Abstract
The CD40 – CD154 dyad is an intensely studied field as is glycosylation status and both impact immunological functions and autoimmune conditions. CD40 has several isoforms, is modified by glycosylation, and trimerizes to form the functional receptor. We described a CD4+CD40+ T cell (Th40) subset which is expanded in autoimmunity and is necessary and sufficient in transferring type I diabetes. Glycosylation impacts immunological events and T cells from autoimmune mouse strains express 30–40% less GlcNAc-branched N-glycans than T cells from non-autoimmune strains, a decrease known to activate T cells. Here we demonstrate that several CD40 receptor constellations exist on CD4 T cells. However, rather than containing different isoforms of CD40 they contain different glycoforms of isoform I. The glycoform profile is dependent on availability of CD154 and autoimmune NOD mice express a high level of a less glycosylated form. Interestingly, CD40 stimulation induces some CD40 receptor constellations that contain TNF-receptors 1 and 2 and targeting of those alters CD40 signaling outcomes in NOD Th40 cells. CD40-stimulation in-vivo of non-autoimmune BALB/c mice expands the Th40 population and alters the CD40 glycoform profile of those cells to appear more like that of autoimmune prone NOD mice.
Further understanding the dynamics and composition of the different CD40 receptor constellations will provide important insights into treatment options in autoimmunity.
Keywords: CD40, glycosylation, autoimmunity, T cells, Th40, TNF-receptor
Introduction
The CD40 – CD154 dyad is intensely studied and known to be critical in the establishment and perpetuation of autoimmunity (Balasa et al., 1997; Durie et al., 1993; Kobata et al., 2000; Munroe and Bishop, 2007; Quezada et al., 2003; Toubi and Shoenfeld, 2004; Vaitaitis et al., 2003; Vaitaitis and Wagner, 2008; Waid et al., 2004; Wang et al., 2002; Yu et al., 2001). CD40 (tnfrsf5) belongs to the TNF-receptor super family and like other TNF-receptor super family members, CD40 molecules multimerize to form the functional receptor (Wyzgol et al., 2009). When engaged, CD40 associates with lipid rafts to interact with the adaptor molecules, TNF receptor associated factors (TRAFs), for downstream signaling (Xie et al., 2006). While the major natural ligand for CD40 is CD154, C4-binding protein (C4BP) and Hsp70 can act as CD40 ligands as well (Brodeur et al., 2003; Wang et al., 2001) and thus there are several interaction modalities. CD154 (tnfsf2) belongs to the TNF super family, is trimerized to be functional and occurs both as a membrane bound and a soluble form (Baccam and Bishop, 1999). This suggests that CD154 may have cytokine functions. Expression of CD154 occurs on activated T cells but has been demonstrated on platelets and macrophages as well as other cell types (Lutgens and Daemen, 2002; Sprague et al., 2007; Toubi and Shoenfeld, 2004). Over-expression of CD154 is associated with many autoimmune conditions (Datta, 1998; Jinchuan et al., 2004; Toubi and Shoenfeld, 2004) and this can lead to persistent CD40-stimulation with expansion of effector T cells thus establishing and perpetuating the disease state (Vaitaitis and Wagner, 2008; Vaitaitis et al., 2010; Wagner, 2009).
CD40 expression has been associated with antigen presenting cells (APC) but in actuality its expression is quite ubiquitous including neural (Suo et al., 2002), endothelial, epithelial (van Kooten and Banchereau, 2000), adipocyte (Poggi et al., 2009) and T cells (Wagner, 2009; Wagner et al., 1999; Wagner et al., 2002). We have identified a CD4+ subset of T cells that expresses CD40, termed Th40 cells. Th40 cell percentage expands with progressive insulitis and Th40 cells are necessary and sufficient in transferring autoimmune Type 1 diabetes (T1D) (Wagner et al., 1999; Wagner et al., 2002; Waid et al., 2008; Waid et al., 2004; Waid et al., 2007). This expansion is likely due to that via persistent CD40-signals to the autoimmune Th40 cells these cells constantly recruit high levels of CD40 and TRAF2 to the raft microdomain and signals through those molecules result in increased survival and proliferation via the induction of high levels of Bcl-XL and cFLIPp43 (Vaitaitis and Wagner, 2008). An important discovery was that the expansion of the Th40 subset (Waid et al., 2004) as well as disease onset (Balasa et al., 1997) in autoimmunity is prevented by blocking CD40 – CD154 interaction.
CD40 is also known to induce recombination activating gene (RAG) 1 and RAG2 expression in Th40 cells which leads to the alteration of TCR expression that could therefore alter antigen recognition in the periphery (Vaitaitis et al., 2003). This then has great implications in autoimmunity since this process may generate autoaggressive T cells that could establish and perpetuate autoimmune disease. CD40 is known to induce a similar process, immunoglobulin class switching, in B cells although this requires the presence of IL-4 (Fuleihan et al., 1993). Yet another function of CD40 is the role as costimulatory molecule inducing cytokine production in CD4 T cells (Baker et al., 2008; Munroe and Bishop, 2007). Beyond the realm of immune cells, endothelial cells are known to induce adhesion molecules in response to CD40-stimulation while fibroblasts induce expression of chemokines (Vogel et al., 2004) and adipocytes induce pro-inflammatory cytokines (Poggi et al., 2009). This then raises the question of how it is possible for CD40 to perform so many different signaling functions. Is there more than one form of CD40 receptor?
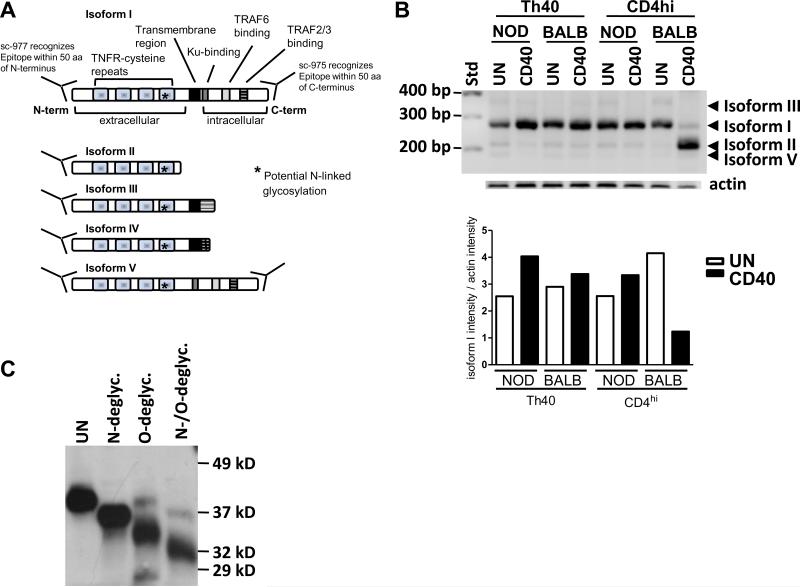
CD40 is a type I glycoprotein and is the product of a single gene, however, five mRNA splice isoforms have been identified in mice (Tone et al., 2001). Isoform I is the full length, 289 amino acid (aa) protein, while in isoform V a 30 aa region comprising the canonical transmembrane region is replaced with a single glutamic acid (Fig. 2A). Isoforms II, III, and IV lack all, or nearly all, of the intracellular, C-terminal portion of the protein which is essential for CD40-mediated signaling. Expression of isoform II has been shown to reduce the amount of signal-transducible isoform I on the surface of the cell (Tone et al., 2001). The deduced amino acid sequence for isoform I predicts a size of 32 kD but glycosylation increases the apparent molecular weight of the protein to about 48 kD (Braesch-Andersen et al., 1989).
Figure 2.
(A) Graphic representation of the five known isoforms of mouse. (B) CD40 Th40 and CD4hi T cells were sorted from 10–16 weeks old NOD and BALB/c female spleens then CD40 was stimulated overnight (CD40) or not (UN). RT-PCR was performed using primers capable of targeting the known five isoforms in mice. Resulting PCR products were isolated and sequenced to confirm identity. Actin was amplified as an internal standard. A graph representing the intensity of isoform I divided by the intensity of actin in the experiment is shown. (C) The lipid raft fraction from NOD Th40 cells was subjected to N-linked, O-linked, or both N-linked and O-linked deglycosylation. Protein was then assayed in a western blot for CD40 using a C-terminal reactive antibody. Experiments in B and C were done three separate times.
Glycosylation is known to impact immunological functions including leukocyte trafficking, regulation of TCR activation threshold, T cell differentiation and polarization into Th1, Th2 or Th17 (Marth and Grewal, 2008). Glycosylation alters the molecular interactions of immune associated molecules at the cell surface (Marth and Grewal, 2008), e.g. galectin-3 interacts with N-glycans on the TCR molecules thereby limiting TCR clustering in response to agonist stimulus (Demetriou et al., 2001). In glycosylation-deficient mice the galectin-3 / TCR interaction is disrupted, effectively lowering the TCR activation threshold (Demetriou et al., 2001). Deficiency in glycosylation has been associated with autoimmune disease in mice (Demetriou et al., 2001; Grigorian et al., 2007; Morgan et al., 2004) and several autoimmune prone mouse strains, including NOD, have been shown to express 30 – 40% less β1, 6GlcNAc-branched N-glycans than non-autoimmune prone strains (Lee et al., 2007). In addition, reduction of GlcNAc-branching by 20 – 25% is sufficient to enhance TCR signaling and T cell proliferation (Grigorian et al., 2007).
Here we show that there is more than one form of CD40 receptor on Th40 cells. We demonstrate that while there is a possibility that different CD40 isoforms multimerize to form those different receptors, it is more likely that different glycoforms of CD40 isoform I are responsible for differential receptor formation. CD40-stimulation in-vivo expands the Th40 population in BALB/c mice to levels seen in autoimmune NOD mice and furthermore, it drives the Th40 population into a CD40 glycoform profile similar to that of autoimmune NOD mice. Conversely, when CD40–CD154 interactions are blocked in NOD mice, not only is the Th40 population contained (Waid et al., 2004) but the CD40 glycoform profile is altered to look more like that of non-autoimmune BALB/c. Availability of CD154 governs the induction of high levels of a less glycosylated form of CD40 isoform I. Interestingly, we reveal an interaction between CD40 and TNF-receptors (TNFR) 1 and 2 and that there is a difference in this association between autoimmune and non-autoimmune conditions. Finally we demonstrate that TNFR1 and/or TNFR2 engagement in addition to CD40 stimulation modulates the outcomes of CD40 signaling in autoimmune derived Th40 cells. Further understanding of the the dynamics in the formation of CD40 receptors, including the multiple CD40 glycoform and hybrid CD40-TNFR1/2 receptors described here, may help to better target and prevent the CD40 signaling dependent induction and perpetuation of autoimmune disease.
Materials and Methods
Mice
NOD and BALB/c mice were from Jackson Laboratories and Taconic and were housed under pathogen free conditions at the University of Colorado Denver, AAALAC-approved facility. The NOD mice consistently achieve >90% diabetes in females by the age of 18 weeks. All experiments were carried out under IACUC-approved protocol.
Antibodies and reagents
Conjugated microbeads for cell-sorting were purchased from Miltenyi Biotec. CD40 antibodies 1C10, 4F11 (Heath et al., 1994), and FGK45 (Rolink et al., 1996) and anti-CD154 antibody, MR1 (Noelle et al., 1992), were produced in house and isotype antibodies were purchased from eBioscience, Inc. Western blot antibodies for CD40 (sc-975 and sc-977), TRAF2 (sc-876), TNFR1 (sc-7865 and sc-1070), TNFR2 (sc-7862 and sc-12751), and TRAP2 (sc-68352) were from Santa Cruz Biotechnology, Inc. All chemicals were from Sigma-Aldrich®.
T cell purification and cell culture
Splenic Th40 and CD4hi T cells from 10 – 16 week old, female NOD or BALB/c mice were sorted using an autoMACS™ (Miltenyi Biotec) as previously described (Vaitaitis and Wagner, 2008) with the exception that directly conjugated CD4-microbeads were used instead of biotinylated CD4-antibody followed by streptavidin-microbeads. Cells were cultured in DMEM containing 10% fetal calf serum and 50 μM β-mercaptoethanol. Cells were CD40 crosslinked using 5 μg/ml each of biotinylated 1C10 and/or 4F11 followed by 1 μg/ml of streptavidin.
In-vivo antibody treatments
To block CD40 – CD154 interactions, 3-week old, female NOD mice were injected intraperitoneally with 50 μg anti-CD154 antibody (MR1) and were boosted with another 50 μg one week later. When mice reached 12 weeks of age spleens were harvested and cells prepared as described (Vaitaitis and Wagner, 2008).
To stimulate CD40 in-vivo, 10-week old, female BALB/c mice were injected intraperitoneally with 50 μg each of anti-CD40 antibodies 1C10 and FGK45. Four to six days after the injection spleens were harvested and cells purified as described (Vaitaitis and Wagner, 2008).
Protein and lipid raft preparations
Preparation of whole cell lysates and detergent-insoluble microdomains (rafts) from equal numbers of Th40 and CD4hi T cells was done as described (Vaitaitis and Wagner, 2008).
Western blot
Proteins were separated on 10% (in the case of CD40, TRAF2, TNFR1 and TNFR2 western blots) or 4–15% (in the case of TRAP2 western blots) polyacrylamide gels then transferred to PVDF membrane (Bio-Rad Laboratories). As an internal standard (except in the case of westerns following immunoprecipitations) the probed membrane was stripped and stained with Coomassie Blue R-250. Analysis of band intensity was done with Kodak 1D densitometry software (Eastman Kodak Company).
Immunoprecipitations
Sorted and treated cells were lysed (20 mM Tris-HCl pH 7.5, 2 mM EDTA, 137 mM NaCl, 0.5% Triton X-100, 1 μg/ml each of leupeptin and aprotinin, 0.2 mM PMSF and 0.4 mM sodium-orthovanadate) then lysates were clarified by centrifuging at 16 K × g for 3 minutes. 1C10-, 4F11-, FGK45-, TNFR1-, or TNFR2-conjugated magnetic beads (conjugated according to manufacturer's protocol; MyOne™ tosylactivated Dynabeads® from Invitrogen) were immediately added to the lysates and incubated for 20–30 minutes, rotating at room temperature. A strong magnet (Invitrogen) was used to wash the beads three times with lysis buffer then the immunoprecipitated material was eluted using 0.1 M sodium citrate at pH 3.0.
RT-PCR
RNA was purified from 100,000 cells in TRIzol (Invitrogen) and cDNA was synthesized using SuperScript™ II (Invitrogen). cDNA representing 1,500 cells (1/67 of resulting cDNA) was used in PCR with CD40-primers capable of detecting the five known isoforms in mice (Tone et al., 2001). Primer-1, 5'-CGGCTTCTTCTCCAATCAGTCATC-3'; Primer-2, 5'-CTTCCATCTCCTGGGGATCTTG-3'. These primers produce bands as follows. Isoform I – 281 bp; isoform II – 219 bp; isoform III – 382 bp; isoform IV – 276 bp; isoform V – 194 bp. Resulting bands were isolated and sequenced at the CU-Cancer Center DNA Sequencing Core (University of Colorado Denver, Anschutz Medical Campus). Actin was amplified as a standard as previously described (Vaitaitis et al., 2003).
Deglycosylation assay
Deglycosylation was done using and following directions in a kit from QA-bio, LLC.
Electrophoretic mobility shift assays (EMSA)
EMSAs were done as described (Vaitaitis and Wagner, 2008) with the exceptions that the consensus Nf-kB oligonucleotide was biotinylated and detection was done by transferring the protein-DNA complexes to PVDF membrane then the bands were visualized by streptavidin-HRP followed by ECL-Plus (GE Healthcare).
T cell proliferation and death assays
T cell proliferation was measured by CFSE dilution and death rates measured by propidium iodide staining as described previously (Vaitaitis and Wagner, 2008). The measurements were confirmed by counting absolute numbers of live versus dead cells in trypan blue.
Flow cytometry
Flow cytometry was done on a FACS Calibur flow cytometer (BD Biosciences) and analysis performed using FlowJo analysis software.
Protein sequencing
Protein sequencing was done at University of Colorado Cancer Center Proteomics Core (University of Colorado Denver, Anschutz Medical Campus) on protein bands isolated from polyacrylamide gel separated CD40 immunoprecipitates.
Data analysis
Data analysis was performed using GraphPad Prism 5 from GraphPad Software, Inc.
Results
NOD Th40 cells have a high ratio of a 43 kD to a 48 kD CD40 band and the smaller band is recruited to the lipid raft microdomain in Th40 cells
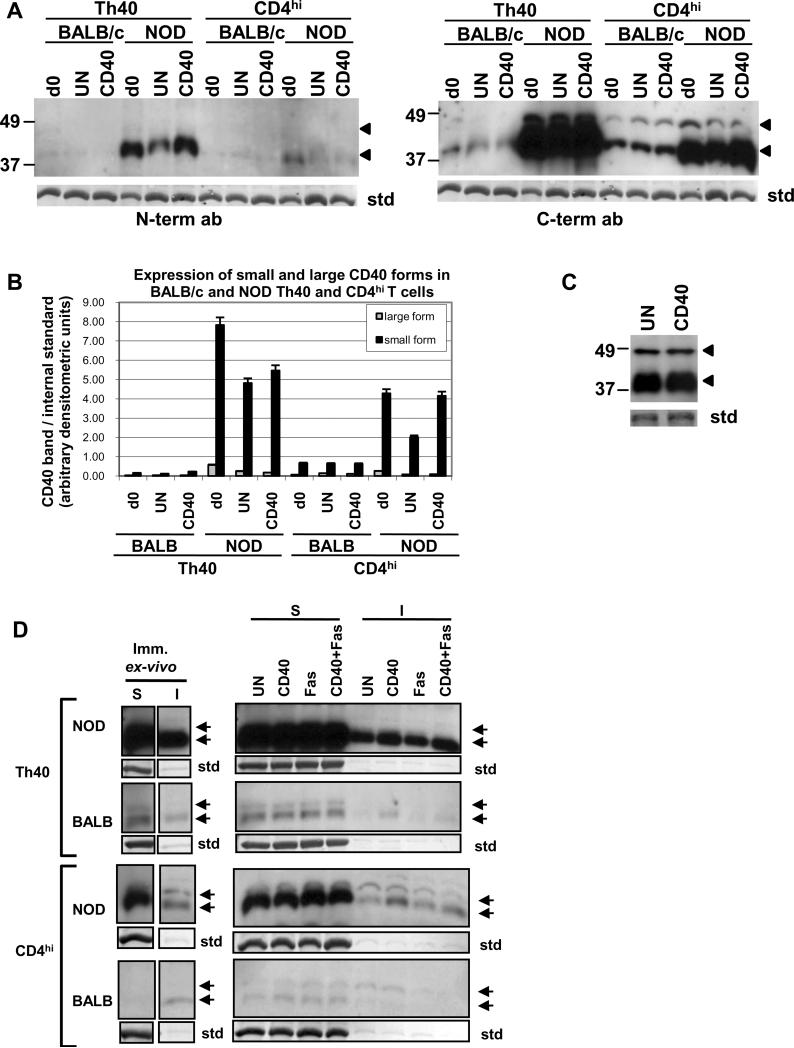
We have shown that Th40 cells from autoimmune prone NOD mice, which are necessary and sufficient to transfer T1D to NOD.scid animals (Wagner et al., 2002; Waid et al., 2008; Waid et al., 2004), express high levels of CD40 in the raft microdomain and that this leads to survival and proliferation of this cell subset in these animals (Vaitaitis and Wagner, 2008). This is not the case for the same subset from non-autoimmune mice. Considering that CD40 is capable of many different types of signaling events we speculated that those different signaling outcomes could be due to that the CD40 receptor on different cells or even on the same cell could be composed of different isoforms multimerizing to form different types of CD40 receptors. Therefore we determined whether, by better separating the protein samples in the 30 – 60 kD range, we could demonstrate more than one protein band reactive with CD40 antibodies. Two bands, at 43 kD and 48 kD, were detectable with a C-terminal and an N-terminal reactive antibody in both BALB/c and NOD T cells with the highest expression in NOD Th40 cells (Fig. 1A and B). In Th40 cells from NOD mice the ratio of 43 kD:48 kD CD40 bands was 13 immediately ex-vivo and when CD40 was engaged over night in-vitro this ratio increased drastically to 32 (Fig. 1A and B). Meanwhile, in non-autoimmune BALB/c Th40 cells, this ratio was 5 in immediately ex-vivo samples and only increased to about 6 when CD40 was engaged over night (Fig. 1A and B). The smaller to larger band ratio was even higher in NOD CD4hi T cells compared to NOD Th40 cells although it should be noted that the overall expression of CD40 in those cells was lower than in the NOD Th40 cells (Fig. 1A and B).
Figure 1.
Th40 and CD4hi T cells were sorted from 10–16 weeks old NOD and BALB/c female spleens. (A) Cells were lysed immediately (d0) or incubated over night in the absence (UN) or presence of CD40 stimulation (CD40) then lysed. Equal amounts of protein were run in western blot and immunodetection was performed with a CD40 N-terminal reactive antibody then the membrane was stripped and reprobed using a C-terminal reactive antibody. (B) Graph representing the amount of small and large CD40 band. Data are represented as mean +/− SEM. (C) Th40 cells from female 4 week old NOD mice were incubated over night in the absence (UN) or presence of CD40 stimulation (CD40). Equal amounts of protein were run in western blot and immunodetection was performed with a CD40 C-terminal reactive antibody. (D) Cells treated as in A were fractioned into soluble (S; cytoplasmic) and insoluble (I; lipid raft) fractions. Western blot was performed using a CD40 C-terminal reactive antibody.
For an internal standard (std) in A and C and D the stripped membrane was stained with coomassie blue and a representative band is shown. Arrows indicate CD40 bands. Experiments were done at least three times.
Although 10–16 week old NOD mice do not exhibit overt diabetes, inflammatory processes are ongoing with e.g. insulitis already established. It is possible that the inflammatory state has an effect on the CD40 expression and therefore we determined whether the hyper-expression of CD40 is present in young, 4 week old mice or not. As shown in figure 1 C, a high level of CD40 expression is present in Th40 cells already at 4 weeks of age. At this age insulitis is minimal and few inflammatory processes ongoing demonstrating that the CD40 hyper-expression is independent of the inflammatory milieu.
CD40 is known to be recruited to lipid raft microdomains where it interacts with signal adaptor molecules for downstream signaling (Vaitaitis and Wagner, 2008) (Munroe and Bishop, 2007; Xie et al., 2006). When raft microdomain preparations were analyzed in western blots we found that in Th40 cells from both NOD and BALB/c the main form associated with this fraction is the 43 kD form while in CD4hi T cells from NOD both the 43 kD and the 48 kD form are recruited (Fig. 1D). Interestingly, although BALB/c CD4hi T cells have a higher amount of the small form of CD40 overall (Fig. 1A and B) only the large form was recruited to the lipid raft (Fig. 1D). NOD Th40 cells had the highest amount of CD40 recruited to the raft as we have reported previously (Vaitaitis and Wagner, 2008).
The 43 kD CD40 band is a glycoform of isoform I
Different possibilities for what constitutes the two CD40 protein bands exist. There are five known CD40 splice isoforms in mice (Tone et al., 2001) (Fig. 2A) and CD40 is known to be glycosylated which alters the apparent size of the protein (Braesch-Andersen et al., 1989) (Fig. 2A). It is also possible that there is proteolytically cleaved CD40 protein that could account for the smaller band. Therefore we addressed which of these possibilities could account for the two bands. First we considered that different isoforms could constitute the two protein bands. In figure 1 we utilized both an N-terminally reactive (sc-977) and a C-terminally reactive (sc-975) CD40 antibody. Both CD40 bands were detectable with those. Only two of the isoforms possess both the extreme N-terminus and the extreme C-terminus in mice, namely isoforms I and V (Tone et al., 2001). Therefore we concluded that if the bands are isoforms of CD40 they must be isoforms I and V. To further address this we performed RT-PCR utilizing primers that will detect all known isoforms of CD40 and sequenced the resulting products. While isoforms I, II, III and V were detectable, the major form detected was isoform I (Fig. 2B). When the cells were CD40 engaged in-vitro, isoform I mRNA expression was increased about 2.5-fold in NOD Th40 cells while no other isoform was increased or became detectable. Interestingly, the isoform II mRNA expression was down-regulated by CD40 engagement. In NOD CD4hi T cells, a very slight increase of the isoform I mRNA was induced by CD40 engagement (Fig. 2B). BALB/c Th40 cells also demonstrated a slight increase in expression of CD40 isoform I mRNA in response to CD40 engagement while the BALB/c CD4hi T cells displayed a decrease. Interestingly, BALB/c CD4hi cells induced an increase in isoform II mRNA expression. It was reported that there are mouse CD40 isoforms that lack exons 2 or 3 (Tone et al., 2001) which could be detectable with the anti-CD40 C-terminal and N-terminal antibodies. However, our mRNA data do not support this possibility since we were not able to detect any mRNA corresponding to such CD40 isoforms when employing RT-PCR primers that would detect such isoforms (data not shown).
The mRNA expression results (Fig. 2B) indicate that isoform V is present at almost non-detectable levels and does not increase in response to CD40 engagement. However, there can be discrepancies between mRNA and protein expression levels. Therefore we employed protein sequencing in an attempt to demonstrate the presence of CD40 isoform V protein. All attempts to identify isoform V failed and only isoform I was identified. Therefore we concluded that the 43 kD CD40 band is not isoform V. We then considered that the 43 kD band could be a proteolytically cleaved form of CD40. In figure 1A we determined that the 43 kD CD40 protein band was reactive with antibodies to both the extreme C-terminus and the extreme N-terminus demonstrating that the 43 kD band is not a proteolytically cleaved product of the 48 kD band. This left us with the possibility that the 43 kD band in figure 1 is the result of an altered post-translational modification of isoform I whose mRNA expression was increased upon CD40 engagement (Fig. 2B). The CD40 protein sequence predicts an N-linked glycosylation site in the extracellular portion of the protein (Fig. 2A) and it has been shown that glycosylation is a feature of the CD40 protein (Braesch-Andersen et al., 1989). Therefore it is possible that the 43 kD band is a less glycosylated form of isoform I. Since in NOD Th40 cells the only detectable band in the lipid raft fraction is the 43 kD band (Fig. 1C) we subjected that fraction to complete deglycosylation followed by western blot for CD40. Virtually all of the 43 kD band was reduced to a band of 32 kD when completely deglycosylated (Fig. 2C), matching the size of CD40 isoform I predicted from its amino acid composition in a non-glycosylated state. A completely deglycosylated isoform V protein would be 28.8 kD. Therefore we concluded that the major CD40 protein expressed in Th40 cells from both autoimmune and non-autoimmune animals is isoform I and that a 43 kD glycoform of that is highly inducible by CD40 engagement.
CD40 signals regulate CD40 expression itself as well as the 43 kD to 48 kD glycoform ratio in Th40 cells
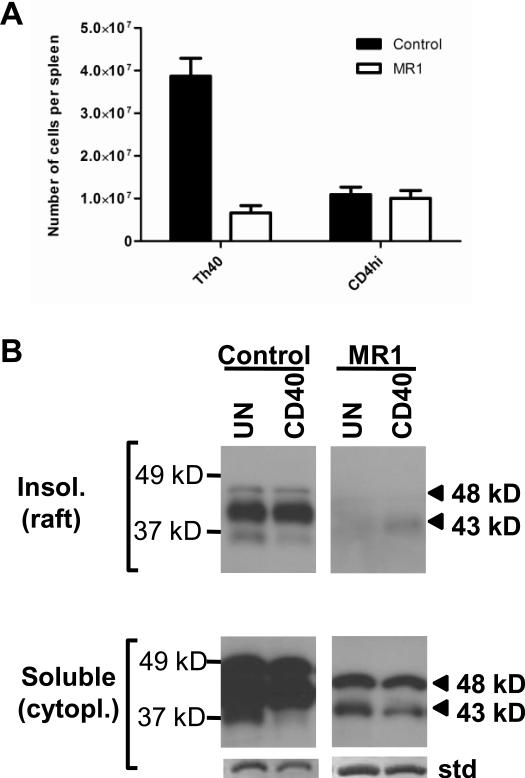
Blocking CD40 – CD154 interactions at 3 weeks of age, but not 9 weeks of age, prevents the onset of diabetes in NOD mice (Balasa et al., 1997; Waid et al., 2004). That block also prevents the expansion of the Th40 cell subset, reestablishing Th40:Treg homeostasis (Waid et al., 2004). Therefore we evaluated whether CD154 block has any effect on the CD40 isoform I glycoform expression in NOD Th40 cells. Treatment of NOD mice at 3 weeks of age with one intraperitoneal injection of anti-CD154 antibody (MR1) followed by a booster one week later prevented the expansion of Th40 cells as we have demonstrated before (Waid et al., 2004) (Fig. 3A). That treatment also altered the CD40 glycoform expression in the NOD Th40 cells to have a profile more like that of non-autoimmune BALB/c mice (compare Fig. 1C and Fig. 3B). This demonstrates that the CD40 receptor composition is dependent on the availability of CD154.
Figure 3.
Splenic Th40 and CD4hi T cells were sorted from control and anti-CD154 treated NOD female mice. (A) Graph depicting absolute numbers of Th40 and CD4hi T cells in the control group (n=5) and the anti-CD154 (MR1) injected group (n=5). Data are represented as mean +/− SEM. (B) Cytoplasmic and lipid raft fractions were prepared from Th40 cells from mice in A then western blot was performed using a CD40 C-terminal reactive antibody. For an internal standard (std) the stripped membrane was stained with coomassie blue and a representative band is shown. Data shown is representative of the results from all the mice in A.
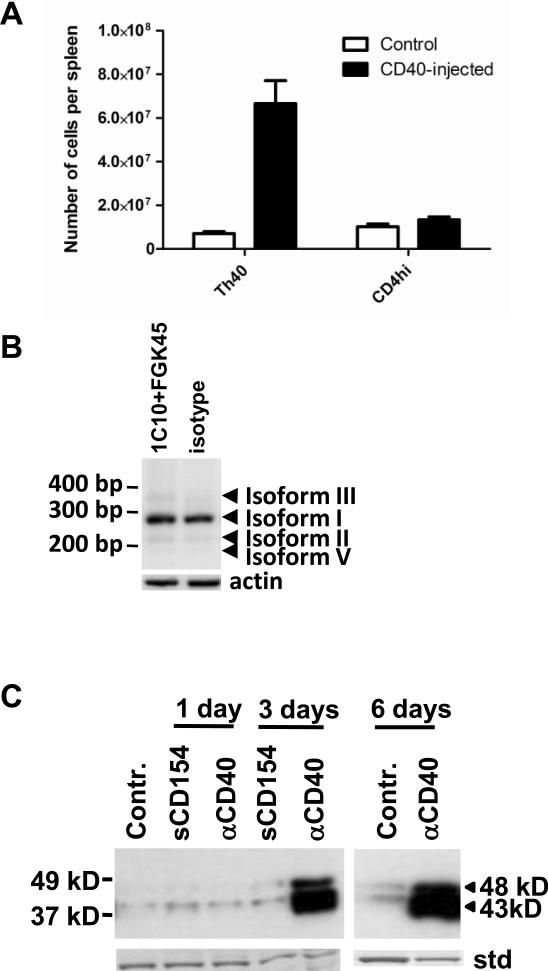
We have shown that while BALB/c mice have Th40 cells in the periphery, that population is contained compared to autoimmune NOD mice (Waid et al., 2004). Blocking CD40 – CD154 interactions in NOD mice contained the Th40 population at levels comparable to BALB/c mice (Waid et al., 2004) which suggests that CD40-signals are directly responsible for the expansion of the Th40 population in-vivo. Therefore we hypothesized that the Th40 population could be expanded in BALB/c mice by stimulation through CD40. BALB/c mice that had been injected intraperitoneally with agonistic CD40 antibodies demonstrated an expansion of the splenic Th40 population similar to numbers seen in autoimmune NOD mice (Fig. 4A). This confirms that excessive CD40 signals allow this population to expand in-vivo.
Figure 4.
10–12 weeks old, female BALB/c mice were injected i.p. with 1C10+FGK45 agonistic CD40 antibodies or with isotype control antibody. Three to six days post-injection, splenic Th40 and CD4hi T cells were purified. (A) Graph representing the absolute numbers of cells in each population. (B) Mice were injected as above then RNA was purified from Th40 cells. RT-PCR was performed using primers capable of targeting the known five CD40 isoforms in mice. (C) Mice were injected as above with the addition of recombinant sCD154 injections then Th40 cells were purified. Equal amounts of protein was loaded in each lane and analyzed in western blot for CD40 using a C-terminal reactive antibody. For an internal standard (std) the stripped membrane was stained with coomassie blue and a representative band is shown. Each experiment was done in at least 6 separate mice. Data in A are represented as mean +/− SEM.
We have shown that CD40 signals induce an increase in CD40 expression itself among other downstream consequences (Vaitaitis and Wagner, 2008). In figure 3A and B we also demonstrate that blocking CD40 signals can alter the 43kD:48 kD ratio of CD40 glycoform expression in NOD mice. Therefore we speculated that injections of agonistic CD40 antibodies into BALB/c mice could induce a higher CD40 expression level in the Th40 cell subset of this non-autoimmune strain. The CD40 isoform mRNA profile did not change in BALB/c mice injected intraperitoneally with agonistic CD40 antibodies and isoform I was still the major isoform expressed (Fig. 4B). However, the mice injected with agonistic CD40 antibodies demonstrated increased CD40 protein expression and a glycoform profile and 43 kD:48 kD band ratio similar to that of autoimmune NOD Th40 cells (Fig. 4C). Although the Th40 cells expanded in response to the injection with agonistic CD40 antibodies (Fig. 4A), the actual qualitative CD40 expression/glycoform profile changed as Th40 cells were purified from the differently treated mice and equal amounts of protein was loaded in each lane in figure 4C. When BALB/c mice were injected with sCD154 there was no effect on the protein expression. This is likely due to that the recombinant sCD154 is not trimerized (data not shown) which is known to be necessary for signaling to occur (Baccam and Bishop, 1999).
Immunoprecipitation of CD40 reveals differences in CD40 receptor composition and a CD40-engagement dependent association between the different protein bands
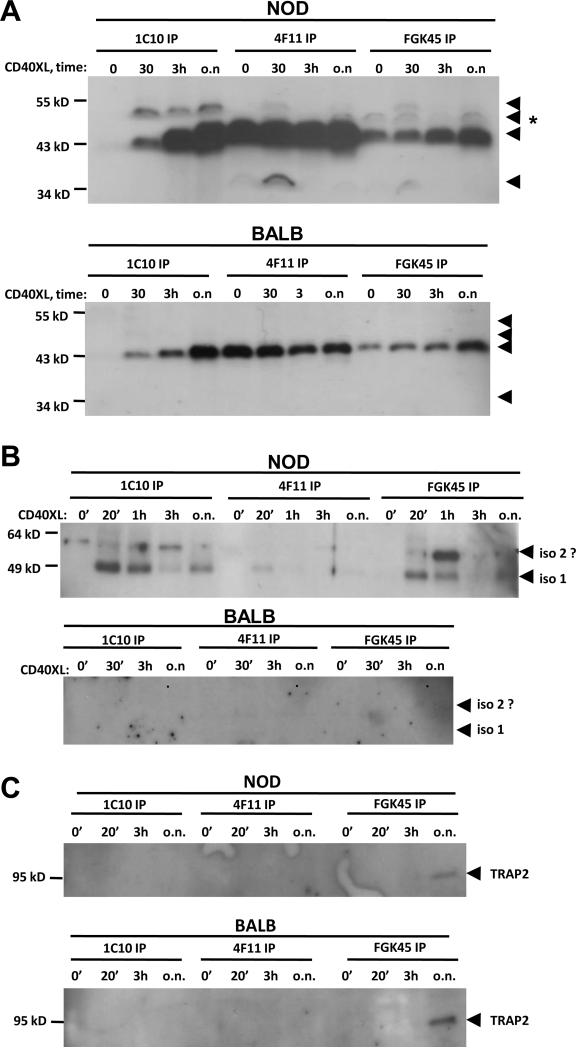
Several different antibodies are utilized in the literature for the detection of CD40. For mouse CD40 studies some of the most commonly utilized antibodies are 1C10, 4F11 (Heath et al., 1994) and FGK45 (Rolink et al., 1996). These antibodies recognize different epitopes on the CD40 receptor and we surmise that those epitopes may be formed by different glycoforms and/or isoforms associating with each other. Therefore we applied those antibodies in immunoprecipitation experiments to evaluate whether they recognize the same CD40 constellations/glycoforms or not. All three antibodies immunoprecipitated a 43 kD band from untreated, immediately ex-vivo, Th40 cells in NOD mice (Fig. 5A). The association of the 43 kD band in the 1C10- and FGK45-constellations was increased upon CD40 stimulation while it remained approximately the same in the 4F11-constellation. The same was evident in BALB/c Th40 cells although the levels were lower. In NOD Th40 cells a 48 kD band was detected which was only weakly detected in the other two constellations. The 48 kD band was not detectable in the BALB/c Th40 cells. This demonstrates that the CD40 receptor composition is dynamic and that actual CD40 engagement has an impact on the multimerization/association of the different constellations. Interestingly, FGK45 immunoprecipitated a CD40 protein band of a different size from NOD Th40 cells, but not BALB/c, which had not previously been noted in our studies, (Fig. 5A; arrow with *). That band also became detectable in the CD40 stimulated 4F11-constellation at the 30 minutes and over-night time points but not at the 3 hour time point. Additionally, in the 4F11- and FGK45-constellations yet another smaller band at approximately 34 kD was detected. It remains to be determined whether the 43 kD and 48 kD bands are the isoform I glycoforms we found in figures 1–3 and whether the new bands are yet other glycoforms of isoform I or whether they are different isoforms that are present in such low abundance that they only become detectable when enriched in immunoprecipitations.
Figure 5.
Th40 cells were sorted from 10 – 16 weeks old NOD or BALB/c female mouse spleens. Cell lysates from cells immediately ex-vivo (0') or CD40 stimulated for 30 minutes, 3 hours and over night (30', 3h, o.n. respectively) were subjected to immunoprecipitations using CD40 antibodies 1C10, 4F11 and FGK45. (A) Western blot for CD40 using a C-terminal reactive antibody. Arrows indicate CD40 bands. (B) Western blot for TRAF2. Arrows indicate TRAF2 bands. (C) Western blot for TRAP2. Experiments were done at least three times.
The different CD40 receptor constellations interact differentially with TRAF2 and TRAP2
CD40 is known to signal through TRAF2 (Xie et al., 2006) and both CD40 and TRAF2 have been shown to colocalize to the lipid raft microdomain of Th40 cells (Vaitaitis and Wagner, 2008). Additionally, TRAF2 coimmunoprecipitates with CD40 in a CD40-stimulus dependent manner (Xie et al., 2006). We analyzed whether TRAF2 coimmunoprecipitated with the three constellations of the CD40 receptor in figure 5A. TRAF2 was coimmunoprecipitated mainly with 1C10 and FGK45 in NOD Th40 cells while in 4F11 there was only a very low level (Fig. 5B). This shows that 4F11 reactive CD40 constellations are less likely to signal through TRAF2 in NOD Th40 cells. No TRAF2 coimmunoprecipitated with CD40 in BALB/c Th40 (Fig. 5B). It is not clear whether the higher band is a different form of TRAF2 or not (Fig. 5B; iso 2?).
When isolating major bands from CD40 immunoprecipitations for protein identification one band exhibited a strong match to TNF-receptor 1 associated protein 2 (TRAP2; data not shown). We confirmed this by western blot on CD40 coimmunoprecipitated protein and found that the FGK45 reactive CD40 constellation in both NOD and BALB/c Th40 cells coimmunoprecipitated TRAP2 but only after overnight stimulation of CD40 (Fig. 5C).
The different CD40 receptor constellations interact with TNFR1 and TNFR2 and engagement of the TNFR1 and TNFR2 molecules impact CD40 signaling outcomes
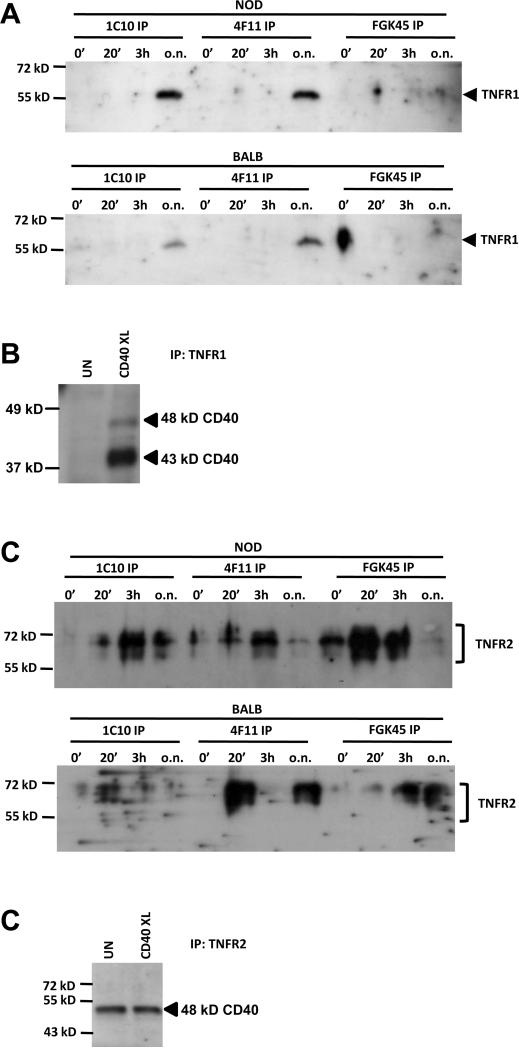
Because of the association of TRAP2 with CD40 (Fig. 5C) we considered that it is possible that different members of the TNF-receptor super family could interact to form hybrid receptors. Therefore we determined whether TNFR1 and TNFR2 coimmunoprecipitate with CD40. A CD40-engagement dependent association of TNFR1 was found in both NOD and BALB/c Th40 cells after overnight CD40-stimulation (Fig. 6A). To confirm this, we performed immunoprecipitation with an antibody to TNFR1 followed by western blot for CD40 on cell lysates from NOD Th40 cells. CD40 was indeed coimmunoprecipitated by the TNFR1 antibody after CD40 stimulation and mainly a 43 kD CD40 band was present (Fig. 6B).
Figure 6.
Th40 cells were sorted from 10 – 16 weeks old NOD or BALB/c female mouse spleens. Cell lysates from cells immediately ex-vivo (0') or CD40 stimulated for 30 minutes, 3 hours and over night (30', 3h, o.n. respectively) were subjected to immunoprecipitations using CD40 antibodies 1C10, 4F11 and FGK45 (in A and C) or an antibody to TNFR1 (in B). (A) Western blot for TNFR1 was performed. (B) Untreated (UN) or CD40-stimulated (CD40XL) cell lysates from NOD Th40 cells were subjected to immunoprecipitation using an antibody to TNFR1. Western blot was performed using a CD40 C-terminal reactive antibody. (C) Western blot for TNFR2 was performed. (D) Untreated (UN) or CD40-stimulated (CD40XL) cell lysates from NOD Th40 cells were subjected to immunoprecipitation using an antibody to TNFR2. Western blot was performed using a CD40 C-terminal reactive antibody. Experiments were done three times.
CD40 and TNFR2 also interacted, especially when CD40 was engaged, in both NOD and BALB/c Th40 cells (Fig. 6C). However, the pattern of interaction differed substantially between Th40 cells from NOD and from BALB/c mice. In NOD Th40 cells TNFR2 coimmunoprecipitated with 1C10, 4F11 and FGK45 reactive CD40 constellations while in BALB/c Th40 cells, mainly 4F11 constellations were associated with TNFR2 (Fig. 6C). To confirm the CD40-TNFR2 coimmunoprecipitation we performed immunoprecipitation with an antibody to TNFR2 followed by western blot for CD40. CD40 was coimmunoprecipitated with TNFR2 in both untreated and CD40 stimulated NOD Th40 cells but only a larger, 48 kD band was detected (Fig. 6D).
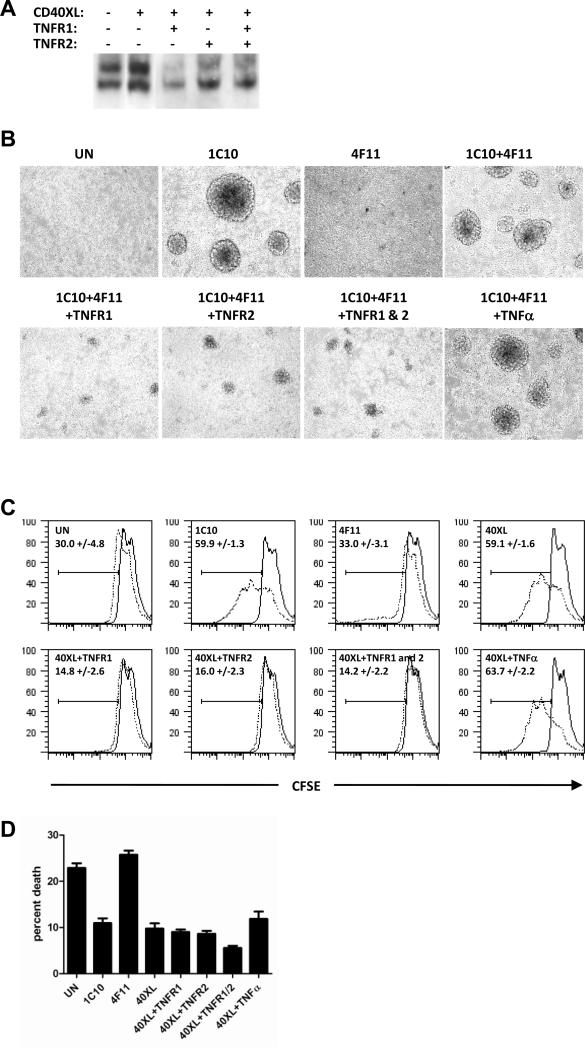
Since TNFR1 and TNFR2 interact with CD40 to form hybrid receptors we determined what the functional outcomes of such receptors are. CD40 stimulation is known to induce Nf-κB DNA-binding activity in T cell clones (Wagner et al., 2002) and NOD Th40 cells (Vaitaitis and Wagner, 2008). Therefore we determined whether engaging TNFR1 and TNFR2 had an effect on Nf-κB DNA-binding activity in NOD Th40 cells. As previously demonstrated, CD40 stimulation induced increased Nf-κB DNA-binding activity (Fig. 7A). However, when TNFR1 and/or TNFR2 were engaged simultaneously with CD40 that increase was abolished (Fig. 7A).
Figure 7.
Th40 cells were sorted from 10 – 16 weeks old NOD or BALB/c female mouse spleens. (A) Th40 cells were CD40 crosslinked (CD40XL; 1C10+4F11) in the absence/presence of TNFR1 and/or TNFR2 crosslinking. Nuclear extracts were prepared and EMSA performed for Nf-kB DNA-binding activity using a consensus Nf-kB oligonucleotide. (B) Th40 cells were crosslinked using indicated combinations of 1C10, 4F11, TNFR1, TNFR2, and TNFα. After 3 days of culture the growth patterns were photographed. (C) Th40 cells were CFSE labeled then treated as in B. Proliferation (CFSE dilution) was measured after one day (solid line) and 4 days (dotted line) of culture. (D) Graph representing percent dead cells in C (measured by staining permeabilized cells with propidium iodide) in each of the conditions at 4 days of culture.
When culturing NOD Th40 cells in the presence of CD40 stimulation we have noted that the cells form large clusters (Fig. 7B; 1C10 and 1C10+4F11). Therefore we determined whether TNFR1 and/or TNFR2 engagement in addition to CD40 stimulation affected the cluster formation. When antibodies to both TNFR1 and TNFR2 were used to crosslink those molecules, the CD40 induced cluster formation was prevented almost completely (Fig. 7B) demonstrating a negative regulation of this CD40 signaling outcome by TNFR1 and TNFR2. Interestingly, the addition of TNFα, the known ligand for TNF-receptors 1 and 2, to the CD40 stimulated Th40 cells did not prevent the cluster formation (Fig. 7B). Additionally, 4F11 crosslinking alone of the NOD Th40 cells did not induce any cluster formation revealing a difference in signaling potential between the 1C10 and 4F11 reactive CD40 receptor constellations (Fig. 7B).
We have previously shown that CD40 stimulation induces proliferation of NOD Th40 cells (Vaitaitis and Wagner, 2008). Therefore we determined whether TNFR1 and TNFR2 engagement in addition to CD40 stimulation had an effect on proliferation. Untreated Th40 cells demonstrated a small amount of spontaneous proliferation (Fig. 7C). However, that proliferation was accompanied by a similar amount of cell death (Fig. 7D). CD40 stimulation induced robust proliferation as reported previously (Vaitaitis and Wagner, 2008) but only when 1C10 was used as the agonistic antibody (Fig. 7C; 1C10 and 40XL (1C10+4F11)). 4F11 crosslinked Th40 cells demonstrated no additional proliferation compared to untreated Th40 cells, again showing that the 4F11 reactive CD40 receptor constellations have a different signaling potential compared to 1C10 reactive ones. However, 4F11 signals induced a small but significant increase in the rate of death (Fig. 7D; p<0.0001, t-test). Both TNFR1 and TNFR2 engagement resulted in the prevention of the CD40 (1C10) induced proliferation (Fig. 7C). Interestingly, the TNFR1 and TNFR2 treatments also prevented much of the spontaneous proliferation seen in the untreated Th40 cells (Fig. 7C; p<0.012 in all cases, t-test). CD40 (1C10) stimulation in the presence of TNFR1 and /or TNFR2 did however rescue the Th40 cells from the spontaneous death seen in untreated cells (Fig. 7D; p<0.0001, t-test).
Discussion
The CD40 – CD154 dyad has long been under investigation as one of the major culprits in the fulmination and progression of autoimmune disease. Many roles have been ascribed the TNF-receptor super family member CD40 yet little is known about how this receptor is assembled, whether there is more than one form of CD40 receptor and what the outcomes of such receptors are. One of the known differences in CD40 signaling lies in the TRAF family association with CD40 (Bishop et al., 2007). Depending on which TRAF family members associate with CD40 in a given cell, the outcome of signaling downstream of the CD40 receptor could be different. Many studies focus on genotype, yet epigenetics, e.g. splice isoforms and post-translational modification, can radically alter proteins and their ability to interact and signal within the cellular milieu. Do isoforms and post-translational modification such as glycosylation have an effect on how CD40 molecules are able to assemble into a functional receptor and on interactions with other proteins? To fully understand all these possibilities may not be possible since carbohydrates can have literally thousands of different conformations; however, in this manuscript we begin to address these issues.
We focused on Th40 cells, which have been shown to be necessary and sufficient in transferring type 1diabetes in the NOD model of that disease. We demonstrate in figures 1 and 2 that while we are able to detect four of five known different CD40 isoforms on the mRNA level, only isoform I is represented on the protein level. We determined that complete deglycosylation of the detected 43 kD band resulted in a 32 kD band corresponding to the non-glycosylated predicted size of isoform I. Therefore we concluded that the major CD40 proteins detected in both NOD and BALB/c Th40 cells are different glycoforms of isoform I. In addition this CD40 isoform is the form thought not only to be able to signal via its C-terminal intracellular domain (as would be possible for isoform V as well) but also to be able to partition into the raft microdomain via its transmembrane region. As this region is missing in isoform V, isoform V cannot associate with the lipid raft. We found both the 43 kD and the 48 kD bands in the raft microdomain of NOD and BALB/c CD4+ T cells indicating that both those bands contain the transmembrane region thus strengthening the argument that they represent different post-translationally modified forms of isoform I.
The raft microdomain is where CD40 interactions with other proteins and TRAFs take place (Bishop et al., 2007; Munroe and Bishop, 2007) and it is also the subcellular fraction to which TRAF2 partitions in the NOD Th40 cells (Vaitaitis and Wagner, 2008). In this manuscript we demonstrate that CD40 coimmunoprecipitates TRAF2 in NOD Th40 cells but not in BALB/c Th40 cells. We have shown previously that CD40 induces cFLIPp43 expression leading to survival and proliferation of NOD Th40 cells but not BALB/c Th40 cells (Vaitaitis and Wagner, 2008). The TRAF2 data presented here demonstrate yet another difference in CD40 signaling between NOD and BALB/c Th40 cells and further explains why BALB/c Th40 cells are not capable of survival and proliferation in response to CD40 signals in-vitro.
In-vivo, CD40 signals appear to be absolutely necessary in order for the Th40 population to expand. Preventing CD40 signaling in NOD mice contained the Th40 population to levels seen in non-autoimmune BALB/c mice. This treatment also prevented the high expression of CD40 and altered the CD40 glycoform profile to look more like that of the non-autoimmune BALB/c mouse. Conversely, excessive CD40 signals in-vivo to BALB/c mice caused the selective expansion of the Th40 cells and those cells increased their overall CD40 expression as well as altered their glycoform profile to appear more like that of autoimmune NOD mice. However, since the CD40 induced proliferation of BALB/c Th40 cells does not take place in-vitro it is likely that there are several steps involved in-vivo. The injection of agonistic CD40 antibody could affect many different cells in-vivo and therefore many inflammatory pathways perhaps leading to the acquisition of a specific CD40 receptor constellation that may be necessary for proliferation to take place in response to CD40 signals. It remains to be determined whether Th40 cells from BALB/c mice injected with agonistic CD40 antibodies would proliferate in response to CD40-stimulation in-vitro.
More intricate possibilities are unveiled by the interaction of CD40 with TNFR1 and TNFR2. Our data reveal that antibody crosslinking of TNFR1 and/or TNFR2 prevents several of the CD40 signaling outcomes in NOD Th40 cells. However, the addition of TNFα does not. This indicates that the actions of the antibodies are likely not exerted via a bona fide TNFR1 or TNFR2 but rather through interfering with CD40 signals through the hybrid receptors.
Glycosylation has been shown to regulate important functions of the immune system such as trafficking of immune cells and the activation threshold of TCR (Marth and Grewal, 2008). It was demonstrated that several autoimmune prone mouse strains, including NOD, express 30 – 40% less β1,6GlcNAc-branched N-glycans than non-autoimmune prone strains (Lee et al., 2007) and that such a reduction is sufficient to enhance TCR signaling and T cell proliferation (Grigorian et al., 2007). The CD40 – CD154 dyad is critically involved in the progression of autoimmune disease (Balasa et al., 1997; Durie et al., 1993; Kobata et al., 2000; Munroe and Bishop, 2007; Quezada et al., 2003; Toubi and Shoenfeld, 2004; Vaitaitis et al., 2003; Vaitaitis and Wagner, 2008; Waid et al., 2004; Wang et al., 2002; Yu et al., 2001) and here we show that high expression of a less glycosylated form of CD40 isoform I is associated with the survival and proliferation of the pathogenic Th40 cells in autoimmune NOD mice establishing CD40 as yet another molecule affected by the glycosylation status of the cell. Furthermore, CD40 signals both in-vivo and in-vitro have a positive feedback on CD40-expression itself inducing expression of the less glycosylated form of CD40 isoform I. Interestingly, blocking of CD154 in-vivo allows for an increase in the 48 kD glycoform of isoform I. Therefore it is possible that, in the absence of persistent CD40-stimulation, the basal expression of CD40 in Th40 cells occurs as the more glycosylated form but when CD40-signals are received and persist, the glycoform expression is changed, ultimately changing the make-up of the CD40 receptor constellations. Signals through the new constellations in turn activate the Th40 cell to survive/proliferate and achieve effector status.
Understanding the dynamics of the different CD40 receptor constellations will be critical to understanding the etiology of autoimmune disease. Also, increased knowledge about the induced hybrid receptors of CD40 with TNFR1 and TNFR2 may hold clues to why treatments to block or signal through the TNF-receptor family members have very different outcomes depending on the disease model and the age of the treatment recipient (Yang et al., 1994). Perhaps combination treatments targeting both CD40 and TNFR1 and/or TNFR2 would be more effective in preventing or ameliorating disease.
Acknowledgements
This work was supported by grants from the American Diabetes Association, the NIDDK, and the Kleberg Foundation awarded to DHW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baccam M, Bishop GA. Membrane-bound CD154, but not CD40-specific antibody, mediates NF-kappaB-independent IL-6 production in B cells. Eur J Immunol. 1999;29:3855–66. doi: 10.1002/(SICI)1521-4141(199912)29:12<3855::AID-IMMU3855>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Baker RL, Wagner DH, Jr., Haskins K. CD40 on NOD CD4 T cells contributes to their activation and pathogenicity. J Autoimmun. 2008;31:385–92. doi: 10.1016/j.jaut.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Balasa B, Krahl T, Patstone G, Lee J, Tisch R, McDevitt HO, Sarvetnick N. CD40 ligand-CD40 interactions are necessary for the initiation of insulitis and diabetes in nonobese diabetic mice. J Immunol. 1997;159:4620–7. [PubMed] [Google Scholar]
- Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Med Biol. 2007;597:131–51. doi: 10.1007/978-0-387-70630-6_11. [DOI] [PubMed] [Google Scholar]
- Braesch-Andersen S, Paulie S, Koho H, Nika H, Aspenstrom P, Perlmann P. Biochemical characteristics and partial amino acid sequence of the receptor-like human B cell and carcinoma antigen CDw40. J Immunol. 1989;142:562–7. [PubMed] [Google Scholar]
- Brodeur SR, Angelini F, Bacharier LB, Blom AM, Mizoguchi E, Fujiwara H, Plebani A, Notarangelo LD, Dahlback B, Tsitsikov E, Geha RS. C4b-binding protein (C4BP) activates B cells through the CD40 receptor. Immunity. 2003;18:837–48. doi: 10.1016/s1074-7613(03)00149-3. [DOI] [PubMed] [Google Scholar]
- Datta SK. Production of pathogenic antibodies: cognate interactions between autoimmune T and B cells. Lupus. 1998;7:591–6. doi: 10.1191/096120398678920703. [DOI] [PubMed] [Google Scholar]
- Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–9. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- Durie FH, Fava RA, Foy TM, Aruffo A, Ledbetter JA, Noelle RJ. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993;261:1328–30. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- Fuleihan R, Ramesh N, Geha RS. Role of CD40-CD40-ligand interaction in Ig-isotype switching. Current Opinion in Immunology. 1993;5:963–7. doi: 10.1016/0952-7915(93)90113-7. [DOI] [PubMed] [Google Scholar]
- Grigorian A, Lee SU, Tian W, Chen IJ, Gao G, Mendelsohn R, Dennis JW, Demetriou M. Control of T Cell-mediated autoimmunity by metabolite flux to N-glycan biosynthesis. J Biol Chem. 2007;282:20027–35. doi: 10.1074/jbc.M701890200. [DOI] [PubMed] [Google Scholar]
- Heath AW, Wu WW, Howard MC. Monoclonal antibodies to murine CD40 define two distinct functional epitopes. Eur J Immunol. 1994;24:1828–34. doi: 10.1002/eji.1830240816. [DOI] [PubMed] [Google Scholar]
- Jinchuan Y, Zonggui W, Jinming C, Li L, Xiantao K. Upregulation of CD40--CD40 ligand system in patients with diabetes mellitus. Clin Chim Acta. 2004;339:85–90. doi: 10.1016/j.cccn.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Kobata T, Azuma M, Yagita H, Okumura K. Role of costimulatory molecules in autoimmunity. Rev Immunogenet. 2000;2:74–80. [PubMed] [Google Scholar]
- Lee SU, Grigorian A, Pawling J, Chen IJ, Gao G, Mozaffar T, McKerlie C, Demetriou M. N-glycan processing deficiency promotes spontaneous inflammatory demyelination and neurodegeneration. J Biol Chem. 2007;282:33725–34. doi: 10.1074/jbc.M704839200. [DOI] [PubMed] [Google Scholar]
- Lutgens E, Daemen MJ. CD40-CD40L interactions in atherosclerosis. Trends Cardiovasc Med. 2002;12:27–32. doi: 10.1016/s1050-1738(01)00142-6. [DOI] [PubMed] [Google Scholar]
- Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8:874–87. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R, Gao G, Pawling J, Dennis JW, Demetriou M, Li B. N-acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. J Immunol. 2004;173:7200–8. doi: 10.4049/jimmunol.173.12.7200. [DOI] [PubMed] [Google Scholar]
- Munroe ME, Bishop GA. A costimulatory function for T cell CD40. J Immunol. 2007;178:671–82. doi: 10.4049/jimmunol.178.2.671. [DOI] [PubMed] [Google Scholar]
- Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992;89:6550–4. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi M, Jager J, Paulmyer-Lacroix O, Peiretti F, Gremeaux T, Verdier M, Grino M, Stepanian A, Msika S, Burcelin R, de Prost D, Tanti JF, Alessi MC. The inflammatory receptor CD40 is expressed on human adipocytes: contribution to crosstalk between lymphocytes and adipocytes. Diabetologia. 2009;52:1152–63. doi: 10.1007/s00125-009-1267-1. [DOI] [PubMed] [Google Scholar]
- Quezada SA, Eckert M, Adeyi OA, Schned AR, Noelle RJ, Burns CM. Distinct mechanisms of action of anti-CD154 in early versus late treatment of murine lupus nephritis. Arthritis Rheum. 2003;48:2541–54. doi: 10.1002/art.11230. [DOI] [PubMed] [Google Scholar]
- Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for S mu-S epsilon heavy chain class switching. Immunity. 1996;5:319–30. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- Sprague DL, Sowa JM, Elzey BD, Ratliff TL. The role of platelet CD154 in the modulation in adaptive immunity. Immunol Res. 2007;39:185–93. doi: 10.1007/s12026-007-0074-3. [DOI] [PubMed] [Google Scholar]
- Suo Z, Wu M, Ameenuddin S, Anderson HE, Zoloty JE, Citron BA, Andrade-Gordon P, Festoff BW. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J Neurochem. 2002;80:655–66. doi: 10.1046/j.0022-3042.2001.00745.x. [DOI] [PubMed] [Google Scholar]
- Tone M, Tone Y, Fairchild PJ, Wykes M, Waldmann H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc Natl Acad Sci U S A. 2001;98:1751–6. doi: 10.1073/pnas.98.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubi E, Shoenfeld Y. The role of CD40-CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity. 2004;37:457–64. doi: 10.1080/08916930400002386. [DOI] [PubMed] [Google Scholar]
- Vaitaitis GM, Poulin M, Sanderson RJ, Haskins K, Wagner DH., Jr. Cutting edge: CD40-induced expression of recombination activating gene (RAG) 1 and RAG2: a mechanism for the generation of autoaggressive T cells in the periphery. J Immunol. 2003;170:3455–9. doi: 10.4049/jimmunol.170.7.3455. [DOI] [PubMed] [Google Scholar]
- Vaitaitis GM, Wagner DH., Jr. High distribution of CD40 and TRAF2 in Th40 T cell rafts leads to preferential survival of this auto-aggressive population in autoimmunity. PLoS One. 2008;3:e2076. doi: 10.1371/journal.pone.0002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitaitis GM, Waid DM, Wagner DH., Jr. The expanding role of TNF-Receptor Super Family member CD40 (tnfrsf5) in Autoimmune Disease: Focus on Th40 cells. Current Immunology Reviews. 2010 [Google Scholar]
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- Vogel JD, West GA, Danese S, De La Motte C, Phillips MH, Strong SA, Willis J, Fiocchi C. CD40-mediated immune-nonimmune cell interactions induce mucosal fibroblast chemokines leading to T-cell transmigration. Gastroenterology. 2004;126:63–80. doi: 10.1053/j.gastro.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Wagner DH., Jr. The co-evolution of our understanding of CD40 and inflammation. Diabetologia. 2009;52:997–9. doi: 10.1007/s00125-009-1357-0. [DOI] [PubMed] [Google Scholar]
- Wagner DH, Jr., Newell E, Sanderson RJ, Freed JH, Newell MK. Increased expression of CD40 on thymocytes and peripheral T cells in autoimmunity: a mechanism for acquiring changes in the peripheral T cell receptor repertoire. Int J Mol Med. 1999;4:231–42. doi: 10.3892/ijmm.4.3.231. [DOI] [PubMed] [Google Scholar]
- Wagner DH, Jr., Vaitaitis G, Sanderson R, Poulin M, Dobbs C, Haskins K. Expression of CD40 identifies a unique pathogenic T cell population in type 1 diabetes. Proc Natl Acad Sci U S A. 2002;99:3782–7. doi: 10.1073/pnas.052247099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waid DM, Vaitaitis GM, Pennock ND, Wagner DH., Jr. Disruption of the homeostatic balance between autoaggressive (CD4+CD40+) and regulatory (CD4+CD25+FoxP3+) T cells promotes diabetes. J Leukoc Biol. 2008;84:431–9. doi: 10.1189/jlb.1207857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waid DM, Vaitaitis GM, Wagner DH., Jr. Peripheral CD4loCD40+ auto-aggressive T cell expansion during insulin-dependent diabetes mellitus. Eur J Immunol. 2004;34:1488–97. doi: 10.1002/eji.200324703. [DOI] [PubMed] [Google Scholar]
- Waid DM, Wagner R, Putnam A, Vaitaitis GM, Pennock ND, Calverley DC, Gottlieb P, Wagner DH., Jr. A Unique T Cell Subset Described as CD4loCD40+ T cells (TCD40) in Human Type 1 Diabetes. Clin Immunol. 2007;124:138–148. doi: 10.1016/j.clim.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Wang X, Huang W, Mihara M, Sinha J, Davidson A. Mechanism of action of combined short-term CTLA4Ig and anti-CD40 ligand in murine systemic lupus erythematosus. J Immunol. 2002;168:2046–53. doi: 10.4049/jimmunol.168.4.2046. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kelly CG, Karttunen JT, Whittall T, Lehner PJ, Duncan L, MacAry P, Younson JS, Singh M, Oehlmann W, Cheng G, Bergmeier L, Lehner T. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity. 2001;15:971–83. doi: 10.1016/s1074-7613(01)00242-4. [DOI] [PubMed] [Google Scholar]
- Wyzgol A, Muller N, Fick A, Munkel S, Grigoleit GU, Pfizenmaier K, Wajant H. Trimer stabilization, oligomerization, and antibody-mediated cell surface immobilization improve the activity of soluble trimers of CD27L, CD40L, 41BBL, and glucocorticoid-induced TNF receptor ligand. J Immunol. 2009;183:1851–61. doi: 10.4049/jimmunol.0802597. [DOI] [PubMed] [Google Scholar]
- Xie P, Hostager BS, Munroe ME, Moore CR, Bishop GA. Cooperation between TNF receptor-associated factors 1 and 2 in CD40 signaling. J Immunol. 2006;176:5388–400. doi: 10.4049/jimmunol.176.9.5388. [DOI] [PubMed] [Google Scholar]
- Yang XD, Tisch R, Singer SM, Cao ZA, Liblau RS, Schreiber RD, McDevitt HO. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994;180:995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Medling B, Yagita H, Braley-Mullen H. Characteristics of inflammatory cells in spontaneous autoimmune thyroiditis of NOD.H-2h4 mice. J Autoimmun. 2001;16:37–46. doi: 10.1006/jaut.2000.0458. [DOI] [PubMed] [Google Scholar]