Abstract
Urinary 1,6-hexamethylene diamine (HDA) may serve as a biomarker for systemic exposure to 1,6-hexamethylene diisocyanate (HDI) in occupationally exposed populations. However, the quantitative relationships between dermal and inhalation exposure to HDI and urine HDA levels have not been established. We measured acid-hydrolyzed urine HDA levels along with dermal and breathing-zone levels of HDI in 48 automotive spray painters. These measurements were conducted over the course of an entire workday for up to three separate workdays that were spaced approximately 1 month apart. One urine sample was collected before the start of work with HDI-containing paints and subsequent samples were collected during the workday. HDA levels varied throughout the day and ranged from nondetectable to 65.9 μg l−1 with a geometric mean and geometric standard deviation of 0.10 μg l−1 ± 6.68. Dermal exposure and inhalation exposure levels, adjusted for the type of respirator worn, were both significant predictors of urine HDA levels in the linear mixed models. Creatinine was a significant covariate when used as an independent variable along with dermal and respirator-adjusted inhalation exposure. Consequently, exposure assessment models must account for the water content of a urine sample. These findings indicate that HDA exhibits a biphasic elimination pattern, with a half-life of 2.9 h for the fast elimination phase. Our results also indicate that urine HDA level is significantly associated with systemic HDI exposure through both the skin and the lungs. We conclude that urinary HDA may be used as a biomarker of exposure to HDI, but biological monitoring should be tailored to reliably capture the intermittent exposure pattern typical in this industry.
Keywords: biomarkers; creatinine; dermal exposure; 1,6-hexamethylene diamine; 1,6-hexamethylene diisocyanate; inhalation exposure; urine analysis
INTRODUCTION
Painters in auto-body shops are exposed to 1,6-hexamethylene diisocyanate (HDI) through inhalation and skin contact during their work activities. After absorption into the body, HDI has been indicated, in vitro, to hydrolyze to 1,6-hexamethylene diamine (HDA) (Berode et al., 1991), additionally 4,4'-methylenediphenyl diisocyanate (MDI) has been observed, in vivo, to hydrolyze to 4,4'-methylenedianiline (MDA) (Sepai et al., 1995). Others have indicated that after diisocyanates are absorbed into the body, they may be acetylated or form protein adducts by conjugation with various macromolecules (Sepai et al., 1995; Pauluhn and Lewalter, 2002; Sennbro et al., 2003; Cocker, 2007). However, upon acid hydrolysis of a urine sample, acetylated HDA and/or HDI-protein adducts may be broken down and released in the form of free HDA (Berode et al., 1991; Sepai et al., 1995; Flack et al., 2010; S. Flack, K. W. Fent, L.G. Gaines et al., submitted for publication). Consequently, HDA in hydrolyzed urine may serve as a biomarker of HDI exposure. A relationship between inhalation HDI exposure and HDA levels in hydrolyzed urine has been indicated in humans (Brorson et al., 1990; Maitre et al., 1996; Liu et al., 2004; Pronk et al., 2006) but information relating dermal HDI exposure to HDA levels in urine is lacking. Significant correlations were observed between airborne toluene diisocyanate (TDI) levels and toluene diamine (TDA) concentrations in hydrolyzed urine (Kaaria et al., 2001a; Sennbro et al., 2004), whereas the association between airborne MDI levels and MDA concentrations in hydrolyzed plasma or urine were much weaker (Kaaria et al., 2001b; Sennbro et al., 2006), indicating the possible contribution of dermal uptake for MDI. Austin (2007) observed increased urinary TDA levels among workers who had direct skin contact with uncured TDI-based foam compared to nonhandlers, even though both groups were exposed to similar airborne levels of TDI. Austin suggested that dermal absorption accounted for this large difference in TDA levels between the two groups but whether the dermal exposure was attributed to hydrolyzed diisocyanate (i.e. diamine) from contaminated surfaces or to the diisocyanate itself could not be discerned. In addition, amines were observed in the hydrolyzed urine of rats following dermal exposure to TDI (Yeh et al., 2008). Although the mechanistic pathway is unknown, there is increasing toxicological and epidemiological evidence that dermal exposure to diisocyanates plays a role in the development of respiratory sensitization and occupational asthma (Bello et al., 2007). It is also noteworthy that HDI was detected on the hands and arms of spray painters who were wearing coveralls and gloves indicating penetration of HDI through the dermal protection (Fent et al., 2009b). Thus, the current scientific evidence stresses the importance of investigating dermal exposure to HDI and its relationship with internal dose levels.
Previous research has led to conflicting estimates of the elimination rate of HDA in urine following inhalation exposure to HDI in humans (Brorson et al., 1990; Tinnerberg et al., 1995; Liu et al., 2004). The elimination rate of amines formed from the respective diisocyanate parent compounds (e.g. HDI, TDI, or MDI) appears to be biphasic, which translates to a fast and a slow-phase elimination (Brorson et al., 1991; Tinnerberg et al., 1995; Lind et al., 1996; Lind et al., 1997). The fast elimination phase likely reflects direct clearance from the plasma and correlates to relatively recent, within a day, exposure (Brorson et al., 1990; Skarping et al., 1991; Tinnerberg et al., 1995; Lind et al., 1996; Dalene et al., 1997; Liu et al., 2004). The slower phase appears to reflect urinary elimination of degradation products of diisocyanate-adducted blood proteins, e.g. albumin, hemoglobin (Sepai et al., 1995). Consequently, this slower phase may indicate cumulative exposure to the respective diisocyanate (Brorson et al., 1991; Lind et al., 1996; Lind et al., 1997). To date, published exposure assessment studies have focused on the fast elimination phase of HDA.
Brorson et al. (1990) calculated the half-life of 1.2 h for subjects exposed to HDI vapor in a test chamber. Tinnerberg et al. (1995) exposed human volunteers to HDI vapor in a test chamber and calculated the half-life as 2.5 h. However, neither Brorson et al. (1990) nor Tinnerberg et al. (1995) controlled for dermal exposure to HDI vapor, which may have biased their results. Liu et al. (2004) calculated a half-life of 2.8 h for subjects exposed to HDI biuret vapor containing a small percentage of HDI monomer through a mouth piece in such a manner that no dermal exposure occurred. To our knowledge, the half-life of HDA resulting exclusively via dermal or inhalation only exposure to HDI has not been established. Determination of the HDA half-life is critical for development of an effective biological monitoring and exposure assessment strategy for workers exposed to HDI as well as to establish a scientifically sound permissible exposure limit value for HDI exposure from both inhalation and dermal routes.
Using data collected in a repeated exposure assessment survey for task-based inhalation and dermal exposure to HDI in 48 automotive spray painters in North Carolina and Washington State, we address the quantitative and time-dependent relationship between dermal and inhalation exposure to HDI and HDA levels in acid-hydrolyzed urine. While it is possible that polymeric forms of HDI could be hydrolyzed or conjugated to HDI/HDA containing species, which after acid hydrolysis of urine could form HDA, no studies have been published to confirm this. On the contrary, Liu et al. (2004) observed that the correlation between inhalation exposure to HDI biuret aerosol and HDA in urine was weak, indicating that HDA levels better reflect HDI monomer exposure rather than oligomers.
MATERIALS AND METHODS
Study population
Spray painters in automotive repair shops who worked with HDI-containing paints were recruited for this study in the Raleigh-Durham area of North Carolina and the Puget Sound area of Washington State. The study population has been described previously (Fent et al., 2009a). Briefly, 11 shops in North Carolina with a total of 15 workers and 25 shops in Washington with a total of 33 workers participated in the study. Two shops had three painters, eight shops had two painters, and the remaining shops had one participating painter per shop. Each exposed worker was monitored on up to three separate occasions over a 9- to 12-month period. Due to attrition, six subjects were monitored once and 15 subjects were monitored twice. All subjects were male and ranged in age from 21 to 59 years, with an average age of 34. Thirty-one subjects identified themselves as white, nine as Hispanic, four as African-American, one as Asian, one as Native American, and two as mixed race. This study was approved by the Institutional Review Board in the Office of Human Research Ethics at the University of North Carolina at Chapel Hill and by the Washington State Institutional Review Board at the Washington State Department of Social and Health Services.
Dermal and breathing-zone air sampling
Personal breathing-zone sampling was used to estimate inhalation exposure to HDI during each paint task, and dermal exposure to HDI was assessed using the tape-strip method previously developed in our laboratory (Fent et al., 2008). The collection and analysis of the tape-strip samples and breathing-zone sampling have been described (Fent et al., 2009a; Fent et al., 2009b). Briefly, on each sampling visit to an auto-body shop, breathing-zone samples were collected during each clear-coat painting task, and dermal tape-strip samples were collected immediately following each task. Tape-strip samples were most commonly taken on the hands and arms, even when the painter wore coveralls and/or gloves during the paint task. The painter was observed during the paint tasks to note the duration of exposure and the type of personal protective equipment worn. The breathing-zone concentrations (BZCs), as described in Fent et al. (2009a), were adjusted over the painting time to provide a time-weighted average concentration for each task. For the analyses presented here, the reported BZC were multiplied by the total exposure time to obtain the total inhalation exposure; heron referred to as BZC. The assigned protection factor (APF) designated by the Occupational Safety and Health Administration (OSHA, 2006) was recorded for each respirator type worn by a worker as follows: none, APF = 1; air purifying (half-face), APF = 10; air purifying (full-face), APF = 50; supplied air (full-face or hood), APF = 1000; powered air purifying respirator (full-face or hood), and APF = 1000. The APF was used to adjust the BZC in order to account for the respiratory protection afforded by the painters’ respirators.
Urine sampling
During each sampling visit to an auto-body shop, one spot urine sample was obtained from each participating painter before the start of work with HDI-containing paints. During the workday, spot urine samples were obtained from the worker each time he urinated. At a minimum, one pre-exposure sample and one end-of-day sample were collected. Urine was collected in sterile, polypropylene urine collection cups (100 ml; SAMCO Bio-Tite; Fisher Scientific, Pittsburgh, PA, USA). After collection, urine was stored in a cooler (4°C) for shipment to the laboratory. Upon arrival, urine samples were transferred into 50 ml polypropylene tubes with flat-top closures (Fisher Scientific brand; Fisher Scientific) for storage at −40°C until analyzed for HDA and creatinine concentration.
An average of 3.5 samples were obtained per worker per day. The maximum number of samples obtained from a worker on a single day was nine. Fifteen samples were obtained from five workers during the normal workday when they were not painting; these workers were employed at shops that had more than one painter.
HDA analysis
Urinary HDA was analyzed using a modified version of the protocol developed by Rosenberg et al. (2002). Briefly, 10 μl of 150 μg l−1 1,7-diaminoheptane (HpDA), an internal standard, was added to 1 ml of urine before hydrolysis at 100°C for 4 h with 100 μl of concentrated sulfuric acid. The samples were neutralized with 4 ml of saturated sodium hydroxide (NaOH), mixed with 0.5 g sodium chloride (NaCl), and extracted three times with 2 ml of toluene. The samples were then derivatized with 20 μl heptafluorobutyric anhydride for 60 min at 55°C. After the samples cooled to room temperature, 1 M potassium phosphate buffer (pH = 7.0, 4 ml) was added to remove excess derivatizing agent. The organic layer was retained, and sodium sulfate was added to remove traces of water from the sample. The organic layer was transferred to a clean vial and dried using a TurboVap® LV Evaporator (Zymark Center, Hopkinton, MA, USA) with a water bath heated to 55°C under a stream of N2 at 2 psi for the first 2 h and then pressure was increased to 5 psi for the last 1 ml to dry. The samples were reconstituted with 200 μl ethyl acetate, sonicated, and transferred to gas chromatography vial inserts. The samples were then dried to completion in a SpeedVac® (Savant Instruments Inc., Holbrook, NY, USA) and reconstituted with 60 μl ethyl acetate. The samples were analyzed by gas chromatography–mass spectrometry (GC–MS; Thermo, Austin, TX, USA) in negative chemical ionization mode with methane as reagent gas (1.8 ml min−1). The GC–MS was operated with the temperatures of the transfer line and ion source at 300 and 150°C, respectively, in splitless injection mode (220°C, 1 μl injection volume, 30 s) using helium as carrier gas (1.0 ml min−1) with the following column temperature program: 50°C for 1 min, increase at 10°C min−1 to 155°C, increase at 5°C min−1 to 185°C, increase at 25°C min−1 to 290°C, and hold at 290°C for 10 min. The HDA and HpDA were quantitated by selective ion monitoring with (HDA at m/z 448 and HpDA at m/z 462).
Standard curves were prepared by spiking pooled urine from four unexposed individuals with HDA. The standard curves consisted of a reagent blank (no HDA or HpDA), a negative control (HpDA but no HDA), and nine different HDA concentrations (0.08, 0.39, 0.78, 1.56, 3.13. 6.25, 9.00, 12.5, and 20.0 μg l−1) with HpDA (1.5 μg l−1). Weighted linear regression was used to construct a standard curve using the HDA/HpDA ratio (Almeida et al., 2002). Different weighting factors (w = x–0.5, x−1, x−2, y–0.5, y−1, y−2, y–1.5; where x = HDA/HpDA instrument response ratio and y = HDA concentration) were evaluated to fit standard curves. The weighting factor that yielded the smallest sum of absolute relative error as a percentage of the nominal concentration was used to fit the standard curve (Almeida et al., 2002). The standard curve was linear from 0 to 20 μg l−1 (w = y−2, R2 = 0.98). The method detection limit (MDL) of 0.04 μg l−1 was calculated using the procedure established by US EPA (2004).
Creatinine analysis
The creatinine concentration in the urine was determined using the Creatinine Companion assay kit (Exocell, Inc., Philadelphia, PA, USA) (Murray, 1987; Exocell, 2004). This method is based on the Jaffe reaction of alkaline picrate with creatinine to form a red Janozski complex. Prior to analysis, samples were diluted in distilled water (1:20), and 20 μl of the diluted samples were aliquoted, in duplicate, into a 96-well microtiter plate along with creatinine standards of 1, 3, and 10 mg dl−1, in duplicate. Two milliliters of 1 N NaOH was added to alkaline picrate reagent, and 100 μl of the solution was added to each well. The plate was incubated at room temperature for 10 min, and the absorbance determined at 500 nm (Emax; Molecular Devices, Sunnyvale, CA, USA). A 100 μl aliquot of the acid reagent provided with the kit was then added to each well, and the absorbance at 500 nm determined after a 5-min incubation at room temperature. The difference between the two absorbance values was recorded for each well. A standard curve was calculated based on the standards and their responses. Unknown samples were evaluated by comparing their responses to the standard curve.
Statistical analysis
The data were analyzed using SAS statistical software (SAS 9.1; SAS Institute Cary, NC, USA) and R software (The R Foundation). Due to the relatively high percentage of nondetectable levels of HDA in the urine samples (38%) as well as HDI in the breathing-zone air (9%) and dermal tape-strip (63%) samples, multiple imputation was used to impute data below the detection limits. For each observation with a nondetectable level, 10 values were imputed. We applied logarithmic transformation to all exposure variables to make them normally distributed before imputation. We imputed from truncated multivariate normal distributions with an upper truncation at the logarithmic transformed limit of detection (LOD) for HDI or MDL for HDA. Several authors, including Lubin et al. (2004), have considered imputation from truncated normal distributions. Our use of a multivariate version of these methods allowed us to control for within-subject correlations (e.g. samples taken). After imputation, all the data were transformed back to their original scale to allow for additional computations. The results of the statistical analyses (PROC MIXED) of the 10 imputed datasets were combined with PROC MIANALYZE to obtain valid estimates and statistical inferences. Averages were computed where PROC MIANALYZE could not be used (i.e. fit statistics).
Data from urine samples obtained after HDI exposure were used in the exposure modeling using mixed effects linear regression analyses. The urine samples collected before the first exposure that occurred on that day were included in descriptive statistics but excluded from the exposure modeling. The urine samples collected from workers on days when they did not paint were also excluded from the exposure modeling but included in descriptive statistics. Each post-exposure urine sample was used as a unique observation in the exposure modeling. Prior to statistical analysis, urine HDA levels were log transformed to satisfy normality assumptions (Shapiro Wilks W > 0.85). Although creatinine levels were approximately normally distributed (W = 0.89), log-transformation of these data improved the normality (W = 0.96). Consequently, when testing creatinine as an independent variable, as opposed to normalization, log-transformed creatinine levels were used in the linear mixed models (Barr et al., 2005).
Dermal exposure level and BZC were used in the analyses separately or jointly to investigate whether both exposure routes contributed to urine HDA levels. Inhalation exposure was examined using two methods: (i) the measured BZCs were used as such to indicate inhalation exposure and (ii) BZCs were adjusted based on the APF in order to investigate the potential effect of respiratory protection on urine HDA levels. The exposure variables were evaluated for potential collinearity by examining the Spearman correlation coefficients among the pairs of variables. None of the variables exceeded our criterion for high correlation (i.e. r > 0.70).
We used multiple linear mixed effects modeling (PROC MIXED) in order to account for the repeated measurements study design and, thus, to obtain both inter- and intra-person variance estimates. The basic multiple linear mixed effects model used was
| (1) |
where Yij represents the natural logarithm of the urine HDA level (the jth measurement obtained for the ith worker), X1ij represents the natural logarithm of the measured BZC unadjusted or adjusted for the respirator APF, X2ij represents the natural logarithm of the measured dermal exposure, X3ij represents the natural logarithm of the creatinine concentration in the urine sample, and αi and ϵij represent the random effects associated with worker (αi for i = 1,2, …, 48 workers) and an error term (ϵij for j = 1,2, …, 16 measurements per worker). Models were constructed using standard regression techniques, and model fit was examined with regression diagnostics, such as residual analysis. The statistical significance was evaluated at α level of 0.10.
Using this model, we assumed that αi and ϵij are mutually independent and normally distributed with means of zero and respective variances and representing the between- and within-worker variance components, where total variance . It is also assumed that Yij is normally distributed with mean and variance . Compound symmetry was used for the covariance structure.
The model was analyzed for either cumulative or effective exposure. The cumulative dermal, BZC, and APF-adjusted BZC (BZC-APF) exposure levels were calculated by summing all the respective exposure levels that occurred before a urine sample was obtained. Thus, for the cumulative exposure model, the exposure variables were calculated by
| (2) |
where Ct is the concentration at time t of the measured dermal, BZC, or BZC-APF level, T is the time of the urine sample collection, and t is the midpoint of exposure period. The exposure period is the duration of the total task, which reflected the time the air-sampling pump was operating. Workers’ exposure during this period was likely intermittent because, in general, the painter would mix paints, enter the booth, apply the first coat, leave the booth, wait for the first coat to dry, and then reenter the booth to apply additional coats. Thus, for the purpose of the exposure models, the entire time the pump was operating is referred to as ‘exposure period’ even though the painter may have not been directly exposed to HDI during the entire time.
The effective exposure was calculated based on the time-decay formula
| (3) |
where C0 is the initial concentration at t = 0 and λ is the decay constant. The decay constant is given by rearranging as
| (4) |
The effective exposure was then calculated by
| (5) |
We used two published half-lives of urinary HDA, 1.2 h and 2.8 h (Brorson et al., 1990; Liu et al., 2004), to estimate a decay constant of 0.01 (70 min) and 0.004 (174 min), respectively, under these conditions and sampling periods. Thus, three types of models were constructed: (A) cumulative exposure, (B) effective exposure with a half-life of 70 min, and (C) an effective exposure with a half-life of 174 min (see Tables 2 and 3).
Table 2.
Summary of (A) cumulative and (B–C) effective linear mixed effects models with creatinine as an independent variable and with estimated HDA half-life (T1/2) of 70 or 174 min for predicting natural log-transformed urine HDA levels in automotive spray painters exposed to HDI
| Exposure model | Exposure variable | Estimate | Standard error | P value | AIC | R2 | |
| A. Cumulative | 1 | lnBZC | 0.157 | 0.069 | 0.022 | 1037 | 0.23 |
| lncreatinine | 1.254 | 0.168 | <0.0001 | ||||
| 2 | lnBZC-APF | 0.179 | 0.055 | 0.001 | 1032 | 0.26 | |
| lncreatinine | 1.290 | 0.167 | <0.0001 | ||||
| 3 | lndermal | 0.151 | 0.058 | 0.010 | 1036 | 0.25 | |
| lncreatinine | 1.256 | 0.168 | <0.0001 | ||||
| 4 | lnBZC | 0.076 | 0.086 | 0.381 | 1038 | 0.25 | |
| lndermal | 0.113 | 0.074 | 0.124 | ||||
| lncreatinine | 1.253 | 0.168 | <0.0001 | ||||
| 5 | lnBZC-APF | 0.139 | 0.065 | 0.032 | 1035 | 0.28 | |
| lndermal | 0.079 | 0.068 | 0.244 | ||||
| lncreatinine | 1.281 | 0.167 | <0.0001 | ||||
| Worker var | 0.930 | ||||||
| Residual var | 1.762 | ||||||
| B. Effective T1/2 = 70 min | 1 | lnBZC | 0.120 | 0.063 | 0.056 | 1039 | 0.22 |
| lncreatinine | 1.270 | 0.168 | <0.0001 | ||||
| 2 | lnBZC-APF | 0.148 | 0.051 | 0.004 | 1034 | 0.25 | |
| lncreatinine | 1.303 | 0.168 | <0.0001 | ||||
| 3 | lndermal | 0.134 | 0.053 | 0.012 | 1037 | 0.24 | |
| lncreatinine | 1.275 | 0.168 | <0.0001 | ||||
| 4 | lnBZC | 0.040 | 0.082 | 0.625 | 1039 | 0.24 | |
| lndermal | 0.112 | 0.070 | 0.108 | ||||
| lncreatinine | 1.275 | 0.168 | <0.0001 | ||||
| 5 | lnBZC-APF | 0.109 | 0.062 | 0.081 | 1037 | 0.27 | |
| lndermal | 0.072 | 0.064 | 0.266 | ||||
| lncreatinine | 1.298 | 0.168 | <0.0001 | ||||
| Worker var | 0.959 | ||||||
| Residual var | 1.769 | ||||||
| C. Effective T1/2 = 174 min | 1 | lnBZC | 0.150 | 0.069 | 0.031 | 1038 | 0.23 |
| lncreatinine | 1.263 | 0.168 | <0.0001 | ||||
| 2 | lnBZC-APF | 0.173 | 0.055 | 0.002 | 1033 | 0.26 | |
| lncreatinine | 1.300 | 0.168 | <0.0001 | ||||
| 3 | lndermal | 0.151 | 0.058 | 0.009 | 1036 | 0.25 | |
| lncreatinine | 1.267 | 0.168 | <0.0001 | ||||
| 4 | lnBZC | 0.068 | 0.086 | 0.431 | 1038 | 0.25 | |
| lndermal | 0.118 | 0.072 | 0.103 | ||||
| lncreatinine | 1.265 | 0.168 | <0.0001 | ||||
| 5 | lnBZC-APF | 0.132 | 0.064 | 0.039 | 1035 | 0.28 | |
| lndermal | 0.083 | 0.067 | 0.214 | ||||
| lncreatinine | 1.293 | 0.167 | <0.0001 | ||||
| Worker var | 0.930 | ||||||
| Residual var | 1.765 |
lnBZC = natural log-transformed BZC; lnBZC-APF = natural log-transformed respirator-adjusted BZC; lndermal = natural log-transformed dermal exposure level; lncreatinine = natural log-transformed creatinine concentration; var = variance.
Table 3.
Summary of (A) cumulative and (B–C) effective linear mixed effects models with estimated HDA half-life (T1/2) of 70 or 174 min for predicting natural log-transformed urine HDA levels normalized with creatinine in automotive spray painters exposed to HDI
| Exposure model | Exposure variable | Estimate | Standard error | P value | AIC | R2 | |
| A. Cumulative | 1 | lnBZC | 0.160 | 0.069 | 0.020 | 1039 | 0.05 |
| 2 | lnBZC-APF | 0.172 | 0.056 | 0.002 | 1035 | 0.08 | |
| 3 | lndermal | 0.153 | 0.058 | 0.009 | 1037 | 0.07 | |
| 4 | lnBZC | 0.079 | 0.087 | 0.364 | 1039 | 0.07 | |
| lndermal | 0.113 | 0.074 | 0.125 | ||||
| 5 | lnBZC-APF | 0.129 | 0.065 | 0.048 | 1036 | 0.10 | |
| lndermal | 0.085 | 0.068 | 0.211 | ||||
| Worker var | 0.931 | ||||||
| Residual var | 1.783 | ||||||
| B. Effective T1/2 = 70 min | 1 | lnBZC | 0.118 | 0.063 | 0.061 | 1040 | 0.04 |
| 2 | lnBZC-APF | 0.139 | 0.051 | 0.007 | 1037 | 0.07 | |
| 3 | lndermal | 0.131 | 0.053 | 0.013 | 1038 | 0.06 | |
| 4 | lnBZC | 0.040 | 0.083 | 0.633 | 1041 | 0.06 | |
| lndermal | 0.109 | 0.070 | 0.118 | ||||
| 5 | lnBZC-APF | 0.098 | 0.063 | 0.120 | 1039 | 0.08 | |
| lndermal | 0.075 | 0.065 | 0.250 | ||||
| Worker var | 0.959 | ||||||
| Residual var | 1.793 | ||||||
| C. Effective T1/2 = 174 min | 1 | lnBZC | 0.150 | 0.069 | 0.031 | 1039 | 0.05 |
| 2 | lnBZC-APF | 0.164 | 0.055 | 0.003 | 1035 | 0.08 | |
| 3 | lndermal | 0.150 | 0.058 | 0.009 | 1037 | 0.06 | |
| 4 | lnBZC | 0.069 | 0.086 | 0.427 | 1040 | 0.07 | |
| lndermal | 0.116 | 0.072 | 0.108 | ||||
| 5 | lnBZC-APF | 0.121 | 0.065 | 0.060 | 1037 | 0.10 | |
| lndermal | 0.087 | 0.067 | 0.194 | ||||
| Worker var | 0.933 | ||||||
| Residual var | 1.788 | ||||||
lnBZC = natural log-transformed BZC; lnBZC-APF = natural log-transformed respirator-adjusted BZC; lndermal = natural log-transformed dermal exposure; var = variance.
In addition, we used multiple linear mixed effects models to investigate the effect of dermal and inhalation exposure on urine HDA levels when (i) creatinine level was used as an independent variable in the model (Barr et al., 2005) and (ii) urine HDA level was normalized with creatinine concentration in the urine sample. The general form of model (ii) was
| (6) |
where Yij represents the natural logarithm of the urine HDA level normalized with creatinine concentration.
In summary, six sets of models were generated: one cumulative exposure model and two effective exposure models where creatinine level was used as an independent variable (models A–C; Table 2) and where HDA level was normalized by creatinine concentration in urine (models A–C; Table 3). Within these six sets of models, five submodels were built: (1) BZC, (2) BZC-APF, or (3) dermal levels as single exposure variables in a model and (4) dermal and BZC levels, or (5) dermal and BZC-APF levels as two exposure variables in a model to determine the significance of these predictors in any given model.
In addition, we used a marginal R2 statistic proposed by Vonesh and Chinchilli (1997) to assess the goodness-of-fit of fixed effects in our linear mixed models. Several R2 statistics have been proposed for assessing the goodness-of-fit of fixed effects for linear mixed models (Xu, 2003; Orelien and Edwards, 2008). However, marginal R2 statistics are more appropriate than conditional R2 statistics for estimating explained variability from fixed effects as marginal R2 statistics do not use random effects in the computation of predicted means that lead to residuals (Orelien and Edwards, 2008). Orelien and Edwards (2008) found this statistic to perform extremely well at differentiating between full and reduced models and not diverging when models were overfitted.
RESULTS
HDA levels ranged from nondetectable (158 samples of 417 analyzed) to 65.9 μg l−1 with an arithmetic mean and standard deviation (SD) of 0.54 μg l−1 and 3.32, respectively (Table 1). The maximum HDA level of 65.9 μg l−1 was 6-fold higher than the next highest level of 10.1 μg l−1. The geometric mean (GM) and geometric standard deviation (GSD) were 0.10 and 6.68 μg l−1, respectively. Creatinine concentrations ranged from 0.094 to 8.31 g l−1 with an arithmetic mean of 1.54 g l−1 (SD = 0.97) and a GM of 1.26 g l−1 (GSD = 2.01). The HDA levels normalized with creatinine concentration ranged from 0 to 21.6 μg g−1 creatinine with a arithmetic mean of 0.003 μg g−1 (SD = 0.012).
Table 1.
Summary of the automotive spray painters exposure to HDI and their urine HDA and creatinine levels
| N | Number of detects | GM | GSD | Range | |
| HDI dermal (ng m−2) | 276 | 101 | 38.3 | 7.95 | <LOD–121 000 |
| HDI BZC (ng) | 272 | 250 | 29.4 | 6.39 | <LOD–1480 |
| HDI BZC–APF (ng) | 272 | 250 | 0.25 | 12.4 | <LOD–148 |
| HDA (μg l−1) | 417 | 259 | 0.10 | 6.68 | <MDL–65.9 |
| HDA first of day (μg l−1) | 120 | 67 | 0.08 | 6.71 | <MDL–7.75 |
| HDA first of Monday (μg l−1) | 19 | 7 | 0.04 | 5.07 | <MDL–0.51 |
| Creatinine (g l−1) | 417 | 417 | 1.26 | 2.01 | 0.09–8.32 |
N = number of measurements; GM = geometric mean; GSD = geometric standard deviation; LOD = 1.68 ng per tape-strip or air filter. BZC refers to the mass of HDI collected on the air filter during the exposure period; MDL = 0.04 μg l−1 for urine HDA.
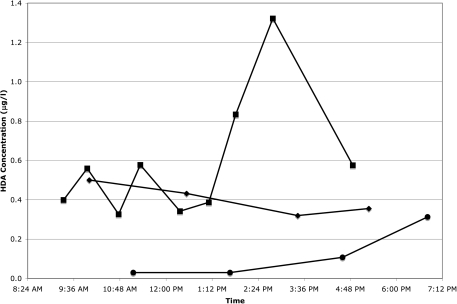
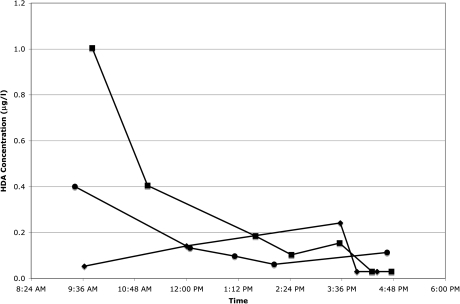
Of the 120 worker-day sample sets, 67 had detectable HDA in the first urine sample of the day (arithmetic mean = 0.34 μg l−1, SD = 0.82). Seven of these occurred on Monday morning (arithmetic mean = 0.12 μg l−1, SD = 0.16). HDI exposure was known to have occurred before the first urine sample was collected in only 3 of the 67 cases. In 43 of the 120 worker-day urine sample sets, the first sample of the day had a higher HDA level (without creatinine normalization) than the last sample of the day. Contrary to our expectations, when HDA levels were examined against collection time for individual subjects, we discovered that HDA levels did not always steadily increase with exposure and time. Figures 1 and 2 are examples of the variation of HDA levels without creatinine normalization during a workday. HDA levels normalized with creatinine concentration showed similar variation (data not shown).
Fig. 1.
Level of HDA in urine samples from a painter in Washington state throughout the workday on Visit 1 (filled circles), Visit 2 (filled diamonds), and Visit 3 (filled squares).
Fig. 2.
Level of HDA in urine samples from painter in Washington State throughout the workday on Visit 1 (filled circles), Visit 2 (filled diamonds), and Visit 3 (filled squares). Worker did not paint on Visit 1.
No HDA was detected in any of the urine samples collected from three of the five workers who were not painting on the day of sampling. One worker had detectable HDA levels (1.43 and 1.04 μg l−1) in both of his urine samples from that day. Another worker had detectable HDA levels in all five of his urine samples from that day, ranging from 0.06 to 0.40 μg l−1 (Fig. 2, Visit 1). The highest HDA level occurred in the first sample of the day at 9:25 am, and the levels steadily decreased during the day to the lowest level of 0.06 μg l−1 at 2:02 pm. The final sample of the day taken at 4:39 pm had a level of 0.11 μg l−1.
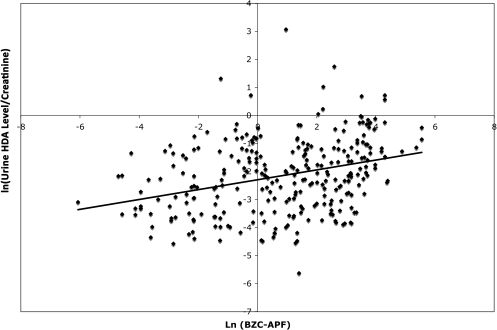
Mixed effects models
The results of the linear mixed effects models with creatinine as an independent variable and creatinine-normalized HDA levels are provided in Tables 2 and 3, respectively. Model A-2 relating BZC-APF to creatinine-normalized HDA is represented graphically in Fig. 3. The respective cumulative (A) and effective exposure (B–C) models with creatinine as an independent variable (Table 2) or creatinine-normalized HDA levels (Table 3) yielded similar results. Creatinine concentration, when used as an independent variable, was always a highly significant variable (P < 0.0001) with parameter estimates ranging from 1.25 to 1.30 (Table 2). The Akaike's Information Criterion (AIC) is also provided for each model; a smaller AIC indicates a better fitting model. The respective models with creatinine as an independent variable or creatinine-normalized HDA levels gave similar AICs. However, the marginal R2 for the models with creatinine as an independent variable were 3- to 4-fold higher (0.22–0.28) than the respective marginal R2 for the models with creatinine-normalized HDA levels (0.04–0.10).
Fig. 3.
The relationship between natural log-transformed BZC–APF exposure and natural log-transformed urine HDA levels adjusted for creatinine in samples collected from 48 spray painters exposed to HDI in North Carolina and Washington State. The line indicates the best-fit linear regression (R2 = 0.10). (Note: Since multiple imputation creates 10 different datasets, for this figure, all nondetectable levels of urine HDA and BZC were assigned a value of the LOD/√2 instead of multiple imputation used for the models.)
Dermal, BZC, and BZC-APF estimates were all significant (P ≤ 0.06) when modeled as single exposure variables in the model (submodels 1–3; Tables 2 and 3). BZC-APF estimates were more significant (P values 0.001–0.007) than BZC (P values 0.02–0.06) indicating that adjustment of BZC with APF provided a better fit for the model. Both dermal and BZC were insignificant predictors of urine HDA level when placed in the same model (P values ≥ 0.1; submodel 4; Tables 2 and 3). The models where BZC-APF was the only exposure variable (submodel 2; Tables 2 and 3) had the lowest AIC, and the dermal (submodel 3; Tables 2 and 3) and BZC-APF and dermal models (submodel 5; Tables 2 and 3) had the next lowest AIC. The models including both dermal and BZC-APF provided the best overall model fit because these models had a low AIC and had the highest R2s. The AIC indicated that the models, which did not include the dermal exposure level, had the tightest fit with an AIC of 2 or 3 points lower than the models including dermal exposure level. However, the models that included the dermal exposure level provided the best goodness-of-fit for the fixed effects based on the R2 value, an index of how well the model explains the dependent variable. The inter- and intraperson variance estimates are provided for these models only (submodel 5; Tables 2 and 3). Interestingly, the parameter estimates and P values for BZC-APF, dermal, and creatinine were almost identical in the cumulative exposure (model A) and the 174 min half-life model (model C; Tables 2 and 3). However, dermal exposure was an insignificant predictor (P values ≥ 0.2) while BZC-APF was always a significant predictor (P values ≤ 0.08) except in the effective exposure model B with creatinine-normalized HDA levels (P value 0.12; Table 3).
We suspected that the insignificance of dermal exposure when modeled jointly with BZC-APF may be due to the large number of nondetectable levels of HDI in the dermal tape-strip samples. Therefore, we investigated these models with a subset of the subjects, who did not wear coveralls or gloves while painting and whose dermal exposure levels were above the LOD. This subset comprised 12 individuals (23 worker days) and a total of 47 urine samples. Interestingly, in the cumulative exposure model, dermal exposure alone with creatinine concentration in the model was significant (P value 0.03; R2 = 0.37) while BZC-APF alone with creatinine concentration was borderline significant (P value 0.10; R2 = 0.33). However, when both dermal and BZC-APF exposure along with creatinine concentration were included in the model (R2 = 0.37), neither dermal (P value 0.21) nor BZC-APF (P value 0.71) exposure was significant.
DISCUSSION
We observed that both dermal and inhalation exposure were significant predictors of urine HDA levels when used as single exposure variables in the multiple linear regression analysis (Tables 2 and 3). However, when BZC-APF and dermal exposure were considered in the same model, BZC-APF was always a significant predictor while dermal exposure was insignificant. This may reflect the fact that 64% of the dermal samples collected were below the LOD compared to 9% of the BZC samples, thereby allowing a more robust estimation of the model fit between BZC and urine HDA levels. On the contrary, in a subset of workers (n = 12) who did not wear gloves or coveralls and whose dermal exposure levels were above the LOD, neither BZC-APF nor dermal exposure was significant when placed into the same model. However, a significant relationship between dermal exposure and a borderline significant relationship between BZC-APF and urine HDA levels were observed in the cumulative exposure model when modeled alone with creatinine concentration.
When interpreting these results, caution is warranted due to the limitations of the methods used to measure dermal and inhalation exposure. First, the method employed to calculate the exposure period may have slightly biased the inhalation exposure estimates. The total task period monitored reflected the duration of time the air-sampling pump was operating and not the actual time worker was in contact with HDI-containing paints. Second, estimation of the inhalation exposure from BZC by adjusting for respirator APF is challenging and does not account for improper fit and/or maintenance. Adjustment with APF provides reliable protection estimates only when a respiratory protection program is employed that includes proper training, fit testing, maintenance, and use requirements (as described in OSHA, 2006). Unfortunately, we did not have means to test the respiratory protection during this study, and it is unknown how rigorously the workers followed these requirements. However, our data indicated that adjustment of inhalation exposure level with the APF provided a better model fit than BZC without adjustment. The APF adjustment used assumes that all similar type respirators provide the same level of protection. For example, it is assumed that all half-face cartridge-type respirators reduce the BZC by a factor of 10. However, there may be considerable variation in the protection level of the individual respirators based on the mask fit, the cartridge change schedule, and even manufacturers. Therefore, the correlation between urine HDA level and inhalation exposure to HDI, adjusted by APF, should be confirmed in an investigation in which the workers’ respiratory protection programs are evaluated. Third, the poor correlation between urine HDA level and dermal HDI exposure may be affected by rapid absorption of HDI through the stratum corneum and/or conjugation of HDI to macromolecules in the skin, and thus contributing to the large number of nondetectable samples collected from the skin even though the tape-strips were collected immediately after each paint task. Further investigation on the dermal absorption of HDI and binding to macromolecules in human skin are warranted to increase our understanding of the contribution of this exposure route to urine HDA levels and potential ill effects due to dermal exposure.
All models in which creatinine concentration was accounted for as an independent variable along with inhalation and dermal exposure showed it to be a highly significant variable (P < 0.0001; Table 2). This indicates that the exposure models need to include creatinine concentration to account for the water content of urine. Furthermore, the parameter estimates and P values of all variables were similar when un-normalized models were compared to the normalized models (Tables 2 and 3). If the creatinine concentration had a parameter estimate of 1 in the model, the model with creatinine as an independent variable would have been equivalent to the model with creatinine-normalized HDA levels. However, the creatinine parameter estimates ranged from 1.25 to 1.30 in the un-normalized models implying that normalization of urine HDA level with creatinine concentration may attenuate the external exposure biomarker relationship. The models with creatinine as an independent variable also had much higher marginal R2s than models with traditional normalization. Since the marginal R2 is computed with only fixed effect components (i.e. no random effect components are included), much of the variance in the urine HDA levels may have been caused by variance in the creatinine concentrations. In the models where creatinine is an independent variable, any variance due to the creatinine is modeled as a fixed effect for urine HDA. However, in the traditional normalization models, any creatinine variance is included in variance for the overall model, and thus not modeled with the fixed effects. Therefore, as proposed by Barr et al. (2005) and as indicated by this study, creatinine concentration should be used as an independent variable in linear mixed models, rather than the traditionally used method of normalization of urine HDA level with creatinine concentration.
Significance of both dermal and BZC-APF exposure increased with increasing half-life estimate for HDA level, and the significances in the 174 min (2.9 h) half-life model (D) were similar to the cumulative exposure model (model A; Tables 2 and 3). A similar half-life was also observed by Liu et al. (2004) who estimated a half-life of 2.8 h for subjects exposed to HDI vapor using a method that precluded dermal exposure. Our observation is also similar to the 2.5 h half-life derived from a study in which both dermal and inhalation exposure occurred (Tinnerberg et al., 1995).
As illustrated in Fig. 2, some workers exhibited decreasing urine HDA levels over the course of a day. The reason for this is unclear but may reflect biphasic elimination kinetics. A slow clearance phase would also explain the detectable HDA levels in the 64 (53%) first urine samples of the day when exposure was not known to have occurred yet. It is possible that some of the first urine samples of the day with detectable HDA level may have reflected HDI exposure received the day before. However, calculation using the GM HDA level of the first urine samples (0.08 μg l−1) along with the estimated half-life of 2.9 h and assuming that the first urine sample was taken at 8 am, point to a urine HDA level of 2.89 μg l−1 at 5 pm the day before. Since the GM HDA level of the last urine samples of the day was 0.14 μg l−1 and 97% of all last urine samples were below 2.89 μg l−1, this would appear to be an unlikely scenario. The results of these calculations are similar for creatinine-normalized HDA levels. In addition, secondary exposure to HDI (i.e. not due to painting) may also contribute to the morning HDA levels. The secondary exposure may be caused by touching contaminated surfaces or entering the mixing area without dermal or inhalation protection. The detectable HDA levels in all the urine samples of two of the five workers who were monitored on days when they were not painting may be explained by either biphasic elimination of HDA or secondary exposure to HDI (i.e. not due to painting).
Further evidence for the biphasic elimination kinetics is provided by the seven workers who had detectable HDA levels in their first urine sample before exposure occurred on a Monday. While it is conceivable that some of the painters worked on Sundays or on weeknights, 36 different subjects had at least one urine sample with detectable HDA before exposure was known to have occurred and, thus, it seems unlikely that this many painters would have had a second employment in another painting facility. As evidenced by Figs. 1 and 2 and the estimated half-life of 2.9 h for HDA, collecting one urine sample at the end of the workday may not provide a reliable exposure estimate for that day. Furthermore, a biphasic elimination pattern is indicated as seen with TDA (Brorson et al., 1991; Tinnerberg et al., 1995; Lind et al., 1996; Lind et al., 1997). Therefore, further studies are needed to investigate the potential biphasic elimination kinetics of HDA in spray painters and to determine the half-life of the slow elimination phase.
There are no regulatory guidelines regarding safe HDA levels in urine in the USA. In the UK, however, a biological monitoring guidance level of 1 μmol HDA per mole creatinine exists (HSE, 2006). Among our subjects, the average HDA level was 0.29 μmol HDA per mole creatinine and the median value was 0.10 μmol HDA per mole creatinine. This indicates that by UK standards, the subjects are adequately protected. It should be noted that this guidance value is based on an recommendation for good occupational hygiene practices achievable by most of UK industry and not based on protection from health effects caused by HDI exposure (HSE, 2006). This guidance document also states that urine samples should be collected immediately after the task or shift. In several diisocyanate studies, only one urine sample per worker was collected at the end of the workday (Maitre et al., 1996; Rosenberg et al., 2002; Sabbioni et al., 2007). Our results indicate that this methodology may miss the timeframe when the highest diamine level after exposure occurs. As indicated by our results and stated in the HSE guidelines, the optimal sampling strategy would be collection and analysis of all urine voids during the day. This, however, may prove to be impractical, and thus collecting a urine sample within 2 h after exposure ceased may be sufficient for monitoring HDA as a biomarker for HDI exposure in an occupational exposure setting.
The quantitative relationship between dermal and inhalation exposure to HDI and urine HDA levels has not been characterized previously. Here, we report a significant association between inhalation and dermal exposure to HDI and urine HDA level in occupationally exposed workers. The results indicate biphasic elimination kinetics with a fast phase of 2.9 h. Further study is necessary to determine the half-life of the slow phase. The results of our investigation indicate that biological monitoring of the workers should be tailored to reliably capture the intermittent exposure pattern typical in this industry as well as the use of personal protective equipment. Furthermore, when urine HDA levels are used as biomarkers of exposure in this occupational group, urine creatinine concentration should be included as an independent variable in subsequent statistical exposure assessment analysis.
FUNDING
National Institute for Occupational Safety and Health (R01-OH007598, T42/CCT422952, and T42 OH008673); National Institute of Environmental Health Sciences (P30ES10126 and T32 ES007018); American Chemistry Council (RSK0015-01).
Acknowledgments
The authors are grateful to the automotive repair shop workers who volunteered to participate in this study and to Diana Ceballos (University of Washington) and Robert Anderson (Safety and Health Assessment and Research for Prevention Program) for their help in collecting the samples in Washington State.
References
- Almeida AM, Castel-Branco MM, Falcao AC. Linear regression for calibration lines revisited: weighting schemes for bioanalytical methods. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;774:215–22. doi: 10.1016/s1570-0232(02)00244-1. [DOI] [PubMed] [Google Scholar]
- Austin S. Biological monitoring of TDI-derived amines in polyurethane foam production. Occup Med (Lond) 2007;57:444–8. doi: 10.1093/occmed/kqm085. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello D, Herrick CA, Smith TJ, et al. Skin exposure to isocyanates: reasons for concern. Environ Health Perspect. 2007;115:328–35. doi: 10.1289/ehp.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berode M, Testa B, Savolainen H. Bicarbonate-catalyzed hydrolysis of hexamethylene diisocyanate to 1,6-diaminohexane. Toxicol Lett. 1991;56:173–8. doi: 10.1016/0378-4274(91)90104-e. [DOI] [PubMed] [Google Scholar]
- Brorson T, Skarping G, Nielsen J. Biological monitoring of isocyanates and related amines. II. Test chamber exposure of humans to 1,6-hexamethylene diisocyanate (HDI) Int Arch Occup Environ Health. 1990;62:385–9. doi: 10.1007/BF00381369. [DOI] [PubMed] [Google Scholar]
- Brorson T, Skarping G, Sango C. Biological monitoring of isocyanates and related amines. IV. 2,4- and 2,6-toluenediamine in hydrolysed plasma and urine after test-chamber exposure of humans to 2, 4- and 2,6-toluene diisocyanate. Int Arch Occup Environ Health. 1991;63:253–9. doi: 10.1007/BF00386374. [DOI] [PubMed] [Google Scholar]
- Cocker J. Biological monitoring for isocyanates. Occup Med (Lond) 2007;57:391–3. doi: 10.1093/occmed/kql148. [DOI] [PubMed] [Google Scholar]
- Dalene M, Skarping G, Lind P. Workers exposed to thermal degradation products of TDI- and MDI-based polyurethane: biomonitoring of 2,4-TDA, 2,6-TDA, and 4,4'-MDA in hydrolyzed urine and plasma. Am Ind Hyg Assoc J. 1997;58:587–91. doi: 10.1080/15428119791012522. [DOI] [PubMed] [Google Scholar]
- EPA. Statistical protocol for the determination of the single-laboratory lowest concentration minimum reporting level (LCMRL) and validation of laboratory performance at or below the minimum reporting level (MRL) US EPA Office of Ground Water and Drinking Water Standards and Risk Management Division; 2004. EPA, Cincinnati, OH. Document # 815-R-05–006. [Google Scholar]
- Exocell. The creatinine companion. Philadelphia, PA: Exocell: 2004. [Google Scholar]
- Fent KW, Gaines LG, Thomasen JM, et al. Quantification and statistical modeling—part I: breathing-zone concentrations of monomeric and polymeric 1,6-hexamethylene diisocyanate. Ann Occup Hyg. 2009a;53:677–89. doi: 10.1093/annhyg/mep046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent KW, Gaines LG, Thomasen JM, et al. Quantification and statistical modeling—part II: dermal concentrations of monomeric and polymeric 1,6-hexamethylene diisocyanate. Ann Occup Hyg. 2009b;53:691–702. doi: 10.1093/annhyg/mep048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent KW, Jayaraj K, Ball LM, et al. Quantitative monitoring of dermal and inhalation exposure to 1,6-hexamethylene diisocyanate monomer and oligomers. J Environ Monit. 2008;10:500–7. doi: 10.1039/b715605g. [DOI] [PubMed] [Google Scholar]
- Flack S, Ball LM, Nylander-French LA. Occupational exposure to HDI: progress and challenges in biomarker analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2010 doi: 10.1016/j.jchromb.2010.01.012. doi:10.1016/j.jchromb.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSE. Urine sampling for isocyanate exposure measurement. Health and Safety Executive; 2006. http://www.hse.gov.uk/pubns/guidance/g408.pdf. Accessed 20 September 2009. [Google Scholar]
- Kaaria K, Hirvonen A, Norppa H, et al. Exposure to 2,4- and 2,6-toluene diisocyanate (TDI) during production of flexible foam: determination of airborne TDI and urinary 2,4- and 2,6-toluenediamine (TDA) Analyst. 2001a;126:1025–31. doi: 10.1039/b102022f. [DOI] [PubMed] [Google Scholar]
- Kaaria K, Hirvonen A, Norppa H, et al. Exposure to 4,4'-methylenediphenyl diisocyanate (MDI) during moulding of rigid polyurethane foam: determination of airborne MDI and urinary 4,4'-methylenedianiline (MDA) Analyst. 2001b;126:476–9. doi: 10.1039/b009549o. [DOI] [PubMed] [Google Scholar]
- Lind P, Dalene M, Skarping G, et al. Toxicokinetics of 2,4- and 2,6-toluenediamine in hydrolysed urine and plasma after occupational exposure to 2,4- and 2,6- toluene diisocyanate. Occup Environ Med. 1996;53:94–9. doi: 10.1136/oem.53.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind P, Dalene M, Tinnerberg H, et al. Biomarkers in hydrolysed urine, plasma and erythrocytes among workers exposed to thermal degradation products from toluene diisocyanate foam. Analyst. 1997;122:51–6. doi: 10.1039/a606148f. [DOI] [PubMed] [Google Scholar]
- Liu Y, Berode M, Stowe MH, et al. Urinary hexane diamine to assess respiratory exposure to hexamethylene diisocyanate aerosol: a human inhalation study. Int J Occup Environ Health. 2004;10:262–71. doi: 10.1179/oeh.2004.10.3.262. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–6. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre A, Berode M, Perdrix A, et al. Urinary hexane diamine as an indicator of occupational exposure to hexamethylene diisocyanate. Int Arch Occup Environ Health. 1996;69:65–8. doi: 10.1007/BF02630741. [DOI] [PubMed] [Google Scholar]
- Murray R. Creatinine. In: Pesce A, Kaplan L, editors. Methods in clinical chemistry. St Louis, MO: CV Mosby Co; 1987. pp. 10–7. [Google Scholar]
- Orelien JG, Edwards LJ. Fixed-effect variable selection in linear mixed models using R2 statistics. Comput Stat Data Anal. 2008;52:1896–907. [Google Scholar]
- OSHA. Respiratory protection–1910.134, Regulation (Standards - 29 CFR) U.S. Department of Labor; 2006. http://www.osha.gov/. Accessed 15 September 2008. [Google Scholar]
- Pauluhn J, Lewalter J. Analysis of markers of exposure to polymeric methylene-diphenyl diisocyanate (pMDI) in rats: a comparison of dermal and inhalation routes of exposure. Exp Toxicol Pathol. 2002;54:135–46. doi: 10.1078/0940-2993-00242. [DOI] [PubMed] [Google Scholar]
- Pronk A, Yu F, Vlaanderen J, et al. Dermal, inhalation and internal exposure to 1,6-HDI and its oligomers in car body repair shop workers and industrial spray painters. Occup Environ Med. 2006;63:624–31. doi: 10.1136/oem.2005.023226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C, Nikkila K, Henriks-Eckerman ML, et al. Biological monitoring of aromatic diisocyanates in workers exposed to thermal degradation products of polyurethanes. J Environ Monit. 2002;4:711–6. doi: 10.1039/b206340a. [DOI] [PubMed] [Google Scholar]
- Sabbioni G, Wesp H, Lewalter J, et al. Determination of isocyanate biomarkers in construction site workers. Biomarkers. 2007;12:468–83. doi: 10.1080/13547500701395636. [DOI] [PubMed] [Google Scholar]
- Sennbro CJ, Lindh CH, Mattsson C, et al. Biological monitoring of exposure to 1,5-naphthalene diisocyanate and 4,4'-methylenediphenyl diisocyanate. Int Arch Occup Environ Health. 2006;79:647–53. doi: 10.1007/s00420-006-0096-5. [DOI] [PubMed] [Google Scholar]
- Sennbro CJ, Lindh CH, Tinnerberg H, et al. Development, validation and characterization of an analytical method for the quantification of hydrolysable urinary metabolites and plasma protein adducts of 2,4- and 2,6-toluene diisocyanate, 1,5-naphthalene diisocyanate and 4,4'-methylenediphenyl diisocyanate. Biomarkers. 2003;8:204–17. doi: 10.1080/1354750031000090660. [DOI] [PubMed] [Google Scholar]
- Sennbro CJ, Lindh CH, Tinnerberg H, et al. Biological monitoring of exposure to toluene diisocyanate. Scand J Work Environ Health. 2004;30:371–8. doi: 10.5271/sjweh.825. [DOI] [PubMed] [Google Scholar]
- Sepai O, Henschler D, Sabbioni G. Albumin adducts, hemoglobin adducts and urinary metabolites in workers exposed to 4,4'-methylenediphenyl diisocyanate. Carcinogenesis. 1995;16:2583–7. doi: 10.1093/carcin/16.10.2583. [DOI] [PubMed] [Google Scholar]
- Skarping G, Brorson T, Sango C. Biological monitoring of isocyanates and related amines. III. Test chamber exposure of humans to toluene diisocyanate. Int Arch Occup Environ Health. 1991;63:83–8. doi: 10.1007/BF00379069. [DOI] [PubMed] [Google Scholar]
- Tinnerberg H, Skarping G, Dalene M, et al. Test chamber exposure of humans to 1,6-hexamethylene diisocyanate and isophorone diisocyanate. Int Arch Occup Environ Health. 1995;67:367–74. doi: 10.1007/BF00381050. [DOI] [PubMed] [Google Scholar]
- Vonesh E, Chinchilli V. Linear and nonlinear models for the analysis of repeated measurements. New York, NY: Marcel Dekker; 1997. [Google Scholar]
- Xu R. Measuring explained variation in linear mixed effects models. Stat Med. 2003;22:3527–41. doi: 10.1002/sim.1572. [DOI] [PubMed] [Google Scholar]
- Yeh HJ, Lin WC, Shih TS, et al. Urinary excretion of toluene diisocyanates in rats following dermal exposure. J Appl Toxicol. 2008;28:189–95. doi: 10.1002/jat.1266. [DOI] [PubMed] [Google Scholar]