Abstract
Background:
Cognitive decline has been reported in Huntington disease (HD), as well as in the period before diagnosis of motor symptoms (i.e., pre-HD). However, the severity, frequency, and characterization of cognitive difficulties have not been well-described. Applying similar cutoffs to those used in mild cognitive impairment (MCI) research, the current study examined the rates of subtle cognitive dysfunction (e.g., dysfunction that does not meet criteria for dementia) in pre-HD.
Methods:
Using baseline data from 160 non–gene-expanded comparison participants, normative data were established for cognitive tests of episodic memory, processing speed, executive functioning, and visuospatial perception. Cutoff scores at 1.5 standard deviations below the mean of the comparison group were then applied to 575 gene-expanded pre-HD participants from the observational study, PREDICT-HD, who were stratified by motor signs and genetic risk for HD.
Results:
Nearly 40% of pre-HD individuals met criteria for MCI, and individuals closer to HD diagnosis had higher rates of MCI. Nonamnestic MCI was more common than amnestic MCI. Single-domain MCI was more common than multiple-domain MCI. Within the nonamnestic single-domain subtype, impairments in processing speed were most frequent.
Conclusions:
Consistent with the Alzheimer disease literature, MCI as a prodromal period is a valid concept in pre-HD, with nearly 40% of individuals showing this level of impairment before diagnosis. Future studies should examine the utility of MCI as a marker of cognitive decline in pre-HD.
GLOSSARY
- AD
= Alzheimer disease;
- BFRT
= Benton Facial Recognition Test;
- DCL
= Diagnostic Confidence Level;
- HD
= Huntington disease;
- HVLT-R
= Hopkins Verbal Learning Test–Revised;
- MCI
= mild cognitive impairment;
- PD
= Parkinson disease;
- SCWT
= Stroop Color Word Test;
- SDMT
= Symbol Digit Modalities Test;
- UHDRS
= Unified Huntington's Disease Rating Scale.
Mild cognitive impairment (MCI) is a transitional stage between normal cognition and dementia.1 It is operationally defined by subjective cognitive complaints, objective deficits (e.g., cognitive scores falling 1.5 or more standard deviations below matched peers), and the absence of dementia and functional impairment.2,3 The concept of MCI has proven valuable because these individuals progress to dementia more quickly than cognitively normal peers.4 Though most commonly associated with Alzheimer disease (AD),4 MCI has been applied to other neurologic conditions. For example, MCI associated with vascular disease5,6 can predict worsening of the disease.7 Individuals with Parkinson disease (PD) can have single or multiple domain MCI,8,9 and they progress to dementia at higher rates than intact peers.10
Cognitive dysfunction is one of the triad of symptoms of manifest Huntington disease (HD), with impairments in attention, verbal fluency, psychomotor speed, executive functioning, memory, and visuospatial functioning. However, the incidence of clinically relevant cognitive impairments is unknown.11–15 Moreover, cognitive changes develop gradually in HD, with some appearing 15 years before motor signs.16 Thus, early identification of MCI is of keen interest in HD, where treatment with neuroprotective agents might delay the progression of cognitive decline. The current study examined the incidence of MCI in a large sample of individuals with the genetic expansion for HD, but who did not show sufficient motor signs for a diagnosis of HD (i.e., pre-HD). Based on studies of other neurologic conditions, it was expected that some pre-HD individuals would display cognitive patterns consistent with MCI, with single-domain being more frequent than multiple-domain MCI.
METHODS
Participants.
Study participants included 160 non–gene-expanded and 575 gene-expanded individuals from the PREDICT-HD study,17 a prospective observational investigation of the earliest signs and symptoms of HD. Dates of data collection were from October 2002 to April 2009. Age- and education-corrected norms were developed using the non–gene-expanded comparison participants. These individuals were a mean of 44.9 years old (SD 8.1; range 30–59), had a mean of 14.5 years of education (SD 2.8; range 8–20), and were predominantly women (70.6%) and white (100%). Comparison participants had a parent with HD, but all had CAG repeat lengths in the unexpanded range (i.e., <30).
To characterize the incidence of MCI in pre-HD, gene-expanded participants were studied. These individuals had CAG repeat lengths in the expanded range (i.e., ≥36) as verified by genetic testing conducted independently from this study. Pre-HD participants were a mean of 42.1 years old (SD 7.4; range 30–59), had a mean of 14.5 years of education (SD 2.6; range 8–20), and were predominantly women (62.3%) and white (97.9%). All gene-expanded participants were classified as pre-HD based on expert raters' assessments of motor signs and functional capacity impairments that were insufficient to merit a diagnosis of HD. Motor signs were evaluated using the total motor score of the Unified Huntington's Disease Rating Scale (UHDRS), in which 31 items (e.g., ocular pursuit, finger taps, chorea) are rated on a 4-point scale ranging from normal to severe impairment. Total motor scores in the pre-HD participants suggested minimal motor signs (mean 4.5, SD 4.7). Consistent with the methods in the PREDICT-HD study, only gene-expanded participants with less than unequivocal signs of HD were included. The Total Functional Capacity score18 of the UHDRS was used to quantify a patient's ability to perform basic and instrumental activities of daily living; the score is derived from reports of the pre-HD participant and his or her companion. Scores range from 0 to 13, with higher scores indicating more intact functioning. Only gene-expanded participants with no functional impairments (i.e., Total Functional Capacity score = 13) were included in the study. Pre-HD participants were stratified in 2 ways. First, using the expert ratings based on the Total Motor Score (i.e., Diagnostic Confidence Level [DCL], item 17 of the UHDRS Motor Assessment), participants were grouped by their likelihood of having HD: normal (DCL0, n = 234), nonspecific motor abnormalities (<50% confidence, DCL1, n = 246), motor abnormalities that may be signs of HD (50%–89%, DCL2, n = 67), or motor abnormalities that are likely signs of HD (90%–98%, DCL3, n = 25). Again, participants with unequivocal signs of HD (≥99%, DCL4) were excluded from these analyses. Second, estimated years to diagnosis of HD was calculated with current age and CAG repeat length.19 The estimated time to HD diagnosis allowed us to stratify across 3 risk periods: near (<9 years to estimated diagnosis, n = 148), mid (9–15 years to estimated diagnosis, n = 214), and far (>15 years to estimated diagnosis, n = 213).
Cognitive measures.
Four cognitive domains were chosen for hypothesis testing because they have all been reported to be affected in manifest HD. Episodic memory was assessed using the Hopkins Verbal Learning Test–Revised (HVLT-R). In this test, participants are given 3 trials to learn a list of 12 related words. After a 20- to 25-minute delay, free recall for the 12 words is assessed. The raw score from the delayed recall trial (number correct) was used. Processing speed was assessed using the Symbol Digit Modalities Test (SDMT). Participants have 90 seconds to use a reference key to pair as many numeric digits with corresponding geometric figures. The number of correctly paired items was used. Executive functioning was assessed by the Stroop Color Word Test (SCWT), which contains 3 trials, each lasting 45 seconds. First, participants name as many colored ink patches (red, blue, and green) as they can. Next, participants read as many color names printed in black ink (“red,” “blue,” and “green”) as they can. Then participants are again instructed to name the color ink, but of incongruous color names (e.g., respond “red” to the word “blue” printed in red ink). In this interference trial, correct responding requires suppression of the overlearned response of reading a word. The number correct from the interference trial was used. Visuospatial perception was assessed with the Benton Facial Recognition Test (BFRT). In the BFRT, participants select photographs of faces that match a target face, but vary in orientation and illumination. The number correct, of a maximum of 27, is recorded. There are no time limits for matching faces. For all 4 cognitive scores (HVLT-R, SDMT, SCWT, BFRT), raw scores are reported and higher scores reflect better performance.
Standard protocol approvals, registrations, and patient consents.
All procedures were approved by local institutional review boards at all PREDICT-HD sites. All study participants provided written informed consent prior to data collection.
Procedures.
Using baseline data from the comparison group, age- and education-corrected normative data were generated for each of the 4 cognitive tests. The age groupings used were 30–39, 40–49, and 50–59. The education groupings used were 12 or fewer years and more than 12 years. Within each age and education group (e.g., 40–49 years of age with more than 12 years education), cutoff scores were determined that fell at 1.5 standard deviations below the mean, which is a common demarcation point for the identification of MCI. The cutoff scores from the comparison group were then applied to the pre-HD participants to determine the frequency of MCI in the large cohort. Amnestic MCI was defined as a memory score (i.e., HVLT-R) falling below the comparison cutoff. Nonamnestic MCI was defined as at least one nonmemory score (i.e., SDMT, SCWT, BFRT) falling below the comparison cutoff. Within the amnestic and nonamnestic categories, single-domain MCI was defined as only 1 cognitive score falling below the comparison cutoff, whereas multiple-domain MCI was defined as 2 or more cognitive scores falling below the comparison cutoffs. χ2 was used to compare groups (comparison and pre-HD) on percentage with MCI subtypes.
RESULTS
Non–gene-expanded comparison participants.
On average, raw scores on the cognitive tests in the non–gene-expanded comparison participants fell within the normal range compared to normative data from test manuals (HVLT-R: mean 10.0, SD 2.0 [47th percentile]; SDMT: mean 53.7, SD 8.8 [55th percentile]; SCWT Interference: mean 46.2, SD 8.8 [55th percentile]; BFRT: mean 23.0, SD 1.9 [71st percentile]). For our entire comparison group, cutoff scores at 1.5 standard deviations below the mean were HVLT-R 7.1, SDMT 40.4, SCWT Interference 33.0, and BFRT 20.2. Note that these cutoffs are based on the entire sample (i.e., collapsed across age and education groupings), and the individual group cutoff scores can be obtained from the first author.
Gene-expanded pre-HD participants.
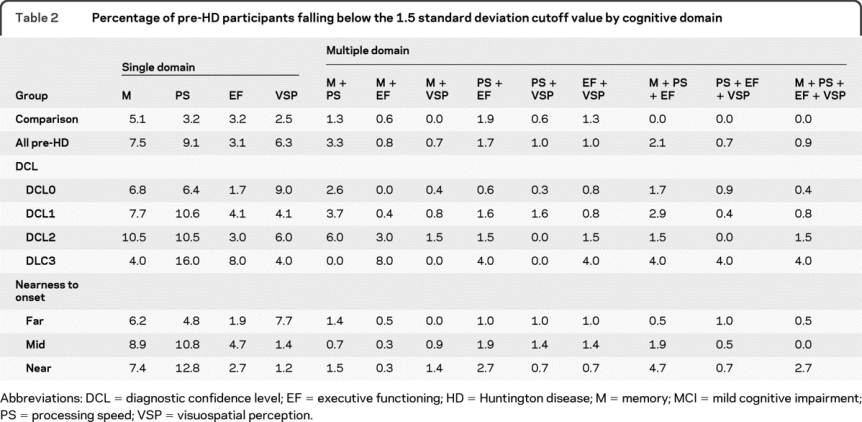
Table 1 summarizes the percentage of pre-HD participants who scored 1.5 or more SDs below our comparison group cutoff for each MCI subtype. When each pre-HD participant was compared to his or her age- and education-corrected cutoff score, 39.8% of the gene-expanded pre-HD participants were identified as having some type of MCI (i.e., at least one cognitive domain falling 1.5 or more standard deviations below the comparison group). The majority of these individuals were classified as having a nonamnestic MCI subtype (18.4%), with fewer having an amnestic subtype (7.5%). Within both amnestic and nonamnestic subtypes, single-domain MCI was much more common than multiple-domain MCI (26.0% vs 13.9%). Within all pre-HD participants, deficits in processing speed were the most common followed by episodic memory and visuospatial processing impairments (table 2). MCI in executive functioning was least common.
Table 1 Percentage of pre-HD participants falling below the 1.5 standard deviation cutoff value by subtype
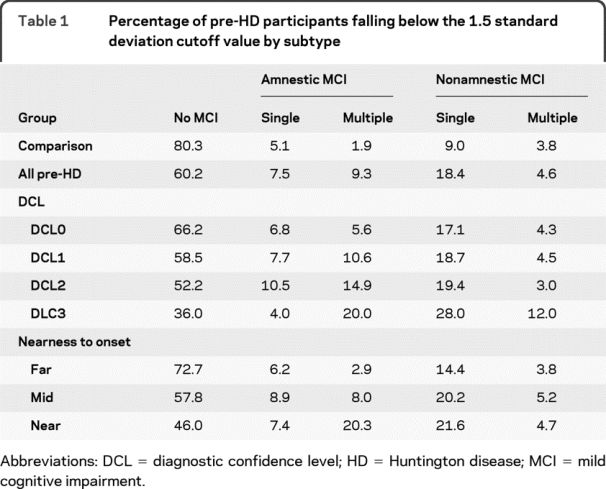
Table 2 Percentage of pre-HD participants falling below the 1.5 standard deviation cutoff value by cognitive domain
Those pre-HD participants who presented with more motor abnormalities (and greater expert rater confidence of emerging HD) had higher rates of MCI (DCL0 = 33.8%, DCL1 = 41.5%, DCL2 = 47.8%, DCL3 = 64.0%, p < 0.0001 for trend). Additional details about the MCI subtypes and cognitive domains affected as they relate to motor abnormalities are presented in tables 1 and 2.
As pre-HD participants got closer to estimated diagnosis based on their genetic risk, the prevalence of MCI increased (far = 27.3%, mid = 42.3%, near = 54.1%; p < 0.0001 for trend). Pre-HD participants who were near (<9 years) or midway (9–15 years) to diagnosis had higher rates of MCI than the comparison group (p < 0.0001), while those who were far (>15 years) from diagnosis did not (p = 0.10). Nearness to diagnosis appeared to lead to different patterns of MCI subtypes. Whereas nonamnestic MCI demonstrated a linear increase with nearness to diagnosis, the amnestic subtype was less linear. Single-domain MCI was more common in both the far and mid pre-HD participants (far: single = 20.6%, multi = 6.7%; mid: single = 29.1%, multi = 13.2%), but single and multiple-domain MCI were equally common in those near to diagnosis (single = 29.1%, multi = 25.0%). In those far from diagnosis, visuospatial perception and episodic memory were the most common single-domain subtypes (see table 2). In those mid and near diagnosis, processing speed and episodic memory were most commonly affected.
DISCUSSION
Cognitive deficits have been widely reported in pre-HD and manifest HD,11–15 but the prevalence of MCI in HD has not been previously examined. In a large cohort of individuals who were estimated to be over 14 years from a motor diagnosis of HD, we found that nearly 40% displayed mild impairments in episodic memory, processing speed, executive functioning, and/or visuospatial perception. These pre-HD individuals met existing criteria for MCI, as they did not have dementia and were not experiencing functional decline, but did show evidence of cognitive deterioration using standard criteria for MCI (i.e., decline in performance by 1.5 or more standard deviations relative to age- and education-matched normative data). The rates of MCI observed in this pre-HD cohort are notably higher than MCI rates reported in non-HD geriatric samples (e.g., 4% of amnestic MCI),20 although this appears to be the first study to examine the prevalence of MCI in a young, pre-HD cohort.
Unlike distributions seen in AD, the nonamnestic MCI subtype was more than twice as frequent as the amnestic subtype in this cohort of pre-HD individuals. In some ways, this is not surprising,21 as few (if any) of these individuals are expected to progress to AD, given their known risk of HD. However, consistent with MCI in other neurologic disorders, single-domain MCI was more common than multiple-domain MCI. Nearly twice as many gene-expanded participants demonstrated isolated cognitive deficits as those with multiple-domain impairments (e.g., single-domain MCI = 25.9%, multiple-domain MCI = 13.9%). We suspect that single-domain MCI may reflect an earlier point in the transition between normal cognition and dementia, and that multiple-domain MCI represents a later point in the progression of cognitive dysfunction.
When individual cognitive domains were examined, processing speed (9.1%) and episodic memory (7.5%) were most commonly affected in pre-HD. This finding is consistent with reports of cognitive deficits in both manifest HD and pre-HD. For example, differences between gene-expanded and non–gene-expanded participants have been observed on a range of cognitive measures, including those assessing processing speed.17 Similarly, multiple studies have identified impairments in learning and memory in patients with HD.14,22 Given the prevalence of deficits in these 2 cognitive domains in our study, they might serve as targets for early intervention in clinical trials. However, it should be noted that only the processing speed measure had a linear trend across all 3 phases of pre-HD (near, mid, far), which might make this the best candidate target for those clinical trials. Somewhat surprisingly, the rate of the executive dysfunction MCI subtype was the lowest of the 4 domain subtypes examined, irrespective of the estimated time to HD diagnosis. Response inhibition on the SCWT does not fully capture the multifaceted domain of executive functioning. Future studies should utilize multiple measures of executive functioning to investigate MCI in this domain.
Not only is MCI relatively common in pre-HD, but it appears associated with onset of HD. As more motor abnormalities were observed in these patients (i.e., higher DCLs), MCI rates increased. For example, 33.8% of individuals rated as motorically normal (i.e., DCL0) were classified with some type of MCI, whereas 64% of individuals with motor signs likely to be HD (i.e., DCL3) had MCI. Such findings indicate that when motor signs are observed in these patients, a referral for a neuropsychological evaluation is also likely needed. MCI risk also appears related to genetic risk of HD, as individuals approaching estimated diagnosis (based on CAG repeat length and current age) had double the rates of MCI (e.g., 27.3% and 54.1% of the far and near participants). These findings indicate that MCI represents a prodromal period in HD, similar to the transitional stage in AD and other neurodegenerative conditions.
Our findings have both clinical and research implications. For health care providers, greater attention needs to be directed toward MCI in pre-HD. Despite being “presymptomatic,” a sizable minority of this relatively young cohort is falling well below expectations in a broad range of cognitive domains. Although statistically significant declines in cognition relative to controls have been reported in gene-expanded and non–gene-expanded individuals,16–17 the clinical significance of these cognitive changes was not examined. This information is vital for development of diagnostic criteria that would better aid in the early identification of the MCI phase of the disease. The need for early identification of MCI is partly driven by the evolution of putative neuroprotective agents that ideally would be administered when pathology is first detected. Additionally, since many pre-HD individuals are working and raising families, they may actually be experiencing some mild functional difficulties in daily life that are not captured by the functional capacity scale of the UHDRS. Interventions, such as cognitive rehabilitation or cognitive-enhancing medications, might be useful in remediating the early effects of HD. From a research standpoint, our results raise the possibility that pre-HD individuals with MCI could represent a subgroup of all pre-HD individuals. Those with MCI might be closer to HD diagnosis. These more-at-risk individuals might be better candidates for disease-modifying clinical trials, especially trials that use cognition as a primary endpoint.
The present study is a first step toward characterizing clinically relevant MCI in pre-HD. Though we examined 4 cognitive domains commonly affected in HD, other cognitive domains (e.g., learning, language, construction, higher level problem-solving) clearly merit study. We also only examined baseline prevalence rates of MCI, whereas future studies should longitudinally investigate the prognostic value of MCI in pre-HD. As with AD, vascular cognitive impairment without dementia, and PD, the concept of MCI in pre-HD is much more valuable if individuals exhibit faster cognitive decline or higher rates of progression to dementia. If MCI-positive individuals convert at higher rates, this information could better inform clinical and personal decision-making by practitioners, patients, and their families. Clinical trials of pre-HD could also be more efficient if samples were enriched with cases of MCI. Likewise, neuroimaging studies of MCI-positive and MCI-negative pre-HD might advance an understanding of the neurobiology of cognitive decline in HD, just as studies of cerebral atrophy and metabolic hypoperfusion have in other etiologies of MCI. Finally, it would be useful to identify the prevalence of MCI in patients with manifest HD.
One notable limitation of the current study was its incomplete classification of MCI. In AD, MCI is typically operationally defined by 4 criteria: subjective cognitive complaint, objective cognitive deficit, absence of functional impairments, and absence of dementia.1,2 Our study provided information about 3 of the criteria, whereas data on subjective cognitive complaints were not collected. Although this leaves us unsure about how much of a concern MCI is in pre-HD, this might be the least valuable diagnostic criterion given that the presence of subjective cognitive complaints tends to be quite variable in MCI and may have less predictive utility than objective cognitive deficits.23 Additionally, individuals with pre-HD and manifest HD may have decreased awareness,24,25 which could render subjective cognitive complaints of limited value in this population. Nonetheless, future studies might also collect subjective information about cognitive functioning from both patients and collateral sources. Another limitation of the current study is in its samples. Individuals who participate in PREDICT-HD might not represent all pre-HD individuals, as only the minority of at-risk individuals get the genetic test for HD. However, this selection bias is largely unavoidable for this type of research. A third limitation of the study was that cognitive domains were only represented by a single neuropsychological test rather than multiple tests that assess that domain; future studies might employ a broader assessment battery. We also did not systematically exclude participants with elevated depression scores. Though increased depression has been linked with MCI in other studies,26,27 and depression can occur in HD, studies of this pre-HD cohort suggest minimal depressive symptoms.17,28 Nonetheless, future studies might examine the relationship between depression and MCI in pre-HD.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by James Mills and Dr. Langbehn.
COINVESTIGATORS
PREDICT-HD Investigators, Coordinators, Motor Raters, Cognitive Raters—Peg Nopoulos, MD, Robert Rodnitzky, MD, Ergun Uc, MD, BA, Leigh Beglinger, PhD, Vincent A. Magnotta, PhD, Stephen Cross, BA, Nicholas Doucette, BA, Andrew Juhl, BS, Jessica Schumacher, BA, Mycah Kimble, BA, Pat Ryan, MS, MA, Jessica Wood, MD, PhD, Eric Epping, MD, PhD, Thomas Wassink, MD, and Teri Thomsen, MD (University of Iowa Hospitals and Clinics, Iowa City); David Ames, MD, Edmond Chiu, MD, Phyllis Chua, MD, Olga Yastrubetskaya, PhD, Joy Preston, Anita Goh, DPsych, and Angela Komiti (The University of Melbourne, Kew, Victoria, Australia); Lynn Raymond, MD, PhD, Rachelle Dar Santos, BSc, Joji Decolongon, MSC, and David Weir, BSc (University of British Columbia, Vancouver, Canada); Adam Rosenblatt, MD, Christopher A. Ross, MD, PhD, Barnett Shpritz, BS, MA, OD, and Claire Welsh (Johns Hopkins University, Baltimore, MD); William M. Mallonee, MD, and Greg Suter, BA (Hereditary Neurological Disease Centre, Wichita, KS); Ali Samii, MD, Hillary Lipe, ARNP, and Kurt Weaver, PhD (University of Washington and VA Puget Sound Health Care System, Seattle, WA); Randi Jones, PhD, Cathy Wood-Siverio, MS, Stewart A. Factor, DO, and Claudia Testa, MD, PhD (Emory University School of Medicine, Atlanta, GA); Roger A. Barker, BA, MBBS, MRCP, Sarah Mason, BSC, Anna Goodman, PhD, and Anna DiPietro (Cambridge Centre for Brain Repair, Cambridge, UK); Elizabeth McCusker, MD, Jane Griffith, RN, and Bernadette Bibb, PhD (Westmead Hospital, Sydney, Australia); Bernhard G. Landwehrmeyer, MD, Daniel Ecker, MD, Patrick Weydt, MD, Michael Orth, MD, PhD, Sigurd Süβmuth, MD, RN, Katrin Barth, RN, and Sonja Trautmann, RN (University of Ulm, Ulm, Germany); Kimberly Quaid, PhD, Melissa Wesson, MS, and Joanne Wojcieszek, MD (Indiana University School of Medicine, Indianapolis); Mark Guttman, MD, Alanna Sheinberg, BA, Adam Singer, and Janice Stober, BA, BSW (Centre for Addiction and Mental Health, University of Toronto, Markham, Canada); Susan Perlman, MD, and Arik Johnson, PsyD (University of California, Los Angeles Medical Center, Los Angeles, CA); Michael D. Geschwind, MD, PhD, and Jon Gooblar (University of California San Francisco); Tom Warner, MD, PhD, Stefan Kloppel, MD, Maggie Burrows, RN, BA, Marianne Novak, MD, Thomasin Andrews, MD, BSC, MRCP, Elisabeth Rosser, MBBS, FRCP, and Sarah Tabrizi, BSC, PhD (National Hospital for Neurology and Neurosurgery, London, UK); Anne Rosser, MD, PhD, MRCP, and Kathy Price, RN (Cardiff University, Cardiff, Wales, UK); Amy Chesire, Frederick Marshall, MD, and Mary Wodarski, BA (University of Rochester, Rochester, NY); Oksana Suchowersky, MD, FRCPC, Sarah Furtado, MD, PhD, FRCPC, and Mary Lou Klimek, RN, BN, MA (University of Calgary, Calgary, Canada); Peter Panegyres, MB, BS, PhD, Carmela Connor, BP, MP, DP, and Elizabeth Vuletich, BSC (Neurosciences Unit, Graylands, Selby-Lemnos & Special Care Health Services, Perth, Australia); Joel Perlmutter, MD, and Stacey Barton, MSW, LCSW (Washington University, St. Louis, MO); Sheila A Simpson, MD, and Daniela Rae, RN (Clinical Genetics Centre, Aberdeen, Scotland, UK); David Craufurd, MD, Ruth Fullam, BSC, and Elizabeth Howard, MD (University of Manchester, Manchester, UK); Pietro Mazzoni, MD, PhD, Karen Marder, MD, MPH, Carol Moskowitz, MS, and Paula Wasserman, MA (Columbia University Medical Center, New York, NY); Diane Erickson, RN, Dawn Miracle, BS, MS, and Rajeev Kumar, MD (Colorado Neurological Institute, Englewood, CO); Vicki Wheelock, MD, Terry Tempkin, RNC, MSN, Nicole Mans, and Kathleen Baynes, PhD (University of California Davis, Sacramento); Joseph Jankovic, MD, Christine Hunter, RN, CCRC, and William Ondo, MD (Baylor College of Medicine, Houston, TX); Justo Garcia de Yebenes, MD, Monica Bascunana Garde, Marta Fatas, and Asuncion Martinez (Hospital Ramón y Cajal, Madrid, Spain); Martha Nance, MD, Dawn Radtke, RN, and David Tupper, PhD (Hennepin County Medical Center, Minneapolis, MN); Wayne Martin, MD, Pamela King, BScN, RN, and Satwinder Sran, BSC (University of Alberta, Edmonton, Canada); Anwar Ahmed, PhD, Stephen Rao, PhD, Christine Reece, BS, Janice Zimbelman, PhD, PT, Alexandra Bea, BA, and Emily Newman, BA (Cleveland Clinic Foundation, Cleveland, OH).
ACKNOWLEDGMENT
The authors thank the following contributors for their support with this research study.
Steering Committee: Jane Paulsen, PhD, Principal Investigator, Eric Epping, MD, PhD, Douglas Langbehn, MD, PhD, Hans Johnson, PhD, Megan Smith, PhD, Janet Williams (University of Iowa Hospitals and Clinics, Iowa City); Elizabeth Aylward, PhD (Seattle Children's Research Institute, Seattle, WA); Kevin Biglan, MD (University of Rochester, Rochester, NY); Blair Leavitt, MD (University of British Columbia, Vancouver, Canada); Marcy MacDonald, PhD (Massachusetts General Hospital, Boston); Martha Nance, MD (Hennepin County Medical Center, Minneapolis, MN); Jean Paul Vonsattel, PhD (Columbia University Medical Center, New York, NY).
Scientific Sections: Biomarkers—Blair Leavitt, MDCM, FRCPC (Chair), Michael Hayden, PhD (University of British Columbia); Stefano DiDonato, MD (Neurological Institute “C. Besta,” Italy); Ken Evans, PhD (Ontario Cancer Biomarker Network); Wayne Matson, PhD (VA Medical Center, Bedford, MA); Asa Peterson, MD, PhD (Lund University, Sweden); Sarah Tabrizi, PhD (National Hospital for Neurology and Neurology and Neurosurgery, London, UK).
Cognitive—Deborah Harrington, PhD (Chair, University of California, San Diego); Tamara Hershey, PhD, Desiree White (Washington University) (Cognitive Science Battery Development); Holly Westervelt, PhD, Jennifer Davis, Pete Snyder, PhD, Geoff Tremont (Chair, Quality Control and Training, Brown University); Megan Smith, PhD (Chair, Administration), David Moser, PhD, Leigh Beglinger, PhD (University of Iowa); Lucette Cysique (St. Vincent's/University of Melbourne, Australia); Carissa Gehl, PhD (VA Medical Center, Iowa City, IA); Robert K. Heaton, David Moore, Joanne Hamilton, David Salmon (University of California, San Diego); Kristy Matheson (University of Aberdeen); Paula Shear (University of Cincinnati); Karen Siedlecki (Fordham University); Glenn Smith (Mayo Clinic); Marleen Van Walsem (EHDN).
Functional Assessment—Janet Williams, PhD (Co-Chair), Leigh Beglinger, PhD, Anne Leserman, MSW LISW, Justin O'Rourke, Bradley Brossman, MA, Eunyoe Ro, MA (University of Iowa); Rebecca Ready, PhD (University of Massachusetts); Anthony Vaccarino, PhD (Ontario Cancer Biomarker Network); Sarah Farias, PhD (University of California, Davis); Noelle Carlozzi, PhD (Kessler Medical Rehabilitation Research & Education Center); Carissa Nehl, PhD (VA Medical Center, Iowa City, IA).
Genetics—Marcy MacDonald, PhD (Co-Chair), Jim Gusella, PhD, Rick Myers, PhD (Massachusetts General Hospital); Michael Hayden, PhD (University of British Columbia); Tom Wassink, MD (Co-Chair), Eric Epping, MD, PhD (University of Iowa).
Imaging—Administrative: Ron Pierson, PhD (Chair), Kathy Jones, Jacquie Marietta, William McDowell, Steve Dunn, Greg Harris, Eun Young Kim, Yong Qiang Zhao (University of Iowa); John Ashburner (Functional Imaging Lab, London); Vince Calhoun (University of New Mexico); Steve Potkin, MD (University of California, Irvine); Klaas Stephan (University College of London); Arthur Toga, PhD (University of California, Los Angeles).
Striatal—Elizabeth Aylward, PhD (Chair, Seattle Children's Research Institute); Kurt Weaver, PhD (University of Washington and VA Puget Sound Health Care System, Seattle, WA).
Surface Analysis—Peg Nopoulos, MD (Chair), Eric Axelson, Jeremy Bockholt, BS (University of Iowa).
Shape Analysis—Christopher A. Ross, MD, PhD (Chair), Michael Miller, PhD, Sarah Reading, MD (Johns Hopkins University); Mirza Faisal Beg, PhD (Simon Fraser University).
DTI—Vincent Magnotta, PhD (Chair), Karl Helmer, PhD (Massachusetts General Hospital); Kelvin Lim, MD (University of Ulm, Germany); Mark Lowe, PhD (Cleveland Clinic); Sasumu Mori, PhD (Johns Hopkins University); Allen Song, PhD (Duke University); Jessica Turner, PhD (University of California, Irvine).
fMRI—Steve Rao (Chair), Erik Beall, PhD, Katherine Koenig, PhD, Mark Lowe, PhD, Michael Phillips, MD, Christine Reece, BS, Jan Zimbelman, PhD, PT (Cleveland Clinic).
Motor—Kevin Biglan, MD (University of Rochester); Karen Marder (Columbia University); Jody Corey-Bloom (University of California, San Diego), all Co-Chairs; Michael Geschwind (University of California, San Francisco); Ralf Reilmann (Muenster, Germany).
Psychiatric—Eric Epping, MD, PhD (Chair), Jess Fedorowicz, MD, Robert Robinson, MD, Megan Smith, PhD (University of Iowa); Karen Anderson, MD (University of Maryland); David Craufurd, MD (Manchester University); Mark Groves, MD (Columbia University); Anthony Vaccarino, PhD, Ken Evans, PhD (Ontario Cancer Biomarker Network); Hugh Rickards, MD (Queen Elizabeth Psychiatric Hospital); Eric van Duijn (Leiden University Medical Center, the Netherlands).
Core Sections: Statistics—Douglas Langbehn, MD, PhD (Chair), James Mills, MEd, MS (University of Iowa); David Oakes, PhD (University of Rochester).
Recruitment/Retention—Martha Nance, MD (Chair, University of Minnesota); Anne Leserman, MSW, LISW, Stacie Vik, BA, Christine Anderson, BA, Nick Doucette, BA, Kelly Herwig, BA, MS, Mycah Kimble, BA, Pat Ryan, MSW, LISW, MA, Jessica Schumacher, BA, Kelli Thumma, BA, Elijah Waterman (University of Iowa); Norm Reynolds (University of Wisconsin, Milwaukee).
Ethics—Cheryl Erwin, JD, PhD (University of Texas Houston, McGovern Center for Health, Humanities, and the Human Spirit); Eric Epping, MD, PhD, Janet Williams, PhD (University of Iowa); Martha Nance, MD (University of Minnesota).
IT/Management—Hans Johnson, PhD (Chair), R.J. Connell, BS, Paul Allen, Sudharshan Reddy Bommu, Rex Gray, Karen Pease, Ben Rogers, Jim Smith, AS, Kent Williams, Shuhua Wu, Roland Zschiegner (University of Iowa).
Program Management: Administrative—Chris Werling-Witkoske (Administrative Chair), Kristine Bjork, BA (Editorial Associate), Ann Dudler (Cognitive Section Project Assistant), Jamy Schumacher (Personnel) (University of Iowa).
Financial—Steve Blanchard, MSHA (Co-Chair), Machelle Henneberry, Kelsey Montross, BA (Accounting) (University of Iowa).
DISCLOSURE
Dr. Duff serves as a grant reviewer for the US Department of Veterans Affairs; serves as an Associate Editor for the Archives of Clinical Neuropsychology and on the editorial board of The Clinical Neuropsychologist; and receives/has received research support from Janssen, Eli Lilly and Company, the NIH (1K23AG028417 [PI] and 1RC1AG035546 [coinvestigator]), and from CHDI Foundation, Inc. Dr. Paulsen receives research support from CHDI Foundation, Inc.; and the NIH (NINDS NS40068 [PI]). Mr. Mills receives research support from the NIH (NINDS NS40068 [Biostatistician]), and from CHDI Foundation, Inc. Dr. Beglinger receives/has received research support from Eli Lilly and Company, Medivation, Inc., the NIH (NIA, R03 AG025850-01 [co-PI], R01 NS052592-02 [site investigator], NCI P20 CA 103672 [PI for pilot grant funds], R01CA122934-01A1 [coinvestigator], NINDS K23 NS055733-01A1 [PI], and NINDS R01NS060118-01A1 [site investigator]), and from CHDI Foundation, Inc. Dr. Moser receives/has received research support from the NIH (NIA 1 R01 AG03417 A2 [PI], 1R01NS055827 - 01A2 [coinvestigator], and NIA 1 K23 AG020649-01A1 [PI]), and from CHDI Foundation, Inc. Dr. Smith receives/has received research support from the NIH (NINDS NS40068 [key personnel] and NINR NR010559-03 [key personnel]) and from CHDI Foundation, Inc. Dr. Langbehn receives/has received research support from Eli Lilly and Company, the NIH (NIDA R01 DAO5821 [Biostatistician, coinvestigator], NINDS 1R01 NS40068-1 [Biostatistician, coinvestigator], NINDS R01 NS054893-01A1 [Biostatistician], and NIH RO1 NG HG003330-01A1 [Biostatistician]), CHDI Foundation, Inc., and from the Michael J. Fox Foundation. Dr. Stout has served as a consultant for and received funding for travel from Medivation, Inc.; serves on the editorial advisory boards of the Journal of the International Neuropsychological Society and Brain Imaging and Behavior; and receives research support from the NIH (NINDS NS40068 [coinvestigator]) and from CHDI Foundation, Inc. Dr. Queller receives/has received research support from the NIH (NINDS NS40068 [research scientist] and from CHDI Foundation, Inc. Dr. Harrington receives research support from the US Department of Veterans Affairs (1IO1CX000146-01 and B501R), the US Office of Naval Research (N00141010072), the NIH (NS040068 [Site PI]), and from the McDonnell Foundation (220020185).
Address correspondence and reprint requests to Dr. Jane Paulsen, The University of Iowa, 1-305 MEB, Iowa City, IA 52242 jane-paulsen@uiowa.edu
Editorial, page 490
e-Pub ahead of print on July 7, 2010, at www.neurology.org.
Study funding: Supported by the NIH (NINDS 40068 [J.P.], NIMH 01579, and NIA 1K23AG028417 [K.D.]); the Roy J. and Lucille Carver Trust; the Howard Hughes Medical Institute; the Huntington Disease Society of America; and CHDI Foundation, Inc. (formerly High Q Foundation).
Disclosure: Author disclosures are provided at the end of the article.
Received December 30, 2009. Accepted in final form March 29, 2010.
REFERENCES
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 2.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240–246. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1133–1142. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 5.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke–Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006;37:2220–2241. [DOI] [PubMed] [Google Scholar]
- 6.Chui HC. Vascular cognitive impairment: Today and tomorrow. Alzheimers Dement 2006;2:185–194. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Moorhouse PK, Song X, et al. Disease progression in vascular cognitive impairment: cognitive, functional and behavioural outcomes in the Consortium to Investigate Vascular Impairment of Cognition (CIVIC) cohort study. J Neurol Sci 2007;252:106–112. [DOI] [PubMed] [Google Scholar]
- 8.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 2009;72:1121–1126. [DOI] [PubMed] [Google Scholar]
- 9.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord 2007;22:1272–1277. [DOI] [PubMed] [Google Scholar]
- 10.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord 2006;21:1343–1349. [DOI] [PubMed] [Google Scholar]
- 11.Beglinger LJ, Nopoulos PC, Jorge RE, et al. White matter volume and cognitive dysfunction in early Huntington's disease. Cogn Behav Neurol 2005;18:102–107. [DOI] [PubMed] [Google Scholar]
- 12.Paulsen JS, Conybeare RA. Cognitive changes in Huntington's disease. Adv Neurol 2005;96:209–225. [PubMed] [Google Scholar]
- 13.Stout JC, Johnson SA. Cognitive impairment and dementia in basal ganglia disorders. Curr Neurol Neurosci Rep 2005;5:355–363. [DOI] [PubMed] [Google Scholar]
- 14.Montoya A, Pelletier M, Menear M, Duplessis E, Richer F, Lepage M. Episodic memory impairment in Huntington's disease: a meta-analysis. Neuropsychologia 2006;44:1984–1994. [DOI] [PubMed] [Google Scholar]
- 15.Henry JD, Crawford JR, Phillips LH. A meta-analytic review of verbal fluency deficits in Huntington's disease. Neuropsychology 2005;19:243–252. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington's disease decades before diagnosis: the PREDICT-HD study. J Neurol Neurosurg Psychiatry 2008;79:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulsen JS, Hayden M, Stout JC, et al. Preparing for preventive clinical trials: the PREDICT-HD study. Arch Neurol 2006;63:883–890. [DOI] [PubMed] [Google Scholar]
- 18.Shoulson I, Kurlan R, Rubin A. Assessment of functional capacity in neurodegenerative disorders: Huntington's disease as a prototype. In: Munsat TL, ed. Quantification of Neurologic Deficits. Boston: Butterworth; 1989:285–309. [Google Scholar]
- 19.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin Genet 2004;65:267–277. [DOI] [PubMed] [Google Scholar]
- 20.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med 2008;148:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 22.Solomon AC, Stout JC, Johnson SA, et al. Verbal episodic memory declines prior to diagnosis in Huntington's disease. Neuropsychologia 2007;45:1767–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts JL, Clare L, Woods RT. Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord 2009;28:95–109. [DOI] [PubMed] [Google Scholar]
- 24.Hoth KF, Paulsen JS, Moser DJ, Tranel D, Clark LA, Bechara A. Patients with Huntington's disease have impaired awareness of cognitive, emotional, and functional abilities. J Clin Exp Neuropsychol 2007;29:365–376. [DOI] [PubMed] [Google Scholar]
- 25.Duff K, Paulsen JS, Beglinger LJ, et al. “Frontal” behaviors before the diagnosis Huntington's disease and their relationship to markers of disease progression: evidence of early lack of awareness. J Neuropsychiatry Clin Neurosci 2010;22:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry 2006;63:273–279. [DOI] [PubMed] [Google Scholar]
- 27.Edwards ER, Spira AP, Barnes DE, Yaffe K. Neuropsychiatric symptoms in mild cognitive impairment: differences by subtype and progression to dementia. Int J Geriatr Psychiatry 2009;24:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC, PREDICT Investigators of the HSG. Psychiatric symptoms in Huntington's disease before diagnosis: the PREDICT-HD Study. Biol Psychiatry 2007;62:1341–1346. [DOI] [PubMed] [Google Scholar]



