Abstract
Cell lines provide a tool for investigating basic biological processes that underlie the complex interactions among the tissues and organs of an intact organism. We compare the evolution of insect and mammalian populations as they progress from diploid cell strains to continuous cell lines, and review the history of the well-characterized Aedes albopictus mosquito cell line, C7-10. Like Kc and S3 cells from Drosophila melanogaster, C7-10 cells are sensitive to the insect steroid hormone, 20-hydroxyecdysone (20E), and express 20E-inducible proteins as well as the EcR and USP components of the ecdysteroid receptor. The decrease in growth associated with 20E treatment results in an accumulation of cells in the G1 phase of the cycle, and a concomitant decrease in levels of cyclin A. In contrast, 20E induces a G2 arrest in a well-studied imaginal disc cell line from the moth, Plodia interpunctella. We hypothesize that 20E-mediated events associated with molting and metamorphosis include effects on regulatory proteins that modulate the mitotic cell cycle and that differences between the 20E response in diverse insect cell lines reflect an interplay between classical receptor-mediated effects on gene expression and non-classical effects on signaling pathways similar to those recently described for the vertebrate steroid hormone, estrogen.
1. Introduction
Insect cells in culture provide opportunities that could not have been envisioned when Goldschmidt (1915) first attempted to observe silkworm spermatogenesis in vitro. Therapeutic applications of mammalian stem cells, whether derived from embryos, adult tissues, somatic cells genetically reprogrammed to express specific transcription factors, or chemically-induced pluripotent stem cells, have stimulated new interest in the regulation of cell growth and differentiation (Lin et al., 2009). Advances in stem cell research have particular relevance to totipotent cells found in insects, such as regenerative cells in insect midgut (Loeb et al., 2001; Micchelli and Perrimon, 2006), which have already been used to elucidate new, chemically-defined factors that affect insect cell growth and differentiation (Goto et al., 2005). Although the most recent technologies for physical manipulation of cultured cells, such as tissue engineering in three dimensions, induced cell differentiation at air-liquid interfaces, and growth of cells in hydrated gels remain to be extended to insect systems, efforts towards implementation of microcarriers for production of viruses (Liu and Wu, 2004) and bioreactors to facilitate production of recombinant proteins (Saarinen and Murhammer, 2000) underscore the importance of understanding how chemically-defined molecules, such as insect hormones, influence growth and differentiation in vitro.
Considerable progress has been made during the five decades since TDC Grace established the first insect cell lines from ovaries of the diapausing silkmoth, Antheraea eucalypti (Grace, 1962). Grace cultured A. eucalypti cells in a medium based on the composition of insect hemolymph (Wyatt, 1956), with the addition of water-soluble vitamins and supplemental hemolymph. Shortly thereafter, Mitsuhashi and Maramorosch (1964) developed a rich medium made from salts, glucose, lactalbumin hydrolysate, yeastolate and serum, which supported growth of leafhopper cells in the absence of supplemental hemolymph, and paved the way for establishment of lines from other small insects. Although many insect cell lines are now maintained in commercially-available media that are better defined chemically, insect cells typically require serum or hydrolysates for optimal growth. Requirements for growth factors, mitogens, hormones and other cell-derived regulatory molecules that would support routine use of chemically-defined media remain to be systematically evaluated, even with the best characterized cell lines from Drosophila melanogaster (Galesi et al., 2007).
Historically, in vitro manipulation of insect tissues progressed in parallel with efforts to identify molecules that regulate insect growth and development. In early ligation experiments with larval insects Kopec (1922) provided key evidence for existence of a molting hormone, now known as the steroid 20-hydroxyecdysone (20E). Purification of the hormone from Bombyx mori pupae (Butenandt and Karlson, 1954) was monitored using a sensitive bioassay based on stimulation of puparium formation in larvae of the fly, Calliphora erythrocephala (Fraenkel, 1935). Despite years of intensive investigation, however, the physiological events that coordinate cell and tissue interactions with mitotic events involved in molting, proliferation, and differentiation of larval tissues and imaginal discs are poorly understood. Here we review our efforts to develop the Aedes albopictus C7-10 mosquito cell line to investigate how 20E, which is readily soluble in culture media, affects specific regulatory events in the mitotic cell cycle.
2. Origin of the Ae. albopictus C7-10 cell line
Given the complexity of holometabolous insects, the survival and proliferation of isolated cells in vitro as continually renewable, mitotically-dividing cells is remarkable. As is the case with mammalian tissues, insect cell lines originate from dissected tissue fragments, which remain metabolically active in a suitable culture medium (for details with insect materials, see Lynn, 2001 and Echalier, 1997). After weeks to months, small populations of dividing cells grow out from the tissue fragments, and become apparent under the light microscope. Echalier's isolation of the Kc line, the first permanent cell line from Drosophila melanogaster, was the fortuitous result of benevolent neglect over several months, due to disruptions caused by a “student revolution” in Paris (Echalier, 1997). In contrast, within a few weeks after initiation of the in vitro culture, Singh (1967) obtained the Ae. albopictus cells that eventually became known as the ATC-15 line, from which the clonal C7-10 line originated.
The ATC-15 Ae. albopictus cell population was established from several hundred freshly hatched larvae, minced with scissors, treated with trypsin, and cultured in Mitsuhashi and Maramorosch (1964) medium with 20% fetal bovine serum (Singh, 1967). A decade later, Sarver and Stollar (1977) adapted ATC-15 cells to Eagle's minimal medium supplemented with nonessential amino acids, glutamine, glucose, vitamins, antibiotics and fetal bovine serum (Shih et al., 1998), and selected a clonal population, LT-C7, which showed cytopathic effects after infection with Sindbis and Vesicular Stomatitis viruses (Sarver and Stollar, 1977; Gillies and Stollar, 1982). Maintaining LT-C7 cells in Eagle's medium, which apart from the serum is chemically defined, facilitated use of isotopes and allowed direct comparisons of arbovirus growth between mosquito and vertebrate cells. Use of Eagle's medium further facilitated selection of mutant cell lines resistant to drugs such as 5-bromodeoxyuridine, ouabain, α-amanitin, methotrexate, and hydroxyurea (reviewed by Fallon and Kurtti, 2005). The C7-10 cells, which we use as our standard, wild-type population, are a subclone of the LT-C7 line. The Ae. albopictus cell line known as C6/36 is a closely-related line that originated from the same Eagle's-adapted ATC-15 cells, selected for high arbovirus virus yield (reviewed by Fallon, 1997).
3. Comparisons between insect and mammalian cell lines
The astute reader will note that Grace used the word “strain,” rather than “cell line,” in the title of his pioneering publication (Grace, 1962). In the 1960's, it was becoming increasingly apparent that with time, cells evolve through a predictable pattern of events, best documented with mammalian cells, that ultimately results in a transition from primary culture to an immortal cell line consisting of transformed cells that exhibit the properties of cells derived directly from malignant tumors (Fig. 1). Insect cell culturists do not rigorously distinguish between cell strains and cell lines, and the transition from diploid to aneuploid karytoype that occurs in mammalian cells does not appear to occur, at least in cell lines from Diptera. In this context, it will be of particular interest to follow the evolution of cell lines from Anopheles mosquitoes, which were established relatively recently for investigation of mosquito immune responses (see Fallon and Sun, 2001).
Fig. 1.
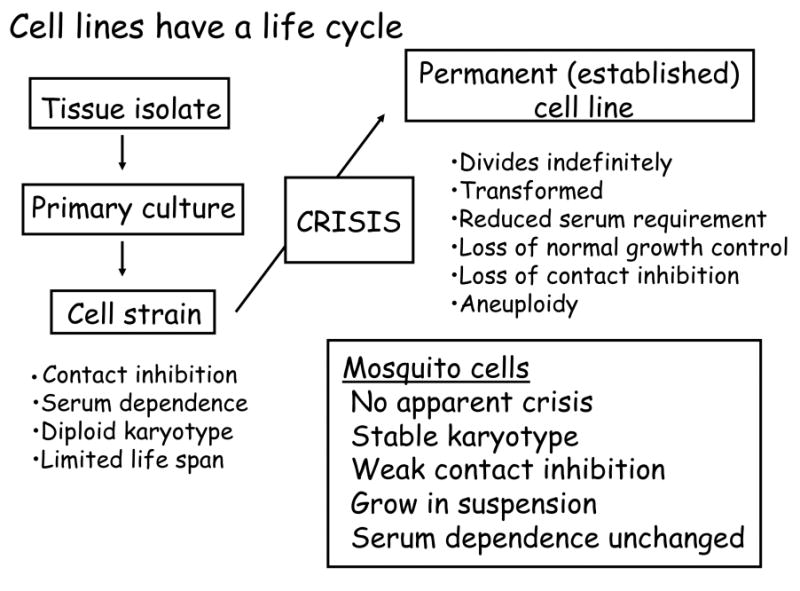
Stages in the evolution of an established cell line from a tissue isolate. Although insects cells have not been studied as carefully as vertebrate cells, they appear not to undergo the crisis, accompanied by senescence and conversion to aneuploidy, that has been documented in mammalian cell lines.
In mammalian terminology, the mitotic cells from a primary culture first give rise to a cell strain—a population of cells that can be reproducibly sub-cultured by dilution over a period of months. Cell strains have characteristic properties: they require serum, have a diploid karyotype, are contact inhibited, and are capable of a limited number of cell divisions, often called the “Hayflick limit” in recognition of the pioneering studies by Leonard Hayflick (Hayflick, 1965). Upon reaching the Hayflick limit, the cell population enters a senescent phase, or crisis, with cessation of growth. In human cell strains, senescence is correlated with a progressive shortening of telomeres at each division cycle, but differences between mouse and human cell lines suggest that telomere shorting is only one of several mechanisms that cells use for tracking the number of divisions (Wright and Shay, 2000). We are not aware of a Hayflick limit having been described for any insect cell line; nor, over the past three decades, have we observed a crisis in C7-10 cells or their clonal derivatives.
As mammalian cell strains senesce, one in roughly 105 to 107 cells bypasses the block to division (Wright and Shay, 2000). Cells that divide beyond the Hayflick limit have mutations in cell cycle or DNA damage checkpoint proteins that in aggregate, allow mitosis to continue despite negative regulatory processes associated with crisis. Cells that bypass crisis are said to be “transformed” in the sense that they divide indefinitely, have reduced requirements for serum, show loss of contact inhibition, are aneuploid, and often form tumors when re-injected into syngeneic animals. Consistent with the absence of a Hayflick limit, C7-10 cells have remained diploid over several decades, without the characteristic transition to the aneuploid karyotype that occurs when mammalian cells evolve from a cell strain to established cell line. Likewise, permanent cell lines from Drosophila typically have stable diploid karyotypes (Echalier, 1997).
Although the C7-10 cells with which we have worked for several decades remain predominantly diploid, and do not exhibit a reduced requirement for fetal bovine serum, they do resemble transformed cells in their relatively low degree of contact inhibition, and their ability to divide in suspension cultures. Moreover, certain mutant cell lines, such as those selected for high resistance to methotrexate, undergo gene amplification, which can be accompanied by distinct changes in ploidy (Shotkoski and Fallon, 1990). In this context, we note that double minute chromosomes have been reported in C6/36 cells (Mukherjee and Herrera, 1985; Monroe et al., 1992), but systematic comparisons between these two cloned cell lines have not been undertaken.
4. The 20E response
With the elucidation of its chemical structure and recognition of its effects on polytene chromosomes, 20E was one of the first insect hormones to be examined for its effects on insect cells in vitro. In pioneering work with Drosophila Kc cells (reviewed by Echalier, 1997), various investigators showed that 20E (β-ecdysone) is more active than its immediate precursor, ecdysone (α-ecdysone). Aside from arresting division, 20E treatment has been associated with changes in cell morphology and mobility; alterations in activities of certain enzymes including acetylcholinesterase, β-galactosidase, dopa-decarboxylase, catalase, superoxide dismutase, and protein kinases; synthesis of ecdysone-inducible proteins, including the small (but not the large) heat shock proteins, as well as actin and tubulin; and changes in cell surface glycoproteins. Although a model that relates these apparently disparate effects of 20E on Drosophila cell lines remains to be proposed, effects of estrogen on cytoskeletal organization have been attributed to non-nuclear effects of steroids, mediated by kinase cascades independent of gene expression and protein synthesis (Sanchez and Simoncini, 2009).
4.1 20E inhibits C7-10 cell growth
As has been shown with Drosophila cells, physiological concentrations of 20E inhibit growth of C7-10 cells (Johnston and Fallon, 1985), and induce synthesis of a small number of proteins (Lan et al., 1993). Inhibition of cell growth by 20E, as well as its effects on cell morphology and cytoskeletal proteins, have also been observed with lepidopteran cell lines, particularly the imaginal disc cell line IAL-PID2 from the moth, Plodia interpunctella (Siaussat et al., 2007, 2008). We note that in Kc and S2 Drosophila cell lines, inhibitory effects on cell growth facilitated selection of 20E-resistant derivatives, which were used in transfection studies to characterize the ecdysteroid receptor as a heterodimeric complex of EcR and Ultraspiracle proteins (Yao et al., 1993). Considerable effort has been directed towards characterization of the ecdysteroid receptor, EcR, and its heterodimeric partner, ultraspiracle (USP), from diverse arthropods, with nearly 100 cloned sequences reported (Nakagawa and Henrich, 2009). As expected, phylogenetic analyses of sequence relationships reflect accepted taxonomic relationships.
4.2 C7-10 cells synthesize 20E-inducible proteins
In contrast to its inhibitory effects on growth overall, 20E-induced expression of the small heat shock proteins in some Drosophila cell lines provided a potential system for investing an up-regulated 20E response in transfected cells. Towards this end, Dobens et al. (1991) developed plasmids containing tandem copies of the 20E response elements (EcRE) upstream of the hsp27 gene encoding small heat-shock protein HSP27. Transfected Drosophila cells showed a remarkable, 20-fold increase in reporter gene expression after treatment with 20E. Our inability to reproduce this robust effect in transfected C7-10 cells prompted a broader analysis of the 20E response in these cells. Using one and two-dimension polyacrylamide gel electrophoresis, we identified eleven 20E-inducible proteins, ranging in size from 22 to 52 kDa, but these proteins did not include HSP27 or other small heat shock proteins (Lan et al., 1993), and their identities remain unknown. We obtained a partial internal amino acid sequence from the 52 kDa protein, which was induced four to six hours after treatment with 20E. An in silico analysis suggested that the 52 kDa protein is a conserved hypothetical protein that occurs in a wide range of species (Eccleston et al., 2002); to date, however, this protein has not been characterized further in either Drosophila or Caenorhabditis elegans, other than to identify possible protease function based on amino acid sequence.
4.3 20E receptors are expressed by C7-10 cells
Our inability to detect, in transfected C7-10 cells, strong inducible reporter gene expression from the Drosophila hsp27 EcRE led us to consider the possibility that 20E-induced inhibition of cell growth represented a pharmacological effect, rather than a receptor-mediated hormone response. Using reverse-transcriptase polymerase chain reaction (RT-PCR) with primers based on Ae. aegypti EcR and USP homologs, we verified that C7-10 cells constitutively expressed the EcR and USP components of the ecdysone receptor (Jayachandran and Fallon, 2000). Although both transcripts were expressed in C7-10 cells, EcR transcripts were expressed at higher levels (or were more stable) than USP transcripts. On northern blots, the major EcR transcript measured 4.2 kb, and a minor transcript measured 6.0 kb. In contrast, USP transcripts were detectable only by more-sensitive RT-PCR methodology.
Western blot analyses further indicated that C7-10 cells express EcR protein. Cell nuclei contained a predominant EcR isoform with a mass of ∼ 80 kDa, and lesser amounts of minor isoforms, with masses of 72, 74, and 75 kDa, while only the 80 and 74 kDa isoforms were detected in the cytoplasm. Not surprisingly, the abundance of these proteins was low, and bands corresponding to the signal on Western blots were not apparent on stained gels. Heat-shock-induced expression of sense and/or antisense EcR constructs clearly influenced recovery of stably transfected cells, suggesting that perturbation of the receptor-mediated pathway disrupts cell growth, even in the absence of 20E. Moreover, antisense-EcR expression increased the relative abundance of a second EcR isoform (which we named EcRb) with eleven amino acid substitutions and a seven amino acid deletion near its C-terminus, relative to the predominant (EcRa) isoform expressed in control cells (Jayachandran and Fallon, 2001).
4.4 Possible binding of mosquito AP-1 to EcRE
Because Raikhel and coworkers successfully used the EcRE from Drosophila hsp27 to characterize an EcRE from the mosquito Ae. aegypti (Miura et al., 1999), and to inhibit receptor binding to the Ae. aegypti vitellogenin EcRE (Wang et al., 1998), we remained puzzled as to why the element did not support 20E-induced expression of a reporter gene in C7-10 cells. Indeed, recovery of stably transfected cells was diminished in a dose-dependent manner when constructs containing the hsp27 EcRE were introduced with Lipofectamine. This effect was stronger with EcRE sequences oriented in the “sense” orientation, relative to control constructs containing antisense EcRE, or lacking the EcRE sequence altogether. Because EcRE constructs contained potential binding sites for the AP-1 (mammalian Activator Protein 1) transcription factor, we hypothesized that the input DNA itself might bind and sequester AP-1, or a related transcription factor. Ap-1 is comprised of the proto-oncogene products JUN and FOS, which form a JUN/JUN homodimer or a JUN/FOS heterodimer. Using a DNA affinity column, we purified a 40 kDa nuclear protein that bound to DNA containing AP-1 sites, and cross-reacted with a heterologous antibody to JUN (Jayachandran and Fallon, 2002). At the time, we were unable to validate the identity of this putative JUN homolog by mass spectrometry. More recent technical advances in proteomics and mosquito genome annotation, coupled with advances in understanding how AP-1 participates in non-classical genomic estrogen response pathways in mammalian systems (Safe and Kim, 2008) suggest that participation of AP-1 in the 20E response may merit further investigation.
5. The Cell Cycle
In traditional cell culture, cells lack the cell-to-cell interactions that might be expected among the epidermal cells of an insect larva, where coordinated behavior is required for a successful molt. Nevertheless, cells in culture can be induced to progress through the mitotic cycle synchronously, and the most robust effect of 20E on C7-10 cells is arrested growth that occurs at a particular phase of the cell cycle (Gerenday and Fallon, 2004). In a population of cultured cells, each individual goes through its mitotic cycle independently of its neighbors. Interactions among proteins known as cyclins, cyclin-dependent kinases and phosphatases, and cyclin-dependent kinase inhibitors regulate normal progression through the cell cycle, and space permits only a brief synopsis here (Fig. 2). Swanhart et al. (2005) provide an excellent review on growth and cell cycle progression in Drosophila.
Fig. 2.
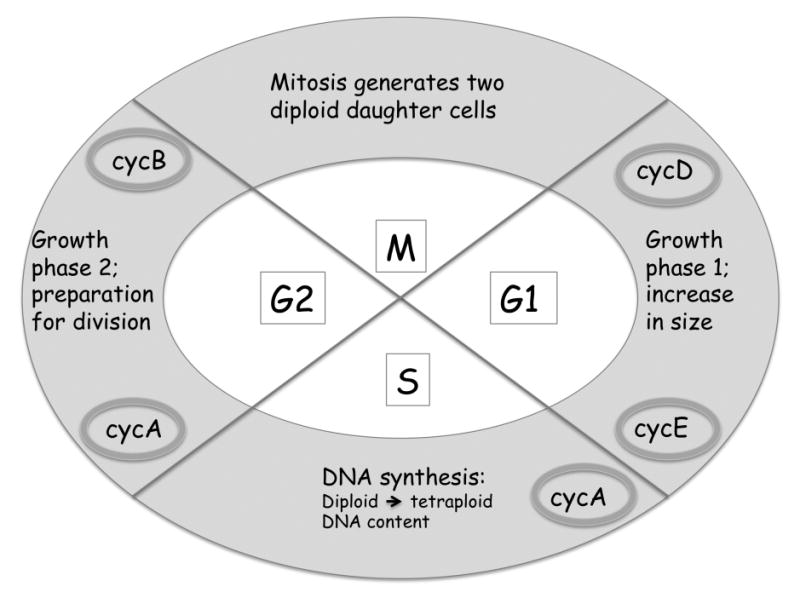
Schematic representation of the cell cycle. M, mitosis; G1, gap 1; S, synthesis; G2, gap2. Predominant cyclins (cyc) are shown in shaded ovals.
After mitosis, a daughter cell enters the G1 phase of the cycle, during which it grows and accumulates metabolites required for DNA synthesis. While the cell is in G1, cyclin D mediated events allow the cell to monitor its external environment, including, in mammalian cells, the presence of estrogen. Cyclins E and A become prominent as cells enter the S, or Synthesis, phase of the cycle, when DNA replication occurs. As synthesis progresses, cyclin E is degraded. Following S, as cells enter G2, mitotic cyclin B accumulates, followed by mitosis. G1 and G2 are “gaps” in the cycle, which prevent DNA replication before mitosis is complete, and prevent mitosis until replication is complete. Typically, cells growing exponentially in culture medium divide asynchronously, and the proportion of cells in any particular phase of the cycle reflects the relative length of that phase, relative to the generation time.
A classical method for synchronizing a cell population with respect to entry into the mitotic cycle involves two successive treatments with hydroxyurea, a reversible inhibitor of the enzyme ribonucleotide reductase (RNR). RNR reduces ribonucleotides to the deoxyribonucleotide precursors required for DNA synthesis. In an initial treatment with hydroxyurea, cells in G2 and in G1 progress through the cycle to the G1/S boundary, and arrest. Cells in S at the time of exposure to hydroxyurea complete synthesis. Removal of hydroxyurea allows the cohort of cells at the G1/S boundary to pass through S in synchrony. After a period of time equivalent to the duration of S, a second treatment with hydroxyurea blocks all cells, including those that were in S at the first treatment, at the G1/S boundary. After release from the second hydroxyurea block, essentially all the cells in the population pass through S in synchrony. Gerenday et al. (1997) describe the conditions for synchronization of C7-10 cells.
Cell cycle synchronization requires basic knowledge of the duration of G1, S and G2, relative to the population doubling time. In the presence of fresh medium, that is, access to unlimited nutrients, cells cycle rapidly; nearly every cell in the population is mitotically active. With increased time, nutrients become depleted, and the population doubling time increases, reflecting heterogeneity in the growth rate and the exit of individual cells from the cell cycle into a quiescent state. C7-10 cells have a generation time of 15 h when they enter exponential growth, which increases to 28 h as the population reaches confluence (Gerenday and Fallon, 1996). Using pulse-chase experiments with [3H]thymidine, we showed that the duration of S is 6-8 h, and G2, 2-2.5 h. Although our understanding of cell cycle events in the context of a 20E response is limited, available data indicate that P. interpunctella imaginal wing cells arrest in G2 and show the expected decrease in cyclin B (Mottier et al., 2004), while C7-10 cells arrest in the G1 phase of the cycle, and show decreased abundance of cyclin A (Gerenday and Fallon, 2004). The G1 arrest in mosquito cells was somewhat unexpected, because cumulative studies on epidermal and imaginal disc cells in insects, and limited studies with insect cell lines, had supported the generalization that insect cells differ from mammalian cells in that the G2 phase of the cycle is longer than G1, and that arrest typically occurs in G2 (Hatt et al., 1994). Further studies using flow cytometry showed that after treatment with 20E, C7-10 cells complete the ongoing cycle before arresting in G1 (Gerenday and Fallon, 2004), and recent data suggest that the mosquito homolog of the cell cycle inhibitory protein, DACAPO, increases after 20E treatment (Gerenday and Fallon, unpublished observations).
6. Concluding Remarks
Approximately 500 insect cell lines have been described, most of which represent Lepidopteran or Dipteran species. Historically, Lepidopteran cell lines came into prominence for production of baculoviruses for biological control, and more recently as protein expression systems, while Drosophila cell lines provided an in vitro adjunct to the classical genetic approaches available with this model organism. Mosquito cell lines provided in important in vitro system for investigation of arbovirus infections.
The changes that enable a primary cell culture to generate cells capable of indefinite growth are poorly understood with insect, relative to mammalian cells, and few rigorous investigations of karyotype stability have been done with Lepidopteran cell lines, which have relatively high numbers of small, holocentric chromosomes that are difficult to distinguish by light microscopy. For cytological studies, Dipteran cell lines have the advantage of small numbers of large chromosomes, which can be easily distinguished with the light microscope. Karyotype analyses with Drosophila and mosquito cell lines suggest that their capacity for unlimited growth does not involve selection for aneuploid cells. That much remains to be learned from insect cells in culture is underscored by the recent recognition of insect stem cells, and their potential for identifying specific molecules that control growth and differentiation. Unfortunately, such investigations require rigorous attention to details of medium composition, and hypotheses that might guide successful discovery of regulatory molecules are difficult to articulate. Technical advances in cell culture, such as three-dimensional systems that foster cell-cell interactions, may allow more sophisticated reconstruction of hormonal cascades that control insect growth and metamorphosis, including possible stimulatory effects of 20E at sub-micromolar concentrations (Cherbas et al., 1980; Echalier, 1997, Chapter 8).
Our own work has focused on the Ae. albopictus cell line called C7-10, which we have used to investigate cell cycle progression in response to 20E. These cells originated from non-feeding neonate larvae (Singh, 1967), and their arrest in the G1 phase of the cycle contrasts with the G2 arrest observed in Plodia interpunctella imaginal wing cells, established from last instar larvae. The extent to which differences in the 20E-mediated cell cycle arrest in these two lines reflect the developmental context of the cells from which the lines originated (see Swanhart et al., 2005) remains to be explored.
The complexity of hormone-regulated events is underscored by the extensive work done with estrogen-mediated proliferation of breast cancer cell lines, which has important therapeutic applications. Estrogen's effects have been shown to involve an interplay between classical “genomic” interactions of ligand-bound receptors to estrogen responsive elements, as well as protein-protein interactions that involve factors such as AP-1, in which the estrogen receptor participates, but does not interact directly with promoter DNA (Safe and Kim, 2008). Estrogen also stimulates signaling pathways involving phosphorylation of downstream effector proteins, whose activity may be localized in the cytoplasm (Fox et al., 2009). As these novel effects of estrogen become better understood, insect endocrinologists can use the information to develop new models for understanding the roles of insect hormones in growth and metamorphosis.
Acknowledgments
AMF thanks Dr. Judy Willis for introducing her to the Society for In vitro Biology (formerly, the Tissue Culture Association) and for unfailing encouragement and support of her scientific career. Work in the Fallon laboratory was supported by grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Butenandt A, Karlson P. Uber die Isolierung eines Metamorphose-Hormone der Insekten in kristallisierter Form. Zeitschrift fur Naturforschung. 1954;96:389–91. [Google Scholar]
- Cherbas L, Yonge CD, Cherbas P, Williams CM. W. Roux's Archives of Developmental Biology. 1980;189:7–15. doi: 10.1007/BF00848562. [DOI] [PubMed] [Google Scholar]
- Dobens L, Rudolph K, Berger EM. Ecdysterone regulatory elements function as both transcriptional activators and repressors. Molecular and Cellular Biology. 1971;11:2318–2323. doi: 10.1128/mcb.11.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston ED, Gerenday A, Fallon AM. Leveraging genomic databases: from an Aedes albopictus cell line to the malaria vector Anopheles gambiae via the Drosophila genome project. Insect Molecular Biology. 2002;11:187–195. doi: 10.1046/j.1365-2583.2002.00324.x. [DOI] [PubMed] [Google Scholar]
- Echalier G. Drosophila Cells in Culture . Academic Press; New York: 1997. p. 702. [Google Scholar]
- Fallon AM. Transfection of cultured mosquito cells. In: Crampton JM, Beard CB, Louis C, editors. Molecular biology of insect disease vectors . Chapman and Hall; New York: 1997. pp. 430–443. [Google Scholar]
- Fallon AM, Kurtti TJ. Cultured cells as a tool for analysis of gene expression. In: Marquardt WC, editor. Biology of disease vectors. 2nd. Elsevier; New York: 2005. pp. 539–549. [Google Scholar]
- Fallon AM, Sun D. Exploration of mosquito immunity using cells in culture. Insect Biochemistry and Molecular Biology. 2001;31:263–78. doi: 10.1016/s0965-1748(00)00146-6. [DOI] [PubMed] [Google Scholar]
- Fox EM, Andrade J, Shupnik MA. Novel actions of estrogen to promote proliferation: Integration of cytoplasmic and nuclear pathways. Steroids. 2009;74:622–627. doi: 10.1016/j.steroids.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel G. Hormone causing pupation in the blowfly Calliphora erythrocephala. (Series B).Proceedings of the Royal Society of London. 1935;118:1. [Google Scholar]
- Gerenday A, Blauwkamp TS, Fallon AM. Synchronization of Aedes albopictus mosquito cells using hydroxyurea. Insect Molecular Biology. 1997;6:191–196. doi: 10.1111/j.1365-2583.1997.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Gerenday A, Fallon AM. Cell cycle parameters in Aedes albopictus mosquito cells. In vitro Cellular and Developmental Biology--Animal. 1996;32:307–312. doi: 10.1007/BF02723064. [DOI] [PubMed] [Google Scholar]
- Gerenday A, Fallon AM. Ecdysone-induced accumulation of mosquito cells in the G1 phase of the cell cycle. Journal of Insect Physiology. 2004;50:831–838. doi: 10.1016/j.jinsphys.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Gillies S, Stollar V. Conditions necessary for inhibition of protein synthesis and production of cytopathic effect in Aedes albopictus cells infected with vesicular stomatitis virus. Molecular and Cellular Biology. 1982;2:66–75. doi: 10.1128/mcb.2.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt RB. Some experiments on spermatogenesis in vitro. Proceedings of the National Academy of Science USA. 1915;1:220–22. doi: 10.1073/pnas.1.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S, Loeb MJ, Takeda M. Bombyxin stimulates proliferation of cultured stem cells derived from Heliothis virescens and Mamestra brassicae larvae. In vitro Cellular and Developmental Biology-Animal. 2005;40:38–42. doi: 10.1290/0312092.1. [DOI] [PubMed] [Google Scholar]
- Grace TDC. Establishment of four strains of cells from insect tissues grown in vitro. Nature. 1962;195:788–789. doi: 10.1038/195788a0. 1962. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Experimental Cell Research. 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Jayachandran G, Fallon AM. Evidence for expression of EcR and USP components of the 20-hydroxyecdysone receptor by a mosquito cell line. Archives of Insect Biochemistry and Physiology. 2000;43:87–96. doi: 10.1002/(SICI)1520-6327(200002)43:2<87::AID-ARCH5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Jayachandran G, Fallon AM. Antisense expression of the 20-hydroxyecdysone receptor (EcR) in transfected mosquito cells uncovers a new EcR isoform that varies at the C-terminal end. In vitro Cellular and Developmental Biology-Animal. 2001;37:522–529. doi: 10.1290/1071-2690(2001)037<0522:aeothr>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Jayachandran G, Fallon AM. Decreased survival of mosquito cells after stable transfection with a Drosophila ecdysteroid response element: Possible involvement of a 40 kDa DNA binding protein. Journal of Insect Science 2:21. 2002 doi: 10.1093/jis/2.1.21. http://Insectscience.org/2.21. [DOI] [PMC free article] [PubMed]
- Johnston AM, Fallon AM. Characterization of the ribosomal proteins from mosquito (Aedes albopictus) cells. European Journal of Biochemistry. 1985;150:507–515. doi: 10.1111/j.1432-1033.1985.tb09051.x. [DOI] [PubMed] [Google Scholar]
- Kopec S. Studies on the necessity of the brain for the inception of insect metamorphosis. Biological Bulletin. 1922;42:323–342. [Google Scholar]
- Lan Q, Gerenday A, Fallon AM. Cultured Aedes albopictus cells synthesize hormone-inducible proteins. In vitro Cell and Developmental Biology—Animal. 1993;29:813–818. doi: 10.1007/BF02634349. [DOI] [PubMed] [Google Scholar]
- Lin T, Ambasudhan R, Yuan Z, Li W, Hilcove S, Abujarour R, Lin X, Hahm HS, Hao E, Hayek A, Ding S. A chemical platform for improved induction of human iPSCs. Nature Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Wu SC. Mosquito and mammalian cells grown on microcarriers for four-serotype dengue virus production: Variations in virus titer, plaque morphology, and replication rate. Biotechnology and Bioengineering. 2004;85:482–488. doi: 10.1002/bit.10918. [DOI] [PubMed] [Google Scholar]
- Loeb MJ, Martin PAW, Hakim RS, Goto S, Takeda M. Regeneration of cultured midgut cells after exposure to sublethal doses of toxin from two strains of Bacillus thuringiensis. Journal of Insect Physiology. 2001;47:599–606. doi: 10.1016/s0022-1910(00)00150-5. [DOI] [PubMed] [Google Scholar]
- Lynn DE. Novel techniques to establish new insect cell lines. In vitro Cellular and Developmental Biology—Animal. 2001;37:319–321. doi: 10.1007/BF02577564. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi J, Maramorosch K. Leafhopper tissue culture: embryonic, nymphal and imaginal tissues from aseptic insects. Contributions of the Boyce Thompson Institute. 1964;22:435–460. [Google Scholar]
- Miura K, Wang SF, Raikhel AS. Two distinct subpopulations of the ecdysone receptor complex in the female mosquito during vitellogenesis. Molecular and Cellular Endocrinology. 1999;156:111–120. doi: 10.1016/s0303-7207(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Monroe TJ, Muhlmann-Diaz MC, Kovach MJ, Carlson JO, Bedford JS, Beaty BJ. Stable transformation of a mosquito cell line results in extraordinarily high copy numbers of the plasmid. Proceedings of the National Academy of Sciences USA. 1992;89:5725–5729. doi: 10.1073/pnas.89.13.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottier V, Siaussat D, Bozzolan F, Auzoux-Bordenave S, Porcheron P, Debernard S. The 20-hydroxyecdysone-induced cellular arrest in G2 phase is preceded by an inhibition of cyclin expression. Insect Biochemistry and Molecular Biology. 2004;34:51–60. doi: 10.1016/j.ibmb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Mukherjee AB, Herrera RJ. Replication pattern of double minutes derived from an insect cell line. Experientia. 1985;41:85–86. doi: 10.1007/BF02005888. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Henrich VC. Arthropod nuclear receptors and their role in molting. FEBS Journal. 2009;276:6128–6157. doi: 10.1111/j.1742-4658.2009.07347.x. [DOI] [PubMed] [Google Scholar]
- Saarinen MA, Murhammer DW. Culture in the rotating-wall vessel affects recombinant protein production capability of two insect cell lines in different manners. In vitro Cellular and Developmental Biology-Animal. 2000;36:362–366. doi: 10.1290/1071-2690(2000)036<0362:CITRWV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. Journal of Molecular Endocrinology. 2008;41:263–275. doi: 10.1677/JME-08-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AM, Simoncini T. Extra-nuclear signaling of the ERa to the actin cytoskeleton in the central nervous system. Steroids. 2009 doi: 10.1016/j.steroids.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Sarver N, Stollar V. Sindbis virus-induced cytopathic effect in clones of Aedes albopictus (Singh) cells. Virology. 1977;80:390–400. doi: 10.1016/s0042-6822(77)80014-7. [DOI] [PubMed] [Google Scholar]
- Shih KM, Gerenday A, Fallon AM. Culture of mosquito cells in Eagle's medium. In vitro Cellular and Developmental Biology—Animal. 1998;34:629–630. doi: 10.1007/s11626-996-0010-1. [DOI] [PubMed] [Google Scholar]
- Shotkoski FA, Fallon AM. Genetic changes in methotrexate-resistant mosquito cells. Archives of insect Biochemistry and Physiology. 1990;15:79–92. doi: 10.1002/arch.940150203. [DOI] [PubMed] [Google Scholar]
- Siaussat D, Bozzolan F, Porcheron P, Debernard S. Identification of steroid hormone signaling pathway in insect cell differentiation. Cellular and Molecular Life Sciences. 2007;64:365–376. doi: 10.1007/s00018-007-6452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siaussat D, Bozzolan F, Porcheron P, Debernard S. The 20-hydroxyecdysone-induced signaling pathway in G2/M arrest of Plodia interpunctella imaginal wing cells. Insect Biochemistry and Molecular Biology. 2008;38:529–539. doi: 10.1016/j.ibmb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Singh KRP. Cell cultures derived from larvae of Aedes albopictus (Skuse) and Aedes aegypti (L) Current Science. 1967;36:506–508. [Google Scholar]
- Swanhart L, Kupsco J, Duronio RJ. Developmental control of growth and cell cycle progression in Drosophila. In: Humphrey T, Brooks G, editors. Methods in Molecular Biology. Vol. 296. 2005. [DOI] [PubMed] [Google Scholar]; Cell cycle control: Mechanisms and Protocols . Humana Press; Totowa, New Jersey: 2005. pp. 69–94. [Google Scholar]
- Wang SF, Miura K, Miksicek RJ, Segraves WA, Raikhel AS. DNA binding and transactivation characteristics of the mosquito ecdysone receptor—ultraspiracle complex. Journal of Biological Chemistry. 1998;273:27531–27540. doi: 10.1074/jbc.273.42.27531. [DOI] [PubMed] [Google Scholar]
- Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nature Medicine. 2000;6:849–51. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]
- Wyatt SS. Culture in vitro of tissue from the silkworm Bombyx mori L. Journal of General Physiology . 1956;39:841–852. doi: 10.1085/jgp.39.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao TP, Froman BM, Jiang Z, Cherbas L, Chen JD, McKeown M, Cherbas P, Evans RM. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature. 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]


