Abstract
Background
Investigations of gene-environment interaction (G × E) in depression have implicated a polymorphism in the promoter region of the serotonin transporter gene (5-HTTLPR) as a moderator of the stress-depression relationship. However, recent evidence for 5-HTTLPR G × E in depression has been inconsistent. The present study examined the moderating effect of the val158met polymorphism in the catechol-O-methyltransferase (COMT) gene on the strength of 5-HTTLPR G × E.
Methods
A community sample of youth (n = 384) was genotyped for 5-HTTLPR and COMT. A multi-method, multi-informant index of chronic family stress was derived from interviews and questionnaires administered at youth age 15. G × G × E was examined in relation to depression diagnoses between ages 15 and 20 and depressive symptoms at age 20.
Results
Significant three-way interactions were observed for both depressive symptoms and diagnoses, such that 5-HTTLPR G × E occurred only in the context of COMT val158 allele homozygosity. For val158 homozygotes, the 5-HTTLPR L allele exerted a protective effect in the face of stress. No genetic main effect or two-way G × E was found for 5-HTTLPR.
Conclusions
Inconsistent 5-HTTLPR G × E findings to date may be partly attributable to unmeasured epistatic effects between 5-HTTLPR and COMT val158met. Identifying the conditions under which 5-HTTLPR G × E is most likely to operate may allow depression prevention and treatment efforts to target youth at highest risk.
Keywords: depression, serotonin transporter gene, catechol-O-methyltransferase, chronic stress, gene-environment interaction
Most depressions are preceded by stressful experiences [1], yet only one in five people becomes depressed following a severe stressor [2]. Considerable attention has focused on identifying biological and psychosocial factors that account for the interindividual variability in depressive responses to stress [3–5]. At the biological level, variation in a functional polymorphism in the promoter region of the serotonin transporter gene (5-HTTLPR) is a putative moderator of the stress-depression relationship. In the original report, Caspi and colleagues [6] observed that the short (S) allele at 5-HTTLPR conferred risk for depression in the face of stress, whereas long (L) allele homozygotes were resilient to stressful life events.
More than 15 studies [reviewed in 7] have provided at least partial support for Caspi et al.’s [6] findings. Recently, however, researchers have cautioned that the 5-HTTLPR gene-stress interaction (G × E) may not be as robust as initially thought [8]. Inconsistent findings have emerged, as various studies failed to find evidence of G × E [9–13], and others reported interaction effects in the opposite direction to that observed by Caspi et al. [14,15]. These discrepancies were highlighted by two recent meta-analyses that concluded 5-HTTLPR G × E has a negligible effect on depression vulnerability [16,17].
Recent reviews, however, have affirmed the validity of G × E modeling and observed multiple replications of the original Caspi et al. findings, noting a number of measurement and methodological requirements that should be met [18–20]. One additional explanation for inconsistent findings is that emotional reactivity to stressful contexts is controlled by multiple genes [21], with some variants augmenting the stress response and others attenuating it. From this perspective, the effect of 5-HTTLPR on vulnerability (or resilience) to depression following exposure to stressors likely depends on variation at other susceptibility loci.
Several studies have implicated gene-gene interplay (i.e., epistasis) involving 5-HTTLPR in serotonin transporter expression [22,23], morphology of limbic circuitry [24], and a variety of behavioral phenotypes, including traits relevant to stress reactivity (e.g., negative affectivity, harm avoidance) [25–28]. These findings suggest that examining epistatic effects may help demarcate the conditions under which 5-HTTLPR G × E is most likely to operate.
Investigations in healthy samples have established an association between the catechol-O-methyltransferase (COMT) gene and sensitivity to experimental stress [29–31], supporting COMT as a plausible candidate for G × E [32]. The COMT gene contains an extensively studied G>A single nucleotide polymorphism (SNP) (rs4680) that results in a valine (val) to methionine (met) amino acid substitution commonly known as val158met. The met158 allele produces a heat-labile enzyme that is associated with a three- to four-fold reduction in degradation of dopamine in the synapse [33].
Inconsistent results from genetic association studies call into question whether COMT exerts a direct effect on risk for depression. Both met158 [34] and val158 [35,36] alleles have been linked to depression, but other studies have reported null associations [37–39]. It has been suggested COMT influences risk for affective disorders in conjunction with environmental stress [40]. The COMT met158 allele is thought to confer vulnerability to stress because it is associated with increased stress hormone release [29,30], as well as greater sensory and affective ratings of pain [31], in response to physical and psychological stressors. Additionally, the met158 variant is consistently linked to increased activation in the amygdala and hippocampus, as measured by functional magnetic resonance imaging (fMRI), following exposure to unpleasant and emotional visual stimuli [41–43]. COMT-mediated dopamine increases in limbic regions are hypothesized to result in enhanced arousal and exaggerated affective responses to environmental stressors [40]. A “case-only” study of Italian inpatients diagnosed with affective disorders [44] offered preliminary evidence that the met158 allele increases susceptibility to depression following adverse events.
The present study examined the moderating effect of COMT genotype on 5-HTTLPR × chronic stress interactions in an Australian community sample of adolescents. Exposure to chronic family stress was indexed by a multi-method, multi-informant composite score from age 15 assessments. Depressive symptoms and diagnoses were assessed five years later at youth age 20. A gene-gene-environment interaction (G × G × E) was predicted such that the strength of 5-HTTLPR G × E would depend on COMT genotype. Specifically, carriers of both the 5-HTTLPR S and COMT met158 alleles were predicted to exhibit the greatest depressive response to chronic stress, whereas those homozygous for L and val158 alleles were predicted to be least reactive to stress.
Materials and Methods
Participants
Participants were recruited from the Mater-University Study of Pregnancy (MUSP), which followed a birth cohort of 7,223 mothers and their offspring born between 1981 and 1984 in Brisbane, Australia [45]. Mothers completed the Delusions-Symptoms-States Inventory [DSSI; 46] during pregnancy, post-partum, 6 months after birth, and 5 years after birth. As described in detail elsewhere [47], the present study selected and followed up 815 of the original families when the child reached age 15, oversampling for mothers with a putative history of depression based on the severity and chronicity of depressive symptoms endorsed on the DSSI whose diagnostic status was later confirmed. The sample studied at age 15 was 92% Caucasian and 8% minority (Asian, Pacific Islander, and Aboriginal), with median family income falling in the lower middle class and mothers’ median education level at grade 10. At youth age 20, all families were invited to participate in a second assessment, yielding a sample of 705 youth and mothers that agreed and completed further interviews and questionnaires [see 48 for details].
Of the 705 youth assessed at age 20, 512 provided blood samples between ages 22–25. Participants not providing DNA either had withdrawn from follow-ups, declined the current study, moved, had major medical problems, or were deceased. The 512 participants in the genotyping sample at ages 22–25 did not differ from the 303 participating at age 15 but not at ages 22–25 in terms of youth depression history by age 15 or maternal history of depression by age 15, χ2 s < 1, ps > .10, but were less likely to be male, χ2(1, 815) = 21.29, p < .01.
The current analyses are based on 384 randomly selected DNA samples from the 512 participants who provided blood because funding was available for only one 384-well genotyping plating. Three samples failed to produce an adequate reading, resulting in current analyses based on 149 males and 232 females, mean age 23.7 (SD = 0.89). Comparing the 381 youth for whom COMT and 5-HTTLPR genotype were determined with the 131 youth whose DNA samples were unanalyzed, there was no difference in maternal depression status, χ2(1, 512) < 1, p > .10, although males were less likely to have their sample analyzed than females, χ2(1, 512) = 16.49, p < .001. Stratified by genotype group, descriptive statistics for the main study variables are presented in Table 1.
Table 1.
Descriptive Statistics for Main Study Variables
| 5-HTTLPR S |
5-HTTLPR L |
|||
|---|---|---|---|---|
| Variable | COMT M | COMT V | COMT M | COMT V |
| N | 205 | 60 | 68 | 29 |
| Gender distribution (males/females) | 75/128 | 20/40 | 32/36 | 11/18 |
| Mean (SD) age 20 BDI-II score | 7.66 (8.02) | 6.74 (8.72) | 7.73 (7.90) | 7.61 (8.58) |
| Mean (SD) age 15 family stress | 0.03 (0.55) | −0.01 (0.58) | −0.15 (0.52) | 0.10 (0.61) |
| No. (%) maternal depression by youth age 15 | 99 (48%) | 20 (33%) | 23 (34%) | 16 (55%) |
| No. (%) with depression diagnoses prior to and including age 15 | 17 (8%) | 5 (8%) | 7 (10%) | 2 (7%) |
| No. (%) with depression diagnoses between years 15 and 20 | 58 (28%) | 17 (28%) | 15 (22%) | 8 (28%) |
N, number of participants; S, S allele carrier; L, L allele homozygote; M, met158 allele carrier; V, val158 allele homozygote
Procedure
At youth age 15, participants were visited in their homes. Interviewers who were blind to the mother’s depression status conducted separate and independent interviews with youth, mothers, and available fathers. The mother-child pairs were again assessed at age 20 and the youth were contacted in 2006 for participation in the genotyping study when they were between ages 22 and 25. Procedures were approved by the Institutional Review Boards of the University of Queensland; University of California, Los Angeles; and Emory University.
Measures
Youth depression symptoms
Self-reported depressive symptoms at age 20 were assessed using the Beck Depression Inventory-II [BDI-II; 49], a well-validated and extensively used measure of severity of depressive symptoms. Reliability for the current sample was α = 0.92.
Youth and mother depression diagnoses
Youth diagnoses of major depression and dysthymic disorder between ages 15 and 20 were assessed using the Structured Clinical Interview for DSM-IV [SCID; 50] administered at age 201. The SCID allowed precise dating of depressive disorders to ensure onsets occurred after the age 15 assessment. Diagnostic reliability estimates based on 10% of the interviews rated by independent judges were κ = 0.83 for current diagnoses of depression and κ = 0.89 for past depression.
Maternal depression during the child’s lifetime prior to age 15, used as a covariate in all analyses, was assessed by SCIDs administered to mothers at youth age 15. Youth current and past depression diagnoses at age 15 were also ascertained using the SCID.
Chronic family stress
A composite family stress variable was derived from a battery of 11 self-report and interview measures completed at age 15 pertaining to marital and parental functioning. A variety of indicators of family stress was collected in order to adequately capture aspects of both the marital and parent-child relationship, as well as incorporate multiple informants (i.e., parent, youth, interviewer) and methods (i.e., self-report questionnaire, interview). Three measures were interviewer-rated scores from the youth and mother versions of the UCLA Chronic Stress Interview [51] covering quality of mother’s marital/romantic relationship, her relationship with the youth, and the youth’s relationship with immediate family members. Intraclass correlations based on independent ratings of these domains were 0.82, 0.82, and 0.76, respectively. Validity data for adults and youth are reported elsewhere [51,52].
To assess overall marital relationship quality, the Satisfaction subscale of the Dyadic Adjustment Scale [DAS; 53] was administered to mothers who were in relationships, as well as husbands when available. Mothers and fathers also completed the Modified Conflict Tactics Scale [MCTS; 54] assessing psychological and physical coercion (e.g., argued heatedly; refused to talk; threw something; hit partner). Cronbach’s α for the DAS and MCTS ranged from 0.80–0.95 and 0.79–0.92, respectively, across parents.
The Children’s Report of Parental Behavior Inventory [CRPBI; 55] was administered to youths to assess the quality of their interactions with both parents. The psychological control versus psychological autonomy and youth’s perception of acceptance versus rejection were the two subscales of the CRPBI used in the current analyses. Internal consistency for each subscale by parent ranged from 0.77–0.91.
Each of these 11 measures was standardized across the sample and non-missing variables for each participant were averaged to form a composite (α = 0.78). Scores ranged from -1.25 to 1.99 (M = 0.01, SD = 0.57), with higher values indicating greater family stress.
Genotyping
Participants who agreed to the blood collection study were mailed consent forms, a blood collection pack, and questionnaires, and were instructed to have the blood drawn at a local pathology lab. The blood samples were picked up by courier from the individual and transported to the Genetic Epidemiological Laboratory of the Queensland Institute of Medical Research, where the genotyping procedures were conducted.
Assays for the COMT val158met SNP were designed using MassARRAY Assay Design software (version 3.0; Sequenom Inc., San Diego, CA) and typed using iPLEX chemistry on a Compact MALDI-TOF Mass Spectrometer (Sequenom). Forward and reverse PCR primers and a primer extension probes were purchased from Bioneer Corporation (Daejeon, Korea). Genotyping was carried out in standard 384-well plates with 12.5 ng genomic DNA used per sample. A modified Sequenom protocol was followed, using half reaction volumes in each of the PCR, SAP and iPLEX stages giving a total reaction volume of 5.5 μL. The iPLEX reaction products were desalted by diluting samples with 18 μL of water and 3 μL SpectroCLEAN resin (Sequenom) and then were applied to a SpectroChip (Sequenom), processed and analyzed on a Compact MALDI-TOF Mass Spectrometer by MassARRAY Workstation software (version 3.3) (Sequenom). Allele calls for 384-well plates were reviewed using the cluster tool in the SpectroTYPER software (version 3.3; Sequenom) to evaluate assay quality. In the present sample, genotype frequencies (and corresponding proportions) at val158met were VV = 91 (.24), VM = 195 (.51), MM = 95 (.25), and were in Hardy-Weinberg Equilibrium, χ2 (1, 381) = 0.04, p = 0.83.
The 5-HTT 43 bp deletion polymorphism was assayed using previously reported methods [56]. Most samples were subject to triplicate gel analysis. A minimum of two independent results in agreement was required for inclusion which gave a final call rate of 96.4%. In the present sample the genotype frequencies (and proportions) were LL = 122 (.32), LS = 178 (.47), and SS = 81 (.21), and in Hardy-Weinberg equilibrium, χ2 (1, 381) = 1.61, p = 0.20.
The minor allele of the rs25531 SNP in the L allele has been reported to render the L allele functionally equivalent to S [23]. This SNP was assayed using the protocol of Wray et al.[56], and the current analyses were performed reclassifying LG alleles as S.
Statistical Analysis
The effects of 5-HTTLPR, COMT, chronic family stress, and their two- and three-way interactions on age 20 depressive symptoms (BDI) were estimated using ordinary least squares (OLS) regression. Gender, maternal depression history, and youth depression history at age 15 were included in the model as covariates. One dummy variable was created to represent the contrast of the LL group versus SS and SL groups, consistent with past research on 5-HTTLPR and stress reactivity [57]. Similarly, met158 carriers were grouped and contrasted with val158 homozygotes in accordance with prior research [e.g., 44].
Given these groupings, four genotype combinations were possible: S allele carriers and met158 allele carriers (hereafter SM), S allele carriers and val158 homozygotes (SV), L homozygotes and met158 allele carriers (LM), and L homozygotes and val158 homozygotes (LV).
Using an estimated effect size of ΔR2 = .02 drawn from previous G × G × E research [58], a Type I error rate of 0.05, and a desired power level of 0.80, power analyses in Stata 10.0 [59] indicated that 324 observations were needed to detect a 3-way interaction between 5-HTTLPR, COMT, and family stress, given that one exists.
The same set of predictors was entered in a logistic regression model with youth depression onset between years 15 and 20 as the outcome variable. OLS and logistic regression parameters were compared to confirm that any observed interactions were not artifacts of measurement scale [32]. For both models, when interactions were detected, simple effects were calculated using the LINCOM command in Stata 10.0 [59]. Simple effect p-values (denoted padj) were adjusted following procedures formulated by Benjamini to control the False Discovery Rate for correlated test statistics [60].
Results
Gene-Environment Correlation (rGE)
Univariate analysis of variance revealed no main effects of 5-HTTLPR, F(1, 356) = 0.57, p = .45, or COMT val158met, F(1, 356) = 1.63, p = .20 on exposure to family stress, nor was there any joint effect of 5-HTTLPR and COMT, F(1, 356) = 1.38, p = .24.
5-HTTLPR × COMT × Family Stress on BDI symptoms
Predictors were entered hierarchically, with main effects on Step 1, two-way interactions on Step 2, and the three-way interaction on Step 3. A significant main effect of chronic family stress was observed on Step 1, whereas neither 5-HTTLPR nor COMT exerted a direct effect on BDI (see Table 2). The addition of the two-way interactions on Step 2 did not account for a significant increment in variance explained, ΔR2 = .006, ΔF(3, 332) = 0.81, p = .49, indicating no G × E. In contrast, on Step 3, the three-way interaction term was significant, b = −11.04, SE = 3.73, p < .01.
Table 2.
Hierarchical Linear Regression Analysis of 5-HTTLPR, COMT val158met, Chronic Family Stress, and Their Interactions Predicting Depressive Symptoms at Age 20
| Step Statistics |
Final Statistics |
||
|---|---|---|---|
| Predictors | ΔR2 | b (SE b) | b (SE b) |
| Step 1 | .18** | ||
| Gender | −1.60 (0.90)* | −1.49 (0.90) | |
| Maternal Depression | 2.49 (0.92)** | 2.66 (0.92)** | |
| Youth Prior Depression | 2.06 (0.48)** | 2.06 (0.48)** | |
| Family Stress | 3.88 (0.81)** | −3.25 (2.51) | |
| 5-HTTLPR | −0.36 (0.97) | −0.60 (1.86) | |
| COMT | 1.25 (1.01) | 1.14 (1.84) | |
| Step 2 | .01 | ||
| Stress × 5-HTTLPR | 2.57 (1.78) | 10.01 (3.07)** | |
| Stress × COMT | 0.47 (1.74) | 8.12 (3.11)** | |
| 5-HTTLPR × COMT | −0.32 (2.23) | −0.16 (2.20) | |
| Step 3 | .02** | ||
| Stress × 5-HTTLPR × COMT | −11.04 (3.74)** | ||
| Total R2 | .21** | ||
p < .05
p < .01
For the LV group, the slope of BDI scores on chronic family stress was not significantly different from 0, b = −3.25, SE = 2.50, padj = .24. All other genotype groups exhibited significant positive slopes of BDI on chronic stress (SV, b = 6.75, SE = 1.80, padj < .05; SM, b = 3.84, SE = 1.06, padj < .05; LM, b = 4.88, SE = 1.88, padj < .05) (see Figure 1). Further, the slope of the LV group was significantly different from the slopes for the other three genotype groups (all padjs < .05) whereas the three groups with nonzero slopes did not differ from each other in terms of strength of the chronic stress-BDI association (all padjs > .10).2
Figure 1.
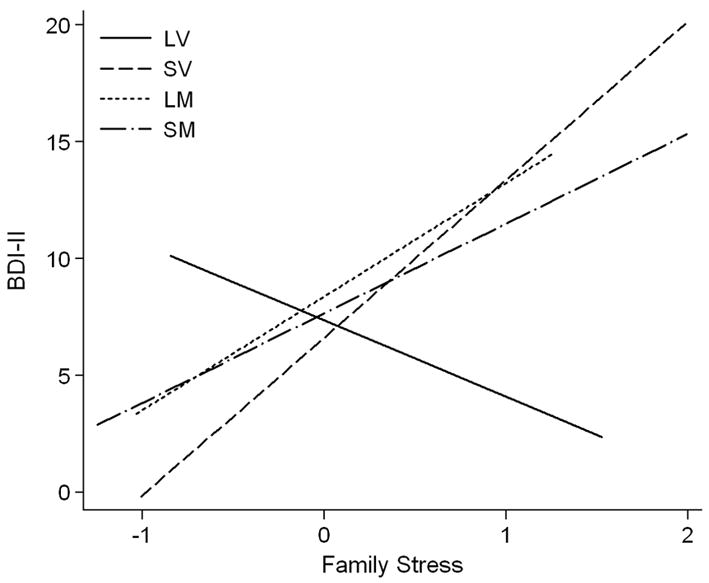
Association between age 20 Beck Depression Inventory-II scores and chronic family stress at age 15 as a function of 5-HTTLPR and COMT genotypes. LV, 5-HTTLPR L homozygotes + COMT val158 homozygotes; LM, 5-HTTLPR L homozygotes + COMT met158 carriers; SV, 5-HTTLPR S carriers + COMT val158 homozygotes; SM, 5-HTTLPR S carriers + COMT met158 carriers; BDI-II, Beck Depression Inventory-II.
5-HTTLPR × COMT × Family Stress on Depression Diagnoses
As in the linear model, variables were entered hierarchically into the regression. In Step 1, 5-HTTLPR and COMT did not significantly affect the probability of being diagnosed with depression between ages 15 and 20, whereas increasing stress was associated with a greater likelihood of depression (see Table 3). On Step 2, there was no effect of the two-way interactions, taken together, on depression risk, χ2 (3) = 0.24, p > .10. Consistent with the OLS model, on Step 3 the three-way interaction term was significantly less than 0, b = −2.40, SE = 1.13, Wald = 4.41, p < .05. As shown in Figure 2, the form of this interaction was nearly identical to that observed in the OLS model. Again, the LV group was resilient to increasing stress, b = −0.62, SE = 0.73, z = −0.85, padj = .39. In contrast to the LV group, all other genotype combinations showed a positive relationship between chronic family stress and likelihood of depression; however, p-values of all the corresponding simple slopes fell short of significance after adjustment for multiple testing (LM, b = 1.30, SE = 0.61, padj = .13; SV, b = 0.83, SE = 0.55, padj = .27; SM, b = 0.37, SE = 0.29, padj = .28).3
Table 3.
Hierarchical Logistic Regression Analysis of 5-HTTLPR, COMT val158met, Chronic Family Stress, and Their Interactions Predicting Depression Diagnosis between Ages 15 and 20
| Step Statistics |
Final Statistics |
|||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | Step χ2 | b | SE | Wald | b | SE | Wald | Model χ2 |
| Step 1 | 34.70** | 34.70** | ||||||
| Gender | −0.62 | 0.27 | 5.19* | −0.67 | 0.27 | 5.89* | ||
| Maternal Depression | 0.56 | 0.26 | 4.64* | 0.57 | 0.26 | 4.73* | ||
| Youth Prior Depression | 0.40 | 0.12 | 10.47** | 0.41 | 0.12 | 10.94** | ||
| Family Stress | 0.48 | 0.23 | 4.61* | −0.62 | 0.73 | 0.72 | ||
| 5-HTTLPR | 0.17 | 0.29 | 0.34 | 0.03 | 0.53 | 0.01 | ||
| COMT | −0.06 | 0.29 | 0.04 | −0.16 | 0.54 | 0.08 | ||
| Step 2 | 0.24 | 34.94** | ||||||
| Stress × 5-HTTLPR | −0.07 | 0.52 | 0.01 | 1.45 | 0.91 | 2.54 | ||
| Stress × COMT | 0.24 | 0.48 | 0.24 | 1.92 | 0.94 | 4.12* | ||
| 5-HTTLPR × COMT | −0.03 | 0.65 | 0.01 | 0.12 | 0.64 | 0.04 | ||
| Step 3 | 4.63* | 39.58** | ||||||
| Stress × 5-HTTLPR × COMT | −2.38 | 1.13 | 4.41* | |||||
p < .05
p < .01
Figure 2.
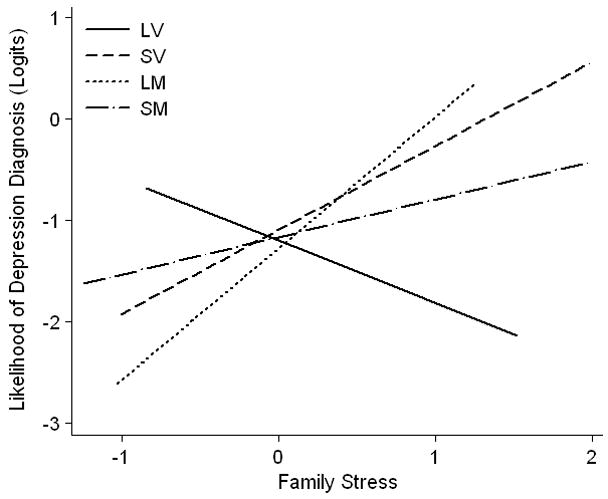
Association between likelihood of depressive disorder diagnosis between ages 15 and 20 and chronic family stress at age 15 as a function of 5-HTTLPR and COMT genotypes. LV, 5-HTTLPR L homozygotes + COMT val158 homozygotes; LM, 5-HTTLPR L homozygotes + COMT met158 carriers; SV, 5-HTTLPR S carriers + COMT val158 homozygotes; SM, 5-HTTLPR S carriers + COMT met158 carriers.
Discussion
The current study examined the joint effect of 5-HTTLPR and COMT val158met on depressive reactions to chronic stress in a sample of young adults. COMT genotype previously linked to stress sensitivity [30,31] and depressive disorders [34–36] was found to alter the strength of 5-HTTLPR G × E. Specifically, 5-HTTLPR G × E was observed only among val158 homozygotes. In the context of val158 homozygosity, the 5-HTTLPR LL genotype was associated with resilience to stress, relative to the SL and SS genotypes. However, in the presence at least one met158 allele, both 5-HTTLPR genotype groups were vulnerable to depressogenic stressors.
These results are consistent with past investigations that have linked the 5-HTTLPR S and COMT met158 variants to emotional reactivity to environmental stress [see 7,44]. The presence of at least one of these susceptibility alleles was sufficient to increase risk for depression in response to stress. On the other hand, the current findings do not support the view that 5-HTTLPR L homozygotes are immune to pathogenic environments. When paired with a met158 allele, L homozygosity provided no protective effect against chronic stressors. This pattern of results may be attributable to heightened affective arousal experienced by carriers of the S or met158 allele when confronted with stressful conditions [40,41]. The presence of either one of these variants has been shown to be sufficient to increase neural and psychophysiological reactivity to aversive cues [40,57].
Contrary to hypotheses, the met158 allele did not potentiate the effect of the S allele on depressive reactivity. Biological mechanisms underlying the lack of additivity between met158 and S are unclear, but may be elucidated by future research into the interaction of serotonergic and dopaminergic neurotransmitter systems [e.g., 61,62].
The current G × G × E findings may be relevant to some discrepancies among existing reports of 5-HTTLPR G × E. Some studies have failed to replicate the G × E observed by Caspi et al. [6], and recent null meta-analytic findings [16,17] have called attention to this inconsistency [cf. 18–20]. Indeed, in the current analyses the moderating effect of 5-HTTLPR was detected only when COMT and 5-HTTLPR were analyzed simultaneously. Thus, one of several possible explanations for conflicting results in the literature is that the nature of 5-HTTLPR G × E is dependent on the background of other genes, including COMT, regulating sensitivity to stress.
The present results should be considered preliminary in light of the limited sample size available for the genetic analyses. The group homozygous for the L and val158 alleles, in particular, was relatively small (n = 29). Thus, it is possible that this study was underpowered to detect a significant effect of family stress in this group, although there was no evidence of a trend in this direction. This is potentially important given that the null stress-depression association in the LV group, relative to the strong depressogenic effect of stress obtained in all other genotype combinations, appeared to account for the observed G × G × E. It is possible that results could differ in larger samples, and conclusions should be considered tentative until replications from large-scale studies, with more representative samples of all genotype combinations, are available.
Several other limitations of the present study should be noted. First, depression outcomes were assessed over a limited age range (i.e., late adolescence). This may be an important qualification of the results in light of apparent fluctuations in G × E across developmental stages [14,63]. Second, the current study controlled for gender, but future studies with sufficiently large samples should explore whether complex gene-gene interactions may apply differently for males and females [64,65]. Third, sample size limitations prevented a thorough investigation of the role of heterozygosity at 5-HTTLPR and COMT. Further research is necessary to determine whether additive, dominant, or recessive models for these loci are most appropriate for G × E designs. Finally, rGE presents potential complications in the interpretation of G × E in studies of measured genes and measured environments [32]. In the present sample, tests for rGE indicated that exposure to chronic family stress was not associated with genotype. However, it is implausible that the family context is entirely independent of genetic contributions, so the possibility remains that some portion of the observed interactions reflects unmeasured G × G.
Future research is necessary to explore the mechanisms, timing, and continuity of stressors in 5-HTTLPR G × E. In the current study, chronic stress was assessed 5 years prior to the self-reported depressive symptoms. The plausibility of G × E operating over this interval is supported by evidence suggesting relatively high stability of chronic stress exposure over time [66]. However, given the association of chronic stress in adolescence with early childhood adversity as well as acute stressors proximal to the age 20 assessment [66], it remains to be seen whether chronic family stress exerts a unique influence in 5-HTTLPR G × E [see 63]. Future studies using psychometrically-sound measures of acute stress and early adversity are needed to explore the range of G × E effects involving 5-HTTLPR and COMT.
In sum, these preliminary results raise the possibility that the effect of 5-HTTLPR on depressive reactivity to life stress may be more reliably detected by simultaneously accounting for variation in other monoaminergic genes that underlie stress reactivity. The current findings of complex gene-environment interplay await replication in future research involving large samples. By identifying the biological and psychological moderators of 5-HTTLPR G × E, the conditions in which G × E is most likely to operate can be better understood.
Acknowledgments
The authors greatly appreciate the assistance of Robyne LeBrocque, Cheri Dalton Comber, and Sascha Hardwicke (project coordinators) and their interview staff. We also thank staff of the Genetic Epidemiological Laboratory of the Queensland Institute of Medical Research: Professor Nick Martin (Head) for cooperation and access, Michael James for 5-HTTLPR genotyping, and Megan Campbell and Dixie Statham who coordinated genetic data collection and analysis. Thanks also to the original MUSP principals, William Bor, MD, Michael O’Callaghan, MD, and Professor Gail Williams. This study was supported by NIMH R01 MH52239 to Brennan, Hammen, and Najman.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
There were only 4 onsets of dysthymia between age 15 and age 20. Results were unaltered when these cases were omitted from the analyses.
Exploratory analyses were performed using an additive model for 5-HTTLPR, coding a single variable with values of 0, 1, and 2 to represent the number of S alleles present. The three-way interaction remained significant in predicting depressive symptoms, b = −6.05, SE = 2.72, z = −2.22, p < .05. The slope of depression on stress was comparable in the SS and SL groups (ps < .05), regardless of COMT genotype, consistent with the dominant model of the 5-HTTLPR S allele adopted in the original analyses.
Follow-up analyses were performed exclusively in a subsample of 354 Caucasian participants (i.e., 93.0% of the genotyped sample) to ensure that ethnic stratification did not affect the findings. Results from the OLS model revealed that the 3-way interaction remained significant, b = −10.35, SE = 4.01, z = 4.02, p < .01. Similarly, the strength of the G × G × E in the logistic model was not affected, b = −2.66, SE = 1.16, z = −2.29, p < .05. Further, the nature of these interactions was equivalent to that observed in the full sample; in all cases the significance of simple slopes was unchanged.
References
- 1.Paykel ES. Life events and affective disorders. Acta Psychiatr Scand. 2003;108:61–66. doi: 10.1034/j.1600-0447.108.s418.13.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown GW, Harris TO. Social Origins of Depression: A Study of Psychiatric Disorder in Women. New York: Free Press; 1978. [Google Scholar]
- 3.Joiner TE, Coyne J, editors. The interactional nature of depression: Advances in interpersonal approaches. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- 4.Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- 5.Thase M. Neurobiological aspects of depression. In: Gotlib I, Hammen C, editors. Handbook of depression. 2. New York: Guildford; 2008. pp. 187–217. [Google Scholar]
- 6.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 7.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- 8.Zammit S, Owen MJ. Stressful life events, 5-HTT genotype and risk of depression. Br J Psychiatry. 2006;188:199–201. doi: 10.1192/bjp.bp.105.020644. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie NA, Whitfield JB, Williams B, et al. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35:101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- 10.Surtees PG, Wainwright NWJ, Willis-Owen SAG, et al. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry. 2006;59:224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Chipman P, Jorm AF, Prior M, et al. No interaction between the serotonin transporter polymorphism (5-HTTLPR) and childhood adversity or recent stressful life events on symptoms of depression: Results from two community surveys. Am J Med Genet B Neuropsychiatr Genet. 2007;144:561–565. doi: 10.1002/ajmg.b.30480. [DOI] [PubMed] [Google Scholar]
- 12.Middeldorp CM, Cath DC, Beem AL, et al. Life events, anxious depression and personality: A prospective and genetic study. Psychol Med. 2008;38(11):1557–1565. doi: 10.1017/S0033291708002985. [DOI] [PubMed] [Google Scholar]
- 13.Power T, Stewart R, Ancelin ML, et al. 5-HTTLPR genotype, stressful life events and late-life depression: No evidence of interaction in a French population [published online ahead of print July 18, 2008] Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Laucht M, Treutlein J, Blomeyer D, et al. Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: Evidence from a high-risk community sample of young adults [published online ahead of print January 20, 2009] Int J Neuropsychopharmacol. 2009 doi: 10.1017/S1461145708009875. [DOI] [PubMed] [Google Scholar]
- 15.Chorbov VM, Lobos EA, Todorov AA, et al. Relationship of 5-HTTLPR genotypes and depression risk in the presence of trauma in a female twin sample. Am J Med Genet B Neuropsychiatr Genet. 2007;144:830–833. doi: 10.1002/ajmg.b.30534. [DOI] [PubMed] [Google Scholar]
- 16.Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munafó MR, Durrant C, Lewis G, Flint J. Gene × environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Caspi A, Hariri AR, Holmes A, et al. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. doi: 10.1176/appi.ajp.2010.09101452. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutter M, Thapar A, Pickles A. Gene-environment interactions: Biologically valid pathway or artifact? Arch Gen Psychiatry. 2009;66:1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- 20.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- 21.Kendler KS. “A gene for…”: The nature of gene action in psychiatric disorders. Am J Psychiatry. 2005;162(7):1243–1252. doi: 10.1176/appi.ajp.162.7.1243. [DOI] [PubMed] [Google Scholar]
- 22.Hu X, Oroszi G, Chun J, et al. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 23.Wendland JR, Martin BJ, Kruse MR, et al. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 24.Pezawas L, Meyer-Lindenberg A, Goldman AL, et al. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol Psychiatry. 2008;13:709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin J, Osher Y, Kotler M, et al. Association between tridimensional personality questionnaire (TPQ) traits and three functional polymorphisms: Dopamine receptor D4 (DRD4), serotonin transporter promoter region (5-HTTLPR) and catechol O-methyltransferase (COMT) Mol Psychiatry. 2000;5:96–100. doi: 10.1038/sj.mp.4000640. [DOI] [PubMed] [Google Scholar]
- 26.Auerbach JG, Faroy M, Ebstein R, et al. The association of the dopamine D4 receptor gene (DRD4) and the serotonin transporter promoter gene (5-HTTLPR) with temperament in 12-month-old infants. J Child Psychol Psychiat. 2001;42(6):777–783. doi: 10.1111/1469-7610.00774. [DOI] [PubMed] [Google Scholar]
- 27.Szekely A, Ronai Z, Nemoda Z, et al. Human personality dimensions of persistence and harm avoidance associated with DRD4 and 5-HTTLPR polymorphisms. Am J Med Genet B Neuropsychiatr Genet. 2004;126B:106–110. doi: 10.1002/ajmg.b.20134. [DOI] [PubMed] [Google Scholar]
- 28.Olsson CA, Byrnes GB, Anney RJL, et al. COMT Val158Met and 5HTTLPR functional loci interact to predict persistence of anxiety across adolescence: results from the Victorian Adolescent Health Cohort Study. Genes Brain Behav. 2008;6:647–652. doi: 10.1111/j.1601-183X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 29.Oswald LM, McCaul M, Choi L, et al. Catechol-O-methyltransferase polymorphism alters hypothalamic-pituitary-adrenal axis responses to naloxone: A preliminary report. Biol Psychiatry. 2004;55:102–105. doi: 10.1016/j.biopsych.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Jabbi M, Kema IP, van der Pompe G, et al. Catechol-o-methyltransferase polymorphism and susceptibility to major depressive disorder modulates psychological stress response. Psychiatr Genet. 2007;17:183–193. doi: 10.1097/YPG.0b013e32808374df. [DOI] [PubMed] [Google Scholar]
- 31.Zubieta J-K, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects μ-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 32.Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arc Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 33.Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 34.Ohara K, Nagai M, Suzuki Y, Ohara K. Low activity allele of catechol-o-methyltransferase gene and Japanese unipolar depression. NeuroReport. 1998;9:1261–1265. doi: 10.1097/00001756-199805110-00009. [DOI] [PubMed] [Google Scholar]
- 35.Hettema JM, An S-S, Bukszar J, et al. Catechol-o-methyltransferase contributes to genetic susceptibility shared among anxiety spectrum phenotypes. Biol Psychiatry. 2008;64:302–310. doi: 10.1016/j.biopsych.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massat I, Souery D, Del-Favero J, et al. Association between COMT(Val158Met) functional polymorphism and early onset in patients with major depressive disorder in a European multicenter genetic association study. Mol Psychiatry. 2005;10:598–605. doi: 10.1038/sj.mp.4001615. [DOI] [PubMed] [Google Scholar]
- 37.Cusin C, Serretti A, Lattuada E, et al. Association of MAO-A, COMT, 5-HT2A, DRD2, and DRD4 polymorphisms with illness time course in mood disorders. Am J Med Genet B Neuropsychiatr Genet. 2002;4:380–390. doi: 10.1002/ajmg.10358. [DOI] [PubMed] [Google Scholar]
- 38.Frisch A, Postilnick D, Rockah R, et al. Association of unipolar major depressive disorder with genes of the sertonergic and dopaminergic pathways. Mol Psychiatry. 1999;4:389–392. doi: 10.1038/sj.mp.4000536. [DOI] [PubMed] [Google Scholar]
- 39.Wray NR, James MR, Dumenil T, et al. Association study of candidate variants of COMT with neuroticism, anxiety and depression. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1314–1318. doi: 10.1002/ajmg.b.30744. [DOI] [PubMed] [Google Scholar]
- 40.Drabant EM, Hariri AR, Meyer-Lindenberg A, et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arc Gen Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- 41.Smolka MN, Schumann G, Wrase J, et al. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25(4):836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smolka MN, Bühler M, Schumann G, et al. Gene-gene effects on central processing of aversive stimuli. Mol Psychiatry. 2007;12:307–317. doi: 10.1038/sj.mp.4001946. [DOI] [PubMed] [Google Scholar]
- 43.Kienast T, Hariri AR, Schlagenhauf F, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nature Neurosci. 2008;11(12):1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- 44.Mandelli L, Serretti A, Marino E, et al. Interaction between serotonin transporter gene, catechol-O-methyltransferase gene and stressful life events in mood disorders. Int J Neuropsychopharmacol. 2007;10:437–447. doi: 10.1017/S1461145706006882. [DOI] [PubMed] [Google Scholar]
- 45.Keeping JD, Najman JM, Morrison J, et al. A prospective longitudinal study of social, psychological, and obstetrical factors in pregnancy: Response rates and demographic characteristics of the 8,556 respondents. Br J Obstet and Gynaecol. 1989;96:289–297. doi: 10.1111/j.1471-0528.1989.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 46.Bedford A, Foulds G. Delusions-Symptoms-States Inventory of Anxiety and Depression. Windsor, England: NFER; 1978. [Google Scholar]
- 47.Hammen C, Brennan PA. Depressed adolescents of depressed and nondepressed mothers: Tests of an interpersonal impairment hypothesis. J Consult Clin Psychol. 2001;69:284–294. doi: 10.1037//0022-006x.69.2.284. [DOI] [PubMed] [Google Scholar]
- 48.Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007;41(3):256–262. doi: 10.1016/j.jadohealth.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beck AT, Steer RA, Brown GK. The Beck Depression Inventory. 2. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 50.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical Interview for DSM-IV Axis I disorders. Washington, D.C: American Psychiatric Press; 1995. [Google Scholar]
- 51.Hammen C, Adrian C, Gordon D, et al. Children of depressed mothers: Maternal strain and symptom predictors of dysfunction. J Abnorm Psychol. 1987;96:190–198. doi: 10.1037//0021-843x.96.3.190. [DOI] [PubMed] [Google Scholar]
- 52.Hammen C, Brennan P, Keenan-Miller D. Patterns of adolescent depression to age 20: The role of maternal depression and youth interpersonal dysfunction. J Abnorm Child Psychol. 2008;36:1189–1198. doi: 10.1007/s10802-008-9241-9. [DOI] [PubMed] [Google Scholar]
- 53.Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. J Marriage Fam. 1976;38:15–28. [Google Scholar]
- 54.Neidig P, Friedman D. Spouse abuse: A treatment program for couples. Champaign, IL: Research Press; 1984. [Google Scholar]
- 55.Schludermann EH, Schludermann SM. Children’s Report of Parent Behavior (CRPBI-108, CRPBI-30) for older children and adolescents (Tech Rep) Winnipeg, Manitoba, Canada: University of Manitoba, Department of Psychology; 1988. [Google Scholar]
- 56.Wray NR, James MR, Gordon SD, et al. Accurate, large-scale genotyping of 5HTTLPR and flanking single nucleotide polymorphisms in an association study of depression, anxiety, and personality measures. Biol Psychiatry. 2009;66:468–476. doi: 10.1016/j.biopsych.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hariri AR, Holmes A. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10(4):182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Lazary J, Lazary A, Gonda X, et al. new evidence for the association of the serotonin transporter gene (SLC6A4) haplotypes, threatening life events, and depressive phenotype. Biol Psychiatry. 2008;64:498–504. doi: 10.1016/j.biopsych.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 59.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 60.Benjamini Y, Drai D, Elmer G, et al. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 61.Jabbi M, Korf J, Kema IP, et al. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Mol Psychiatry. 2007;12:483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- 62.Zangen A, Nakash R, Overstreet DH, Yadid G. Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology. 2001;155:434–439. doi: 10.1007/s002130100746. [DOI] [PubMed] [Google Scholar]
- 63.Brown GW, Harris TO. Depression and the serotonin transporter 5-HTTLPR polymorphism: A review and a hypothesis concerning gene-environment interaction. J Affect Disord. 2008;111:1–12. doi: 10.1016/j.jad.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Craddock N, Owen MJ, O’Donovan MC. The catechol-O-methyltransferase (COMT) gene as a candidate for psychiatric phenotypes: Evidence and lessons. Mol Psychiatry. 2006;11:446–458. doi: 10.1038/sj.mp.4001808. [DOI] [PubMed] [Google Scholar]
- 65.Sjöberg RL, Nilsson KW, Nordquist N, et al. Development of depression: Sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. Int J Neuropsychopharm. 2006;9:443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- 66.Hazel NA, Hammen C, Brennan P, Najman J. Continued stress exposure accounts for the association between early adversity and adolescent depression. Psychol Med. 2008;38:581–589. doi: 10.1017/S0033291708002857. [DOI] [PubMed] [Google Scholar]


