Abstract
In order to study the effect of the temporal synergism of neural oscillations on reproductive regulation and the response of RFamide-related peptide-3 (RFRP-3; a mammalian ortholog of avian gonadotropin-inhibitory hormone), expression of immunoreactive RFRP-3 in the neurons of the dorsomedial nucleus of the hypothalamus was monitored in sexually immature and mature laboratory mice (study I). In study II, the effects of serotonin and dopamine precursors (5-hydroxytryptophan and L-dihydroxyphenylalanine; injected daily, 8 or 12 h apart, for 13 days in 3-week-old mice) on testicular activity and immunoreactive RFRP-3 neurons were studied until 24 days after treatment. Results indicate high levels of expression of immunoreactive RFRP-3 in the sexually immature and 8-hour mice (simulating gonadal suppression), while a low level was noted in mature and 12-hour mice (simulating gonadal stimulation). These findings not only suggest the modulation of gonadal development in mice (during the course of puberty attainment) by changing the temporal phase relation of serotonergic and dopaminergic oscillations (as in some seasonally breeding species), but also demonstrate an inverse correlation of RFRP-3 neurons and gonadal activity in both control and experimental conditions.
Key Words: 5-Hydroxytryptophan, L-DOPA, RFamide-related peptide-3, Gonadotropin-inhibitory hormone, Testosterone, Reproduction, Mice
Introduction
A number of studies have reported that the temporal phase relation of circadian serotonergic and dopaminergic oscillations affects gonadal development in many seasonally breeding birds and mammals [1,2,3,4,5,6]. Administration of a dopamine precursor (L-dihydroxyphenylalanine; L-DOPA) 12 h after the injection of a serotonin precursor (5-hydroxytryptophan; 5-HTP) in a 24-hour period (12-hour relation) has been reported to induce breeding conditions in these species, as well as the advancement of puberty (in the Japanese quail) [7]. On the other hand, if these drugs are injected 8 h apart (8-hour relation), this may lead to gonadal suppression/non-breeding conditions, while other relations (0, 4, 16 and 20 h) have been found to be ineffective. In the Indian palm squirrel, Funambulus pennantii, the 12-hour phase relation of serotonergic and dopaminergic oscillations not only extends the breeding season, but may also eliminate annual reproductive regression [8]. On the other hand, in this subtropical mammalian species, an 8-hour phase relation stimulates non-breeding conditions out of season [9]. On the basis of the previously mentioned studies, it was assumed that there must be a pacemaker system that has serotonergic and dopaminergic components. This assumption is further supported by the report of seasonal alterations in the phase relations of circadian serotonin and dopamine rhythms in the right and left suprachiasmatic nucleus (SCN) of the Syrian hamster [10]. The manipulation of these rhythms by the administration of 5-HTP and L-DOPA at different intervals in a 24-hour period is known to alter seasonal scotosensitive and/or scotorefractory conditions in hamsters [2] and photosensitive and/or photorefractory conditions in quails [11]. Moreover, control animals are also reported to have a different circadian phase relation of serotonin and dopamine oscillations during different breeding conditions [12,13]. Our recent report in mice indicated that 8- and 12-hour relations of 5-HTP and L-DOPA administration affect gonadal growth, while other relations (0, 4, 16 and 20 h) are ineffective [14].
An RFamide (Arg-Phe-NH2) peptide identified in the quail brain inhibits gonadotropin release; this was named gonadotropin-inhibitory hormone (GnIH), a novel hypothalamic dodecapeptide (SIKPSAYLPLRF-NH2) [15]. This inhibitory peptide is a regulator of pituitary gonadotropin release, both in vitro and in vivo, in birds and mammals [15,16,17,18,19,20,21]. In mammals, cDNAs that encode the 2 biologically active GnIH orthologous peptides RFRP-1 and RFRP-3 contain a C-terminal LPXRF-amide motif and have been detected in the brain [17,22]. Intracerebroventricular infusion (i.c.v.) or intraperitoneal (i.p.) administration of deduced human RFRP-1 increased prolactin release in the rat [23]. By contrast, i.c.v. injections of RFRP-3 reduced plasma levels of luteinizing hormone (LH) in rats [24]. When i.c.v. or i.p. injections were used, GnIH also reduced plasma LH levels in Syrian hamsters [17], while, in the same species, immediate early gene expression is also reduced in RFRP cells during the LH surge [22,25]. It was also found that peripheral administration of the deduced ovine ortholog RFRP-3 reduces the amplitude of LH pulses in sheep, and LH and FSH release in vitro. Both avian GnIH and its mammalian ortholog RFRP-3 are considered to be functional orthologs, and act to inhibit gonadotropin release [18,22,23,24,25,26]. The location of GnIH/RFRP neuronal cell bodies has been studied in several mammals, and appears to be the dorsomedial hypothalamus (DMH) in mice [27].
Compared to bird GnIH, there is limited experimental information clarifying the functional significance of RFRP-3 in mammals and its potential role as a key neuropeptide involved in mammalian reproduction. Hence, in this study, our first objective was to compare RFRP-3 expression in prepubertal and postpubertal mice, and to correlate this with gonadal status and activity pattern (study I). The second objective was to study the effect of 5-HTP and L-DOPA injections given at 8- or 12-hour intervals (the conditions that simulated non-breeding/breeding, respectively) on the testicular development and RFRP-3 neurons of the laboratory mice (study II). These findings indicate an inverse correlation in the expression of RFRP-3 neurons and gonadal function (in control as well as experimental conditions) for the first time in mice.
Materials and Methods
Male laboratory mice (Mus musculus) of the Parkes strain were obtained from a colony maintained in our laboratory. The mice were housed under hygienic conditions in a well-ventilated photoperiodically controlled room (light:dark 12:12), and were provided with commercial food (Pashu Aahar Kendra, Varanasi, India) and tap water ad libitum. All the studies were conducted in accordance with institutional practices, and within the framework of the revised Animals (Scientific Procedures) Act of 2002 of the Government of India.
Study I
Three-week-old sexually immature (prepubertal) and 13-week-old sexually mature (postpubertal) normal mice (n = 5 in each group) were weighed and anesthetized with diethyl ether. Blood was collected from the heart into a heparinized tube, followed by whole body perfusion. Mice were perfused transcardially with phosphate-buffered saline (PBS) followed by Zamboni's fixative [4% paraformaldehyde in 0.1 M sodium phosphate buffer; pH 7.4]. The testes were excised and post-fixed in the same fixative overnight for histological studies. Twenty-four hours after fixation, the testes were dehydrated in a graded series of alcohol, treated with xylene, embedded in paraffin wax, and 6-μm-thick sections were cut by a Weswox rotary microtome (Western Electric & Scientific Works, Ambala Cantt, India), and stained with silver nitrate [28]. The brain was also dissected and post-fixed to be processed for the immunohistochemistry of RFRP-3.
Study II
Three-week-old prepubertal mice (12–14 g), acclimatized to a continuous condition of light (LLdim) for 2 days, were weighed and randomly divided into 3 groups (n = 5 per group). Mice of the control group received 2 daily injections of normal saline. The 2 precursor drugs, 5-HTP and L-DOPA, were prepared daily (fine suspension in normal saline) and injected intraperitoneally (5 mg/100 g body weight/day) in 0.1 ml solution over a period of 13 days. Mice of both study groups received the serotonin precursor (5-HTP) at 08:00, and the dopamine precursor (L-DOPA) at different time intervals in the 2 groups, i.e. at 16:00 or 20:00, so as to establish 8- or 12-hour phase relations, respectively, between the 2 injections. These doses of 5-HTP and L-DOPA (Sigma Aldrich, St. Louis, Mo., USA) have been reported to increase the brain content of serotonin and dopamine, respectively, in rats [29,30,31]. Since the neurotransmitters serotonin and dopamine cannot cross the blood-brain barrier, but their precursors can, 5-HTP and L-DOPA were used [31].
During the treatment period, mice were maintained under continuous light (LLdim) so as to avoid possible photoperiodic interference of the light-dark cycle with the entrainment of neural oscillations by the administration of the drugs. After an injection period of 13 days, all the groups were transferred back to a 12-hour light:12-hour dark photocycle (lights on at 08:00 and off at 20:00 by an automatic timer) to allow them to mature further under controlled conditions; the mice were weighed weekly. Twenty-four days after the last injection, when the mice were 58 days old, they were anesthetized and treated as described for study I. The length and width of the left testis was measured in situ with dial calipers, and the testicular volume was calculated using Bissonett's formula (4/3) π ab2 (a = 1/2 of the long axis; b = 1/2 of the short axis) [8,9]. Histological sections of the testis were viewed under a microscope (Axioskop 2 Plus; Carl Zeiss, Oberkochen, Germany), and images were captured with a digital camera. Seminiferous tubule diameter was determined in randomly selected sections (10 per mouse testis) by using the image analyzer software Motic Images 2000 version 1.3.
To determine the percentage of affected seminiferous tubules, all the tubules from 10 randomly selected sections of the testis from 5 mice of each group were counted [32]. The seminiferous tubules were considered affected if they showed any of the following details: intraepithelial vacuolation; exfoliation of germ cells; degenerated appearance of germ cells; loosening of germinal epithelium; tubules showing presence of spermatids of different stages of the spermatogenic cycle in the same tubule; marginal condensation of chromatin in round spermatids; occurrence of germ cells and tubules lined with only Sertoli cells and rare germ cells [33]. Following quantification/scoring of the spermatogenesis (where germ cell association or stages were divided into 3 main stages: early stage (I–VI); middle stage, i.e. the stage just before sperm release (VII–VIII); and late stage (IX–XII) [34]), it was found that the percentage of affected seminiferous tubules increases during suppression/atrophy of the testicular function when the number of germ cells of the middle stage decreases [14].
Radioimmunoassay
A radioimmunoassay (RIA) of plasma testosterone was performed using a commercial RIA kit (Immunotech, Marseille, France) according to the manufacturer's instructions. The antiserum used in the assay was specific for testosterone; cross-reactivity was less than 0.03% with estradiol, 0.03% with progesterone, 0.01% with dehydroepiandrosterone, and 0.6% with androstenedione. Sensitivity of the assay was 0.025 ng/ml. The intra- and inter-assay coefficients of variation were 14.8% and 15%, respectively.
Immunohistochemistry of RFRP-3
Immunohistochemistry was performed using the method of Sternberger and Sternberger [35] with some modifications [36]. Brains, dissected after whole body perfusion, were post-fixed as described in study I, and then passed through a graded series of alcohol and embedded in paraffin wax. Coronal sections of the brain (6 μm) were deparaffinized in xylene, rehydrated and rinsed in PBS. For the immunohistochemistry of RFRP-3, endogenous peroxidase activity was eliminated from the sections by incubation with 0.3% H2O2 in absolute methanol for 20 min. After blocking nonspecific binding components with 5% normal goat serum in PBS for 1 h at room temperature, the sections were immersed in the primary antiserum raised against quail GnIH [14] at a dilution of 1:1,000 for 16–20 h at 4°C. Slides were rinsed in PBS (0.02 M, pH 7.4) and incubated with 1:1,000 dilution of horseradish-peroxidase-conjugated secondary antibody, followed by another set of washes. Sections were then incubated for 1 h in avidin-biotin complex (Vectastain Elite Kit, Vector Laboratories, Burlingame, Calif., USA). The resulting complex was visualized using 0.03% 3,3′-diaminobenzidine in 0.05 M Tris-HCl (pH 7.4) with 0.03% H2O2 for 10–20 min. After washing with Tris-HCl, the staining was stopped by washing in distilled water. Sections were dehydrated through an ethanol series, cleaned in xylene and mounted using DPX (a mixture of distyrene, a plasticizer and xylene). Slides were viewed under a Carl Zeiss Axioskop 2 Plus microscope, and images were captured using a digital camera. For negative control, slides were treated with PBS only instead of GnIH antiserum (specific for RFRP-3, the specificity has been checked by a competitive ELISA).
Image Analysis
The total number of immunoreactive RFRP-3 cell bodies in the DMH was counted automatically using a ×20 objective lens and MacBiophotonics ImageJ software. The sections were divided into series, and just 1 of these was counted. Once images of these series containing RFRP-3 neurons were captured, background threshold levels were adjusted to allow for automatic counting of ir-neurons (not fibers) in these sections by the software package. In addition, the ImageJ software placed a dot on each neuron counted, allowing the observer to verify accuracy during the counting process.
For measurement of the RFRP-3 neuronal area (visible immunoreactive area), sections containing the highest density of RFRP-3 cells were selected. Using a ×100 objective lens in 1 plane of focus only, 5 cells nearest to the centre of the screen that were in focus had their perimeters traced using a mouse with image analyzer software (Motic Images 2000 version 1.3). The fibers sprouting from the cell body were excluded by continuing tracing in an arc defined by the perimeter of the cell body on either side of the region that the fibers emanated from (i.e. continuing as if the fiber was not present and the cell body was uniform in shape at the fiber's origin). This provided a measure of the area of each cell, which was stored and used in the later analysis. If the whole perimeter of a cell body was not clearly visible, then that cell was not measured. Up to 50 randomly selected cells in each brain were measured in this way. For analysis, the cell body area was averaged within a group.
Statistical Analyses
For study I, data was analyzed using a non-parametric Mann-Whitney test for RFRP-3 neurons. For study II, one-way ANOVA followed by Dunnett's test were conducted to assess the effects of treatments on different parameters/variables (such as body weight, testicular volume, seminiferous tubule diameter, affected seminiferous tubules – based on testicular histology). RFRP-3 neuronal area and numbers were analyzed by two-way ANOVA with replication followed by post-hoc measurement (Duncan's test) in both studies. Significance was assumed at the level of p < 0.05.
Results
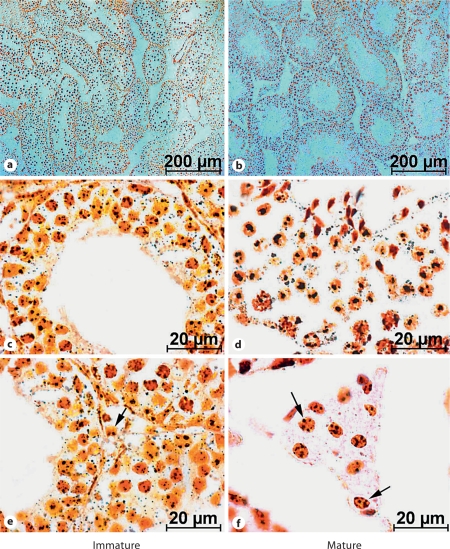
Histologically, transverse sections of the testes of immature mice showed only spermatogonial cells and spermatocytes in the smaller seminiferous tubules. Contrarily, the complete spermatogenic complement was present in the enlarged seminiferous tubules of the testes of mature mice with spermatozoa attached to the spermatids. In the triangular intertubular spaces, only a few dispersed Leydig cells were seen in immature mice testes, while those cells showing hypertrophy were more abundant and arranged in compact clumps in mature mice (fig. 1).
Fig. 1.
a, b Silver-stained transverse section of the testis. c, d High magnification of the seminiferous tubule. Note an increased size of seminiferous tubules (showing full breeding condition with all the stages of spermatogenesis with spermatozoa) in the sexually mature mice, as compared to the immature mice. e, f Increased number and active condition of Leydig cells (arrows) in the intertubular space of mature mouse testis.
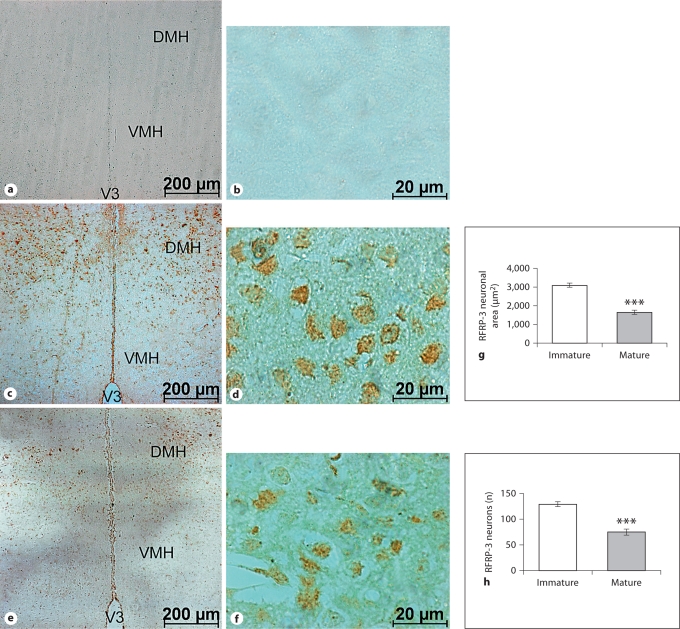
Coronal sections passing through the DMH nuclei showed dense populations of RFRP-3 neurons in the sexually immature mice compared to the mature mice. The number of ir-neurons and immunoreactivity were also higher in immature mice compared to controls. The negative control slide (without primary antibody) showed the specificity of the immunostaining. The area of the RFRP-3 neurons also decreased in mature mice when compared to immature ones (fig. 2).
Fig. 2.
Low- and high-magnification coronal sections through the DMH showing the negative control (i.e. not treated with GnIH antiserum; a, b), and the localization of ir-RFRP-3 neurons in the sexually immature (c, d) and mature (e, f) mice. V3 = Third ventricle; VMH = ventromedial hypothalamus; DMH = dorsomedial hypothalamus. The histograms show ir-RFRP-3 neuronal area (g) and the number of ir-RFRP-3 neurons in the DMH (h). Values are means ± SE. ∗∗∗ p <0.001 vs. immature mice.
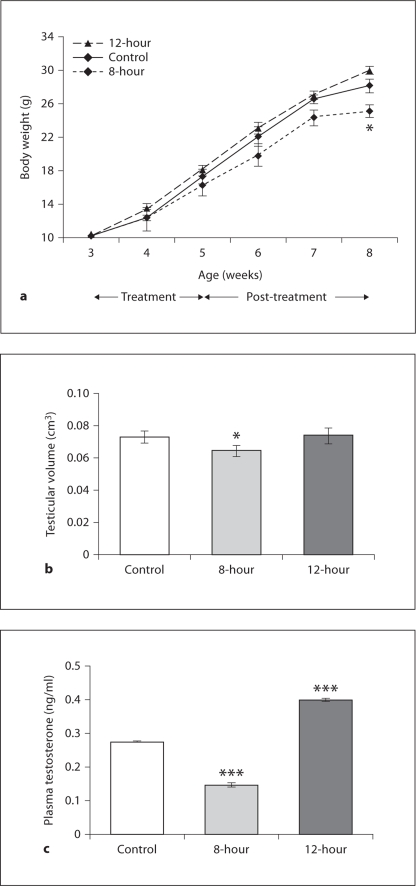
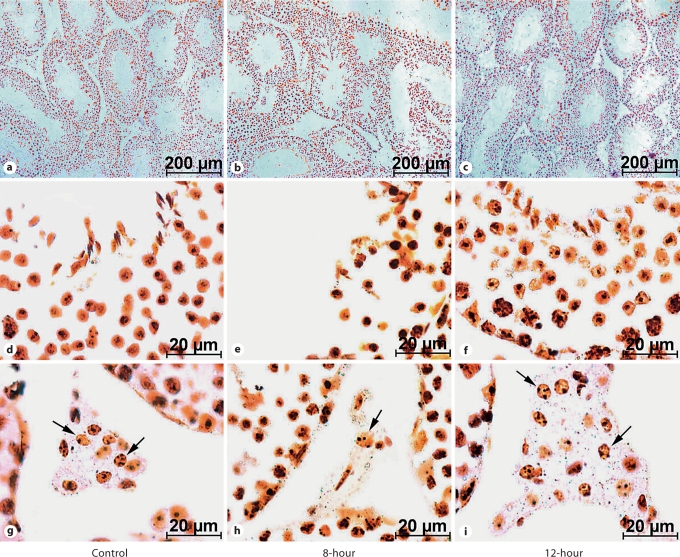
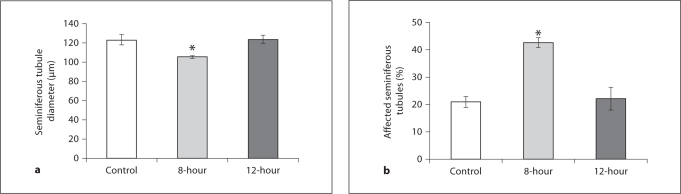
In study II, body weight gradually increased in all the groups with age; however, at the termination of the study, it was lower in 8-hour mice as compared to the controls (fig. 3a). Testicular volume decreased in 8-hour mice, but no change was observed in the 12-hour mice when compared to controls (fig. 3b). In contrast, plasma testosterone concentration decreased in 8-hour and increased in 12-hour mice (fig. 3c). Further, when compared to the control mice, testicular histology of the 8-hour mice showed degenerated structures. Some seminiferous tubules showed complete absence of a defined structure, and some tubules appeared atrophic, showing intraepithelial vacuolation. Other tubules were apparently still normal, but with reduced layers of the seminiferous epithelium containing pycnotic nuclei. Presence of desquamation in the enlarged lumen and enlarged interstitial spaces was also apparent in the testis of 8-hour mice. The seminiferous tubules of the 12-hour mice showed full breeding condition, with all the successive stages of transformation of spermatogonia into spermatozoa. In contrast to the control and 12-hour mice (where the Leydig cells were more abundant and arranged in compact clumps), in the testis of 8-hour mice, only a few dispersed Leydig cells with small and pycnotic nuclei were seen in the triangle intertubular region between the seminiferous tubules (fig. 4). When analyzed quantitatively, a significant reduction was noted in the diameter of the seminiferous tubules of 8-hour mice testes compared to controls (fig. 5a). Further, the percentage of affected seminiferous tubules in the testes of 8-hour mice was significantly higher than in control mice (fig. 5b).
Fig. 3.
Body weight response (a), testicular volume (b) and plasma testosterone concentrations (c). Values are means ± SE. ∗ p < 0.05, ∗∗∗ p < 0.001 vs. control.
Fig. 4.
Silver-stained transverse sections of the testes of mice under low (a–c) and high magnification (d–i). Note atrophic changes in 8-hour mice (b, e) compared to the control group (a, d), i.e. suppression of gametogenic activity with few layers of spermatogonia having pycnotic nuclei and enlarged/empty lumen in the seminiferous tubules. An increased degree of spermatogenic activity is evident in 12-hour mice (c, f). An increased number and hypertrophy of Leydig cells (arrows) can be seen in 12-hour mice testes (i), while reduced numbers and atrophy are seen in 8-hour mice (h), compared to the control (g).
Fig. 5.
Seminiferous tubule diameter (a) and percentage (b) of affected tubules in the testis of 8- and 12-hour mice (for group details, see fig. 3). Values are means ± SE. ∗ p < 0.05 vs. control.
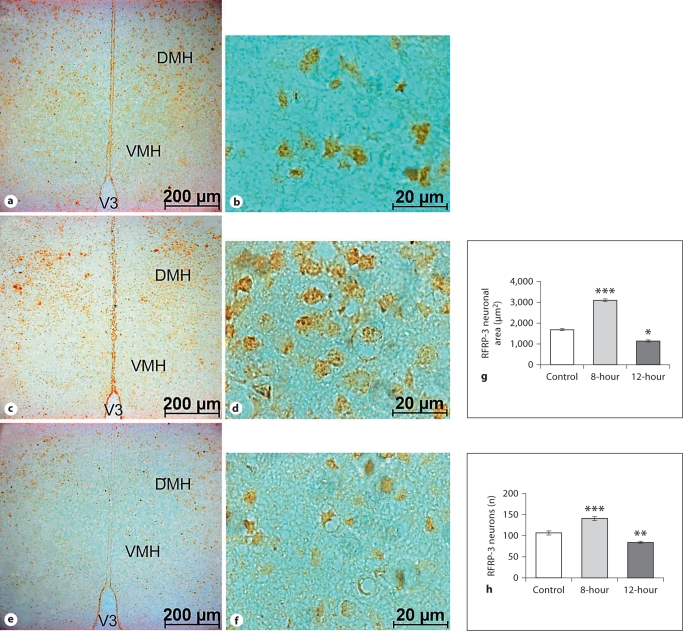
Coronal sections of the brain showed increased immunostaining in the DMH neurons of 8-hour mice, which decreased in the 12-hour mice, compared to the control. Furthermore, significant increases in the neuronal area and number were also observed in the 8-hour mice, while these decreased in the 12-hour mice, compared to the control (fig. 6).
Fig. 6.
Low- and high-magnification coronal sections through the DMH showing ir-RFRP-3 neurons. Compared to the control (a, b), there is an increased number and size of ir-neurons in 8-hour mice (c, d) and a decrease in 12-hour mice (e, f). Increased immunoreactivity is also evident in the RFRP-3 neurons of 8-hour mice, a decrease in the 12-hour mice, as compared to the control. V3 = Third ventricle; VMH = ventromedial hypothalamus; DMH = dorsomedial hypothalamus. Histograms show the RFRP-3 neuronal area (g) and the number of RFRP-3 neurons in the DMH (h). Values are means ± SE. ∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001 vs. control.
Discussion
GnIH, a hypothalamic peptide, is well-established as a regulator of gonadotropin secretion from the pituitary gland. GnIH and its orthologs, the RFamide-related peptides, directly inhibit gonadotropin synthesis and release from the pituitary gland in birds and mammals [15,17,22]. To study the functional aspects of RFRP-3 during different reproductive conditions, the expression and correlation of RFRP-3 with gonadal function was studied in control (prepubertal and postpubertal) and simulated breeding conditions in male mice. The present findings indicate that administration of 5-HTP and L-DOPA at specific time intervals (8 or 12 h) not only altered the testicular activity (spermatogenesis and plasma testosterone profile), but also induced simultaneous changes in the RFRP-3 neurons of DMH nuclei.
The present findings indicate that mice treated with 5-HTP and L-DOPA at 8-hour intervals, in addition to a decrease in body mass, showed suppression of testicular growth, spermatogenesis, steroidogenic activity (Leydig cell atrophy) and plasma testosterone concentration, when compared to the control. On the other hand, an increased degree of testicular activity was noted in the 12-hour mice. These gonadal responses indicate that in continuous breeders the 8-hour relation of serotonergic and dopaminergic oscillations may lead to the suppression of gonadal growth and related changes, while the 12-hour relation may accelerate gonadal growth. In spite of the fact that both groups of mice received the same dose of 5-HTP and L-DOPA, the response was different (almost opposite) between the groups. Hence, it is quite reasonable to suggest that temporal phase relation of neural oscillations, as in the case of seasonal gonadal development, may also influence reproductive development in prepubertal mice as a function of their time relation. It is also obvious that the 12-hour relation of circadian serotonergic and dopaminergic oscillations, when established in mice at an early stage, accelerated the rate of gonadal growth and spermatogenesis. It is quite possible that with this short-term treatment, if started during the early stage of development, an 8-hour relation may completely mask the development of the reproductive system and the 12-hour relation may induce either precocious sexual maturity and/or hypergonadal function.
Varying effects of 5-HTP and L-DOPA induced by different time intervals appear to be physiological and not pharmacological effects because: (1) in spite of receiving the same dose of the neurotransmitter precursors, the response was different in the 2 groups of mice as a function of their time relation, and (2) in addition to affecting testicular function, 8- and 12-hour relations also induced opposite effects on the RFRP-3 neurons. Combining these 2 responses together, it is clear that decreased gonadal activity in mice correlates with increased RFRP-3 neuronal activity in DMH nuclei. This correlation and gonadotropin inhibitory activity of RFRP-3 neurons is strengthened by the observations of study I, where sexually mature mice exhibited fewer (and a decreased size of) RFRP-3 neurons in contrast to sexually immature mice. An inverse relationship between RFRP-3 neurons and gonadal activity in both control and experimental conditions is quite evident. However, it may still be suggested (albeit based on indirect evidence [24,37,38]) that an observed alteration in RFRP-3 neuronal activity following the administration of 5-HTP and L-DOPA at specific time intervals may be one of the variables affecting gonadal function in mice. Further, the role of the circadian mechanism (temporal phase relation of circadian neural oscillations/activities) in the reproductive regulation (gonad and/or RFRP-3 neurons) observed in the present study is strengthened by the recent report of Gibson et al. [25]. These authors provided evidence for incorporating the RFRP system into the conceptual framework for the ovulatory machinery of some rodents, and perhaps other species. They suggested a neural route of communication from the SCN clock to an inhibitory peptidergic pathway. Together, these findings point to novel circadian control of the RFRP system and its potential participation in the circuitry controlling ovulatory function.
RFRP-3, similarly to GnIH, may inhibit gonadal development and maintenance through a decrease in pituitary gonadotropin synthesis and release, either directly or through GnRH neurons. Since increased gonadal activity of 12-hour mice is correlated with suppressed RFRP-3 expression, and 8-hour mice showed decreased gonadal activity and a simultaneous increase in RFRP-3 neurons, the concept of an inverse relation between the 2 components (gonad and RFRP-3 activity) is strengthened. Moreover, since such a relationship exists under both control (normal gonadal maturation) and experimental (simulated breeding) conditions, it is quite reasonable to suggest that the circadian phase relation of neural oscillations, which regulates gonadal development (whether seasonal or during the attainment of puberty), is likely to produce its effect at the CNS/hypothalamic level, and RFRP-3 appears to be the main candidate for mediating such effects. Stress-induced suppression of sexual behavior in the adult male rat is reported to involve RFRP-3 neurons [39], and may (indirectly) support our findings of the inverse correlation of RFRP-3 activity and reproductive functions.
An electrophysiological study of GnRH neurons in male and female mice showed that RFRP-3 could exert rapid inhibitory effects on the firing rate of 41% of GnRH neurons, with a small population of GnRH neurons (12%) showing an increased firing rate [26]. This study strongly suggests that, at least in mice, RFRP-3 may act on GnRH cells to induce an overall inhibitory influence. This report along with the report of Kirby et al. [39] also strengthens our suggestion that the 8-hour induced gonado-suppressive effect may be mediated through RFRP-3 neurons, and, in prepubertal mice, higher expression of this peptide may be one of the underlying reason for reproductive quiescence.
In the context of circadian organization, it is also interesting to note that peaks of circadian hypothalamic serotonin and/or dopamine content occur at different times (different acrophase) in the breeding and non-breeding conditions of the Syrian hamster [10], quail [12] and cat fish [40]. Moreover, when such circadian studies were performed in simulated breeding conditions, the phase relation of the circadian hypothalamic serotonin and dopamine peaks stayed constant. The 2 neurotransmitter peaks of increased amplitudes occurred at 12-hour intervals in reproductively stimulated 12-hour or pinealectomized quails, while the peaks of significantly lower amplitudes occurred at the same time in reproductively quiescent 8-hour or melatonin-treated quails [13]. In the present study also, injections of 5-HTP and L-DOPA given at 8-hour intervals were expected to induce a specific phase relation between hypothalamic serotonergic and dopaminergic peaks/oscillations, which (as a function of their time relation) would interfere with testicular function through the neuroendocrine axis. The findings suggest the involvement of RFRP-3 in this regulation. This assumption is based on the fact that the expression of RFRP-3 in the hypothalamus increased during gonadal suppression not only in the 8-hour mice (in which spermatogenic activity was lower than in the control), but it was also higher in the sexually immature mice than in adults. On the other hand, the testicular activity increased in 12-hour mice, showing weak immunostaining of RFRP-3 as compared to the control, as well as in the normal adult sexually mature mice compared to the sexually immature mice.
These findings clearly indicate that the phase angle between neural oscillations may influence reproductive development in prepubertal mice as a function of their time relation, and suggest the influence of circadian organization in the development of the neuroendocrine-gonadal axis during sexual maturity, as observed in day-old quail chicks [7]. Thus, the basic mechanism may be the same in some respects during the first maturation of the gonadal axis (attainment of puberty) and during recurrent seasonal gonadal development and quiescence.
In summary, the present study provides evidence of an inverse correlation between gonadal function and RFRP-3 neurons of mice, in both control and experimental conditions. These findings also indicate that the temporal phase relation between the circadian serotoninergic and dopaminergic oscillations may modulate gonadal development during the process of sexual maturity in laboratory mice. Further, there are many inhibitory neuroendocrine mechanisms involved in the prepubertal and drug-induced (8-hour relation) reproductive suppression, and RFRP-3 appears to be one of these factors. Additional studies involving administration of RFRP-3 in adult or 12-hour mice and its antagonist in prepubertal or 8-hour mice may strengthen the evidence supporting this suggestion.
Acknowledgements
This work was supported by funds from the Council of Scientific and Industrial Research, New Delhi, India, to C.M.C. (research project 37/1284/07/EMR-II). The authors gratefully acknowledge Dr. A.K. Saxena (Centre of Experimental Medicine & Surgery, Institute of Medical Sciences, Banaras Hindu University) for the silver staining. A Senior Research Fellowship to S.S. from the Indian Council of Medical Research is also acknowledged.
References
- 1.Miller LJ, Meier AH. Circadian neurotransmitter activity resets the endogenous annual cycle in a migratory sparrow. Biol Rhythm Res. 1983;14:85–94. [Google Scholar]
- 2.Wilson JM, Meier AH. Resetting the annual cycle with timed daily injections of 5-hydroxytryptophan and L-dihydroxyphenylalanine in Syrian hamsters. Chronobiol Int. 1989;6:113–132. doi: 10.3109/07420528909064621. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi CM, Bhatt R. The effects of different temporal relationships of 5-hydroxytryptophan (5-HTP) and L-dihydroxyphenylalanine (L-DOPA) on reproductive and metabolic responses of migratory red headed bunting (Emberiza bruniceps) J Interdiscipl Cycle Res. 1990;21:129–139. [Google Scholar]
- 4.Chaturvedi CM, Jaiwal R. Temporal synergism of neurotransmitter affecting drugs and seasonal reproductive responses of Indian palm squirrel, Funambulus pennanti. J Neural Transm. 1990;81:31–40. doi: 10.1007/BF01245443. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi CM, Prasad SK. Timed daily injections of neurotransmitter precursor alter the gonadal and body weights of Spotted munia, Lonchura punctulata maintained under short daily photoperiods. J Exp Zool. 1991;260:194–201. [Google Scholar]
- 6.Kumar P, Chaturvedi CM. Correlation of nitric oxide (NO) activity and gonadal function in Japanese quail, Coturnix coturnix japonica following temporal phase relation of serotonergic and dopaminergic oscillations. Anim Reprod Sci. 2008;106:48–64. doi: 10.1016/j.anireprosci.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Phillips D, Chaturvedi CM. Functional maturation of neuroendocrine gonadal axis is altered by specific phase relations of circadian neurotransmitter activity in Japanese quail. Biomed Environ Sci. 1995;8:367–377. [PubMed] [Google Scholar]
- 8.Jaiwal R, Chaturvedi CM. Elimination of testicular regression by 12 h temporal relationship of serotonergic and dopaminergic activity in Indian palm squirrel, Funanbulus pennanti. J Neural Transm. 1991;84:45–52. doi: 10.1007/BF01249108. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi CM, Singh AB. Suppression of annual testicular development in Indian palm squirrel, Funanbulus Pennanti by 8 h temporal relationship of serotonin and dopamine precursor drugs. J Neural Transm. 1992;88:53–60. doi: 10.1007/BF01245036. [DOI] [PubMed] [Google Scholar]
- 10.Wilson JM, Meier AH. Seasonal changes in the circadian rhythms of serotonin and dopamine concentration in right and left supra chiasmatic nuclei (SCN) of Syrian hamster. Chronobiology. 1987;14:255. [Google Scholar]
- 11.Chaturvedi CM, Tiwari AC, Kumar P. Effect of temporal synergism of neural oscillations on photorefractoriness in Japanese quail (Coturnix coturnix japonica) J Exp Zool A Comp Exp Biol. 2006;305:3–12. doi: 10.1002/jez.a.236. [DOI] [PubMed] [Google Scholar]
- 12.Tiwari AC, Kumar P, Singh S, Sharma D, Chaturvedi CM. Reproductive phase dependent temporal co-relation and circadian variation in hypothalamic concentration of serotonin, dopamine and peripheral thyroxine level in Japanese quail following 5-HTP and L-DOPA administration at specific time interval. Biol Rhythm Res. 2006;37:73–86. [Google Scholar]
- 13.Kumar P, Pati AK, Mohan J, Sastry KVH, Tyagi JS, Chaturvedi CM. Effects of simulated hypo- and hyper-reproductive conditions on the characteristics of circadian rhythm in hypothalamic concentration of serotonin and dopamine and in plasma levels of thyroxine, triiodothyronine, and testosterone in Japanese quail, Coturnix coturnix japonica. Chronobiol Int. 2009;26:28–46. doi: 10.1080/07420520802697882. [DOI] [PubMed] [Google Scholar]
- 14.Sethi S, Chaturvedi CM. Temporal synergism of neurotransmitters (serotonin and dopamine) affects testicular development in mice. Zoology. 2009;112:461–470. doi: 10.1016/j.zool.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Comm. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 16.Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K. Gonadotropin inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology. 2006;147:1187–1194. doi: 10.1210/en.2005-1178. [DOI] [PubMed] [Google Scholar]
- 17.Kriegsfeld LJ, Feng-Mei D, Bentley GE, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsutsui K, Ubuka T, Yin H, Osugi T, Ukena K, Bentley GE, Ciccone N, Inoue K, Chowdhury VS, Sharp PJ, Wingfield JC. Mode of action and functional significance of avain gonadotropin-inhibitory hormone (GnIH): a review. J Exp Zool A Comp Exp Biol. 2006;305:801–806. doi: 10.1002/jez.a.305. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsui K, Ukena K. Hypothalamic LPXRF-amide peptides in vertebrates: identification, localization and hypophysiotropic activity (review) Peptides. 2006;29:1121–1129. doi: 10.1016/j.peptides.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Tsutsui K, Bentley GE, Ubuka T, Saigoh E, Yin H, Osugi T, Inoue K, Chowdhury VS, Ukena K, Ciccone N, Sharp PJ, Wingfield JC. The general and comparative biology of gonadotropin-inhibitory hormone (GnIH) Gen Comp Endocrinol. 2007;153:365–370. doi: 10.1016/j.ygcen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Tsutsui K. A new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): biosynthesis, mode of action and functional significance. Prog Neurobiol. 2009;88:76–88. doi: 10.1016/j.pneurobio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, Millar RP, Bentley GE. Potent action of RFRP-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149:5811–5821. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- 23.Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2:703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 24.Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol. 2008;199:105–112. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- 25.Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3 (RFRP-3), a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone (GnRH) neuron firing in the mouse. Endocrinology. 2009;150:2799–2804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 27.Ukena K, Tsutsui K. Distribution of novel RFamide-related peptide-like immunoreactivity in the mouse central nervous system. Neurosci Lett. 2001;300:153–156. doi: 10.1016/s0304-3940(01)01583-x. [DOI] [PubMed] [Google Scholar]
- 28.Danscher G. Light and electron microscopic localization of silver in biological tissue. Histochemistry. 1981;71:177–186. doi: 10.1007/BF00507822. [DOI] [PubMed] [Google Scholar]
- 29.Fuxe K, Butcher LL, Engel J. DL-5-hydroxytryptophan induced changes in central monoamine neurons after peripheral decarboxylase inhibition. J Pharmacol. 1971;23:420–424. doi: 10.1111/j.2042-7158.1971.tb08673.x. [DOI] [PubMed] [Google Scholar]
- 30.Ternaux JP, Bioreu A, Bourgoin S, Hanon M, Hery F, Glowlnski J. In vivo release of 5-HT in the lateral ventricle of the rat: effects of 5-hydroxytryptophan and tryptophan. Brain Res. 1975;101:533–548. doi: 10.1016/0006-8993(76)90476-5. [DOI] [PubMed] [Google Scholar]
- 31.Bianchine JR. Drugs for Parkinson's disease: centrally acting muscle relaxant. In: Gilman AG, Goodman LS, editors. Logical Basis of Therapeutics. New York: Macmillan; 1980. pp. 475–493. [Google Scholar]
- 32.Singh SK, Chakravarty S. Antispermatogenic and antifertility effect of 20, 25-diazacholesterol dihydrochloride in mice. Reprod Toxicol. 2003;17:37–44. doi: 10.1016/s0890-6238(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 33.Singh SK. Testicular toxicity of some chemical agents. In: Joshi SC, Ansari AS, editors. Advances in Reproductive Toxicology. Jaipur: Pointer; 2006. pp. 138–145. [Google Scholar]
- 34.Russell LD, Ettlin RA, Hikim APS, Clegg ED. Histology and Histopathological Evaluation of the Testis. Clearwater: Cache River Press; 1990. [Google Scholar]
- 35.Sternberger LA, Sternberger NH. The unlabeled antibody method: comparison of peroxidase-antiperoxidase with avidin-biotin complex by a new method of quantification. J Histochem Cytochem. 1986;34:599–605. doi: 10.1177/34.5.3517144. [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi CM, Newton BW, Cornett LE, Koike TI. An in situ hybridization and immunohistochemical study of vasotocin neurons in the hypothalamus of water-deprived chickens. Peptides. 1994;15:1179–1187. doi: 10.1016/0196-9781(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 37.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51:171–180. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke IJ, Qi Y, Sari IP, Smith JT. Evidence that RF-amide related peptides are inhibitors of reproduction in mammals. Front Neuroendocrinol. 2009;30:371–378. doi: 10.1016/j.yfrne.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci. 2009;106:11324–11329. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senthilkumaran B, Joy KP. Effects of ovariectomy and oestradiol replacement on hypothalamic serotonergic and monoamine oxidase activity in the catfish, Heteropneustes fossilis: a study correlating plasma oestradiol and gonadotrophin levels. J Endocrinol. 1994;142:193–203. doi: 10.1677/joe.0.1420193. [DOI] [PubMed] [Google Scholar]