Abstract
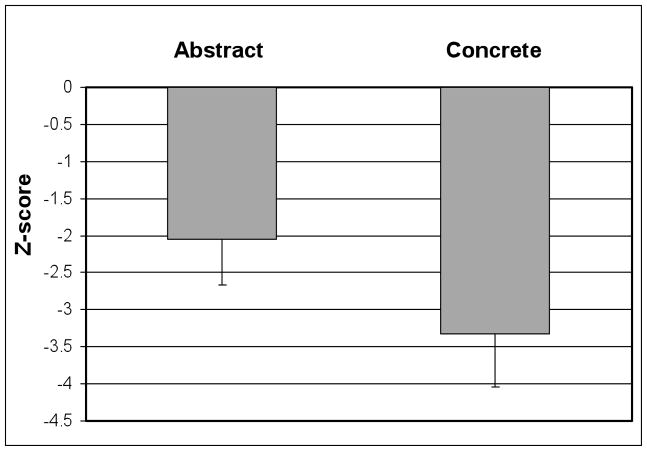
Patients with semantic dementia (SD) have a striking impairment in semantic memory, but the basis for this deficit is unclear. We examined semantic memory for concrete and abstract verbs with a two-alternative, forced-choice measure of lexical semantic associative knowledge. Patients with SD had significantly greater difficulty with concrete verbs (z = −3.33) than abstract verbs (z = −2.05), a “reversal of the concreteness effect” that was present in a majority of individual patients. The subgroup of SD patients with imaging had significant cortical thinning in the anterior and inferolateral portions of the temporal lobes. These areas of visual association cortex may be important for storing and processing visual features for word meaning. Moreover, poor performance with concrete relative to abstract verbs correlated with cortical thinning of the right anterior temporal lobe in SD, suggesting that this region may contribute to storing and processing visual semantic features. These observations raise the possibility that degraded visual feature knowledge contributes in part to the impaired comprehension of concrete words in SD.
Keywords: semantic, semantic dementia, verb
INTRODUCTION
Semantic memory is the long-term representation of the meaning of words, objects, actions and the like. Functional neuroimaging investigations of semantic memory have provided much evidence in support of a “sensory-motor” approach to the study of the semantic system (reviewed in Barsalou, 2008; Barsalou, Simmons, Barbey, & Wilson, 2003; Martin, 2007; Pulvermuller, 2005). According to a strong version of this approach, representations of word meaning rely on sensory-perceptual and motor-action features that constitute the word’s referent. For example, an apple is a small, red, roundish, sweet-smelling, pulpy-textured object, and the representation of these features is critical in understanding APPLE. Other objects are distinguished from APPLE, partly because they differ in their sensory-motor feature associations. Moreover, each of these features is represented in a region of the brain that is in or adjacent to the region that processes the perception of that feature. Thus the color feature of APPLE is represented in the ventral temporal-occipital portion of visual association cortex where color is processed. The shape feature of APPLE is represented more anteriorly in visual association cortex where shape is processed. And the size feature of APPLE is represented in the inferior parietal region that contributes to processing spatial properties of objects. Evidence to support this approach comes from fMRI studies that show activation of different brain regions for each of the perceptual features associated with a concept (Binder, Liebenthal, Possing, Medler, & Ward, 2004; Binder, Westbury, McKiernan, Possing, & Medler, 2005; Chao, Haxby, & Martin, 1999; Chao & Martin, 2000; Hauk, Johnsrude, & Pulvermuller, 2004; Kan, Barsalou, Solomon, & Thompson-Schill, 2003; Kellenbach, Brett, & Patterson, 2001; Simmons, Martin, & Barsalou, 2005; Simmons et al., 2007). From this perspective, the neural representation of a concept like APPLE relies on the activity of brain regions where its perceptual features are stored.
As with all functional neuroimaging studies of healthy participants, the fMRI evidence in support of the sensory-motor approach is correlational. It is therefore crucial to look for converging data from patients with semantic deficits for concrete concepts like APPLE. Patient studies consistent with these fMRI observations would suggest a necessary role for specific brain regions in conceptual representations of object and action concepts. An important source of such data are patients with semantic dementia (SD). As implied by the name of this syndrome, SD appears to cause a profound deficit in semantic memory (Hodges & Patterson, 2007). In characterizing the semantic memory deficit in SD, some previous studies have demonstrated a “reversal of the concreteness effect” (Breedin, Saffran, & Coslett, 1995; Cipolotti & Warringtin, 1995; Reilly, Cross, Troiani, & Grossman, 2007; Warrington, 1975, 1981; Yi, Moore, & Grossman, 2007), although this has not been observed by all investigators (Jefferies et al, 2009). This is a deficit for words with concrete referents relative to words with abstract referents, and it is a reversal of the behavioral performance pattern overwhelmingly observed in healthy individuals (known as the “concreteness effect”). The “concreteness effect” is a robust behavioral finding of faster and more accurate performance for concrete words than for abstract words on a range of lexical and semantic tasks. This effect is highly consistent in healthy participants (reviewed in Paivio, 1991) and can be more pronounced in some brain-damaged patient groups (Coltheart, 1980; Goodglass, Hyde, & Blumstein, 1969; Jefferies, Baker, Doran, & Lambon Ralph, 2007). “Reversal of the concreteness effect” in SD may stem from degraded visual feature representations in temporal cortex. Indeed, patients with SD typically exhibit disease in anterior and inferior regions of the left and right temporal lobes that may encompass visual association cortex (Gorno-Tempini, Dronkers, Rankin, Ogar, Phengrasamy, Rosen, et al. 2004; Grossman McMillan, Moore, Ding, Glosser, Work, et al., 2004; Mummery, Patterson, Price, & Hodges,, 2000).
Other work in SD has suggested that the anterior temporal lobes contain an amodal “hub” that integrates the sensory-perceptual and motor-action features of a concept in semantic memory (Patterson, Nestor & Rogers, 2007). While the amodal hub may be a crucial component of the semantic system, the existence of such a hub does not contradict the main argument of the sensory-motor approach to semantic memory. This is because the semantic features that the hub integrates are represented in perceptual and motor regions of cortex, just as they are from the sensory-motor approach.
In a previous study we demonstrated a “reversal of the concreteness effect” for concrete verbs in a group of SD patients (Yi, Moore, & Grossman, 2007). Given the typical pattern of cortical disease in SD, we speculated that this finding was due to the degradation of visual-perceptual features that contribute to knowledge of concrete words. The present study reproduces the finding of a concrete relative to abstract verb deficit in SD patients using a new task. We assessed verbs rather than nouns because the previous study demonstrated greater sensitivity on concreteness assessments for verbs. We found that SD patients have a significantly greater impairment for concrete than abstract verbs on a simple test of associative knowledge. Moreover, in the present study we extend our previous observations by examining cortical atrophy quantitatively using structural MRI in the subgroup of SD patients with imaging data. In addition to demonstrating the typical pattern of anterior temporal atrophy, we found that poor performance with concrete relative to abstract verbs correlated with cortical atrophy of the right anterior temporal lobe. These findings suggest that the semantic memory deficit in SD is due in part to the degraded representation of visual features of concepts. These findings offer converging evidence for the sensory-motor theory of semantic memory.
METHODS
Participants
We studied 11 participants diagnosed with a relatively mild neurodegenerative disease in the Department of Neurology at the Hospital of the University of Pennsylvania. These patients were diagnosed with SD, a form of frontotemporal dementia (FTD), according to a modification of published criteria by a cognitive neurologist with experience assessing dementia patients (McKhann et al., 2001; Neary et al., 1998). These patients presented with naming difficulty, frequent circumlocutions and word-finding pauses in their spontaneous speech, together with comprehension difficulty for single words and objects. Diagnosis was confirmed in a consensus conference based on a review of a semi-structured history, a comprehensive mental status exam, and a complete neurological exam by at least two independent, trained reviewers. If the reviewers disagreed in their diagnosis, consensus was established through open discussion. A control group of 16 age- and education-matched healthy adult volunteers was also studied. Table 1 summarizes the demographic and clinical characteristics of the participants. All participants and their legal representatives participated in an informed consent procedure approved by the IRB at the University of Pennsylvania.
TABLE 1.
CLINICAL AND DEMOGRAPHIC CHARACTERISTICS OF PARTICIPANTS
| Semantic Dementia | Healthy Seniors | |
|---|---|---|
| n | 11 | 16 |
| Age (yrs)1 | 69.4 (9.0) | 72.3 (7.9) |
| Education (yrs)1 | 15.7 (2.8) | 15.9 (1.9) |
| MMSE (max = 30)1, 2 | 21.1 (6.4) | 29.1 (1.1) |
| Pyramid and Palm Tree (% correct)3 | 83.8 (10.7) | 96.9 (1.4) |
| Semantic Categorization (% correct)3 | 74.8 (16.4) | 93.3 (5.4) |
NOTE
Patients and controls were matched for age (t(25)=0.90; ns) and education (t(25)=0.16; ns), but patients were more impaired than controls according to the MMSE ((t(25)=3.92; p<.005).
Due to an oversight, MMSE was not collected at the time of assessment in one patient.
Performance in patients differs from controls on the cognitive measures at least at the p<0.05 level, according to Neuman-Keuls procedure.
We also examined performance on a general measure of dementia severity and two measures of semantic memory for objects. These included:
Mini Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975), a general measure of dementia severity;
Pyramid and Palm Trees Associative Knowledge (P&P) (Howard & Patterson, 1992), a two-alternative, forced-choice measure of object associative knowledge; we report percent correct for the word form;
Semantic Categorization test (Grossman et al., 1997), a true/false judgment of whether a printed noun or a photograph of an object is a member of a target superordinate; we report percent correct.
As summarized in Table 1, patients with SD were significantly impaired on these measures relative to controls.
Materials
We evaluated verb associative knowledge using concrete verbs (n=20) and abstract verbs (n=20) from the Verb Similarity Test (VST) (Price & Grossman, 2005). The VST uses a forced-choice, two-alternative format identical to the Pyramids and Palm Trees Test (PPT), a measure of noun associative knowledge (Howard & Patterson, 1992). This task presents a target word centrally above two choice words. The participant is required to choose the word most similar in meaning to the target. The forced-choice responses for the task were identified from a local normative database in which native English-speaking undergraduate students (n=86) were asked to listen to each target verb and immediately list at least five associated verbs. The participants were provided with practice examples (e.g., The verb sneeze might generate the verbs blow, sniff…) prior to administration of the task. The correct answer and competing foils were selected according to the frequency of the response to each target: The correct answer occurred very frequently but the foil occurred rarely. Imageability, age of acquisition and familiarity ratings for all target verbs were obtained from 11 control adults using Likert scales. The stimuli from the VST with the 20 highest and 20 lowest imageability ratings were used as the concrete and abstract verbs, respectively, in this task. Concrete verbs had significantly higher imageability ratings than abstract verbs [t(38) = 16.5; p<0.001]. Concrete and abstract verbs did not differ significantly for familiarity, but concrete verbs were acquired significantly earlier than abstract verbs [t(38)=2.63, p<0.05]. We confirmed that the stimuli were verbs with a frequency count sensitive to major grammatical subcategory (Francis & Kucera, 1982), where they had at least a 5:1 verb/noun ratio. Test trials of the task with normal controls demonstrated that performance was significantly above chance for each of the 40 items. Appendix A summarizes the final set of VST items classified by semantic category. In the version of these stimuli prepared for administration to patients, abstract and concrete items were randomly ordered and presented in a fixed order. The right vs. left location of the correct responses was prepared in a pseudo-random manner so that half of the correct responses for each type of verb were on the left and half on the right.
APPENDIX A.
VERB SIMILARITY JUDGMENT TASK STIMULI
| Target | Imageability Group | Frequency Group | Judgment A | Judgment B |
|---|---|---|---|---|
| Hop | c | lo | Jump | Tumble |
| Capture | c | lo | Apprehend | Manage |
| Peek | c | lo | Glare | Glance |
| Thank | a | hi | Acknowledge | Realize |
| Cling | c | lo | Rub | Adhere |
| Rescue | c | hi | Nourish | Save |
| Confirm | a | hi | Verify | Finish |
| Assess | a | lo | Predict | Evaluate |
| Admire | a | hi | Envy | Like |
| Put | a | hi | Place | Recline |
| Shake | c | hi | Turn | Vibrate |
| Advise | a | hi | Offer | Direct |
| Notice | a | hi | Look | Recognize |
| Peer | a | lo | Squint | Poke |
| Bring | c | hi | Deliver | Transfer |
| Collect | c | hi | Gather | Grab |
| Fall | c | hi | Flip | Descend |
| Stumble | c | lo | Falter | Injure |
| Slump | c | lo | Surround | Slouch |
| Mash | c | lo | Crush | Stomp |
| Perceive | a | lo | Stare | Detect |
| Mold | c | hi | Shape | Carve |
| Flatter | a | lo | Praise | Bless |
| Analyze | a | hi | Suspect | Examine |
| Condemn | a | lo | Punish | Scare |
| Amend | a | hi | Raise | Correct |
| Creep | c | lo | Sneak | Drag |
| Inspire | a | lo | Motivate | Promote |
| Endorse | a | hi | Help | Approve |
| Flee | c | hi | Disappear | Escape |
| Soar | c | lo | Float | Fly |
| Sense | a | lo | Perceive | Locate |
| Scurry | c | lo | Scamper | Wobble |
| Think | a | hi | Guess | Wonder |
| Limp | c | lo | Shuffle | Hobble |
| Yearn | a | lo | Obtain | Strive |
| Listen | a | hi | Hear | Obey |
| Inject | c | hi | Push | Pat |
| Detect | a | lo | Hunt | Identify |
| Emerge | c | hi | Arise | Mount |
NOTE:
Imageability groups: c=Concrete and a=Abstract. Frequency groups: lo=Low Frequency and hi=High Frequency. Correct answers are shown in italics.
Procedure
The 40 item VST was preceded by a brief practice session. For both the practice and VST, participants were presented with a written target word and instructed to indicate, from two written choices, the word most similar in meaning to the target. Each stimulus was presented on a separate sheet of paper in a triad format with the target word positioned in the center and the two options below. Participants were instructed to point to the word most similar in meaning to the target. For a subset of participants, the VST was administered on a computer using E-prime software.
Imaging Procedure
High resolution structural MRI scans were available for a subset of 5 SD patients to establish cortical thickness using a diffeomorphic registration-based cortical thickness measure (Das et al., 2009). This method uses a continuous one-to-one correspondence between the gray matter–white matter interface and the estimated gray matter–cerebrospinal fluid interface given by a diffeomorphic mapping in the image space, and defines thickness in terms of a distance measure between the interfaces of this sheet-like structure. We set a statistical threshold for identifying significant gray matter thinning in each of the three subgroups at the p<0.001 level (uncorrected). We assessed gray matter thinning of the patient group relative to 16 age-matched controls using an independent samples t-test in SPM5 with a voxel height threshold of p<.001 (uncorrected) and a cluster extent of 100 adjacent voxels to ensure the reliability of thinning in this region. We related cortical thinning to behavioral performance (difference in abstract z-scores minus concrete z-scores) in regions masked by the patient group cortical thinning results using a multiple regression model in SPM5 with a voxel height threshold of p<.05 and a cluster extent of 100 adjacent voxels. Masking the regression analysis to include only regions of significant cortical thinning in SD allows us to restrict our analysis to regions for which we have a priori hypotheses.
RESULTS
Behavioral results
SD patients (78.0 ±15% correct) were significantly impaired on this simple measure of verb associative knowledge in comparison to controls (93.4 ±6.3% correct) [t(25) = 3.67; p=0.001]. We converted patient performance to z-scores based on controls’ performance. Behavioral results of individual patients are summarized in Table 2. Using a z-score of -1.96 (equivalent to p<0.05, two-tailed), we found significantly abnormal performance in 7 (64%) of 11 SD patients.
TABLE 2.
INDIVIDUAL SD PATIENT PERFORMANCE
| SD Patient | Percent correct (z-score) | |||
|---|---|---|---|---|
| Abstract | Concrete | Low Frequency | High Frequency | |
| 1 | 80(−1.31) | 85(−2.00) | 70(−4.68) | 95(0.46) |
| 2 | 55(−4.38) | 60(−6.44) | 60(−6.48) | 55(−4.08) |
| 3 | 60(−3.76) | 70(−4.66) | 65(−5.58) | 65(−2.95) |
| 4 | 90(−0.08) | 100(0.67) | 100(0.73) | 90(−0.11) |
| 5 | 80(−1.31) | 80(−2.89) | 85(−1.97) | 75(−1.81) |
| 6 | 50(−4.99) | 60(−6.44) | 50(−8.29) | 60(−3.52) |
| 7 | 65(−3.15) | 70(−4.66) | 60(−6.48) | 75(−1.81) |
| 8 | 100(1.15) | 90(−1.11) | 100(0.73) | 90(−0.11) |
| 9 | 100(NA) | 100(NA) | 100(NA) | 100(NA) |
| 10 | 80(−1.31) | 80(−2.89) | 80(−2.88) | 80(−1.24) |
| 11 | 80(−1.31) | 80(−2.89) | 100(0.73) | 60(−3.52) |
We analyzed participant performance accuracy across semantic categories of verbs. Controls showed a concreteness effect, with better performance on concrete verbs (96.3% ±5.6 correct) compared to abstract verbs (90.6% ±8.1 correct) [t(15) = 3.58; p=0.003]. This advantage for concrete stimuli over abstract stimuli was seen in 15 (94%) of 16 controls. We converted patient performance to z-scores based on control performance in each category so that we could compare patient performance on concrete verbs and abstract verbs in a manner that minimizes any effect of the discrepancy in controls between concrete verbs and abstract verbs. One patient performed at ceiling on the task and was therefore not included in these z-score analyses. The findings are summarized in Figure 1. Within SD patients, performance on concrete verbs was significantly impaired relative to abstract verbs [t(10) = 4.77; p=.001]. Inspection of individual patient z-scores revealed worse performance with concrete verbs compared to abstract verbs in 9 (90%) of 10 SD patients. Examining raw scores in participants who did not perform at ceiling, we found a loss of the concreteness effect in 4 (40%) of 10 SD patients, although we did not find an interaction of group (patient/control) by word category (abstract/concrete) [F(1,25) = 1.02, n.s.). The group of SD patients who did not show concreteness effects in their raw scores were found to have significantly longer disease durations than patients showing the concreteness effect [t(8) = 2.82; p<.05].
FIGURE 1.
PERFORMANCE ON ABSTRACT VERBS AND CONCRETE VERBS IN SEMANTIC DEMENTIA
We conducted an analysis to determine if the lexical frequencies of the stimuli could better account for patient performance. High frequency and low frequency categories were created by using a median split that grouped stimuli into the 20 highest and 20 lowest average frequency trials (Francis & Kucera, 1982). Behavioral data are summarized in Table 2. As with the concrete and abstract groupings, we converted patient performance to z-scores based on control performance, leaving out one patient whose performance was at ceiling. For SD patients there was no significant difference in performance for low frequency verbs relative to high frequency verbs [t(9) = 1.62; n.s.].
Imaging results
Imaging data are summarized in Table 3. Figure 2A shows significant cortical thinning in the subset of SD patients for whom MRI scans were available involving anterior and inferolateral temporal regions bilaterally.
TABLE 3.
PEAK VOXELS IN SIGNIFICANTLY ATROPHIC CLUSTERS IN PATIENTS COMPARED TO HEALTHY SENIORS AND IN CLUSTERS OF SIGNIFICANT CORRELATION OF TASK PERFORMANCE WITH CORTICAL ATROPHY
| PEAK ANATOMIC LOCUS (Brodmann Area) | MNI COORDINATES | Z-SCORE | ||
|---|---|---|---|---|
| X | Y | Z | ||
|
SD Atrophy | ||||
| L. anterior temporal (38) | −28 | 12 | −48 | 5.89 |
| R. ventral temporal (20) | 28 | −24 | −36 | 4.90 |
| L. medial frontal (11) | −4 | 40 | −28 | 4.66 |
| L. cerebellum | −28 | −46 | −38 | 3.83 |
|
Correlation of Abstract-Concrete with Atrophy in SD | ||||
| R. ventral temporal (20) | 56 | −16 | −38 | 4.05 |
FIGURE 2.
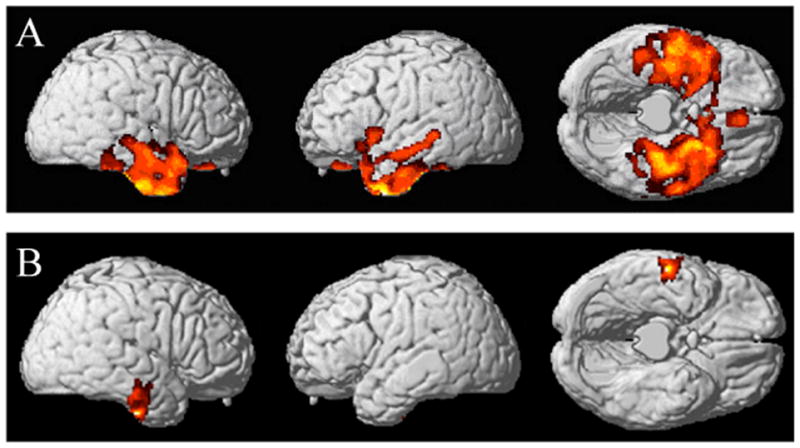
A. CORTICAL ATROPHY IN SEMANTIC DEMENTIA
B. REVERSAL OF THE CONCRETENESS EFFECT RELATED TO CORTICAL, ATROPHY IN SEMANTIC DEMENTIA
We also examined whether relative difficulty with concrete verbs in SD was related to cortical thinning. The results of these analyses are summarized in Table 3. Figure 2B shows that the size of the difference in abstract z-scores minus concrete z-scores correlated significantly with the degree of cortical atrophy in a portion of the right anterior temporal lobe in SD. This means that, for SD patients, the size of the reversal of the concreteness effect (as reflected in the abstract-concrete difference scores) increases with the degree of thinning of right anterolateral temporal cortex.
DISCUSSION
We found that patients with SD are significantly impaired on a simple measure of associative knowledge involving verbs. Moreover, these patients were significantly more impaired in their performance with concrete verbs than abstract verbs. This pattern was seen in a majority of individual SD patients. Quantitative assessment of cortical thinning in SD revealed significant disease involving visual association cortex in the anterior, lateral and ventral portions of the temporal lobes. Relatively greater difficulty with concrete verbs compared to abstract verbs was related to thinning of right anterior temporal cortex. These findings raise the possibility that semantic memory difficulty in SD is due in part to degradation of visual-perceptual feature knowledge associated with concrete concepts represented in the anterolateral temporal lobe, particularly on the right. This evidence is consistent with much of the functional neuroimaging work supporting the sensory-motor approach to semantic memory.
As expected, control performance on the verb similarity task revealed a strong concreteness effect, with significantly greater accuracy for concrete than for abstract verbs in assessments of the entire group as well as in individual participant evaluations. Looking at raw scores in the patient group, we saw a loss of the concreteness effect overall, however, there was no interaction of word category by subject group. Given the overwhelming robustness of the concreteness effect in healthy participants, we accept loss of the concreteness effect, or similar relative performance on abstract and concrete conditions, as abnormal in SD. In individual patients, the raw scores show a loss of the concreteness effect in 4 out of 10 SD patients. Moreover, we found that these 4 patients had significantly longer disease durations than the other 6 patients. Thus, disease progression in SD and its effects on visual association cortex of the temporal lobes may bring about a loss of the concreteness effect through greater damage to visual-perceptual feature representations. It is important to point out that a raw score analysis of SD patient performance does not account for the difference in task difficulty (i.e. the concreteness effect) for the concrete and abstract word conditions observed in controls. In order to account for this behavioral difference in controls we converted patient performance to z-scores. This analysis allows us to relate patient performance to controls in both the concrete and abstract word conditions and reveals that small concreteness effects in the raw scores of impaired patients are substantially different from the behavioral patterns typically seen in controls. From this z-score analysis we find that SD patients are significantly more impaired on concrete verbs relative to controls than they are on abstract verbs relative to controls. This shows that SD patients have a greater relative deficit for concrete verbs than abstract verbs.
These results are consistent with previous findings of “reversal of the concreteness effect,” that is, more difficulty understanding concrete words than abstract words, in SD and another temporal lobe disease group, herpes simplex encephalitis (Sirigu, Duhamel, & Poncet, 1991; Warrington & Shallice, 1984). The earliest demonstration of this phenomenon was provided by Warrington (1975). Several single cases and brief series have been published over the subsequent 3 decades (Breedin et al., 1995; Cipolotti & Warringtin, 1995; Sirigu et al., 1991; Warrington, 1981; Warrington & Shallice, 1984). These reports describe more difficulty identifying, reading and understanding concrete words than abstract words. More recently, we used a multiple-choice word-description matching task to demonstrate “reversal of the concreteness effect” in a larger group of SD patients (Yi et al., 2007). We also found that these patients are as impaired on a multiple-choice word-video matching task as they are on the word-description task for concrete words. In the present study, we extended our results by examining the anatomic distribution of cortical atrophy associated with concrete word difficulty in patients with SD. This revealed disease that is typical of SD, involving anterior temporal neocortex. This has been demonstrated repeatedly in imaging studies of SD (Gorno-Tempini et al., 2004; Grossman et al., 2004; Mummery et al., 2000). Moreover, these studies show lateral and ventral atrophy extending posteriorly in the temporal lobe, as we found.
The hypothesis that the semantic impairment in SD is due to degraded perceptual feature representations is consistent with the sensory-motor theory of semantic memory (Barsalou, 2008; Barsalou et al., 2003; Martin, 2007; Pulvermuller, 2005). According to the sensory-motor approach, the relatively greater decline in concrete than abstract knowledge in SD stems from disease in visual association cortex, which degrades the representation of visual-perceptual feature knowledge needed for concrete concepts. The anatomic distribution of disease in SD involves visual association cortex. These temporal areas are associated with the so-called ventral stream that is important for processing features such as shape and color (Ungerleider, Mishkin, Ingle, Goodale, & Mansfield, 1982). Most evidence for a semantic memory deficit in SD in fact comes from demonstrations of difficulty with visual-perceptual material (Ikeda, Patterson, Graham, Ralph, & Hodges, 2006; Lambon Ralph, McClelland, Patterson, Galton, & Hodges, 2001). Although some studies point to difficulty with non-visual features in SD (Bozeat et al., 2000; Bozeat et al., 2002; Coccia, Bartolini Luzzi, Provinciali, & Lambon Ralph, 2004; Luzzi, Snowden, Neary, Coccia, Provinciali, & Lambon Ralph, 2007), these studies largely examined patients with longer disease duration who presumably have more extensive cortical disease that may compromise other modality-specific association regions such as auditory association cortex in more dorsal regions of the temporal lobe. Moreover, these studies often employed cross-modality tasks (i.e. sound-picture matching or sound-word matching) that confound conclusions about modality-specific deficits.
It is important to point out that this sensory-motor account is not incompatible with “amodal” approaches to semantic memory such as the integrative hub hypothesis (Patterson et al., 2007). For example, theories hypothesizing an integrating role for an amodal component in semantic memory also propose that knowledge of features associated with concrete concepts are represented in modality-specific association cortex (Koenig & Grossman, 2007; Patterson et al., 2007). This explanation leaves open the possibility that damage to an integrative hub may coincide with damage to visual feature representations in the temporal lobe, resulting in an amodal semantic deficit along with a disproportionate impairment for semantic concepts that depend critically on visual feature knowledge. It is also worthwhile considering the possibility that visual feature representations are essential for object concepts and that degrading these features in SD could result in a multimodal semantic impairment because non-visual features are insufficient for maintaining object representations.
Prior work in SD has emphasized that the semantic memory deficit in these patients leaves them susceptible to difficulty with low frequency words (Bird, Lambon Ralph, Patterson, & Hodges, 2000; Patterson, 2006, 2007). A recent study highlights behavioral impairments for both low frequency and low imageability words in SD, arguing that “reversal of the concreteness effect” in SD is an anomalous finding (Jefferies, Patterson, Jones, & Lambon Ralph, 2009). It is worth noting, however, that the words in the low imageability condition of this study were longer in letter length than words in the high imageability condition. Given that picture naming accuracy has previously been shown to correlate with word length in a group of SD patients (Lambon Ralph, Graham, Ellis, & Hodges, 1998), it is possible that word length had an impact on performance on the 4-item synonym judgment task in Jefferies et al., 2009. Moreover, looking at individual patients, this study did find 3 instances of loss or reversal of the concreteness effect when comparing patient performance on high imageability with medium imageability words and 1 instance when comparing medium imageability with low imageability words. We examined the effect of frequency on patient performance in our study by dividing the stimuli into high and low frequency groups. Using a z-score analysis based on control performance, we found no relationship between word frequency and performance accuracy in SD, replicating our previous observations that imageability effects are stronger in the SD group than frequency effects (Yi et al., 2007).
A number of previous reports described a deficit for verbs in SD (Bird, Howard, & Franklin, 2000; Bird, Lambon Ralph et al., 2000; Bozeat et al., 2002; Yi et al., 2007). There are several factors that may contribute to difficulty with verbs. One possibility is related to the extension of progressive disease into association regions of neocortex that are important for representing motion and action knowledge (Avants, Anderson, Grossman, & Gee, 2007), or the degradation of white matter projections that integrate visual-perceptual feature knowledge in visual association cortex with motion-related areas in lateral temporal-occipital areas and action representations in premotor areas (Asmuth et al., 2008; Borroni, Brambati, Agosti, Gipponi, Bellelli, Gasparotti, et al., 2007). Another contributing factor may be that verb concepts are poorly organized hierarchically (Miller & Fellbaum, 1991). Compensatory strategies for word comprehension are less likely to be as effective in this context since there are fewer features shared across overlapping concepts. By comparison, noun concepts are better organized hierarchically and are able to take advantage of features shared across overlapping concepts (Gonnerman et al., 1997).
It is noteworthy that our analyses relating reversal of the concreteness effect directly to cortical thinning in SD demonstrated an association with the right temporal lobe. A previous study also suggested that right temporal lobe disease plays an important role in the semantic deficits of SD patients (Lambon Ralph et al, 2001). One possibility is that visual perceptual feature knowledge underlying concrete concepts like actions and objects is preferentially represented in the right temporal lobe. From this perspective, the typical natural history of SD involves, initially, a deficit with lexical retrieval and impairments involving the names of concrete concepts in association with left temporal disease (Grossman et al., 2004). As the disease progresses, additional involvement of the right temporal lobe results in difficulty with concrete concepts (Avants et al., 2007). This would be consistent with evidence from functional neuroimaging suggesting that, while abstract word meanings recruit left temporal cortex alone, concrete word meaning recruit bilateral temporal cortices (Binder et al., 2005).
This study reproduces the “reversal of the concreteness effect” in SD, a finding that has previously been interpreted as consistent with the sensory-motor account of semantic memory. We provide strong evidence in support of that claim by directly relating this behavioral effect with thinning of a portion of visual association cortex in the right temporal lobe. This connection demonstrates the critical role of visual association cortex in representing the meanings of concrete words.
Acknowledgments
This work was supported in part by the National Institutes of Health (AG17586, AG15116, NS44266, NS53488). Portions of this work were presented at the annual meeting of the American Academy of Neurology, Chicago, April, 2008.
References
- Asmuth J, Zhang H, Grossman M. DTI analysis of white matter deficits in frontotemporal lobar degeneration. Neurology. 2008;70:A452. [Google Scholar]
- Avants B, Anderson C, Grossman M, Gee JC. Spatiotemporal normalization for longitudinal analysis of gray matter atrophy in frontotemporal dementia. Medical Image Computing and Computer Assisted Intervention. 2007;4792:303–310. doi: 10.1007/978-3-540-75759-7_37. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review of Psychology. 2008;59(1):617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Kyle Simmons W, Barbey AK, Wilson CD. Grounding conceptual knowledge in modality-specific systems. Trends in Cognitive Sciences. 2003;7(2):84–91. doi: 10.1016/s1364-6613(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Binder JR, Liebenthal E, Possing ET, Medler DA, Ward D. Neural correlates of sensory and decision processes in auditory object identification. Nature Neuroscience. 2004;7:295–301. doi: 10.1038/nn1198. [DOI] [PubMed] [Google Scholar]
- Binder JR, Westbury C, McKiernan KA, Possing ET, Medler DA. Distinct brain systems for processing concrete and abstract concepts. Journal of Cognitive Neuroscience. 2005;905:917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Bird H, Howard D, Franklin S. Why is a verb like an inanimate object? Grammatical category and semantic category deficits. Brain and Language. 2000;72:246–309. doi: 10.1006/brln.2000.2292. [DOI] [PubMed] [Google Scholar]
- Bird H, Lambon Ralph MA, Patterson K, Hodges JR. The rise and fall of verb frequency and imageability: Noun and verb production in semantic dementia. Brain and Language. 2000;73:17–49. doi: 10.1006/brln.2000.2293. [DOI] [PubMed] [Google Scholar]
- Borroni B, Brambati SM, Agosti C, Gipponi S, Bellelli G, Gasparotti R, et al. Evidence of White Matter Changes on Diffusion Tensor Imaging in Frontotemporal Dementia. Archives of Neurology. 2007;64:246–251. doi: 10.1001/archneur.64.2.246. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Hodges JR. When objects lose their meaning: What happens to their use? Cognitive, Affective, and Behavioral Neuroscience. 2002;2:236–251. doi: 10.3758/cabn.2.3.236. [DOI] [PubMed] [Google Scholar]
- Breedin SD, Saffran EM, Coslett HB. Reversal of a concreteness effect in a patient with semantic dementia. Cognitive Neuropsychology. 1995;11:617–660. [Google Scholar]
- Chao LL, Haxby J, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neuroscience. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Warringtin EK. Semantic memory and reading abilities: A case report. Journal of the International Neuropsychological Society. 1995;1:104–110. doi: 10.1017/s1355617700000163. [DOI] [PubMed] [Google Scholar]
- Coccia M, Bartolini M, Luzzi S, Provinciali L, Lambon Ralph MA. Semantic memory is an amodal, dynamic system: Evidence from the interaction of naming and object use in semantic dementia. Cognitive Neuropsychology. 2004;21:513–527. doi: 10.1080/02643290342000113. [DOI] [PubMed] [Google Scholar]
- Coltheart M. Deep dyslexia: A review of the syndrome. In: Coltheart M, Patterson K, Marshall JC, editors. Deep dyslexia. London: Routledge and Kegan Paul; 1980. [Google Scholar]
- Folstein MF, Folstein SF, McHugh PR. "Mini Mental State." A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. The frequency analysis of English usage. Boston: Houghton-Mifflin Co; 1982. [Google Scholar]
- Gonnerman LM, Andersen ES, Devlin JT, Kempler D, Seidenberg MS. Double dissociation of semantic categories in Alzheimer’s disease. Brain and Language. 1997;57:254–279. doi: 10.1006/brln.1997.1752. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Hyde MR, Blumstein S. Frequency, picturability and availability of nouns in aphasia. Cortex. 1969;5:104–119. doi: 10.1016/s0010-9452(69)80022-5. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Anderson C, Khan A, Avants B, Elman L, McCluskey L. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71(18):1396–1401. doi: 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What’s in a name: Voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia, and corticobasal degeneration. Brain. 2004;127:628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Grossman M, White-Devine T, Payer F, Onishi K, D’Esposito M, Robinson KM, et al. Constraints on the cerebral basis for semantic processing from neuroimaging studies of Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:152–158. doi: 10.1136/jnnp.63.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermuller F. Somatotopic Representation of Action Words in Human Motor and Premotor Cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Semantic dementia: A unique clinicopathological syndrome. Lancet Neurology. 2007;6:1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and Palm Trees: A Test of Semantic Access from Pictures and Words. Nature Publishing Group; 1992. [Google Scholar]
- Ikeda M, Patterson K, Graham KS, Ralph MAL, Hodges JR. A horse of a different colour: Do patients with semantic dementia recognise different versions of the same object as the same? Neuropsychologia. 2006;44:566–575. doi: 10.1016/j.neuropsychologia.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Baker SS, Doran M, Lambon Ralph MA. Refractory effects in stroke aphasia: A consequence of poor semantic control. Neuropsychologia. 2007;45:1065–1079. doi: 10.1016/j.neuropsychologia.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Jones RW, Lambon Ralph MA. Comprehension of concrete and abstract words in semantic dementia. Neuropsychology. 2009;23(4):492–9. doi: 10.1037/a0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Barsalou LW, Solomon K, Thompson-Schill SL. Role of mental imagery in a property-verification task: fMRI evidence for perceptual representations of conceptual knowledge. Cognitive Neuropsychology. 2003;20:525–540. doi: 10.1080/02643290244000257. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Large, colorful, and noisy? Attribute- and modality-specific activations during retrieval of perceptual attribute knowledge. Cognitive, Affective, and Behavioral Neuroscience. 2001;1:207–221. doi: 10.3758/cabn.1.3.207. [DOI] [PubMed] [Google Scholar]
- Koenig P, Grossman M. Neural basis of semantic memory. Cambridge, UK: Cambridge University Press; 2007. Process and content in semantic memory. [Google Scholar]
- Lambon Ralph MA, Graham KS, Ellis AW, Hodges JR. Naming in semantic dementia-what matters? Neuropsychologia. 1998;36(8):775–784. doi: 10.1016/s0028-3932(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13:341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- McKhann G, Trojanowski JQ, Grossman M, Miller BL, Dickson D, Albert M. Clinical and pathological diagnosis of frontotemporal dementia: Report of a work group on frontotemporal dementia and Pick’s disease. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Miller GA, Fellbaum C. Semantic networks of English. Cognition. 1991;41:197–229. doi: 10.1016/0010-0277(91)90036-4. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Hodges JR. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47:36–45. [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Paivio A. Mental representations: A dual coding approach. Oxford: Oxford University Press; 1986. [Google Scholar]
- Paivio A. Dual coding theory: Retrospect and current status. Canadian Journal of Psychology. 1991;45:255–287. [Google Scholar]
- Patterson K. ‘Pre-semantic’ cognition in semantic dementia: six deficits in search of an explanation. Journal of Cognitive Neuroscience. 2006;18:169–183. doi: 10.1162/089892906775783714. [DOI] [PubMed] [Google Scholar]
- Patterson K. The reign of typicality in semantic memory. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:813–821. doi: 10.1098/rstb.2007.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Price C, Grossman M. Verb agreements during on-line sentence processing in Alzheimer’s disease and frontotemporal dementia. Brain and Language. 2005;94:217–232. doi: 10.1016/j.bandl.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F. Brain mchanisms linking language and action. Nature Reviews Neuroscience. 2005;6:576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Reilly J, Cross K, Troiani V, Grossman M. Single-word semantic judgments in semantic dementia: Do phonology and grammatical class count? Aphasiology. 2007;21:558–569. [Google Scholar]
- Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cerebral Cortex. 2005;15:1602. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, Barsalou LW. A common neural substrate for perceiving and knowing about color. Neuropsychologia. 2007;45(12):2802–2810. doi: 10.1016/j.neuropsychologia.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Poncet M. The role of sensorimotor experience in object recognition. Brain. 1991;114:2555–2573. doi: 10.1093/brain/114.6.2555. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M, Ingle DJ, Goodale MA, Mansfield RJW. Analysis of visual behavior. Cambridge: MIT Press; 1982. Two cortical visual systems; pp. 549–580. [Google Scholar]
- Warrington EK. Concrete word dyslexia. British Journal of Psychology. 1981;72:175–196. doi: 10.1111/j.2044-8295.1981.tb02175.x. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category-specific semantic impairments. Brain. 1984;107:829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Yi HA, Moore P, Grossman M. Reversal of the concreteness effect for verbs in semantic dementia. Neuropsychology. 2007;21:9–19. doi: 10.1037/0894-4105.21.1.9. [DOI] [PubMed] [Google Scholar]