Abstract
Ribozymes must fold into compact, native structures to function properly in the cell. The first step in forming the RNA tertiary structure is the neutralization of the phosphate charge by cations, followed by collapse of the unfolded molecules into more compact structures. The specificity of the collapse transition determines the structures of the folding intermediates and the folding time to the native state. However, the forces that enable specific collapse in RNA are not understood. Using time-resolved SAXS, we report that upon addition of 5 mM Mg2+ to the Azoarcus group I ribozyme, up to 80% of chains form compact structures in less than 1 millisecond. In 1 mM Mg2+, the collapse transition produces extended structures that slowly approach the folded state, while ≥ 1.5 mM Mg2+ leads to an ensemble of random coils that fold with multi-stage kinetics. Increased flexibility of molecules in the intermediate ensemble correlates with a Mg2+-dependent increase in the fast folding population and a previously unobserved crossover in the collapse kinetics. Partial denaturation of the unfolded RNA with urea also increases the fraction of chains following the fast-folding pathway. These results demonstrate that the preferred collapse mechanism depends on the extent of Mg2+-dependent charge neutralization, and that non-native interactions within the unfolded ensemble contribute to the heterogeneity of the ribozyme folding pathways at the very earliest stages of tertiary structure formation.
Introduction
Understanding the physical basis of events early in the folding process is crucial for mapping out the dynamics of RNAs. Unlike proteins, which fold in response to the hydrophobic effect, RNA folding is enabled by neutralization of the phosphate anionic charge by multivalent cations such as Mg2+ 1,2. Charge neutralization triggers the formation of compact intermediates that are more flexible, with a shorter persistence length 3. The compact intermediates subsequently undergo a further conformational search leading to the native state 4,5.
A central question is how physical and chemical forces during counterion-induced collapse influence the outcomes of the folding process. Many conformational states are accessible at the beginning of the collapse transition from the unfolded state, and stochastic fluctuations among these cause individual molecules to fold along different paths 6. The ensuing competition among parallel folding trajectories has been confirmed by many ensemble and single molecule experiments 7,8. The observed folding kinetics and the fraction of RNA that reaches the native state in a biologically meaningful time depend on the specificity of the early transitions, because different intermediate ensembles with different structures experience different energy barriers to folding 9,10.
Previous studies of ribozyme folding suggest that neutralization of the phosphate charge by counterions results in non-specific relaxation of the unfolded state, followed by sequence-specific folding to more compact and native-like structures 11-16. In particular, small-angle X-ray scattering (SAXS) and footprinting studies of the Tetrahymena group I ribozyme showed that deletion of key tertiary interactions had no effect on the initial relaxation and contraction of the unfolded state (τ ~ 15 ms) 14,17. However, these mutations blocked slow steps (τ ~ 0.1-100 s) that reflect tertiary folding and reorganization of the misfolded ribozyme core 18,19.
By contrast, the Azoarcus group I ribozyme and the catalytic domain of Bacillus subtilis RNase P experience a specific equilibrium collapse transition to native-like intermediates in the same Mg2+ concentration range as neutralization of the phosphate charge 13,15,20, suggesting the native topology is established early in the folding process for these RNAs. Consistent with this hypothesis, these ribozymes refold rapidly under native conditions (τ = 5 - 50 ms; 37 °C) 21-24. These observations support theoretical predictions that the yield of native RNA is highest when the collapse transition is specifically nucleated by a subset of native interactions 6.
To determine whether counterion-induced collapse and nucleation of native-like structures can occur simultaneously in RNA, we studied the collapse transition kinetics of the Azoarcus group I ribozyme by stopped-flow SAXS. SAXS is well suited to studying the folding-driven structural changes because it provides information about the global structure, even when the conformations are dynamic or irregular 25. Time-resolved SAXS using stopped-flow mixers or continuous flow microfluidic chambers have been used to study the kinetics of protein folding 26,27 and RNA folding 14,17,28. and virus assembly 29.
Taking advantage of a small volume mixer and fast X-ray detector, we show that the Azoarcus ribozyme exhibits multiple folding pathways with different kinetics, and that partitioning between these pathways depends strongly on Mg2+ concentration. The rapid pathway has a collapse time less than 1 ms, similar to the estimated 1 ms collapse time of the RNase P catalytic domain 13,15,20 but at least 10 times shorter than previously reported for the Tetrahymena ribozyme 14,17,30. More RNA partitions into the fast phase when the RNA interactions are partially denatured by urea, suggesting that the intrinsic heterogeneity of the unfolded ensemble underlies the various folding mechanisms, and that phases slower than 1 ms arise from the rearrangement of non-native structures 21.
Experimental Section
RNA samples
The 195 nucleotide Azoarcus ribozyme was prepared by large-scale in vitro transcription of pAz-IVS DNA as previously described 23,31. The RNA was concentrated (~10 mg/mL) and exchanged 3-4 times with 20 mM Tris-HCl (pH 7.5) using Centricon-30 concentrators (Amicon). The RNA samples were filtered through a 0.2 μm membrane (Millipore).
SAXS measurements
Time-revolved SAXS experiments were performed with a Bio-Logic SFM-400 stopped-flow mixer (Fig. 1A) coupled with a Pilatus 100K photon counting detector (Dectris) at BioCAT beamline 18-D at the Advanced Photon Source (Argonne National Laboratory). A 0.6 ms dead time was achieved by installing a micro-volume mixer (MEC 22998, Bio-Logic) in the SFM and confirmed by time-resolved UV-absorption measurements (MOS-200) of the reduction of 2,6-dichlorophenolindophenol by ascorbic acid. The trigger for the stopped flow plungers was synchronized with the photon detector on the basis of the time-resolved X-ray absorption tests of a ZnCl2 solution.
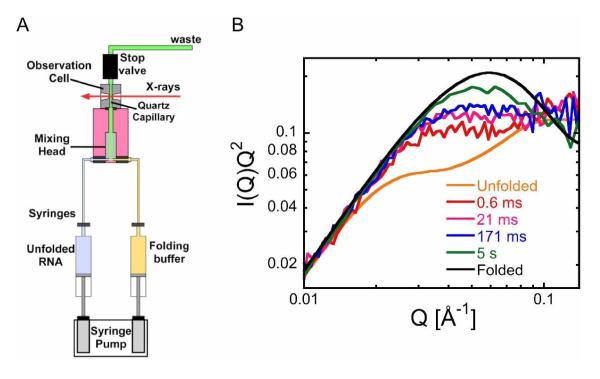
Figure 1. Time-resolved SAXS of Azoarcus ribozyme folding.
(A) Schematic view of stopped-flow mixer (SFM400). Syringes were loaded with unfolded RNA (1 mg/mL after mixing) in 20 mM Tris-HCl and folding buffer containing MgCl2. The dead time (~0.6 ms) was minimized by a high flow rate and short distance from the small-volume mixer to the observation point. (B) Kratky plots of real-time folding data in 1.5 mM MgCl2. Curves at 5 s (green) were in 5 mM MgCl2. For time-resolved measurements (≤ 200 ms), 20 identical 1 ms datasets were averaged. Scattering data up to 5 s were acquired for 50 ms and averaged over 4 shots. For unfolded RNA in 20 mM Tris-HCl (orange) and folded RNA in 5 mM MgCl2 (black) at equilibrium, data were collected for 1.6 s (4 times).
Folding experiments were carried out at 32 °C by mixing equal volumes of 2 mg/ml unfolded RNA in 20 mM Tris-HCl and folding buffer containing 2X MgCl2 in 20 mM Tris-HCl. The unfolded RNA was incubated 5-10 min at 50 °C prior to the experiment, which we previously found minimizes misfolding and aggregation 23. In some experiments, 3 M urea was added to the RNA and the folding buffer before mixing. Time-resolved scattering data was acquired for 1 ms intervals and read out in 4 ms. The high flux of the X-ray beam (~ 1013 photons per second) and the sensitivity of the detector provided reasonable scattering profile over a Q range of 0.006 to 0.25 Å−1 even with very short acquisition times. For each condition, 15 – 20 identical shots were averaged to obtain reasonable scattering statistics. Scattering data at folding intervals longer than 1 s were acquired for 50 ms and averaged over 4 shots. SAXS measurements of equilibrium titrations of 1 mg/mL ribozyme with Mg2+ (Fig. S1) were carried out as previously described 31.
SAXS data analysis
Following background subtraction, the scattering intensities, I(Q), were converted into real-length pair distance distribution functions, P(r), by indirect inverse Fourier transform using GNOM 32. The radius of gyration, Rg, was obtained from the P(r) representing the best fit to I(Q) as previously described 31. Fractional saturation of the folding transition, representing the amount of remaining unfolded RNA, ΦU, was estimated from , in which Rg,U (unfolded) and Rg,F (folded) are obtained from RNAs equilibrated for 30 min in 0 and 10 mM MgCl2.
Results
Rapid collapse of the Azoarcus ribozyme in Mg2+
The collapse transition under equilibrium conditions produces a large change in the scattering curve of the Azoarcus ribozyme, reflecting a decrease in Rg from 60 Å for the unfolded RNA in no MgCl2 to 31 Å for the folded RNA in 5 mM MgCl2 (Fig. S1) 31. In the range of 0.5-2 mM MgCl2, the Azoarcus ribozyme forms an intermediate (IC) that is as compact as the native state and contains the core helices and some tertiary interactions 23,31,33. At 32 °C in 1 mg/mL RNA, the midpoint for the transition to IC is 0.88 mM MgCl2 (Fig. S1), which is 40% less than the 1.5 mM Mg2+ needed to equal the number of charges on the phosphates (3 mM).
We used stopped-flow SAXS to follow the collapse process in real time (Fig. 1A and Methods). Unfolded RNA in 20 mM Tris-HCl and MgCl2 solution were mixed with a dead time of 0.6 ms, and data were acquired for 1 ms every 5 ms, starting at 0.6 ms and continuing to 200 ms. Scattering data were also acquired in 5 mM MgCl2 at folding times up to 10 min.
The rapid compaction of the RNA with folding time can be seen in Kratky plots of the scattering intensity (Fig. 1B). The Kratky plot of unfolded ribozyme increases over the intermediate Q range 0.04 to 0.1 Å−1 (Fig. 1B and Fig. S2), reflecting the predominantly extended conformations characteristic of the unfolded RNA at low ionic strength. After only 0.6 ms folding time in 1.5 mM Mg2+, a plateau in the intermediate Q region reveals evidence of a collapse transition in the sub-millisecond timescale. After 21 ms, a plateau in the Kratky plot is consistent with random coil-like structures 34. As expected, the Kratky plot of the folded RNA exhibits a maximum at Q = 0.06 Å−1, reflecting a relatively homogeneous and compact structure (Fig. 1B).
As the Mg2+ concentration in the folding reaction is raised from 1 to 5 mM, the RNA becomes progressively more compact within the first millisecond of the folding reaction (Fig. S2A). In 1 mM Mg2+, which is near the midpoint of the equilibrium folding curve, the lack of a defined peak in the Kratky plots at 11 and 51 ms indicates the system contains a mixture of disordered structures. In contrast, the early intermediates in 2 and 5 mM Mg2+ are much more compact. This difference is even greater at 171 ms, at which point the Kratky plot of RNA refolded in 5 mM Mg2+ displays a well developed peak at Q ~ 0.04 Å-1 (Fig. S2D). In general, the changes in the scattering curves indicate that local interactions measured at wide scattering angles (Q ~ 0.1 Å−1), and the global fold measured at narrow scattering angles (Q < 0.05 Å−1), form on the same time scales for this ribozyme.
Structure of early folding intermediates
The average flexibility and extension of the RNA chains following initial collapse was analyzed by comparing the exponent, ν, for I ~ Q−ν over the range 0.05 Å−1 < Q < 0.1 Å−1 (Fig. 2 and Fig. S3). This region of the scattering curves is more sensitive to the orientations of neighboring segments in the RNA than the Guiner region, Q < 0.05 Å−1, which reports the global shape. The value of ν ~ 1.2 of the unfolded RNA is characteristic of rigid worm-like polymers (Fig. 2), in agreement with our previous result that the unfolded RNA has an extended structure (lP ≈ 21 Å) at low ionic strength 3. For the fully folded ribozyme, ν = 3 (Fig. 2), consistent with a collapsed chain (lP ≈ 10 Å).
Figure 2. Structure of early folding ensembles in Mg2+.

Time-dependent exponents, ν, in different MgCl2 were determined from linear fits to log [I(Q) ~ Q−ν] vs log Q for 0.05 < Q < 0.1 Å−1 (see Fig. S3). Error bars are from the statistics of the fit. Dashed line corresponds to worm-like chain (ν = 1); Dotted line corresponds to random coil-like chain (ν = 2). For an ideal sphere, ν = 4. The circled symbols represent ν values at equilibrium.
The ν values as a function of folding time are plotted in Fig. 2 for each Mg2+ concentration. When the ribozyme was folded in 1 mM Mg2+, the RNA chains remain extended (ν = 1.3 to 1.5 at 170 ms and 1.75 at 10 min) compared with a random-coil (ν ~ 2), perhaps due to incomplete neutralization of the phosphate charges in 1 mM Mg2+. Above 1 mM Mg2+, the RNA population after 0.6 ms of folding became increasingly flexible and compact, with ν values rising to 1.65 for 1.5 mM Mg2+, 1.85 for 2 mM Mg2+ and 2.3 for 5 mM Mg2+ (Fig. 2 and Fig S2). Thus, when there are a sufficient number of counterions, a random-coil state is achieved rapidly.
From these results, we conclude that the chain flexibility and the degree of compaction in the first few milliseconds of folding are remarkably sensitive to the association of Mg2+ ions with the RNA. A comparison of the right and left sides of Figure 2 shows that the early collapse transition (and thus the folding kinetics) is more sensitive to Mg2+ than is the stability of the native state. When reactions in 1.5 mM and 5 mM MgCl2 are compared, values of ν differ by 0.65 at 0.6 ms but only by 0.4 at 171 ms and 0.5 at 10 min.
Partitioning of multiphase folding kinetics
To examine the kinetic pathways for collapse and tertiary folding, the progress of the folding reaction was determined from the P(r) and Rg for each Mg2+ concentration. Distance distribution functions, P(r), obtained from the indirect inverse Fourier transform of I(Q) were consistent with the formation of more globular structures over time (Fig. 3 inset), and revealed a sharp decrease in Rg, within the first 0.6 ms of folding (Fig. 3). These data show that most of the ribozyme population has collapsed into compact structures by 1 ms in 5 mM MgCl2.
Figure 3. Multi-stage collapse kinetics.

Decrease in Rg over time from the unfolded state (60.5 Å) to the folded state (31 Å) during refolding in 5 mM MgCl2. Error bars represent the uncertainty in Rg from the data inversion. Solid line is the best fit to a triple exponential decay function (τ1 ≤ 0.2 ms, τ2 = 20 ms, τ3 = 170 s; see Table S1). Blue bar, 5-20 ms time window in which tertiary interactions are detected by hydroxyl radical footprinting 22. Inset: Pair distance distribution function, P(r), for RNA folding in 5 mM MgCl2 at the times shown.
In addition to the sub-millisecond collapse that accounts for most of the decrease in Rg, we also observed an additional fast transition around 20 ms, and a very slow transition between 100 and 300 s. The multiphase folding kinetics measured by stopped-flow SAXS were qualitatively similar to observations from stopped-flow fluorescence and hydroxyl radical footprinting at 37 °C 21. Thus, folding of the Azoarcus ribozyme involves at least three kinetic phases and stretches over timescales from ≤10−3 to ~103 seconds.
Partitioning of the RNA population among different folding processes was evaluated by linking the folding kinetics to the thermodynamic stability of the folded RNA. We previously showed that the change in X-ray scattering by the Azoarcus ribozyme with increasing Mg2+ concentration can be described by an equilibrium between unfolded and compact states of the RNA 15,31. We used this two-state model to obtain the average fraction of unfolded chains, ΦU(t), at each folding time (Fig. S1; Methods). The decrease in ΦU(t) over the first 200 ms was fit to a double exponential decay function,
in which τ1 and τ2 are the time constants associated with the initial collapse and the second transition, and P1 and P2 are their partition coefficients, respectively.
As seen above from the change in ν (Fig. 2), the extent of collapse in the first 0.6 ms rose significantly as the Mg2+ concentration in the folding reaction was increased (Fig. 4A). In 1 mM Mg2+, the initial stage of collapse (P1) accounted for 30% of the RNA population within 0.6 ms, while in 10 mM Mg2+, the Azoarcus ribozyme was 90% folded in this time (Fig. 4B). Although the native structure is fully stable above 5 mM MgCl2, 10% of the RNA remained unfolded after 200 ms (Fig. 4B). This presumably reflects RNA chains that are kinetically trapped in misfolded states 21.
Figure 4. Mg2+ determines partitioning of collapse kinetics.
(A) Fraction of unfolded Azoarcus ribozyme, ΦU(t), refolded in 1, 1.5, 2, 5 and 10 mM MgCl2. Solid lines represent the best fit to Eq. 1; fit parameters are in Table S1. See Fig. S4A,B for further data. (B) Partitioning of RNA population into burst (P1; blue) and second phase (P2; red) within 200 ms. 1-ΦU,E is the fraction of folded RNA at equilibrium. The difference between 1-ΦU,E (orange) and P1 + P2 (green) represents long-lived misfolded RNAs. Error bars are from the uncertainty of fits in (A).
The second process, with τ2 = 20-40 ms, accounted for another 18% of the unfolded fraction (Fig. 4B). Its appearance in 1.5-2 mM Mg2+ correlated with the crossover to random coil behavior (ν = 2; Fig. 2), suggesting that it represents a process that is only populated when the chains are able to explore compact configurations. As the RNA tertiary interactions became fully stable in 5 mM Mg2+, the amplitude of the second phase decreased and finally vanished from the observation window.
Choice of folding pathways linked to structure of unfolded state
Multi-stage collapse is thought to arise from partitioning of the RNA population into parallel folding trajectories, some of which involve kinetically trapped intermediates 10. Alternatively, the kinetic phases might represent sequential steps between stable intermediates 9,14. To determine whether competition from non-native interactions impedes early folding steps of the Azoarcus group I ribozyme, we examined the effect of urea on the collapse kinetics.
Urea was expected to smooth out the free energy landscape of the unfolded RNA ensemble, which contains some secondary structure 23. In agreement with the idea that urea destabilizes interactions in the unfolded state, the Rg of the unfolded ribozyme was 67.5 Å in 3 M urea, 7 Å larger than the unfolded RNA without urea (Fig. S1 inset). The midpoint of the folding equilibrium shifted to 1.24 mM Mg2+ in 3 M urea, which was 1.4 times higher than without urea (Fig. S1). Because urea did not change the cooperativity of the folding transition (n = 2.3), its destabilizing effect on the tertiary structure could be compensated by raising the Mg2+ concentration 1.4-fold.
Pre-incubation of the unfolded RNA in urea increased the fraction of fast-collapsing RNA about 50%, compared to isostable urea-free reactions (Fig. 5). Thus, urea not only accelerates remodeling of misfolded RNAs in the late stages of folding, it also perturbs the initial collapse process. Urea also increased the rate of the second process slightly (Table S1). These results suggested that the slower P2 phase arose from partitioning into ensembles that experience higher energy barriers along the folding trajectory, presumably due to interference from non-native interactions. If the kinetic phases represented sequential folding, we would have expected urea to increase the folding times without changing the amplitudes of each phase. Even in 3 M urea, about 10% of the RNA population requires more than a minute to fold (Fig. 5B).
Figure 5. Urea increases the fraction of rapidly compacting RNA.
Time-dependent folding of 3 M urea-relaxed ribozyme compared with urea-free ribozyme. (A) Fraction unfolded Azoarcus ribozyme as in Fig. 4A. Solid lines, no urea; dashed lines, with 3 M urea. Colors denote conditions of isostability determined from equilibrium titrations (Fig. S1). See Fig. S4C for further data. (B) Partition factors for first two phases (P1 and P2) with urea (red) and without urea (blue). The Mg2+ concentration axis for urea-free reactions (top) is shifted by a factor of 1.4 relative to the lower x axis.
Discussion
RNA folding pathways are often heterogeneous, spanning many timescales and involving native and non-native intermediates 2,4. This heterogeneity can arise very early in the folding process due to stable local interactions in the unfolded RNA ensemble 35,36. The specificity of the folding trajectories influences RNA self-assembly, ligand recognition and regulation, yet the physical forces driving the nucleation and collapse of RNA tertiary structure are poorly understood.
Our time-resolved SAXS results show that the Azoarcus ribozyme forms compact structures in less than a millisecond under native conditions, about 10 fold faster than the earliest reported transitions of the Tetrahymena ribozyme 14,17,30. The structures formed within this time window are compact, consistent with specific folding of the RNA. Importantly, partitioning of the Azoarcus ribozyme among different collapse processes depends critically on the Mg2+ concentration. Below, we discuss how electrostatic interactions determine the topology of the RNA chains at an early stage of the folding process and the search for native-like conformations.
Our SAXS, fluorescence and footprinting results are all consistent with the early formation of native structure and suggest that the timescales for collapse and tertiary folding overlap in this RNA (blue bar; Fig. 3). We observe at least three different time-dependent processes by stopped-flow SAXS, with time constants τ1 ≤ 1 ms, τ2 = 20 to 40 ms and τ3 ≈ 170 s. These global structural changes agree well with earlier stopped-flow fluorescence and kinetic hydroxyl radical footprinting results probing the formation of local tertiary interactions, after accounting for differences in temperature and Mg2+ concentration 21,22. In 15 mM Mg2+, footprinting showed that tertiary interactions form throughout the wild type ribozyme within 5-20 ms at 37 °C 22. Two fast transitions were also detected by stopped-flow fluorescence (τ1 ≤ 10 ms and τ2 = 30 ms) and were assigned to formation of the native-like IC intermediate and the folded state, respectively 21.
Previous biochemical experiments also showed that 5-15% of the Azoarcus ribozyme population folded through slow pathways (1 s and 100 s at 37 °C) that involve reorganization of interactions near the central triple helix and the P3/P7 pseudoknot 21. Refolding of such misfolded intermediates explains the very slow transitions (τ3 ~ 170 s) observed here.
The heterogeneity of the collapse kinetics in the Azoarcus ribozyme can be explained by partitioning of the RNA population into the parallel folding pathways (Fig. 6). This partitioning model is supported by footprinting and non-denaturing gel electrophoresis experiments showing that most of the RNA forms all of the tertiary contacts in 5-20 ms, yet a small amount remains unfolded up to 5 min 21. This model implies that the structural collapse probed by scattering profiles mostly reflects the native-like intermediates, and the various kinetic phases represent subpopulations (U1 U2, U3) that fold through different transition states (Fig. 6).
Figure 6.
Folding nucleation and collapse of the Azoarcus ribozyme. Parallel folding from subpopulations of the unfolded state, U1, U2, and U3, leads to heterogeneous folding kinetics. Direct folding to the native state (U1) is favored by higher Mg2+ concentration or by partially denaturing amounts of urea. U2 is populated only when Mg2+ concentration is higher than the midpoint of the equilibrium folding transition (0.88 mM) and has a cusp at 1.5 - 2 mM Mg2+. Left and middle cartoons represent hypothetical structures in the unfolded and non-native relaxed coil ensembles. Average persistence lengths (lp) are from fits to P(r) as previously described 3.
This picture is remarkably consistent with theoretical predictions that folding proceeds rapidly to the native state when the collapse transition is nucleated by specific native interactions 6,37. When a protein or RNA is driven into compact structures before it has the opportunity to search out the correct topology, the initial collapse transition becomes non-specific, producing structures that are mostly non-native. Theoretical models suggest that non-specific collapse is followed by a diffusive search across low energy barriers for more compact structures, and subsequently by activated transitions from metastable misfolded states 6. The smoothness of the free energy landscape determines the extent to which individual molecules fold along different paths and the barriers to folding 10,38.
Accordingly, we postulate that the fastest collapse transitions in our experiments represent molecules (U1) that fold directly to the native structure, while the second phase (τ2 ~ 20 ms) represents a subpopulation (U2) in which the initial collapse is non-specific, and the ensuing search for stably folded and native-like structures is impeded by incorrect local contacts. The slowest phase represents the small population (U3) that becomes trapped in long-lived misfolded structures. Since urea increased the size of the initial burst (P1) and reduced partitioning into the second phase (P2), the ensemble of initial structures is a critical factor driving the population of the folding landscape and the flux through discrete folding pathways. A similar effect was observed in the Tetrahymena ribozyme, that folds slowly in Mg2+ from an unfolded state in low ionic strength, but exhibits a higher flux through fast folding pathways when the RNA is prestructured in Na+ 9,39,40.
An alternative explanation for multiphasic collapse kinetics is stepwise folding through a series of intermediate states. This model, however, cannot explain why urea increased the folding rate (τ2) and changed the partition factors P1 and P2. In addition, the plateaus in the SAXS progress curves at Rg = 35 and 40 Å are inconsistent with scattering from pure populations of known equilibrium folding intermediates (Rg = 33 Å and ~ 55 Å) 21,31. By the time the plateaus in the kinetic experiments are reached, our footprinting and fluorescence results show that some of the population has already folded completely. Overall, the data are better explained by parallel folding trajectories (Fig. 6) than by sequential folding.
A major conclusion from our results is that the partitioning among different folding pathways depends on interactions of the RNA with counterions. First, we observe that Mg2+ increases the fraction of chains that form compact structures within 1 ms. Mg2+ may favor specific nucleation of the correct fold by stabilizing long-range tertiary interactions 22, or simply by reducing the RNA persistence length. Second, the non-native collapse (τ2 = 20 ms) emerges in Mg2+ concentrations above the midpoint of the equilibrium titration (0.88 mM Mg2+) and maximizes near 1.5 mM Mg2+, in which electrostatic neutralization is complete. Flux through this pathway correlates with the crossover from extended intermediates in 1 mM Mg2+ to random-coil configurations in 2 mM Mg2+. Mg2+ has the same effect on the folding kinetics when the unfolded RNA is partially denatured with urea (Fig 5B).
We propose that Mg2+ concentrations sufficient to fully stabilize the folded RNA (≥ 1.5 mM) allow the RNA to form compact structures in the absence of specific long-range interactions, resulting in a subpopulation that must search for a stable fold after collapse has occurred 6. Whether this effect requires multivalent counterions remains to be determined. Urea favors the specific collapse pathway by relaxing non-native interactions that may be present in the unfolded ensemble, and by increasing the number of energetically favorable contacts needed to drive a chain through the collapse transition. Urea also lowers the energetic barrier of the conformational search in the second phase by destabilizing incorrect structures. These effects of urea occur early during folding, and are distinct from the previously documented ability of denaturants to accelerate slow refolding transitions by raising the free energy of metastable misfolded states 41,42.
In contrast to the Azoarcus ribozyme, the Tetrahymena ribozyme predominantly folds through misfolded intermediates 41. To assess whether this difference can be discerned from the collapse kinetics, we compared the Kratky plots for the two RNAs in 5 mM Mg2+. The scattering profile of the Azoarcus ribozyme after 0.6 ms of folding (Fig. S1A) contained a more pronounced peak than reported for the Tetrahymena ribozyme after 7 ms of folding time, and is more comparable with Tetrahymena ribozyme after 44 ms 17. Not only does the Azoarcus ribozyme collapse and fold about than 10 times faster than the Tetrahymena ribozyme (in the first two phases), but the initial collapsed population (or fractional weight) is about two times larger for Azoarcus ribozyme than for Tetrahymena ribozyme. Thus, the overall time needed to form compact structures in the population and the heterogeneity of the collapse kinetics correlates with the propensity of each ribozyme to partition into native structures instead of non-native structures.
Conclusion
Our time-resolved structural studies demonstrate that native-like conformations of the Azoarcus ribozyme form within the same time window as the initial collapse transition in Mg2+, but that the heterogeneity of the folding kinetics depends strongly on the presence of counterions and the average compactness (or flexibility) of the polynucleotide chain. Our results are best explained by subpopulations that fold either through rapid and direct nucleation of the tertiary interactions, non-specific collapse followed by short-range structural rearrangements, or refolding of long-lived, misfolded structures. The folding landscape can be smoothed by destabilizing interactions in the unfolded state, however, partitioning among these paths is still influenced by Mg2+ concentration. These observations demonstrate the importance of electrostatic interactions for the nucleation of long-range tertiary structure in RNA.
Supplementary Material
Acknowledgments
The authors thank D. Thirumalai for stimulating discussion, R. Behrouzi for preparation of RNA samples and M. Mayerle for preparation of T7 RNA polymerase. This work was supported by NIST and the NIH (GM60819 to S.W.). Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under contract No. W-31-109-ENG-38. BioCAT is a National Institutes of Health-supported Research Center RR-08630. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Supporting information available. Time constants, equilibrium titration results, Kratky plots and further time-resolved SAXS data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Draper DE. RNA. 2004;10:335. doi: 10.1261/rna.5205404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Thirumalai D, Hyeon C. Biochemistry. 2005;44:4957. doi: 10.1021/bi047314+. [DOI] [PubMed] [Google Scholar]
- (3).Caliskan G, Hyeon C, Perez-Salas U, Briber RM, Woodson SA, Thirumalai D. Phys. Rev. Lett. 2005;95:4. doi: 10.1103/PhysRevLett.95.268303. [DOI] [PubMed] [Google Scholar]
- (4).Sosnick TR. Protein Sci. 2008;17:1308. doi: 10.1110/ps.036319.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Woodson SA. Curr. Opin. Chem. Biol. 2005;9:104. doi: 10.1016/j.cbpa.2005.02.004. [DOI] [PubMed] [Google Scholar]
- (6).Thirumalai D, Lee N, Woodson SA, Klimov DK. Annu. Rev. Phys. Chem. 2001;52:751. doi: 10.1146/annurev.physchem.52.1.751. [DOI] [PubMed] [Google Scholar]
- (7).Zhuang XW. Annu. Rev. Biophys. Biomolec. Struct. 2005;34:399. doi: 10.1146/annurev.biophys.34.040204.144641. [DOI] [PubMed] [Google Scholar]
- (8).Li PTX, Vieregg J, Tinoco I. Annu. Rev. Biochem. 2008;77:77. doi: 10.1146/annurev.biochem.77.061206.174353. [DOI] [PubMed] [Google Scholar]
- (9).Russell R, Zhuang XW, Babcock HP, Millett IS, Doniach S, Chu S, Herschlag D. Proc. Natl. Acad. Sci. U. S. A. 2002;99:155. doi: 10.1073/pnas.221593598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Thirumalai D, Woodson SA. Accounts Chem. Res. 1996;29:433. [Google Scholar]
- (11).Das R, Travers KJ, Bai Y, Herschlag D. J. Am. Chem. Soc. 2005;127:8272. doi: 10.1021/ja051422h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Deras ML, Brenowitz M, Ralston CY, Chance MR, Woodson SA. Biochemistry. 2000;39:10975. doi: 10.1021/bi0010118. [DOI] [PubMed] [Google Scholar]
- (13).Fang XW, Littrell K, Yang X, Henderson SJ, Siefert S, Thiyagarajan P, Pan T, Sosnick TR. Biochemistry. 2000;39:11107. doi: 10.1021/bi000724n. [DOI] [PubMed] [Google Scholar]
- (14).Kwok LW, Shcherbakova I, Lamb JS, Park HY, Andresen K, Smith H, Brenowitz M, Pollack L. J. Mol. Biol. 2006;355:282. doi: 10.1016/j.jmb.2005.10.070. [DOI] [PubMed] [Google Scholar]
- (15).Moghaddam S, Caliskan G, Chauhan S, Hyeon C, Briber RM, Thirumalai D, Woodson SA. J. Mol. Biol. 2009;393:753. doi: 10.1016/j.jmb.2009.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Pljevaljcic G, Klostermeier D, Millar DP. Biochemistry. 2005;44:4870. doi: 10.1021/bi047772i. [DOI] [PubMed] [Google Scholar]
- (17).Russell R, Millettt IS, Tate MW, Kwok LW, Nakatani B, Gruner SM, Mochrie SGJ, Pande V, Doniach S, Herschlag D, Pollack L. Proc. Natl. Acad. Sci. U. S. A. 2002;99:4266. doi: 10.1073/pnas.072589599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Pan J, Woodson SA. J. Mol. Biol. 1998;280:597. doi: 10.1006/jmbi.1998.1901. [DOI] [PubMed] [Google Scholar]
- (19).Sclavi B, Sullivan M, Chance MR, Brenowitz M, Woodson SA. Science. 1998;279:1940. doi: 10.1126/science.279.5358.1940. [DOI] [PubMed] [Google Scholar]
- (20).Fang XW, Thiyagarajan P, Sosnick TR, Pan T. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8518. doi: 10.1073/pnas.142288399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Chauhan S, Behrouzi R, Rangan P, Woodson SA. J. Mol. Biol. 2009;386:1167. doi: 10.1016/j.jmb.2008.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Chauhan S, Woodson SA. J. Am. Chem. Soc. 2008;130:1296. doi: 10.1021/ja076166i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Rangan P, Masquida B, Westhof E, Woodson SA. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1574. doi: 10.1073/pnas.0337743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Fang XW, Pan T, Sosnick TR. Nat. Struct. Biol. 1999;6:1091. doi: 10.1038/70016. [DOI] [PubMed] [Google Scholar]
- (25).Lipfert J, Doniach S. Annu. Rev. Biophys. Biomolec. Struct. 2007;36:307. doi: 10.1146/annurev.biophys.36.040306.132655. [DOI] [PubMed] [Google Scholar]
- (26).Arai M, Ikura T, Semisotnov GV, Kihara H, Amemiya Y, Kuwajima K. J. Mol. Biol. 1998;275:149. doi: 10.1006/jmbi.1997.1456. [DOI] [PubMed] [Google Scholar]
- (27).Zhu L, Qin ZJ, Zhou JM, Kihara H. Biochimie. 2004;86:127. doi: 10.1016/j.biochi.2003.12.002. [DOI] [PubMed] [Google Scholar]
- (28).Das R, Kwok LW, Millett IS, Bai Y, Mills TT, Jacob J, Maskel GS, Seifert S, Mochrie SGJ, Thiyagarajan P, Doniach S, Pollack L, Herschlag D. J. Mol. Biol. 2003;332:311. doi: 10.1016/s0022-2836(03)00854-4. [DOI] [PubMed] [Google Scholar]
- (29).Canady MA, Tsuruta H, Johnson JE. J. Mol. Biol. 2001;311:803. doi: 10.1006/jmbi.2001.4896. [DOI] [PubMed] [Google Scholar]
- (30).Schlatterer JC, Kwok LW, Lamb JS, Park HY, Andresen K, Brenowitz M, Pollack L. J. Mol. Biol. 2008;379:859. doi: 10.1016/j.jmb.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Chauhan S, Caliskan G, Briber RM, Perez-Salas U, Rangan P, Thirumalai D, Woodson SA. J. Mol. Biol. 2005;353:1199. doi: 10.1016/j.jmb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- (32).Semenyuk AV, Svergun DI. J. Appl. Crystallogr. 1991;24:537. [Google Scholar]
- (33).Perez-Salas UA, Rangan P, Krueger S, Briber RM, Thirumalai D, Woodson SA. Biochemistry. 2004;43:1746. doi: 10.1021/bi035642o. [DOI] [PubMed] [Google Scholar]
- (34).Roe R. In: Methods of X-ray and Neutron Scattering in Polymer Science. Roe R, editor. Oxford University Press; New York: 2000. p. 155. [Google Scholar]
- (35).Ditzler MA, Rueda D, Mo JJ, Hakansson K, Walter NG. Nucleic Acids Res. 2008;36:7088. doi: 10.1093/nar/gkn871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Heilman-Miller SL, Woodson SA. J. Mol. Biol. 2003;328:385. doi: 10.1016/s0022-2836(03)00304-8. [DOI] [PubMed] [Google Scholar]
- (37).Guo ZY, Thirumalai D. Biopolymers. 1995;36:83. [Google Scholar]
- (38).Socci ND, Onuchic JN, Wolynes PG. Proteins. 1998;32:136. [PubMed] [Google Scholar]
- (39).Shcherbakova I, Gupta S, Chance MR, Brenowitz M. J. Mol. Biol. 2004;342:1431. doi: 10.1016/j.jmb.2004.07.092. [DOI] [PubMed] [Google Scholar]
- (40).Heilman-Miller SL, Pan J, Thirumalai D, Woodson SA. J. Mol. Biol. 2001;309:57. doi: 10.1006/jmbi.2001.4660. [DOI] [PubMed] [Google Scholar]
- (41).Pan J, Thirumalai D, Woodson SA. J. Mol. Biol. 1997;273:7. doi: 10.1006/jmbi.1997.1311. [DOI] [PubMed] [Google Scholar]
- (42).Pan T, Fang X, Sosnick T. J. Mol. Biol. 1999;286:721. doi: 10.1006/jmbi.1998.2516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.