (Taylor and Francis Group, 2004); has served as a consultant for Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim, Novartis, Ipsen, Schering-Plough Corp., and Adamas Pharmaceuticals; receives research support from the Institute for Neurodegenerative Disorders; and holds stock options in Adamas Pharmaceuticals.
Address correspondence and reprint requests to Dr. Daniel Weintraub, 3615 Chestnut St., #330, Philadelphia, PA 19104 daniel.weintraub@uphs.upenn.edu
Study funding: Supported by the NIH NIMH 067894 (D.W.).
Disclosure: Author disclosures are provided at the end of the article.
Received December 30, 2009. Accepted in final form April 9, 2010.
REFERENCES
- 1.Starkstein SE, Mayberg HS, Leiguarda R, et al. A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 1992;55:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole SA, Woodard JL, Juncos JL, et al. Depression and disability in Parkinson's disease. J Neuropsychiatry Clin Neurosci 1996;8:20–25. [DOI] [PubMed] [Google Scholar]
- 3.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry 2000;69:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aarsland D, Larsen JP, Tandberg E, et al. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J Am Geriatr Soc 2000;48:938–942. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Recognition and treatment of depression in Parkinson's disease. J Geriatr Psychiatry Neurol 2003;16:178–183. [DOI] [PubMed] [Google Scholar]
- 6.Weintraub D, Morales KH, Moberg PJ, et al. Antidepressant studies in Parkinson's disease: a review and meta-analysis. Mov Disord 2005;20:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson's disease and depression. Neurology 2009;72:886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devos D, Dujardin K, Poirot I, et al. Comparison of desipramine and citalopram treatments for depression in Parkinson's disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2008;23:850–857. [DOI] [PubMed] [Google Scholar]
- 9.Rommelfanger KS, Weinshenker D. Norepinephrine: the redheaded stepchild of Parkinson's disease. Biochem Pharmacol 2007;74:177–190. [DOI] [PubMed] [Google Scholar]
- 10.Raisman R, Briley M, Langer SZ. Specific tricyclic antidepressant binding sites in rat brain. Nature 1979;281:148–150. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter LL, Milosavljevic N, Schecter JM, Tyrka AR, Price LH. Augmentation with open-label atomoxetine for partial or nonresponse to antidepressants. J Clin Psychiatry 2005;66:1234–1238. [DOI] [PubMed] [Google Scholar]
- 12.Lemke MR. Effect of reboxetine on depression in Parkinson's disease patients. J Clin Psychiatry 2002;63:300–304. [DOI] [PubMed] [Google Scholar]
- 13.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson's disease. Arch Neurol 1999;56:33–39. [DOI] [PubMed] [Google Scholar]
- 14.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996;26:477–486. [DOI] [PubMed] [Google Scholar]
- 15.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163:28–40. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 17.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, ed. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986:165–173. [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 19.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 20.Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci 1992;4:134–139. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 22.Guy W. ECDEU Assessment Manual for Psychopharmacology–Revised, DHEW Publ. No. (ADM) 76-338 ed. Rockville, MD: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 23.Olin JT, Schneider LS, Katz IR, et al. Provisional diagnostic criteria for depression of Alzheimer disease. Am J Geriatr Psychiatry 2002;10:125–128. [PubMed] [Google Scholar]
- 24.Marsh L, McDonald WM, Cummings JL, et al. Provisional diagnostic criteria for depression in Parkinson's disease: report of an NINDS/NIMH work group. Mov Disord 2006;21:148–158. [DOI] [PubMed] [Google Scholar]
- 25.Lichstein KL, Durrence H, Riedel BW, Bayen UJ. Primary versus secondary insomnia in older adults: subjective sleep and daytime functioning. Psychol Aging 2001;16:264–271. [DOI] [PubMed] [Google Scholar]
- 26.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson's disease. Neurology 2009;73:1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen J, Aabro E, Gulmann N, et al. Anti-depressive treatment in Parkinson's disease: a controlled trial of the effect of nortriptyline in patients with Parkinson's disease treated with l-dopa. Acta Neurol Scand 1980;62:210–219. [DOI] [PubMed] [Google Scholar]
- 28.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basilis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 2003;60:337–341. [DOI] [PubMed] [Google Scholar]
- 29.Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol 2008;6:254–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lile JA, Durell TM, Glaser PEA, Stooops WW, Rush CR. Discriminative-stimulus, self-reported, performance, and cardiovascular effects of atomoxetine in methylphenidate-trained humans. Exp Clin Psychopharmacol 2006;14:136–147. [DOI] [PubMed] [Google Scholar]
- 31.Adler CH, Caviness JN, Hentz JG, et al. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson's disease. Mov Disord 2003;18:287–293. [DOI] [PubMed] [Google Scholar]
- 32.Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson's disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry 2005;76:1636–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science 1999;283:549–554. [DOI] [PubMed] [Google Scholar]
- 34.Zweig RM, Cardillo JE, Cohen M, Giere S, Hedreen JC. The locus ceruleus and dementia in Parkinson's disease. Neurology 1993;43:986. [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain SR, del Campo N, Dowson J, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry 2007;62:977–984. [DOI] [PubMed] [Google Scholar]
- 36.Marsh L, Biglan K, Gerstenhaber M, Williams JR. Atomoxetine for the treatment of executive dysfunction in Parkinson's disease: a pilot open-label study. Mov Disord 2009;24:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahl SM. Brainstorms: neurotransmission of cognition, part 3: mechanism of action of selective NRIs: both dopamine and norepinephrine increase in prefrontal cortex. J Clin Psychiatry 2003;64:230–231. [PubMed] [Google Scholar]
- 38.Zgaljardic DJ, Borod JC, Foldi NS, et al. An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson's disease. J Clin Exp Neuropsychol 2006;28:1127–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]




