Abstract
The goal of this study was to evaluate which anthropometric measure (human body measurement) best predicts insulin resistance measured by the insulin sensitivity index (SI) and the homeostasis model of assessment of insulin resistance (HOMA-IR) in nondiabetic patients with schizophrenia patients treated with clozapine or olanzapine.
Methods
We conducted a cross-sectional study of nondiabetic subjects with schizophrenia being treated with olanzapine or clozapine using a frequently sampled intravenous glucose tolerance test, nutritional assessment, and anthropometric measures to assess the relationship between anthropometric measures and insulin resistance.
Results
No difference was found between the groups treated with clozapine and olanzapine in age, gender, race, body mass index (BMI), waist circumference (WC), lipid levels, HOMA-IR, or SI. The disposition index (SI × the acute insulin response to glucose), which measures how the body compensates for insulin resistance to maintain a normal glucose level, was significantly lower in the group treated with clozapine than in the group treated with olanzapine (1067 ± 1390 vs. 2521 ± 2805; p = 0.013), suggesting that the subjects treated with clozapine had a reduced compensatory response to IR compared with the subjects treated with olanzapine. In the clozapine group, both higher WC and BMI were significantly associated with elevated HOMA-IR and lower SI; however, WC was a stronger correlate of IR than BMI, as measured by SI (−0.50 vs. −0.40). In the olanzapine group, neither WC nor BMI was significantly associated with any measure of glucose metabolism.
Conclusions
In this study, WC was the single best anthropometric surrogate for predicting IR in patients treated with clozapine but not olanzapine. The results suggest that WC may be a valuable screening tool for predicting IR in patients with schizophrenia being treated with clozapine who are at relatively higher risk of developing the metabolic syndrome, type 2 diabetes mellitus, and associated cardiovascular disease.
Keywords: schizophrenia, waist circumference, insulin resistance, glucose metabolism, metabolic syndrome, diabetes mellitus, second-generation (atypical) antipsychotics, clozapine, olanzapine
INTRODUCTION
Evidence suggests that treatment with second-generation (atypical) antipsychotic drugs, particularly clozapine and olanzapine, is strongly associated with the development of insulin resistance (IR), type 2 diabetes mellitus,1–7 dyslipidemia,4,6–10 and obesity.6,7,11–14 These metabolic abnormalities along with hypertension15 are major modifiable cardiovascular risk factors, and the key components of metabolic syndrome, which is highly predictive of overt type 2 diabetes mellitus and cardiovascular diseases.16 Consequently, the prevalence of metabolic syndrome and cardiovascular diseaeses in the population with schizophrenia who take second-generation antipsychotics is much higher than in the general population.13,17–22 In 2005, McEvoy et al.23 found that the age-adjusted prevalence of metabolic syndrome in patients with schizophrenia was 40.9%, based on the criteria from the third report of the National Cholesterol Education Program’s Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (NCEP ATP III), and or 42.7%, based on the criteria of the American Heart Association (AHA). In 2002, Ford et al. reported that the age-adjusted prevalence of metabolic syndrome in the general population was 23.7% using the NCEP ATP III criteria.24 This study also found that the Mexican American group had the highest age-adjusted prevalence (31.9%) of metabolic syndrome, and that the age-adjusted prevalence was higher in Mexican American and African American women than men. They also found that the prevalence of metabolic syndrome increased with age in all racial groups.24
In 2001, NCEP ATP III highlighted the importance of assessment and early treatment for each component of the metabolic syndrome by formulating working criteria, which adopted waist circumference (WC) as the best surrogate for visceral adipose tissue (VAT). VAT as measured by WC has been shown to have a stronger association with cardiometabolic risk factors than general adiposity as measured by body mass index (BMI).25–34 Other findings suggest that both general adiposity and VAT, either acting additively35–41 or independently,42 are strong predictors not only of IR36–38,43 but also of type 2 diabetes mellitus,35,42,44 atherogenic dyslipidemia,41 and hypertension.44 Obesity per se may not, however, be sufficient to cause the metabolic syndrome.45 Many studies show that IR with resulting hyperinsulinemia precedes the development of various metabolic abnormalities even after adjusting for obesity.46–48 IR also develops the inflammatory milieu for cardiovascular disease.43,49,50 IR may be observed in the population of individuals with normal weight and, conversely, substantial numbers of individuals who are overweight or obese may remain insulin sensitive. Similarly, metabolic benefit and decreased risk of cardiovascular disease (CVD) following weight loss occur primarily in those overweight or obese individuals who are also insulin resistant.51 Thus, while obesity is correlated with IR, both may act independently52 or IR may accentuate the effect of obesity53 in contributing to the risk of CVD. Nevertheless, VAT best predicts the degree of IR54 and derangements in plasma glucose-insulin homeostasis32,33 regardless of total body fat mass.
Following the Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes, the American Diabetic Association in 2004 noted that the etiology of the increased prevalence of type 2 diabetes mellitus in psychiatric disorders could be due to the weight gain associated with certain second-generation antipsychotic agents.55 A recent study, however, suggests that, despite these guidelines, monitoring for obesity and metabolic abnormalities such as elevated glucose and lipid levels was poor during a mean 600 ± 235 day follow-up period.56
Several studies suggest that obesity due to second generation antipsychotic drugs such as clozapine and olanzapine is an independent factor that contributes to the development of other cardiometabolic risks, such as type 2 diabetes mellitus, dyslipidemia, and hypertension.7,13,20,21,57 Similarly, other findings suggest that these drugs are associated with glucose abnormalities that vary in severity independent of adiposity.3,58,59 The usefulness of anthropometric measures (human body measurements) in predicting obesity-related metabolic side effects such as IR associated with antipsychotic agents in patients with schizophrenia is still uncertain. One study found that both BMI and WC significantly predicted abnormalities in glucose homeostasis measured by a frequently sampled intravenous glucose tolerance test (FSIVGTT) in patients with schizophrenia taking olanzapine, risperidone, ziprasidone, or first-generation antipsychotics.60 Clozapine and olanzapine have been found to be more strongly associated with IR and weight gain than any other antipsychotics.3,13,22 The goal of the study described in this paper was to evaluate which anthropometric measure best predicts IR, as measured by FSIVGTT and the homeostasis model of assessment of insulin (HOMA-IR), in nondiabetic patients with schizophrenia being treated with clozapine or olanzapine.
METHODS AND MATERIALS
The study was approved by the institutional review boards of the Massachusetts General Hospital and the Massachusetts Department of Mental Health. Fifty-seven outpatients with schizophrenia who had been taking either clozapine or olanzapine for a minimum of 1 year with well-established adherence to the medication were recruited from an urban mental health center. Patients were excluded on the basis of current substance abuse; diabetes mellitus; thyroid disease; pregnancy; significant medical illness including severe cardiovascular, hepatic, or renal diseases; or unstable psychiatric illness. Patients treated with the following medications known to affect glucose tolerance were also excluded: birth control pills containing norgestrel, steroids, beta-blockers, anti-inflammatory drugs (including aspirin and ibuprofen), thiazide diuretics, agents that induce weight loss, and valproate sodium. After providing written informed consent, demographic data (e.g., age, gender, race, age of illness onset, family history of diabetes) were recorded and information on habitual physical activity behaviors was documented using a self-administered questionnaire. Subjects then underwent a diagnostic evaluation by a research psychiatrist using the Structured Clinical Interview for DSM-IV.61 Subjects were given a diet plan calculated to maintain body weight and to provide a minimum of 250 gm of carbohydrate for each of the 3 days prior to the FSIVGTT. Subjects were also instructed to fast for the 12 hours preceding the FSIVGTT and not to take their morning medications the day of the test. Family, residential program staff, and outreach workers assisted subjects in maintaining high carbohydrate intake and then guaranteeing fasting. Subjects were admitted to the clinical research center at 6:45 A.M. on the morning of the test. A complete nutritional assessment was conducted at admission and immediately prior to the initiation of the FSIVGTT.
Anthropometric Measurements
Height was measured using a Harpenden stadiometer, which was calibrated on a weekly basis. Subjects were weighed on a digital electronic scale, and weight was recorded to the nearest 0.1 kg. BMI was calculated by weight (kg)/height (m2). Waist circumferences were measured at the narrowest waist, umbilicus waist, iliac waist, and broadest hip (buttocks). Waist-hip ratio was calculated as the iliac waist measurement relative to the widest hip circumference. A quantitative activity questionnaire (Modifiable Activity Questionnaire) was used to assess both leisure and occupational activity components62
Frequently Sampled Intravenous Glucose Tolerance Test
Subjects with possible diabetes mellitus (fasting plasma glucose level ≥ 126 mg/dL [6.99 mmol/L]) at baseline were dropped from the study. Those who had a fasting plasma glucose level below 126 mg/dL underwent a FSIVGTT. The FSIVGTT procedure involved placing two intravenous catheters in antecubital veins (1 in each arm). Baseline blood samples were drawn for fasting plasma glucose and serum insulin levels, basic chemistry profiles, lipid profile, complete blood count, and serum clozapine or olanzapine concentrations 10 minutes prior to the glucose infusion (time −10 minutes). Glucose 0.3 gm/kg in normal saline was then administered intravenously for 30 seconds (time 0). Blood samples of approximately 2 mL each were drawn at −10, −5, 0, 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, 18, 20, 21, 22, 23, 24, 25, 27, 30, 35, 40, 45, 50, 55, 60, 65, 70, 80, 90, 100, 110, 120, 140, 160, and 180 minutes for measurement of plasma glucose and serum insulin concentrations.63–66 Twenty minutes after the glucose infusion, 0.05 units/kg Humulin insulin (Eli Lilly, Indianapolis, IN) was administered intravenously for 45 seconds. Vital signs and plasma glucose concentrations were monitored throughout the procedure.
Minimal Model Calculation
Insulin sensitivity index (SI), glucose effectiveness or utilization (SG), and the acute insulin response to glucose (AIRG) were calculated based on plasma glucose and serum insulin values using the Minimal Model (MINMOD) Millenium computer program developed by Richard Bergman, PhD.64,65,67 The SI represents the increase in net fractional glucose clearance rate per unit change in serum insulin concentration after the intravenous glucose load. The SG represents the net fractional glucose clearance rate due to the increase in glucose independent of any increase in circulating insulin concentrations above baseline. The AIRG measures the acute (0–10 minutes) beta-cell response to a glucose load calculated by the areas under the curve that were higher than basal insulin values. The AIRG was assessed as the incremental area under the curve (calculated by the trapezoid rule) from 0 to 10 minutes of the FSIVGTT. The disposition index, which equals SI × AIRG), an index of beta-cell function that takes account of prevailing insulin sensitivity and exploits the hyperbolic relationship between the two63,68 was calculated by the method described by Kahn et al.68 The HOMA-IR is an alternative method for assessing insulin resistance and beta-cell function on the basis of known relationships between fasting plasma glucose and serum insulin concentrations. The HOMA-IR was calculated using the following formula: fasting serum insulin concentration × fasting plasma glucose concentration/22.5.69,70 The HOMA-IR was calculated by taking the mean of 3 fasting values (times −10, −5, and 0).
Laboratory Assays
Laboratory assays were performed by the chemistry laboratory and the Mallinckrodt General Clinical Research Center Core Laboratory of Massachusetts General Hospital. Insulin immunometric assays were performed using an Immulite Analyzer (Diagnostic Product Corp; Los Angeles, Calif) with an intra-assay coefficient of variation of 4.2% to 7.6%. Fasting plasma glucose level was measured with a hexokinase reagent kit (A-gent glucose test; Abbott, South Pasadena, Calif). Glucose assays were run in duplicate, and the intra-assay coefficient of variation ranged from 2% to 3%. Fasting total plasma cholesterol and triglyceride levels were measured enzymatically71 with an intra-assay coefficient of variation of 1.7% to 2.7% and 0.9% to 1.2%, respectively. The high-density lipoprotein cholesterol fraction was measured after precipitation of low-density and very low–density lipoproteins with dextran sulfate-magnesium72 with an intra-assay coefficient of variation of 0.89% to 1.82%. Low-density lipoprotein cholesterol values were estimated indirectly for participants with plasma triglyceride levels less than 400 mg/dL (4.52 mmol/L).73
Statistical analysis
The data were analyzed using SPSS (version 13.0, SPSS Inc., Chicago). Descriptive statistics were employed to describe demographics, anthropometric, and laboratory measures. HOMA-IR was not distributed normally and was, therefore, log transformed before analysis. Stepwise multiple linear regression was used to examine whether one or more anthropometric measures might predict IR as measured by HOMA-IR or SI when other confounding variables were also considered. The criteria of p = 0.05 for a variable to enter and p ≥ 0.10 for a variable to be removed were used in the multiple linear regression analysis. Pearson correlation coefficients were used to quantify relations between glucose metabolism measures and anthropometric measures. Further; partial correlation coefficients were used to examine anthropometric correlates of glucose metabolism after controlling for potential covariates. For all statistical analyses, a p value less than 0.05 (2 tailed) was used to test for statistical significance.
RESULTS
Of the 57 subjects, 35 were treated with clozapine (duration ranging from 24 to 168 months, median 72 months; daily dose range 100–600 mg, median 350 mg) and 22 subjects were treated with olanzapine (duration ranging from 12 to 108 months, median 25 months; daily dose range 5–30 mg, median 15 mg). There was a significant difference in length of treatment between clozapine and olanzapine (p < 0.001). Demographic and clinical characteristics of the study sample are shown in Table 1. There was no difference between the clozapine and olanzapine groups in age, gender, race, BMI, WC, lipid levels, SI, SG, and activity levels as measured with the Modifiable Activity Questionnaire. The disposition index was significantly lower in the group treated with clozapine compared with the group treated with olanzapine (1067 ± 1390 vs. 2521 ± 2805; p = 0.013), suggesting that the subjects treated with clozapine had a reduced compensatory to IR compared with the subjects treated with olanzapine.
Table 1.
Demographic and clinical characteristics of non-diabetic subjects treated with clozapine or olanzapine.
| Entire sample (N=57) |
Clozapine (N=35) |
Olanzapine (N=22) |
Group Comparison | p value | |
|---|---|---|---|---|---|
| Age | 42 ± 10 | 41 ± 10 | 44 ± 11 | t(55)= 1.32 | 0.194 |
| Age of illness onset(years) | 23±9 | 21±7 | 24±10 | t(30)=0.88 | 0.386 |
| Gender, N (%) | X2(1)= 1.01 | 0.314 | |||
| Male | 46 (81) | 28(80) | 18(82) | ||
| Female | 11(19) | 7(20) | 4 (18) | ||
| Race, N (%) | X2(2)= 5.66 | 0.059 | |||
| Caucasian | 48 (84) | 32(91) | 16 (73) | ||
| Black | 8(14) | 2 (6) | 6 (27) | ||
| Hispanic | 1 (2) | 1 (3) | 0 (0) | ||
| Family history of diabetes | X2(1)= 2.55 | 0.111 | |||
| Yes | 23 (40) | 17(49) | 6 (27) | ||
| No | 34 (60) | 18(51) | 16(73) | ||
| Exposure to CLZ or OLZ (months) | 63 (41) | 83 (37) | 32 (25) | t(51)=−6.08 | <.001 |
| MAQ total score | 13.7±11.2 | 15.2±9.7 | 11.1±13.2 | t(55)= −1.30 | 0.198 |
| MAQ leisure | 7.7±7.5 | 8.7±5.9 | 6.2±9.4 | t(55)= −1.23 | 0.225 |
| MAQ occupation | 6.1±8.9 | 6.5±8.3 | 5.3±10 | t(54)= −0.49 | 0.623 |
| Weight(kg) | 82±15 | 82±16 | 83±14 | t(55)= 0.26 | 0.797 |
| BMI(kg/m2) | 27.4±4.8 | 27.7±5 | 26.8±4.6 | t(55)= −0.68 | 0.498 |
| Waist circumference(cm) | 99.7±10.7 | 100.1±10.2 | 99.2±11.5 | t(53)= −0.30 | 0.763 |
| Waist-Hip ratio | 0.97±0.08 | 0.99±0.08 | 0.96±0.07 | t(48)= −1.22 | 0.228 |
| Total cholesterol(mg/dL) | 173±37 | 170±31 | 181±48 | t(49)= 1.05 | 0.301 |
| HDL-C(mg/dL) | 36±13 | 36±13 | 35±13 | t(49)= −0.03 | 0.975 |
| LDL-C(mg/dL) | 98±33 | 94±31 | 104±38 | t(44)= 0.88 | 0.386 |
| Triglycerides(mg/dL) | 197±130 | 203±136 | 184±119 | t(48)=−0.46 | 0.651 |
| Fasting plasma glucose(mg/dL) | 95±10 | 95±8 | 96±13 | t(55)=0.07 | 0.943 |
| Fasting serum insulin(µIU/L) | 9.3±6.6 | 9.0±6.3 | 9.6±7.2 | t(55)=0.31 | 0.754 |
| HOMA-IR | 2.2±1.6 | 2.2±1.6 | 2.3±1.7 | t(55)= 0.96 | 0.845 |
| SI (× 10−4 min−1 per µU/mL) | 3.8±3.2 | 3.7±3.6 | 4.1±2.3 | t(55)= 0.43 | 0.673 |
| SG(min−1) | 0.0160±0.0062 | 0.0160±0.0057 | 0.0161±0.0069 | t(52)= 0.03 | 0.975 |
| AIRG (AUG, µU/mL per 10min) | 503±496 | 396±475 | 653±497 | t(51)= 1.90 | 0.063 |
| DI | 1638±2163 | 1067±1390 | 2521±2805 | t(54)= 2.60 | 0.013 |
Note: MAQ: Modified activity questionnaire; BMI: Body mass index; HDL-C: High density lipoprotein cholesterol; LDL-C: Low density lipoprotein cholesterol; HOMA-IR: Homeostasis model of assessment of insulin resistance; SI: Insulin sensitivity index; SG: Glucose effectiveness; AIRG: Acute insulin response to glucose; DI: Disposition index
At first, a stepwise multiple linear regression model was developed for the entire sample to identify relevant predictors of IR as measured by HOMA-IR (log transformed) and SI. Various characteristics that might be related to IR, such as antipsychotic agent used (i.e., clozapine or olanzapine in this study), age, gender, race, duration of treatment with antipsychotic agent (clozapine or olanzapine), as well as WC, BMI, and waist-hip ratio were considered as candidate predictors for the regression model. For both outcome measures (HOMA-IR and SI), only WC was able to enter into the regression model (Table 2). WC explained 11% of variance of HOMA-IR and 17% of variance of SI. Both regression models indicated that a greater WC predicts increased IR.
Table 2.
Stepwise regression analysis: Anthropometric correlates of insulin resistance in subjects with schizophrenia treated with clozapine or olanzapine (N = 57).
| Predictor variables entered | R2 change | df | F change | p | |
|---|---|---|---|---|---|
| Model 1 | |||||
| Outcome variable = HOMA-IR (log transformed) | |||||
| Waist circumference | 0.11 | 1,55 | 5.53 | 0.023 | |
| Model 1 summary: Final regression model equation with unstandardized coefficients: HOMA-IR (log transformed) = −0.60 + 0.01 by waist circumference (cm); standard errors corresponding to unstandardized coefficients are 0.36 and 0.004 for constant and waist circumference, respectively. | |||||
| Model 2 | |||||
| Outcome variable = SI | |||||
| Waist circumference | 0.17 | 1,55 | 9.23 | 0.004 | |
| Model 2 summary: Final regression model equation with unstandardized coefficients: SI = 15.98 − 0.12 by waist circumference (cm); standard errors corresponding to unstandardized coefficients are 3.30 and 0.03 for constant and waist circumference, respectively | |||||
Notes: The following variables were evaluated as possible candidates for both regression models: age, gender, race, antipsychotic agent used, duration of exposure to clozapine or olanzapine, body mass index, waist circumference, waist/hip ratio. Only waist circumference met criteria to enter the regression analysis
Waist circumference is taken from umbilicus waist measures;
Waist-hip ratio was calculated as iliac waist measure relative to the broadest hip circumference
x
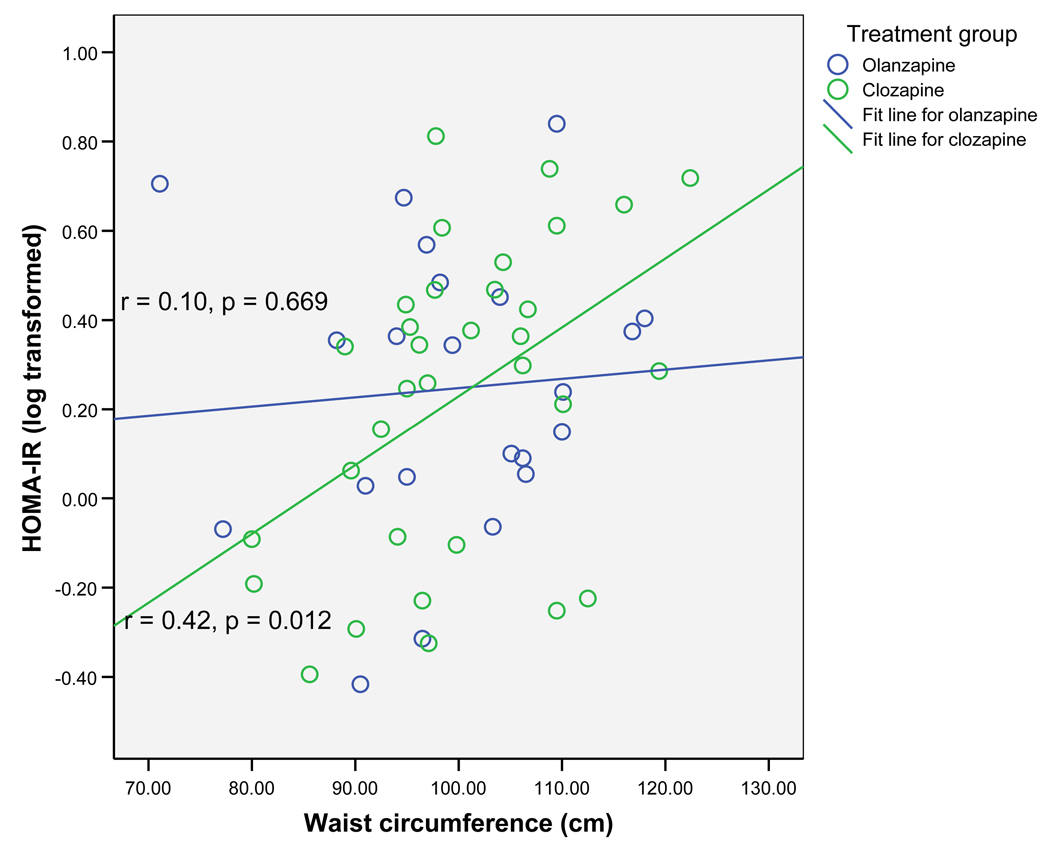
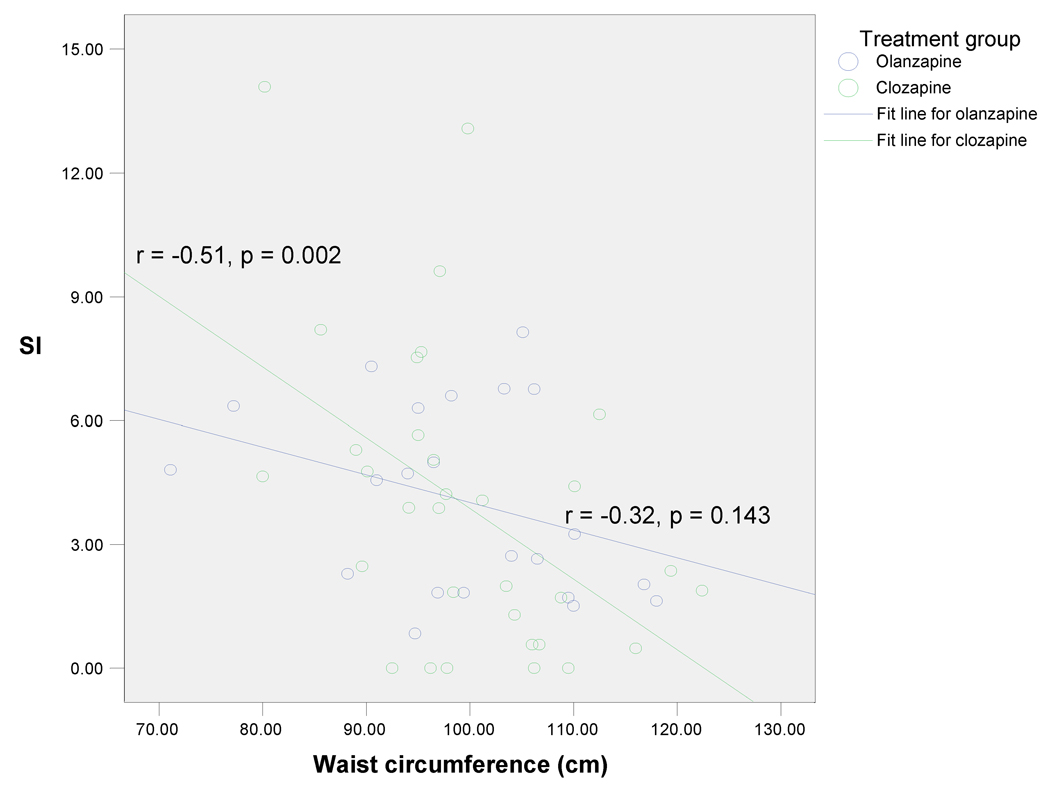
Pearson correlation coefficients between anthropometric measures and glucose metabolism measures were next examined within the two treatment groups (Table 3). In the clozapine group, both higher WC and BMI were significantly associated with elevated HOMA-IR, lower SI, and elevated AIRG (p < 0.05 for all). However, WC was a stronger correlate of IR than BMI, as measured by SI (−0.51 vs. −0.42). In contrast, in the olanzapine group, neither WC nor BMI was significantly associated with any measure of glucose metabolism (p > 0.05 for all) (Table 3). Figure 1 illustrates that WC was positively correlated with HOMA-IR; the finding was significant in the clozapine group (r = 0.42, p = 0.012) but not in the olanzapine group (r = 0.10, p = 0.669). Likewise, as shown in Figure 2, WC was negatively correlated with SI; the finding was again significant in the clozapine group (r= −0.51, p=0.002) but not in the olanzapine group (r = −0.32, p=0.143). Waist hip ratio was not associated with any measure of glucose metabolism in either group (p > 0.05 for all). The findings from this analysis suggest that, in patients treated with clozapine, increase in WC is associated with a proportional increase in IR, but that such an association does not exist for patients treated with olanzapine.
Table 3.
Correlations between anthropometric and glucose metabolism measures in 57 subjects with schizophrenia.
| BMI | Waist circumference | Waist-hip ratio | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Clozapine group (N=35) | ||||||
| HOMA-IR (log transformed) | 0.41 | 0.014 | 0.42 | 0.012 | − 0.24 | 0.166 |
| SI | − 0.42 | 0.013 | − 0.51 | 0.002 | 0.06 | 0.718 |
| SG | 0.15 | 0.410 | 0.17 | 0.359 | − 0.21 | 0.260 |
| AIRG | 0.41 | 0.022 | 0.40 | 0.024 | 0.03 | 0.868 |
| Disposition index | − 0.11 | 0.545 | − 0.20 | 0.254 | − 0.22 | 0.207 |
| Olanzapine group (N=22) | ||||||
| HOMA-IR (log transformed) | 0.03 | 0.905 | 0.10 | 0.669 | − 0.15 | 0.508 |
| SI | − 0.26 | 0.249 | − 0.32 | 0.143 | − 0.13 | 0.567 |
| SG | 0.16 | 0.488 | − 0.01 | 0.959 | 0.06 | 0.788 |
| AIRG | − 0.04 | 0.874 | 0.10 | 0.670 | 0.05 | 0.843 |
| Disposition index | − 0.10 | 0.668 | − 0.11 | 0.623 | − 0.06 | 0.804 |
BMI: body mass index; HOMA-IR: homeostasis model of assessment of insulin resistance; SI: insulin sensitivity index; SG: glucose effectiveness; AIRG: acute insulin response to glucose.
Figure 1.
Relationship between waist circumference and homeostasis model of assessment of insulin (HOMA-IR)
Figure 2.
Relationship between waist circumference and insulin sensitivity index (SI)
To further validate the findings discussed above, a partial correlation analysis was performed within the treatment groups after controlling again for potential confounding variables including age, gender, race, duration of treatment with antipsychotic agent, and family history of diabetes (Table 4). In the clozapine group, higher WC was still significantly associated with lower SI and elevated HOMA-IR (p < 0.05 for both). Moreover, between WC and BMI, WC seemed to correlate more strongly than BMI for predicting IR as measured by HOMA-IR (log transformed) (r = 0.48 vs. r = 0.46) and SI (r = −0.50 vs. r = −0.40) in the clozapine group. In the olanzapine group, neither WC nor BMI was significantly associated with any measure of glucose metabolism (p > 0.05 for all). Waist-hip ratio was also not correlated with any measure of glucose metabolism in either group (p > 0.05 for all).
Table 4.
Partial correlations between anthropometric and glucose metabolism measures after controlling for age, gender, race, duration of exposure to clozapine or olanzapine, and family history of diabetes.
| BMI | Waist circumference | Waist-hip ratio | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Clozapine group (N=35) | ||||||
| HOMA-IR (log transformed) | 0.46 | 0.054 | 0.48 | 0.046 | 0.29 | 0.251 |
| SI | − 0.40 | 0.098 | − 0.50 | 0.033 | − 0.10 | 0.682 |
| SG | − 0.09 | 0.733 | −0.03 | 0.896 | 0.21 | 0.399 |
| AIRG | 0.38 | 0.123 | 0.32 | 0.202 | 0.09 | 0.720 |
| Disposition index | − 0.02 | 0.926 | − 0.10 | 0.694 | − 0.11 | 0.676 |
| Fasting glucose | 0.20 | 0.438 | 0.17 | 0.509 | 0.21 | 0.934 |
| Fasting insulin | 0.34 | 0.172 | 0.39 | 0.106 | 0.15 | 0.564 |
| Olanzapine group (N=22) | ||||||
| HOMA-IR (log transformed) | − 0.05 | 0.858 | .004 | 0.990 | − 0.42 | 0.104 |
| SI | − 0.17 | 0.541 | − 0.25 | 0.360 | − 0.02 | 0.934 |
| SG | − 0.10 | 0.701 | − 0.10 | 0.713 | − 0.00 | 0.987 |
| AIRG | − 0.13 | 0.645 | 0.03 | 0.904 | − 0.28 | 0.294 |
| Disposition index | − 0.08 | 0.782 | − 0.11 | 0.675 | − 0.12 | 0.653 |
| Fasting glucose | − 0.03 | 0.927 | − 0.03 | 0.626 | − 0.37 | 0.802 |
| Fasting insulin | − 0.13 | 0.635 | −0.07 | 0.795 | − 0.14 | 0.165 |
BMI: body mass index; HOMA-IR: homeostasis model of assessment of insulin resistance; SI: insulin sensitivity index; SG: glucose effectiveness; AIRG: acute insulin response to glucose.
DISCUSSION
In our study, WC was the single best anthropometric surrogate to predict IR in patients treated with clozapine. Neither WC, BMI, nor waist-hip ratio was found to be a strong predictor of IR in subjects treated with olanzapine. The association between WC and IR in the clozapine but not the olanzapine group raises the possibility that a different anthropometric measure may predict increased IR for these two antipsychotic drugs or that different mechanisms are involved in the IR that can occur in association with these two drugs. The findings support the use of WC as a valuable screening tool to predict IR in patients with schizophrenia being treated with clozapine who are at relatively higher risk of developing the metabolic syndrome, type 2 diabetes mellitus, and associated CVD.1,2,6,12
The side-effect profile of clozapine has always created dilemmas for clinicians concerning the therapeutic use of this highly effective drug. Agranulocytosis, the once fearsome side effect of clozapine, is now largely preventable by screening with regular blood tests. The various metabolic abnormalities that constitute the metabolic syndrome caused by clozapine can also be screened for using criteria proposed by NCEP ATP III. Of the five criteria, WC can be easily measured at the clinic with less expertise required. WC is regarded as the best measure of VAT,26,28,30 which is a highly sensitive predictor of IR.54 IR has also been regarded as playing the key role in the development of other metabolic abnormalities.46,48 Although obesity contributes to the development of IR,22 generally not all overweight and obese individuals are insulin resistant, while even an individual with normal weight can have IR.51 There is a documentation that non-obese patients with schizophrenia treated with clozapine and olanzapine have developed IR.3,58
A strong relationship between WC and IR has been reported in the general population, and the stepwise multiple linear regression models in our study indicated that greater WC predicted increased IR regardless of whether the patient was treated with clozapine or olanzapine. However, further analysis comparing anthropometric measures and IR in the two groups showed that WC, and BMI to lesser extent, but not waist-hip ratio were strongly correlated with IR only in the clozapine group. Based on the results of the regression model, it is possible that the effect of olanzapine on glucose metabolism may be consistent through a range of waist measurements (i.e., individuals treated with olanzapine may have the same degree of IR regardless of weight gain and waist measurement). It is also possible that a significant correlation could not be detected in the small sample of only 22 subjects treated with olanzapine in this study (having comparatively less abnormal glucose homeostasis than the clozapine subjects). It is also possible that WC may not be a sensitive measure for predicting IR in patients treated with olanzapine. Finally, it is also possible that olanzapine may cause IR independent of obesity.
This study had a number of limitations. Because drug treatment was not randomized and assessment was cross-sectional, the finding of an association between olanzapine and clozapine treatment, impairment of glucose metabolism, and anthropometric measures cannot be conclusively established as a causal relationship. In addition, the exclusion of subjects on various medications may limit the generalizability of our findings. We also could not factor in the effects of previous treatment with other drugs. In addition, there was a statistically significant difference in duration of treatment with each antipsychotic drug. While the duration of treatment with each drug was different, the two groups were similar in age and age of onset. Despite the difference in duration of treatment, there was no difference between groups on any of the measure that assess weight and adiposity (BMI, WC, waist-hip ratio) or metabolic outcomes including lipid levels and measures of IR (SI, HOMA-IR, SG). As supported by the literature, the effect these drugs have on insulin sensitivity appears to occur rather early in treatment, so that there was sufficient time for this effect to appear in both groups. However, there was a difference between the two groups in disposition index, which may, in fact, reflect duration of treatment, as disposition index represents the ability of beta cells to respond to IR. The olanzapine group appeared to have a more robust disposition index in response to IR compared with the clozapine group, which may reflect the shorter mean duration of treatment with olanzapine.
Future studies that include larger samples, unmedicated patients, and varying durations of prospective antipsychotic exposure can address some of the limitations of this study. At present, we recommend monitoring VAT by measuring WC along with monitoring of fasting glucose, lipid levels, and blood pressure for patients with schizophrenia being treated with clozapine. Clinicians may also want to consider simple measures, such as monitoring belt loop size, as a surrogate for direct waist measurements, for patients or clinicians who are uncomfortable with the waist measurement procedure.
Acknowledgments
Funding: Stanley Foundation, (DCH); National Institute of Health (NIH/NCRR) Grant 5MO1RR01066-24 (General Clinical Research Center), and a NARSAD New Investigator Award (DCH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Study sponsors were not involved in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the report for publication. The authors have listed the following disclosures: Dr. Henderson has received honoraria or research support over the past year from Takeda, Bristol-Myers Squibb, Pfizer, Solvay, Janssen, and COVANCE. Dr. Goff has received honoraria or research support over the past year from Wyeth, Eli Lilly, Pfizer, Novartis, Indevus, Lundbeck, and Schering-Plough. Dr. Copeland has received an honorarium from Eli Lilly. Dr. Fan has received research support from Eli Lilly. Dr. Freudenreich has received research support from Cephalon. Dr. Evins has received research support from Pfizer, GlaxoSmithKline, and National Institute on Drug Abuse (NIDA) CDDC. Dr. Cather, Dr. Sharma and Ms. Borba report no disclosures.
References
- 1.Kamran A, Doraiswamy PM, Jane JL, et al. Severe hyperglycemia associated with high doses of clozapine. Am J Psychiatry. 1994;151:1395. doi: 10.1176/ajp.151.9.1395a. [DOI] [PubMed] [Google Scholar]
- 2.Koller E, Schneider B, Bennett K, et al. Clozapine-associated diabetes. Am J Med. 2001;111:716–723. doi: 10.1016/s0002-9343(01)01000-2. [DOI] [PubMed] [Google Scholar]
- 3.Henderson DC, Cagliero E, Copeland PM, et al. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: A frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry. 2005;62:19–28. doi: 10.1001/archpsyc.62.1.19. [DOI] [PubMed] [Google Scholar]
- 4.Lindenmayer JP, Czobor P, Volavka J, et al. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry. 2003;160:290–296. doi: 10.1176/appi.ajp.160.2.290. [DOI] [PubMed] [Google Scholar]
- 5.Sernyak MJ, Leslie DL, Alarcon RD, et al. Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry. 2002;159:561–566. doi: 10.1176/appi.ajp.159.4.561. [DOI] [PubMed] [Google Scholar]
- 6.Henderson DC, Cagliero E, Gray C, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: A five-year naturalistic study. Am J Psychiatry. 2000;157:975–981. doi: 10.1176/appi.ajp.157.6.975. [DOI] [PubMed] [Google Scholar]
- 7.Newcomer JW. Abnormalities of glucose metabolism associated with atypical antipsychotic drugs. J Clin Psychiatry. 2004;65:36–46. [PubMed] [Google Scholar]
- 8.Olfson M, Marcus SC, Corey-Lisle P, et al. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163:1821–1825. doi: 10.1176/ajp.2006.163.10.1821. [DOI] [PubMed] [Google Scholar]
- 9.Casey DE. Dyslipidemia and atypical antipsychotic drugs. J Clin Psychiatry. 2004;65:27–35. [PubMed] [Google Scholar]
- 10.Wirshing DA, Boyd JA, Meng LR, et al. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry. 2002;63:856–865. doi: 10.4088/jcp.v63n1002. [DOI] [PubMed] [Google Scholar]
- 11.Lamberti JS, Bellnier T, Schwarzkopf SB. Weight gain among schizophrenic patients treated with clozapine. Am J Psychiatry. 1992;149:689–690. doi: 10.1176/ajp.149.5.689. [DOI] [PubMed] [Google Scholar]
- 12.Umbricht DS, Pollack S, Kane JM. Clozapine and weight gain. J Clin Psychiatry. 1994;55:157–160. [PubMed] [Google Scholar]
- 13.Newcomer JW. Metabolic considerations in the use of antipsychotic medications: A review of recent evidence. J Clin Psychiatry. 2007;68:20–27. [PubMed] [Google Scholar]
- 14.Wirshing DA, Wirshing WC, Kysar L, et al. Novel antipsychotics: Comparison of weight gain liabilities. J Clin Psychiatry. 1999;60:358–363. [PubMed] [Google Scholar]
- 15.Henderson DC, Daley TB, Kunkel L, et al. Clozapine and hypertension: A chart review of 82 patients. J Clin Psychiatry. 2004;65:686–689. doi: 10.4088/jcp.v65n0514. [DOI] [PubMed] [Google Scholar]
- 16.Wannamethee SG, Shaper AG, Lennon L, et al. Metabolic syndrome vs Framingham risk score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 17.Bobes J, Arango C, Aranda P, Carmena, et al. Cardiovascular and metabolic risk in outpatients with schizophrenia treated with antipsychotics: Results of the CLAMORS Study. Schizophr Res. 2007;90:162–173. doi: 10.1016/j.schres.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Osby U, Correia N, Brandt L, et al. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res. 2000;45:21–28. doi: 10.1016/s0920-9964(99)00191-7. [DOI] [PubMed] [Google Scholar]
- 19.Casey DE, Haupt DW, Newcomer JW, et al. Antipsychotic-induced weight gain and metabolic abnormalities: Implications for increased mortality in patients with schizophrenia. J Clin Psychiatry. 2004;65:4–18. quiz 19–20. [PubMed] [Google Scholar]
- 20.Newcomer JW. Metabolic risk during antipsychotic treatment. Clin Ther. 2004;26:1936–1946. doi: 10.1016/j.clinthera.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13:S170–S177. [PubMed] [Google Scholar]
- 22.Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry. 2006;51:480–491. doi: 10.1177/070674370605100803. [DOI] [PubMed] [Google Scholar]
- 23.McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80:19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 25.Ho SC, Chen YM, Woo JL, et al. Association between simple anthropometric indices and cardiovascular risk factors. Int J Obes Relat Metab Disord. 2001 Nov;25(11):1689–1697. doi: 10.1038/sj.ijo.0801784. [DOI] [PubMed] [Google Scholar]
- 26.Rankinen T, Kim SY, Perusse L, Despres JP, Bouchard C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord. 1999;23:801–809. doi: 10.1038/sj.ijo.0800929. [DOI] [PubMed] [Google Scholar]
- 27.Reeder BA, Senthilselvan A, Despres JP, et al. Canadian Heart Health Surveys Research Group. The association of cardiovascular disease risk factors with abdominal obesity in Canada. CMAJ. 1997;157:S39–S45. [PubMed] [Google Scholar]
- 28.Onat A, Avci GS, Barlan MM, et al. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord. 2004;28:1018–1025. doi: 10.1038/sj.ijo.0802695. [DOI] [PubMed] [Google Scholar]
- 29.Aekplakorn W, Kosulwat V, Suriyawongpaisal P. Obesity indices and cardiovascular risk factors in Thai adults. Int J Obes (Lond) 2006;30:1782–1790. doi: 10.1038/sj.ijo.0803346. [DOI] [PubMed] [Google Scholar]
- 30.Jia WP, Lu JX, Xiang KS, et al. Prediction of abdominal visceral obesity from body mass index, waist circumference and waist-hip ratio in Chinese adults: Receiver operating characteristic curves analysis. Biomed Environ Sci. 2003;16:206–211. [PubMed] [Google Scholar]
- 31.Lemieux S, Prud'homme D, Tremblay A, et al. Anthropometric correlates to changes in visceral adipose tissue over 7 years in women. Int J Obes Relat Metab Disord. 1996;20:618–624. [PubMed] [Google Scholar]
- 32.Lemieux S, Prud'homme D, Bouchard C, et al. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64:685–693. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 33.Lemieux S, Prud'homme D, Nadeau A, et al. Seven-year changes in body fat and visceral adipose tissue in women.Association with indexes of plasma glucose-insulin homeostasis. Diabetes Care. 1996;19:983–991. doi: 10.2337/diacare.19.9.983. [DOI] [PubMed] [Google Scholar]
- 34.Shen W, Punyanitya M, Chen J, et al. Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity (Silver Spring) 2006;14:727–736. doi: 10.1038/oby.2006.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meisinger C, Doring A, Thorand B, et al. Body fat distribution and risk of type 2 diabetes in the general population: Are there differences between men and women? The MONICA/KORA Augsburg cohort study. Am J Clin Nutr. 2006;84:483–489. doi: 10.1093/ajcn/84.3.483. [DOI] [PubMed] [Google Scholar]
- 36.Farin HM, Abbasi F, Reaven GM. Body mass index and waist circumference correlate to the same degree with insulin-mediated glucose uptake. Metabolism. 2005;54:1323–1328. doi: 10.1016/j.metabol.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 37.Farin HM, Abbasi F, Reaven GM. Body mass index and waist circumference both contribute to differences in insulin-mediated glucose disposal in nondiabetic adults. Am J Clin Nutr. 2006;83:47–51. doi: 10.1093/ajcn/83.1.47. [DOI] [PubMed] [Google Scholar]
- 38.Farin HM, Abbasi F, Reaven GM. Comparison of body mass index versus waist circumference with the metabolic changes that increase the risk of cardiovascular disease in insulin-resistant individuals. Am J Cardiol. 2006;98:1053–1056. doi: 10.1016/j.amjcard.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Wannamethee SG, Shaper AG, Morris RW, et al. Measures of adiposity in the identification of metabolic abnormalities in elderly men. Am J Clin Nutr. 2005;81:1313–1321. doi: 10.1093/ajcn/81.6.1313. [DOI] [PubMed] [Google Scholar]
- 40.Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord. 2001;25:1047–1056. doi: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
- 41.Dalton M, Cameron AJ, Zimmet PZ, et al. Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med. 2003;254:555–563. doi: 10.1111/j.1365-2796.2003.01229.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Rimm EB, Stampfer MJ, et al. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 43.Wannamethee SG, Lowe GD, Shaper AG, et al. The metabolic syndrome and insulin resistance: Relationship to haemostatic and inflammatory markers in older non-diabetic men. Atherosclerosis. 2005;181:101–108. doi: 10.1016/j.atherosclerosis.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 44.Gupta R, Rastogi P, Sarna M, et al. Body-mass index, waist-size, waist-hip ratio and cardiovascular risk factors in urban subejcts. J Assoc Physicians India. 2007;55:621–627. [PubMed] [Google Scholar]
- 45.Grundy SM. What is the contribution of obesity to the metabolic syndrome? Endocrinol Metab Clin North Am. 2004;33:267–282. doi: 10.1016/j.ecl.2004.03.001. table of contents. [DOI] [PubMed] [Google Scholar]
- 46.Haffner SM, Valdez RA, Hazuda HP, et al. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992;41:715–722. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 47.Kitabchi AE, Temprosa M, Knowler WC, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: Effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest. 2006;116:1813–1822. doi: 10.1172/JCI29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207–223. doi: 10.1210/rp.59.1.207. [DOI] [PubMed] [Google Scholar]
- 50.Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med. 2005;47:201–210. [PubMed] [Google Scholar]
- 51.Reaven G. All obese individuals are not created equal: Insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diab Vasc Dis Res. 2005;2:105–112. doi: 10.3132/dvdr.2005.017. [DOI] [PubMed] [Google Scholar]
- 52.McLaughlin T, Allison G, Abbasi F, et al. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism. 2004;53:495–499. doi: 10.1016/j.metabol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 53.Park SH, Lee WY, Lee YS, et al. The relative effects of obesity and insulin resistance on cardiovascular risk factors in nondiabetic and normotensive men. Korean J Intern Med. 2004;19:75–80. doi: 10.3904/kjim.2004.19.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan DC, Watts GF, Sussekov AV, et al. Adipose tissue compartments and insulin resistance in overweight-obese Caucasian men. Diabetes Res Clin Pract. 2004;63:77–85. doi: 10.1016/j.diabres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 56.Mackin P, Bishop DR, Watkinson HM. A prospective study of monitoring practices for metabolic disease in antipsychotic-treated community psychiatric patients. BMC Psychiatry. 2007;7:28. doi: 10.1186/1471-244X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newcomer JW. Antipsychotic medications: Metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68:8–13. [PubMed] [Google Scholar]
- 58.Newcomer JW, Haupt DW, Fucetola R, et al. Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry. 2002;59:337–345. doi: 10.1001/archpsyc.59.4.337. [DOI] [PubMed] [Google Scholar]
- 59.Haupt DW, Newcomer JW. Hyperglycemia and antipsychotic medications. J Clin Psychiatry. 2001;62:15–26. discussion 40–1. [PubMed] [Google Scholar]
- 60.Haupt DW, Fahnestock PA, Flavin KA, et al. Adiposity and insulin sensitivity derived from intravenous glucose tolerance tests in antipsychotic-treated patients. Neuropsychopharmacology. 2007;32:2561–2569. doi: 10.1038/sj.npp.1301392. [DOI] [PubMed] [Google Scholar]
- 61.Spitzer RL, Williams JB, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 62.Pereira MA, FitzerGerald SJ, Gregg EW, et al. A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc. 1997;29:S1–S205. [PubMed] [Google Scholar]
- 63.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: Measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- 65.Bergman RN, Watanabe R, Rebrin K, et al. Toward an integrated phenotype in pre-NIDDM. Diabet Med. 1996;13:S67–S77. [PubMed] [Google Scholar]
- 66.Henderson DC, Copeland PM, Borba CP, et al. Glucose metabolism in patients with schizophrenia treated with olanzapine or quetiapine: A frequently sampled intravenous glucose tolerance test and minimal model analysis. J Clin Psychiatry. 2006;67:789–797. doi: 10.4088/jcp.v67n0513. [DOI] [PubMed] [Google Scholar]
- 67.Bergman RN, Prager R, Volund A, et al. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest. 1987;79:790–800. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 69.Perez-Martin A, Raynaud E, Hentgen C, et al. Simplified measurement of insulin sensitivity with the minimal model procedure in type 2 diabetic patients without measurement of insulinemia. Horm Metab Res. 2002;34:102–106. doi: 10.1055/s-2002-20524. [DOI] [PubMed] [Google Scholar]
- 70.Hermans MP, Levy JC, Morris RJ, et al. Comparison of insulin sensitivity tests across a range of glucose tolerance from normal to diabetes. Diabetologia. 1999;42:678–687. doi: 10.1007/s001250051215. [DOI] [PubMed] [Google Scholar]
- 71.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 72.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 73.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]