Abstract
Phospholipase C-γ1 (PLC-γ1) is a multiple-domain protein and plays an important role in epidermal growth factor (EGF)-induced cell mitogenesis, but the underlying mechanism is unclear. We have previously demonstrated that PLC-γ1 is required for EGF-induced mitogenesis of squamous cell carcinoma (SCC) cells, but the mitogenic function of PLC-γ1 is independent of its lipase activity. Earlier studies suggest that the Src homology 3 (SH3) domain of PLC-γ1 possesses mitogenic activity. In the present study, we sought to determine the role of the SH3 domain of PLC-γ1 in EGF-induced SCC cell mitogenesis. We examined the effect of overexpression of PLC-γ1, a catalytically active PLC-γ1 mutant lacking the SH3 domain or a catalytically inactive PLC-γ1 mutant lacking the X domain on EGF-induced SCC4 (tongue squamous cell carcinoma) cell mitogenesis. We found that overexpression of PLC-γ1 enhanced EGF-induced SCC4 cell mitogenesis. This enhancement was abolished by deletion of the SH3 domain but not by deletion of the X catalytic domain. These data suggest that the SH3 domain, but not the catalytic domain, is required for PLC-γ1 to mediate EGF-induced SCC4 cell mitogenesis.
Keywords: Epidermal growth factor receptor, phospholipase C-γ1, squamous cell carcinoma, mitogenesis, SH3 domain, catalytic domain
1. Introduction
The epidermal growth factor receptor (EGFR) plays a key role in cancer cell proliferation. Therefore, elucidation of the pathway for EGFR-stimulated proliferation is important and might lead to development of new targets for cancer therapy. Activation of EGFR by its ligand such as epidermal growth factor (EGF) or transforming growth factor α (TGF-α) initiates multiple downstream signaling pathways [1]. One of the pathways involves phospholipase C-γ1 (PLC-γ1) [1]. Unlike other phospholipase C (PLC) isozymes, PLC-γ1 contains two Src homology 2 (SH2) domains and one Src homology 3 (SH3) domain between X and Y catalytic domains [2]. In response to EGFR ligands, the SH2 domains of PLC-γ1 interact with EGFR. This interaction allows tyrosine phosphorylation and activation of the catalytic domain of PLC-γ1 by the tyrosine kinase EGFR [3, 4]. The catalytic activation of PLC-γ1 leads to the phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis which generates two intracellular second messengers, inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). DAG is a physiologic activator of protein kinase C (PKC), and IP3 induces the release of calcium from intracellular stores. The intracellular calcium release and PKC activation are essential for regulation of many cellular activities [5–7]. The SH3 domain is a protein-interaction module which has been found to play an important role in a variety of biological processes by binding with proline-rich ligands [8]. Although PLC-γ1 has been shown to mediate EGF-induced cell mitogenesis, the underlying mechanism is unclear. The PLC-γ1 SH3 domain has been reported to possess mitogenic activity [9, 10]. However, microinjection of the PLC-γ1 SH3 domain fails to enhance EGF-induced mitogenesis [11]. We have previously shown that EGF-induced mitogenesis of squamous cell carcinoma (SCC) cells requires PLC-γ1 but not the catalytic activity of PLC [12], suggesting that regions other than the catalytic domain of PLC-γ1 may be responsible for EGF-induced SCC mitogenesis. In the present study, we examined the effect of overexpression of PLC-γ1 or PLC-γ1 mutant lacking the SH3 or X domain on EGF-induced SCC cell mitogenesis. We found that overexpression of PLC-γ1 enhanced EGF-induced mitogenesis, but this enhancement was abolished by deletion of the SH3 domain but not by deletion of the catalytic domain. These observations provide direct evidence for requirement of the SH3 domain, but not the catalytic domain, for PLC-γ1 to mediate EGF-induced SCC cell mitogenesis.
2. Materials and Methods
2.1. Cell Culture
Human tongue squamous cell carcinoma SCC4 cells obtained from ATCC were grown in the SCC medium composed of 1:1 mixture of Ham’s F12 and Dulbecco’s Modified Eagle’s Medium with 10% fetal bovine serum. Cells were serum-starved for 24 hrs before treatment with EGF.
2.2. DNA constructs and transfection
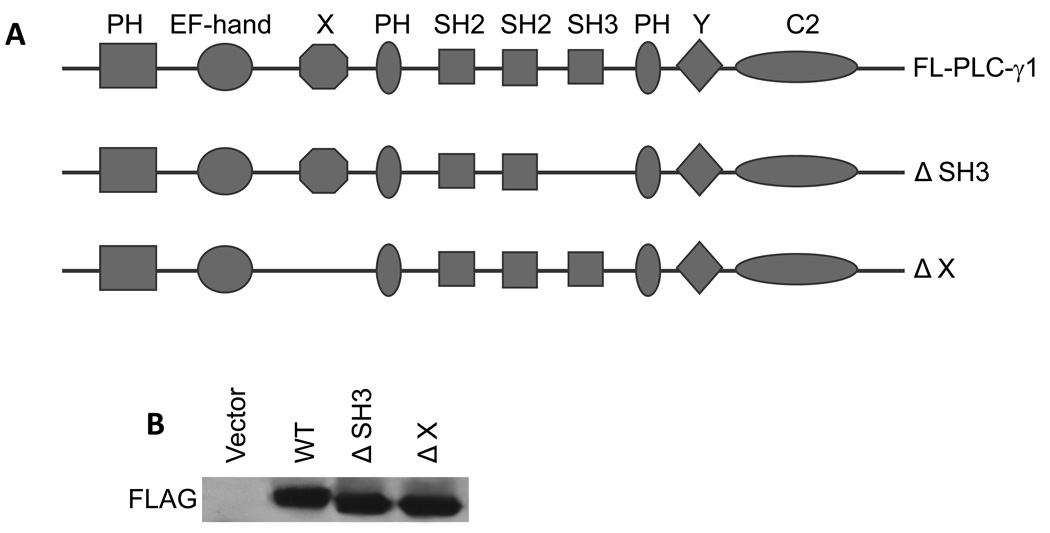
SCC4 cells were transiently transfected in suspension with pcDNA 3.1 containing the full-length human PLC-γ1 cDNA (a gift from Dr. John Imboden, San Francisco General Hospital) or the various mutants tagged with a FLAG epitope (Figure 1) using a polybrene/glycerol method [13]. The pcDNA3.1 vector contains the neomycin (G418) resistant gene to allow selection. The transfected cells were selected by 4-day incubation in 300 µM G418 starting 2 days after transfection to enrich transfected cells as we previously described [14,15].
Fig. 1.
Schematic diagram of full-length and truncated PLC-γ1-FLAG-tagged fusion constructs. (A) The constructs of the full-length PLC-γ1 cDNA (FL-PLC-γ1), PLC-γ1 mutant lacking the SH3 domain (ΔSH3) or PLC-γ1 mutant lacking the X catalytic domain (ΔX). (B) SCC4 cells were transfected with various constructs as indicated. Transfected cells were selected by 4-day incubation in 300 µM G418. The total cell lysates were isolated. The level of the FLAG fusion protein was determined by immunoblotting with anti-FLAG antibody.
2.3. Immunoblotting
The total cell lysates were isolated using radioimmunoprecipitation assay lysis buffer containing 50 mM HEPES, pH 7.4, 1% Triton X-100, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, and Complete Protease Inhibitor Cocktail Tablets (Roche Applied Science, Indianapolis, IN). The protein concentration of the lysate was measured by bicinchoninic acid protein assay kits (Thermo Fisher Scientific, Rockford, IL). Equal amounts of protein were electrophoresed by the reducing SDS-polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene fluoride microporous membranes (Immobilon-P, 0.45 µm; Millipore Billerica, MA). After incubation in blocking buffer (100 mM Tris base, 150 mM NaCl, 5% nonfat milk, and 0.5% Tween-20) for 1 h at room temperature, blots were then incubated overnight at 4 °C with a monoclonal antibody against the FLAG peptide at a dilution of 1:1000 (Agilent Technologies, Santa Clara, CA).
2.4. PLC-γ1 activity assay
PLC-γ1 activity was determined by measuring accumulation of IP3 according to the experimental procedure described [16]. SCC4 cells in 150 mm dishes were washed with PBS containing 0.1% sodium orthovanadate and 0.1% sodium fluoride, and then incubated with 1% NP-40 containing Phosphatase Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN) and Complete Protease Inhibitor Cocktail Tablets (Roche Applied Science) for 5 min. Cells were scraped into microfuge tubes and incubated at 4 °C on a rotator for 1 h. Sixty micrograms of protein from the supernatant collected after centrifugation was incubated with 2 µg polyclonal antibody against the C-terminus of PLC-γ1 (BD Biosciences, San Jose, CA) at 4 °C overnight, and then with 20 µl UltraLink Immobilized Protein G (Thermo Fisher Scientific) at 4 °C for 1 h. After centrifugation, the pellet was washed with the reaction buffer (10 mM HEPES, pH 7.0, 10 mM NaCl, 120 mM KCl, 2 mM EGTA, 0.05% deoxycholate, 5 µg/ml bovine serum albumin and 10 µM CaCl2) and resuspended in 200 µl reaction buffer. In triplicates, 50 µl of the suspension was incubated with sonicated vesicles containing [3H]-PIP2 (PerkinElmer Life Science, Waltham, MA), phosphatidylcholine (Sigma Aldrich Corporation, St. Louis, MO) and phosphatidylserine (Sigma Aldrich Corporation) in a molar ratio of 1:3:3 in 100 µl reaction buffer. The reaction was ended by adding 200 µl of 10% trichloroacetic acid at 5 min and 200 µl of 10% bovine serum albumin. The radioactivity of supernatant after centrifugation was determined by a scintillation counter and normalized to the protein content in the immunoprecipitates [17–19].
2.5. Mitogenesis Assay
Cell mitogenesis was measured by [3H]-thymidine incorporation as previously described [12]. Briefly, keratinocytes were incubated with 1 µCi/well of [3H]-thymidine (PerkinElmer Life Science) for 8 hours. Cells were then washed with refrigerated PBS, treated with 5% trichloroacetic acid (ice cold) for 30 min, washed again with PBS, and solubilized in 0.5 ml 0.5 N NaOH/0.5% SDS. The cell-associated radioactivity was then measured by liquid scintillation counting and normalized to DNA content. Six wells were harvested for each treatment; three wells were used for [3H]-thymidine incorporation, and the remaining three wells were used to determine the DNA content.
3. Results and Discussion
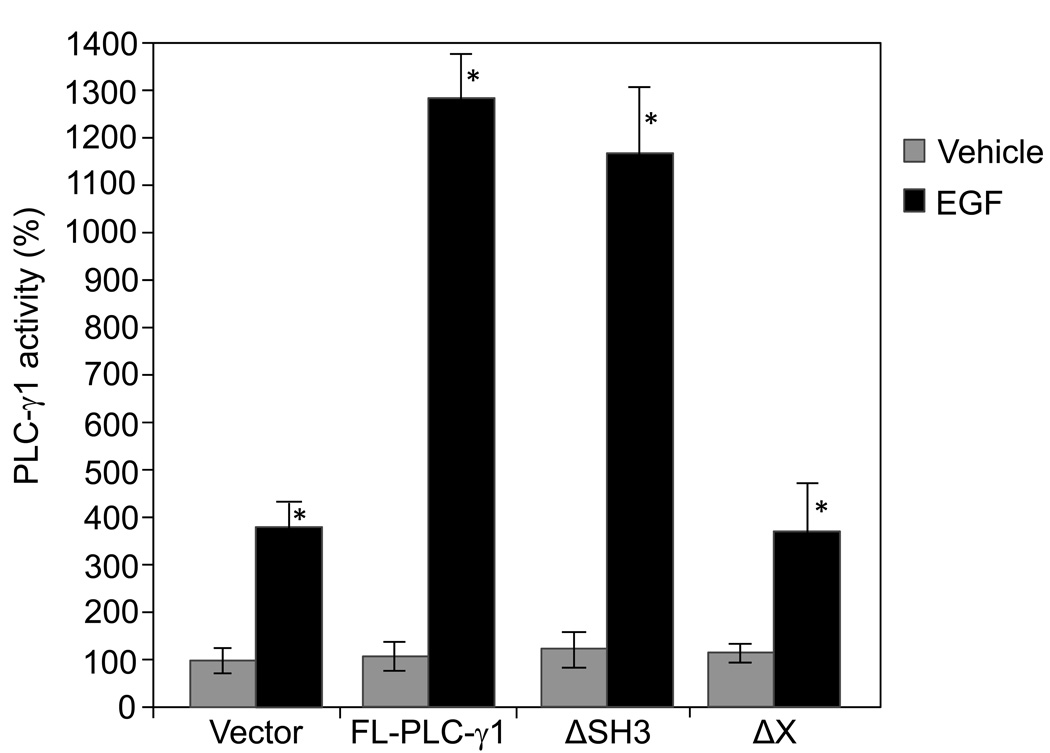
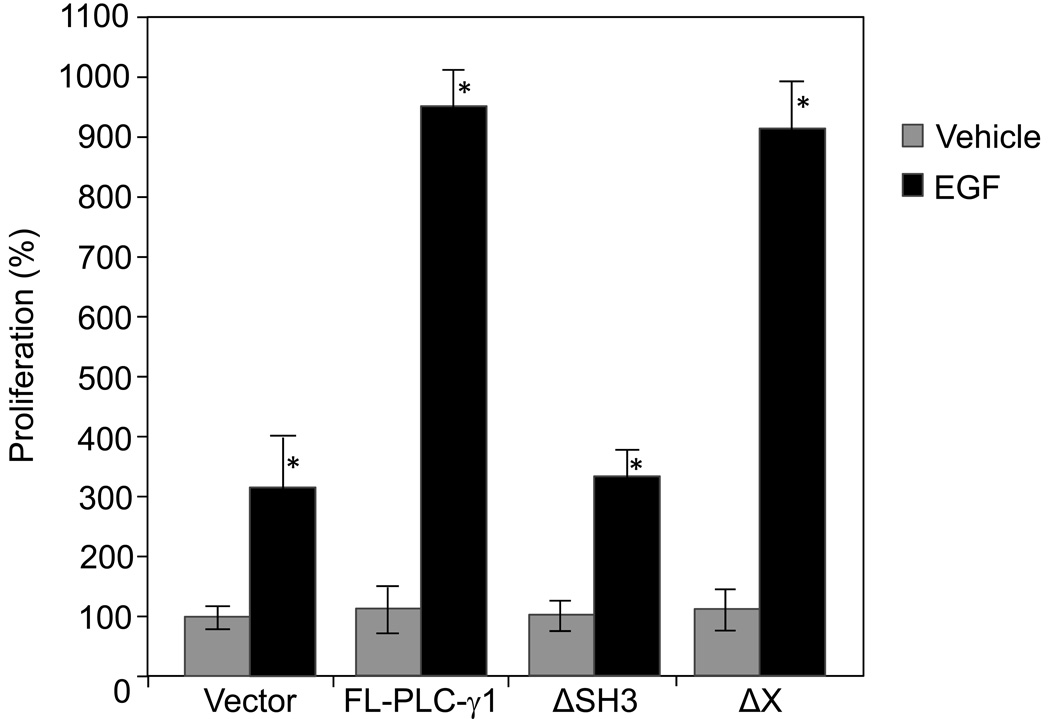
We have previously demonstrated that PLC-γ1 is required for EGF-induced SCC cell mitogenesis and the mitogenic function of PLC-γ1 is independent of its lipase activity [12], suggesting that regions other than the catalytic domain mediate EGF-induced SCC mitogenesis. Earlier studies have shown that microinjection of the PLC-γ1 SH3 domain into fibroblasts induces cell mitogenesis [9, 10]. The present study was undertaken to determine whether the SH3 domain of PLC-γ1 plays a role in EGF-induced SCC mitogenesis. A full-length human PLC-γ1 (FL-PLC-γ1) cDNA, a PLC-γ1 mutant lacking the SH3 domain (ΔSH3), and a PLC-γ1 mutant lacking the X catalytic domain were used in this study. All the cDNA inserts in the expression vector pcDNA 3.1 were tagged at the N-terminal end with a FLAG epitope which allows observations of the overexpressed PLC-γ1. The constructs were separately transfected into SCC4 cells (Fig. 1A). Transfected cells were then stimulated by EGF at a concentration of 50 ng/ml. The expression of the full-length PLC-γ1 or PLC-γ1 mutants was confirmed by immunoblotting with anti-FLAG antibody (Fig. 1B). All the constructs were found to express comparable levels of FLAG-PLC-γ1. As expected, the truncated PLC-γ1 proteins (ΔSH3 and ΔX) were slightly smaller than FL-PLC-γ1 (Fig. 1B). The catalytic activity of endogenous and transfected PLC-γ1 and mutants in SCC4 cells was confirmed by PLC-γ1 activity assay following PLC-γ1 immunoprecipitation. All of these constructs showed comparable levels of PLC-γ1 activity when transfected into SCC4 cells under serum-starved conditions. EGF treatment for 2 minutes induced activity of endogenous PLC-γ1 in vector-transfected cells (Fig. 2). Overexpression of FL-PLC-γ1 enhanced EGF-induced PLC-γ1 activation. Deletion of the X domain abolished the enhancement of EGF-induced PLC-γ1 activation. However, deletion of the SH3 domain did not alter the enhancement (Fig. 2). In parallel to PLC-γ1 activity, all of these constructs showed comparable levels of mitogenesis when transfected into SCC4 cells under serum-starved conditions. Overexpression of FL-PLC-γ1 enhanced EGF-induced mitogenesis. However, this enhancement was abolished by deletion of the SH3 domains but not affected by deletion of the X catalytic domain (Fig. 3). These data suggest that overexpression of PLC-γ1 enhances EGF-induced mitogenesis via the SH3 domain but not the catalytic domain and the lipase activity.
Fig. 2.
EGF-induced PLC-γ1 activation in SCC4 cells transfected with various PLC-γ1 constructs. SCC4 cells were transfected with various PLC-γ1 constructs as indicated. Transfected cells were selected by 4-day incubation in 300 µM G418, and then treated with EGF (50 ng/ml) for 2 minutes. Cells were harvested and total cell lysates were isolated. PLC-γ1 in the total cell lysates was immunoprecipitated using an antibody against the C-terminus of PLC-γ1, and PLC-γ1 activity was assayed. The results are expressed as percentages of the values obtained with vector-transfected/vehicle-treated cells. Data are mean ± SD of triplicates within a single representative experiments, *P< 0.01 (highly significantly different from the vehicle treated cells). Results shown are representative of three independent experiments.
Fig. 3.
EGF-induced mitogenesis of SCC4 cells transfected with various PLC-γ1 constructs. SCC4 cells were transfected with various PLC-γ1 constructs as indicated. Transfected cells were selected by 4-day incubation in 300 µM G418, and then treated with EGF (50 ng/ml) for 24 hours. Cell mitogenesis was determined by [3H]-thymidine incorporation assay. The results are expressed as percentages of the values obtained with vector-transfected/vehicle-treated cells. Data are mean ± SD of triplicates within a single representative experiment, *p< 0.01 (highly significantly different from the vehicle treated cells). Results shown are representative of three independent experiments.
The results of the present study are in line with our previous findings demonstrating a lipase independent role for PLC-γ1 in mediating EGF-induced SCC cell mitogenesis [12]. The mitogenic activity of the SH3 domain has been shown by two earlier studies. In these studies, the mitogenesis is initiated by microinjection of a peptide containing the SH3 domain of PLC-γ1 into fibroblasts in the presence of serum [9, 10]. However, in a subsequent study Wang et al found that microinjection of a peptide containing the SH3 domain of PLC-γ1 into MDCK epithelial cells fails to enhance EGF-induced mitogenesis [11]. Our results indicate that the SH3 domain within the context of the PLC-γ1 protein structure mediates EGF-induced SCC cell mitogenesis. Thus, the SH3 domain is required but may not be sufficient to enhance EGF-induced SCC mitogenesis.
The SH3 domain of PLC-γ1 has been reported to serve as a guanine nucleotide exchange factor that activates GTPases PIKE (phosphatidylinositol 3-kinase enhancer) [20] and dynamin-1 [21]. PIKE mediates activation of nuclear phosphatidylinositol 3-kinase (PI3K) by nerve growth factor, which results in mitogenesis. Dynamin-1 is required for EGFR endocytosis, which plays an important role in EGFR-induced mitogenesis. However, PIKE and dynamin-1 are found to be expressed only in neuronal cells [20, 21]. Further investigation is required to determine the precise mechanism by which the SH3 domain of PLC-γ1 mediates EGF-induced SCC cell mitogenesis.
Research highlights.
Overexpression of phospholipase C-γ1 enhances epidermal growth factor-induced squamous cell carcinoma cell migration.
The Src homology 3 domain of phospholipase C-γ1 is required for epidermal growth factor-induced squamous cell carcinoma cell migration.
The catalytic domain of phospholipase C-γ1 is dispensable for epidermal growth factor-induced squamous cell carcinoma cell migration.
Acknowledgments
This work was supported by Grants 1R03DE018001 and 1R21DE019529-01A2 from the National Institutes of health.
Abbreviations
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- SCC
squamous cell carcinoma
- PLC
phospholipase C
- PLC-γ1
phospholipase C-γ1
- PI3K
phosphoinositide 3-kinases
- PIP2
phosphatidylinositol 4,5-bisphosphate
- IP3
inositol 1,4,5-trisphosphate
- DAG
diacylglycerol
- PKC
protein kinase C
- SH2
Src homology 2
- SH3
Src homology 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637–643. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 2.Williams RL, Katan M. Structural views of phosphoinositide-specific phospholipase C: signalling the way ahead. Structure. 1996;4:1387–1394. doi: 10.1016/s0969-2126(96)00146-3. [DOI] [PubMed] [Google Scholar]
- 3.Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter G, Ji Q. Phospholipase C-γ as a signal-transducing element. Exp Cell Res. 1999;253:15–24. doi: 10.1006/excr.1999.4671. [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 6.Majerus PW, Connolly TM, Deckmyn H, Ross TS, Bross TE, Ishii H, Bansal VS, Wilson DB. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986;234:1519–1526. doi: 10.1126/science.3024320. [DOI] [PubMed] [Google Scholar]
- 7.Rhee SG, Suh PG, Ryu SH, Lee SY. Studies of inositol phospholipid-specific phospholipase C. Science. 1989;244:546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- 8.Mayer BJ. SH3 domains: complexity in moderation. J Cell Sci. 2001;114:1253–1263. doi: 10.1242/jcs.114.7.1253. [DOI] [PubMed] [Google Scholar]
- 9.Smith MR, Liu YL, Kim SR, Bae YS, Kim CG, Kwon KS, Rhee SG, Kung HF. PLC-γ1 Src homology domain induces mitogenesis in quiescent NIH 3T3 fibroblasts. Biochem and Biophys Res Commun. 1996;222:186–193. doi: 10.1006/bbrc.1996.0719. [DOI] [PubMed] [Google Scholar]
- 10.Huang PS, Davis L, Huber H, Goodhart PJ, Wegrzyn RE, Oliff A, Heimbrook DC. An SH3 domain is required for the mitogenic activity of microinjected phospholipase C-γ1. FEBS Lett. 1995;358:287–292. doi: 10.1016/0014-5793(94)01453-8. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Gluck S, Zhang L, Moran MF. Requirement for phospholipase C-γ1 enzymatic activity in growth factor-induced mitogenesis. Mol Cell Biol. 1998;18:590–597. doi: 10.1128/mcb.18.1.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Z, Chen Y, Liao EY, Jiang Y, Liu FY, Pennypacker SD. Phospholipase C-γ1 is Required for the Epidermal Growth Factor Receptor-induced Squamous Cell Carcinoma Cell Mitogenesis. Biochem Biophys Res Commun. 2010;397:296–300. doi: 10.1016/j.bbrc.2010.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang CK, Connolly D, Blumenberg M. Comparison of methods for transfection of human epidermal keratinocytes. J Invest Dermatol. 1991;97:969–973. doi: 10.1111/1523-1747.ep12491889. [DOI] [PubMed] [Google Scholar]
- 14.Xie Z, Bikle DD. Phospholipase C-γ1 is required for calcium-induced keratinocyte differentiation. J Biol Chem. 1999;274:20421–20424. doi: 10.1074/jbc.274.29.20421. [DOI] [PubMed] [Google Scholar]
- 15.Xie Z, Bikle DD. Inhibition of 1,25-dihydroxyvitamin-D-induced keratinocyte differentiation by blocking the expression of phospholipase C-γ1. J Invest Dermatol. 2001;117:1250–1254. doi: 10.1046/j.0022-202x.2001.01526.x. [DOI] [PubMed] [Google Scholar]
- 16.Sun H-Q, LIn K-M, Yin HL. Gelsolin modulates phospholipase C activity in vivo through phospholipid binding. J Cell Biol. 1997;138:811–820. doi: 10.1083/jcb.138.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Z, Singleton PA, Bourguignon LY, Bikle DD. Calcium-induced human keratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-γ1. Mol Biol Cell. 2005;16:3236–3246. doi: 10.1091/mbc.E05-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-γ1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- 19.Xie Z, Chang SM, Pennypacker SD, Liao EY, Bikle DD. Phosphatidylinositol-4-phosphate 5-kinase 1α mediates extracellular calcium-induced keratinocyte differentiation. Mol Biol Cell. 2009;20:1695–1704. doi: 10.1091/mbc.E08-07-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ, Joseph Hurt K, Bae YS, Suh P-G, Snyder SH. Phospholipase C-γ1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature. 2002;415:541–544. doi: 10.1038/415541a. [DOI] [PubMed] [Google Scholar]
- 21.Choi JH, Park JB, Bae SS, Yun S, Kim HS, Hong WP, Kim IS, Kim JH, Han MY, Ryu SH, Patterson RL, Snyder SH, Suh PG. Phospholipase C-γ1 is a guanine nucleotide exchange factor for dynamin-1 and enhances dynamin-1-dependent epidermal growth factor receptor endocytosis. J Cell Sci. 2004;117:3785–3795. doi: 10.1242/jcs.01220. [DOI] [PubMed] [Google Scholar]